Abstract
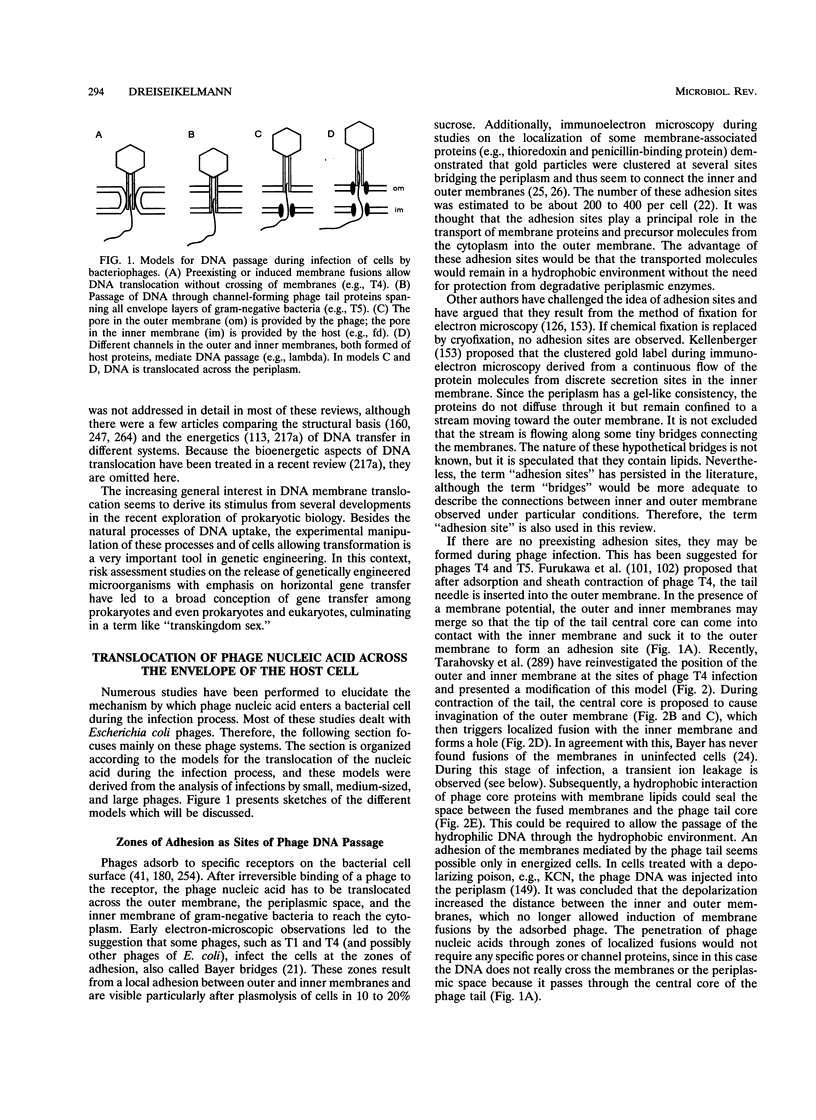
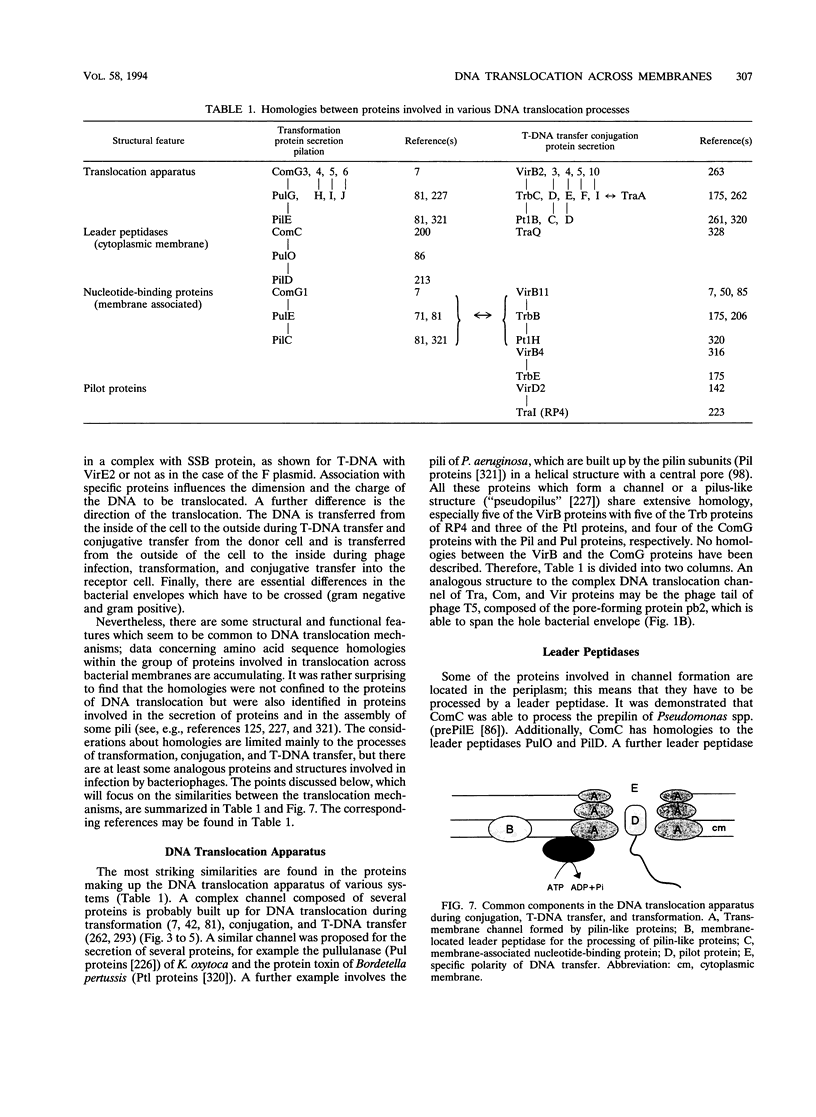
DNA translocation across bacterial membranes occurs during the biological processes of infection by bacteriophages, conjugative DNA transfer of plasmids, T-DNA transfer, and genetic transformation. The mechanism of DNA translocation in these systems is not fully understood, but during the last few years extensive data about genes and gene products involved in the translocation processes have accumulated. One reason for the increasing interest in this topic is the discussion about horizontal gene transfer and transkingdom sex. Analyses of genes and gene products involved in DNA transfer suggest that DNA is transferred through a protein channel spanning the bacterial envelope. No common model exists for DNA translocation during phage infection. Perhaps various mechanisms are necessary as a result of the different morphologies of bacteriophages. The DNA translocation processes during conjugation, T-DNA transfer, and transformation are more consistent and may even be compared to the excretion of some proteins. On the basis of analogies and homologies between the proteins involved in DNA translocation and protein secretion, a common basic model for these processes is presented.
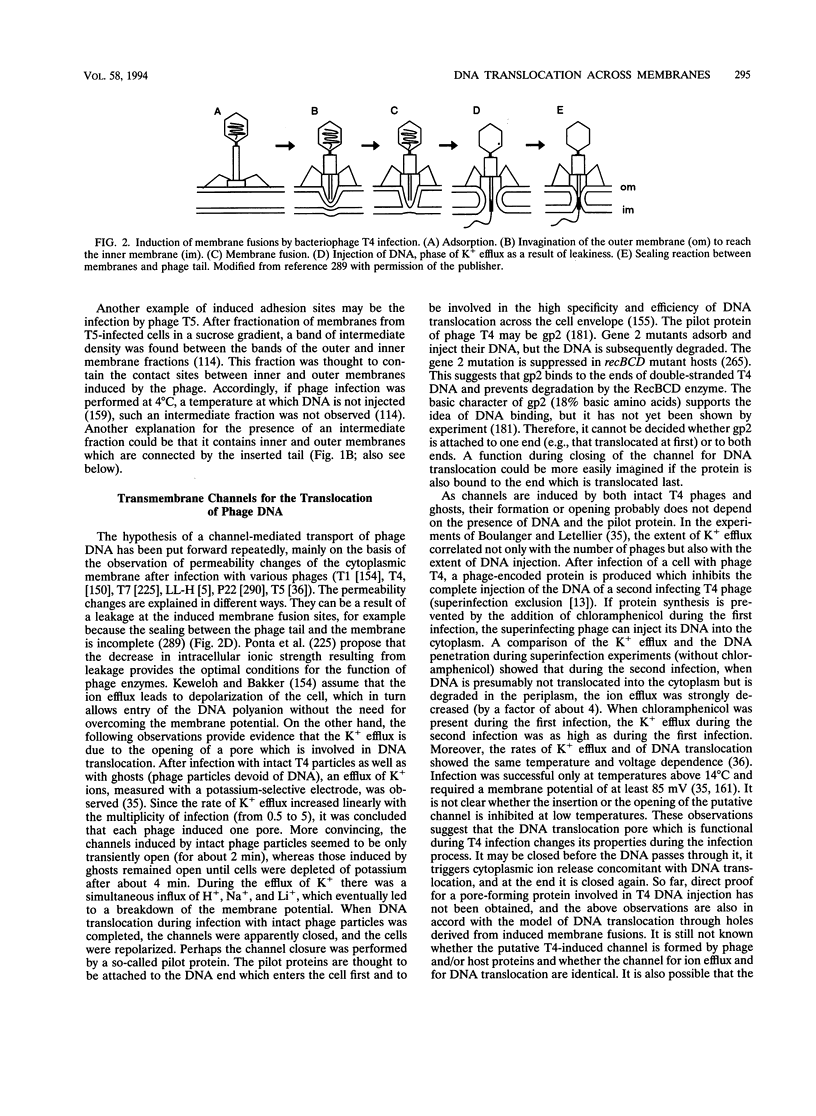
Full text
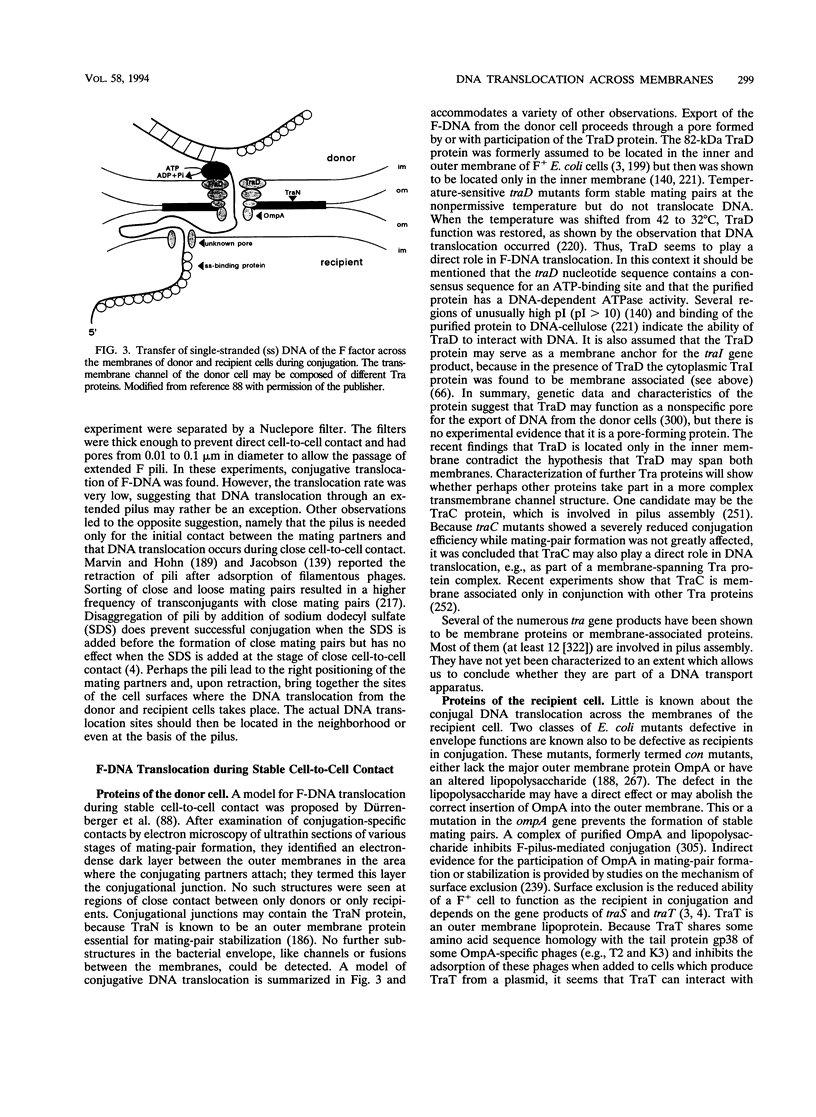
PDF



















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON T. F. Recombination and segregation in Escherichia coli. Cold Spring Harb Symp Quant Biol. 1958;23:47–58. doi: 10.1101/sqb.1958.023.01.007. [DOI] [PubMed] [Google Scholar]
- Abdel-Monem M., Taucher-Scholz G., Klinkert M. Q. Identification of Escherichia coli DNA helicase I as the traI gene product of the F sex factor. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4659–4663. doi: 10.1073/pnas.80.15.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abo T., Inamoto S., Ohtsubo E. Specific DNA binding of the TraM protein to the oriT region of plasmid R100. J Bacteriol. 1991 Oct;173(20):6347–6354. doi: 10.1128/jb.173.20.6347-6354.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achtman M., Manning P. A., Edelbluth C., Herrlich P. Export without proteolytic processing of inner and outer membrane proteins encoded by F sex factor tra cistrons in Escherichia coli minicells. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4837–4841. doi: 10.1073/pnas.76.10.4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achtman M., Morelli G., Schwuchow S. Cell-cell interactions in conjugating Escherichia coli: role of F pili and fate of mating aggregates. J Bacteriol. 1978 Sep;135(3):1053–1061. doi: 10.1128/jb.135.3.1053-1061.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alatossava T., Jütte H., Seiler H. Transmembrane cation movements during infection of Lactobacillus lactis by bacteriophage LL-H. J Gen Virol. 1987 Jun;68(Pt 6):1525–1532. doi: 10.1099/0022-1317-68-6-1525. [DOI] [PubMed] [Google Scholar]
- Albano M., Breitling R., Dubnau D. A. Nucleotide sequence and genetic organization of the Bacillus subtilis comG operon. J Bacteriol. 1989 Oct;171(10):5386–5404. doi: 10.1128/jb.171.10.5386-5404.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albano M., Dubnau D. A. Cloning and characterization of a cluster of linked Bacillus subtilis late competence mutations. J Bacteriol. 1989 Oct;171(10):5376–5385. doi: 10.1128/jb.171.10.5376-5385.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albano M., Hahn J., Dubnau D. Expression of competence genes in Bacillus subtilis. J Bacteriol. 1987 Jul;169(7):3110–3117. doi: 10.1128/jb.169.7.3110-3117.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albright L. M., Yanofsky M. F., Leroux B., Ma D. Q., Nester E. W. Processing of the T-DNA of Agrobacterium tumefaciens generates border nicks and linear, single-stranded T-DNA. J Bacteriol. 1987 Mar;169(3):1046–1055. doi: 10.1128/jb.169.3.1046-1055.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alt-Moerbe J., Rak B., Schröder J. A 3.6-kbp segment from the vir region of Ti plasmids contains genes responsible for border-sequence-directed production of T region circles in E. coli. EMBO J. 1986 Jun;5(6):1129–1135. doi: 10.1002/j.1460-2075.1986.tb04337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alt-Mörbe J., Heinemeyer W., Schröder J. The virD genes from the vir region of the Ti plasmid: T-region border dependent processing steps in different rec mutants of Escherichia coli. Gene. 1990 Nov 30;96(1):43–49. doi: 10.1016/0378-1119(90)90339-s. [DOI] [PubMed] [Google Scholar]
- Amábile-Cuevas C. F., Chicurel M. E. Bacterial plasmids and gene flux. Cell. 1992 Jul 24;70(2):189–199. doi: 10.1016/0092-8674(92)90095-t. [DOI] [PubMed] [Google Scholar]
- Anderson C. W., Eigner J. Breakdown and exclusion of superinfecting T-even bacteriophage in Escherichia coli. J Virol. 1971 Dec;8(6):869–886. doi: 10.1128/jvi.8.6.869-886.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arwert F., Venema G. Transformation in Bacillus subtilis. Fate of newly introduced transforming DNA. Mol Gen Genet. 1973;123(2):185–198. doi: 10.1007/BF00267334. [DOI] [PubMed] [Google Scholar]
- Bakkeren G., Koukolíková-Nicola Z., Grimsley N., Hohn B. Recovery of Agrobacterium tumefaciens T-DNA molecules from whole plants early after transfer. Cell. 1989 Jun 2;57(5):847–857. doi: 10.1016/0092-8674(89)90799-x. [DOI] [PubMed] [Google Scholar]
- Barany F., Kahn M. E. Comparison of transformation mechanisms of Haemophilus parainfluenzae and Haemophilus influenzae. J Bacteriol. 1985 Jan;161(1):72–79. doi: 10.1128/jb.161.1.72-79.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barany F., Kahn M. E., Smith H. O. Directional transport and integration of donor DNA in Haemophilus influenzae transformation. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7274–7278. doi: 10.1073/pnas.80.23.7274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouki R., Smith H. O. Reexamination of phenotypic defects in rec-1 and rec-2 mutants of Haemophilus influenzae Rd. J Bacteriol. 1985 Aug;163(2):629–634. doi: 10.1128/jb.163.2.629-634.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battisti L., Green B. D., Thorne C. B. Mating system for transfer of plasmids among Bacillus anthracis, Bacillus cereus, and Bacillus thuringiensis. J Bacteriol. 1985 May;162(2):543–550. doi: 10.1128/jb.162.2.543-550.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer M. E. Adsorption of bacteriophages to adhesions between wall and membrane of Escherichia coli. J Virol. 1968 Apr;2(4):346–356. doi: 10.1128/jvi.2.4.346-356.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer M. E. Areas of adhesion between wall and membrane of Escherichia coli. J Gen Microbiol. 1968 Oct;53(3):395–404. doi: 10.1099/00221287-53-3-395. [DOI] [PubMed] [Google Scholar]
- Bayer M. E., Bayer M. H., Lunn C. A., Pigiet V. Association of thioredoxin with the inner membrane and adhesion sites in Escherichia coli. J Bacteriol. 1987 Jun;169(6):2659–2666. doi: 10.1128/jb.169.6.2659-2666.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer M. E. Zones of membrane adhesion in the cryofixed envelope of Escherichia coli. J Struct Biol. 1991 Dec;107(3):268–280. doi: 10.1016/1047-8477(91)90052-x. [DOI] [PubMed] [Google Scholar]
- Bayer M. H., Keck W., Bayer M. E. Localization of penicillin-binding protein 1b in Escherichia coli: immunoelectron microscopy and immunotransfer studies. J Bacteriol. 1990 Jan;172(1):125–135. doi: 10.1128/jb.172.1.125-135.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck E., Zink B. Nucleotide sequence and genome organisation of filamentous bacteriophages fl and fd. Gene. 1981 Dec;16(1-3):35–58. doi: 10.1016/0378-1119(81)90059-7. [DOI] [PubMed] [Google Scholar]
- Beijersbergen A., Dulk-Ras A. D., Schilperoort R. A., Hooykaas P. J. Conjugative Transfer by the Virulence System of Agrobacterium tumefaciens. Science. 1992 May 29;256(5061):1324–1327. doi: 10.1126/science.256.5061.1324. [DOI] [PubMed] [Google Scholar]
- Benz R., Bauer K. Permeation of hydrophilic molecules through the outer membrane of gram-negative bacteria. Review on bacterial porins. Eur J Biochem. 1988 Sep 1;176(1):1–19. doi: 10.1111/j.1432-1033.1988.tb14245.x. [DOI] [PubMed] [Google Scholar]
- Berger B. R., Christie P. J. The Agrobacterium tumefaciens virB4 gene product is an essential virulence protein requiring an intact nucleoside triphosphate-binding domain. J Bacteriol. 1993 Mar;175(6):1723–1734. doi: 10.1128/jb.175.6.1723-1734.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham D. P., Barnhart B. J. Inhibition of transformation by antibodies against competent Haemophilus influenzae. J Gen Microbiol. 1973 Apr;75(2):249–258. doi: 10.1099/00221287-75-2-249. [DOI] [PubMed] [Google Scholar]
- Boeke J. D., Model P., Zinder N. D. Effects of bacteriophage f1 gene III protein on the host cell membrane. Mol Gen Genet. 1982;186(2):185–192. doi: 10.1007/BF00331849. [DOI] [PubMed] [Google Scholar]
- Botstein D., Waddell C. H., King J. Mechanism of head assembly and DNA encapsulation in Salmonella phage p22. I. Genes, proteins, structures and DNA maturation. J Mol Biol. 1973 Nov 15;80(4):669–695. doi: 10.1016/0022-2836(73)90204-0. [DOI] [PubMed] [Google Scholar]
- Boulanger P., Letellier L. Characterization of ion channels involved in the penetration of phage T4 DNA into Escherichia coli cells. J Biol Chem. 1988 Jul 15;263(20):9767–9775. [PubMed] [Google Scholar]
- Boulanger P., Letellier L. Ion channels are likely to be involved in the two steps of phage T5 DNA penetration into Escherichia coli cells. J Biol Chem. 1992 Feb 15;267(5):3168–3172. [PubMed] [Google Scholar]
- Bourdineaud J. P., Howard S. P., Lazdunski C. Localization and assembly into the Escherichia coli envelope of a protein required for entry of colicin A. J Bacteriol. 1989 May;171(5):2458–2465. doi: 10.1128/jb.171.5.2458-2465.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley D. E. Morphological and serological relationships of conjugative pili. Plasmid. 1980 Sep;4(2):155–169. doi: 10.1016/0147-619x(80)90005-0. [DOI] [PubMed] [Google Scholar]
- Bradley D. E., Whelan J. Escherichia coli tolQ mutants are resistant to filamentous bacteriophages that adsorb to the tips, not the shafts, of conjugative pili. J Gen Microbiol. 1989 Jul;135(7):1857–1863. doi: 10.1099/00221287-135-7-1857. [DOI] [PubMed] [Google Scholar]
- Bradshaw H. D., Jr, Traxler B. A., Minkley E. G., Jr, Nester E. W., Gordon M. P. Nucleotide sequence of the traI (helicase I) gene from the sex factor F. J Bacteriol. 1990 Jul;172(7):4127–4131. doi: 10.1128/jb.172.7.4127-4131.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitling R., Dubnau D. A membrane protein with similarity to N-methylphenylalanine pilins is essential for DNA binding by competent Bacillus subtilis. J Bacteriol. 1990 Mar;172(3):1499–1508. doi: 10.1128/jb.172.3.1499-1508.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton C. C., Jr The properties of sex pili, the viral nature of "conjugal" genetic transfer systems, and some possible approaches to the control of bacterial drug resistance. CRC Crit Rev Microbiol. 1971 May;1(1):105–160. doi: 10.3109/10408417109104479. [DOI] [PubMed] [Google Scholar]
- Brisson-Noël A., Arthur M., Courvalin P. Evidence for natural gene transfer from gram-positive cocci to Escherichia coli. J Bacteriol. 1988 Apr;170(4):1739–1745. doi: 10.1128/jb.170.4.1739-1745.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant J. L., Jr, King J. DNA injection proteins are targets of acridine-sensitized photoinactivation of bacteriophage P22. J Mol Biol. 1984 Dec 25;180(4):837–863. doi: 10.1016/0022-2836(84)90260-2. [DOI] [PubMed] [Google Scholar]
- Buchwald M., Murialdo H., Siminovitch L. The morphogenesis of bacteriophage lambda. II. Identification of the principal structural proteins. Virology. 1970 Oct;42(2):390–400. doi: 10.1016/0042-6822(70)90282-5. [DOI] [PubMed] [Google Scholar]
- Casjens S. R., Hendrix R. W. Locations and amounts of major structural proteins in bacteriophage lambda. J Mol Biol. 1974 Sep 15;88(2):535–545. doi: 10.1016/0022-2836(74)90500-2. [DOI] [PubMed] [Google Scholar]
- Chelsky D., Ralph R., Jonak G. Sequence requirements for synthetic peptide-mediated translocation to the nucleus. Mol Cell Biol. 1989 Jun;9(6):2487–2492. doi: 10.1128/mcb.9.6.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie P. J., Ward J. E., Jr, Gordon M. P., Nester E. W. A gene required for transfer of T-DNA to plants encodes an ATPase with autophosphorylating activity. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9677–9681. doi: 10.1073/pnas.86.24.9677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie P. J., Ward J. E., Winans S. C., Nester E. W. The Agrobacterium tumefaciens virE2 gene product is a single-stranded-DNA-binding protein that associates with T-DNA. J Bacteriol. 1988 Jun;170(6):2659–2667. doi: 10.1128/jb.170.6.2659-2667.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citovsky V., DE Vos G., Zambryski P. Single-Stranded DNA Binding Protein Encoded by the virE Locus of Agrobacterium tumefaciens. Science. 1988 Apr 22;240(4851):501–504. doi: 10.1126/science.240.4851.501. [DOI] [PubMed] [Google Scholar]
- Citovsky V., Wong M. L., Zambryski P. Cooperative interaction of Agrobacterium VirE2 protein with single-stranded DNA: implications for the T-DNA transfer process. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1193–1197. doi: 10.1073/pnas.86.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citovsky V., Zambryski P. Transport of nucleic acids through membrane channels: snaking through small holes. Annu Rev Microbiol. 1993;47:167–197. doi: 10.1146/annurev.mi.47.100193.001123. [DOI] [PubMed] [Google Scholar]
- Citovsky V., Zupan J., Warnick D., Zambryski P. Nuclear localization of Agrobacterium VirE2 protein in plant cells. Science. 1992 Jun 26;256(5065):1802–1805. doi: 10.1126/science.1615325. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Gawron-Burke C. Conjugative transposons and the dissemination of antibiotic resistance in streptococci. Annu Rev Microbiol. 1986;40:635–659. doi: 10.1146/annurev.mi.40.100186.003223. [DOI] [PubMed] [Google Scholar]
- Clewell D. B. Movable genetic elements and antibiotic resistance in enterococci. Eur J Clin Microbiol Infect Dis. 1990 Feb;9(2):90–102. doi: 10.1007/BF01963632. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Weaver K. E. Sex pheromones and plasmid transfer in Enterococcus faecalis. Plasmid. 1989 May;21(3):175–184. doi: 10.1016/0147-619x(89)90041-3. [DOI] [PubMed] [Google Scholar]
- Clément J. M., Hofnung M. Gene sequence of the lambda receptor, an outer membrane protein of E. coli K12. Cell. 1981 Dec;27(3 Pt 2):507–514. doi: 10.1016/0092-8674(81)90392-5. [DOI] [PubMed] [Google Scholar]
- Cohen A., Fisher W. D., Curtiss R., 3rd, Adler H. I. DNA isolated from Escherichia coli minicells mated with F+ cells. Proc Natl Acad Sci U S A. 1968 Sep;61(1):61–68. doi: 10.1073/pnas.61.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concino M. F., Goodgal S. H. DNA-binding vesicles released from the surface of a competence-deficient mutant of Haemophilus influenzae. J Bacteriol. 1982 Oct;152(1):441–450. doi: 10.1128/jb.152.1.441-450.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concino M. F., Goodgal S. H. Haemophilus influenzae polypeptides involved in deoxyribonucleic acid uptake detected by cellular surface protein iodination. J Bacteriol. 1981 Oct;148(1):220–231. doi: 10.1128/jb.148.1.220-231.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danner D. B., Deich R. A., Sisco K. L., Smith H. O. An eleven-base-pair sequence determines the specificity of DNA uptake in Haemophilus transformation. Gene. 1980 Nov;11(3-4):311–318. doi: 10.1016/0378-1119(80)90071-2. [DOI] [PubMed] [Google Scholar]
- Das A. Agrobacterium tumefaciens virE operon encodes a single-stranded DNA-binding protein. Proc Natl Acad Sci U S A. 1988 May;85(9):2909–2913. doi: 10.1073/pnas.85.9.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash P. K., Traxler B. A., Panicker M. M., Hackney D. D., Minkley E. G., Jr Biochemical characterization of Escherichia coli DNA helicase I. Mol Microbiol. 1992 May;6(9):1163–1172. doi: 10.1111/j.1365-2958.1992.tb01555.x. [DOI] [PubMed] [Google Scholar]
- Davidoff-Abelson R., Dubnau D. Kinetic analysis of the products of donor deoxyribonucleate in transformed cells of Bacillus subtilis. J Bacteriol. 1973 Oct;116(1):154–162. doi: 10.1128/jb.116.1.154-162.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deich R. A., Smith H. O. Mechanism of homospecific DNA uptake in Haemophilus influenzae transformation. Mol Gen Genet. 1980 Feb;177(3):369–374. doi: 10.1007/BF00271475. [DOI] [PubMed] [Google Scholar]
- Delius H., Mantell N. J., Alberts B. Characterization by electron microscopy of the complex formed between T4 bacteriophage gene 32-protein and DNA. J Mol Biol. 1972 Jun 28;67(3):341–350. doi: 10.1016/0022-2836(72)90454-8. [DOI] [PubMed] [Google Scholar]
- Di Laurenzio L., Frost L. S., Paranchych W. The TraM protein of the conjugative plasmid F binds to the origin of transfer of the F and ColE1 plasmids. Mol Microbiol. 1992 Oct;6(20):2951–2959. doi: 10.1111/j.1365-2958.1992.tb01754.x. [DOI] [PubMed] [Google Scholar]
- Dorward D. W., Garon C. F. DNA Is Packaged within Membrane-Derived Vesicles of Gram-Negative but Not Gram-Positive Bacteria. Appl Environ Microbiol. 1990 Jun;56(6):1960–1962. doi: 10.1128/aem.56.6.1960-1962.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorward D. W., Garon C. F. DNA-binding proteins in cells and membrane blebs of Neisseria gonorrhoeae. J Bacteriol. 1989 Aug;171(8):4196–4201. doi: 10.1128/jb.171.8.4196-4201.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorward D. W., Garon C. F., Judd R. C. Export and intercellular transfer of DNA via membrane blebs of Neisseria gonorrhoeae. J Bacteriol. 1989 May;171(5):2499–2505. doi: 10.1128/jb.171.5.2499-2505.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas C. J., Halperin W., Nester E. W. Agrobacterium tumefaciens mutants affected in attachment to plant cells. J Bacteriol. 1982 Dec;152(3):1265–1275. doi: 10.1128/jb.152.3.1265-1275.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas C. J., Staneloni R. J., Rubin R. A., Nester E. W. Identification and genetic analysis of an Agrobacterium tumefaciens chromosomal virulence region. J Bacteriol. 1985 Mar;161(3):850–860. doi: 10.1128/jb.161.3.850-860.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau D., Cirigliano C. Fate of transforming deoxyribonucleic acid after uptake by competent Bacillus subtilis: size and distribution of the integrated donor segments. J Bacteriol. 1972 Aug;111(2):488–494. doi: 10.1128/jb.111.2.488-494.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau D. Genetic competence in Bacillus subtilis. Microbiol Rev. 1991 Sep;55(3):395–424. doi: 10.1128/mr.55.3.395-424.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau D. The regulation of genetic competence in Bacillus subtilis. Mol Microbiol. 1991 Jan;5(1):11–18. doi: 10.1111/j.1365-2958.1991.tb01820.x. [DOI] [PubMed] [Google Scholar]
- Duckworth D. H., Dunn G. B. Membrane protein biosynthesis in T5 bacteriophage-infected Escherichia coli. Arch Biochem Biophys. 1976 Feb;172(2):319–328. doi: 10.1016/0003-9861(76)90083-7. [DOI] [PubMed] [Google Scholar]
- Dums F., Dow J. M., Daniels M. J. Structural characterization of protein secretion genes of the bacterial phytopathogen Xanthomonas campestris pathovar campestris: relatedness to secretion systems of other gram-negative bacteria. Mol Gen Genet. 1991 Oct;229(3):357–364. doi: 10.1007/BF00267456. [DOI] [PubMed] [Google Scholar]
- Dupuy B., Taha M. K., Possot O., Marchal C., Pugsley A. P. PulO, a component of the pullulanase secretion pathway of Klebsiella oxytoca, correctly and efficiently processes gonococcal type IV prepilin in Escherichia coli. Mol Microbiol. 1992 Jul;6(14):1887–1894. doi: 10.1111/j.1365-2958.1992.tb01361.x. [DOI] [PubMed] [Google Scholar]
- Dürrenberger F., Crameri A., Hohn B., Koukolíková-Nicola Z. Covalently bound VirD2 protein of Agrobacterium tumefaciens protects the T-DNA from exonucleolytic degradation. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9154–9158. doi: 10.1073/pnas.86.23.9154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dürrenberger M. B., Villiger W., Bächi T. Conjugational junctions: morphology of specific contacts in conjugating Escherichia coli bacteria. J Struct Biol. 1991 Oct;107(2):146–156. doi: 10.1016/1047-8477(91)90018-r. [DOI] [PubMed] [Google Scholar]
- Dürwald H., Hoffmann-Berling H. Endonuclease-I-deficient and ribonuclease I-deficient Escherichia coli mutants. J Mol Biol. 1968 Jul 14;34(2):331–346. doi: 10.1016/0022-2836(68)90257-x. [DOI] [PubMed] [Google Scholar]
- Eisenstadt E., Lange R., Willecke K. Competent Bacillus subtilis cultures synthesize a denatured DNA binding activity. Proc Natl Acad Sci U S A. 1975 Jan;72(1):323–327. doi: 10.1073/pnas.72.1.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engström P., Zambryski P., Van Montagu M., Stachel S. Characterization of Agrobacterium tumefaciens virulence proteins induced by the plant factor acetosyringone. J Mol Biol. 1987 Oct 20;197(4):635–645. doi: 10.1016/0022-2836(87)90470-0. [DOI] [PubMed] [Google Scholar]
- Erni B., Zanolari B., Kocher H. P. The mannose permease of Escherichia coli consists of three different proteins. Amino acid sequence and function in sugar transport, sugar phosphorylation, and penetration of phage lambda DNA. J Biol Chem. 1987 Apr 15;262(11):5238–5247. [PubMed] [Google Scholar]
- Feucht A., Schmid A., Benz R., Schwarz H., Heller K. J. Pore formation associated with the tail-tip protein pb2 of bacteriophage T5. J Biol Chem. 1990 Oct 25;265(30):18561–18567. [PubMed] [Google Scholar]
- Filali Maltouf A., Labedan B. Host cell metabolic energy is not required for injection of bacteriophage T5 DNA. J Bacteriol. 1983 Jan;153(1):124–133. doi: 10.1128/jb.153.1.124-133.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filichkin S. A., Gelvin S. B. Formation of a putative relaxation intermediate during T-DNA processing directed by the Agrobacterium tumefaciens VirD1,D2 endonuclease. Mol Microbiol. 1993 May;8(5):915–926. doi: 10.1111/j.1365-2958.1993.tb01637.x. [DOI] [PubMed] [Google Scholar]
- Flannagan S. E., Clewell D. B. Conjugative transfer of Tn916 in Enterococcus faecalis: trans activation of homologous transposons. J Bacteriol. 1991 Nov;173(22):7136–7141. doi: 10.1128/jb.173.22.7136-7141.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkhard W., Leonard K. R., Malsey S., Marvin D. A., Dubochet J., Engel A., Achtman M., Helmuth R. X-ray diffraction and electron microscope studies on the structure of bacterial F pili. J Mol Biol. 1979 May 15;130(2):145–160. doi: 10.1016/0022-2836(79)90423-6. [DOI] [PubMed] [Google Scholar]
- Fujii T., Naka D., Toyoda N., Seto H. LiCl treatment releases a nickase implicated in genetic transformation of Streptococcus pneumoniae. J Bacteriol. 1987 Nov;169(11):4901–4906. doi: 10.1128/jb.169.11.4901-4906.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa H., Kuroiwa T., Mizushima S. DNA injection during bacteriophage T4 infection of Escherichia coli. J Bacteriol. 1983 May;154(2):938–945. doi: 10.1128/jb.154.2.938-945.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa H., Mizushima S. Roles of cell surface components of Escherichia coli K-12 in bacteriophage T4 infection: interaction of tail core with phospholipids. J Bacteriol. 1982 May;150(2):916–924. doi: 10.1128/jb.150.2.916-924.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fürste J. P., Pansegrau W., Ziegelin G., Kröger M., Lanka E. Conjugative transfer of promiscuous IncP plasmids: interaction of plasmid-encoded products with the transfer origin. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1771–1775. doi: 10.1073/pnas.86.6.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghai J., Das A. The virD operon of Agrobacterium tumefaciens Ti plasmid encodes a DNA-relaxing enzyme. Proc Natl Acad Sci U S A. 1989 May;86(9):3109–3113. doi: 10.1073/pnas.86.9.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietl C., Koukolíková-Nicola Z., Hohn B. Mobilization of T-DNA from Agrobacterium to plant cells involves a protein that binds single-stranded DNA. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9006–9010. doi: 10.1073/pnas.84.24.9006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser-Wuttke G., Keppner J., Rasched I. Pore-forming properties of the adsorption protein of filamentous phage fd. Biochim Biophys Acta. 1989 Nov 3;985(3):239–247. doi: 10.1016/0005-2736(89)90408-2. [DOI] [PubMed] [Google Scholar]
- Goodgal S. H. DNA uptake in Haemophilus transformation. Annu Rev Genet. 1982;16:169–192. doi: 10.1146/annurev.ge.16.120182.001125. [DOI] [PubMed] [Google Scholar]
- Goodman S. D., Scocca J. J. Identification and arrangement of the DNA sequence recognized in specific transformation of Neisseria gonorrhoeae. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6982–6986. doi: 10.1073/pnas.85.18.6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves J. F., Biswas G. D., Sparling P. F. Sequence-specific DNA uptake in transformation of Neisseria gonorrhoeae. J Bacteriol. 1982 Dec;152(3):1071–1077. doi: 10.1128/jb.152.3.1071-1077.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray C. W., Brown R. S., Marvin D. A. Adsorption complex of filamentous fd virus. J Mol Biol. 1981 Mar 15;146(4):621–627. doi: 10.1016/0022-2836(81)90050-4. [DOI] [PubMed] [Google Scholar]
- Guihard G., Boulanger P., Letellier L. Involvement of phage T5 tail proteins and contact sites between the outer and inner membrane of Escherichia coli in phage T5 DNA injection. J Biol Chem. 1992 Feb 15;267(5):3173–3178. [PubMed] [Google Scholar]
- Hahn J., Albano M., Dubnau D. Isolation and characterization of Tn917lac-generated competence mutants of Bacillus subtilis. J Bacteriol. 1987 Jul;169(7):3104–3109. doi: 10.1128/jb.169.7.3104-3109.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington L. C., Rogerson A. C. The F pilus of Escherichia coli appears to support stable DNA transfer in the absence of wall-to-wall contact between cells. J Bacteriol. 1990 Dec;172(12):7263–7264. doi: 10.1128/jb.172.12.7263-7264.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwieg E., Bazinet C., King J. The DNA injection apparatus of phage p22. Biophys J. 1986 Jan;49(1):24–26. doi: 10.1016/S0006-3495(86)83579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemann J. A. Genetics of gene transfer between species. Trends Genet. 1991 Jun;7(6):181–185. doi: 10.1016/0168-9525(91)90433-q. [DOI] [PubMed] [Google Scholar]
- Heinemann J. A., Sprague G. F., Jr Bacterial conjugative plasmids mobilize DNA transfer between bacteria and yeast. Nature. 1989 Jul 20;340(6230):205–209. doi: 10.1038/340205a0. [DOI] [PubMed] [Google Scholar]
- Heller K. J. Molecular interaction between bacteriophage and the gram-negative cell envelope. Arch Microbiol. 1992;158(4):235–248. doi: 10.1007/BF00245239. [DOI] [PubMed] [Google Scholar]
- Heller K. J., Schwarz H. Irreversible binding to the receptor of bacteriophages T5 and BF23 does not occur with the tip of the tail. J Bacteriol. 1985 May;162(2):621–625. doi: 10.1128/jb.162.2.621-625.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix R. W., Casjens S. R. Protein cleavage in bacteriophage lambda tail assembly. Virology. 1974 Sep;61(1):156–159. doi: 10.1016/0042-6822(74)90250-5. [DOI] [PubMed] [Google Scholar]
- Herrera-Estrella A., Van Montagu M., Wang K. A bacterial peptide acting as a plant nuclear targeting signal: the amino-terminal portion of Agrobacterium VirD2 protein directs a beta-galactosidase fusion protein into tobacco nuclei. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9534–9537. doi: 10.1073/pnas.87.24.9534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs M., Mattick J. S. Common components in the assembly of type 4 fimbriae, DNA transfer systems, filamentous phage and protein-secretion apparatus: a general system for the formation of surface-associated protein complexes. Mol Microbiol. 1993 Oct;10(2):233–243. doi: 10.1111/j.1365-2958.1993.tb01949.x. [DOI] [PubMed] [Google Scholar]
- Hoffman B., Levine M. Bacteriophage P22 virion protein which performs an essential early function. I. Analysis of 16-ts mutants. J Virol. 1975 Dec;16(6):1536–1546. doi: 10.1128/jvi.16.6.1536-1546.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman B., Levine M. Bacteriophage P22 virion protein which performs an essential early function. II. Characterization of the gene 16 function. J Virol. 1975 Dec;16(6):1547–1559. doi: 10.1128/jvi.16.6.1547-1559.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooykaas P. J. Transformation of plant cells via Agrobacterium. Plant Mol Biol. 1989 Sep;13(3):327–336. doi: 10.1007/BF00025321. [DOI] [PubMed] [Google Scholar]
- Howard E. A., Winsor B. A., De Vos G., Zambryski P. Activation of the T-DNA transfer process in Agrobacterium results in the generation of a T-strand-protein complex: Tight association of VirD2 with the 5' ends of T-strands. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4017–4021. doi: 10.1073/pnas.86.11.4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard E. A., Zupan J. R., Citovsky V., Zambryski P. C. The VirD2 protein of A. tumefaciens contains a C-terminal bipartite nuclear localization signal: implications for nuclear uptake of DNA in plant cells. Cell. 1992 Jan 10;68(1):109–118. doi: 10.1016/0092-8674(92)90210-4. [DOI] [PubMed] [Google Scholar]
- Hui F. M., Morrison D. A. Genetic transformation in Streptococcus pneumoniae: nucleotide sequence analysis shows comA, a gene required for competence induction, to be a member of the bacterial ATP-dependent transport protein family. J Bacteriol. 1991 Jan;173(1):372–381. doi: 10.1128/jb.173.1.372-381.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihler G., Rupp W. D. Strand-specific transfer of donor DNA during conjugation in E. coli. Proc Natl Acad Sci U S A. 1969 May;63(1):138–143. doi: 10.1073/pnas.63.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ippen-Ihler K. A., Minkley E. G., Jr The conjugation system of F, the fertility factor of Escherichia coli. Annu Rev Genet. 1986;20:593–624. doi: 10.1146/annurev.ge.20.120186.003113. [DOI] [PubMed] [Google Scholar]
- Jacobson A. Role of F pili in the penetration of bacteriophage fl. J Virol. 1972 Oct;10(4):835–843. doi: 10.1128/jvi.10.4.835-843.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalajakumari M. B., Manning P. A. Nucleotide sequence of the traD region in the Escherichia coli F sex factor. Gene. 1989 Sep 30;81(2):195–202. doi: 10.1016/0378-1119(89)90179-0. [DOI] [PubMed] [Google Scholar]
- Jarchow E., Grimsley N. H., Hohn B. virF, the host-range-determining virulence gene of Agrobacterium tumefaciens, affects T-DNA transfer to Zea mays. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10426–10430. doi: 10.1073/pnas.88.23.10426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaswal R. K., Veluthambi K., Gelvin S. B., Slightom J. L. Double-stranded cleavage of T-DNA and generation of single-stranded T-DNA molecules in Escherichia coli by a virD-encoded border-specific endonuclease from Agrobacterium tumefaciens. J Bacteriol. 1987 Nov;169(11):5035–5045. doi: 10.1128/jb.169.11.5035-5045.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joenje H., Venema G. Different nuclease activities in competent and noncompetent Bacillus subtilis. J Bacteriol. 1975 Apr;122(1):25–33. doi: 10.1128/jb.122.1.25-33.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadesch T. R., Williams R. C., Chamberlin M. J. Electron microscopic studies of the binding of Escherichia coli RNA polymerase to DNA. I. Characterization of the non-specific interactions of holoenzyme with a restriction fragment of bacteriophage T7 DNA. J Mol Biol. 1980 Jan 5;136(1):65–78. doi: 10.1016/0022-2836(80)90366-6. [DOI] [PubMed] [Google Scholar]
- Kahn M. E., Barany F., Smith H. O. Transformasomes: specialized membranous structures that protect DNA during Haemophilus transformation. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6927–6931. doi: 10.1073/pnas.80.22.6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn M. E., Maul G., Goodgal S. H. Possible mechanism for donor DNA binding and transport in Haemophilus. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6370–6374. doi: 10.1073/pnas.79.20.6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn M. E., Smith H. O. Transformation in Haemophilus: a problem in membrane biology. J Membr Biol. 1984;81(2):89–103. doi: 10.1007/BF01868974. [DOI] [PubMed] [Google Scholar]
- Kahn M., Concino M., Gromkova R., Goodgal S. DNA binding activity of vesicles produced by competence deficient mutants of Haemophilus. Biochem Biophys Res Commun. 1979 Apr 13;87(3):764–772. doi: 10.1016/0006-291x(79)92024-2. [DOI] [PubMed] [Google Scholar]
- Kalasauskaite E. V., Kadisaite D. L., Daugelavicius R. J., Grinius L. L., Jasaitis A. A. Studies on energy supply for genetic processes. Requirement for membrane potential in Escherichia coli infection by phage T4. Eur J Biochem. 1983 Jan 17;130(1):123–130. [PubMed] [Google Scholar]
- Kalasauskaite E., Grinius L., Kadisaite D., Jasaitis A. Electrochemical H+ gradient but not phosphate potential is required for Escherichia coli infection by phage T4. FEBS Lett. 1980 Aug 11;117(1):232–236. doi: 10.1016/0014-5793(80)80952-5. [DOI] [PubMed] [Google Scholar]
- Kanemoto R. H., Powell A. T., Akiyoshi D. E., Regier D. A., Kerstetter R. A., Nester E. W., Hawes M. C., Gordon M. P. Nucleotide sequence and analysis of the plant-inducible locus pinF from Agrobacterium tumefaciens. J Bacteriol. 1989 May;171(5):2506–2512. doi: 10.1128/jb.171.5.2506-2512.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellenberger E. The 'Bayer bridges' confronted with results from improved electron microscopy methods. Mol Microbiol. 1990 May;4(5):697–705. doi: 10.1111/j.1365-2958.1990.tb00640.x. [DOI] [PubMed] [Google Scholar]
- Keweloh H., Bakker E. P. Permeability changes in the cytoplasmic membrane of Escherichia coli K-12 early after infection with bacteriophage T1. J Bacteriol. 1984 Oct;160(1):347–353. doi: 10.1128/jb.160.1.347-353.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn B., Abdel-Monem M., Hoffmann-Berling H. DNA helicases. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):63–67. doi: 10.1101/sqb.1979.043.01.011. [DOI] [PubMed] [Google Scholar]
- Kuldau G. A., De Vos G., Owen J., McCaffrey G., Zambryski P. The virB operon of Agrobacterium tumefaciens pTiC58 encodes 11 open reading frames. Mol Gen Genet. 1990 Apr;221(2):256–266. doi: 10.1007/BF00261729. [DOI] [PubMed] [Google Scholar]
- LACKS S. Molecular fate of DNA in genetic transformation of Pneumococcus. J Mol Biol. 1962 Jul;5:119–131. doi: 10.1016/s0022-2836(62)80067-9. [DOI] [PubMed] [Google Scholar]
- LEVINE J. S., STRAUSS N. LAG PERIOD CHARACTERIZING THE ENTRY OF TRANSFORMING DEOXYRIBONUCLEIC ACID INTO BACILLUS SUBTILIS. J Bacteriol. 1965 Feb;89:281–287. doi: 10.1128/jb.89.2.281-287.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labedan B. A very early step in the T5 DNA injection process. Virology. 1976 Dec;75(2):368–375. doi: 10.1016/0042-6822(76)90035-0. [DOI] [PubMed] [Google Scholar]
- Labedan B., Heller K. B., Jasaitis A. A., Wilson T. H., Goldberg E. B. A membrane potential threshold for phage T4 DNA injection. Biochem Biophys Res Commun. 1980 Mar 28;93(2):625–630. doi: 10.1016/0006-291x(80)91124-9. [DOI] [PubMed] [Google Scholar]
- Lacks S., Greenberg B., Neuberger M. Identification of a deoxyribonuclease implicated in genetic transformation of Diplococcus pneumoniae. J Bacteriol. 1975 Jul;123(1):222–232. doi: 10.1128/jb.123.1.222-232.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacks S., Greenberg B., Neuberger M. Role of a deoxyribonuclease in the genetic transformation of Diplococcus pneumoniae. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2305–2309. doi: 10.1073/pnas.71.6.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacks S., Greenberg B. Single-strand breakage on binding of DNA to cells in the genetic transformation of Diplococcus pneumoniae. J Mol Biol. 1976 Feb 25;101(2):255–275. doi: 10.1016/0022-2836(76)90376-4. [DOI] [PubMed] [Google Scholar]
- Lacks S., Neuberger M. Membrane location of a deoxyribonuclease implicated in the genetic transformation of Diplococcus pneumoniae. J Bacteriol. 1975 Dec;124(3):1321–1329. doi: 10.1128/jb.124.3.1321-1329.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacks S. Uptake of circular deoxyribonucleic acid and mechanism of deoxyribonucleic acid transport in genetic transformation of Streptococcus pneumoniae. J Bacteriol. 1979 May;138(2):404–409. doi: 10.1128/jb.138.2.404-409.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahue E. E., Matson S. W. Purified Escherichia coli F-factor TraY protein binds oriT. J Bacteriol. 1990 Mar;172(3):1385–1391. doi: 10.1128/jb.172.3.1385-1391.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanni Y. T. First-step-transfer deoxyribonucleic acid of bacteriophage T5. Bacteriol Rev. 1968 Sep;32(3):227–242. doi: 10.1128/br.32.3.227-242.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson T. G., Goodgal S. H. Donor DNA processing is blocked by a mutation in the com101A locus of Haemophilus influenzae. J Bacteriol. 1992 May;174(10):3392–3394. doi: 10.1128/jb.174.10.3392-3394.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson T. G., Goodgal S. H. Sequence and transcriptional regulation of com101A, a locus required for genetic transformation in Haemophilus influenzae. J Bacteriol. 1991 Aug;173(15):4683–4691. doi: 10.1128/jb.173.15.4683-4691.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessl M., Balzer D., Pansegrau W., Lanka E. Sequence similarities between the RP4 Tra2 and the Ti VirB region strongly support the conjugation model for T-DNA transfer. J Biol Chem. 1992 Oct 5;267(28):20471–20480. [PubMed] [Google Scholar]
- Levengood-Freyermuth S. K., Click E. M., Webster R. E. Role of the carboxyl-terminal domain of TolA in protein import and integrity of the outer membrane. J Bacteriol. 1993 Jan;175(1):222–228. doi: 10.1128/jb.175.1.222-228.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levengood S. K., Beyer W. F., Jr, Webster R. E. TolA: a membrane protein involved in colicin uptake contains an extended helical region. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):5939–5943. doi: 10.1073/pnas.88.14.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levengood S. K., Webster R. E. Nucleotide sequences of the tolA and tolB genes and localization of their products, components of a multistep translocation system in Escherichia coli. J Bacteriol. 1989 Dec;171(12):6600–6609. doi: 10.1128/jb.171.12.6600-6609.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg A. A. Bacteriophage receptors. Annu Rev Microbiol. 1973;27:205–241. doi: 10.1146/annurev.mi.27.100173.001225. [DOI] [PubMed] [Google Scholar]
- Lipinska B., Rao A. S., Bolten B. M., Balakrishnan R., Goldberg E. B. Cloning and identification of bacteriophage T4 gene 2 product gp2 and action of gp2 on infecting DNA in vivo. J Bacteriol. 1989 Jan;171(1):488–497. doi: 10.1128/jb.171.1.488-497.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Londoño-Vallejo J. A., Dubnau D. comF, a Bacillus subtilis late competence locus, encodes a protein similar to ATP-dependent RNA/DNA helicases. Mol Microbiol. 1993 Jul;9(1):119–131. doi: 10.1111/j.1365-2958.1993.tb01674.x. [DOI] [PubMed] [Google Scholar]
- Luckey M., Nikaido H. Specificity of diffusion channels produced by lambda phage receptor protein of Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jan;77(1):167–171. doi: 10.1073/pnas.77.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maneewannakul S., Kathir P., Ippen-Ihler K. Characterization of the F plasmid mating aggregation gene traN and of a new F transfer region locus trbE. J Mol Biol. 1992 May 20;225(2):299–311. doi: 10.1016/0022-2836(92)90923-8. [DOI] [PubMed] [Google Scholar]
- Manning P. A., Pugsley A. P., Reeves P. Defective growth functions in mutants of Escherichia coli K12 lacking a major outer membrane protein. J Mol Biol. 1977 Oct 25;116(2):285–300. doi: 10.1016/0022-2836(77)90217-0. [DOI] [PubMed] [Google Scholar]
- Manoil C., Rosenbusch J. P. Conjugation-deficient mutants of Escherichia coli distinguish classes of functions of the outer membrane OmpA protein. Mol Gen Genet. 1982;187(1):148–156. doi: 10.1007/BF00384398. [DOI] [PubMed] [Google Scholar]
- Marvin D. A., Hohn B. Filamentous bacterial viruses. Bacteriol Rev. 1969 Jun;33(2):172–209. doi: 10.1128/br.33.2.172-209.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson S. W., Morton B. S. Escherichia coli DNA helicase I catalyzes a site- and strand-specific nicking reaction at the F plasmid oriT. J Biol Chem. 1991 Aug 25;266(24):16232–16237. [PubMed] [Google Scholar]
- Matson S. W., Nelson W. C., Morton B. S. Characterization of the reaction product of the oriT nicking reaction catalyzed by Escherichia coli DNA helicase I. J Bacteriol. 1993 May;175(9):2599–2606. doi: 10.1128/jb.175.9.2599-2606.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazodier P., Davies J. Gene transfer between distantly related bacteria. Annu Rev Genet. 1991;25:147–171. doi: 10.1146/annurev.ge.25.120191.001051. [DOI] [PubMed] [Google Scholar]
- McBride K. E., Knauf V. C. Genetic analysis of the virE operon of the Agrobacterium Ti plasmid pTiA6. J Bacteriol. 1988 Apr;170(4):1430–1437. doi: 10.1128/jb.170.4.1430-1437.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy D. Cloning of the rec-2 locus of Haemophilus influenzae. Gene. 1989 Jan 30;75(1):135–143. doi: 10.1016/0378-1119(89)90390-9. [DOI] [PubMed] [Google Scholar]
- Melchers L. S., Maroney M. J., den Dulk-Ras A., Thompson D. V., van Vuuren H. A., Schilperoort R. A., Hooykaas P. J. Octopine and nopaline strains of Agrobacterium tumefaciens differ in virulence; molecular characterization of the virF locus. Plant Mol Biol. 1990 Feb;14(2):249–259. doi: 10.1007/BF00018565. [DOI] [PubMed] [Google Scholar]
- Merryweather A., Barth P. T., Wilkins B. M. Role and specificity of plasmid RP4-encoded DNA primase in bacterial conjugation. J Bacteriol. 1986 Jul;167(1):12–17. doi: 10.1128/jb.167.1.12-17.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer R. R., Laine P. S. The single-stranded DNA-binding protein of Escherichia coli. Microbiol Rev. 1990 Dec;54(4):342–380. doi: 10.1128/mr.54.4.342-380.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkley E. G., Jr, Willetts N. S. Overproduction, purification and characterization of the F traT protein. Mol Gen Genet. 1984;196(2):225–235. doi: 10.1007/BF00328054. [DOI] [PubMed] [Google Scholar]
- Mohan S., Aghion J., Guillen N., Dubnau D. Molecular cloning and characterization of comC, a late competence gene of Bacillus subtilis. J Bacteriol. 1989 Nov;171(11):6043–6051. doi: 10.1128/jb.171.11.6043-6051.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. A., Baker M. F. Competence for genetic transformation in pneumococcus depends on synthesis of a small set of proteins. Nature. 1979 Nov 8;282(5735):215–217. doi: 10.1038/282215a0. [DOI] [PubMed] [Google Scholar]
- Morrison D. A., Guild W. R. Breakage prior to entry of donor DNA in Pneumococcus transformation. Biochim Biophys Acta. 1973 Apr 11;299(4):545–556. doi: 10.1016/0005-2787(73)90226-8. [DOI] [PubMed] [Google Scholar]
- Morrison D. A., Mannarelli B. Transformation in pneumococcus: nuclease resistance of deoxyribonucleic acid in the eclipse complex. J Bacteriol. 1979 Nov;140(2):655–665. doi: 10.1128/jb.140.2.655-665.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. A. Transformation in pneumococcus: existence and properties of a complex involving donor deoxyribonucleate single strands in eclipse. J Bacteriol. 1977 Nov;132(2):576–583. doi: 10.1128/jb.132.2.576-583.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motallebi-Veshareh M., Balzer D., Lanka E., Jagura-Burdzy G., Thomas C. M. Conjugative transfer functions of broad-host-range plasmid RK2 are coregulated with vegetative replication. Mol Microbiol. 1992 Apr;6(7):907–920. doi: 10.1111/j.1365-2958.1992.tb01541.x. [DOI] [PubMed] [Google Scholar]
- Mulder J. A., Venema G. Isolation and partial characterization of Bacillus subtilis mutants impaired in DNA entry. J Bacteriol. 1982 Apr;150(1):260–268. doi: 10.1128/jb.150.1.260-268.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder J. A., Venema G. Transformation-deficient mutants of Bacillus subtilis impaired in competence-specific nuclease activities. J Bacteriol. 1982 Oct;152(1):166–174. doi: 10.1128/jb.152.1.166-174.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méjean V., Claverys J. P. Polarity of DNA entry in transformation of Streptococcus pneumoniae. Mol Gen Genet. 1988 Aug;213(2-3):444–448. doi: 10.1007/BF00339614. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Song S. A., Shaltiel L., Nurminen M. Outer membrane of Salmonella XIV. Reduced transmembrane diffusion rates in porin-deficient mutants. Biochem Biophys Res Commun. 1976 May 23;76(2):324–330. doi: 10.1016/0006-291x(77)90728-8. [DOI] [PubMed] [Google Scholar]
- Notani N. K., Setlow J. K., Joshi V. R., Allison D. P. Molecular basis for the transformation defects in mutants of Haemophilus influenzae. J Bacteriol. 1972 Jun;110(3):1171–1180. doi: 10.1128/jb.110.3.1171-1180.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick S. L., Baldeschwieler J. D. Fluorescence measurement of the kinetics of DNA injection by bacteriophage lambda into liposomes. Biochemistry. 1988 Oct 4;27(20):7919–7924. doi: 10.1021/bi00420a050. [DOI] [PubMed] [Google Scholar]
- Nunn D. N., Lory S. Product of the Pseudomonas aeruginosa gene pilD is a prepilin leader peptidase. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3281–3285. doi: 10.1073/pnas.88.8.3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohki M., Tomizawa J. Asymmetric transfer of DNA strands in bacterial conjugation. Cold Spring Harb Symp Quant Biol. 1968;33:651–658. doi: 10.1101/sqb.1968.033.01.074. [DOI] [PubMed] [Google Scholar]
- Okamoto S., Toyoda-Yamamoto A., Ito K., Takebe I., Machida Y. Localization and orientation of the VirD4 protein of Agrobacterium tumefaciens in the cell membrane. Mol Gen Genet. 1991 Aug;228(1-2):24–32. doi: 10.1007/BF00282443. [DOI] [PubMed] [Google Scholar]
- Ou J. T., Anderson T. F. Role of pili in bacterial conjugation. J Bacteriol. 1970 Jun;102(3):648–654. doi: 10.1128/jb.102.3.648-654.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmen R., Driessen A. J., Hellingwerf K. J. Bioenergetic aspects of the translocation of macromolecules across bacterial membranes. Biochim Biophys Acta. 1994 Jan 4;1183(3):417–451. doi: 10.1016/0005-2728(94)90072-8. [DOI] [PubMed] [Google Scholar]
- Palmen R., Vosman B., Buijsman P., Breek C. K., Hellingwerf K. J. Physiological characterization of natural transformation in Acinetobacter calcoaceticus. J Gen Microbiol. 1993 Feb;139(2):295–305. doi: 10.1099/00221287-139-2-295. [DOI] [PubMed] [Google Scholar]
- Palva E. T., Westermann P. Arrangement of the maltose-inducible major outer membrane proteins, the bacteriophage lambda receptor in Escherichia coli and the 44 K protein in Salmonella typhimurium. FEBS Lett. 1979 Mar 1;99(1):77–80. doi: 10.1016/0014-5793(79)80253-7. [DOI] [PubMed] [Google Scholar]
- Panicker M. M., Minkley E. G., Jr DNA transfer occurs during a cell surface contact stage of F sex factor-mediated bacterial conjugation. J Bacteriol. 1985 May;162(2):584–590. doi: 10.1128/jb.162.2.584-590.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panicker M. M., Minkley E. G., Jr Purification and properties of the F sex factor TraD protein, an inner membrane conjugal transfer protein. J Biol Chem. 1992 Jun 25;267(18):12761–12766. [PubMed] [Google Scholar]
- Pansegrau W., Balzer D., Kruft V., Lurz R., Lanka E. In vitro assembly of relaxosomes at the transfer origin of plasmid RP4. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6555–6559. doi: 10.1073/pnas.87.17.6555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pansegrau W., Ziegelin G., Lanka E. Covalent association of the traI gene product of plasmid RP4 with the 5'-terminal nucleotide at the relaxation nick site. J Biol Chem. 1990 Jun 25;265(18):10637–10644. [PubMed] [Google Scholar]
- Piechowska M., Fox M. S. Fate of transforming deoxyribonucleate in Bacillus subtilis. J Bacteriol. 1971 Nov;108(2):680–689. doi: 10.1128/jb.108.2.680-689.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponta H., Altendorf K. H., Schweiger M., Hirsch-Kaufmann M., Pfennig-Yeh M. L., Herrlich P. E. coli membranes become permeable to ions following T7-virus-infection. Mol Gen Genet. 1976 Dec 8;149(2):145–150. doi: 10.1007/BF00332882. [DOI] [PubMed] [Google Scholar]
- Possot O., d'Enfert C., Reyss I., Pugsley A. P. Pullulanase secretion in Escherichia coli K-12 requires a cytoplasmic protein and a putative polytopic cytoplasmic membrane protein. Mol Microbiol. 1992 Jan;6(1):95–105. doi: 10.1111/j.1365-2958.1992.tb00841.x. [DOI] [PubMed] [Google Scholar]
- Pugsley A. P., Dupuy B. An enzyme with type IV prepilin peptidase activity is required to process components of the general extracellular protein secretion pathway of Klebsiella oxytoca. Mol Microbiol. 1992 Mar;6(6):751–760. doi: 10.1111/j.1365-2958.1992.tb01525.x. [DOI] [PubMed] [Google Scholar]
- Pugsley A. P. The complete general secretory pathway in gram-negative bacteria. Microbiol Rev. 1993 Mar;57(1):50–108. doi: 10.1128/mr.57.1.50-108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puvanesarajah V., Schell F. M., Stacey G., Douglas C. J., Nester E. W. Role for 2-linked-beta-D-glucan in the virulence of Agrobacterium tumefaciens. J Bacteriol. 1985 Oct;164(1):102–106. doi: 10.1128/jb.164.1.102-106.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puyet A., Greenberg B., Lacks S. A. Genetic and structural characterization of endA. A membrane-bound nuclease required for transformation of Streptococcus pneumoniae. J Mol Biol. 1990 Jun 20;213(4):727–738. doi: 10.1016/S0022-2836(05)80259-1. [DOI] [PubMed] [Google Scholar]
- Rees C. E., Wilkins B. M. Protein transfer into the recipient cell during bacterial conjugation: studies with F and RP4. Mol Microbiol. 1990 Jul;4(7):1199–1205. doi: 10.1111/j.1365-2958.1990.tb00695.x. [DOI] [PubMed] [Google Scholar]
- Rees C. E., Wilkins B. M. Transfer of tra proteins into the recipient cell during bacterial conjugation mediated by plasmid ColIb-P9. J Bacteriol. 1989 Jun;171(6):3152–3157. doi: 10.1128/jb.171.6.3152-3157.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusch R. N. Biological complexes of poly-beta-hydroxybutyrate. FEMS Microbiol Rev. 1992 Dec;9(2-4):119–129. doi: 10.1111/j.1574-6968.1992.tb05829.x. [DOI] [PubMed] [Google Scholar]
- Reusch R. N., Hiske T. W., Sadoff H. L. Poly-beta-hydroxybutyrate membrane structure and its relationship to genetic transformability in Escherichia coli. J Bacteriol. 1986 Nov;168(2):553–562. doi: 10.1128/jb.168.2.553-562.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusch R. N., Sadoff H. L. D-(-)-poly-beta-hydroxybutyrate in membranes of genetically competent bacteria. J Bacteriol. 1983 Nov;156(2):778–788. doi: 10.1128/jb.156.2.778-788.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusch R. N., Sadoff H. L. Putative structure and functions of a poly-beta-hydroxybutyrate/calcium polyphosphate channel in bacterial plasma membranes. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4176–4180. doi: 10.1073/pnas.85.12.4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reygers U., Wessel R., Müller H., Hoffmann-Berling H. Endonuclease activity of Escherichia coli DNA helicase I directed against the transfer origin of the F factor. EMBO J. 1991 Sep;10(9):2689–2694. doi: 10.1002/j.1460-2075.1991.tb07812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ried G., Henning U. A unique amino acid substitution in the outer membrane protein OmpA causes conjugation deficiency in Escherichia coli K-12. FEBS Lett. 1987 Nov 2;223(2):387–390. doi: 10.1016/0014-5793(87)80324-1. [DOI] [PubMed] [Google Scholar]
- Riede I., Eschbach M. L. Evidence that TraT interacts with OmpA of Escherichia coli. FEBS Lett. 1986 Sep 15;205(2):241–245. doi: 10.1016/0014-5793(86)80905-x. [DOI] [PubMed] [Google Scholar]
- Roessner C. A., Ihler G. M. Formation of transmembrane channels in liposomes during injection of lambda DNA. J Biol Chem. 1986 Jan 5;261(1):386–390. [PubMed] [Google Scholar]
- Roessner C. A., Ihler G. M. Proteinase sensitivity of bacteriophage lambda tail proteins gpJ and pH in complexes with the lambda receptor. J Bacteriol. 1984 Jan;157(1):165–170. doi: 10.1128/jb.157.1.165-170.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenshine I., Tchelet R., Mevarech M. The mechanism of DNA transfer in the mating system of an archaebacterium. Science. 1989 Sep 22;245(4924):1387–1389. doi: 10.1126/science.2818746. [DOI] [PubMed] [Google Scholar]
- Rosenthal A. L., Lacks S. A. Complex structure of the membrane nuclease of Streptococcus pneumoniae revealed by two-dimensional electrophoresis. J Mol Biol. 1980 Aug 5;141(2):133–146. doi: 10.1016/0022-2836(80)90381-2. [DOI] [PubMed] [Google Scholar]
- Rossi L., Hohn B., Tinland B. The VirD2 protein of Agrobacterium tumefaciens carries nuclear localization signals important for transfer of T-DNA to plant. Mol Gen Genet. 1993 Jun;239(3):345–353. doi: 10.1007/BF00276932. [DOI] [PubMed] [Google Scholar]
- Russel M. Filamentous phage assembly. Mol Microbiol. 1991 Jul;5(7):1607–1613. doi: 10.1111/j.1365-2958.1991.tb01907.x. [DOI] [PubMed] [Google Scholar]
- Russel M., Whirlow H., Sun T. P., Webster R. E. Low-frequency infection of F- bacteria by transducing particles of filamentous bacteriophages. J Bacteriol. 1988 Nov;170(11):5312–5316. doi: 10.1128/jb.170.11.5312-5316.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRAUSS N. CONFIGURATION OF TRANSFORMING DEOXYRIBONUCLEIC ACID DURING ENTRY INTO BACILLUS SUBTILIS. J Bacteriol. 1965 Feb;89:288–293. doi: 10.1128/jb.89.2.288-293.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint N., De E., Julien S., Orange N., Molle G. Ionophore properties of OmpA of Escherichia coli. Biochim Biophys Acta. 1993 Jan 18;1145(1):119–123. doi: 10.1016/0005-2736(93)90388-g. [DOI] [PubMed] [Google Scholar]
- Scandella D., Arber W. An Escherichia coli mutant which inhibits the injection of phage lambda DNA. Virology. 1974 Apr;58(2):504–513. doi: 10.1016/0042-6822(74)90084-1. [DOI] [PubMed] [Google Scholar]
- Scandella D., Arber W. Phage lambda DNA injection into Escherichia coli pel- mutants is restored by mutations in phage genes V or H. Virology. 1976 Jan;69(1):206–215. doi: 10.1016/0042-6822(76)90207-5. [DOI] [PubMed] [Google Scholar]
- Schandel K. A., Maneewannakul S., Ippen-Ihler K., Webster R. E. A traC mutant that retains sensitivity to f1 bacteriophage but lacks F pili. J Bacteriol. 1987 Jul;169(7):3151–3159. doi: 10.1128/jb.169.7.3151-3159.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schandel K. A., Muller M. M., Webster R. E. Localization of TraC, a protein involved in assembly of the F conjugative pilus. J Bacteriol. 1992 Jun;174(11):3800–3806. doi: 10.1128/jb.174.11.3800-3806.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab M., Gruber H., Högenauer G. The TraM protein of plasmid R1 is a DNA-binding protein. Mol Microbiol. 1991 Feb;5(2):439–446. doi: 10.1111/j.1365-2958.1991.tb02127.x. [DOI] [PubMed] [Google Scholar]
- Scott J. R., Kirchman P. A., Caparon M. G. An intermediate in transposition of the conjugative transposon Tn916. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4809–4813. doi: 10.1073/pnas.85.13.4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. R. Sex and the single circle: conjugative transposition. J Bacteriol. 1992 Oct;174(19):6005–6010. doi: 10.1128/jb.174.19.6005-6010.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto H., Lopez R., Tomasz A. Cell surface-located deoxyribonucleic acid receptors in transformable pneumococci. J Bacteriol. 1975 Jun;122(3):1339–1350. doi: 10.1128/jb.122.3.1339-1350.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw C. H., Watson M. D., Carter G. H., Shaw C. H. The right hand copy of the nopaline Ti-plasmid 25 bp repeat is required for tumour formation. Nucleic Acids Res. 1984 Aug 10;12(15):6031–6041. doi: 10.1093/nar/12.15.6031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasu K., Kado C. I. Membrane location of the Ti plasmid VirB proteins involved in the biosynthesis of a pilin-like conjugative structure on Agrobacterium tumefaciens. FEMS Microbiol Lett. 1993 Aug 1;111(2-3):287–294. doi: 10.1111/j.1574-6968.1993.tb06400.x. [DOI] [PubMed] [Google Scholar]
- Shirasu K., Kado C. I. The virB operon of the Agrobacterium tumefaciens virulence regulon has sequence similarities to B, C and D open reading frames downstream of the pertussis toxin-operon and to the DNA transfer-operons of broad-host-range conjugative plasmids. Nucleic Acids Res. 1993 Jan 25;21(2):353–354. doi: 10.1093/nar/21.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasu K., Morel P., Kado C. I. Characterization of the virB operon of an Agrobacterium tumefaciens Ti plasmid: nucleotide sequence and protein analysis. Mol Microbiol. 1990 Jul;4(7):1153–1163. doi: 10.1111/j.1365-2958.1990.tb00690.x. [DOI] [PubMed] [Google Scholar]
- Silverstein J. L., Goldberg E. B. T4 DNA injection. II. Protection of entering DNA from host exonuclease V. Virology. 1976 Jul 1;72(1):212–223. doi: 10.1016/0042-6822(76)90324-x. [DOI] [PubMed] [Google Scholar]
- Singh R. N. Number of deoxyribonucleic acid uptake sites in competent cells of Bacillus subtilis. J Bacteriol. 1972 Apr;110(1):266–272. doi: 10.1128/jb.110.1.266-272.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skurray R. A., Hancock R. E., Reeves P. Con--mutants: class of mutants in Escherichia coli K-12 lacking a major cell wall protein and defective in conjugation and adsorption of a bacteriophage. J Bacteriol. 1974 Sep;119(3):726–735. doi: 10.1128/jb.119.3.726-735.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. O., Danner D. B., Deich R. A. Genetic transformation. Annu Rev Biochem. 1981;50:41–68. doi: 10.1146/annurev.bi.50.070181.000353. [DOI] [PubMed] [Google Scholar]
- Smith H., Wiersma K., Bron S., Venema G. Transformation in Bacillus subtilis: purification and partial characterization of a membrane-bound DNA-binding protein. J Bacteriol. 1983 Oct;156(1):101–108. doi: 10.1128/jb.156.1.101-108.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H., Wiersma K., Venema G., Bron S. Transformation in Bacillus subtilis: a 75,000-dalton protein complex is involved in binding and entry of donor DNA. J Bacteriol. 1984 Mar;157(3):733–738. doi: 10.1128/jb.157.3.733-738.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H., Wiersma K., Venema G., Bron S. Transformation in Bacillus subtilis: further characterization of a 75,000-dalton protein complex involved in binding and entry of donor DNA. J Bacteriol. 1985 Oct;164(1):201–206. doi: 10.1128/jb.164.1.201-206.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H., de Vos W., Bron S. Transformation in Bacillus subtilis: properties of DNA-binding-deficient mutants. J Bacteriol. 1983 Jan;153(1):12–20. doi: 10.1128/jb.153.1.12-20.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachel S. E., Nester E. W., Zambryski P. C. A plant cell factor induces Agrobacterium tumefaciens vir gene expression. Proc Natl Acad Sci U S A. 1986 Jan;83(2):379–383. doi: 10.1073/pnas.83.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachel S. E., Timmerman B., Zambryski P. Activation of Agrobacterium tumefaciens vir gene expression generates multiple single-stranded T-strand molecules from the pTiA6 T-region: requirement for 5' virD gene products. EMBO J. 1987 Apr;6(4):857–863. doi: 10.1002/j.1460-2075.1987.tb04831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachel S. E., Zambryski P. C. Agrobacterium tumefaciens and the susceptible plant cell: a novel adaptation of extracellular recognition and DNA conjugation. Cell. 1986 Oct 24;47(2):155–157. doi: 10.1016/0092-8674(86)90437-x. [DOI] [PubMed] [Google Scholar]
- Stachel S. E., Zambryski P. C. virA and virG control the plant-induced activation of the T-DNA transfer process of A. tumefaciens. Cell. 1986 Aug 1;46(3):325–333. doi: 10.1016/0092-8674(86)90653-7. [DOI] [PubMed] [Google Scholar]
- Steck T. R., Close T. J., Kado C. I. High levels of double-stranded transferred DNA (T-DNA) processing from an intact nopaline Ti plasmid. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2133–2137. doi: 10.1073/pnas.86.7.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steck T. R., Kado C. I. Virulence genes promote conjugative transfer of the Ti plasmid between Agrobacterium strains. J Bacteriol. 1990 Apr;172(4):2191–2193. doi: 10.1128/jb.172.4.2191-2193.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steck T. R., Lin T. S., Kado C. I. VirD2 gene product from the nopaline plasmid pTiC58 has at least two activities required for virulence. Nucleic Acids Res. 1990 Dec 11;18(23):6953–6958. doi: 10.1093/nar/18.23.6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart G. J., Carlson C. A. The biology of natural transformation. Annu Rev Microbiol. 1986;40:211–235. doi: 10.1146/annurev.mi.40.100186.001235. [DOI] [PubMed] [Google Scholar]
- Strauss N. Further evidence concerning the configuration of transforming deoxyribonucleic acid during entry into Bacillus subtilis. J Bacteriol. 1966 Feb;91(2):702–708. doi: 10.1128/jb.91.2.702-708.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara E., Nikaido H. Pore-forming activity of OmpA protein of Escherichia coli. J Biol Chem. 1992 Feb 5;267(4):2507–2511. [PubMed] [Google Scholar]
- Sun T. P., Webster R. E. fii, a bacterial locus required for filamentous phage infection and its relation to colicin-tolerant tolA and tolB. J Bacteriol. 1986 Jan;165(1):107–115. doi: 10.1128/jb.165.1.107-115.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szmelcman S., Hofnung M. Maltose transport in Escherichia coli K-12: involvement of the bacteriophage lambda receptor. J Bacteriol. 1975 Oct;124(1):112–118. doi: 10.1128/jb.124.1.112-118.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarahovsky Y. S., Khusainov A. A., Deev A. A., Kim Y. V. Membrane fusion during infection of Escherichia coli cells by phage T4. FEBS Lett. 1991 Sep 2;289(1):18–22. doi: 10.1016/0014-5793(91)80899-e. [DOI] [PubMed] [Google Scholar]
- Ter-Nikogosian V. A., Vartanian M. K., Trchunian A. A. Indutsirovannye bakteriofagami izmeneniia membrannogo potentsiala i transporta ionov cherez membranu S. typhimurium LT2. Biofizika. 1991 Mar-Apr;36(2):281–285. [PubMed] [Google Scholar]
- Thomas D., Prevelige P., Jr A pilot protein participates in the initiation of P22 procapsid assembly. Virology. 1991 Jun;182(2):673–681. doi: 10.1016/0042-6822(91)90608-e. [DOI] [PubMed] [Google Scholar]
- Thomashow M. F., Karlinsey J. E., Marks J. R., Hurlbert R. E. Identification of a new virulence locus in Agrobacterium tumefaciens that affects polysaccharide composition and plant cell attachment. J Bacteriol. 1987 Jul;169(7):3209–3216. doi: 10.1128/jb.169.7.3209-3216.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorstenson Y. R., Kuldau G. A., Zambryski P. C. Subcellular localization of seven VirB proteins of Agrobacterium tumefaciens: implications for the formation of a T-DNA transport structure. J Bacteriol. 1993 Aug;175(16):5233–5241. doi: 10.1128/jb.175.16.5233-5241.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomb J. F. A periplasmic protein disulfide oxidoreductase is required for transformation of Haemophilus influenzae Rd. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10252–10256. doi: 10.1073/pnas.89.21.10252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomb J. F., Barcak G. J., Chandler M. S., Redfield R. J., Smith H. O. Transposon mutagenesis, characterization, and cloning of transformation genes of Haemophilus influenzae Rd. J Bacteriol. 1989 Jul;171(7):3796–3802. doi: 10.1128/jb.171.7.3796-3802.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomb J. F., el-Hajj H., Smith H. O. Nucleotide sequence of a cluster of genes involved in the transformation of Haemophilus influenzae Rd. Gene. 1991 Jul 31;104(1):1–10. doi: 10.1016/0378-1119(91)90457-m. [DOI] [PubMed] [Google Scholar]
- Toro N., Datta A., Carmi O. A., Young C., Prusti R. K., Nester E. W. The Agrobacterium tumefaciens virC1 gene product binds to overdrive, a T-DNA transfer enhancer. J Bacteriol. 1989 Dec;171(12):6845–6849. doi: 10.1128/jb.171.12.6845-6849.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro N., Datta A., Yanofsky M., Nester E. Role of the overdrive sequence in T-DNA border cleavage in Agrobacterium. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8558–8562. doi: 10.1073/pnas.85.22.8558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres O. R., Korman R. Z., Zahler S. A., Dunny G. M. The conjugative transposon Tn925: enhancement of conjugal transfer by tetracycline in Enterococcus faecalis and mobilization of chromosomal genes in Bacillus subtilis and E. faecalis. Mol Gen Genet. 1991 Mar;225(3):395–400. doi: 10.1007/BF00261679. [DOI] [PubMed] [Google Scholar]
- Traxler B. A., Minkley E. G., Jr Evidence that DNA helicase I and oriT site-specific nicking are both functions of the F TraI protein. J Mol Biol. 1988 Nov 5;204(1):205–209. doi: 10.1016/0022-2836(88)90609-2. [DOI] [PubMed] [Google Scholar]
- Traxler B. A., Minkley E. G., Jr Revised genetic map of the distal end of the F transfer operon: implications for DNA helicase I, nicking at oriT, and conjugal DNA transport. J Bacteriol. 1987 Jul;169(7):3251–3259. doi: 10.1128/jb.169.7.3251-3259.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umlauf B., Dreiseikelmann B. Cloning, sequencing, and overexpression of gene 16 of Salmonella bacteriophage P22. Virology. 1992 Jun;188(2):495–501. doi: 10.1016/0042-6822(92)90503-h. [DOI] [PubMed] [Google Scholar]
- Vagner V., Claverys J. P., Ehrlich S. D., Méjean V. Direction of DNA entry in competent cells of Bacillus subtilis. Mol Microbiol. 1990 Oct;4(10):1785–1788. doi: 10.1111/j.1365-2958.1990.tb00557.x. [DOI] [PubMed] [Google Scholar]
- Van Alphen L., Havekes L., Lugtenberg B. Major outer membrane protein d of Escherichia coli K12. Purification and in vitro activity of bacteriophages k3 and f-pilus mediated conjugation. FEBS Lett. 1977 Mar 15;75(1):285–290. doi: 10.1016/0014-5793(77)80104-x. [DOI] [PubMed] [Google Scholar]
- Venema G. Bacterial transformation. Adv Microb Physiol. 1979;19:245–331. doi: 10.1016/s0065-2911(08)60200-3. [DOI] [PubMed] [Google Scholar]
- Vijayakumar M. N., Morrison D. A. Localization of competence-induced proteins in Streptococcus pneumoniae. J Bacteriol. 1986 Mar;165(3):689–695. doi: 10.1128/jb.165.3.689-695.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosman B., Kuiken G., Kooistra J., Venema G. Transformation in Bacillus subtilis: involvement of the 17-kilodalton DNA-entry nuclease and the competence-specific 18-kilodalton protein. J Bacteriol. 1988 Aug;170(8):3703–3710. doi: 10.1128/jb.170.8.3703-3710.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. E., Saraste M., Runswick M. J., Gay N. J. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1(8):945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Herrera-Estrella A., Van Montagu M. Overexpression of virD1 and virD2 genes in Agrobacterium tumefaciens enhances T-complex formation and plant transformation. J Bacteriol. 1990 Aug;172(8):4432–4440. doi: 10.1128/jb.172.8.4432-4440.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Herrera-Estrella L., Van Montagu M., Zambryski P. Right 25 bp terminus sequence of the nopaline T-DNA is essential for and determines direction of DNA transfer from agrobacterium to the plant genome. Cell. 1984 Sep;38(2):455–462. doi: 10.1016/0092-8674(84)90500-2. [DOI] [PubMed] [Google Scholar]
- Wang K., Stachel S. E., Timmerman B., VAN Montagu M., Zambryski P. C. Site-Specific Nick in the T-DNA Border Sequence as a Result of Agrobacterium vir Gene Expression. Science. 1987 Jan 30;235(4788):587–591. doi: 10.1126/science.235.4788.587. [DOI] [PubMed] [Google Scholar]
- Ward J. E., Akiyoshi D. E., Regier D., Datta A., Gordon M. P., Nester E. W. Characterization of the virB operon from an Agrobacterium tumefaciens Ti plasmid. J Biol Chem. 1988 Apr 25;263(12):5804–5814. [PubMed] [Google Scholar]
- Ward J. E., Jr, Dale E. M., Binns A. N. Activity of the Agrobacterium T-DNA transfer machinery is affected by virB gene products. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9350–9354. doi: 10.1073/pnas.88.20.9350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. E., Jr, Dale E. M., Christie P. J., Nester E. W., Binns A. N. Complementation analysis of Agrobacterium tumefaciens Ti plasmid virB genes by use of a vir promoter expression vector: virB9, virB10, and virB11 are essential virulence genes. J Bacteriol. 1990 Sep;172(9):5187–5199. doi: 10.1128/jb.172.9.5187-5199.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. E., Jr, Dale E. M., Nester E. W., Binns A. N. Identification of a virB10 protein aggregate in the inner membrane of Agrobacterium tumefaciens. J Bacteriol. 1990 Sep;172(9):5200–5210. doi: 10.1128/jb.172.9.5200-5210.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters V. L., Hirata K. H., Pansegrau W., Lanka E., Guiney D. G. Sequence identity in the nick regions of IncP plasmid transfer origins and T-DNA borders of Agrobacterium Ti plasmids. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1456–1460. doi: 10.1073/pnas.88.4.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters V. L., Strack B., Pansegrau W., Lanka E., Guiney D. G. Mutational analysis of essential IncP alpha plasmid transfer genes traF and traG and involvement of traF in phage sensitivity. J Bacteriol. 1992 Oct;174(20):6666–6673. doi: 10.1128/jb.174.20.6666-6673.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster R. E. The tol gene products and the import of macromolecules into Escherichia coli. Mol Microbiol. 1991 May;5(5):1005–1011. doi: 10.1111/j.1365-2958.1991.tb01873.x. [DOI] [PubMed] [Google Scholar]
- Weiss A. A., Johnson F. D., Burns D. L. Molecular characterization of an operon required for pertussis toxin secretion. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2970–2974. doi: 10.1073/pnas.90.7.2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitchurch C. B., Hobbs M., Livingston S. P., Krishnapillai V., Mattick J. S. Characterisation of a Pseudomonas aeruginosa twitching motility gene and evidence for a specialised protein export system widespread in eubacteria. Gene. 1991 May 15;101(1):33–44. doi: 10.1016/0378-1119(91)90221-v. [DOI] [PubMed] [Google Scholar]
- Willetts N., Skurray R. The conjugation system of F-like plasmids. Annu Rev Genet. 1980;14:41–76. doi: 10.1146/annurev.ge.14.120180.000353. [DOI] [PubMed] [Google Scholar]
- Willetts N., Wilkins B. Processing of plasmid DNA during bacterial conjugation. Microbiol Rev. 1984 Mar;48(1):24–41. doi: 10.1128/mr.48.1.24-41.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winans S. C., Allenza P., Stachel S. E., McBride K. E., Nester E. W. Characterization of the virE operon of the Agrobacterium Ti plasmid pTiA6. Nucleic Acids Res. 1987 Jan 26;15(2):825–837. doi: 10.1093/nar/15.2.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winans S. C. Two-way chemical signaling in Agrobacterium-plant interactions. Microbiol Rev. 1992 Mar;56(1):12–31. doi: 10.1128/mr.56.1.12-31.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. H., Ippen-Ihler K. Nucleotide sequence of traQ and adjacent loci in the Escherichia coli K-12 F-plasmid transfer operon. J Bacteriol. 1989 Jan;171(1):213–221. doi: 10.1128/jb.171.1.213-221.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky M. F., Porter S. G., Young C., Albright L. M., Gordon M. P., Nester E. W. The virD operon of Agrobacterium tumefaciens encodes a site-specific endonuclease. Cell. 1986 Nov 7;47(3):471–477. doi: 10.1016/0092-8674(86)90604-5. [DOI] [PubMed] [Google Scholar]
- Young C., Nester E. W. Association of the virD2 protein with the 5' end of T strands in Agrobacterium tumefaciens. J Bacteriol. 1988 Aug;170(8):3367–3374. doi: 10.1128/jb.170.8.3367-3374.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambryski P. Basic processes underlying Agrobacterium-mediated DNA transfer to plant cells. Annu Rev Genet. 1988;22:1–30. doi: 10.1146/annurev.ge.22.120188.000245. [DOI] [PubMed] [Google Scholar]
- Zambryski P., Tempe J., Schell J. Transfer and function of T-DNA genes from agrobacterium Ti and Ri plasmids in plants. Cell. 1989 Jan 27;56(2):193–201. doi: 10.1016/0092-8674(89)90892-1. [DOI] [PubMed] [Google Scholar]
- Ziegelin G., Pansegrau W., Strack B., Balzer D., Kröger M., Kruft V., Lanka E. Nucleotide sequence and organization of genes flanking the transfer origin of promiscuous plasmid RP4. DNA Seq. 1991;1(5):303–327. doi: 10.3109/10425179109020786. [DOI] [PubMed] [Google Scholar]
- Zoon K. C., Habersat M., Scocca J. J. Synthesis of envelope polypeptides by Haemophilus influenzae during development of competence for genetic transformation. J Bacteriol. 1976 Jul;127(1):545–554. doi: 10.1128/jb.127.1.545-554.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoon K. C., Scocca J. J. Constitution of the cell envelope of Haemophilus influenzae in relation to competence for genetic transformation. J Bacteriol. 1975 Aug;123(2):666–677. doi: 10.1128/jb.123.2.666-677.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Enfert C., Reyss I., Wandersman C., Pugsley A. P. Protein secretion by gram-negative bacteria. Characterization of two membrane proteins required for pullulanase secretion by Escherichia coli K-12. J Biol Chem. 1989 Oct 15;264(29):17462–17468. [PubMed] [Google Scholar]



