Abstract
Glioblastoma multiforme is one of the most common and aggressive primary brain tumors in adults. High glutamate levels are thought to contribute to glioma growth. While research has focused on understanding glutamate signaling in glioma cells, little is known about the role of glutamate between glioma and astrocyte interactions. To study the relationship between astrocytes and tumor cells, the CNS-1 rodent glioma cell line was used. We hypothesized increased glutamate uptake by astrocytes would negatively affect CNS-1 cell growth. Primary rodent astrocytes and CNS-1 cells were co-cultured for 7 days in a Boyden chamber in the presence of 5 mM glutamate. Cells were treated with propentofylline, an atypical synthetic methylxanthine known to increase glutamate transporter expression in astrocytes. Our results indicate astrocytes can increase glutamate uptake through the GLT-1 transporter, leading to less glutamate available for CNS-1 cells, ultimately resulting in increased CNS-1 cell apoptosis. These data suggest that astrocytes in the tumor microenvironment can be targeted by the drug, propentofylline.
Keywords: glioma, methylxanthine, GLT-1, U-251
INTRODUCTION
Glutamate levels are tightly regulated in the brain under normal physiological conditions. It is a key molecule involved in metabolism and is the most abundant excitatory neurotransmitter in the nervous system. Glutamate transporters maintain the extracellular glutamate levels below excitotoxic amounts, preventing neuronal cell death [1]. Excitatory amino-acid transporter-2, EAAT2 (GLT-1 in rodents), accounts for over 90% of this regulation [2, 3], and astrocytes account for most of the EAAT2 expression in the human brain.
Glioblastoma multiforme (GBM) is characterized by high CNS glutamate levels. Microdialysis in both human and rodent models have identified glutamate levels ranging up to 200 mM in the brain with a glioma tumor [4, 5]. Recently, research has focused on understanding the biological significance of high glutamate levels in glioma. Glutamate release from glioma cells occurs though the XC- amino acid transporter and acts in an autocrine and paracrine manner [6, 7]. High glutamate levels are known to increase glioma cell invasion, migration and growth [8]. Studies in animal models have demonstrated that blockade of glutamate uptake in tumor cells by glutamate receptor antagonists results in decreased tumor growth [9–11]. Glutamate receptors, such as calcium-permeable alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors (AMPA-R), are highly expressed in human glioma cell lines. They are activated by released glutamate, and are essential for cell migration [7, 12].
Glutamate transporters, contrary to receptors, are mostly expressed in glial cells and are involved in rapid removal of glutamate from the extracellular space. Reduced expression of glutamate transporters is present in human glioma tissue, which may also contribute to high glutamate levels [5, 6, 13]. Interestingly, an inverse relationship has been shown between the expression of the glutamate transporter EEAT2 (which is highly expressed in neighboring astrocytes compared to glioma cells) and tumor grade [14]. While research has focused on understanding glutamate signaling in glioma cells, there has been little investigation into the relationship of glioma and glutamate in the tumor microenvironment beyond its cytotoxic effects. In this study we investigated the relationship between primary rodent astrocytes and CNS-1 cells under the effects of the drug propentofylline (PPF), an atypical synthetic methylxanthine [1-(50-oxohexyl)-3-methyl-7-propylxanthine]. Our laboratory has previously shown that i.p. injection of PPF decreases tumor growth in a rodent glioma model by directly targeting the tumor microenvironment [15]. We demonstrated that PPF targets microglia; however, PPF is also known to have an effect on astrocytes in vivo and in vitro. Our lab has previously demonstrated that PPF increases the expression of glutamate transporters (GLT-1 and GLAST), resulting in an increase in glutamate clearance [16]. In the present study, we investigate the effects of PPF on astrocytes using a rodent glioma cell line. CNS-1 cells were chosen for their fast growth rate, invasive nature, and histological similarities to human GBM when administered into the rat striatum [17]. Our results demonstrate that astrocytes increase glutamate uptake through the GLT-1 transporter, leading to less glutamate available for CNS-1 cells, ultimately resulting in increased CNS-1 cell apoptosis.
METHODS
Cell Culture
Highly purified astrocyte cultures were prepared as previously described [16]. Briefly, cortices were harvested from postnatal day 2–3 (P2-P3) Lewis rat pups, minced and incubated with Trypsin/EDTA (Mediatech Manassas, VA). The supernatant was then replaced with DMEM (Mediatech, Manassas VA) supplemented with 10% fetal bovine serum (Hyclone Logan, UT), 1.1% GlutaMax (Invitrogen Carlsbad, CA), and 1% penicillin/streptomycin (100 U/ml penicillin, 100 μg/ml streptomycin, Mediatech, Manassas, VA) containing 2000 units DNase (Sigma St Louis, MO). The tissue was mechanically disrupted by trituration, the cell suspensions were centrifuged, and the cells resuspended in media without DNase. A small aliquot of cells were stained for trypan blue exclusion for counting, then cells were plated at 1 x 106 cells per 75 cm2 flask. Cultures were maintained at 37°C with 5% CO2, and media was changed every 3–4 days. After 14 days in vitro (DIV 14) astrocytes were harvested by gently shaking flasks by hand for 1 min to remove microglia. Flasks were then vigorously shaken with PBS for 1 min, and then remaining adhered cells were trypsinized and collected. The resulting cells were found to be >95% astrocytes by staining with GFAP antibody (1:500, Sigma St Louis, MO) and goat anti-mouse Alexa Fluor™-555 secondary antibody. Cells were used immediately for experiments. The U-251 cell line was cultured in astrocyte media as described above. Human astrocytes were obtained from ScienCell (Carlsbad, CA) and cultured in astrocyte media (ScienCell Carlsbad, CA).
Trypan Blue Staining
Astrocytes were cultured for 3 or 7 days at 3 x 105 cells/well in 12 transwell plates containing astrocyte media (DMEM (Mediatech, Manassas VA) supplemented with 10% fetal bovine serum (Hyclone Logan, UT), 1.1% GlutaMax (Invitrogen Carlsbad, CA), and 1% penicillin/streptomycin (100 U/ml penicillin, 100 μg/ml streptomycin, Mediatech, Manassas, VA)) with 5 mM glutamate. Cells were collected by scraping. Aliquots of 10 μL were collected from each well and counted under the hemocytometer. Three samples per well were counted and then averaged.
Small interference RNA knockdown
Small interference RNA (siRNA) oligonucleotides specific for GLT-1 (#1: UAACUUCAUGACAAUCUCGTT, #2:UCGUGGACAUGUAAUAUACAA) were validated by and purchased from Ambion (Grand Island, NY). Small interference RNA (siRNA) oligonucleotides specific for GLAST (#1: GCAUGUGCUUCCAAUAUGA, #2:UACAUAUUGGAAGCACAUGCCCACGA, #3: CCCGCUUCCUGCUCAAUGGUAA) were validated by and purchased from Invitrogen (Grand Island, NY). Transient transfection was carried out using iFect (Neuromics Edina, MN) as previously described [18]. Briefly, astrocytes were plated at 3 x 105 cells/well in a 12 well plate. Once cells had adhered, they were transfected with 1 μg siRNA. Control samples were treated with an empty vector siRNA (Sigma St Louis, MO) or with iFect reagent alone. Cells were left in astrocyte media containing 5mM glutamate (10% fetal bovine serum (Hyclone Logan, UT), 1.1% GlutaMax (Invitrogen Carlsbad, CA), and 1% penicillin/streptomycin (100 U/ml penicillin, 100 μg/ml streptomycin, Mediatech, Manassas, VA)) at 37°C with 5% CO2 overnight and then used the following day for experiments. For experiments requiring knockdown for 7 days, astrocytes were treated with siRNA twice (day 0 and day 3).
Quantitative RT-PCR
Total RNA was isolated from astrocyte cultures using the Qiagen RNeasy mini-kit (Qiagen, Valencia, CA), according to the manufacturer’s protocol for isolation of total RNA from animal cells. Reverse transcription (RT) was carried out using QuantiTect reverse transcription kit (Qiagen, Valencia, CA) according to the vendor’s protocol. Real-Time RT-PCR reactions were carried out in a total reaction volume of 25 μL containing a final concentration of 1.5 U Platinum Taq DNA polymerase (Invitrogen); 20 mM Tris HCl (pH 8.4); 50 mM KCl; 3 mM MgCl2; 200 μM dGTP, dCTP, and dATP; 400 μM dUTP and 1 U of UDG (uracil DNA glycosylase); 900 nM of forward and reverse primers; 300 nM Taqman probe; and 5 μL of cDNA (50 ng) from the RT step. Primer and probe sequences for the genes of interest (GLT-1, GLAST, and GAPDH) were purchased from Invitrogen (Grand Island, NY). The iCycler™ Multicolor Real-Time PCR detection system (Bio-Rad) was used to quantify PCR product. The fluorescence and threshold values (CT) obtained were used to compare the relative amount of target mRNA in experimental groups to those of controls using the method [19]. Each experiment was run thrice and samples were run in duplicate. For each sample, the mean CT value for the control gene (GAPDH) was then subtracted from the mean CT value for the gene of interest (GLT-1, GLAST) to obtain a ΔCT value. The ΔCT values for the control group (untreated) were then averaged and subtracted from the ΔCT for the experimental groups to obtain the ΔΔCT. The relative fold change from control was then expressed by calculation of for each sample and the results are expressed as the group mean fold change ± SEM relative to GAPDH.
Flow Cytometry
CNS-1 cells were first labeled with 10μM cytofluorescein ester (CFSE) (Sigma St Louis, MO) at a concentration of 3 x 105 cells per 1,000 μl of PBS at 37°C for 10 minutes. CNS-1 cells were then incubated at 37°C in 24-well plates for 3 days (Falcon Franklin Lakes, NJ). For FACS staining, cells were trypsinized, washed and stained on ice in PBS for 30 minutes. Fc receptors were blocked using FBS for 15 minutes before staining. For apoptosis, Annexin V FITC and PI apoptosis kit was used for staining (eBioscience San Diego, CA). All flow cytometry experiments were performed on a FACSCanto (BD Bioscience Franklin Lakes, NJ).
Immunocytochemistry
Primary rat astrocytes were plated onto sterile 18-mm glass cover slips at the bottom of a Boyden chamber with CNS-1 cells cultured on top of the transwell chamber. Cover slips were collected on different days. After three washes in PBS, cells were permeabilized in 5% glacial acetic acid/95% ethanol (acid–alcohol) for 10 min. After washing, cells were incubated in a 1% normal goat serum for 30 min and then 1hr at room temperature in primary mouse anti-GFAP (1:500, Sigma St Louis, MO) and rat anti-CDllb (1:500, Abcam Cambridgeshire, UK). The following day cells were washed and then incubate for 2 hrs at room temperature with goat anti-mouse Alexa Fluor™-555, goat anti-rat Alexa Fluor™-488 (all at 1:250). Finally, cells were post-fixed in acid–alcohol and mounted with Vectashield (Vector Labs, Burlingame, CA) containing 4′,6-diamidino-2-phenylindole dihydrochloride hydrate (DAPI, Sigma, St Louis, MO) to visualize cell nuclei. The sections were examined with an Olympus fluorescence microscope, and images were captured with a Q-Fire cooled camera (Olympus, Melville, NY). Confocal microscopy was performed using a Zeiss LSM 510 Meta confocal microscope (Carl Zeiss AG, Oberkochen, Germany; Englert Cell Analysis Laboratory, Dartmouth Medical School). Merged color images were processed using Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA).
Glutamate Levels
Rodent or human astrocytes, CNS-1 cells, U-251 cells or co cultures were cultured in 96 well flat bottom plates. Cells were plated in complete media with 5 mM glutamate as described in the Cell Culture subsection above. At various time points, supernatant was collected and analyzed for residual glutamate using the protocol described by the manufacturers (Amplex® Red Glutamic Acid/Glutamate Oxidase Kit, Invitrogen Grand Island, NY). A Typhoon 9410 (GE New York, NY) was used to image fluorescence and ImageJ (NIH) software was used to calculate glutamate concentrations based on a known glutamate concentration curve.
Statistical Analysis
All values are expressed as mean ± S.E.M. Statistical analyses were performed with GraphPad Prism 4 (GraphPad Software Inc. La Jolla, CA) with the significance level set at p<0.05. One-way ANOVA with Dunnett’s post-hoc analysis was used for all apoptosis experiments.
MATERIALS
Propentofylline was purchased from Toronto Research Chemicals (North York, ON) and concentrations were obtained by dissolving PPF in PBS.
RESULTS
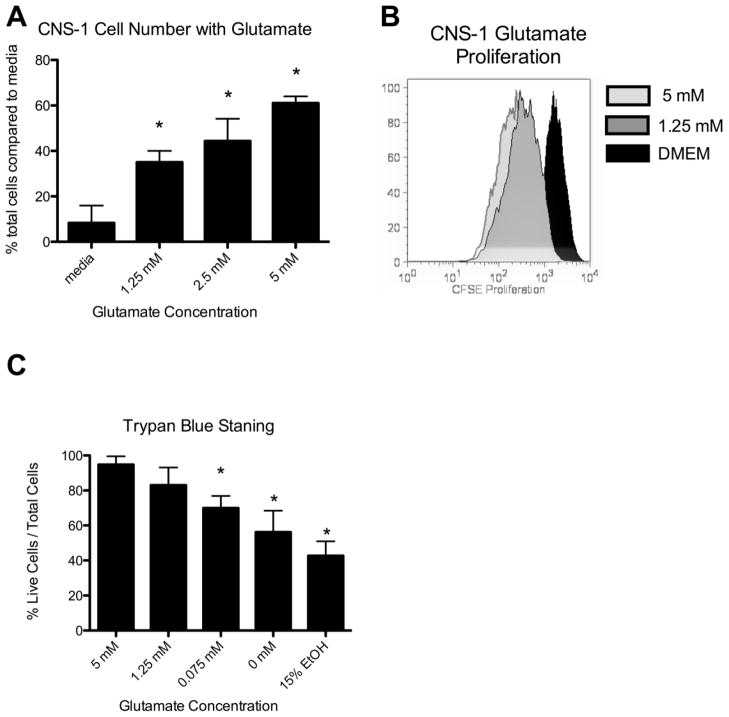
CNS-1 cells proliferate and preferentially grow in glutamate containing media
It has been previously reported in both animal and human cells lines that glutamate increases glioma tumor cell proliferation [9]. To study the relationship of astrocytes and glioma cells, we first needed to confirm that glutamate increases proliferation of CNS-1 cells. With increased glutamate levels in the media, the number of CNS-1 cells significantly (p<0.05) increased after 3 days compared to no glutamate present (Fig. 1a). This was further confirmed by CFSE proliferation, which demonstrated an increase of CNS-1 cell proliferation in the presence of 5 mM glutamate compared to no glutamate (Fig. 1b).
Figure 1.
CNS-1 cells proliferate and preferentially grow in glutamate containing media. (a) Graphical representation of the percent of live CNS-1 cells following 3 days of growth in varying glutamate concentrations determined by trypan blue staining. Increasing glutamate resulted in a significant increase in CNS-1 cell number (* = p < 0.05). (b) CNS-1 cells were CFSE labeled on day 0 and analyzed by FACS analysis following 3 days of growth in varying glutamate concentrations. (c) Graphical representation of the percent of live CNS-1 cells following 7 days of growth in 5 mM glutamate, replaced with varying glutamate concentrations overnight and analyzed by trypan blue staining on day 8. Removal of glutamate resulted in a significant decrease in CNS-1 viability (* = p < 0.05).
While glioma cell lines can be grown without additional glutamate in the media in vitro, growth is inhibited when glutamate uptake is blocked in vivo. To test if CNS-1 cells develop a dependent growth in the presence of glutamate, CNS-1 cells were grown in 5mM glutamate for 7 days and then replaced with media containing lower or no glutamate and assessed for cell viability with trypan blue staining. A significant (p<0.05) decrease in the percentage of CNS-1 viable cells occurred when glutamate was removed after seven days (Fig. 1c). The decrease in cell viability was dose dependent in response to a decrease in glutamate concentration, further supporting preferred and dependent growth in the presence of glutamate (Fig. 1c).
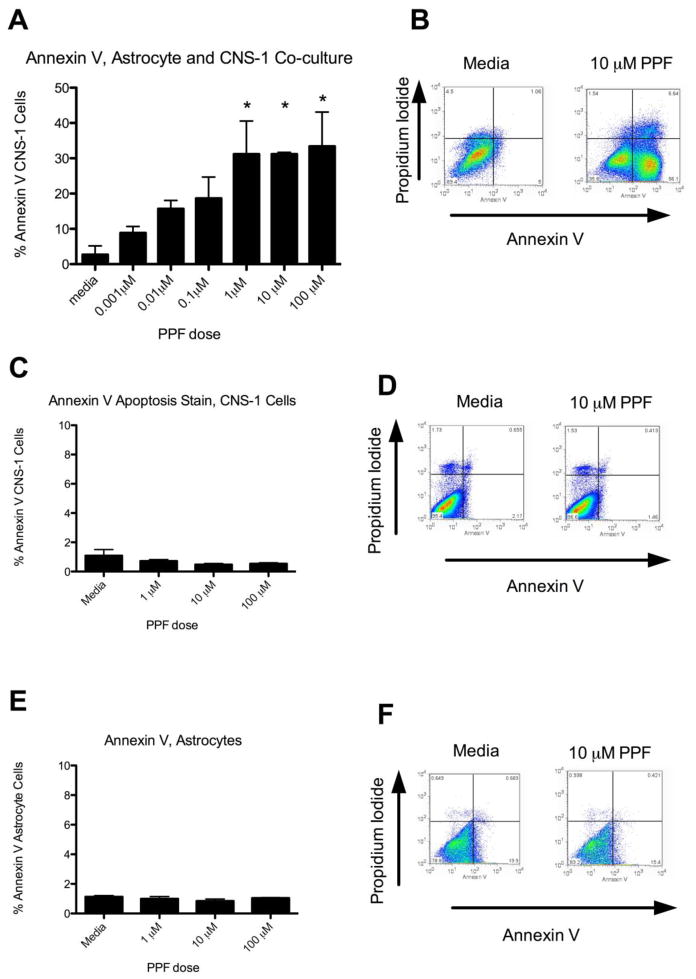
Propentofylline increases Annexin V expression on CNS-1 cells when co-cultured with astrocytes
The relationship between astrocytes and CNS-1 cells in the presence of glutamate was examined following PPF treatment. Primary rodent astrocytes and CNS-1 cells were co-cultured for 7 days in a Boyden chamber in the presence of 5 mM glutamate and treated with PPF. An increase in Annexin V+ CNS-1 cells occurred with increasing doses of PPF (0.001 μM – 100 μM). A significant increase in Annexin V apoptosis was achieved at 1 μM PPF (p<0.05) (Fig. 2a). Of note, a significant increase in apoptosis with PPF treatment did not occur until day 5 in culture, with the highest increase at day 7, supporting the need for CNS-1 cells to first develop growth dependency in the presence of glutamate (Supplemental Fig. 1b). Apoptosis with PPF treatment was not seen when astrocytes or CNS-1 cells were cultured alone, indicating both primary astrocytes and CNS-1 cells must be present for PPF’s effects (Fig. 2c,e). This was further confirmed by immunocytochemistry staining performed on different days to rule out the possibility of a contaminating cell population in the primary astrocytes (Supplemental Fig. 1 a).
Figure 2.
Propentofylline increases Annexin V expression on CNS-1 cells when co-cultured with astrocytes. (a) Graphical representation of percent Annexin V+ CNS-1 cells co-cultured with astrocytes for 7 days and treated with PPF. (* = p < 0.05) (b) Representative FACS plots (media and 10 μM PPF) of Annexin V and Propidium Iodide staining in CNS-1 cells. (c) Graphical representation of percent Annexin V+ CNS-1 cells cultured alone for 7 days and treated with PPF. (d) Representative FACS plots (media and 10 μM PPF) of Annexin V and Propidium Iodide staining in CNS-1 cells. (e) Graphical representation of percent of Annexin V+ primary astrocytes cultured alone for 7 days and treated with PPF. (f) Representative FACS plots (media and 10 μM PPF) of Annexin V and Propidium Iodide staining in primary astrocytes. Data are representative of 3 replicates/experimental group; experiment was repeated three times.
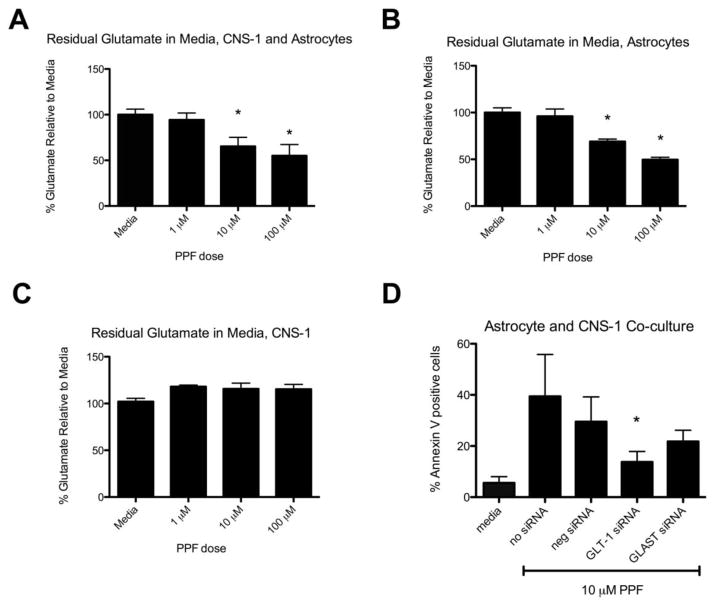
Propentofylline decreases residual glutamate in media
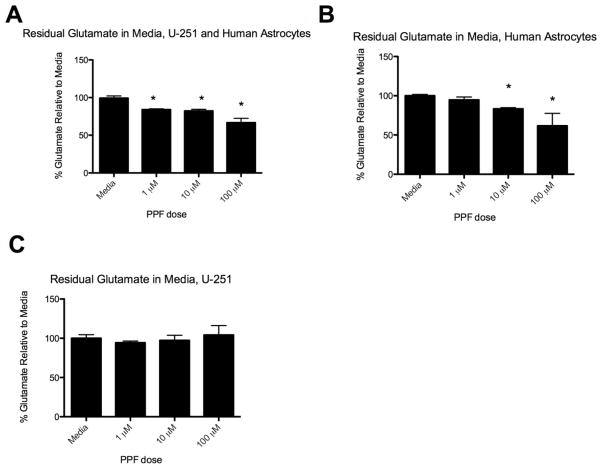
To determine whether the observed increase in apoptosis corresponds with a decrease in glutamate, glutamate levels were tested after 7 days of PPF treatment in 5 mM glutamate. A significant decrease in residual glutamate was observed in astrocyte and CNS-1 co-cultures (Fig. 3a). An almost 50% reduction occurred with 10 μM PPF compared to media (Fig. 3a). A similar percent reduction occurred when astrocytes were cultured alone with PPF treatment (Fig. 3b). There was no decrease in residual glutamate in media when CNS-1 cells were cultured alone with PPF treatment, indicating PPF’s primary effect was on astrocytes and not CNS-1 cells (Fig. 3c).
Figure 3.
Propentofylline decreases residual glutamate in media. (a) Graphical representation of percent glutamate remaining in media compared to untreated (media) group in astrocyte and CNS-1 co-culture treated with varying PPF dosages (* = p < 0.05). (b) Graphical representation of percent glutamate remaining in media compared to untreated (media) group in astrocyte cultures treated with varying PPF doses (* = p < 0.05). (c) Graphical representation of percent glutamate remaining in media compared to untreated (media) group in CNS-1 culture treated with varying PPF dosages. Data are representative of 3 replicates/experimental group; experiment was repeated twice. (d) Propentofylline’s effects on apoptosis are inhibited with GLT-1 blockade in astrocytes. Astrocytes were treated with GLT-1 or GLAST siRNA while co-cultured with CNS-1 cells for 7 days in 5 mM glutamate and treated with PPF (10 μM). Apoptosis of CNS-1 cells was significantly reduced with GLT-1 blockade in astrocytes (* = p < 0.05). Data are representative of 3 replicates/experimental group; experiment was repeated twice.
Propentofylline’s effects on apoptosis are inhibited with GLT-1 blockade in astrocytes
Our laboratory has previously shown that PPF increases glutamate uptake in primary rat astrocytes by increasing GLT-1 and GLAST expression both by mRNA and western blot analyses. To investigate whether an increase in GLT-1 and/or GLAST expression in primary astrocytes results in decreased glutamate levels in media and ultimately CNS-1 cell apoptosis, astrocytes were treated with siRNA against GLT-1 or GLAST (Supplemental Fig. 2). Combined siRNA treatment was found to be the most effective knockdown of GLT-1 and GLAST expression and subsequently used for the apoptosis study (Supplemental Fig. 2). After siRNA knockdown of GLT-1 and GLAST, astrocytes were then co-cultured with CNS-1 cells and treated with 10 μM PPF for 7 days. Cells treated in media (without PPF treatment) were significantly lower in Annexin V+ staining compared to both negative siRNA and no siRNA with PPF treatment (P<0.05) (Fig. 3d). When GLT-1 was knocked down in astrocytes, apoptosis by Annexin V+ was significantly reduced compared to negative siRNA (*p<0.05) (Fig. 3d). Although GLAST inhibition showed a trend towards a decrease in apoptosis, it was not statistically significant (Fig. 3d).
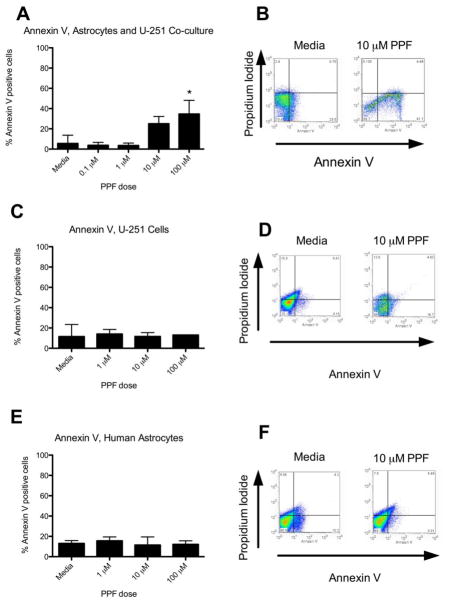
Propentofylline increases Annexin V expression on U-251 cells and decreases residual glutamate when co-cultured with human astrocytes
While the CNS-1 cell line may display many similarities with human glioma there are several differences between human and rodent glutamate transporters [17, 20]. To test the potential translation to humans, we co-cultured adult human astrocytes with U-251 cells in 5 mM glutamate and treated with PPF. PPF significantly increased Annexin V+ staining in U-251 cells (Fig. 4a). This effect was not seen when astrocytes or U-251 cells were cultured alone (Fig. 4c,e). Furthermore, PPF decreased residual glutamate in media when human astrocytes were cultured alone or with U-251 cells (p<0.05) (Fig. 5a,b). This effect did not occur when U-251 cells were cultured alone with PPF (Fig. 5c).
Figure 4.
Propentofylline increases Annexin V expression on U251 cells when co-cultured with human astrocytes. (a) Graphical representation of percent Annexin V+ U-251 cells co-cultured with human astrocytes for 7 days and treated with PPF. (* = p < 0.05) (b) Representative FACS plots (media and 10 μM PPF) of Annexin V and Propidium Iodide staining in U-251 cells and human astrocytes. (c) Graphical representation of percent Annexin V+ U-251 cells cultured alone for 7 days and treated with PPF. (d) Representative FACS plots (media and 10 μM PPF) of Annexin V and Propidium Iodide staining in U-251 cells. (e) Graphical representation of percent Annexin V+ primary human astrocytes cultured alone for 7 days and treated with PPF. (f) Representative FACS plots (media and 10 μM PPF) of Annexin V and Propidium Iodide staining in primary human astrocytes. Data are representative of 3 replicates/experimental group; experiment was repeated twice.
Figure 5.
Propentofylline decreases residual glutamate in the media of human astrocytes. (a) Graphical representation of percent glutamate remaining in media compared to untreated (media) group in astrocyte and U251 co-culture treated with varying PPF dosages (* = p < 0.05). (b) Graphical representation of percent glutamate remaining in media compared to untreated (media) group in astrocyte culture treated with varying PPF dosages (* = p < 0.05). (c) Graphical representation of percent glutamate remaining in media compared to untreated (media) group in U251 culture treated with varying PPF dosages. Data are representative of 3 replicates/experimental group; experiment was repeated twice.
DISCUSSION
In this study we demonstrate that increasing glutamate uptake by GLT-1 in neighboring astrocytes increases Anenxin V expression in CNS-1. Propentofylline has previously been reported to increase GLT-1 in astrocytes and we further report that primary astrocytes are preferentially targeted compared to the tumor cell lines. This may be due to the low expression of GLT-1 in glioma cell lines [14, 21]. Microarray analysis of glioma tissue further supports this observation of low GLT-1 expression in tumor cells [14]. By increasing glutamate transporter expression using PPF, we demonstrate a decrease in media glutamate levels. Our data further support the role of glutamate in glioma cell proliferation and growth. Since GLT-1 is responsible for over 90% of glutamate regulation in the brain, it is not surprising that this glutamate transporter has a more influential role in inducing CNS-1 cells apoptosis, compared to GLAST. In further support of this, immunohistochemistry and sub-cellular fractionation of human glioma tissue demonstrate reduced GLT-1, but normal GLAST levels in glioma patients [13]. This paper focused on the role of GLT-1 and GLAST, which are the primary glutamate transporters expressed in glial cells. Future directions include addressing the role of glutamate transporters expressed in neurons such as EAAT3, EAAT4 and EEAT4 and PPF’s ability to target expression of these transporters.
Glutamate increases glioma proliferation and their invasive potential in vitro [11, 22]. Importantly, blockade of glutamate uptake in animal models induces cell death [10]. Beyond increasing glioma growth, high glutamate levels result in increased cytotoxicity of neighboring cells, creating more space for tumor cells to invade and resulting in neuronal death. Impairment of glutamate transporters, which occurs under disease conditions, may contribute to high glutamate levels. Antisense oligonucleotides against GLT-1 as well as GLT-1 knockout mice demonstrate increased neuronal damage as a result of high glutamate [23, 24]. Reduced expression of glutamate transporters is present in human glioma tissue [5, 6, 13]. Targeting glutamate levels in glioma patients may inhibit tumor growth as well as decrease neuronal death.
We demonstrate here that PPF decreases residual glutamate in media. The use of rodent cells is a clear limitation in this study. While animal cell lines may share many features with human glioma cells, they do not represent the heterogeneity expressed in human tissue. Induction of apoptosis with PPF was confirmed using human astrocytes and a human glioma cell line, however, this work was performed in vitro. Our data supports the concept that targeting glutamate levels in the tumor environment can lead to tumor cell death. We believe PPF can be a novel therapy for glioma and this paper suggests decreasing glutamate levels in the tumor microenvironment as one of PPF’s mechanisms. Further work must be conducted to support the role of GLT-1 as a novel therapeutic target. Future experiments will investigate the role of GLT-1 in astrocytes in vivo and further elicit the apoptosis signaling pathway. The mouse model lacking GLT-1 receptors will be used to study the impact of GLT-1 in the tumor microenvironment on glioma growth [27]. Astrocytes are only one component of the tumor microenvironment and other cell types such as microglia and endothelial cells can influence glutamate levels [25, 26]. This work also establishes the need to further investigate the mechanisms and role of primary astrocytes in glioma invasion and migration.
In summary, these results further supports the dynamic relationship between astrocytes and glioma cells and demonstrate that astrocytes can be preferentially targeted to increase glutamate uptake from glioma cells. We have previously demonstrated that PPF decreases tumor growth in the CNS-1 rat glioma model by decreasing microglial migration and expression of MMP-9 [15]. Here we present another mechanism of PPF’s action in astrocytes. While past research has identified glutamatergic receptors to be involved in glutamate signaling in glioma cells, we present a decrease in glutamate is also achieved by targeting GLT-1 in astrocytes with PPF.
Supplementary Material
Propentofylline effects on astrocyte and CNS-1 co-culture. (a) Immunocytochemistry of astrocytes cultured in the bottom of a Boyden chamber (CNS-1 cells cultured in top of transwell chamber) on day 1, 3, 5 and 7. Blue = DAPI, red = GFAP, green = Iba1. Scale bar = 40 μm. (b) Graphical representation of percent of Annexin V+ CNS-1 cells co-cultured with astrocytes and treated with 10 μM PPF on day 1, 3, 5 and 7 (* = p < 0.05). (c) Graphical representation of percent glutamate remaining in media compared to untreated (media) group in astrocyte and CNS-1 co-culture, treated with 10 μM PPF on day 1, 3, 5 and 7 (* = p < 0.05).
Astrocytes were treated with GLT-1 and GLAST siRNA, cultured in 5 mM glutamate for 7 days, then analyzed for mRNA expression by qRT-PCR (* = p < 0.05, compared to media, ** = p < 0.05, compared to 100 μM PPF).
Acknowledgments
We thank Dr. Nicholas Schworak for the use of the iCycler™, Yingna Liu, Dr Edgar Romero Sandoval, Dr Simon Hillier and Dr Pablo Valdes for their comments on the manuscript. Grant support for this research was through NIH/NIDA and the PhRMA Foundation Pre-Doctoral Fellowship.
Footnotes
Disclosure: The authors declare no conflict of interest.
References
- 1.Erecinska M, Silver IA. Metabolism and role of glutamate in mammalian brain. Prog Neurobiol. 1990;35:245–296. doi: 10.1016/0301-0082(90)90013-7. [DOI] [PubMed] [Google Scholar]
- 2.Hediger MA. Glutamate transporters in kidney and brain. Am J Physiol. 1999;277:F487–492. doi: 10.1152/ajprenal.1999.277.4.F487. [DOI] [PubMed] [Google Scholar]
- 3.Amara SG, Fontana AC. Excitatory amino acid transporters: keeping up with glutamate. Neurochem Int. 2002;41:313–318. doi: 10.1016/s0197-0186(02)00018-9. [DOI] [PubMed] [Google Scholar]
- 4.Schunemann DP, Grivicich I, Regner A, Leal LF, de Araujo DR, Jotz GP, Fedrigo CA, Simon D, da Rocha AB. Glutamate promotes cell growth by EGFR signaling on U-87MG human glioblastoma cell line. Pathol Oncol Res. 2010;16:285–293. doi: 10.1007/s12253-009-9223-4. [DOI] [PubMed] [Google Scholar]
- 5.Roslin M, Henriksson R, Bergstrom P, Ungerstedt U, Bergenheim AT. Baseline levels of glucose metabolites, glutamate and glycerol in malignant glioma assessed by stereotactic microdialysis. J Neurooncol. 2003;61:151–160. doi: 10.1023/a:1022106910017. [DOI] [PubMed] [Google Scholar]
- 6.Ye ZC, Sontheimer H. Glioma cells release excitotoxic concentrations of glutamate. Cancer Res. 1999;59:4383–4391. [PubMed] [Google Scholar]
- 7.Lyons SA, Chung WJ, Weaver AK, Ogunrinu T, Sontheimer H. Autocrine glutamate signaling promotes glioma cell invasion. Cancer Res. 2007;67:9463–9471. doi: 10.1158/0008-5472.CAN-07-2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piao Y, Lu L, de Groot J. AMPA receptors promote perivascular glioma invasion via beta1 integrin-dependent adhesion to the extracellular matrix. Neuro Oncol. 2009;11:260–273. doi: 10.1215/15228517-2008-094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takano T, Lin JH, Arcuino G, Gao Q, Yang J, Nedergaard M. Glutamate release promotes growth of malignant gliomas. Nat Med. 2001;7:1010–1015. doi: 10.1038/nm0901-1010. [DOI] [PubMed] [Google Scholar]
- 10.Ishiuchi S, Tsuzuki K, Yoshida Y, Yamada N, Hagimura N, Okado H, Miwa A, Kurihara H, Nakazato Y, Tamura M, Sasaki T, Ozawa S. Blockage of Ca(2+)-permeable AMPA receptors suppresses migration and induces apoptosis in human glioblastoma cells. Nat Med. 2002;8:971–978. doi: 10.1038/nm746. [DOI] [PubMed] [Google Scholar]
- 11.Rzeski W, Turski L, Ikonomidou C. Glutamate antagonists limit tumor growth. Proc Natl Acad Sci U S A. 2001;98:6372–6377. doi: 10.1073/pnas.091113598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Groot JF, Piao Y, Lu L, Fuller GN, Yung WK. Knockdown of GluR1 expression by RNA interference inhibits glioma proliferation. J Neurooncol. 2008;88:121–133. doi: 10.1007/s11060-008-9552-2. [DOI] [PubMed] [Google Scholar]
- 13.Ye ZC, Rothstein JD, Sontheimer H. Compromised glutamate transport in human glioma cells: reduction-mislocalization of sodium-dependent glutamate transporters and enhanced activity of cystine-glutamate exchange. J Neurosci. 1999;19:10767–10777. doi: 10.1523/JNEUROSCI.19-24-10767.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Groot JF, Liu TJ, Fuller G, Yung WK. The excitatory amino acid transporter-2 induces apoptosis and decreases glioma growth in vitro and in vivo. Cancer Res. 2005;65:1934–1940. doi: 10.1158/0008-5472.CAN-04-3626. [DOI] [PubMed] [Google Scholar]
- 15.Jacobs VL, Landry RP, Liu Y, Romero-Sandoval EA, De Leo JA. Propentofylline decreases tumor growth in a rodent model of glioblastoma multiforme by a direct mechanism on microglia. Neuro Oncol. 2011 doi: 10.1093/neuonc/nor194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tawfik VL, Lacroix-Fralish ML, Bercury KK, Nutile-McMenemy N, Harris BT, Deleo JA. Induction of astrocyte differentiation by propentofylline increases glutamate transporter expression in vitro: heterogeneity of the quiescent phenotype. Glia. 2006;54:193–203. doi: 10.1002/glia.20365. [DOI] [PubMed] [Google Scholar]
- 17.Kruse CA, Molleston MC, Parks EP, Schiltz PM, Kleinschmidt-DeMasters BK, Hickey WF. A rat glioma model, CNS-1, with invasive characteristics similar to those of human gliomas: a comparison to 9L gliosarcoma. J Neurooncol. 1994;22:191–200. doi: 10.1007/BF01052919. [DOI] [PubMed] [Google Scholar]
- 18.Tsui CC, Shankland SJ, Pierchala BA. Glial cell line-derived neurotrophic factor and its receptor ret is a novel ligand-receptor complex critical for survival response during podocyte injury. J Am Soc Nephrol. 2006;17:1543–1552. doi: 10.1681/ASN.2005080835. [DOI] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Williams SM, Sullivan RK, Scott HL, Finkelstein DI, Colditz PB, Lingwood BE, Dodd PR, Pow DV. Glial glutamate transporter expression patterns in brains from multiple mammalian species. Glia. 2005;49:520–541. doi: 10.1002/glia.20139. [DOI] [PubMed] [Google Scholar]
- 21.Vanhoutte N, Abarca-Quinones J, Jordan BF, Gallez B, Maloteaux JM, Hermans E. Enhanced expression of the high affinity glutamate transporter GLT-1 in C6 glioma cells delays tumour progression in rat. Exp Neurol. 2009;218:56–63. doi: 10.1016/j.expneurol.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 22.D’Onofrio M, Arcella A, Bruno V, Ngomba RT, Battaglia G, Lombari V, Ragona G, Calogero A, Nicoletti F. Pharmacological blockade of mGlu2/3 metabotropic glutamate receptors reduces cell proliferation in cultured human glioma cells. J Neurochem. 2003;84:1288–1295. doi: 10.1046/j.1471-4159.2003.01633.x. [DOI] [PubMed] [Google Scholar]
- 23.Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, Iwama H, Nishikawa T, Ichihara N, Kikuchi T, Okuyama S, Kawashima N, Hori S, Takimoto M, Wada K. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science. 1997;276:1699–1702. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- 25.Pascual O, Ben Achour S, Rostaing P, Triller A, Bessis A. Microglia activation triggers astrocyte-mediated modulation of excitatory neurotransmission. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1111098109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takayasu M, Dacey RG., Jr Effects of inhibitory and excitatory amino acid neurotransmitters on isolated cerebral parenchymal arterioles. Brain Res. 1989;482:393–396. doi: 10.1016/0006-8993(89)91207-9. [DOI] [PubMed] [Google Scholar]
- 27.Maragakis NJ, Rothstein JD. Glutamate transporters: animal models to neurologic disease. Neurobiol Dis. 2004;15:461–473. doi: 10.1016/j.nbd.2003.12.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Propentofylline effects on astrocyte and CNS-1 co-culture. (a) Immunocytochemistry of astrocytes cultured in the bottom of a Boyden chamber (CNS-1 cells cultured in top of transwell chamber) on day 1, 3, 5 and 7. Blue = DAPI, red = GFAP, green = Iba1. Scale bar = 40 μm. (b) Graphical representation of percent of Annexin V+ CNS-1 cells co-cultured with astrocytes and treated with 10 μM PPF on day 1, 3, 5 and 7 (* = p < 0.05). (c) Graphical representation of percent glutamate remaining in media compared to untreated (media) group in astrocyte and CNS-1 co-culture, treated with 10 μM PPF on day 1, 3, 5 and 7 (* = p < 0.05).
Astrocytes were treated with GLT-1 and GLAST siRNA, cultured in 5 mM glutamate for 7 days, then analyzed for mRNA expression by qRT-PCR (* = p < 0.05, compared to media, ** = p < 0.05, compared to 100 μM PPF).