Abstract
Background
Surgical site infections (SSIs) are difficult to treat and are associated with substantially longer hospital stay, higher treatment cost, morbidity and mortality, particularly when the etiological agent is multidrug-resistant (MDR). To address the limited data in Uganda on SSIs, we present the spectrum of bacteria isolated from hospitalized patients, the magnitude and impact of MDR bacterial isolates among patients with SSIs.
Methods
A descriptive cross sectional study was conducted from September 2011 through April 2012 involving 314 patients with SSIs in the obstetrics & gynecology, general surgery and orthopedic wards at Mulago National Hospital in Kampala, Uganda. Wound swabs were taken and processed using standard microbiological methods. Clinico-demographic characteristics of patients were obtained using structured questionnaires and patients’ files.
Results
Of the 314 enrolled patients with SSIs (mean age 29.7 ±13.14 years), 239 (76.1%) were female. More than half of the patients were from obstetrics and gynecology (62.1%, 195/314). Of 314 wound swabs taken, 68.8% (216/314) were culture positive aerobically, yielding 304 bacterial isolates; of which 23.7% (72/304) were Escherichia coli and 21.1% (64/304) were Staphylococcus aureus. More than three quarters of Enterobacteriaceae were found to be extended spectrum beta lactamase (ESBL) producers and 37.5% of S. aureus were Methicillin resistant S. aureus (MRSA). MDR occurred in 78.3% (238/304) of the isolates; these were more among Gram-negative bacteria (78.6%, 187/238) compared to Gram-positive bacteria (21.4%, 51/238), (p-value < 0.0001, χ2 = 49.219). Amikacin and imepenem for ESBL-producing Enterobacteriacea and vancomycin for MRSA showed excellent performance except that they remain expensive drugs in Uganda.
Conclusion
Most SSIs at Mulago National Hospital are due to MDR bacteria. Isolation of MRSA and ESBL-producing Enterobacteriaceae in higher proportions than previously reported calls for laboratory guided SSIs- therapy and strengthening of infection control surveillance in this setting.
Keywords: Antimicrobial resistance, Surgical patients, Uganda
Background
Surgical site infections (SSIs) are the infections involving skin, subcutaneous tissue and organs/spaces opened or manipulated during an operation, occurring within 30 days after the procedure or within one year if orthopedic implant is in situ [1,2]. SSIs account for approximately 15.0% and rank third among all types of nosocomial infections in the USA [2,3]. Infection rates among operated patients varies with hospital settings reflecting infection control practices as well as factors related to the agent, environment and the host [4]; for example, infection rates reported were less than 3.0% in German and France, 16.9% in Rio de Janeiro, Brazil and 26.0% in Mwanza, Tanzania [5-8]. SSIs can involve any surgical procedure ranging from obstetrics & gynecology, general surgery and orthopedic [6,9,10].
The most predominant bacteria in hospital-acquired SSIs are Staphylococcus aureus, Enterococcus spp, Pseudomonas aeruginosa, Escherichia coli, and other Enterobacteriaceae; of these, single bacterial isolates are common whereas 9.0% to 27.0% of bacterial isolates from different surgical sites are attributed to polymicrobial [7-9,11]. These infections pose therapeutic challenges and are associated with substantially longer duration of hospital stay, increased hospital cost, higher morbidity and mortality [5,12], particularly when the agents are Methicillin resistant S. aureus (MRSA), Extended spectrum beta lactamase (ESBL) producing Enterobacteriaceae and/or other agents collectively referred to as multidrug-resistant (MDR) [11,13,14]. Studies from developing countries have shown high level of resistance (ranging from 50 to 100%) to the commonly used antibiotics like ampicillin, trimethoprim – sulphamethoxazole, gentamicin, chloramphenicol and third generation cephalosporins among S. aureus, E. coli, and P. aeruginosa[8,15] as opposed to low rates of resistance ranging from 0-50% in developed countries [16]. In both settings however, substantial rates of resistance to oxacillin, erythromycin and clindamycin reported for S. aureus, ranged from 10-60% [8,9,15,16] whereas vancomycin (for S. aureus and other Gram-positive bacteria), amikacin, piperacillin-tazobactam and imepenem (for E. coli, P. aeruginosa and other Gram-negative bacteria) showed resistant rates of less than 25% [17,18].
It is well known that specific therapeutic options to patients with SSIs are largely dependent on data from antimicrobial sensitivity tests generated by clinical laboratories or sound epidemiological data from ongoing nosocomial infection surveillance [6,11,19].
In Uganda, about 10% of the surgical procedures become septic accounting for an increasing morbidity and mortality, with the commonest organism isolated being S. aureus[20-22]; however data on the spectrum of bacteria isolated from hospitalized patients and their antimicrobial susceptibility patterns to guide SSI-therapy in Mulago National Hospital remains scanty. Furthermore, the magnitude and impact of MDR bacteria from SSIs are unknown. Thus, this study aimed at addressing these areas. Data herein will be crucial in guiding SSIs-therapy and will form a baseline for nosocomial SSIs surveillance.
Methods
Study design and sampling process
This descriptive cross-sectional study was conducted at Mulago National Hospital in Kampala, Uganda. The hospital is located on Mulago Hill in the northern part of the city of Kampala and is the largest hospital in Uganda with an estimated 1,500 beds.
The study was conducted for a period of 8 months from September 2011 to April 2012 and involved 314 patients with clinical SSIs who consented to participate. The patients were from obstetrics & gynecology, general surgery and orthopedic wards. All patients with SSIs occurring within 30 days after the operative procedure or within one year if orthopedic implant was in situ were included, whereas surgical patients with community-acquired pyogenic infections such as abscess, furuncle and carbuncles; patients with infection of an episiotomy; and patients with open fractures were excluded from the study.
Study clearance and ethical considerations
The study got ethical clearance from the Institutional Review Board (IRB) of Makerere University College of Health Sciences (# REC REF 2011–183), Mulago Hospital Research Committee (MREC #125) and the Uganda National Council for Science and Technology (UNCST) (REF # HS 1080). A written informed consent from each patient/caretaker and assent for minors (11 to 17years) were obtained whereas for each minor (<11 years), consent was obtained from his/her parent or caretaker. All patient information was kept confidential and anonymous using codes.
Data collection and laboratory procedures
Demographic and clinical characteristics from patients were collected using structured questionnaire and from patients’ files (see Additional file 1). The infected site was cleaned using normal saline and sterile gauze then, from each patient, two wound swabs were collected using sterile cotton swabs in Amies transport media (Biolab, HUNGARY®).
Isolate identification
Wound swabs were processed in the bacteriology laboratory of the Department of Medical Microbiology, Makerere University College of Health Sciences, within 2 hours of collection. The first wound swab was used to make Gram stain smears while the second one was inoculated into blood agar, MacConkey agar, and mannitol-salt agar and incubated at 35-37°C for 24–48 hours. Identification of bacteria was based on conventional physiological and biochemical methods such as Gram stain, catalase reaction, coagulase test, DNase test, hemolytic activity on sheep blood agar plates, bacitracin, optochin and trimethoprim-sulphamethoxazole (SXT) antimicrobial identification disks and bile esculin test for Gram-positive bacteria. Gram-negative bacteria were identified based on colony morphology on blood agar and MacConkey agar, followed by biochemical reactions namely oxidase, triple sugar iron (TSI), sulphur indole and motility (SIM), citrate, and urease tests [23].
Drug susceptibility tests
Following identification of the bacterial isolates, a standard disc diffusion technique for drug susceptibility test (DST) was performed as recommended by Clinical and Laboratory Standard Institute (CLSI) [24]. For Gram-positive bacteria, discs (Biolab®, HUNGARY) tested were ampicillin (10 μg), oxacillin (1 μg), trimethoprim-sulphamethoxazole (1.25/23.75 μg), tetracycline (30 μg), ciprofloxacin (5 μg), chloramphenicol (30 μg), gentamicin (10 μg) [high level gentamicin (120 μg) for Enterococcus spp], erythromycin (15 μg), clindamycin (2 μg), and vancomycin (30 μg). For Gram-negative bacteria discs (Biolab®, HUNGARY) tested included ampicillin (10 μg), piperacillin(100 μg), piperacillin-tazobactam (100/10 μg), amoxicillin-clavulanic acid (20/10 μg), trimethoprim-sulphamethoxazole (1.25/23.75 μg), tetracycline (30 μg), ciprofloxacin (5 μg), chloramphenicol (30 μg), gentamicin (10 μg), amikacin (30 μg), ceftriaxone (30 μg), ceftazidime (30 μg), cefepime (30 μg), and imipenem (10 μg). These were incubated at 35-37°C for 24 hours.
Isolates which were not identifiable by the standard conventional methods, colistin DST for all Acinetobacter spp and P. aeruginosa as well as vancomycin DST for all S. aureus were confirmed using the Phoenix Automated instrument® (Becton-Dickson, Sparks Maryland) as per manufacturer’s instruction.
For determining inducible clindamycin resistance, clindamycin disk (2 μg) and erythromycin disk (15 μg) were placed side by side approximately 15-26 mm apart. Flattening of the zone of inhibition adjacent to the erythromycin disk was regarded as a positive D-test. As recommended by CLSI, isolates were screened for ESBL production using the double disc method and MRSA was identified by the use of cefoxitin disc (30 μg) [24,25]. MDR was defined as an isolate with resistance to three or more antimicrobial classes [26].
Results on isolate identity and antimicrobial susceptibility patterns were promptly reported to the attending doctor for patient care.
Quality control
Reference strains S. aureus ATCC 25923 and Staphylococcus epidermidis ATCC 12228 for Gram-positive bacteria and E. coli ATCC 25922 and P. aeruginosa ATCC 27853 for Gram-negative bacteria were used to quality-control microbiological procedures such as Gram staining , growth of bacteria on respective media, microscopy, biochemical identification tests and drug susceptibility testing.
Data analysis
Variables from the clinical and demographic data in the questionnaire and laboratory data were entered into Excel®, cleaned and exported to STATA software version 11 (College Station, Texas, USA) for analysis according to the objectives of the study. Continuous variables were described as mean (± standard deviation). Categorical variables were described as proportion and were analyzed to compare the significance of difference in distribution by using Chi square test or Fischer’s exact test where appropriate. To determine factors associated with bacteria isolation from SSI, we used univariate followed by multivariate logistic regression analysis. At univariate level all factors which had a p-value of less than 0.05 were subjected to multivariate analysis. The strength of association between factors and outcome was measured using odds ratio with respective 95% confidence interval. Factors with p-value of less than 0.05 on multivariate logistic regression analysis were considered as independent association of bacteria isolation from SSI.
Results
This study enrolled 314 patients with clinical SSIs. Among these, 239 (76.1%) were female. The overall mean age was 29.7 ± 13.14 years (minimum 12 and maximum 83 years). More than half of the patients were from obstetrics and gynecology wards, 62.1% (195/314), whereas 33.1% (104/314) and 4.8% (15/314) were from general surgery and orthopedic wards respectively. The most common surgical procedures were caesarean section 46.2% (145/314) and laparotomy 42.7% (134/314); open reduction and internal fixation (ORIF) accounted for 3.5% (11/314) while other surgical procedures contributed 7.6% (24/314).
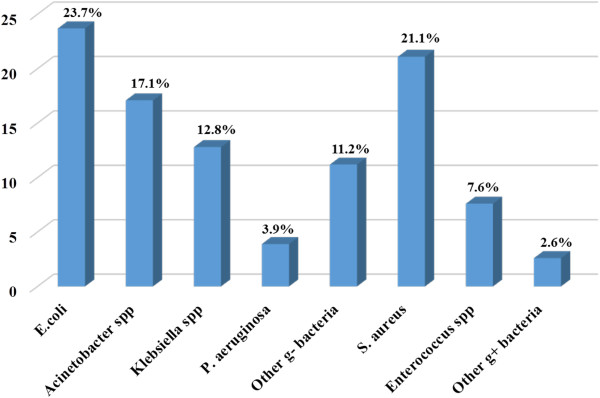
Of the 314 non-repeat wound swabs collected, 216 (68.8%) were culture positive aerobically. The most predominant bacterial isolates were E. coli, 23.7% (72/304) and S. aureus, 21.1% (64/304) (see Figure 1). Single bacterial isolates were recovered from 137 (63.4%) patients whereas 79 (36.6%) had polymicrobial infections.
Figure 1.
Proportion of bacterial isolates from patients with surgical site infections (N = 304). Other g- bacteria: Enterobacter cloacae (10), Proteus mirabilis (7), Morganella morganii (6), Providencia spp (5), Citrobacter freundii (4), Serratia marcescens (1) and Leclercia adecarboxylata (1); Other g + bacteria: Streptococcus pyogenes (3), Streptococcus agalactiae (1), and Streptococcus spp (4).Acinetobacter spp: Acinetobacter baumanii (48) and Acinetobacter baumanii-calcoaceticus complex (4); Klebsiella spp: Klebsiella pneumonia (35) and Klebsiella oxytoca (4); Enterococcus spp: Enterococcus faecalis (21) and Enterococcus faecium (2).
All P. aeruginosa isolates were sensitive to colistin, whereas 3.9% (2/52) of Acinetobacter spp were resistant to colistin. All Gram-negative bacteria were susceptible to imipemen, except 3.9% (2/52) of Acinetobacter spp. All S. aureus isolates were sensitive to vancomycin, whereas one Enterococcus spp [4.4% (1/23)] was resistant to vancomycin i.e. VRE (see Table 1).
Table 1.
Antimicrobial resistance pattern among isolates from surgical site infections
| Antibiotics |
Bacteria isolates (N = 304) |
|||||||
|---|---|---|---|---|---|---|---|---|
| E.coli (n = 72) | Klebsiella spp (n = 39) | Acinetobacter spp (n = 52) | P.aeruginosa (n = 12) | Other g- (n = 34) | S. aureus (n = 64) | Enterococcus spp (n = 23) | Other g + (n = 08) | |
|
Ampicillin |
100.0% |
100.0% |
NA |
NA |
97.1% |
100.0% |
30.0% |
50.0% |
|
Piperacillin |
NA |
NA |
100.0% |
66.7% |
NA |
NA |
NA |
NA |
|
TZP* |
30.6% |
38.5% |
53.8% |
16.7% |
32.4% |
NA |
NA |
NA |
|
AMC+ |
90.3% |
100.0% |
NA |
NA |
94.1% |
NA |
NA |
NA |
|
TMP-SMX × |
86.1% |
92.3% |
98.0% |
100.0% |
94.1% |
89.1% |
NA |
80.0% |
|
Tetracycline |
72.2% |
76.9% |
65.4% |
100.0% |
82.4% |
42.2% |
74.0% |
62.5% |
|
Ciprofloxacin |
72.2% |
66.6% |
77.0% |
16.7% |
47.1% |
29.7% |
60.9% |
50.0% |
|
Chloramphenicol |
41.7% |
71.8% |
NA |
NA |
70.6% |
15.6% |
30.4% |
37.5% |
|
Gentamicin |
54.2% |
76.9% |
88.5% |
16.7% |
76.5% |
18.8% |
21.7%** |
50.0% |
|
Amikacin |
4.2% |
2.6% |
32.7% |
8.3% |
0.0% |
NA |
NA |
NA |
|
Ceftriaxone |
77.8% |
92.3% |
NA |
NA |
67.6% |
NA |
NA |
NA |
|
Ceftazidime |
77.8% |
87.2% |
90.4% |
25.0% |
61.8% |
NA |
NA |
NA |
|
Cefepime |
69.4% |
87.2% |
61.5% |
16.7% |
38.2% |
NA |
NA |
NA |
|
Imipenem |
0.0% |
0.0% |
3.9% |
0.0% |
0.0% |
NA |
NA |
NA |
|
Erythromycin |
NA |
NA |
NA |
NA |
NA |
46.9 % |
65.2% |
50.0% |
|
Clindamycin |
NA |
NA |
NA |
NA |
NA |
40.6% |
NA |
62.5% |
| Vancomycin | NA | NA | NA | NA | NA | 0.0% | 4.4% | 0.0% |
*Piperacillin-tazobactam, +Amoxicillin-clavulanate, ×Trimethoprim-sulphamethoxazole, ** High level resistance screening, NA: Not applicable, spp: species, g+: Gram-positive bacteria [Streptococcus pyogenes (3), Streptococcus agalactiae (1), and Streptococcus spp (4)] and g-: Gram-negative bacteria [Enterobacter cloacae (10), Proteus mirabilis (7), Morganella morganii (6), Providencia spp (5), Citrobacter freundii (4), Serratia marcescens (1) and Leclercia adecarboxylata (1)].
More than three quarters of Enterobacteriaceae were phenotypically found to be ESBL producers [E. coli, 79.2% (57/72); Klebsiella spp, 92.3% (36/39) and other Enterobacteriaceae, 73.5% (25/34)]. The proportion of MRSA among S. aureus isolates was 37.5% (24/64).
Of the 304 isolates recovered from SSIs, 78.3% (238/304) were MDR, these were found significantly more among Gram-negative bacteria (78.6%, 187/238) compared to Gram-positive bacteria (21.4%, 51/238) (p-value < 0.0001, χ2 = 49.219).
In the univariate analysis, bacterial isolation from SSIs among patients was associated with age greater than 40 years, sex, ward type, operation (whether elective or emergency), duration from admission to operation, duration from admission to discharge, type of antibiotic chemoprophylaxis given, duration of postoperative antibiotics and outcome (see Table 2).
Table 2.
Association of clinico-demographic characteristics of patients with and without bacterial isolates from surgical site infections
| Variable | Bacteria isolated from SSI (n = 216) | No bacteria isolated from SSI (n = 98) | OR [95% CI] | P-value | |
|---|---|---|---|---|---|
|
Age (years) |
≤ 19 |
38 (67.9%) |
18 (32.1%) |
1 |
|
|
20 – 39 |
133 (63.6%) |
76 (36.4%) |
0.83(0 .42- 1.61) |
0.5577 |
|
|
40 – 59 |
31 (91.2%) |
3 (8.8%) |
4.89( 1.24- 27.87) |
0.0112 |
|
|
≥ 60 |
14 (93.3%) |
1 (6.7%) |
6.63( 0.86- 295.67) |
0.0478 |
|
|
Sex |
Male |
61 (81.3%) |
14 (18.7%) |
1 |
|
|
Female |
155 (64.9%) |
84 (35.1%) |
0.42(0 .21- 0.82) |
0.0072 |
|
|
Ward type |
Obst & Gyn |
115 (59.0%) |
80 (41%) |
1 |
|
|
General Surgery |
89 (85.6%) |
15 (14.4%) |
4.13( 2.17- 8.22) |
0.0000 |
|
|
Orthopedic |
12 (80.0%) |
3 (20.0%) |
2.78(0.72- 15.79) |
0.1085 |
|
|
Operation |
Elective |
57 (83.8%) |
11 (16.2%) |
1 |
|
|
Emergency |
159 (64.6%) |
87 (35.4%) |
0.35( 0.16- 0.73) |
0.0025 |
|
|
Duration from admission to operation |
≤ 1 day |
147 (65.3%) |
78 (34.7%) |
1 |
|
|
> 1 days |
69 (77.5%) |
20 (22.5%) |
1.83( 1.01- 3.42) |
0.0356 |
|
|
Duration from admission to discharge |
≤ 14days |
48 (58.5%) |
34 (41.5%) |
1 |
|
|
> 14days |
110 (82.7%) |
23 (17.3%) |
3.39( 1.73- 6.68) |
0.0001 |
|
|
Antibiotic chemoprophylaxis |
CRO* |
124 (62.9%) |
73 (37.1%) |
1 |
|
|
CRO* + MZ** |
70 (76.9%) |
21 (23.1%) |
1.96( 1.08- 3.65) |
0.0187 |
|
|
Others× |
22 (84.6%) |
4 (15.4%) |
3.24( 1.04- 13.37) |
0.0289 |
|
|
Duration of Postoperative antibiotics |
≤ 3days |
48 (50.5%) |
47 (49.5%) |
1 |
|
|
> 3days |
168 (76.7%) |
51 (23.3%) |
3.23( 1.87- 5.54) |
0.0001 |
|
|
Outcome |
Improved |
147 (72.1%) |
57 (27.9%) |
1 |
|
| Died | 10 (100.0%) | 0 (0.0%) | - | 0.042 | |
*Ceftriaxone, **Metronidazole, ×Ampicloxacillin, Ciprofloxacin & Gentamicin.
On multivariate logistic regression analysis, longer duration from admission to discharge, longer duration of postoperative antibiotics and outcome were statistically associated with bacteria isolation from SSIs (see Table 3). Of ten patients who died, 70% (7/10) had ESBL producing Enterobacteriaceae, four of which were E. coli.
Table 3.
Multivariate logistic regression analysis for factors associated with bacterial isolation from SSIs
| Variable | OR | 95% CI | p-value |
|---|---|---|---|
| Age (years) |
1.16 |
0.74 - 1.80 |
0.524 |
| Sex |
1.41 |
0.49 - 4.02 |
0.522 |
| Wards |
0.44 |
0.18 - 1.10 |
0.078 |
| Operation |
0.62 |
0.24 - 1.65 |
0.343 |
| Duration from admission to operation |
0.89 |
0.40 - 1.97 |
0.773 |
| Duration from admission to discharge |
1.93 |
1.01 - 3.72 |
0.047 |
| Antibiotic chemoprophylaxis |
1.27 |
0.77 - 2.12 |
0.351 |
| Duration of postoperative antibiotics |
2.52 |
1.45 - 4.36 |
0.001 |
| Outcome | 0.52 | 0.30 - 0.91 | 0.021 |
Discussion
Of the 314 patients with clinical SSIs enrolled in this study; the mean age (29.7 ± 13.14 years), higher female proportion (76.1%) and preponderance of admission in obstetrics and gynecology wards (62.1%) was similar to another study [11], but the proportion of female in other studies were between 30% to 60% and wards of admission varied reflecting the underlying surgical procedures [8,9,13]. Caesarean section and laparotomy accounted for more than three quarters of all surgical procedures in this study whereas other similar studies have also found these procedures to be quite common [8,11,27]. The predominance of SSIs cases in obstetrics and gynecology wards is quite alarming and thus, a need to institute stringent infection prevention and control measures in this setting, more especially in emergency surgeries which accounted for more SSIs cases as opposed to elective surgeries.
As noted from other studies [8,9,11,28], S. aureus and E. coli were the most common bacterial isolates from SSIs. However their sources remain unknown from the present study though other studies have documented both endogenous and exogenous sources from hospital environment could be potential niches [29,30]. Similar to other related studies [9,18,31], high level of resistance was found among commonly used antibiotics like ampicillin, trimethoprim-sulphamethoxazole, and tetracycline in both Gram-positive and Gram-negative bacteria. Gram-negative bacteria showed more resistance to gentamicin, ciprofloxacin and chloramphenicol as compared to Gram-positive bacteria. With exception of Acinetobacter spp, all Gram-negative bacteria displayed low resistance rates to piperacillin-tazobactam, amikacin and imipenem. There were also low resistance rates of Acinetobacter spp and P. aeruginosa to colistin. These findings are similar to another study [17]. The present study has shown that the rates of resistance to erythromycin (46.9%) among S. aureus was relatively low compared to that of Enterococcus spp (65.2%), with excellent performance of vancomycin on both S. aureus and Enterococcus spp. These findings are in agreement to another study [18]. This study found more MDR among Gram-negative bacteria than Gram-positive bacteria; of these the proportion of MRSA (37.5%) among S. aureus isolates was more than previously reported (25.0% and 31.5%) from Uganda [9,21] and other countries [8,16,18]. Thus, while β-lactamase-resistant antibiotics such as cloxacillin could still be effective in this setting, they are likely to be ineffective against the 38% of isolates that were confirmed as MRSA. The fact that we found no vancomycin resistance among S. aureus isolates shows that this drug remains the last resort in systemic infections caused by MRSA in this setting. Previous studies [11,17] have shown low rates (14% to 22%) of ESBL-producers among Enterobacteriaceae isolates but the present study and another similar study in the same region [8] have shown ESBL-producers to account more than three quarter of Enterobacteriaceae. This can be attributed to the empirical use of third generation cephalosporins (usually in combination with another drug such as gentamicin) in almost all hospitalized patients and lack of antimicrobial resistance surveillance in surgical wards at Mulago National Hospital. This is a major threat to patient care as ESBL production renders use of these ceftriaxone or ceftazidime useless. Absence of resistance to imepenem among these isolates is however a good finding, except that imepenem remains an expensive drug.
Multivariate logistic regression analysis of clinical and demographic characteristics of patients with SSIs in this study showed that longer duration from admission to discharge, longer duration of postoperative antibiotics and outcome (death) were associated with bacteria isolation from SSIs. These findings have also been shown in other similar studies [5,11,27].
Limitation
The study did not isolate strict anaerobes, which could have increased the number of bacterial isolates currently reported as negative cultures. This was because of lack of standardized in-house detection methods and lack of anaerobic detection panels in the Phoenix Automated instrument (Becton-Dickson, Sparks Maryland) that we used.
Conclusion
Most SSIs at Mulago National Hospital are due to MDR bacteria, these are significantly more among Gram-negative than Gram-positive bacteria. Isolation of MRSA and ESBL-producing Enterobacteriaceae in higher proportions than previously reported calls for enhanced antibiotic stewardship including laboratory guided SSIs-therapy and strengthening of infection control surveillance by identifying sources of these MDR isolates. In the light of these findings, there is a need to investigate whether there is clonal spread of the predominant bacteria within/or among surgical wards at Mulago National Hospital.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
Conceived and designed the experiments: JS, DPK, and FB. Specimen collection: JS. Supervised the clinical component of research: PM. Performed the experiments: JS. Supervised the laboratory component of research: CFN, DPK, and FB. Analyzed the data: JS, DPK, AK, and FB. Contributed reagents, materials and analysis tools: JS, CFN, HK, and MLJ. Wrote the manuscript: JS, CFN, DPK, PM, MLJ, HK, AK, and FB. All authors have read and approved the final manuscript.
Supplementary Material
Appendix i. Questionnaire.
Contributor Information
Jeremiah Seni, Email: senijj80@gmail.com.
Christine F Najjuka, Email: najjukafc@gmail.com.
David P Kateete, Email: davidkateete@gmail.com.
Patson Makobore, Email: patsonmakobore@yahoo.com.
Moses L Joloba, Email: moses.joloba@case.edu.
Henry Kajumbula, Email: jumbic@hotmail.com.
Antony Kapesa, Email: anthony1kapesa@gmail.com.
Freddie Bwanga, Email: fxb18@case.edu.
Acknowledgements
The authors would like to thank patients and health workers in all surgical wards; Emmanuel Aboce, Tonny Lugya, and Hannington Baluku for excellent technical assistance; Willy Ssengooba for statistical inputs, and all staffs in the Department of Medical Microbiology for their support. This work was funded by Catholic University of Health and Allied Sciences Bugando, Mwanza-Tanzania to JS.
References
- Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Am J Infect Control. 1992;20(5):271–274. doi: 10.1016/S0196-6553(05)80201-9. [DOI] [PubMed] [Google Scholar]
- Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control. 1999;27(2):97–132. doi: 10.1016/S0196-6553(99)70088-X. quiz 133–134; discussion 196. [DOI] [PubMed] [Google Scholar]
- Emori TG, Gaynes RP. An overview of nosocomial infections, including the role of the microbiology laboratory. Clin Microbiol Rev. 1993;6(4):428–442. doi: 10.1128/cmr.6.4.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiano G, Pezzolla A, Filograna MA, Ferrarese F. [Risk factors of surgical wound infection] Ann Ital Chir. 2004;75(1):11–16. [PubMed] [Google Scholar]
- Astagneau P, Rioux C, Golliot F, Brucker G. Morbidity and mortality associated with surgical site infections: results from the 1997–1999 INCISO surveillance. J Hosp Infect. 2001;48(4):267–274. doi: 10.1053/jhin.2001.1003. [DOI] [PubMed] [Google Scholar]
- Barwolff S, Sohr D, Geffers C, Brandt C, Vonberg RP, Halle H, Ruden H, Gastmeier P. Reduction of surgical site infections after Caesarean delivery using surveillance. J Hosp Infect. 2006;64(2):156–161. doi: 10.1016/j.jhin.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Santos KR, Fonseca LS, Bravo Neto GP, Gontijo Filho PP. Surgical site infection: rates, etiology and resistance patterns to antimicrobials among strains isolated at Rio de Janeiro University Hospital. Infection. 1997;25(4):217–220. doi: 10.1007/BF01713147. [DOI] [PubMed] [Google Scholar]
- Mawalla B, Mshana SE, Chalya PL, Imirzalioglu C, Mahalu W. Predictors of surgical site infections among patients undergoing major surgery at Bugando Medical Centre in Northwestern Tanzania. BMC Surgery. 2011;11:21. doi: 10.1186/1471-2482-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anguzu JR, Olila D. Drug sensitivity patterns of bacterial isolates from septic post-operative wounds in a regional referral hospital in Uganda. Afr Health Sci. 2007;7(3):148–154. doi: 10.5555/afhs.2007.7.3.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse JD, Friedman ND, Kirkland KB, Richardson WJ, Sexton DJ. The impact of surgical-site infections following orthopedic surgery at a community hospital and a university hospital: adverse quality of life, excess length of stay, and extra cost. Infect Control Hosp Epidemiol. 2002;23(4):183–189. doi: 10.1086/502033. [DOI] [PubMed] [Google Scholar]
- Fehr J, Hatz C, Soka I, Kibatala P, Urassa H, Smith T, Mshinda H, Frei R, Widmer A. Risk factors for surgical site infection in a Tanzanian district hospital: a challenge for the traditional National Nosocomial Infections Surveillance system index. Infect Control Hosp Epidemiol. 2006;27(12):1401–1404. doi: 10.1086/509855. [DOI] [PubMed] [Google Scholar]
- Kirkland KB, Briggs JP, Trivette SL, Wilkinson WE, Sexton DJ. The impact of surgical-site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol. 1999;20(11):725–730. doi: 10.1086/501572. [DOI] [PubMed] [Google Scholar]
- Engemann JJ, Carmeli Y, Cosgrove SE, Fowler VG, Bronstein MZ, Trivette SL, Briggs JP, Sexton DJ, Kaye KS. Adverse clinical and economic outcomes attributable to methicillin resistance among patients with Staphylococcus aureus surgical site infection. Clin Infect Dis. 2003;36(5):592–598. doi: 10.1086/367653. [DOI] [PubMed] [Google Scholar]
- Wassef MA, Hussein A, Abdul Rahman EM, El-Sherif RH. A prospective surveillance of surgical site infections: study for efficacy of preoperative antibiotic prophylaxis. African Journal of Microbiology Research. 2012;6(12):3072–3078. [Google Scholar]
- Amare B, Abdurrahman Z, Moges B, Ali J, Muluken L, Alemayehu M, Yifru S, Sendek B, Belyhun YFM. et al. Postoperative surgical site bacterial infections and drug susceptibility patterns at Gondar University Teaching Hospital, Northwest Ethiopia. J Bacteriol Parasitol. 2011;2:126. [Google Scholar]
- Throckmorton AD, Baddour LM, Hoskin TL, Boughey JC, Degnim AC. Microbiology of surgical site infections complicating breast surgery. Surg Infect (Larchmt) 2010;11(4):355–359. doi: 10.1089/sur.2009.029. [DOI] [PubMed] [Google Scholar]
- Hawser SP, Bouchillon SK, Hoban DJ, Badal RE. Epidemiologic trends, occurrence of extended-spectrum beta-lactamase production, and performance of ertapenem and comparators in patients with intra-abdominal infections: analysis of global trend data from 2002–2007 from the SMART study. Surg Infect (Larchmt) 2010;11(4):371–378. doi: 10.1089/sur.2009.057. [DOI] [PubMed] [Google Scholar]
- Kownhar H, Shankar EM, Vignesh R, Sekar R, Velu V, Rao UA. High isolation rate of Staphylococcus aureus from surgical site infections in an Indian hospital. J Antimicrob Chemother. 2008;61(3):758–760. doi: 10.1093/jac/dkm519. [DOI] [PubMed] [Google Scholar]
- Krukerink M, Kievit J, de Mheen PJ M-v. Evaluation of routinely reported surgical site infections against microbiological culture results: a tool to identify patient groups where diagnosis and treatment may be improved. BMC Infect Dis. 2009;9:176. doi: 10.1186/1471-2334-9-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olaro C. Study to assess the risk factors for postoperative complications following abdominal surgery in Mulago Hospital. Kampala: Makerere University; 1999. [Google Scholar]
- Ojulong J, Mwambu TP, Joloba M, Bwanga F, Kaddu-Mulindwa DH. Relative prevalence of methicilline resistant Staphylococcus aureus and its susceptibility pattern in Mulago Hospital, Kampala, Uganda. Tanzan J Health Res. 2009;11(3):149–153. doi: 10.4314/thrb.v11i3.47703. [DOI] [PubMed] [Google Scholar]
- Kitara DL, Kakande I, Mugisa BD, Obol JH. The postoperative complications prediction in Mulago Hospital using POSSUM scoring system. East and Central African Journal of Surgery. 2010;15(2):90–96. [Google Scholar]
- Koneman EW, Allen SD, Janda WM, Schreckenberger PC, Winn WC. . 5. Philadelphia, Pa: Lippincott, Williams & Wilkins Publishers; 1997. Color atlas and textbook of diagnostic microbiology. [Google Scholar]
- CLSI. Perfomance standars for antimicrobial susceptibility testing; twenty first information supplement, vol. CLSI document M100-S21. Wayne, PA: Clinical and Laboratory Standards Institute; 2011. [Google Scholar]
- Livermore DM, Brown DF. Detection of beta-lactamase-mediated resistance. J Antimicrob Chemother. 2001;48(Suppl 1):59–64. doi: 10.1093/jac/48.suppl_1.59. [DOI] [PubMed] [Google Scholar]
- D'Agata EM. Rapidly rising prevalence of nosocomial multidrug-resistant, F-negative bacilli: a 9-year surveillance study. Infect Control Hosp Epidemiol. 2004;25(10):842–846. doi: 10.1086/502306. [DOI] [PubMed] [Google Scholar]
- Razavi SM, Ibrahimpoor M, Sabouri Kashani A, Jafarian A. Abdominal surgical site infections: incidence and risk factors at an Iranian teaching hospital. BMC Surg. 2005;5:2. doi: 10.1186/1471-2482-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasatpibal N, Jamulitrat S, Chongsuvivatwong V. Standardized incidence rates of surgical site infection: a multicenter study in Thailand. Am J Infect Control. 2005;33(10):587–594. doi: 10.1016/j.ajic.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Wenzel RP, Perl TM. The significance of nasal carriage of Staphylococcus aureus and the incidence of postoperative wound infection. J Hosp Infect. 1995;31(1):13–24. doi: 10.1016/0195-6701(95)90079-9. [DOI] [PubMed] [Google Scholar]
- Atata RF Ibrahim YKE Olurinola PF Giwa A Akanbi AA II Sani AA Clinical bacterial isolates from hospital environment as agents of surgical wound nosocomial infections Journal of Pharmacy & Bioresources 201072146–155.23898072 [Google Scholar]
- Adegoke AA, Mvuyo T, Okoh AI, Steve J. Studies on multiple antibiotic resistant bacterial isolated from surgical site infection. Scientific Research and Essays. 2010;5(24):3876–3881. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix i. Questionnaire.