Abstract
Objective
Intimal hyperplasia (IH) is the cause of most failed arteriovenous fistulas (AVFs), resulting in repeat procedures and leading to increased utilization of scarce health care resources. Our laboratory has previously demonstrated the role of supplemental oxygen in preventing IH and smooth muscle cell proliferation (SMCp) at an artery-to-graft anastomosis and at the deployment site of an intra-arterial stent. This study examines the effect of supplemental oxygen in preventing IH and SMCp in an AVF in a rabbit model.
Methods
Ninety-six rabbits were randomized into four groups: group 1, control; group 2, no surgery with supplemental oxygen; group 3, AVF without supplemental oxygen; and group 4, AVF with supplemental oxygen. Rabbits receiving supplemental oxygen received 30% oxygen for up to 42 days. Specimens were collected in all groups at days 1, 3, 7, 21, 42, and 90. IH and SMCp were measured at the AVF site as well as in the artery and vein proximal and distal to the AVF.
Results
IH was first noted at day 7 and significantly increased through day 90 at all locations in the nonoxygen-supplemented groups. No significant IH was noted in the oxygen-supplemented group at any location or any time point. SMCp was noted at day 3 through day 21 in the nonoxygen-supplemented group, whereas almost no SMCp was noted in the oxygen-supplemented group at any location or time point.
Conclusions
Without oxygen supplementation, SMCp begins at day 3 and is no longer noted at day 21 after creation of an AVF, whereas IH begins by day 7 and increases at least through day 90 after creation of an AVF. Forty-two days of 30% supplemental oxygen inhibits IH and SCMp after creation of an AVF. These data suggest a role for the short-term administration of low-dose O2 to prevent both IH and SMCp after creation of an AVF that may prolong patency and function.
Vascular access is the “lifeline” for end-stage renal disease (ESRD) patients who receive hemodialysis. Following the “Fistula First Initiative” launched nationwide in 2004, fistulas are considered the gold standard for establishing access to a patient’s circulatory system to provide life-sustaining dialysis.1 Complications of vascular access are not only a major cause of morbidity in hemodialysis patients but also a major fiscal drain for the ESRD program.
Although arteriovenous fistula (AVF) is the preferred vascular access, its patency at 1 year is estimated to be only 62%.2 The most common cause of AVF failure is thrombosis, usually caused by venous stasis associated with venous stenotic lesions.3 Data suggest that AVFs fail secondary to venous stenosis caused by intimal hyperplasia (IH)4 with cellular proliferation and matrix deposition.5 An effective method to control IH is currently unknown. We previously established that vessel wall hypoxia has a causative role in IH. Our laboratory showed that artery wall hypoxia plays a critical role in IH and smooth muscle cell proliferation (SMCp).6–11 Our laboratory also demonstrated that artery wall hypoxia occurs at an arterial anastomosis, and a return to normal artery wall oxygen concentration is noted after 42 days. We have also shown that artery wall hypoxia can be reversed and IH and SMCp inhibited by the administration of 40% supplemental oxygen for 42 days immediately after creation of an arteryto-prosthetic graft anastomosis.8,9 Our work has also revealed that supplemental oxygen can reverse artery wall hypoxia and prevent IH and SMCp at the deployment site of an intra-arterial stent.10,11
Semenza12 has demonstrated that hypoxia is the primary stimulus that modulates the expression of various proteins that are responsible for mediating angiogenesis, matrix deposition, cellular proliferation, and migration. Semenza’s work clearly demonstrates modulating oxygen delivery to the vessel wall should have an effect on the development of IH.
Strategies to prevent IH, including pharmacologic therapy, antioxidant therapy, the local infusion of heparin, and gene-directed therapy by adenoviral vectors,13–16 have yet to be widely used and accepted. One possible impediment to the widespread use of these various therapies shown to be effective in in vitro and in animal studies is the expense, complexity, and the practical utility of applying these therapies daily. Conversely, oxygen is widely available and has a long history of relative safety. Oxygen, as a preventive therapy for IH, has not been fully explored. The primary aim of our study is to test the hypothesis that 30% supplemental oxygen will inhibit both SMCp and IH at an AVF.
METHODS
All of the animal procedures in this study were conducted in accordance with animal use protocols approved by the University of Minnesota and the Minneapolis VA Health Care System (MVAHCS) Institutional Animal Care and Use Committees (IACUC).
Animal model
We chose 96 male New Zealand White (NZW) rabbits (6–7 pounds; Bakkom Rabbitry, Red Wing, Minn) for our animal model because we have long used NZW rabbits in our studies on IH. Four rabbits each were randomized into one of four experimental groups that varied by six different time points (day 1, 3, 7, 21, 42, and 90):
Group 1: control group—these rabbits had no surgery and received no supplemental oxygen.
Group 2: control group with oxygen—these rabbits had no surgery but received supplemental oxygen.
Group 3: AVF with no supplemental oxygen—these rabbits underwent AVF placement but received no supplemental oxygen.
Group 4: AVF with supplemental oxygen—these rabbits underwent AVF placement and received supplemental oxygen.
Oxygen administration
Rabbits were assigned to the control chamber (21% oxygen environment) or to the oxygen chamber (30% oxygen environment). These chambers were designed to regulate the environmental oxygen content at 21% or 30%, maintain constant relative humidity between 55% and 65%, and keep the ambient temperature between 55°F and 72°F. All of the rabbits were conditioned to the normoxic 21% oxygen environment chamber for 1 week before the study and fed standard rabbit chow and water during the course of the study. Rabbits in the supplemental oxygen groups were exposed to oxygen for up to 42 days based on previous work completed in our laboratory.6,9 The oxygen-supplemented rabbits were exposed to a normoxic environment (21% oxygen) for 1 hour/d to allow for daily oxygen chamber care such as food, water and bedding changes.
Creation of the AVF
After 1 week of acclimatization, rabbits were anesthetized with ketamine (40 mg/kg) and xylazine (5mg/kg) intramuscularly and intubated with a 3-mm endotracheal tube. A 24-gauge intravenous catheter was placed in the marginal ear vein and the rabbits were placed on a circulating water heating pad to maintain a normal core body temperature. Isoflurane (1%-1.5%) inhalation anesthesia was used to maintain rabbits under general anesthesia perioperatively.
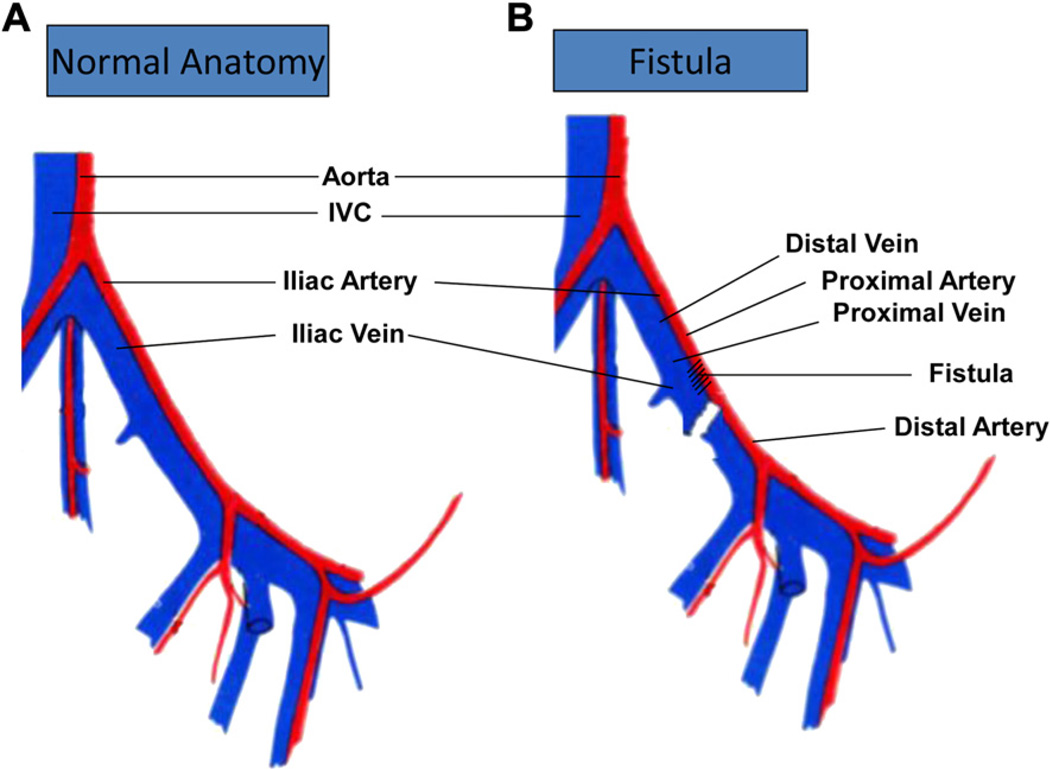
Under loupe magnification and aseptic conditions, a midline incision was used to expose the infrarenal aorta and the left common iliac artery and vein. The rabbit was heparinized with 200 U/kg of intravenous heparin 3 minutes before clamping the left common iliac artery and vein proximally and distally. The iliac vein was transected and the distal end oversewn. An arteriotomy was created in the common iliac artery, and the iliac vein was anastomosed to the iliac artery in an end-to-side manner with two 7-0 Prolene (Ethicon, Somerville, NJ) sutures in a running fashion from heel to toe (Fig 1). Blood loss was replaced with intravenous normal saline.
Fig 1.
Creation of the arteriovenous fistula (AVF). A, Describes the normal anatomy of the iliac artery and vein and its surrounding vessels. B, After clamping the left common iliac artery and vein proximally and distally, the iliac vein was transected and the distal end oversewn. An arteriotomy was created in the common iliac artery, and the iliac vein was anastomosed to iliac artery in an end-to-side manner. The artery proximal to the fistula was considered the proximal artery, and the artery distal to the fistula was considered the distal artery. The vein immediately adjacent to the fistula was considered proximal vein and the vein distal to the fistula was the distal vein. IVC, Inferior vena cava.
Administration of 5-bromo-2′-deoxyuridine and vessel harvesting for histologic analysis
Tissue was evaluated from four rabbits in each group, at 1, 3, 7, 21, 42, and 90 days. Tissue evaluation was performed at the following five sites: fistula, proximal artery, proximal vein, distal artery, and distal vein. At 24 hours before the rabbits were euthanized, 5-bromo-2’-deoxyuridine (BrdU; 100 mg/kg) was administered intraperitoneally. Rabbits were euthanized with an overdose of pentobarbital.
After euthanasia, the right iliac artery and vein were cannulated with 18-gauge catheters, and the iliac artery was flushed under physiologic pressures (80–100 mm Hg) with normal saline while the venous catheter drained the total body blood volume into an empty intravenous bag. Once a clear effluent was seen, the artery was perfusion fixed with Karnovsky’s solution for 10 minutes,17 and the vessels were harvested en bloc. The harvested specimen was placed in 10% formalin and sent for histologic preparation.
These specimens were placed in paraffin and sectioned through the anastomosis for a total of nine sections, 5 µm apart, for each vessel. The sections were individually stained with (1) hematoxylin and eosin (routine histology), (2) trichrome (stains collagen), (3) fibrin (stains thrombus), (4) Verhoeff-van Gieson (stains elastic fibers), (5) α-actin (stains SMCs), (6) anti-Factor VIII (stains endothelial cells), (7) and anti-BrdU antibodies (to evaluate proliferation).
BrdU staining protocol
The BrdU staining was performed using a Zymed BrdU staining kit (Zymed Laboratories, San Francisco, Calif) according to the manufacturer’s protocol. Each specimen was embedded in paraffin, and 4-µm-thick sections were cut on a microtome. Nine sections were cut transversely at each of the five sites, and the Zymed protocol was subsequently followed without modification.
Morphometric analysis
Cells that incorporated BrdU were identified as having a distinctive brown pigment within their nuclei. A Zeiss microscope with a digitized camera attachment (Oberkochen, Germany) fed images directly into a personal computer-based microprocessor. An image analysis (Bioquant 8.40.20 BQ-XI; Bioquant Image Analysis Corp, Nashville, Tenn) morphometrics program was used to count all BrdU-labeled cells and all other cells within a microscopic field. This process was repeated until the entire circumference of the vessel wall was evaluated. Cellular proliferation was defined as all cells positively staining for BrdU divided by all cells present within the vessel wall. Cellular proliferation is reported in percent units (positively stained BrdU cells/all cells).
The image analysis morphometrics program was also used to calculate IH, which is reported as ratio of intimal area (IA) to media area (MA). The digital camera was calibrated by measuring a known distance on the microscope stage. The intimal and medial boundaries were marked, and the IA and the MA were then automatically calculated. The standardization of IA-to-MA ratio was performed to eliminate any variation in fixation and vessel size between rabbits. The final derivation of IH and SMCp were calculated as an average of all nine sections from each of the five sites.
Statistical analysis
All data are expressed as mean ± standard error of the mean. Analysis of variance was used for comparisons between three or more groups and the Student two-tailed t-test was used for comparisons between two groups. A P value of ≤.05 was considered significant.
RESULTS
There was no BrdU staining activity or intimal thickness noted in group 1 or 2 at any recorded time point (Figs 2 and 3).
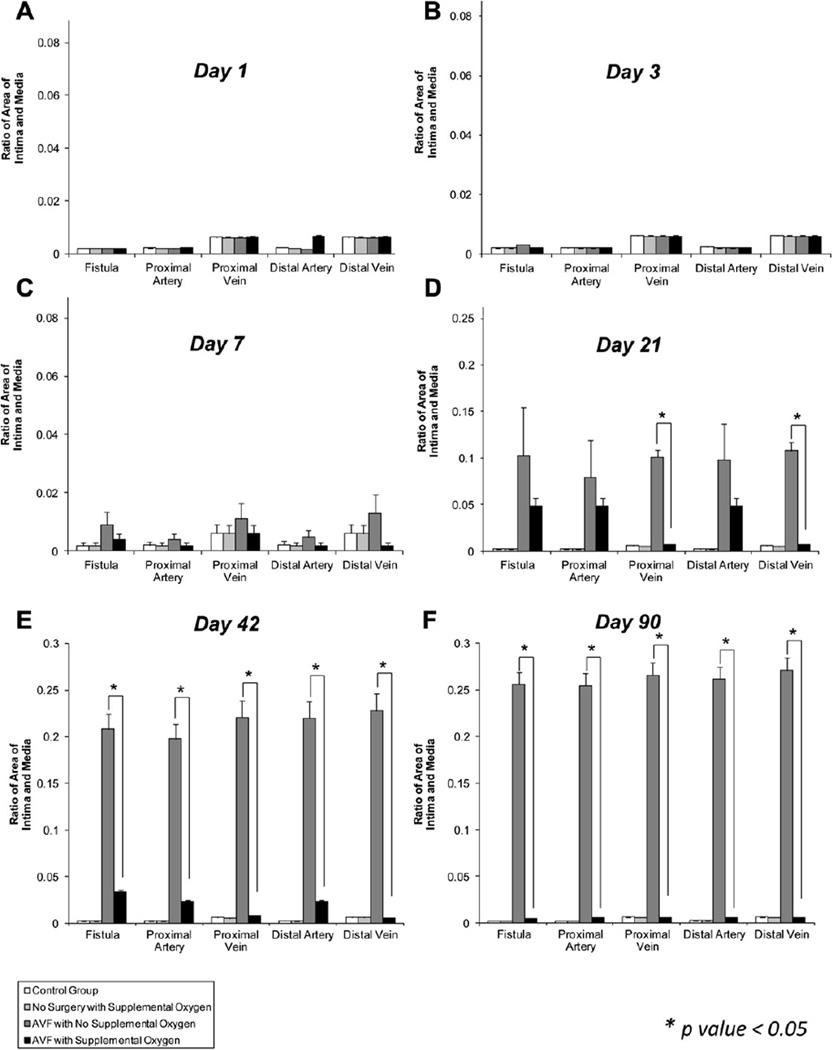
Fig 2.
Effect of supplemental oxygen on intimal hyperplasia (IH) at the arteriovenous fistula (AVF). A, No change in IH with supplemental oxygen at day 1. B, No change in IH with supplemental oxygen at day 3. C, Supplemental oxygen shows a trend toward inhibiting IH at all sites at day 7. D, Supplemental oxygen significantly inhibits IH at the proximal vein and distal vein at day 21. E, Supplemental oxygen inhibits IH at all sites at day 42. F, Supplemental oxygen inhibits IH at all sites at day 90. Data are expressed as the ratio of intimal area (IA) to media area (MA). Data are expressed as mean ± standard error of the mean. P ≤ .05 was considered significant.
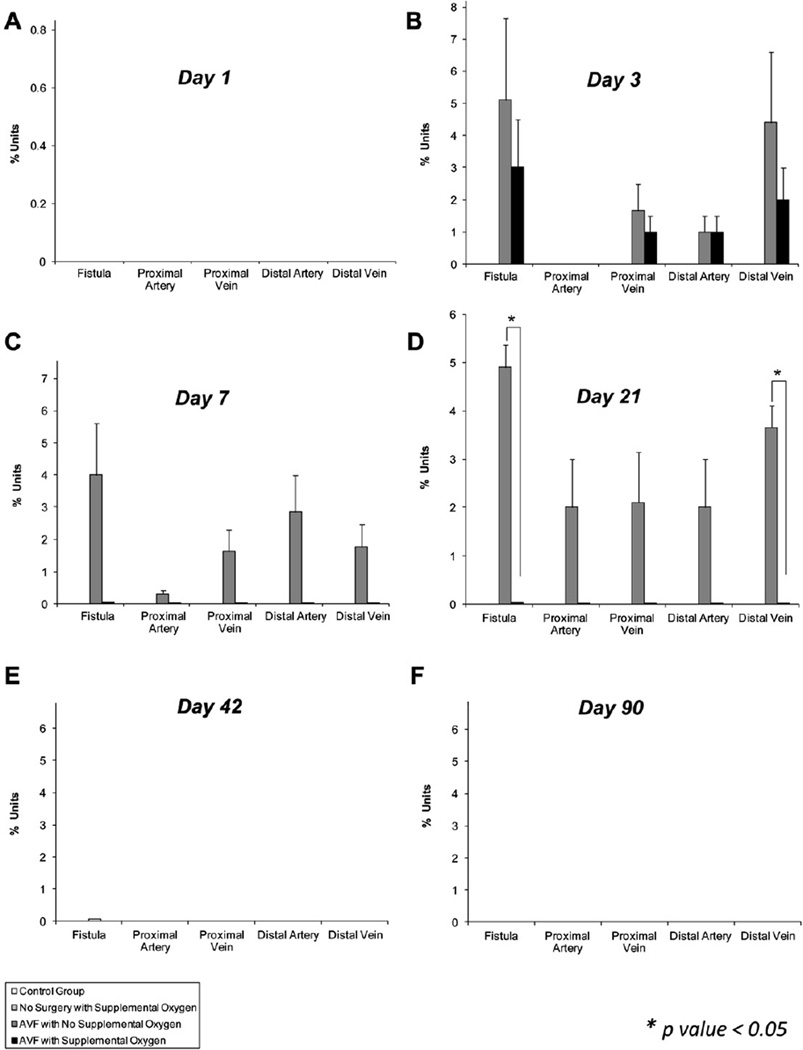
Fig 3.
Effect of supplemental oxygen on smooth muscle cell proliferation (SMCp). A, No significant proliferation was observed on day 1 in any treatment group. On day (B) 3 and (C) 7, the arteriovenous fistula (AVF) operation markedly increased the 5-bromo-2′-deoxyuridine (BrdU) staining, suggestive of proliferation at all sites. However, it was not significantly altered with oxygen supplementation. D, On day 21, the AVF operation markedly increased BrdU staining at all sites, and this proliferation was significantly decreased by oxygen supplementation at the site of the fistula and the distal vein. On day (E) 42 and (F) 90, there was no BrdU staining, suggesting absence of proliferation. This suggests that SMCp is a self-limiting event. Data are expressed as percent units (positively stained BrdU cells/all cells). Data are expressed as mean ± standard error of the mean. P ≤ .05 was considered significant.
For IH results, no change was seen in any group in days 1 and 3 (Fig 2, A and B). There was a nonsignificant increase in IH at all sites when comparing the nonoxygen-supplemented surgical group (group 3) with the oxygen-supplemented surgical group (group 4) on day 7 (Fig 2, C). There was a significant increase in IH at the proximal and distal vein sites in group 3 compared with group 4, whereas there were nonsignificant increases in IH at all other sites when comparing group 3 with group 4 on day 21 (Fig 2, D). On days 42 and 90, there was a significant increase in IH at all locations in group 3 compared with group 4 (Figs 2, E and F, and 4, A–D).
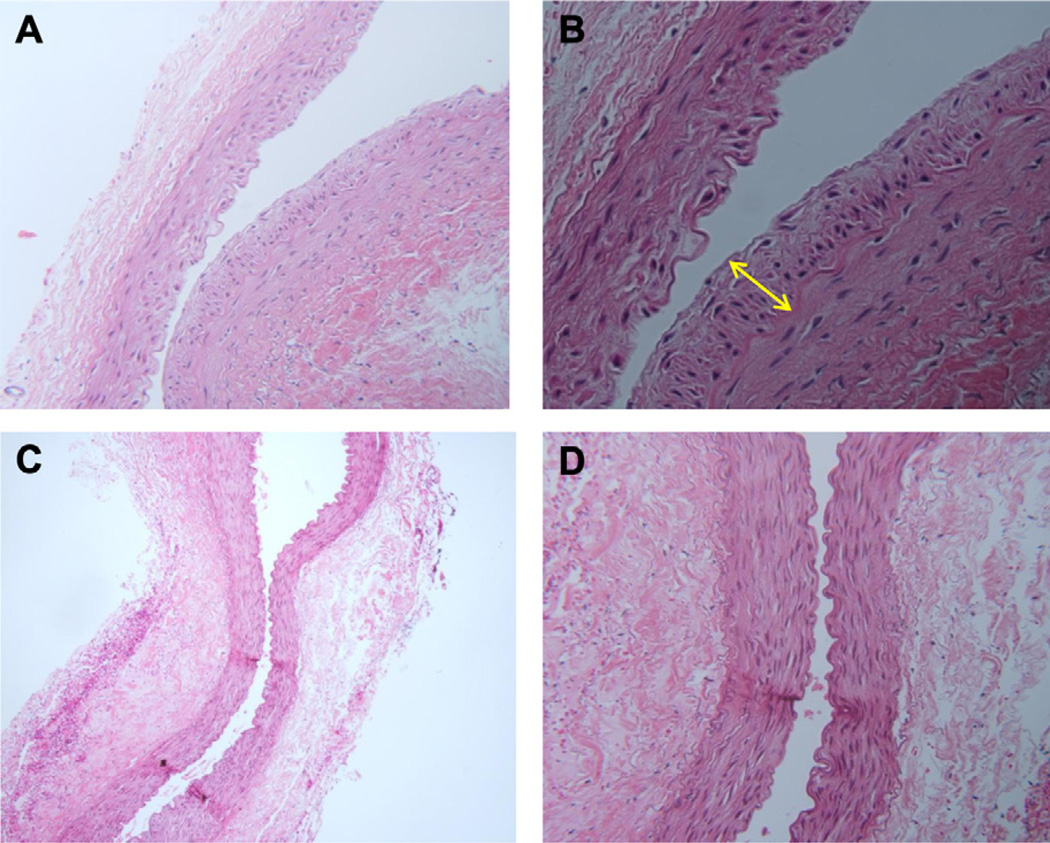
Fig 4.
Intimal hyperplasia (IH) at the distal vein. A and B, A 90-day nonoxygen-treated rabbit (hematoxylin and eosin stain, original magnification, ×100, ×200). The yellow arrow represents the increased area of intima in a nonoxygen-treated vein. C and D, A 90-day oxygen-treated rabbit (hematoxylin and eosin, original magnification, ×100, ×200).
During SMCp evaluation, BrdU staining was not increased in any group on day 1 (Fig 3, A). There was a nonsignificant increase in staining noted at the AVF site and at the distal vein site in group 3 compared with group 4 on day 3 (Fig 3, B). A nonsignificant increase in staining was noted at all sites, except the proximal artery site, in group 3 compared with group 4 on day 7 (Fig 3, C). A significant increase in was staining noted at the AVF and distal vein sites in group 3 compared with group 4, whereas a nonsignificant increase was noted at all other sites when group 3 was compared with group 4 on day 21 (Figs 3, D and 5, A–D). At days 42 and 90, there was no significant staining activity in any group at any location (Fig 3, E and F).
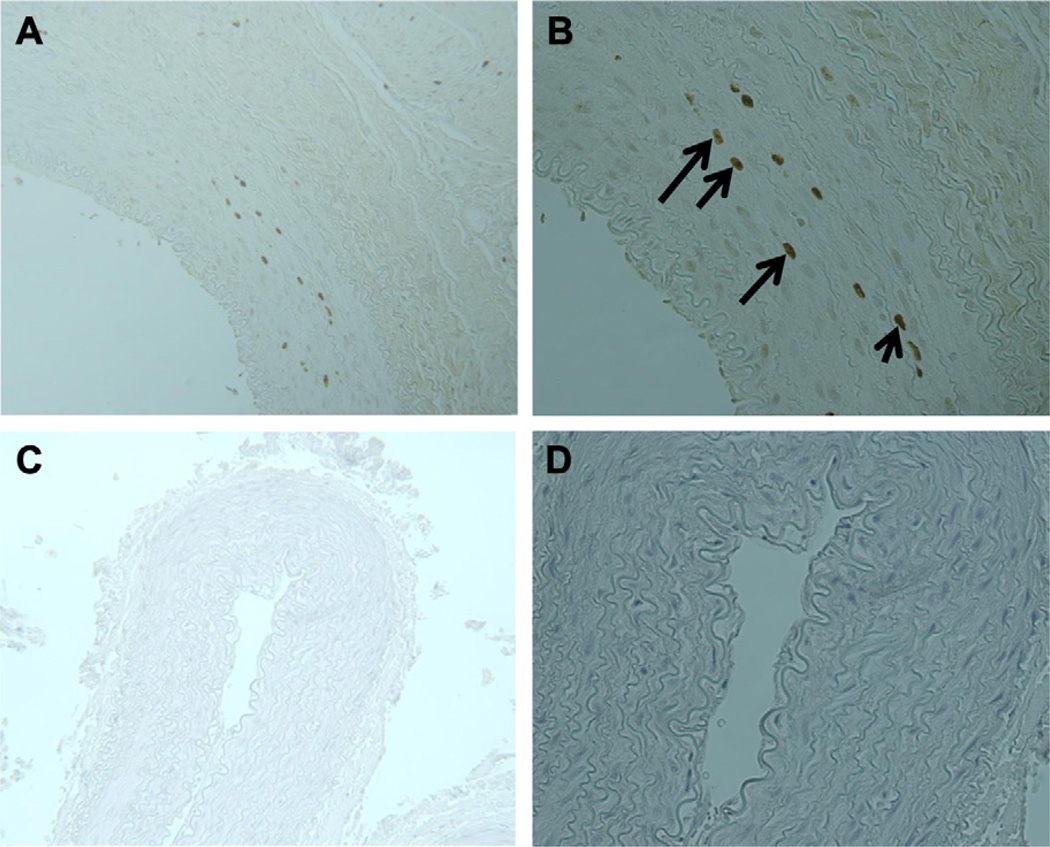
Fig 5.
Cellular proliferation at arteriovenous fistula (AVF). Immunoperoxidase staining of 5-bromo-2′-deoxyuridine( BrdU)-labeled cells. The black arrows identify darkly stained nuclei, which represent BrdU-postive staining cells. A and B, A 21-day nonoxygen-treated rabbit (biotinylated mouse anti-BrdU, original magnification, ×100, ×200). C and D, A 21-day oxygen-treated rabbit (biotinylated mouse anti-BrdU, original magnification, ×100, ×200).
DISCUSSION
This study is the first to demonstrate that the short-term administration of 30% supplemental oxygen inhibits the formation of IH and SMCp at the site of an AVF in an animal model. We clearly demonstrate that IH begins to develop not only at the site of an AVF but also in the artery and vein proximal and distal to the AVF. IH begins at day 7 and continues to increase through day 90 after AVF placement in group 3. SMCp is first noted at day 3, persists through day 21, and is not noted at days 42 and 90. IH continues to increase in group 3 at all locations through day 90, whereas BrdU staining peaks at day 21 and then is no longer present at days 42 or 90. However, one of the limitations in this study includes the ability to evaluate the changes in IH and SMCp along more continuous time points, in particular between days 21 and 42 to evaluate when SMCp proliferation stops.
Findings also support our hypothesis that IH and SMCp are both inhibited by the administration of 30% supplemental oxygen at all locations and at all time-points. Interestingly, the data demonstrate that SMCp begins early (day 3) and is self-limited, in that no SMCp is noted after day 21 in group 3 or 4. These data also demonstrate that IH only occurs after SMCp and IH persists long after SMCp ceases. It would seem that the process of IH, once started, continues without SMCp and may result in the pathologic vessel changes of IH with clinical implications. Our data clearly show that 30% supplemental oxygen will inhibit SMCp and IH and that the effect persists long after the oxygen is discontinued. These data definitively demonstrate that SMCp and IH can be inhibited at the site of an AVF and at locations in the artery proximal to the AVF and in the vein distal to the AVF with the administration of low-dose (30%) supplemental oxygen for up to 21 days. The clinical and research implications of these findings can be powerful.
After the National Kidney Foundation Kidney Disease Outcomes Quality Initiative was published, a slow but continuous change began in the standard of care for dialysis access patients. The new standard of care, not only in the United States but also worldwide, is that patients requiring a surgical dialysis access procedure undergo placement of an AVF first as opposed to a prosthetic vascular access graft. To date, much of the experimental research performed in hemodialysis vascular access has been performed in artery-to-graft or vein-to-graft anastomosis. Although we have clinically noticed stenoses downstream of AVF, and despite research evaluating the effects of hypoxia on veins, the mechanisms underlying the formation of venous stenosis in vascular anastomosis remain poorly understood. Many factors have been hypothesized to be involved in the formation of venous stenosis, including hypoxia. Hypoxia activates several cytokines that are implicated in the angiogenic process and has also been shown to be associated with hemodialysis access failure.18 Our aim with this study was to determine that there was indeed an effect with oxygen in this AVF model. Our future studies will evaluate and address the mechanism of processes we see in this study.
Clinically, the data from this study would suggest that SMC cellular activity is greatest between days 3 and 21 after creation of an AVF and that the administration of 30% supplemental oxygen for 21 days may be all that is needed to inhibit the formation of IH at an AVF and its adjacent artery and vein, thereby improving not only patency and function of an AVF but also patient outcomes and quality of life, while reducing health care costs.
With the reduction in time of administration and concentration of oxygen, we can reduce the chance of experiencing some problems that nasal cannula oxygen users encounter, such as dry or bloody noses and skin irritation, along with changes that could result from free oxygen radicals that may result from the use of supplemental oxygen. And in comparison with other therapies that last 3, 6, or 12 months, and even others that may be life-long, we think that a minimum of 21 days of supplemental oxygen to treat the burst of SMCp is relatively short term.
In addition, our findings add to the body of knowledge already published by our laboratory that demonstrate the key role oxygen plays in many forms of artery wall pathology, including atherosclerosis, IH after the creation of an artery-to-prosthetic vascular graft anastomosis, IH at the deployment of an intra-arterial stent, as well as studies demonstrating an effect on veins. For a number of years, our laboratory used 40% oxygen because other studies have demonstrated that is a safe concentration, and we reduced the oxygen to 30% to see if we could identify an optimal concentration. Our previous assumption that 40% supplemental oxygen is required to mediate IH and improve outcomes has been supplanted by the results we have achieved using 30% supplemental oxygen.
CONCLUSIONS
We propose that areas for future research include defining the mechanisms underlying the ability of oxygen to inhibit SMCp and reduce IH. Also, the exact duration and concentration of supplemental oxygen needs to be carefully defined. Other animal models should be studied to determine if the same results of controlling IH and SMCp at the site of other arterial and venous interventions, including at the deployment site of an intra-arterial stent, are achieved.
Finally, a human clinical trial will be necessary to determine if these same effects are noted in the human model of an AVF. Given the large numbers of ESRD patients who undergo AVF placement, as well as the high failure of these AVF secondary to IH, such a clinical trial is clearly feasible. A shorter duration of treatment with lower oxygen concentrations would result in higher levels of patient compliance and potentially higher success rates. Translation to human condition has widespread implications for a safe, costeffective treatment for patients undergoing all forms of arterial and venous interventions.
Clinical Relevance.
Our study demonstrates that intimal hyperplasia (IH) at the site of an arteriovenous fistula (AVF), as well as IH in the artery proximal to the AVF and IH in the vein distal to the AVF, can be inhibited by the short-term administration of 30% supplemental oxygen. Given the large numbers of end-stage renal disease patients who undergo AVF placement and the high failure of AVFs secondary to IH, this study has widespread implications for the treatment of patients undergoing all forms of arterial and venous interventions and should be translated to the human condition.
Acknowledgments
Supported by National Institutes of Health Grant 5R01-HL-076316.
We thank Paul A. Silvagni, DVM, PhD (W. L. Gore & Associates, Flagstaff, Ariz) for helping us with histologic slide preparation, Leslie Dickinson (Minneapolis VA Health Care System) for animal care and operative assistance, Michael Kuskowski, PhD, for help with statistical analysis, and Connie Lindberg for editorial assistance.
Footnotes
Author conflict of interest: none.
Presented at the 2012 Vascular Annual Meeting of the Society for Vascular Surgery, National Harbor, Md, June 7–9, 2012.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
AUTHOR CONTRIBUTIONS
Conception and design: CL, DG, JW, SR, SS
Analysis and interpretation: CL, JW, SR, SS
Data collection: CL, JW, SR, SS
Writing the article: CL, DG, JW, SR, SS
Critical revision of the article: DG, SS
Final approval of the article: CL, DG, JW, SR, SS
Statistical analysis: Not applicable
Obtained funding: SS
Overall responsibility: SS
REFERENCES
- 1.Fistula First. [Accessed April 10, 2012];Fistula First National Vascular Access Improvements Initiative. Available at: http://www.fistulafirst.org/.
- 2.Rooijens PM, Tordoir JM, Stijnen T, Burgmans JPJ, Smet de AA, Yo TI. Radiocephalic wrist arteriovenous fistula for hemodialysis: meta-analysis indicates a high primary failure rate. Eur J Vasc Endovasc Surg. 2004;28:583–589. doi: 10.1016/j.ejvs.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 3.Palder SB, Kirkman RL, Whitemore MD, Hakim RM, Lazarus JM, Tilney NL. Vascular access for hemodialysis. Ann Surg. 1985;202:235–239. doi: 10.1097/00000658-198508000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sullivan KL, Besarab A, Bonn J, Shapiro MJ, Gardiner GA, Jr, Moritz MJ. Hemodynamics of failing dialysis grafts. Radiology. 1993;186:867–872. doi: 10.1148/radiology.186.3.8430200. [DOI] [PubMed] [Google Scholar]
- 5.Rekhter M, Nicholls S, Ferguson M, Gordon D. Cell proliferation in human arteriovenous fistulas used for hemodialysis. Arterioscler Thromb. 1993;13:609–617. doi: 10.1161/01.atv.13.4.609. [DOI] [PubMed] [Google Scholar]
- 6.Lee ES, Bauer GE, Caldwell MP, Santilli SM. Association of artery wall hypoxia and cellular proliferation at a vascular anastomosis. J Surg Res. 2000;91:32–37. doi: 10.1006/jsre.2000.5891. [DOI] [PubMed] [Google Scholar]
- 7.Santilli SM, Wernsing SE, Lee ES. Transarterial wall oxygen gradients at a prosthetic vascular graft to artery anastomosis in the rabbit. J Vasc Surg. 2000;31:1229–1239. doi: 10.1067/mva.2000.104590. [DOI] [PubMed] [Google Scholar]
- 8.Santilli SM, Wernsing SE, Lee ES. The effect of supplemental oxygen on the transarterial wall oxygen gradients at a prosthetic vascular graft to artery anastomosis in the rabbit. Ann Vasc Surg. 2001;15:435–442. doi: 10.1007/s100160010119. [DOI] [PubMed] [Google Scholar]
- 9.Lee ES, Caldwell MP, Tretinyak AS, Santilli SM. Supplemental oxygen controls cellular proliferation and anastomotic intimal hyperplasia at a vascular graft-to-artery anastomosis in the rabbit. J Vasc Surg. 2001;33:608–613. doi: 10.1067/mva.2001.113495. [DOI] [PubMed] [Google Scholar]
- 10.Tretinyak AS, Lee ES, Uema KM, d’Audiffret AC, Caldwell MP, Santilli SM. Supplemental oxygen reduces intimal hyperplasia after intraarterial stenting in the rabbit. J Vasc Surg. 2002;35:982–987. doi: 10.1067/mva.2002.123090. [DOI] [PubMed] [Google Scholar]
- 11.Santilli SM, Tretinyak AS, Lee ES. Transarterial wall oxygen gradients at the deployment site of an intra-arterial stent in the rabbit. Am J Physiol Heart Circ Physiol. 2000;279:H1518–H1525. doi: 10.1152/ajpheart.2000.279.4.H1518. [DOI] [PubMed] [Google Scholar]
- 12.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 13.Clowes AW, Reidy MA. Prevention of stenosis after vascular reconstruction: Pharmacologic control of intimal hyperplasia-a review. J Vasc Surg. 1991;13:885–891. doi: 10.1067/mva.1991.27929. [DOI] [PubMed] [Google Scholar]
- 14.Davies MG, Dalen H, Austerheim AM, Gulbrandsen TF, Svendsen E, Hagen PO. Suppression of intimal hyperplasia in experimental vein grafts by oral 1-arginine supplementation and single ex vivo immersion in deferoxamine manganese. J Vasc Surg. 1996;23:410–420. doi: 10.1016/s0741-5214(96)80005-x. [DOI] [PubMed] [Google Scholar]
- 15.Chen C, Lumsden AB, Hanson SR. Local infusion of heparin reduces anastomotic neointimal hyperplasia in aortoiliac expanded polytetra-fluoroethylene bypass grafts in baboons. J Vasc Surg. 2000;31:354–363. doi: 10.1016/s0741-5214(00)90165-4. [DOI] [PubMed] [Google Scholar]
- 16.Kibbe MR, Nie S, Yoneyama T, Hatakeymna K, Lizonova A, Kovesdi I, et al. Optimization of ex vivo inducible nitric oxide synthase gene transfer to vein grafts. Surgery. 1999;126:323–329. [PubMed] [Google Scholar]
- 17.Barbarsch D, Lorenzar J. Arteriosclerosis and hypoxia. J Atheroscler Res. 1969;9:283–294. doi: 10.1016/s0368-1319(69)80023-2. [DOI] [PubMed] [Google Scholar]
- 18.Misra S, Fu A, Pugionni A, et al. Increased expression of hypoxia inducible factor-1α in a porcine model of chronic renal insufficiency with arteriovenous polytetrafluoroethylene grafts. J Vasc Interv Radiol. 2008;19:260–265. doi: 10.1016/j.jvir.2007.10.029. [DOI] [PubMed] [Google Scholar]