Abstract
Objective
Examine anti-citrullinated protein/peptide antibodies (ACPA) reactivity and determine associations between ACPA and other rheumatoid arthritis (RA)-related autoantibodies and clinically-assessed swollen or tender joints in first-degree relatives (FDRs) without 1987 and 2010 American College of Rheumatology classified RA.
Methods
A bead-based assay measured 16 separate ACPA in sera from 111 FDRs (Ab+) who were positive on at least one visit for any of 5 RA-related autoantibodies (RF, anti-CCP2, and RF isotypes), and 99 FDRs (Ab−) who were never autoantibody positive. Cut-offs for positivity for each ACPA were determined using receiver operating characteristic curves of data from 200 RA cases and 98 blood-bank controls, wherein positivity for ≥ 9 ACPA had 92% specificity and 62% sensitivity for RA. In FDRs, we assessed ACPA reactivity and examined associations between ACPA (number positive and positivity for ≥ 9 ACPA) and RA-related characteristics.
Results
Four of 7 anti-CCP2 positive and 8% of anti-CCP2 negative FDRs were positive for ≥ 9 ACPA. After adjusting for age, gender, ethnicity and pack-years of smoking, increasing number of ACPA was directly associated with having ≥ 1 tender joint on exam (OR=1.18, 95% CI 1.04–1.34), with the greatest risk seen in FDRs positive for ≥ 9 ACPA (OR=5.00, 95% CI 1.37–18.18).
Conclusions
RA-free FDRs demonstrate reactivity to multiple ACPA, even in those negative for rheumatoid factor and anti-CCP2, and increasing ACPA may be associated with signs of joint inflammation. Prospective evaluation of the relationship between these findings and progression of classifiable RA is warranted.
Keywords: pre-clinical RA, autoantibodies, ACPA, rheumatoid arthritis
Rheumatoid arthritis (RA) is a chronic systemic inflammatory disease of unknown etiology that leads to joint damage, significant disability and reduced life expectancy (1). Nearly 70% of cases of established rheumatoid arthritis (RA) are characterized by the presence of autoantibodies, either rheumatoid factor (RF) or antibodies to citrullinated protein antigens (ACPA), of which anti-cyclic citrullinated peptide (CCP) antibodies are the most specific clinical test currently available. The presence of RF and anti-CCP is routinely tested for and can aid in making a diagnosis of RA; however, the prospective sensitivity and specificity of these tests are still uncertain in clinically unaffected populations (2, 3). In addition, ACPA antibodies recognize many citrullinated epitopes, thereby limiting the ability to make inferences about the type and expansion of unique ACPA responses (4, 5).
Development of RA has not been associated with recognition of a specific citrullinated epitope, although seropositive arthralgia patients with an expanded ACPA repertoire have a higher risk of developing arthritis (6), and a recent study indicated specific patterns prior to symptom onset may exist (7). While the full extent of reactivity is unknown, ACPA have been shown to bind to citrullinated epitopes on fibrinogen, alpha-enolase, vimentin, collagen type II, histones, and biglycan (4, 7–16). ACPA likely play a role in the pathogenesis of rheumatoid arthritis. In murine models of arthritis, ACPA induce disease (17), increase disease severity (18), and enhance tissue injury (5). ACPA have been shown to activate complement through both the classical and alternative pathways (19), are found in circulating immune complexes (20), and stimulate macrophage production of tumor necrosis factor-alpha through Toll-like receptor 4 and Fc gamma receptor (21, 22). ACPA are highly specific for the diagnosis of RA and are present in the blood for a significant period of time prior to symptom onset, as demonstrated by previous biobank studies examining ACPA in stored samples obtained from individuals who subsequently developed signs and symptoms and were diagnosed with RA (23–25). In addition, spreading of ACPA to additional citrullinated epitopes can occur years prior to diagnosis (8, 9, 11), with increasing titers nearer disease onset (8, 23, 24), suggesting an expansion of autoimmunity in early RA development that, if fully understood, may provide insight into the earliest antigenic targets important in disease pathogenesis.
First-degree relatives (FDRs) of individuals with RA are at increased risk of developing RA (26). As these individuals do not have clinically apparent disease but are at increased risk for future RA, they are an informative population in which to study relationships between RA-related autoantibodies, epidemiologic exposures and potential etiologies of RA (27–34). Previous ACPA studies in unaffected family members have indicated an increased prevalence of positivity to ACPA compared to healthy control subjects (27, 35). When characterization of the ACPA epitope response was performed on a subset of the subjects studied, few unaffected relatives showed any reaction to the eight citrullinated epitopes studied (35), which were abnormal in patients with established RA, suggesting that evolution of ACPA reactivity is an important part of a transition from asymptomatic autoimmunity to symptomatic inflammatory arthritis.
The goals of these analyses were to examine whether ACPA array testing detects autoimmunity in individuals at risk of RA beyond testing with anti-CCP2 and RF, and whether this autoimmunity is biologically relevant as indicated by joint findings such as tenderness and swelling that may be indicative of early inflammatory arthritis. Specifically, in our well-characterized cohort of FDRs without classified RA, we examined evidence of reactivity to a panel of ACPA that has been shown to measure epitope spreading prior to RA diagnosis (8). In addition, we examined the association of ACPA with RA-related characteristics, including positivity for RA-related autoantibodies available through standard clinical testing, that is, RF by several methods and anti-CCP2, as well as independently assessed swollen or tender joints on examination.
Materials and Methods
Study Population
Studies of the Etiology of Rheumatoid Arthritis (SERA) is designed to examine the role of environmental and genetic factors in the development and progression of RA-related autoimmunity and to explore pre-clinical immunological changes and other relevant phenotypes (32). Probands with RA are identified from academic centers, Veterans’ hospitals, and private and public sector rheumatology clinics at sites based in Denver, New York, Chicago, Omaha (as center of the Rheumatoid Arthritis Investigational Network [RAIN]), Seattle and Los Angeles. Probands must meet ≥ 4 1987 American College of Rheumatology (ACR) classification criteria for RA based on chart review, or have a diagnosis of RA from a board-certified rheumatologist (36).
FDRs of the probands with RA are recruited into SERA, as their risk for RA is estimated to be increased 3–9-fold over the general population (26). The definition of FDR includes parent, full sibling or offspring of a proband. Recruitment of FDRs occurs through their probands or responses to advertising. FDRs are eligible to participate in the study if they do not have a diagnosis of RA and are ≥ 18 years old. At the initial research visit, FDRs complete disease and exposure assessment questionnaires, undergo a standardized interview and 68-count joint examination by a trained study physician or nurse, and have blood drawn. FDRs determined to have RA based on 1987 ACR Criteria (36) at the time of their initial visit are excluded from the SERA longitudinal cohort. All eligible FDRs are invited for longitudinal follow-up, including blood draws, exam, interview and questionnaires; FDRs that are found to be positive for any RA-related autoantibody at any visit are seen annually, and autoantibody negative FDRs are seen every other year.
As of October 2009, 1421 FDRs had been evaluated at least once in SERA. From this cohort, 236 were positive for any of 5 RA-associated autoantibodies (RF, RF-isotypes – IgM, IgG, and IgA, or anti-CCP2 autoantibody) on at least one of their visits, while 1185 were autoantibody negative. We selected 113 FDRs who had been both autoantibody positive and seen for at least 2 visits (Ab+ FDRs), for a total of 297 visits; one FDR had errors on their ACPA assay, leaving 112 Ab+ FDRs for analysis. We also selected one visit from 100 FDRs who had never been autoantibody positive at any visit (Ab− FDRs), frequency matched to the 113 autoantibody positive FDRs on age, gender, and ethnicity. This method of FDR selection allowed us to maximize the number of visits positive for either autoantibody phenotype analyzed. While no FDRs are included in SERA if they met 1987 classification criteria for RA at their initial visit, four FDRs were positive for RA using the 2010 ACR/EULAR criteria on at least one of their visits. Therefore, to ensure that the population we examined was truly RA-free, we removed all visits where the FDR met 2010 ACR/EULAR classification criteria. This removed 5 visits from our analyses, and left 111 Ab+ FDRs with 292 visits and 99 Ab− FDRs available for analysis.
Autoantibody Studies
All samples were tested for rheumatoid factor (RF), RF isotypes RF-IgM, -IgG, and -IgA, and anti-cyclic citrullinated peptide (anti-CCP) autoantibody. Rheumatoid factor (IU/ml) was measured by nephelometry using the Dade Behring BN100 system. RF IgM, IgG, and IgA (IU/mL) were measured using ELISA (Quanta Lite™) kits to manufacturer’s specifications (INOVA Diagnostics, Inc, San Diego, California); anti-CCP (U/ml) was measured using anti-CCP2 ELISA assay (Diastat, Axis-Shield Diagnostics, Ltd., Dundee, Scotland, United Kingdom). We established a dichotomous cut-off level for each of the RF assays according to the 1987 ACR RA criteria specifying a positive RF level if present in <5% of 491 blood donor controls, separate from the SERA FDR population (36). Anti-CCP was considered positive using the manufacturer’s recommendation of greater than 5 U/ml. Positivity for the high-risk autoantibody profile, which our prior studies have determined to be 96% specific for future RA (37) and which other studies support (23, 24, 38–40), was defined as being positive for anti-CCP2 autoantibody and/or 2 of the RF isotypes: IgM, IgG, or IgA.
To identify autoantibody reactivities to specific citrullinated antigens, a novel multiplex platform was developed (41) using 16 citrullinated autoantigens and three native proteins that are not targeted in RA as background controls (8). This array uses a custom Bio-Plex™ (BioRad, Hercules, CA) bead-based autoantibody assay in which antigens are conjugated to spectrally-distinct beads. Protein antigens were coupled to beads using N-hydroxysuccinimide ester chemistry, and peptide antigens synthesized with C- terminal biotin (by Fmoc chemistry) and coupled to avidin-coated beads. Pooled beads were mixed with serum samples and diluents and incubated at room temperature. After washing, anti-human IgG antibody conjugated to phycoerythrin (PE) was added to the dyed beads and incubated at room temperature. After another wash, the bead mixture was passed through a laser detector (Luminex 200, Austin, TX) that identifies beads based on the fluorescence of the dyes. The amount of antibody bound to each bead was determined by the fluorescence of PE.
Determination of Cutoffs for ACPA Positivity
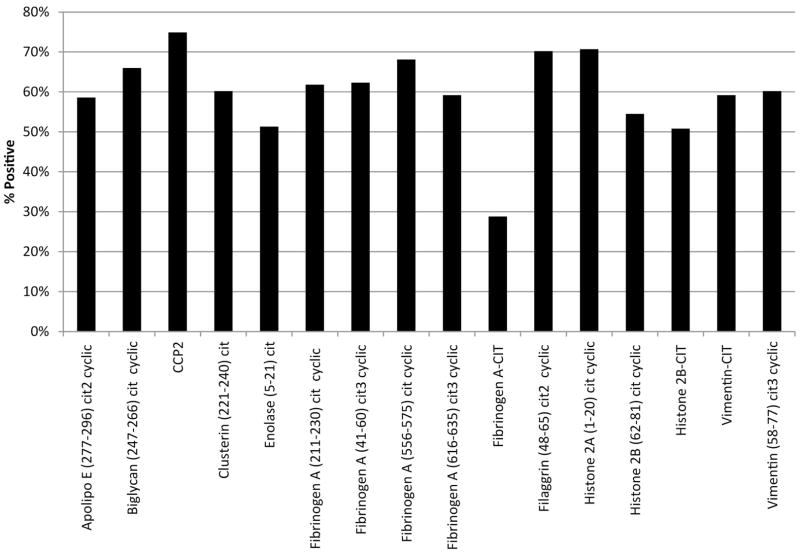
Cutoffs for positivity for each of the ACPA individually were developed using receiver operating characteristic (ROC) curves of data from 200 RA probands and 98 blood donor controls. Values that gave greater than 90% specificity for RA were used as cutoffs to define positivity of individual ACPA. Positivity for the epitopes in the RA probands ranged from 29%–75% (Figure 1). We also used a ROC curve to determine a cutoff for total number of positive ACPAs (e.g. 0, 1, 2, etc.) that would give greater than 90% specificity for RA.
Figure 1.
Percent of RA Probands Positive for Each ACPA. Prevalence ranges between 29% for citrullinated Fibrinogen A to 75% for CCP2, with the majority of probands with RA positivity for greater than 50% of each of the epitopes.
Statistical Analysis
All analyses were conducted in SAS (SAS Version 9.3, Cary, North Carolina). Associations were examined with predictor variables being the number of positive ACPA as an ordinal measure (ranging from 0–16), and a dichotomous measure of positivity for ≥ 9 ACPA, an ACPA count which demonstrated 92% specificity and 62% sensitivity for RA in ROC analysis. Our outcomes were 1) the presence of RF by nephelometry, 2) the presence of RF isotypes IgG, IgM, or IgA individually 3) the presence of the high-risk profile (positive for anti-CCP2 and/or 2 RF isotypes), and 4) the presence of swollen or tender joints on examination (≥ 1 affected joint). Exclusions for swollen or tender joint on examination were made for injury, degenerative joint disease and findings in the first metatarsophalangeal joint, which is more commonly affected by osteoarthritis rather than RA. These exclusions were considered negative for swollen or tender joints. As there were few anti-CCP2 positive FDRs (n=7), we did not analyze anti-CCP2 positivity as an independent outcome.
Relationships between ACPA and our dichotomous outcomes of RF positivity, RF-isotype positivity, high-risk profile positivity, ≥ 1 swollen joint, and ≥ 1 tender joint were calculated using a non-linear mixed model accounting for multiple visits per subject (1 visit per autoantibody negative FDR, and an average 2.62 visits per autoantibody positive FDR). This logistic regression model with random subject effects assumed a logit link function and an outcome with a Bernouilli distribution conditional on subjects. Mixed models account for multiple visits per subject by distinguishing variability between subjects from variability between repeated measurements on the same subject. These models estimate these two components separately and then using functions of these variance estimates as weights to provide the best statistically available method to adjust for differing numbers of visits per subject. Results are reported as odds ratios (OR) and 95% confidence intervals (CI). All multivariate analyses were adjusted for age, gender, ethnicity, and pack years of smoking.
Results
Characteristics of the study population at the first visit are presented in Table 1. Among autoantibody positive FDRs (n=111), mean age was 49.3 ± 17.3 years, while among autoantibody negative FDRs (n=99), mean age was 49.8 ± 17.6 years (p=0.83). Both groups were 75% female and 82% non-Hispanic white (p=1.0). Thirty-two percent of autoantibody positive FDRs and 38% of autoantibody negative FDRs had ever smoked (p=0.32), while approximately 9% of both groups were current smokers. Among autoantibody positive FDRs, 9.9% had at least one swollen joint and 6.3% had at least one tender joint; among autoantibody negative FDRs, 4.0% had at least one swollen joint and 1.0% had at least one tender joint. Of the autoantibody positive FDRs, 30.6% were positive for RF by nephelometry, 17.1% were positive for the high-risk profile, and 6.3% were positive for anti-CCP2 autoantibodies.
Table 1.
Characteristics of Antibody Positive and Antibody Negative First Degree Relatives at the First Visit.
| Ab+ FDRs (n=111) | Ab− FDRs (n=99) | p-value | |
|---|---|---|---|
| Age in years: mean ± SD | 49.3 ± 17.3 | 49.8 ± 17.8 | 0.83 |
| Female gender (n, %) | 83 (74.7%) | 74 (74.8%) | 1.0 |
| Non-Hispanic white ethnicity (n, %) | 91 (82.0%) | 82 (82.8%) | 0.68 |
| Ever smoker (n, %) | 35 (31.5%) | 38 (38.1%) | 0.32 |
| Current smoker (n, %) | 10 (9.1%) | 9 (9.3%) | 0.96 |
| RF neph positive (n, %) | 34 (30.6%) | 0 (0.0%) | N/A |
| RF IgM positive (n, %) | 27 (24.3%) | 0 (0.0%) | N/A |
| RF IgG positive (n, %) | 37 (33.3%) | 0 (0.0%) | N/A |
| RF IgA positive (n, %) | 21 (18.9%) | 0 (0.0%) | N/A |
| Anti-CCP positive (n, %) | 7 (6.3%) | 0 (0.0%) | N/A |
| High-risk profile positive (n, %) | 19 (17.1%) | 0 (0.0%) | N/A |
| Number of ACPA: mean ± SD | 2.3 ± 4.0 | 1.4 ± 2.8 | 0.08 |
| ACPA ≥9 (n, %) | 14 (12.6%) | 7 (7.1%) | 0.18 |
| Swollen joints (≥1) on exam (n, %) | 11 (9.9%) | 4 (4.0%) | 0.10 |
| Tender joints (≥1) on exam (n, %) | 7 (6.3%) | 1 (1.0%) | 0.045 |
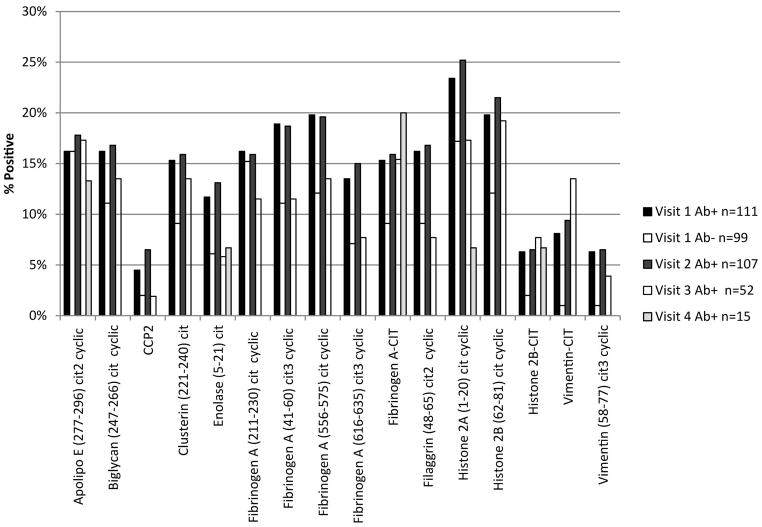
Fourteen Ab+ FDRs (12.6%) and 7 Ab− FDRs (7.1%) were positive for at least 9 APCA (p=0.18) (Table 1). As an entire group, Ab− FDRs were positive for a mean of 1.4 ± 2.8 ACPA, only one ACPA less than the mean in Ab+ FDRs (2.3 ± 4.0) (p=0.08). A greater proportion of Ab+ FDRs were positive for citrullinated clusterin 5 (231–250), and citrullinated cyclic vimentin (58–77) compared with Ab− FDRs (p=0.002 and p=0.045, respectively). The percent of FDRs positive for each ACPA by visit are shown in Figure 2.
Figure 2.
Percent of FDRs Positive for Each ACPA by Visit. The percent positive remains relatively stable over successive visits. Only three individuals had a fifth visit, and are therefore not included in this figure due to small numbers.
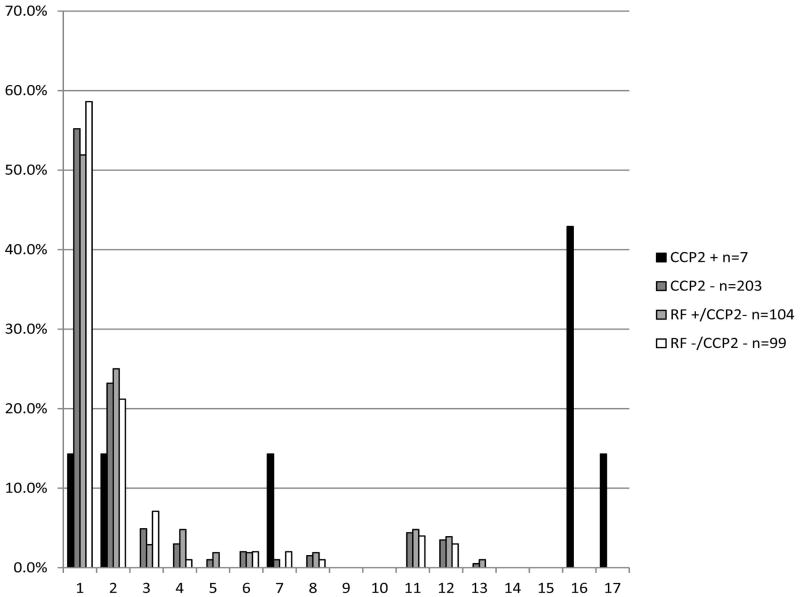
We then examined ACPA positivity by positivity for RF by all assays, as well as anti-CCP2 positivity, as measured by ELISA. FDRs in our study who were anti-CCP2 positive were positive for a mean of 9.7 ± 7.2 ACPA, while those who were anti-CCP2 negative were positive for a mean of 1.6 ± 3.0 (p<0.0001). Of the seven anti-CCP2 positive FDRs, 57.1% were positive for at least 9 ACPA (Figure 3). While 55% of anti-CCP2 negative FDRs were not positive for any ACPA, it is notable that approximately 8% of subjects were positive for nine or more ACPA. Within the anti-CCP2 negative group, FDRSs who were RF positive were positive for a mean of 1.8 ± 3.2 ACPA, while FDRs who were RF negative were positive for a mean of 1.4 ± 2.8 ACPA (p=0.39). Seven percent of RF negative individuals were positive for at least 9 ACPA, while 9.7% of RF positive individuals were positive for at least 9 ACPA (Figure 3).
Figure 3.
Proportion of FDRs Positive for Each Number of ACPA by CCP2 and RF Status at the First Visit. For individuals negative for both anti-CCP2 and RF, 41.4% were positive for at least one ACPA. 57.1% of anti-CCP2 positive FDRs and 8.4% of anti-CCP2 negative FDRs were positive for 9 or more ACPA. Of the anti-CCP2 negative FDRs, 9.7% of RF positive FDRs and 7.1% of RF negative FDRs were positive for at least 9 ACPA.
We then examined associations with RF by nephelometry, RF isotypes and the high-risk profile. Using a non-linear mixed model to account for multiple visits per person, and adjusting for age, gender, ethnicity and pack-years of smoking, increasing number of ACPA was significantly associated with being positive for RF by nephelometry, RF-IgA, and the high-risk autoantibody profile (Table 2). Those positive for at least 9 ACPA had a 6-fold increased risk of having the high-risk profile than those with < 9 ACPA, and a 4.5-fold increased risk of being positive for RF-IgM. Removal of the anti-CCP2 positive individuals attenuated these results towards the null. The greatest effect was on the high-risk profile, which includes anti-CCP2 positivity in its definition (Table 2).
Table 2.
Association Between Increasing Number of ACPA and RA-Related Characteristics.
| Outcome Variables
| |||||||
|---|---|---|---|---|---|---|---|
| RF Positivity OR (95% CI) |
RF-IgG Positivity OR (95% CI) |
RF-IgM Positivity OR (95% CI) |
RF-IgA Positivity OR (95% CI) |
High-Risk Profile OR (95% CI) |
≥1 Swollen Joint OR (95% CI) |
≥1 Tender Joint OR (95% CI) |
|
| Model 1: | |||||||
| Number of ACPA | 1.13 (1.0001–1.27) | 1.00 (0.90–1.11) | 1.16 (0.98–1.37) | 1.14 (1.01–1.29) | 1.21 (1.08–1.35) | 1.11 (0.96–1.28) | 1.18 (1.04–1.34) |
| ACPA ≥9 | 0.55 (0.03–8.83) | 1.30 (0.44–3.82) | 4.55 (1.09–18.91) | 3.24 (0.89–11.76) | 5.79 (1.83–18.35) | 3.64 (0.90–14.63) | 5.00 (1.37–18.18) |
| Model 2: | |||||||
| Number of ACPA | 1.01 (0.86–1.18) | 0.98 (0.86–1.11) | 1.14 (0.94–1.39) | 0.98 (0.83–1.15) | 1.02 (0.88–1.19) | 1.13 (0.95–1.34) | 1.22 (1.06–1.41) |
| ACPA ≥9 | 0.10 (0.002–5.03) | 1.23 (0.35–4.36) | 4.01 (0.83–19.23) | 0.79 (0.15–4.10) | 1.63 (0.40–6.60) | 3.94 (0.88–17.57) | 5.32 (1.32–21.32) |
All models are adjusted for age, gender, ethnicity and pack years, and account for multiple visits per subject.
Model 1 analyses include all individuals.
Model 2 analyses were run after removal of the 7 CCP positive individuals.
Lastly, we looked at possible signs of inflammation. Adjusting for age, gender, ethnicity and pack-years of smoking, increasing number of ACPA was associated with having at least one tender joint on exam (OR=1.18, 95% CI 1.04–1.34). Being positive for at least 9 ACPA indicated significantly greater odds of having at least one tender joint on exam (OR=5.00, 95% CI 1.37–18.18) compared to those who were positive for < 9 ACPA. These associations remained statistically significant after removal of the anti-CCP2 positive individuals (Table 2).
Discussion
In FDRs without 1987 and 2010 ACR classified RA, reactivity to multiple ACPA epitopes is evident, and increasing number of ACPA is associated with having at least one tender joint. A trend was observed for increasing number of ACPA to be associated with having at least one swollen joint, although this was not significant. Being positive for a greater number of ACPA is associated with being positive for RF in general, and with the IgA isotype specifically, and for the high-risk autoantibody profile (i.e., positive for anti-CCP2 and/or 2 RF isotypes). Moreover, those who were positive for at least 9 ACPA had even greater associations with RF-IgM and the high-risk profile, as well as tenderness on joint examination.
Of note, these results indicate the ACPA array utilized herein can detect autoantibodies in individuals that are negative for RF and anti-CCP2 assays. Therefore, this method may be a more sensitive measure than the commonly clinically-available tests for RF and CCP in detecting RA-related autoimmunity in at-risk individuals. Importantly, FDRs without classifiable RA who demonstrate reactivity with this ACPA testing to a broad array of citrullinated epitopes may also have more affected joints as detected by tenderness on exam, supporting that this ACPA array is associated with potentially biologically-meaningful inflammatory processes, if indeed this joint tenderness indicates joint inflammation. While the association with joint tenderness remained after removal of the CCP2 positive individuals, we did not have an appropriate number of Ab− FDRs with ≥ 1 tender joint to determine if this association could be seen in this group alone. Further testing of subsequent visits in this population will be informative to determine whether such an association exists.
A similar proportion of our Ab− population (7.1%) was positive for ≥ 9 ACPA compared to the blood donor population (7.9%) we used in the ROC analysis to determine the ACPA cutoffs. In addition, the blood donor population had a similar mean number of ACPA (1.5 ± 3.1) to that of the Ab− controls. This may indicate that the Ab− FDRs are not exhibiting any expanded ACPA reactivity. However, it should be noted that we do not know the disease status of the blood donor population, and cannot rule out the possibility that some of them are diagnosed with RA, are exhibiting joint inflammation, or are themselves at high-risk for RA. In fact, 2.3% of the blood donor population was positive for CCP2, of which 50% were positive for ≥ 9 ACPA. Limiting this to the blood donor population that is Ab− indicates that 6.3% would be positive for ≥ 9 ACPA. Efforts to test this assay on a truly disease-free, similarly clinically characterized healthy population without familial RA risk to determine what proportion is positive for ≥ 9 ACPA should be pursued.
It is of interest that there were not greater differences in the mean number of ACPA between our Ab+ and Ab− FDRs. The only significant differences were that a greater proportion of Ab+ FDRs were positive for citrullinated clusterin 5 (231–250), and citrullinated cyclic vimentin (58–77) compared with Ab-FDRs. While we only had one autoantibody negative visit for this analysis, it will be informative to observe over successive visits in these Ab− FDRs whether the number of ACPA and percent positive for each epitope remains stable or whether a greater expansion is observed compared to the Ab+ FDRs. Evaluating the number of positive ACPA may also be a way to identify those who are anti-CCP2 antibody negative who are more likely to progress to RA, although this has yet to be demonstrated. Both Ab+ and Ab− FDRs demonstrated reactivity to a wide range of citrullinated epitopes, raising the question of whether they are already showing epitope spreading, as seen in several studies (8, 9, 11).
As in previous studies (6, 8, 11), the ACPA response seems to be driven by the breadth of the response, as shown by the number of ACPA, not by a specific epitope, which may vary between individuals. A specific citrullinated antigen that may be an initial target of autoimmunity may exist, but the correct epitope may not currently be included on this array. In addition, the possibility exists that there may be different initial epitopes for different subjects and that it is the reactivity to any citrullinated antigen rather than a specific citrullinated antigen that is the important first step in RA-related autoimmunity. This is in agreement with a recent study, which indicated the initial ACPA response is restricted but becomes more specific with higher antibody levels nearer symptom onset (7). That being said, there were several interesting trends in reactivity. In general, histone 2A (1–20) cit cyclic was among the most prevalent for both Ab+ and Ab− FDRs. Positivity for a few of the fibrinogen epitopes was high among the Ab+ FDRs, while clusterin (221–240) cit was prevalent among Ab− FDRs. Histone 2A and some of the fibrinogen antigens were prevalent among the CCP2+ individuals. Aside from CCP2, the prevalent epitopes in the RA probands were histone 2A (1–20) cit cyclic and fillagrin (48–65) cit2 cyclic.
This panel included antigens implicated in the literature (6, 9, 11, 12, 14–16, 35, 42–45) and those identified through proteomic screens (46) as targets in RA. While CCP2 and citrullinated-fillagrin may act as surrogate antigens for other RA-autoantibodies, and antibodies targeting citrullinated-fillagrin may cross-react with citrullinated-fibrinogen (14), we chose to examine epitopes that have been associated with RA or that could potentially act as early antigenic targets. Although cross-reactivity among epitopes may occur, we excluded any markers with close correlation to each other using a matrix approach. The majority of ACPA in this array appear to display distinct specificities, as noted in other studies (4, 47). Antibodies targeting citrullinated proteins may also target non-citrullinated epitopes; however, marker optimization studies in the development of this assay demonstrated minimal reactivity of the arginine containing version of most peptides relative to the citrulline versions, indicating little cross-reactivity.
While we found reactivity to multiple specific citrullinated proteins in our FDRs, a study in North American Natives (NAN) found that unaffected family members with a positive anti-CCP test did not show positivity for the limited number of ACPA epitopes using peptides derived from vimentin, fibrinogen and enolase (35). Potential reasons for these differences include that our study’s ACPA testing used a greater number of antigens and epitopes derived from these antigens, including whole proteins representing and peptides derived from vimentin, fibrinogen and enolase that may have resulted in greater autoantibody detection. In addition, we used a microarray that enabled measurement of multiple ACPA while the NAN study used an ELISA that measured antibodies against a restricted set of ACPA, which likely provided our study with a greater sensitivity for ACPA. It should be noted that while the array used in this current study has been previously studied (8, 41), the ELISA and ACPA used in the NAN study have also been well-characterized and validated, and may exhibit greater specificity for RA. These issues will need to be evaluated in further comparative studies.
Although half of our FDRs who were anti-CCP2 negative were not positive for any ACPA, approximately 8% were positive for nine or more ACPA. This is in agreement with studies that found specific ACPA to be positive in individuals with CCP-negative RA (42, 47–50). When the anti-CCP2 positive individuals were removed from the analyses, number of ACPA and having at least 9 ACPA were no longer significantly associated with the high-risk profile, RF-positivity by nephelometry, RF-IgM and RF-IgA, indicating that those who are anti-CCP2 positive may be driving the association with being autoantibody positive.
To our knowledge, this is the first study to find an association between increasing number of ACPA and having at least one swollen or tender joint in RA-free FDRs of RA patients. While all of our FDRs do not have 1987 and 2010 classifiable RA at the time of this study, these results could indicate that some individuals within our cohort are showing early signs of RA, and may be further in the pre-clinical state than previous studies of unaffected family members. Similar to the study finding seropositive arthralgia patients with an extended ACPA repertoire have higher risk of developing arthritis (6), FDRs with increased reactivity to multiple epitopes may already be progressing to RA. While no individuals in the cohort exhibited RA at the first visit, follow-up SERA visits found that 4 individuals met the 2010 ACR/EULAR Classification Criteria (51) on at least one of their subsequent visits; unfortunately this is not enough cases to analyze as an outcome. To ensure our population was RA-free, we removed the visits where the FDRs met 2010 ACR criteria and all subsequent visits. Of these 4 individuals, 3 were Ab+, one was anti-CCP2+, and one was positive for at least 9 ACPA. All 4 had at least one swollen joint, and 3 had at least one tender joint. Removal of the visits from these four individuals from the cohort did not significantly alter our inference with regard to tender joints on examination. While a previous study indicated no difference in joint swelling or tenderness between anti-CCP positive and negative individuals with RA (52), another found that in those with early non-treated RA, ACPA positivity was a good predictor of joint damage (53); therefore, the expanded ACPA profile in our study may indicate differences in pre-disease state.
In conclusion, in this study of FDRs without 1987 and 2010 classifiable RA, an increasing number of ACPA was associated with RA-related disease characteristics, including positivity for RF, RF isotypes, the high-risk autoantibody profile (i.e. positive for anti-CCP2 and/or 2 RF isotypes), and swollen or tender joints on exam. In addition, 8% of our cohort that was negative for all other antibodies (RF and CCP2) was positive for ≥ 9 ACPA, providing evidence that expansion of the ACPA testing may be warranted in examining autoimmunity in those who may be presenting with pre-clinical RA.
Acknowledgments
Funding: Funding for this research was made possible by grants from the NIAMS (R01 AR051394 and T32 AR007534-25) and NIAID (Autoimmunity Prevention Center U19 AI050864), the ACR Research and Education Foundation Within Our Reach: Finding a Cure for Rheumatoid Arthritis campaign (II-T-11-14), and the Walter S. and Lucienne Driskill Foundation.
References
- 1.Gabriel SE, Crowson CS, Kremers HM, Doran MF, Turesson C, O’Fallon WM, et al. Survival in rheumatoid arthritis: a population-based analysis of trends over 40 years. Arthritis Rheum. 2003;48(1):54–8. doi: 10.1002/art.10705. [DOI] [PubMed] [Google Scholar]
- 2.Nishimura K, Sugiyama D, Kogata Y, Tsuji G, Nakazawa T, Kawano S, et al. Meta-analysis: diagnostic accuracy of anti-cyclic citrullinated peptide antibody and rheumatoid factor for rheumatoid arthritis. Ann Intern Med. 2007;146(11):797–808. doi: 10.7326/0003-4819-146-11-200706050-00008. [DOI] [PubMed] [Google Scholar]
- 3.Taylor P, Gartemann J, Hsieh J, Creeden J. A systematic review of serum biomarkers anti-cyclic citrullinated Peptide and rheumatoid factor as tests for rheumatoid arthritis. Autoimmune Dis. 2011;2011:815038. doi: 10.4061/2011/815038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ioan-Facsinay A, el-Bannoudi H, Scherer HU, van der Woude D, Menard HA, Lora M, et al. Anti-cyclic citrullinated peptide antibodies are a collection of anti-citrullinated protein antibodies and contain overlapping and non-overlapping reactivities. Ann Rheum Dis. 2011;70(1):188–93. doi: 10.1136/ard.2010.131102. [DOI] [PubMed] [Google Scholar]
- 5.Kuhn KA, Kulik L, Tomooka B, Braschler KJ, Arend WP, Robinson WH, et al. Antibodies against citrullinated proteins enhance tissue injury in experimental autoimmune arthritis. J Clin Invest. 2006;116(4):961–73. doi: 10.1172/JCI25422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van de Stadt LA, van der Horst AR, de Koning MH, Bos WH, Wolbink GJ, van de Stadt RJ, et al. The extent of the anti-citrullinated protein antibody repertoire is associated with arthritis development in patients with seropositive arthralgia. Ann Rheum Dis. 2011;70(1):128–33. doi: 10.1136/ard.2010.132662. [DOI] [PubMed] [Google Scholar]
- 7.Brink M, Hansson M, Mathsson L, Jakobsson PJ, Holmdahl R, Hallmans G, et al. Multiplex analyses of antibodies against citrullinated peptides in individuals prior to development of rheumatoid arthritis. Arthritis Rheum. 2013 doi: 10.1002/art.37835. [DOI] [PubMed] [Google Scholar]
- 8.Sokolove J, Bromberg R, Deane KD, Lahey LJ, Derber LA, Chandra PE, et al. Autoantibody epitope spreading in the pre-clinical phase predicts progression to rheumatoid arthritis. PLoS One. 2012;7(5):e35296. doi: 10.1371/journal.pone.0035296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Woude D, Rantapaa-Dahlqvist S, Ioan-Facsinay A, Onnekink C, Schwarte CM, Verpoort KN, et al. Epitope spreading of the anti-citrullinated protein antibody response occurs before disease onset and is associated with the disease course of early arthritis. Ann Rheum Dis. 2010;69(8):1554–61. doi: 10.1136/ard.2009.124537. [DOI] [PubMed] [Google Scholar]
- 10.Suwannalai P, Willemze A, van Toorn L, Stoeken-Rijsbergen G, Levarht N, Drijfhout JW, et al. The fine specificity of IgM anti-citrullinated protein antibodies (ACPA) is different from that of IgG ACPA. Arthritis Res Ther. 2011;13(6):R195. doi: 10.1186/ar3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van de Stadt LA, de Koning MH, van de Stadt RJ, Wolbink G, Dijkmans BA, Hamann D, et al. Development of the anti-citrullinated protein antibody repertoire prior to the onset of rheumatoid arthritis. Arthritis Rheum. 2011;63(11):3226–33. doi: 10.1002/art.30537. [DOI] [PubMed] [Google Scholar]
- 12.Kinloch A, Tatzer V, Wait R, Peston D, Lundberg K, Donatien P, et al. Identification of citrullinated alpha-enolase as a candidate autoantigen in rheumatoid arthritis. Arthritis Res Ther. 2005;7(6):R1421–9. doi: 10.1186/ar1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koivula MK, Heliovaara M, Ramberg J, Knekt P, Rissanen H, Palosuo T, et al. Autoantibodies binding to citrullinated telopeptide of type II collagen and to cyclic citrullinated peptides predict synergistically the development of seropositive rheumatoid arthritis. Ann Rheum Dis. 2007;66(11):1450–5. doi: 10.1136/ard.2006.062919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Masson-Bessiere C, Sebbag M, Girbal-Neuhauser E, Nogueira L, Vincent C, Senshu T, et al. The major synovial targets of the rheumatoid arthritis-specific antifilaggrin autoantibodies are deiminated forms of the alpha- and beta-chains of fibrin. J Immunol. 2001;166(6):4177–84. doi: 10.4049/jimmunol.166.6.4177. [DOI] [PubMed] [Google Scholar]
- 15.Takizawa Y, Suzuki A, Sawada T, Ohsaka M, Inoue T, Yamada R, et al. Citrullinated fibrinogen detected as a soluble citrullinated autoantigen in rheumatoid arthritis synovial fluids. Ann Rheum Dis. 2006;65(8):1013–20. doi: 10.1136/ard.2005.044743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vossenaar ER, Despres N, Lapointe E, van der Heijden A, Lora M, Senshu T, et al. Rheumatoid arthritis specific anti-Sa antibodies target citrullinated vimentin. Arthritis Res Ther. 2004;6(2):R142–50. doi: 10.1186/ar1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uysal H, Bockermann R, Nandakumar KS, Sehnert B, Bajtner E, Engstrom A, et al. Structure and pathogenicity of antibodies specific for citrullinated collagen type II in experimental arthritis. J Exp Med. 2009;206(2):449–62. doi: 10.1084/jem.20081862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuhn KA, Cozine CL, Tomooka B, Robinson WH, Holers VM. Complement receptor CR2/CR1 deficiency protects mice from collagen-induced arthritis and associates with reduced autoantibodies to type II collagen and citrullinated antigens. Mol Immunol. 2008;45(10):2808–19. doi: 10.1016/j.molimm.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 19.Trouw LA, Haisma EM, Levarht EW, van der Woude D, Ioan-Facsinay A, Daha MR, et al. Anti-cyclic citrullinated peptide antibodies from rheumatoid arthritis patients activate complement via both the classical and alternative pathways. Arthritis Rheum. 2009;60(7):1923–31. doi: 10.1002/art.24622. [DOI] [PubMed] [Google Scholar]
- 20.Zhao X, Okeke NL, Sharpe O, Batliwalla FM, Lee AT, Ho PP, et al. Circulating immune complexes contain citrullinated fibrinogen in rheumatoid arthritis. Arthritis Res Ther. 2008;10(4):R94. doi: 10.1186/ar2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clavel C, Nogueira L, Laurent L, Iobagiu C, Vincent C, Sebbag M, et al. Induction of macrophage secretion of tumor necrosis factor alpha through Fcgamma receptor IIa engagement by rheumatoid arthritis-specific autoantibodies to citrullinated proteins complexed with fibrinogen. Arthritis Rheum. 2008;58(3):678–88. doi: 10.1002/art.23284. [DOI] [PubMed] [Google Scholar]
- 22.Sokolove J, Zhao X, Chandra PE, Robinson WH. Immune complexes containing citrullinated fibrinogen costimulate macrophages via Toll-like receptor 4 and Fcgamma receptor. Arthritis Rheum. 2011;63(1):53–62. doi: 10.1002/art.30081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rantapaa-Dahlqvist S, de Jong BA, Berglin E, Hallmans G, Wadell G, Stenlund H, et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 2003;48(10):2741–9. doi: 10.1002/art.11223. [DOI] [PubMed] [Google Scholar]
- 24.Nielen MM, van Schaardenburg D, Reesink HW, van de Stadt RJ, van der Horst-Bruinsma IE, de Koning MH, et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum. 2004;50(2):380–6. doi: 10.1002/art.20018. [DOI] [PubMed] [Google Scholar]
- 25.Majka DS, Deane KD, Parrish LA, Lazar AA, Baron AE, Walker CW, et al. Duration of preclinical rheumatoid arthritis-related autoantibody positivity increases in subjects with older age at time of disease diagnosis. Ann Rheum Dis. 2008;67(6):801–7. doi: 10.1136/ard.2007.076679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deighton CM, Wentzel J, Cavanagh G, Roberts DF, Walker DJ. Contribution of inherited factors to rheumatoid arthritis. Ann Rheum Dis. 1992;51(2):182–5. doi: 10.1136/ard.51.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arlestig L, Mullazehi M, Kokkonen H, Rocklov J, Ronnelid J, Dahlqvist SR. Antibodies against cyclic citrullinated peptides of IgG, IgA and IgM isotype and rheumatoid factor of IgM and IgA isotype are increased in unaffected members of multicase rheumatoid arthritis families from northern Sweden. Ann Rheum Dis. 2011 doi: 10.1136/annrheumdis-2011-200668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deane KD, Norris JM, Holers VM. Preclinical rheumatoid arthritis: identification, evaluation, and future directions for investigation. Rheum Dis Clin North Am. 2010;36(2):213–41. doi: 10.1016/j.rdc.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.del Puente A, Knowler WC, Pettitt DJ, Bennett PH. The incidence of rheumatoid arthritis is predicted by rheumatoid factor titer in a longitudinal population study. Arthritis Rheum. 1988;31(10):1239–44. doi: 10.1002/art.1780311004. [DOI] [PubMed] [Google Scholar]
- 30.Demoruelle MK, Weisman MH, Simonian PL, Lynch DA, Sachs PB, Pedraza IF, et al. Brief Report: Airways abnormalities and rheumatoid arthritis-related autoantibodies in subjects without arthritis: Early injury or initiating site of autoimmunity? Arthritis Rheum. 2012;64(6):1756–61. doi: 10.1002/art.34344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feser M, Derber LA, Deane KD, Lezotte DC, Weisman MH, Buckner JH, et al. Plasma 25,OH vitamin D concentrations are not associated with rheumatoid arthritis (RA)-related autoantibodies in individuals at elevated risk for RA. J Rheumatol. 2009;36(5):943–6. doi: 10.3899/jrheum.080764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kolfenbach JR, Deane KD, Derber LA, O’Donnell C, Weisman MH, Buckner JH, et al. A prospective approach to investigating the natural history of preclinical rheumatoid arthritis (RA) using first-degree relatives of probands with RA. Arthritis Rheum. 2009;61(12):1735–42. doi: 10.1002/art.24833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silman AJ, Hennessy E, Ollier B. Incidence of rheumatoid arthritis in a genetically predisposed population. Br J Rheumatol. 1992;31(6):365–8. doi: 10.1093/rheumatology/31.6.365. [DOI] [PubMed] [Google Scholar]
- 34.Suwannalai P, van de Stadt LA, Radner H, Steiner G, El-Gabalawy HS, Zijde CM, et al. Avidity maturation of anti-citrullinated protein antibodies in rheumatoid arthritis. Arthritis Rheum. 2012;64(5):1323–8. doi: 10.1002/art.33489. [DOI] [PubMed] [Google Scholar]
- 35.Ioan-Facsinay A, Willemze A, Robinson DB, Peschken CA, Markland J, van der Woude D, et al. Marked differences in fine specificity and isotype usage of the anti-citrullinated protein antibody in health and disease. Arthritis Rheum. 2008;58(10):3000–8. doi: 10.1002/art.23763. [DOI] [PubMed] [Google Scholar]
- 36.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 37.Deane KD, O’Donnell CI, Hueber W, Majka DS, Lazar AA, Derber LA, et al. The number of elevated cytokines and chemokines in preclinical seropositive rheumatoid arthritis predicts time to diagnosis in an age-dependent manner. Arthritis Rheum. 2010;62(11):3161–72. doi: 10.1002/art.27638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jonsson T, Steinsson K, Jonsson H, Geirsson AJ, Thorsteinsson J, Valdimarsson H. Combined elevation of IgM and IgA rheumatoid factor has high diagnostic specificity for rheumatoid arthritis. Rheumatol Int. 1998;18(3):119–22. doi: 10.1007/s002960050069. [DOI] [PubMed] [Google Scholar]
- 39.Jonsson T, Valdimarsson H. Is measurement of rheumatoid factor isotypes clinically useful? Ann Rheum Dis. 1993;52(2):161–4. doi: 10.1136/ard.52.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vallbracht I, Rieber J, Oppermann M, Forger F, Siebert U, Helmke K. Diagnostic and clinical value of anti-cyclic citrullinated peptide antibodies compared with rheumatoid factor isotypes in rheumatoid arthritis. Ann Rheum Dis. 2004;63(9):1079–84. doi: 10.1136/ard.2003.019877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chandra PE, Sokolove J, Hipp BG, Lindstrom TM, Elder JT, Reveille JD, et al. Novel multiplex technology for diagnostic characterization of rheumatoid arthritis. Arthritis Res Ther. 2011;13(3):R102. doi: 10.1186/ar3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mathsson L, Mullazehi M, Wick MC, Sjoberg O, van Vollenhoven R, Klareskog L, et al. Antibodies against citrullinated vimentin in rheumatoid arthritis: higher sensitivity and extended prognostic value concerning future radiographic progression as compared with antibodies against cyclic citrullinated peptides. Arthritis Rheum. 2008;58(1):36–45. doi: 10.1002/art.23188. [DOI] [PubMed] [Google Scholar]
- 43.Lundberg K, Kinloch A, Fisher BA, Wegner N, Wait R, Charles P, et al. Antibodies to citrullinated alpha-enolase peptide 1 are specific for rheumatoid arthritis and cross-react with bacterial enolase. Arthritis Rheum. 2008;58(10):3009–19. doi: 10.1002/art.23936. [DOI] [PubMed] [Google Scholar]
- 44.Iobagiu C, Magyar A, Nogueira L, Cornillet M, Sebbag M, Arnaud J, et al. The antigen specificity of the rheumatoid arthritis-associated ACPA directed to citrullinated fibrin is very closely restricted. J Autoimmun. 2011;37(4):263–72. doi: 10.1016/j.jaut.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 45.Verpoort KN, Cheung K, Ioan-Facsinay A, van der Helm-van Mil AH, de Vries-Bouwstra JK, Allaart CF, et al. Fine specificity of the anti-citrullinated protein antibody response is influenced by the shared epitope alleles. Arthritis Rheum. 2007;56(12):3949–52. doi: 10.1002/art.23127. [DOI] [PubMed] [Google Scholar]
- 46.Monach PA, Hueber W, Kessler B, Tomooka BH, BenBarak M, Simmons BP, et al. A broad screen for targets of immune complexes decorating arthritic joints highlights deposition of nucleosomes in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2009;106(37):15867–72. doi: 10.1073/pnas.0908032106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Snir O, Widhe M, Hermansson M, von Spee C, Lindberg J, Hensen S, et al. Antibodies to several citrullinated antigens are enriched in the joints of rheumatoid arthritis patients. Arthritis Rheum. 2010;62(1):44–52. doi: 10.1002/art.25036. [DOI] [PubMed] [Google Scholar]
- 48.Liu X, Jia R, Zhao J, Li Z. The role of anti-mutated citrullinated vimentin antibodies in the diagnosis of early rheumatoid arthritis. J Rheumatol. 2009;36(6):1136–42. doi: 10.3899/jrheum.080796. [DOI] [PubMed] [Google Scholar]
- 49.Nicaise Roland P, Grootenboer Mignot S, Bruns A, Hurtado M, Palazzo E, Hayem G, et al. Antibodies to mutated citrullinated vimentin for diagnosing rheumatoid arthritis in anti-CCP-negative patients and for monitoring infliximab therapy. Arthritis Res Ther. 2008;10(6):R142. doi: 10.1186/ar2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pratesi F, Tommasi C, Anzilotti C, Puxeddu I, Sardano E, Di Colo G, et al. Antibodies to a new viral citrullinated peptide, VCP2: fine specificity and correlation with anti-cyclic citrullinated peptide (CCP) and anti-VCP1 antibodies. Clin Exp Immunol. 2011;164(3):337–45. doi: 10.1111/j.1365-2249.2011.04378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, 3rd, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–81. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 52.van der Helm-van Mil AH, Verpoort KN, Breedveld FC, Toes RE, Huizinga TW. Antibodies to citrullinated proteins and differences in clinical progression of rheumatoid arthritis. Arthritis Res Ther. 2005;7(5):R949–58. doi: 10.1186/ar1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meyer O, Labarre C, Dougados M, Goupille P, Cantagrel A, Dubois A, et al. Anticitrullinated protein/peptide antibody assays in early rheumatoid arthritis for predicting five year radiographic damage. Ann Rheum Dis. 2003;62(2):120–6. doi: 10.1136/ard.62.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]