Abstract
Juvenile idiopathic arthritis (JIA) is one of the most common chronic diseases of childhood. Although the pathophysiology behind this disease is poorly understood, there are effective treatments for JIA based on the subtype of disease. Treatment options include non-steroidal anti-inflammatory drugs, intraarticular glucocorticoid injections, and traditional disease-modifying anti-rheumatic drugs such as methotrexate. In the past decade, the use of biologic therapy in JIA, including tumor necrosis factor inhibitors, lnterleukin-1 inhibitors, and lnterleukin-6 inhibitors, has dramatically increased with promising outcomes.
Keywords: Juvenile idiopathic arthritis, Treatment, Non-steroidal anti-inflammatory agents, Intra-articular injections, Glucocorticoids, Antirheumatic agents, Immunosuppressive agents, Methotrexate, Sulfasalazine, Biological agents, Tumor necrosis factor-alpha, Recombinant fusion proteins, Monoclonal antibodies, Immunoconjugates, lnterleukin-1, Interleukin 1 receptor antagonist protein, lnterleukin-6
Introduction
Juvenile idiopathic arthritis (JIA) is a chronic inflammatory disease of unknown etiology. JIA is defined by the International League of Associations for Rheumatology (ILAR) as arthritis that begins before the age of 16 and persists for at least 6 weeks with exclusion of other known conditions [1]. JIA is the most common pediatric autoimmune musculoskeletal condition and is estimated to affect 1 in 1,000 children [2]. JIA is classified based on age of onset, number and type of joints involved, presence of serologic findings, and presence of systemic signs and symptoms.
The categories of JIA include: systemic, oligoarticular, polyarticular, enthesitis-related, psoriatic, and undifferentiated (Table 1). Systemic JIA is characterized by arthritis with characteristic fever that can occur in association with an evanescent erythematous rash, generalized lymphadenopathy, hepatomegaly, splenomegaly, and serositis. Children with oligoarticular JIA have arthritis in four or fewer joints while polyarticular JIA is characterized by arthritis in five or more joints. Oligoarticular JIA can be divided into two subcategories: those with four or fewer joints involved throughout the course of the disease have persistent oligoarticular JIA, whereas those with arthritis affecting more than four joints after the first 6 months of disease have extended oligoarticular JIA. Polyarticular JIA is further classified based on the presence or absence of serum rheumatoid factor. Psoriatic arthritis is defined as arthritis and psoriasis in a child, or arthritis and dactylitis, nail pitting, onycholysis, or psoriasis in a first-degree relative. Enthesitis-related arthritis (ERA) is arthritis and enthesitis (inflammation at the sites of tendinous or ligamentous attachments to the bone) or arthritis with sacroiliac joint involvement, HLA-B27 positivity, acute anterior uveitis, or a first-degree relative with the mentioned conditions. Arthritis that does not fulfill any of these categories or fits more than one category is known as undifferentiated arthritis [1].
Table 1.
Definition and classification of juvenile idiopathic arthritis
| General definition | |
| Arthritis of unknown etiology that begins before the age of 16 and persists for ≥6 weeks while excluding other known conditions | |
| Category | Definition |
| Systemic arthritis |
Arthritis in ≥1 joint(s) with or preceded by fever of ≥2 weeks’ duration that is documented to be daily for ≥3 days and accompanied by ≥1 of the following:
|
| Oligoarthritis | Arthritis in 1–4 joints during the first 6 months of disease
|
| Polyarthritis (rheumatoid factor negative) |
Arthritis in ≥5 joints during first 6 months of disease, negative rheumatoid factor |
| Polyarthritis (rheumatoid factor positive) |
Arthritis in ≥5 joints during first 6 months of disease, ≥2 tests positive for rheumatoid factor ≥3 months apart during first 6 months of disease |
| Psoriatic arthritis |
Arthritis and psoriasis, or arthritis and ≥2 of the following:
|
| Enthesitis- related arthritis |
Arthritis and enthesitis, or arthritis or enthesitis with ≥2 of the following:
|
| Undifferentiated arthritis |
Arthritis that fulfills criteria for no category or ≥2 categories |
(Modified from the International League of Associations for Rheumatology [1])
Treatment of JIA is tailored to control disease and limit disability while balancing this with excessive immunosuppression and side effects from these medications. The effectiveness of antirheumatic drugs in JIA clinical trials is measured by a core set of variables defined by the American College of Rheumatology (ACR) [3]. The six core measures are: physician global assessment of disease activity, parent/patient global assessment of well being, functional assessment, active joint count, restricted joint count, and erythrocyte sedimentation rate (ESR). A definition of improvement in disease activity was developed based on these six measures. The American College of Rheumatology Pediatric (ACR Pedi) 30 response is defined as a minimum of 30 % improvement from baseline in a minimum of three out of six measures, with a worsening by greater than 3 0% in no more than one measure. The ACR Pedi 50 and 70 response requires a 50 and 70 % improvement, respectively, in at least three out of six measures, with a worsening of 30 % in no more than one component. Only the ACR Pedi 30 response has been prospectively validated [3].
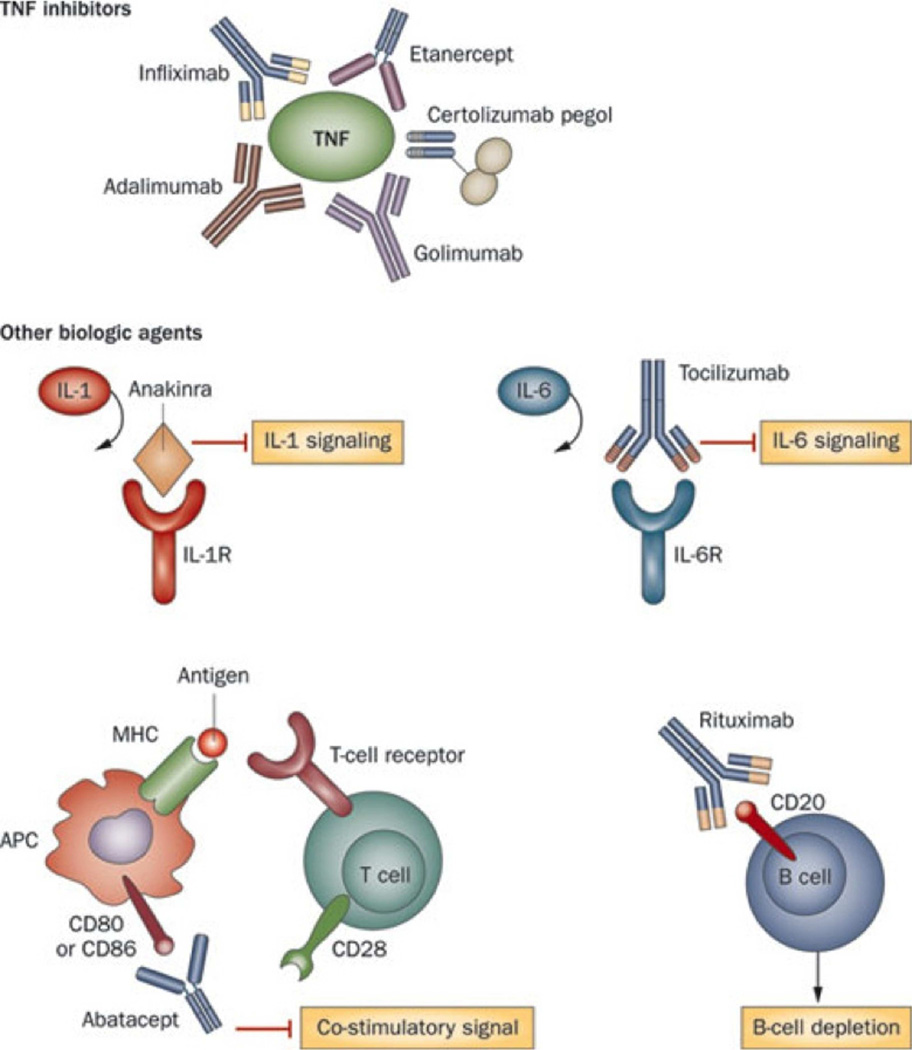
In the past, therapy for JIA has depended on nonsteroidal medications, traditional disease-modifying antirheumatic drugs (DMARDs) like methotrexate and sulfasalazine, and prednisone. Generally, the more joints involved or greater systemic symptoms, the greater amounts of immunosuppressant necessary and greater side effects. Historically, few treatment options were available for children with disease refractory to these medications. The recent development of biologic therapies, which selectively block specific targets of the inflammatory pathway, have revolutionized the treatment of JIA (Fig. 1; Table 2). Biologic agents are not only used for refractory cases of arthritis, but they have also become first-line therapies for certain types of JIA.
Figure 1.
Summary of biologic therapies used in the treatment of juvenile idiopathic arthritis. (From Woodrick and Ruderman [4]; copyright 2011, Nature Publishing Group; reprinted with permission)
Table 2.
DMARDs for treatment of juvenile idiopathic arthritis
| Medication | Mechanism of action | Route | FDA approveda |
|---|---|---|---|
| Abatacept | Binds to CD80/CD86 and inhibits T cell costimulatory signal |
IV, SQ | Yes |
| Adalimumab | TNF inhibitor | SQ | Yes |
| Anakinra | IL-1 inhibitor | SQ | No |
| Canakinumab | IL-1 inhibitor | SQ | No |
| Etanercept | TNF inhibitor | SQ | Yes |
| Infliximab | TNF inhibitor | IV | No |
| Leflunomide | Pyrimidine synthesis inhibitor | Oral | No |
| Methotrexate | Dihydrofolate reductase inhibitor; inhibits DNA synthesis; many others |
Oral, SQ | Yes |
| Rilonacept | IL-1 inhibitor | IV | No |
| Rituximab | Binds CD20 on B cells | IV | No |
| Sulfasalazine | Unknown | Oral | Yes |
| Tociluzimab | IL-6 inhibitor | IV | Yes |
| Tofacitinib | Janus kinase inhibitor | Oral | No |
TNF tumor necrosis factor, IL interleukin, IV intravenous, SQ subcutaneous
For treatment of juvenile idiopathic arthritis
Nonsteroidal anti-inflammatory drugs (NSAIDs)
NSAIDs continue to be the most commonly used medications in arthritis. NSAIDs have both analgesic and anti-inflammatory properties by blocking prostaglandin formation via inhibition of cyclooxygenase-1 and cyclooxygenase-2. Initiation of NSAID monotherapy for 1–2 months is indicated in most cases of oligoarticular and polyarticular JIA per 2011 American College of Rheumatology treatment recommendations [5••]. NSAIDs can be given throughout the treatment period in addition to a non-biologic or biologic DMARD. A randomized, double-blind clinical trial in children with JIA comparing two common NSAIDs—Naproxen and Meloxicam—found no statistically significant differences in response rates according to ACR Pedi 30 [6]. For unknown reasons, patients may respond to one NSAID after the failure of another; therefore, a trial of multiple NSAIDs may be indicated. The primary side effects of traditional NSAIDs involve gastrointestinal symptoms. Overall, NSAIDs are well tolerated in the pediatric population causing minimal toxicity and less serious complications compared to adults [7–9].
Glucocorticoids
Intra-articular glucocorticoid injections
Glucocorticoid joint injections are often recommended for active arthritis in oligoarticular JIA as first-line therapy or second-line treatment following a trial of NSAIDs [5••, 10]. Intra-articular steroid injections are also indicated as needed during treatment with non-biologic or biologic DMARDs if there are only a few active joints in patients with any JIA subtype [5••]. Clinical improvement following a joint injection is expected for at least 4 months and may be repeated after this time interval [5••]. Median time to relapse following a glucocorticoid joint injection in a 2008 study by Marti et al. was 23.1 months [11]. Long-acting corticosteroids are used for intra-articular injections, and triamcinolone hexacetonide was found to be more effective than triamcinolone acetonide in a double-blind trial [12]. Anesthesia may be needed during this procedure, especially in younger patients and when multiple joints are being injected. Possible negative effects of this intervention include subcutaneous or skin atrophy, skin discoloration, and systemic effects of corticosteroids [10, 11, 13].
Systemic glucocorticoids
Systemic corticosteroids have been used for decades in the treatment of JIA [14, 15]. The use of systemic steroids is predominantly limited to patients with systemic JIA because of the significant side effects of long-term steroid use. Steroids are typically used in conjunction with other DMARDs. There is no standardized treatment dosing for corticosteroids; however, consensus algorithms regarding dosing and weaning of this medication have been created for systemic JIA [16••, 17]. The ACR does not include glucocorticoids as recommended treatment for non-systemic JIA; however, they may be indicated in conjunction with other DMARDs for aggressive polyarticular disease [5••, 18,19]. Side effects, especially with chronic use, are significant, and include growth suppression, osteoporosis, immunosuppression, mood disturbance, hypertension, cataracts, and metabolic effects [14].
Non-Biologic DMARDs
Methotrexate
Methotrexate continues to be a first-choice agent for children with JIA. It is a folic acid analogue that competitively inhibits dihydrofolate reductase that interferes with purine biosynthesis and DNA production. Methotrexate also leads to increased adenosine levels, which is hypothesized to be one of its anti-inflammatory mechanisms of action [14]. Methotrexate is indicated as initial treatment for oligoarticular and polyarticular JIA if high disease activity and poor prognostic features are present [5••]. However, this medication is often given as second-line therapy following a trial of NSAID monotherapy and/or following intra-articular joint injections. Methotrexate is also a part of a consensus treatment plan for new-onset systemic JIA [16••].
Methotrexate was found to be an effective therapy for children with JIA with at least three active joints following a double-blind, placebo-controlled trial published in 1992 [20]. Improvement was noted in up to 63 % of patients receiving methotrexate, which was statistically significant compared to placebo. A second randomized, placebo-controlled trial assessing extended oligoarticular and systemic JIA patients also demonstrated that methotrexate is an effective therapy [21].
Methotrexate is generally well tolerated, and its side effects may be related to folate antagonism. Gastrointestinal complaints including abdominal discomfort and nausea are the most common complaints [14]. Other negative effects can include oral ulcers and increased risk for infection given its immunosuppressive effects. Methotrexate can lead to liver and hematologic toxicity, which is why the ACR recommends checking a complete blood count, liver transaminases, and a creatinine at baseline and at least every 12 weeks while on therapy [22].
Leflunomide
Leflunomide inhibits pyrimidine synthesis which leads to decreased proliferation of activated lymphocytes. It also decreases cytokine production, inhibits tyrosine kinase, and inhibits leukocyte-endothelial adhesion leading to its immunomodulatory properties [14]. A randomized, controlled trial comparing the effectiveness of leflunomide to methotrexate in JIA demonstrated that the ACR Pedi 30 responses were significantly higher for the methotrexate group (89 %) compared to the leflunomide group (68 %) [23]. Another open-label study indicated leflunomide might be effective therapy for polyarticular JIA patients who failed methotrexate or were intolerant to that medication with an ACR Pedi 30 response of 52 % at 26 weeks [24]. The ACR treatment recommendations consider leflunomide an appropriate alternative to methotrexate in treatment of patients with polyarthritis [5••]. Patients taking leflunomide should have routine monitoring of complete blood count, liver transaminases, and creatinine given potential toxicities [22].
Sulfasalazine
Sulfasalazine is a 5-aminosalicylic acid analogue linked to a sulfonamide. Anti-inflammatory properties may be related to inhibition of bacterial growth, interference with production of prostaglandins and leukotrienes, and accumulation of adenosine [14]. Sulfasalazine is a recommended treatment for enthesitis-related JIA following a NSAID trial and/or glucocorticoid joint injection [25, 5••]. A randomized, placebo-controlled study demonstrated the safety and efficacy of sulfasalazine in the treatment of children with oligoarticular and polyarticular JIA [26]. Significance between treatment groups was noted for overall articular severity score, global assessments, and laboratory parameters. These groups of patients were reevaluated 7–10 years later to determine if early treatment with sulfasalazine led to sustained benefits [27]. After adjusting for compliance, the initial sulfasalazine group was 4.2 times more likely to have ACR Pedi 30 response compared to the initial placebo group. In one study of children with JIA, 29 % developed side effects leading to discontinuation of the medication [26]. Toxicities can include gastrointestinal effects, rash, cytopenias, and oral ulcers. Laboratory monitoring of complete blood count, liver transaminases, and creatinine is indicated to monitor for toxicities while on sulfasalazine [22].
Other
Other disease modifying drugs include azathioprine, cyclosporine, hydroxychloroquine, penicillamine, tacrolimus, and thalidomide [28–35]. These medications are typically only used with refractory disease or in special indications such as in patients with macrophage activation syndrome, a complication of systemic JIA. There are no clear recommendations for initiation of these medications in treatment of JIA [5••, 16••].
Biologic DMARDs
Tumor Necrosis Factor (TNF) inhibitors
The era of biologic therapy has greatly changed the treatment and outcomes of JIA, and TNF-α inhibitors are the best studied in JIA. TNF-α is a pleiotropic, highly pro-inflammatory cytokine involved in the pathogenesis of numerous autoimmune diseases [36, 37]. Three different TNF inhibitors—Etanercept, Infliximab, and Adalimumab—have been studied in JIA, and more agents in this medication class have been used in adult rheumatoid arthritis and inflammatory bowel disease. TNF inhibitors are recommended as second- or third-line therapy following at least 3 months of methotrexate for patients with continued active disease in oligoarticular or polyarticular JIA [5••]. Also, patients with active sacroiliitis may proceed to anti-TNF therapy after NSAID trial if high disease activity and poor prognostic features are present [5••]. TNF inhibitors are not recommended in any of the consensus treatment plans for new-onset systemic JIA, although they may be indicated if patients have refractory disease with active arthritis [16••, 5••].
Two recent studies have looked at early aggressive initiation of TNF-inhibitors in polyarticular JIA. In 2011, Tynjälä et al. performed a randomized clinical trial comparing three treatment groups: infliximab and methotrexate; methotrexate alone; and methotrexate, sulfasalazine, and hydroxychloroquine (combination DMARDs) [38]. The primary endpoint of ACR Pedi 75 was achieved in 100 % of the infliximab group, 65 % of the combination DMARD group, and 50 % of the methotrexate group. The achievement of inactive disease was also statistically significant, favoring the infliximab group. A similar study by Wallace et al. published in 2012 compared methotrexate, etanercept, and prednisolone to methotrexate alone in a randomized, double-blind, placebo-controlled study in patients with polyarticular JIA [19]. Although no statistical differences were found at 6 months, a trend indicated a better response rate for clinical inactive disease in the TNF inhibitor group (40 %) compared to the methotrexate alone group (23 %).
Safety concerns for TNF inhibitors are primarily related to their immunosuppressive effects. Patients are at increased risk of infection and reactivation of tuberculosis; therefore, recommendations are to perform tuberculosis screening prior to initiation of treatment or when switching between TNF inhibitors [5••, 39]. Other potential adverse events include increased risk for demyelinating disease, leukocytoclastic vasculitis, and drug-induced lupus [14]. Another concern is the increased risk of malignancy, which prompted the Food and Drug Administration to add a black box warning to this class of medications in 2009 [40]. However, a recent study published in Arthritis & Rheumatism demonstrated no relationship of medication use in JIA to development of cancer [41]. Using national Medicaid data, the authors concluded that children with JIA, regardless of treatment, had an increased rate of malignancy.
Etanercept
Etanercept is a soluble chimeric protein containing the human TNF receptor p75 fused to the IgG Fc domain [14]. It was the first TNF inhibitor studied in JIA patients. Multiple studies over the years have suggested JIA patients taking etanercept have an approximately 70 % response rate [14]. The first randomized, double-blind trial of etanercept treatment for polyarticular JIA refractory to methotrexate was published in 2000 [42]. The primary endpoint of disease flare was seen in 81 % of placebo patients at an average of 28 days compared to 28 % of etanercept patients at an average of 116 days. Both the percentage of flare and time to flare were statistically significant. A modified ACR Pedi 30 was obtained in 80 % of etanercept patients compared to 35 % of patients who received placebo. To assess long-term effectiveness, a 3-year open-label study compared methotrexate, etanercept, and methotrexate-etanercept combination [43]. All three groups demonstrated improvement in physician’s global assessment scores and total active joint scores that remained throughout study period. Although no significant differences were noted, the authors suggest this may be secondary to a majority of patients in the two etanercept groups having more severe disease because they were nonresponsive to methotrexate. A study by Otten et al. described factors that influence response rates of etanercept [44]. Increased response was associated with DMARD use prior to etanercept initiation, younger age of disease onset, and low baseline disability scores. Decreased response rates were associated with systemic JIA and female sex. A recently published cohort evaluated safety and efficacy of etanercept in JIA patients under 4 years old [45]. After a mean of 23 months, 80 % had ACR Pedi 30 response, 72 % had ACR Pedi 50 response, and 64 % had ACR Pedi 70 response.
Infliximab
Infliximab is a monoclonal antibody that can bind to both soluble and membrane-bound TNF-α [14]. As opposed to etanercept and adalimumab, infliximab is administered intravenously. Since infliximab is not an entirely humanized monoclonal antibody, combination treatment with methotrexate is recommended to help decrease antibody formation against the drug. Ruperto et al. published a randomized, placebo-controlled trial in 2007 on polyarticular JIA patients receiving infliximab and methotrexate [46]. The ACR Pedi 30 did not differ significantly between the groups; however, during open-label treatment extension at week 204, 44 % of the remaining patients on infliximab met ACR Pedi 30 response criteria [46]. In addition to the side effects mentioned above, infusion reactions occur fairly frequently [47].
Adalimumab
Adalimumab is a fully humanized recombinant IgG monoclonal antibody that binds to TNF. A randomized, double-blind, placebo-controlled trial was performed using adalimumab with or without methotrexate in polyarticular JIA patients [48]. All patients received adalimumab for the first 16 weeks of the trial; ACR Pedi 30 response rate was 94 % in patients also taking methotrexate and 74 % in patients not receiving methotrexate. After withdrawal phase at 48 weeks, the ACR Pedi 30, 50, 70, and 90 responses demonstrated statistically significant improvements in the adalimumab plus methotrexate group. There was no statistical difference between the adalimumab group not taking methotrexate and the placebo group at this time period.
Other
There have been no published studies of certolizumab and golimumab, two newer anti-TNF agents, specifically for the treatment of JIA; however, randomized, controlled trials have been done in adults with rheumatoid arthritis. Certolizumab is a pegylated humanized antibody with specificity for TNF-α. A Cochrane systematic review discussed five randomized, controlled trials comparing certolizumab to placebo or methotrexate in rheumatoid arthritis [49]. Collectively, the ACR 50 improved statistically at 24 and 52 weeks in the certolizumab group. Golimumab is a recombinant human monoclonal antibody that binds to TNF-α. A systematic review of four randomized, controlled trials assessed golimumab versus placebo or other DMARD in rheumatoid arthritis [50]. The golimumab with methotrexate group was 2.6 times more likely to reach ACR 50 response versus methotrexate alone.
Abatacept
Abatacept is a soluble fusion protein of CTLA-4 with the Fc portion of IgG that binds to CD80/CD86 and inhibits a costimulatory signal necessary for T cell activation [14]. Abatacept is recommended for polyarticular disease when a TNF inhibitor does not control disease after 4 months of therapy [5••]. It also is indicated after a second TNF inhibitor is trialed and the patient continues to have moderate to high disease activity. A randomized, double-blind, placebo-controlled withdrawal trial studied abatacept for treatment of children with JIA intolerant to or refractory to at least one DMARD [51]. After the open-label component where all patients received abatacept for 4 months, 72 % achieved an ACR Pedi 30 response. Responders that received abatacept had a significantly decreased rate of disease flares and a significant improvement in quality of life scales compared to the placebo group [52]. After an open-label extension trial at 21 months, 90 % of patients who went onto randomization and continued on abatacept with or without methotrexate achieved the ACR Pedi 30. Abatacept was found to be well tolerated in this long-term study. Five out of 153 children had six serious infections, and five patients had acute infusion reactions [53].
IL-1 inhibitors
lnterleukin-1 (IL-1) plays a central role in inflammation, induction of an acute phase response, and bone erosion and occurs in two forms (IL-lα and IL-1β) [54]. IL-1 signaling is inhibited by a naturally occurring receptor blocking molecule, IL-1 receptor antagonist (IL-1RA). Deficiency in IL-1RA results in an autoinflammatory syndrome characterized by neonatal onset rash, systemic inflammation, arthritis, osteopenia, and lytic bone lesions demonstrating the effects of IL-1 [55, 56]. IL-1 inhibitors prevent interaction of the receptor with IL-1 to inhibit this inflammatory response. Although IL-1 blockade has been shown to be effective in polyarticular arthritis in adults, it is particularly effective in systemic JIA [57]. Anti-IL-1 treatment is one of the four consensus treatment plans for new-onset systemic JIA [16••]. Side effects of this medication class primarily include injection site reactions and increased risk of infection [14, 58–60, 61•].
Anakinra
Anakinra, a human recombinant IL-1 receptor antagonist, is the best studied IL-1 inhibitor [14]. A randomized, double-blind, placebo-controlled trial using anakinra for systemic JIA was published in 2010 [58]. Primary outcome was response at one month characterized by ACR Pedi 30, resolution of systemic symptoms, and decrease in ESR and C-reactive protein (CRP) by at least 50 %. Eight of 12 patients receiving anakinra achieved this response compared to 1 of 12 patients in the placebo group. After switching from placebo to anakinra at 1 month, 9 of 10 patients became responders at month 2. A multicenter series assessed 46 patients who received anakinra as first-line therapy for systemic JIA, in conjunction with steroids and/or additional DMARDs [59]. Overall, approximately 60 % of patients achieved complete response with normalization of labs and no need for additional medication. Fever and rash resolved in greater than 95 % of patients at 1 month and 61 % of patients had no active arthritis after an average follow-up time of 14.5 months.
Canakinumab
Canakinumab is a humanized monoclonal antibody that selectively blocks IL-1β [14]. Canakinumab was given in a multi-center open-label study to patients with systemic JIA and active systemic features [60]. Sixty percent of the 25 patients achieved the adapted ACR Pedi 30 which includes absence of fever in addition to the six other measures. Another recent article discusses two randomized, double-blind, placebo-controlled trials of canakinumab in systemic JIA patients with active disease [61•]. After a single dose of canakinumab, the adapted ACR Pedi 30 response was 84 % in the treatment group and 10 % in the placebo group. The second trial followed 32 weeks of open-label canakinumab treatment, where responders from the first trial were randomized, and time to flare was the primary outcome analyzed. After the withdrawal phase, 74 % of the canakinumab group had no flare versus 25 % of patients in placebo group.
Rilonacept
Rilonacept is a fusion protein between the Fc portion of IgG and the IL-1 receptor that blocks the interaction of IL-1 with cell surface receptors thus preventing IL-1 signaling [14]. Preliminary data are available from a double-blind, placebo-controlled study of systemic JIA patients, which showed Improvement in all six core ACR variables [62]. Currently, the RAPPORT study, a multicenter, randomized, placebo-controlled study of rilonacept in the treatment of systemic JIA, is underway to further analyze efficacy and safety of this medication [63].
Tocilizumab
Tocilizumab is an anti-interleukin-6 (anti-IL-6) receptor monoclonal antibody. IL-6 is the third major proinflammatory cytokine, and high serum concentrations have been associated with disease activity in systemic JIA [60]. Tocilizumab with or without glucocorticoids is recommended as one of the four consensus treatment plans for patients with new-onset systemic JIA [16••]. In a randomized, double-blind, placebo-controlled trial, 91 % of refractory systemic JIA patients achieved an ACR Pedi 30 response after the 6-week open-trial phase during which three doses of tocilizumab were given [64]. An extension open-label study assessed patients at week 144 with 83.9 % achieving ACR Pedi 30 and 57.1 % with inactive disease [65]. A randomized trial of tocilizumab for systemic JIA was recently published by De Benedetti et al. [66•]. After 12 weeks, the tocilizumab group achieved the adapted ACR Pedi 30 in 85 % of patients versus 24 % in the placebo group. Approximately half of patients in the treatment arm had no active arthritis at week 52, and the same percentage of patients were able to discontinue glucocorticoids by this time interval. Side effects included increased risk of infection, neutropenia, and transaminase elevation [64, 66•].
Tofacitinib
Tofacitinib (CP-690,550) was recently approved for treatment of rheumatoid arthritis [67]. Tofacitinib is a Janus kinase (JAK) inhibitor that blocks signaling of multiple cytokines [68]. A placebo-controlled trial of tofacitinib in rheumatoid arthritis was published by Fleischmann et al [69]. At 3 months, the primary outcome of ACR 20 was achieved in 59.8–65.7 % of patients receiving different doses of tofacitinib versus 26.7 % of patients in the placebo group. Adverse events included headache, upper respiratory infection, elevated low-density lipoprotein, and neutropenia [69]. A phase one trial in patients with JIA is currently recruiting participants to assess the pharmacokinetics, safety, and tolerability of tofacitinib [70]. A long-term open-label study of tofacitinib is also enrolling JIA patients [71].
Rituximab
Rituximab is a monoclonal antibody that binds CD20 on B cells leading to removal of B cells from circulation [14]. Rituximab is recommended for polyarticular disease refractory to a TNF inhibitor and abatacept if high disease activity is present [5••]. A multicenter, randomized, double-blind, placebo-controlled trial assessed the efficacy of rituximab plus methotrexate in rheumatoid arthritis patients with inadequate response to anti-TNF medications [72]. The rituximab group demonstrated significant decreases in ACR 20, 50, and 70 compared to the placebo group. Alexeeva et al. evaluated the efficacy and safety of repeat courses of rituximab treatment in severe refractory JIA [73]. ACR Pedi 30 response at week 24 was 98 % and ACR Pedi 70 response at week 96 was 93 %. Side effects include infusion reactions, neutropenia, and decreased immunoglobulins [14, 73].
Conclusions
Advances in the understanding of immunity and inflammation have led to novel therapies for the treatment of JIA. The use of biologic therapies has improved the outcomes of patients with JIA while reducing side effects. Novel biologic therapies continue to be produced, raising the possibility of more effective and targeted therapies for JIA in the future.
Footnotes
Disclosure Julia G. Harris declares that she has no conflict of interest.
Elizabeth A. Kessler declares that she has no conflict of interest.
James W. Verbsky declares that he has no conflict of interest.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Petty R, Southwood T, Manners P, et al. International League of Associations for Rheumatology classifications of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31:390–392. [PubMed] [Google Scholar]
- 2.Helmick C, Felson D, Lawrence R, et al. National Arthritis Data Workgroup: Estimates of the prevalence of arthritis and other rheumatic conditions in the United States Part I. Arthritis Rheum. 2008;58:15–25. doi: 10.1002/art.23177. [DOI] [PubMed] [Google Scholar]
- 3.Giannini E, Ruperto N, Ravelli A, et al. Preliminary definition of improvement in juvenile arthritis. Arthritis Rheum. 1997;40:1202–1209. doi: 10.1002/1529-0131(199707)40:7<1202::AID-ART3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 4.Woodrick RS, Ruderman EM. Safety of biologic therapy in rheumatoid arthritis. Nat Rev Rheumatol. 2011;7:639–652. doi: 10.1038/nrrheum.2011.145. [DOI] [PubMed] [Google Scholar]
- 5. Beukelman T, Patkar NM, Saag KG, et al. 2011 American College of Rheumatology Recommendations for the Treatment of Juvenile Idiopathic Arthritis: Initiation and Safety Monitoring of Therapeutic Agents for the Treatment of Arthritis and Systemic Features. Arthritis Care Res. 2011;63:465–482. doi: 10.1002/acr.20460. This article summarizes the American College of Rheumatology treatment recommendations for juvenile idiopathic arthritis.
- 6.Ruperto N, Nikishina I, Pachanov ED, et al. A Randomized, Double-Blind Clinical Trial of Two Doses of Meloxicam Compared With Naproxen in Children With Juvenile Idiopathic Arthritis. Arthritis Rheum. 2005;52:563–572. doi: 10.1002/art.20860. [DOI] [PubMed] [Google Scholar]
- 7.Dowd JE, Cimaz R, Fink CW. Nonsteroidal anti-inflammatory drug-induced gastroduodenal injury in children. Arthritis Rheum. 1995;38:1225–1231. doi: 10.1002/art.1780380908. [DOI] [PubMed] [Google Scholar]
- 8.Ardoin SP, Sundy JS. Update on nonsteroidal anti-inflammatory drugs. Curr Opin Rheumatol. 2006;18:221–226. doi: 10.1097/01.bor.0000218940.04613.cc. [DOI] [PubMed] [Google Scholar]
- 9.Vora SS, Bengtson CE, Syverson GD, Nocton JJ. An evaluation of the utility of routine laboratory monitoring of juvenile idiopathic arthritis (JIA) patients using non-steroidal anti-inflammatory drugs (NSAIDs): a retrospective review. Pediatr Rheumatol Online J. 2010;8:11. doi: 10.1186/1546-0096-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Padeh S, Passwell JH. Intraarticular corticosteroid injection in the management of children with chronic arthritis. Arthritis Rheum. 1998;41:1210–1214. doi: 10.1002/1529-0131(199807)41:7<1210::AID-ART10>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 11.Marti P, Molinari L, Bolt IB, Seger R, Saurenmann RK. Factors Influencing the efficacy of intraarticular steroid injections in patients with juvenile idiopathic arthritis. Eur J Pediatr. 2008;167:425–430. doi: 10.1007/s00431-007-0525-9. [DOI] [PubMed] [Google Scholar]
- 12.Zulian F, Martini G, Gobber D, et al. Triamcinolone acetonide and hexacetonide intra-articular treatment of symmetrical joints in juvenile idiopathic arthritis: a double-blind trial. Rheumatology. 2004;43:1288–1291. doi: 10.1093/rheumatology/keh313. [DOI] [PubMed] [Google Scholar]
- 13.Goldzweig O, Carrasco R, Hashkes PJ. Systemic adverse events following intraarticular corticosteroid injections for the treatment of juvenile idiopathic arthritis: Two patients with dermatologic adverse events and review of the literature. Semin Arthritis Rheu. 2012 doi: 10.1016/j.semarthrit.2012.12.006. In press. [DOI] [PubMed] [Google Scholar]
- 14.Ilowite NT, Laxer RM. Pharmacology and Drug Therapy. In: Cassidy JT, Petty RE, Laxer RM, Lindsley CB, editors. Textbook of Pediatric Rheumatology. 6th Edition. Philadelphia: Saunders Elsevier; 2011. pp. 71–126. [Google Scholar]
- 15.Schaller JG. Corticosteroids in juvenile rheumatoid arthritis. Arthritis Rheum. 1977;20:537–543. [PubMed] [Google Scholar]
- 16. Dewitt EM, Kimura Y, Beukelman T, et al. Consensus Treatment Plans for New-Onset Systemic Juvenile Idiopathic Arthritis. Arthrit Care Res. 2012;64:1001–1010. doi: 10.1002/acr.21625. This article summarizes consensus treatment plans from the Childhood Arthritis and Rheumatology Research Alliance for new-onset systemic juvenile idiopathic arthritis.
- 17.Ilowite NT, Sandborg CI, Feldman BM, et al. Algorithm development for corticosteroid management in systemic juvenile idiopathic arthritis trial using consensus methodology. Pediatr Rheumatol Online J. 2012;10:31. doi: 10.1186/1546-0096-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prieur AM. The place of corticosteroid therapy in juvenile chronic arthritis in 1992. J Rheumatol. 1993;37:32–34. [PubMed] [Google Scholar]
- 19.Wallace CA, Giannini EH, Spalding SJ, et al. Trial of Early Aggressive Therapy in Polyarticular Juvenile Idiopathic Arthritis. Arthritis Rheum. 2012;64:2012–2021. doi: 10.1002/art.34343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giannini EH, Brewer EJ, Kuzmina N, et al. Methotrexate in Resistant Juvenile Rheumatoid Arthritis. Results of the U.S.A.-U.S.S.R Double-Blind, Placebo-Controlled Trial. N Engl J Med. 1992;326:1043–1049. doi: 10.1056/NEJM199204163261602. [DOI] [PubMed] [Google Scholar]
- 21.Woo P, Southwood TR, Prieur A-M, et al. Randomized, Placebo-Controlled, Crossover Trial of Low-Dose Oral Methotrexate in Children with Extended Oligoarticular or Systemic Arthritis. Arthritis Rheum. 2000;43:1849–1857. doi: 10.1002/1529-0131(200008)43:8<1849::AID-ANR22>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 22.Saag KG, Teng GG, Patkar NM, et al. American College of Rheumatology 2008 Recommendations for the Use of Nonbiologic and Biologic Disease-Modifying Antirheumatic Drugs in Rheumatoid Arthritis. Arthritis Rheum. 2008;59:762–784. doi: 10.1002/art.23721. [DOI] [PubMed] [Google Scholar]
- 23.Silverman E, Mouy R, Spiegel L, et al. Leflunomide or Methotrexate for Juvenile Rheumatoid Arthritis. N Engl J Med. 2005;352:1655–1666. doi: 10.1056/NEJMoa041810. [DOI] [PubMed] [Google Scholar]
- 24.Silverman E, Spiegel L, Hawkins D, et al. Long-Term Open-Label Preliminary Study of the Safety and Efficacy of Leflunomide in Patients With Polyarticular-Course Juvenile Rheumatoid Arthritis. Arthritis Rheum. 2005;52:554–562. doi: 10.1002/art.20861. [DOI] [PubMed] [Google Scholar]
- 25.Burgos-Vargas R, Vazquez-Mellado J, Pacheco-Tena C, et al. A 26 week randomized, double blind, placebo controlled exploratory study of sulfasalazine in juvenile onset spondyloarthropathies. Ann Rheum Dis. 2002;61:941–942. doi: 10.1136/ard.61.10.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Rossum MAJ, Fiselier TJW, Franssen MJAM, et al. Sulfasalazine in the Treatment of Juvenile Chronic Arthritis. Arthritis Rheum. 1998;41:808–816. doi: 10.1002/1529-0131(199805)41:5<808::AID-ART6>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 27.Van Rossum MAJ, van Soesbergen RM, Boers M, et al. Long-term outcome of juvenile idiopathic arthritis following a placebo-controlled trial: sustained benefits of early sulfasalazine treatment. Ann Rheum Dis. 2007;66:1518–1524. doi: 10.1136/ard.2006.064717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Savolainen HA, Kautiainen H, Isomäki H, et al. Azathioprine in patients with juvenile chronic arthritis: a longterm followup study. J Rheumotol. 1997;24:2444–2450. [PubMed] [Google Scholar]
- 29.Lin YT, Yang YH, Tsai MJ, Chiang BL. Long-term effects of azathioprine therapy for juvenile idiopathic arthritis. J Formos Med Assoc. 2000;99:330–335. [PubMed] [Google Scholar]
- 30.Ruperto N, Ravelli A, Castell E, et al. Cyclosporine A in juvenile idiopathic arthritis. Results of the PRCSG/PRINTO phase IV post marketing surveillance study. Clin Exp Rheumatol. 2006;24:599–605. [PubMed] [Google Scholar]
- 31.Brewer EJ, Giannini EH, Kuzmina N, Alekseev L. Penicillamine and hydroxychloroquine in the treatment of severe juvenile rheumatoid arthritis. N Engl J Med. 1986;314:1269–1276. doi: 10.1056/NEJM198605153142001. [DOI] [PubMed] [Google Scholar]
- 32.Kvien TK, Høyeraal HM, Sanstad B. Slow Acting Antirheumatic Drugs in Patients with Juvenile Rheumatoid Arthritis – Evaluated in a Randomized, Parallel 50-Week Clinical Trial. J Rheumatol. 1985;12:533–539. [PubMed] [Google Scholar]
- 33.Tanaka H, Tsugawa K, Suzuki K, et al. Treatment of difficult cases of systemic-onset juvenile idiopathic arthritis with tacrolimus. Eur J Pediatr. 2007;166:1053–1055. doi: 10.1007/s00431-006-0380-0. [DOI] [PubMed] [Google Scholar]
- 34.Lehman TJ, Schechter SJ, Sundel RP, et al. Thalidomide for severe systemic onset juvenile rheumatoid arthritis: A multicenter study. J Pediatr. 2004;145:856–857. doi: 10.1016/j.jpeds.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 35.Garcia-Carrasco M, Fuentes-Alexandro S, Escárcega RO, et al. Efficacy of thalidomide in systemic onset juvenile rheumatoid arthritis. Joint Bone Spine. 2007;74:500–503. doi: 10.1016/j.jbspin.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 36.Shenoi S, Wallace CE. Tumor necrosis factor inhibitors in the management of juvenile idiopathic arthritis: an evidence-based review. Paediatr Drugs. 2010;12:367–377. doi: 10.2165/11532610-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 37.Alonso-Ruiz A, Pijoan JI, Ansuategui E, et al. Tumor necrosis factor alpha drugs in rheumatoid arthritis: systematic review and metaanalysis of efficacy and safety. BMC Musculoskelet Disord. 2008;9:52. doi: 10.1186/1471-2474-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tynjälä P, Vähäsalo P, Tarkiainen M, et al. Aggressive Combination Drug Therapy in Very Early Polyarticular Juvenile Idiopathic Arthritis (ACUTE-JIA): a multicenter randomized open-label clinical trial. Ann Rheum Dis. 2011;70:1605–1612. doi: 10.1136/ard.2010.143347. [DOI] [PubMed] [Google Scholar]
- 39.Singh JA, Furst DE, Bharat A, et al. 2012 Update of the 2008 American College of Rheumatology Recommendations for the Use of Disease-Modifying Antirheumatic Drugs and Biologic Agents in the Treatment of Rheumatoid Arthritis. Arthrit Care Res. 2012;64:625–639. doi: 10.1002/acr.21641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.U.S. Food and Drug Administration: FDA. [Accessed January 2013];Cancer Warnings Required for TNF Blockers. Available at www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucml75803.htm.
- 41.Beukelman T, Haynes K, Curtis JR, et al. Rates of Malignancy Associated With Juvenile Idiopathic Arthritis and Its Treatment. Arthritis Rheum. 2012;64:1263–1271. doi: 10.1002/art.34348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lovell DJ, Giannini EH, Reiff A, et al. Etanercept in Children with Polyarticular Juvenile Rheumatoid Arthritis. N Engl J Med. 2000;342:763–769. doi: 10.1056/NEJM200003163421103. [DOI] [PubMed] [Google Scholar]
- 43.Giannini EH, llowite NT, Lovell DJ, et al. Long-Term Safety and Effectiveness of Etanercept in Children With Selected Categories of Juvenile Idiopathic Arthritis. Arthritis Rheum. 2009;60:2794–2804. doi: 10.1002/art.24777. [DOI] [PubMed] [Google Scholar]
- 44.Otten MH, Prince FHM, Armbrust W, et al. Factors Associated With Treatment Response to Etanercept in Juvenile Idiopathic Arthritis. JAMA. 2011;306:2340–2347. doi: 10.1001/jama.2011.1671. [DOI] [PubMed] [Google Scholar]
- 45.Bracaglia C, Buonuomo PS, Tozzi AE, et al. Safety and Efficacy of Etanercept in a Cohort of Patients with Juvenile Idiopathic Arthritis Under 4 Years of Age. J Rheumatol. 2012;39:1287–1290. doi: 10.3899/jrheum.111555. [DOI] [PubMed] [Google Scholar]
- 46.Ruperto N, Lovell DJ, Cuttica R, et al. A Randomized, Placebo-Controlled Trial of Infliximab Plus Methotrexate for the Treatment of Polyarticular-Course Juvenile Rheumatoid Arthritis. Arthritis Rheum. 2007;56:3096–3106. doi: 10.1002/art.22838. [DOI] [PubMed] [Google Scholar]
- 47.Ruperto N, Lovell DJ, Cuttica R, et al. Long-term efficacy and safety of infliximab plus methotrexate for the treatment of polyarticular-course juvenile rheumatoid arthritis: findings from an open-label treatment extension. Ann Rheum Dis. 2010;69:718–722. doi: 10.1136/ard.2009.100354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lovell DJ, Ruperto N, Goodman S, et al. Adalimumab with or without Methotrexate in Juvenile Rheumatoid Arthritis. N Engl J Med. 2008;359:810–820. doi: 10.1056/NEJMoa0706290. [DOI] [PubMed] [Google Scholar]
- 49.Ruiz Garcia V, Jobanputra P, Burls A, et al. Certolizumab pegol (CDP870) for rheumatoid arthritis in adults. Cochrane Database of Systematic Reviews. 2011;2:CD007649. doi: 10.1002/14651858.CD007649.pub2. [DOI] [PubMed] [Google Scholar]
- 50.Singh JA, Noorbaloochi S, Singh G. Golimumab for Rheumatoid Arthritis: A Systematic Review. J Rheumatol. 2010;37:1096–1104. doi: 10.3899/jrheum.091466. [DOI] [PubMed] [Google Scholar]
- 51.Ruperto N, Lovell DJ, Quartier P, et al. Abatacept in children with juvenile idiopathic arthritis: a randomised, double-blind, placebo-controlled withdrawal trial. Lancet. 2008;372:383–391. doi: 10.1016/S0140-6736(08)60998-8. [DOI] [PubMed] [Google Scholar]
- 52.Ruperto N, Lovell DJ, Li T, et al. Abatacept Improves Health-Related Quality of Life, Pain, Sleep Quality, and Daily Participation in Subjects With Juvenile Idiopathic Arthritis. Arthritis Care Res. 2010;62:1542–1551. doi: 10.1002/acr.20283. [DOI] [PubMed] [Google Scholar]
- 53.Ruperto N, Lovell DJ, Quartier P, et al. Long-term Safety and Efficacy of Abatacept in Children with Juvenile Idiopathic Arthritis. Arthritis Rheum. 2010;62:1792–1802. doi: 10.1002/art.27431. [DOI] [PubMed] [Google Scholar]
- 54.Moltó A, Olivé A. Anti-ll-1 molecules: new comers and new indicators. Joint Bone Spine. 2010;77:102–107. doi: 10.1016/j.jbspin.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 55.Reddy S, Jia S, Geoffrey R, et al. An autoinflammatory disease due to homozygous deletion of the IL1RN locus. N Engl J Med. 2009;360:2438–2444. doi: 10.1056/NEJMoa0809568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aksentijevich I, Masters SL, Ferguson PJ, et al. An autoinflammatory disease with deficiency of the interleukin-1-receptor antagonist. N Engl J Med. 2009;360:2426–2437. doi: 10.1056/NEJMoa0807865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ilowite N, Porras O, Reiff A, et al. Anakinra in the treatment of polyarticular-course juvenile rheumatoid arthritis: safety and preliminary efficacy results of a randomized multicenter study. Clin Rheumatol. 2009;28:129–137. doi: 10.1007/s10067-008-0995-9. [DOI] [PubMed] [Google Scholar]
- 58.Quartier P, Allantaz F, Cimaz R, et al. A multicentre, randomised, double-blind, placebo-controlled trial with the interleukin-1 receptor antagonist anakinra in patients with systemic-onset juvenile idiopathic arthritis (ANAJIS trial) Ann Rheum Dis. 2011;70:747–754. doi: 10.1136/ard.2010.134254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nigrovic PA, Mannion M, Prince FHM, et al. Anakinra as First-Line Disease-Modifying Therapy in Systemic Juvenile Idiopathic Arthritis. Arthritis Rheum. 2011;63:545–555. doi: 10.1002/art.30128. [DOI] [PubMed] [Google Scholar]
- 60.Ruperto N, Quartier P, Wulffraat M, et al. A Phase II, Multicenter, Open-Label Study Evaluating Dosing and Preliminary Safety and Efficacy of Canakinumab in Systemic Juvenile Idiopathic Arthritis With Active Systemic Features. Arthritis Rheum. 2012;64:557–567. doi: 10.1002/art.33342. [DOI] [PubMed] [Google Scholar]
- 61. Ruperto N, Brunner HI, Quartier P, et al. Two Randomized Trials of Canakinumab in Systemic Juvenile Idiopathic Arthritis. N Engl J Med. 2012;367:2396–2406. doi: 10.1056/NEJMoa1205099. This article discusses two trials of canakinumab, a new IL-1 inhibitor, for treatment in systemic juvenile idiopathic arthritis.
- 62.Lovell DJ, Giannini EH, Kimura Y, et al. Preliminary evidence for sustained bioactivity of IL-1 trap (rilonacept), a long acting IL-1 inhibitor, in systemic juvenile idiopathic arthritis (SJIA) Arthritis Rheum. 2007;56:S515. [Google Scholar]
- 63.National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) ClinicalTrials.gov [Internet] Bethesda (MD: National Library of Medicine (US); 2000. Randomized Placebo Phase Study of Rilonacept in the Treatment of Systemic Juvenile Idiopathic Arthritis (RAPPORT) [cited 2013 Jan 30]. Available from: http://clinicaltrials.gov/ct2/show/NCT00534495 NLM Identifier: NCT00534495. [Google Scholar]
- 64.Yokota S, Imagawa T, Mori M, et al. Efficacy and safety of tocilizumab in patients with systemic-onset juvenile idiopathic arthritis: a randomized, double-blind, placebo-controlled, withdrawal phase III trial. Lancet. 2008;371:998–1006. doi: 10.1016/S0140-6736(08)60454-7. [DOI] [PubMed] [Google Scholar]
- 65.Yokota S, Imagawa T, Mori M, et al. Long-term treatment of systemic juvenile idiopathic arthritis with tocilizumab: results of an open-label extension study in Japan. Ann Rheum Dis. 2012;0:1–2. doi: 10.1136/annrheumdis-2012-202310. [DOI] [PubMed] [Google Scholar]
- 66. De Benedetti F, Brunner HI, Ruperto N, et al. Randomized Trial of Tociluzimab in Systemic Juvenile Idiopathic Arthritis. N Engl J Med. 2012;367:2385–2395. doi: 10.1056/NEJMoa1112802. This article discusses a randomized trial of tociluzimab, an IL-6 inhibitor, which demonstrated a significant improvement in treatment of systemic juvenile idiopathic arthritis in the tociluzimab group compared to the placebo group.
- 67.U.S. Food and Drug Administration. [Accessed January 2013];FDA Approves Xeljanz for rheumatoid arthritis. Available at http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm327152.htm.
- 68.LaBranche TP, Jesson Ml, Radi ZA, et al. JAK Inhibition With Tofacitinib Suppresses Arthritis Joint Structural Damage Through Decreased RANKL Production. Arthritis Rheum. 2012;64:3531–3542. doi: 10.1002/art.34649. [DOI] [PubMed] [Google Scholar]
- 69.Fleischmann R, Kremer J, Cush J, et al. Placebo-Controlled Trial of Tofacitinib Monotherapy in Rheumatoid Arthritis. N Engl J Med. 2012;367:495–507. doi: 10.1056/NEJMoa1109071. [DOI] [PubMed] [Google Scholar]
- 70.Pfizer . ClinicalTrials.gov [Internet] Bethesda (MD: National Library of Medicine (US); 2000. An Open-Label Multiple Dose Study To Evaluate The Pharmacokinetics, Safety and Tolerability Of CP-690,550 In Pediatric Patients From 2 To Less Than 18 Years Of Age With Juvenile Idiopathic Arthritis (JIA) [cited 2013 Jan 30] Available from: http://clinicaltrials.gov/ct2/show/NCT01513902 NLM Identifier: NCT01513902. [Google Scholar]
- 71.Pfizer . ClinicalTrials.gov [Internet] Bethesda (MD: National Library of Medicine (US); 2000. A Long-Term, Open-Label Follow-Up Study Of CP-690,550 For Treatment Of Juvenile Idiopathic Arthritis (JIA) [cited 2013 Jan 30] Available from: http://clinicaltrials.gov/ct2/show/NCT01500551 NLM Identifier: NCT01500551. [Google Scholar]
- 72.Cohen SB, Emery P, Greenwald MW, et al. Rituximab for Rheumatoid Arthritis Refractory to Anti-Tumor Necrosis Factor Therapy. Arthritis Rheum. 2006;54:2793–2806. doi: 10.1002/art.22025. [DOI] [PubMed] [Google Scholar]
- 73.Alexeeva EI, Valieva SI, Bzarova TM, et al. Efficacy and safety of repeat courses of rituximab treatment in patients with severe refractory juvenile idiopathic arthritis. Clin Rheumatol. 2011;30:1163–1172. doi: 10.1007/s10067-011-1720-7. [DOI] [PubMed] [Google Scholar]