Summary
Adult stem cells reside in local microenvironments (niches) that produce signals regulating the outcome of stem cell divisions and stem cell-niche interactions. Limited space and signals in the niche often force stem cells to compete with one another. While previous studies have uncovered several examples of genetically distinct stem cells competing for niche access, recent studies demonstrate that genetically equivalent stem cells compete under normal conditions, resulting in dynamic stem cell behavior in the niche. New work in multiple vertebrate and invertebrate tissues shows that stem cell competition occurs continuously, and mutations disrupting the balance between competing stem cells can cause diseases and defects in the niche. This review discusses recent insights into stem cell competition in mammals and Drosophila.
Keywords: Competition, Stem cell, Niche, Cancer
Stem Cell Asymmetry in the Niche
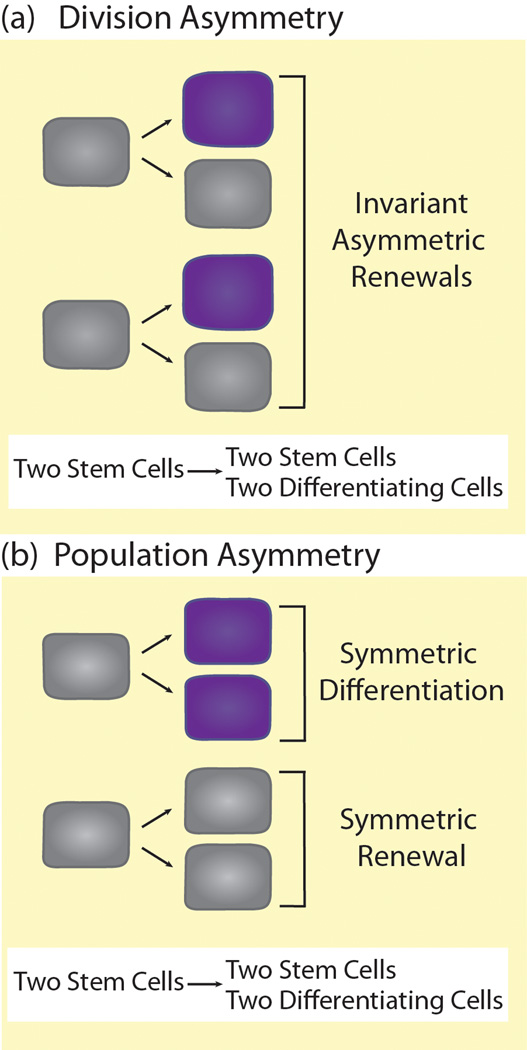
Adult stem cells (Glossary) or tissue-specific stem cells are studied extensively because of their vast regenerative potential. Organisms rely on many types of tissue-specific stem cells to repair and replenish organs and tissues. Populations of stem cells reside in specific microenvironments (niches) that produce distinct signals for stem cell maintenance [1]. Upon division, stem cells can produce two types of progeny: new stem cells and differentiating daughter cells. In theory, a stable number of stem cells can be maintained over time using two biological strategies: division asymmetry or population asymmetry [2]. With division asymmetry, individual stem cells undergo invariant asymmetric divisions to produce one stem cell daughter and one differentiating daughter (Figure 1a). With population asymmetry, the frequency of divisions resulting in two stem cells (symmetric renewals) is roughly equal to the frequency of divisions resulting in two differentiating cells (symmetric differentiations) within the stem cell population (Figure 1b). While either of these theoretical strategies keeps the overall stem cell number stable, recent live imaging and lineage tracing studies reveal the true dynamics of stem cell populations in intact niches [4]. Even in niches where individual stem cells primarily undergo asymmetric divisions, stem cells are lost from the niche and must be replaced through symmetric divisions or dedifferentiation of differentiating progeny [3–6]. Therefore, in practice, all stem cells in niches appear to regulate their maintenance at the population level to some extent.
Figure 1. Maintenance of stem cell populations by division asymmetry or population asymmetry.
(a) With division asymmetry, stem cells (gray) always divide to produce one stem cell daughter (gray) and one differentiating daughter cell (purple). The same number of stem cells is present both before and after division. (b) With population asymmetry, stem cells undergo either symmetric renewal to produce two stem daughters (gray) or symmetric differentiation to produce two differentiating daughters (purple) at equal frequencies. Overall, as the stem cell population divides, the number of stem cells remains constant.
Because niches typically produce restricted maintenance signals and sometimes consist of limited physical space, stem cells are constantly competing with their neighbors for niche occupancy [7,8]. In this review, we discuss two types of stem cell competition. Under normal conditions, stem cells are lost and replaced stochastically in a process termed neutral competition. Loss and replacement levels are balanced to maintain a stable stem cell population, and no stem cell has a long-term advantage over any other [2] (Figure 2a). Although it has not been empirically proven, neutral competition could be controlled by transient signaling fluctuations in the stem cell microenvironment [2]. While no examples have been described in niches, stochastic signaling fluctuations do affect random fate decisions in many tissues including the Drosophila eye [9]. The second type of stem cell competition discussed here is competition between two unequal stem cell populations; we refer to this as non-neutral competition. Inducing certain mutations in a fraction of stem cells in a niche can give those stem cells a competitive advantage or disadvantage over neighboring non-mutant stem cells [8]. Although mutant stem cells with a competitive advantage can take over a niche, they can also decrease the fitness of that niche, disrupting normal function and leading to diseases like cancer [10]. This makes stem cell competition an important area of study in both development and disease. Here, we discuss recent progress in understanding both neutral and non-neutral stem cell competition, highlighting new discoveries in basic and translational research.
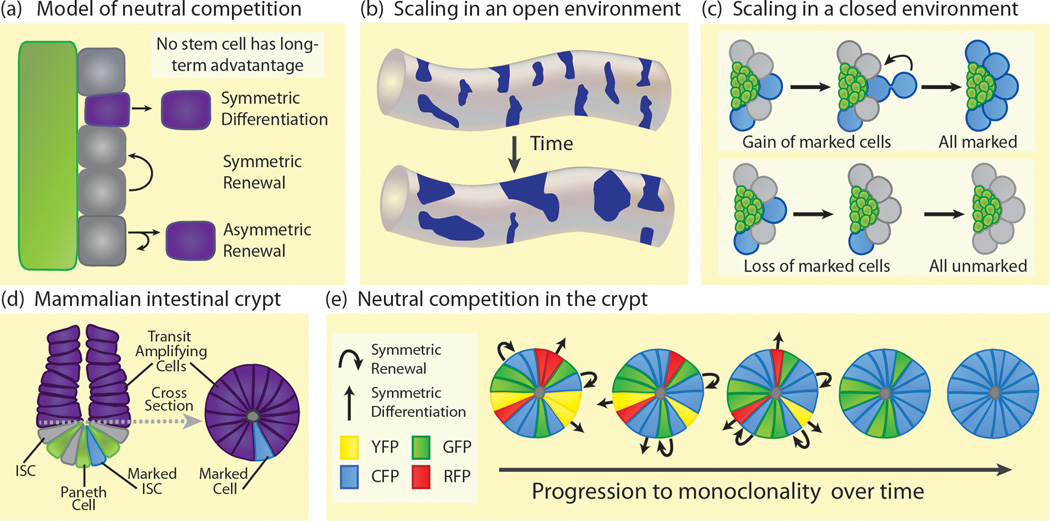
Figure 2. Neutral competition in stem cell niches.
(a) Neutral competition generally describes the process by which stem cells (gray) are stochastically lost from the niche (green) and then replaced. Under neutral competition, all stem cells are equally likely to be lost from the niche. Symmetric differentiation results in loss of a stem cell while symmetric renewal results in gain of a stem cell. Asymmetric renewals give one self-renewing stem cell daughter and one differentiating daughter (purple) and result in no net loss or gain in stem cells. The rates of symmetric differentiations and symmetric renewals must be balanced to ensure the maintenance of a stable stem cell population. (b) In an opened environment (eg. mammalian seminiferous tubule), marked stem cell clones (dark blue) decrease in number over time but increase in average size exhibiting a mathematically defined scaling behavior. Stem cell clones in an opened environment retain scaling behavior over an extended time period. (c) In a closed environment (green), marked stem cell clones (blue) can undergo symmetric renewals to increase the frequency of marked stem cells in the niche (upper panel) or symmetric differentiations to decrease the frequency of marked stem cells (lower panel). Clones exhibit scaling behavior in the short term until the niche becomes entirely populated with marked cells (upper) or unmarked cells (lower). (d) The small intestinal crypt contains Paneth cells (green) at the base, interspersed with Lgr5+ ISCs (gray). ISCs produce transit amplifying progeny (purple) that migrate up the crypt and then up the villi (not shown) to regenerate the intestinal epithelial layer. A cross section of the crypt just above the stem cell compartment (dashed line) shows a ring of transit amplifying cells (right image). These are progeny from multiple ISCs. If an ISC is genetically marked (blue), this mark will subsequently appear in the transit amplifying stem cell progeny. (blue, in cross section). Since crypts are three dimensional, the marked transit amplifying cells are easier to analyze in cross section and give an accurate read-out of the proportion of labeled ISCs. (e) Multicolor labeling of the ISC niche confers permanent fluorescent labels of four different colors, at random, to the ISC population. Initially, cells in the transit amplifying cross section (as shown in d) are heterogeneously labeled (top). As ISCs either symmetrically differentiate (straight arrow) and leave the niche or symmetrically renew (curved arrow) to produce replacement stem cells, labeling in the cross section becomes more homogeneous. Eventually, the niche will become monoclonal with regard to a label (bottom). This path to homogeneity is random with regard to which label becomes dominant. Marked clones exhibit scaling behavior until the crypt reaches monoclonality.
Neutral Stem Cell Competition
Even before the concept of the niche was defined, dividing stem cells were thought to face competing self-renewal and differentiation signals [11]. These signals were envisioned as balanced, maintaining a steady-state population of stem cells while allowing production of adequate differentiating progeny. Decades later, rigorous analyses have shown the dynamic interactions of stem cells in their intact niches. Live imaging in Drosophila testes shows that stem cell loss and replacement occur constantly in the intact niche [4], while mathematical analysis indicates that this loss and replacement happens stochastically in many niches [2]. Permanent marking of a stem cell and its progeny (or clone) allows for the tracking of a stem cell lineage. If a marked stem cell is lost through symmetric differentiation or gained through symmetric renewal, there is a commensurate shrinking or expansion of that clone. Mathematical analysis of clone behavior shows that all stem cells in a given niche are equally likely to undergo symmetric renewal or be lost (for an in-depth mathematical explanation, refer to Klein and colleagues [2]). Neutral competition has been observed in stem cell populations in the mouse epidermis, the testis and gut in both mouse and Drosophila, and even in a fraction of cancer stem cells within solid tumors [2,4,12–18]. We highlight several examples of neutral competition below, discussing possible points of regulation.
Neutral Competition in the Drosophila Testis
The Drosophila testis stem cell niche was the first niche where stem cell loss and replacement were carefully quantified in a live tissue. In the Drosophila testis, germline stem cells (GSCs) adhere to a cluster of non-mitotic somatic cells called the hub, which serves as a physical anchor and signaling niche [19]. GSCs undergo stereotypically oriented asymmetric divisions, with GSCs remaining at the hub and differentiating daughter cells displaced from the hub [20]. Differentiating daughter cells undergo several mitotic amplifying divisions to produce syncytial clusters of spermatogonia before differentiating into spermatocytes and, eventually, sperm. Live imaging of cultured Drosophila testes shows that in young, healthy niches, GSCs divide with an asymmetric outcome about 80% of the time. GSCs also symmetrically renew and symmetrically differentiate, with each of these two outcomes occurring about 10% of the time [4]. Thus, the testis niche is in a constant state of flux, with GSC loss and replacement occurring at approximately equal rates. Although GSC divisions are nearly always oriented perpendicular to the hub, occasionally connected GSC-daughter pairs reorient prior to cytokinesis so that both cells contact the hub and remain in the niche as stem cells. While no GSCs in the niche appear to have any long-term advantage, perhaps short-term fluctuations in niche signaling unequally affect the stem cell population, determining which cells are lost and which produce replacement stem cells. For example, protein-starved males exhibit decreased insulin signaling and decreased GSC number [21,22]. However, GSC symmetric renewals also increase following protein starvation, indicating that as GSCs are lost from the niche, neighboring GSCs produce replacement stem cells [4]. Levels of BMP signaling ligands, which are important for GSC self-renewal, also decrease upon protein starvation [23], and JAK-STAT signaling levels in testis decline with age, correlating with a slight decrease in GSC number [24,25]. Since insulin, BMP and JAK-STAT signaling have all been implicated in stem cell maintenance, fluctuations in these signals following meals, aging or even mating could affect neutral competition in the testis [24,26–28].
Neutral Competition in the Mammalian Testis
Similar to the Drosophila testis, stem cells in the mammalian testis behave according to the principles of neutral competition. The mammalian testis is comprised of long, convoluted seminiferous tubules associated with interstitial tissue and blood vessels [29]. Spermatogonia, including a population of spermatogonial stem cells (SSCs), are located along the basal lamina of the seminiferous tubules and are enriched in areas where the tubules associate with vasculature [30]. Spermatogonia differentiate into meiotic spermatocytes and migrate toward the lumen of the tubule as they mature into spermatozoa. Mathematical analysis of labeled SSC clones demonstrates stochastic loss and replacement of stem cells in the mammalian testis [14]. Over time, the number of clones decreases while the average clone size increases [31] (Figure 2c). Plotting clone size distribution, these data fit a Gaussian distribution predicated by a neutral competition model [2,14]. Since SSCs are located along the length of the seminiferous tubules with no fixed geographic boundaries, we refer to this tissue as an opened stem cell environment. Labeled stem cells from one clone can be lost and replaced by neighboring unlabeled stem cells leading to the shrinking or disappearance of a marked clone; alternatively, unlabeled stem cells can be lost and replaced by neighboring labeled stem cells leading to expansion of a marked clone (Figure 2c). In tissues undergoing neutral competition, this dynamic property of clonal expansion and shrinking occurs predictably in the stem cell population. When the average clone size distribution is plotted, the data acquire a scaling property that follows a defined mathematical function consistent with a neutral competition model. When stem cells reside in an opened environment like the mammalian testis, scaling will continue over the long term [2]. However, when stem cells are restricted by some geographic boundary to a specific location, called a closed stem cell environment, only short-term scaling of clonally marked stem cell populations can occur. For example, in the Drosophila testis, stem cells are physically restricted by the position of the hub, and scaling behavior of stem cell clones is only observed until the entire GSC population becomes either marked or unmarked (Figure 2c).
Neutral Competition in the Drosophila Intestine
In addition to the mammalian testis, neutral competition has been modeled in the Drosophila and mammalian intestines. Drosophila intestinal stem cells (ISCs) are organized into discrete nests scattered at regular intervals along the posterior midgut. Each nest contains one ISC and one or two non-mitotic, undifferentiated daughters called enteroblasts (EBs). [32]. ISCs express the Notch ligand, Delta, which activates Notch signaling in EBs, inducing them to differentiate into either secretory enteroendocrine cells or absorptive enterocytes, the latter comprising the bulk of the midgut [33]. Permanent labeling of ISCs and subsequent mathematical analysis demonstrates that ISCs are also stochastically lost and replaced, consistent with the principles of neutral competition [15]. Since ISCs are located in a geometrically opened tube structure where ISCs from one nest can enter another, permanently marked ISC clones exhibit long-term scaling behavior, as seen in SSC clones in the mammalian testis [14,15].
What controls the balance of stem cell loss and replacement in the midgut? Manipulating Notch signaling levels leads to an altered ratio of ISCs to EBs [15]. Lower Notch levels increase the ISC to EB ratio, indicating increased symmetric renewals, while higher Notch levels decrease the ISC to EB ratio, indicating decreased symmetric renewals. These experiments hint that temporary fluctuations in Notch signaling in the midgut may generate the stochastic stem cell behavior described as neutral competition. Nutritional status also affects the division outcome of ISCs. When ISC division outcome is monitored following feeding in a defined region of the midgut called the distal hairpin, symmetric renewals increase in proportion to asymmetric renewals. The opposite trend is observed following periods of fasting [34]. This indicates that, as in the Drosophila testis, the nutritional status of the midgut affects stem cell replacement rate, a key factor in neutral competition. Especially in the midgut, which can expand or shrink significantly depending on nutrient availability, increased symmetric renewals would increase stem cell number and provide a mechanism for increasing organ size [34]. It will be interesting to determine if ISCs in all regions of the midgut behave similarly to ISCs in the distal hairpin region following nutrient stimulation. ISC proliferation is also modulated by JAK-STAT signaling, especially following cellular damage [35]. Since tissue regeneration following damage likely requires increased symmetric renewals, similar to what is observed following fasting and feeding, perhaps JAK-STAT signaling plays a role in increasing the frequency of ISC symmetric renewals under some regenerative conditions.
Neutral Competition in the Mammalian Intestine
While the mammalian testis and the Drosophila midgut contain geometrically opened environments, the stem cell environment of the mammalian small intestine is closed. Stem cells in the small intestine reside in invaginations called crypts which are located between small protrusions called villi [36]. The stem cells in this tissue have defined geographic boundaries in that stem cells located in one crypt cannot enter another [37]. At the base of each crypt, slow cycling ISCs give rise to mitotic amplifying daughter cells which migrate in an epithelial sheet out of the crypt, terminally differentiating into short-lived intestinal epithelial cells (Figure 2d). The stem cells are believed to be a population of cells at the base of each crypt expressing the marker Lgr5 and intermingled with terminally differentiated Paneth cells that serve as the stem cell niche [38].
Like ISCs in the Drosophila gut, mammalian ISCs follow the principles of neutral competition, undergoing constant turnover while maintaining total stem cell number. This was elegantly shown using permanent genetic marking to label ISCs randomly with one of four different colored markers [16,17]. Over time, instead of maintaining markers of all colors, each crypt became populated by stem cells of a single color (Figure 2e). Since intestinal crypts are geometrically closed, the loss of a marked stem cell decreases the proportion of that color marker in the niche whereas symmetric renewal of a marked stem cell increases the proportion of that color. As the proportion of a color increases in a crypt, there is an increased likelihood that a lost stem cell will be replaced by a cell of that dominant color, eventually leading to monoclonality of the crypt [2, 17]. Because this is a stochastic process, each crypt independently progresses toward a final color as it approaches monoclonality. As with the Drosophila testis, ISC clones undergo scaling in the short term but as all stem cells in the closed niche acquire a uniform color, scaling ceases.
Since stem cell gain and loss remain equal during neutral competition, regulation of stem cell population size remains a major question in stem cell biology. Competition of stem cells for limited self-renewal signals and/or niche space likely serves to restrict the size of the stem cell population. In the small intestine, Paneth cells likely serve as niche cells, although this is somewhat controversial as, in vivo, Paneth cells are not strictly required for ISC maintenance [39,40]. Other studies show that Paneth cells limit ISC number [38]. Mutations decreasing Paneth cell number lead to a commensurate decrease in ISCs. If Paneth cells recover to normal levels, ISCs also recover, indicating that stem cells are competing for limited space or signals. Stem cell number will never increase beyond a certain number; this number is related to the number of Paneth cells present, indicating that neutral competition is likely the effect of many potential stem cells competing for limited niche resources. This is likely a common feature of stem cell niches as genetically increasing or decreasing niche size in the Drosophila ovary or the mammalian bone marrow also leads to a corresponding increase or decrease in stem cell number [41,42].
Non-Neutral Stem Cell Competition
General cell competition, first described in the Drosophila wing disc, occurs when two genetically distinct populations of cells in a tissue have different growth rates [43]. The faster growing cells (winner cells) overtake and compromise the viability of slower growing cells (loser cells) [8]. Non-neutral stem cell competition is similar to general cell competition in that one population of stem cells in a tissue gains a long-term advantage over another population and overtakes a niche. Disadvantaged “loser” stem cells are forced out of the niche and differentiate [44]. Non-neutral competition can occur between genetically distinct stem cells of the same type or two different types of stem cells in the same niche [44]. Non-neutral competition between different types of stem cells disrupts the normal ratio of cell types in the niche. Early studies in the Drosophila ovary demonstrated competition in both germline and epithelial stem cell populations [45–48]. For comprehensive review of stem cell competition in the Drosophila ovary, refer to Sahai-Hernandez and colleagues [49]. Understanding non-neutral stem cell competition has important ramifications when considering procedures like bone marrow transplants, where donor hematopoietic stem cells must compete for the host bone marrow niche in order to engraft efficiently [50]. Currently, some of the best-understood examples of non-neutral competition come from niches in Drosophila.
Non-Neutral Competition in the Drosophila Testis
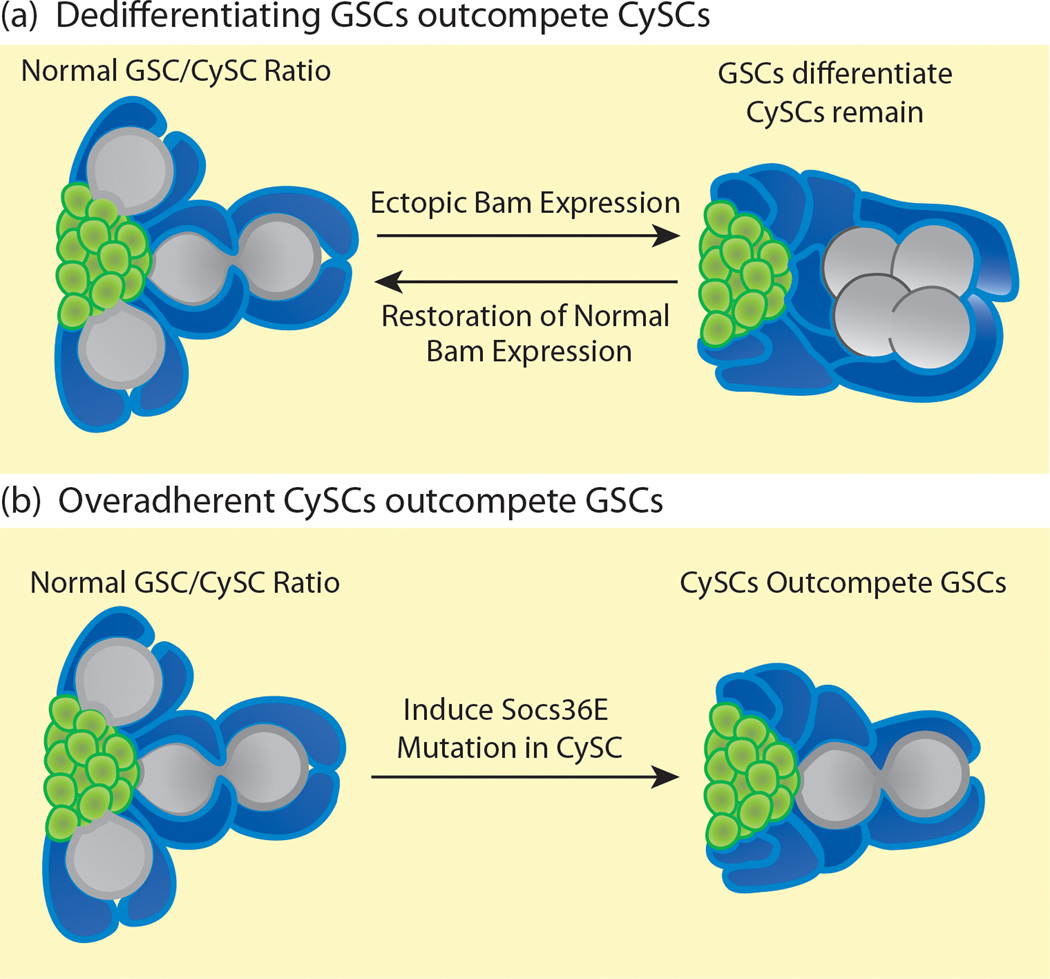
The Drosophila testis contains two populations of stem cells that can compete with each other in a non-neutral manner. In addition to GSCs, a second population of stem cells, somatic cyst stem cells (CySCs), is maintained around the hub. CySCs send maintenance signals to the GSCs and divide asymmetrically to produce non-mitotic cyst cell daughters that support the differentiating germline [19]. Niches are highly plastic, and if normal signals are experimentally disrupted leading to an imbalance of stem cell types in the niche, the imbalance is actively corrected through non-neutral competition following restoration of normal signaling. For example, in the Drosophila testis, differentiation of GSCs is inhibited by repression of the differentiation factor Bag-of-marbles (Bam) [51]. Short-term global activation of Bam causes GSCs to differentiate into spermatogonia, leaving only CySCs around the hub. However, when normal Bam expression is restored, remaining spermatogonial cysts dedifferentiate, physically separating and migrating back to the niche to form new GSCs that outcompete the CySCs to reattach to the hub. This continues until the wild type GSC to CySC ratio is restored [52]. Spermatogonia must be present for dedifferentiation to occur, and JAK-STAT signaling is required for efficient dedifferentiation, suggesting that spermatogonia with the highest levels of JAK-STAT signaling are most competent to dedifferentiate and outcompete CySCs. Since CySCs normally have lower levels of JAK-STAT signaling than GSCs, perhaps this signaling difference helps GSCs to compete with CySCs for occupancy in the niche.
Mutations in the JAK-Stat inhibitor Socs36E also disrupt the balance of stem cell types in the testis niche. When Socs36E is depleted, the ratio of stem cell types in the testis niche shifts towards the CySCs [53,54]. Although the number of CySCs remains constant, the number of GSCs drops and CySCs make increased contact with the hub (Figure 3b). As expected, the level of JAK-STAT signaling in the CySCs increases in Socs36E mutants but, surprisingly, β-integrin levels also increase, indicating that adhesion mediates the ability of CySCs to outcompete GSCs [53]. Decreasing integrin levels partially rescues this competition phenotype, indicating a requirement for integrin in Socs36E-mediated stem cell competition. The purpose of Socs36E-mediated competition is probably to modulate CySC adhesion levels and assure that a proper GSC to CySC ratio is maintained in the testis. Since these stem cell populations function together, retaining the correct GSC to CySC ratio may ensure that the tissue maintains peak efficiency.
Figure 3. Non-neutral competition in the Drosophila testis niche.
(a) In a wild type testis stem cell niche (left image), a one-to-two ratio of GSCs (gray) to CySCs (blue) is maintained around the hub (green). Following global ectopic Bam expression (right image), GSCs differentiate into spermatogonia (gray clusters), leaving CySCs (blue) completely surrounding the hub. Following restoration of normal Bam expression, spermatogonial cysts dedifferentiate into GSCs which outcompete CySCs, restoring the wild type GSC to CySC ratio around the hub (left image). (b) The wild type ratio of GSCs to CySCs (left image) is also disrupted when a Socs36E mutation is induced in the CySCs. The CySCs become more adherent and outcompete neighboring GSCs for space around the hub, decreasing the GSC to CySC ratio (right image).
Non-Neutral Competition in the Mammalian Testis
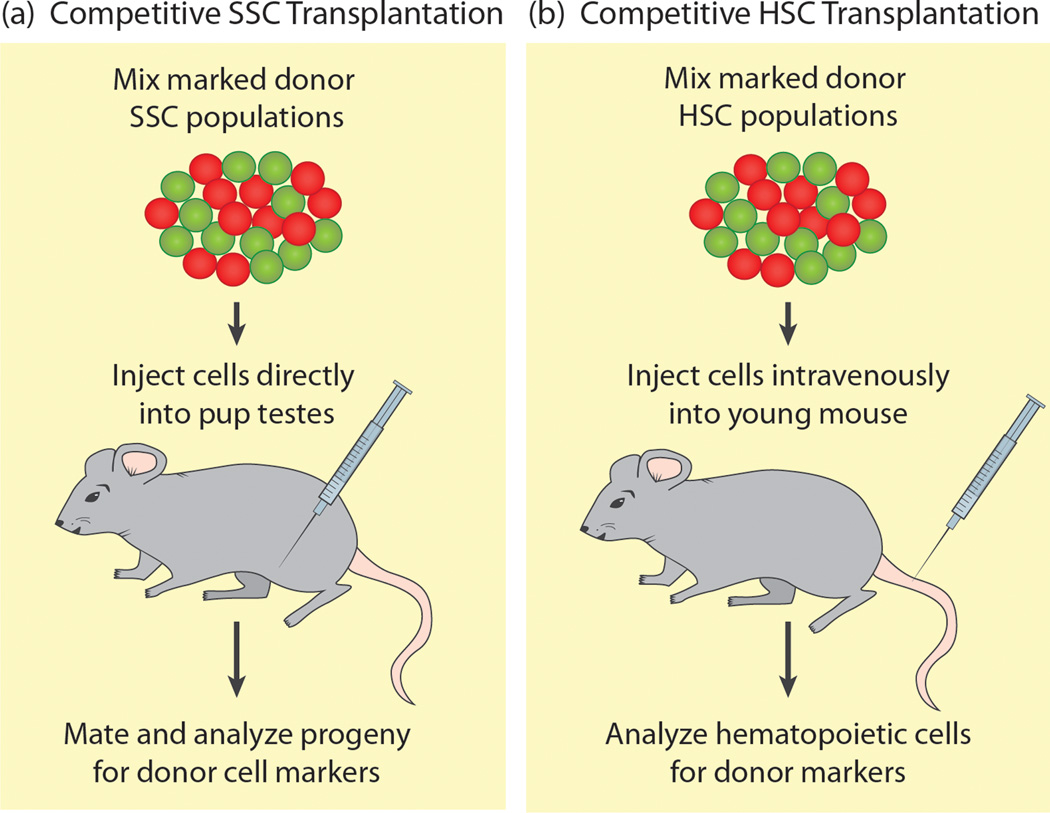
Genetic analysis of stem cell competition is more challenging in mammals than in flies, but transplantation assays have been used for decades to study how distinct donor stem cell populations compete for niche occupancy [55]. While the competitive transplantation assay was first used in the hematopoietic stem cell niche, its use in the mammalian testis is more recent. In early testis studies, SSCs isolated from neonate, pup and adult donor mice were transplanted into recipient testes, and SSCs of increasing donor age could more effectively outcompete endogenous SSCs to colonize recipient testes [56]. More recently, a competitive transplantation assay was used to test whether certain mutations affect SSC competition [57]. Two differentially marked donor populations were mixed and transplanted into an irradiated host to compete for niche occupancy (Figure 4a). This assay identified p27, a cyclin-dependent kinase inhibitor, as important for SSC competition in the testis niche. While p27 mutant SSCs can successfully engraft and produce differentiated progeny under non-competitive conditions, p27 mutant SSCs cannot compete with wild type SSCs for access to the testis niche, probably due to decreased self-renewal ability [57]. This SSC competitive repopulation assay will be a useful tool to identify additional factors required for non-neutral SSC competition in the mammalian testis.
Figure 4. Competitive Repopulation Assays.
(a) Marked spermatogonial stem cells of two different genotypes (red and green) are isolated from donor animals, mixed together and injected into the testes of host mouse pups lacking SSCs. Pups are raised, mated, and the progeny are analyzed for markers specific to the donor SSC populations. The proportion of each class of progeny indicates how efficiently each type of donor SSC can compete for niche access in the testis. (b) Marked hematopoietic stem cells of two different genotypes (red and green) are isolated from donor mice, mixed and injected intravenously into irradiated host mice. Contents of the bone marrow niche and the peripheral blood are analyzed to determine how efficiently each type of donor HSC can competitively engraft and produce functional blood cells.
Non-Neutral competition in the Mammalian Hematopoietic Niche
Competitive transplantation assays are used to identify factors crucial for hematopoietic stem cell competition [55] (Figure 4b). The hematopoietic stem cell (HSC) niche resides in the bone marrow where osteoblasts signal to slow-cycling HSCs, which can be induced to proliferate and differentiate into numerous types of blood cells (reviewed by [58]). For transplanted HSCs to be competitive, they must be able to effectively migrate or “home” into the niche, adhere and self-renew. Defects in any of these properties lead to HSCs that cannot efficiently compete for niche occupancy.
Recently the axon guidance receptor Robo4 was shown to play a role HSC adhesion to the niche. In competitive repopulation assays, Robo4 mutant HSCs cannot effectively adhere and compete poorly for niche occupancy, even though the mutant HSCs can differentiate normally [59]. Interestingly, in Robo4 mutant cells, there is a compensatory increase in expression of CXCR4, a chemokine receptor required for HSC homing and self-renewal [59,60]. HSCs lacking both CXCR4 and Robo4 are completely lost, indicating that for HSCs to non-neutrally compete for their niche, they require not only homing signals to find the niche but also adhesive signals to stay in the niche [59]. Competitive repopulation assays have also identified adhesive integrin molecules as important factors in non-neutral HSC competition. While several integrin complexes are required for competitive HSC adhesion to the niche, other integrin complexes can act as signaling molecules in HSCs [58]. For example, αvβ3 integrin functions as a signaling complex in the niche to control HSC proliferation, and is required for effective HSC competition. αvβ3 levels are controlled primarily by the JAK-STAT signaling pathway. The cytokine ligand thrombopoietin, the thrombopoietin receptor c-Mpl, and STAT5, which are all required for HSCs to effectively compete for niche access [61–63], all regulate the expression of αvβ3 integrin. Given that JAK-STAT signaling also affects integrin expression levels and stem cell competition in the Drosophila testis [53], perhaps this pathway mediates integrin expression in other stem cell niches and affects stem cell competition more generally.
DNA damage and cellular stress also lead to non-neutral competition among HSCs. In competitive repopulation assays, irradiated HSCs, which have high p53 expression, are outcompeted by non-irradiated HSCs. p53 can activate the DNA repair pathway, cell cycle arrest, and, under dire circumstances, apoptosis [64]. HSCs with low p53 expression lack cell cycle checkpoints and display increased expression of signaling molecules such as cytokines and adhesion molecules that are associated with increased competitiveness [59,64]. p53-induced competition depends on the relative levels of p53 expression; low p53-expressing HSCs have a distinct competitive advantage, while both high p53-expressing HSCs and HSCs with no p53 expression are at a comparative disadvantage. The presence of competitive stem cells in a niche, through the mechanisms discussed here, can be advantageous, leading to a robust population of well-maintained stem cells. Unfortunately, if stem cells become so competitive for the niche that they cease to function normally, these advantages can quickly lead to dangerous consequences. For example, since p53 is a known tumor suppressor, HSCs with low functioning p53 may, initially, be very competitive; however, complete loss of p53 function can cause HSCs to overproliferate, eventually leading to the progression of cancer [65] (Box 1).
Box 1: Cancer Cell Competition in Niches.
Cell competition is a recurring theme in cancer biology, particularly in blood cancers like multiple myeloma (MM) and acute myeloid leukemia (AML). When these diseases develop, cancer cells compete with normal HSCs, forcing them out of the niche (reviewed by [66]). Often, cancer cells use the same pathways as HSCs to interact with the niche. Both MM and AML cells use CXCR4 to home into the niche and outcompete endogenous stem cells [67]. In addition to homing, CXCR4 also induces quiescence, shielding a population of cancer cells from chemotherapies which preferentially eradicate proliferating cells. Treatments with CXCR4 inhibitors can mobilize both HSCs and cancerous cells from the niche, inducing them to proliferate and making them susceptible to conventional cancer treatments [68–70]. MM and AML cells also upregulate adhesion molecules including several integrin complexes to increase their ability to adhere to the niche and outcompete less adherent HSCs [71]. Increased signaling by both chemokines and cytokines leads to this increase in integrin signaling. In this way, cancer cells use multiple mechanisms to hijack and colonize the HSC niche.
Metastasis is an additional unfortunate consequence of stem cell competition. Bone marrow is an excellent target for circulating cancer cells. Prostate cancer cells that metastasize to the bone marrow often use CXCR4 signaling to home into the bone marrow niche, outcompeting endogenous HSCs. CXCR4 inhibition can prevent prostate cancer from invading the bone marrow niche in mice [72]. Metastatic prostate cancer cells also upregulate integrins and can compete with HSCs for binding receptors including Annexin-2, which HSCs use to interact with their niche [73]. Once these cancer cells invade the niche and proliferate, they produce signals that induce differentiation of endogenous HSCs, allowing cancer cells to further outcompete HSCs from their niche [74, 75]. Since cancer cells divide rapidly, they can efficiently evolve and hijack the mechanisms used by endogenous stem cells to interact with the niche. These niche interaction mechanisms are always in place, and cancer cells take advantage of this as they establish a foothold in the niche.
Concluding Remarks
Stem cell niches are dynamic signaling environments, and stem cells compete in multiple ways for niche occupancy. Neutral competition studies show that stem cells are constantly vying for occupancy in the niche, and are randomly lost and replaced under normal conditions. Complementary non-neutral competition studies uncover signals that allow distinct populations of stem cells to outcompete their neighbors. In reality, both neutral and non-neutral stem cell competition likely occur in normally functioning niches. Since, in a normal niche, stem cells can acquire defects, these defective stem cells are non-neutrally competed out of the niche by neighboring stem cells [76]. In this way, stem cell competition helps to maintain overall stem cell fitness in an aging niche. Neutral competition is particularly important in niches like the Drosophila testis where two types of stem cells coexist in a specific, balanced ratio. Mathematical analysis of a niche with two stem cell populations has not been attempted but could provide great insight into how this balance is controlled. Perhaps the signals identified in non-neutral competition studies overlap with the signaling fluctuations that affect the dynamics of neutral competition. In general, signals that modulate stem cell adhesion, self-renewal and proliferation seem to control most competitive stem cell interactions. While one can imagine that small increases in these signals could increase the fitness of a stem cell population, too much can lead to an imbalance of cell types, tumorigenesis and metastasis. Finding a balance ensures the maintenance of a healthy stem cell population.
Highlights.
Genetically equal stem cells compete for niche occupancy in multiple tissues
Stochastic competition for niche access is important for stem cell fitness
Mutated stem cells can gain a competitive advantage or disadvantage
Stem cells with a competitive advantage can cause diseases or niche defects
Acknowledgements
We thank L. Greenspan, Q. Ma, Z. Stine, K. Ramachandran and M. de Cuevas for helpful comments. We apologize to colleagues whose work was not cited due to space limitations. Research in the laboratory is supported by NIH grants R01HD040307 and R01HD052937 to ELM.
Glossary: Defining Stem Cell Competition
- Adult stem cell
Cell that can produce both self-renewing stem cells that remain in the niche and differentiating progeny that repair and regenerate tissues
- Niche
Local microenvironment consisting of signals and anatomical structures that support a specific population of stem cells
- Division asymmetry
Strategy to maintain a fixed number of stem cells in which every stem cell in a population undergoes invariant asymmetric divisions
- Population asymmetry
Strategy to maintain a fixed number of stem cells in which a group of stem cells undergoes symmetric renewals and symmetric differentiations at roughly equal rates
- Asymmetric division
Cell division resulting in two daughters cells with different fates; with regard to stem cells, division resulting in one stem cell daughter and one differentiating daughter
- Symmetric renewal
Stem cell division resulting in two stem cell daughters
- Symmetric differentiation
Stem cell division resulting in two differentiating daughters
- Stem cell competition
The process of multiple stem cells vying for limited resources including signals and space in their niche
- Neutral stem cell competition
Stochastic loss and replacement of stem cells in a tissue with no stem cell having a distinct, long-term advantage over another
- Non-neutral stem cell competition
Competition between two unequal populations of stem cells in a niche, with one population gaining an advantage over the other
- Opened stem cell environment
Tissue in which stem cells and their progeny are not restricted by fixed geographic boundaries and the scaling behavior of clones in the tissue occurs over an extended period
- Scaling
Mathematical function used here to describe the behavior of a population of marked stem cell clones under neutral competition in which the average clone number in a tissue decreases over time while the average clone size increases
- Closed stem cell environment
Tissue in which stem cells are restricted by fixed geographic boundaries and the scaling behavior of clones in the tissue only occurs for a limited period of time until an endpoint is reached
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Morrison SJ, Spradling AC. Stem Cells and Niches: Mechanisms That Promote Stem Cell Maintenance throughout Life. Cell. 2008;132:598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klein AM, Simons BD. Universal patterns of stem cell fate in cycling adult tissues. Development. 2011;138:3103–3111. doi: 10.1242/dev.060103. [DOI] [PubMed] [Google Scholar]
- 3.Xie T, Spradling AC. A niche maintaining germ line stem cells in the Drosophila ovary. Science. 2000;290:328–330. doi: 10.1126/science.290.5490.328. [DOI] [PubMed] [Google Scholar]
- 4.Sheng XR, Matunis E. Live imaging of the Drosophila spermatogonial stem cell niche reveals novel mechanisms regulating germline stem cell output. Development. 2011;138:3367–3376. doi: 10.1242/dev.065797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng J, et al. Centrosome misorientation reduces stem cell division during ageing. Nature. 2008;456:599–604. doi: 10.1038/nature07386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakagawa T, et al. Functional Hierarchy and Reversibility Within the Murine Spermatogenic Stem Cell Compartment. Science. 2010;328:62–67. doi: 10.1126/science.1182868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore KA, Lemischka IR. Stem Cells and Their Niches. Science. 2006;311:1880–1885. doi: 10.1126/science.1110542. [DOI] [PubMed] [Google Scholar]
- 8.Johnston LA. Competitive Interactions Between Cells: Death, Growth, and Geography. Science. 2009;324:1679–1682. doi: 10.1126/science.1163862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Losick R, Desplan C. Stochasticity and Cell Fate. Science. 2008;320:65–68. doi: 10.1126/science.1147888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vivarelli S, et al. Cell wars: regulation of cell survival and proliferation by cell competition. Essays in Biochemistry. 2012;53:69–82. doi: 10.1042/bse0530069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lajtha LG. Kinetic models of hemopoietic stem cell populations. In Vitro. 1969;4:14–21. [Google Scholar]
- 12.Clayton E, et al. A single type of progenitor cell maintains normal epidermis. Nature. 2007;446:185–189. doi: 10.1038/nature05574. [DOI] [PubMed] [Google Scholar]
- 13.Doupe DP, et al. The Ordered Architecture of Murine Ear Epidermis Is Maintained by Progenitor Cells with Random Fate. Developmental Cell. 2010;18:317–323. doi: 10.1016/j.devcel.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 14.Klein AM, et al. Mouse Germ Line Stem Cells Undergo Rapid and Stochastic Turnover. Cell Stem Cell. 2010;7:214–224. doi: 10.1016/j.stem.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 15.Navascues Jd, et al. Drosophila midgut homeostasis involves neutral competition between symmetrically dividing intestinal stem cells. The EMBO Journal. 2012;31:2473–2485. doi: 10.1038/emboj.2012.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopez-Garcia C, et al. Intestinal Stem Cell Replacement Follows a Pattern of Neutral Drift. Science. 2010;330:822–825. doi: 10.1126/science.1196236. [DOI] [PubMed] [Google Scholar]
- 17.Snippert HJ, et al. Intestinal Crypt Homeostasis Results from Neutral Competition between Symmetrically Dividing Lgr5 Stem Cells. Cell. 2010;143:134–144. doi: 10.1016/j.cell.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 18.Driessens G, et al. Defining the mode of tumour growth by clonal analysis. Nature. 2012;488:527–530. doi: 10.1038/nature11344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cuevas Md, Matunis EL. The stem cell niche: lessons from the Drosophila testis. Development. 2011;138:2861–2869. doi: 10.1242/dev.056242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamashita YM, et al. Orientation of asymmetric stem cell division by the APC tumor suppressor and centrosome. Science. 2003;301:1547–1550. doi: 10.1126/science.1087795. [DOI] [PubMed] [Google Scholar]
- 21.McLeod CJ, et al. Stem Cell Dynamics in Response to Nutrient Availability. Current Biology. 2010;20:2100–2105. doi: 10.1016/j.cub.2010.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ueishi S, et al. Male Germline Stem Cell Division and Spermatocyte Growth Require Insulin Signaling in Drosophila. Cell Structure and Function. 2009;34:61–69. doi: 10.1247/csf.08042. [DOI] [PubMed] [Google Scholar]
- 23.Ballard SL, et al. Gbb/BMP signaling is required to maintain energy homeostasis in Drosophila. Developmental Biology. 2010;337:375–385. doi: 10.1016/j.ydbio.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boyle M, et al. Decline in Self-Renewal Factors Contributes to Aging of the Stem Cell Niche in the Drosophila Testis. Cell Stem Cell. 2007;1:470–478. doi: 10.1016/j.stem.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Toledano H, et al. The let-7-Imp axis regulates ageing of the Drosophila testis stem-cell niche. Nature. 2012;485:605–610. doi: 10.1038/nature11061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tulina N, Matunis E. Control of Stem Cell Self-Renewal in Drosophila Spermatogenesis by JAK-STAT Signaling. Science. 2001;294:2546–2549. doi: 10.1126/science.1066700. [DOI] [PubMed] [Google Scholar]
- 27.Kiger AA, et al. Stem Cell Self-Renewal Specified by JAK-STAT Activation in Response to a Support Cell Cue. Science. 2001;294:2542–2545. doi: 10.1126/science.1066707. [DOI] [PubMed] [Google Scholar]
- 28.Kawase E, et al. Gbb/Bmp signaling is essential for maintaining germline stem cells and for repressing bam transcription in the Drosophila testis. Development. 2004;131:1365–1375. doi: 10.1242/dev.01025. [DOI] [PubMed] [Google Scholar]
- 29.Yoshida S. Stem cells in mammalian spermatogenesis. Development, Growth & Differentiation. 2011;52:311–317. doi: 10.1111/j.1440-169X.2010.01174.x. [DOI] [PubMed] [Google Scholar]
- 30.Yoshida S, et al. A vasculature-associated niche for undifferentiated spermatogonia in the mouse testis. Science. 2007;317:1722–1726. doi: 10.1126/science.1144885. [DOI] [PubMed] [Google Scholar]
- 31.Nakagawa T, et al. Functional Identification of the Actual and Potential Stem Cell Compartments in Mouse Spermatogenesis. Developmental Cell. 2007;12:195–206. doi: 10.1016/j.devcel.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 32.Ohlstein B, Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006;439:470–474. doi: 10.1038/nature04333. [DOI] [PubMed] [Google Scholar]
- 33.Ohlstein B, Spradling A. Multipotent Drosophila Intestinal Stem Cells Specify Daughter Cell Fates by Differential Notch Signaling. Science. 2007;315:988–992. doi: 10.1126/science.1136606. [DOI] [PubMed] [Google Scholar]
- 34.O'Brien LE, et al. Altered Modes of Stem Cell Division Drive Adaptive Intestinal Growth. Cell. 2011;147:603–614. doi: 10.1016/j.cell.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang H, et al. Cytokine/Jak/Stat Signaling Mediates Regeneration and Homeostasis in the Drosophila Midgut. Cell. 2009;137:1343–1355. doi: 10.1016/j.cell.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simons BD, Clevers H. Stem cell self-renewal in intestinal crypt. Experimental Cell Research. 2011;317:2719–2724. doi: 10.1016/j.yexcr.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 37.Potten CS, et al. The stem cells of small intestinal crypts: where are they? Cell Proliferation. 2009;42:731–750. doi: 10.1111/j.1365-2184.2009.00642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sato T, et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415–418. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim TH, et al. Intact function of Lgr5 receptor-expressing intestinal stem cells in the absence of Paneth cells. Proc Natl Acad Sci U S A. 2012;109:3932–3937. doi: 10.1073/pnas.1113890109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Durand A, et al. Functional intestinal stem cells after Paneth cell ablation induced by the loss of transcription factor Math1 (Atoh1) Proc Natl Acad Sci U S A. 2012;109:8965–8970. doi: 10.1073/pnas.1201652109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang J, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425 doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 42.Song X, et al. Notch signaling controls germline stem cell niche formation in the Drosophila ovary. Development. 2007;134:1071–1080. doi: 10.1242/dev.003392. [DOI] [PubMed] [Google Scholar]
- 43.Morata Gs, Ripoll P. Minutes: Mutants of Drosophila autonomously affecting cell division rate. Developmental Biology. 1975;42:211–221. doi: 10.1016/0012-1606(75)90330-9. [DOI] [PubMed] [Google Scholar]
- 44.Zhao R, Xi R. Stem Cell Competition for Niche Occupancy: Emerging Themes and Mechanisms. Stem Cell Reviews and Reports. 2010;6:345–350. doi: 10.1007/s12015-010-9128-3. [DOI] [PubMed] [Google Scholar]
- 45.Jin Z, et al. Differentiation-Defective Stem Cells Outcompete Normal Stem Cells for Niche Occupancy in the Drosophila Ovary. Cell Stem Cell. 2008;2:39–49. doi: 10.1016/j.stem.2007.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nystul T, Spradling A. An Epithelial Niche in the Drosophila Ovary Undergoes Long-Range Stem Cell Replacement. Cell Stem Cell. 2007;1:277–285. doi: 10.1016/j.stem.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 47.Wang ZA, Kalderon D. Cyclin E-dependent protein kinase activity regulates niche retention of Drosophila ovarian follicle stem cells. Proceedings of the National Academy of Sciences. 2009;106:21701–21706. doi: 10.1073/pnas.0909272106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang ZA, et al. Drosophila follicle stem cells are regulated by proliferation and niche adhesion as well as mitochondria and ROS. Nature Communications. 2012;3 doi: 10.1038/ncomms1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sahai-Hernandez P, et al. Drosophila models of epithelial stem cells and their niches. Wiley Interdisciplinary Reviews: Developmental Biology. 2012;1:447–457. doi: 10.1002/wdev.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Copley MR, et al. Hematopoietic Stem Cell Heterogeneity Takes Center Stage. Cell Stem Cell. 2012;10:690–697. doi: 10.1016/j.stem.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 51.Schulz C, et al. A Misexpression Screen Reveals Effects of bag-of-marbles and TGFBeta Class Signaling on the Drosophila Male Germ-Line Stem Cell Lineage. Genetics. 2004;167:707–723. doi: 10.1534/genetics.103.023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sheng XR, et al. Dedifferentiating Spermatogonia Outcompete Somatic Stem Cells for Niche Occupancy in the Drosophila Testis. Cell Stem Cell. 2009;5:191–203. doi: 10.1016/j.stem.2009.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Issigonis M, et al. JAK-STAT Signal Inhibition Regulates Competition in the Drosophila Testis Stem Cell Niche. Science. 2009;326:153–156. doi: 10.1126/science.1176817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Singh SR, et al. Competitiveness for the niche and mutual dependence of regulated by the JAK/STAT signaling. Journal of Cellular Physiology. 2009;223:500–510. doi: 10.1002/jcp.22073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harrison DE. Competitive repopulation: a new assay for long-term stem cell functional capacity. Blood. 1980;55:77–81. [PubMed] [Google Scholar]
- 56.Shinohara T, et al. Germ line stem cell competition in postnatal mouse testes. Biology of reproduction. 2002;66:1491–1497. doi: 10.1095/biolreprod66.5.1491. [DOI] [PubMed] [Google Scholar]
- 57.Kanatsu-Shinohara M, et al. Transmission distortion by loss of p21 or p27 cyclin-dependent kinase inhibitors following competitive spermatogonial transplantation. Proc Natl Acad Sci U S A. 2010;107:6210–6215. doi: 10.1073/pnas.0914448107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Celso CL, Scadden DT. The haematopoietic stem cell niche at a glance. Journal of Cell Science. 2012;124:3529–3535. doi: 10.1242/jcs.074112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith-Berdan S, et al. Robo4 Cooperates with Cxcr4 to Specify Hematopoietic Stem Cell Localization to Bone Marrow Niches. Cell Stem Cell. 2011;8:72–83. doi: 10.1016/j.stem.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peled A, et al. Dependence of Human Stem Cell Engraftment and Repopulation of NOD/SCID Mice on CXCR4. Science. 1999;283:845–848. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- 61.Umemoto T, et al. Integrin-alpha v beta 3 regulates thrombopoietin-mediated maintenance of hematopoietic stem cells. Blood. 2012;119:83–94. doi: 10.1182/blood-2011-02-335430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yoshihara H, et al. Thrombopoietin/MPL Signaling Regulates Hematopoietic Stem Cell Quiescence and Interaction with the Osteoblastic Niche. Cell Stem Cell. 2007;1:685–697. doi: 10.1016/j.stem.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 63.Wang Z, et al. Conditional deletion of STAT5 in adult mouse hematopoietic stem cells causes loss of quiescence and permits efficient nonablative stem cell replacement. Blood. 2009;113:4856–4865. doi: 10.1182/blood-2008-09-181107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bondar T, Medzhitov R. p53-Mediated Hematopoietic Stem and Progenitor Cell Competition. Cell Stem Cell. 2010;6:309–322. doi: 10.1016/j.stem.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Asai T, et al. The p53 tumor suppressor protein regulates hematopoietic stem cell fate. Journal of Cellular Physiology. 2011;226:2215–2221. doi: 10.1002/jcp.22561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Noll JE, et al. Tug of war in the haematopoietic stem cell niche: do myeloma plasma cells compete for the HSC niche? Blood Cancer Journal. 2012;2 doi: 10.1038/bcj.2012.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Burger JA, Burkle A. The CXCR4 chemokine receptor in acute and chronic leukaemia: a marrow homing receptor and potential therapeutic target. British Journal of Haematology. 2007;137:288–296. doi: 10.1111/j.1365-2141.2007.06590.x. [DOI] [PubMed] [Google Scholar]
- 68.Azab AK, et al. CXCR4 inhibitor AMD3100 disrupts the interaction of multiple myeloma cells with the bone marrow microenvironment and enhances their sensitivity to therapy. Blood. 2009;113:4341–4351. doi: 10.1182/blood-2008-10-186668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nervi B, et al. Chemosensitization of acute myeloid leukemia (AML) following mobilization by the CXCR4 antagonist AMD3100. Blood. 2009;113:6206–6214. doi: 10.1182/blood-2008-06-162123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shiozawa Y, et al. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. Journal of Clinical Investigation. 2011;121:1298–1312. doi: 10.1172/JCI43414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shain KH, et al. Beta1 Integrin Adhesion Enhances IL-6-Mediated STAT3 Signaling in Myeloma Cells: Implications for Microenvironment Influence on Tumor Survival and Proliferation. Cancer Research. 2009;69:1009–1015. doi: 10.1158/0008-5472.CAN-08-2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Domanska UM, et al. CXCR4 Inhibition with AMD3100 Sensitizes Prostate Cancer to Docetaxel Chemotherapy. Neoplasia (New York, N.Y.) 2012;14:709–718. doi: 10.1593/neo.12324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sun YX, et al. Expression and activation of alpha v beta 3 integrins by SDF-1/CXC12 increases the aggressiveness of prostate cancer cells. The Prostate. 2007;67:61–73. doi: 10.1002/pros.20500. [DOI] [PubMed] [Google Scholar]
- 74.Bruns I, et al. Multiple myeloma-related deregulation of bone marrowderived CD34+ hematopoietic stem and progenitor cells. Blood. 2012;120:2620–2630. doi: 10.1182/blood-2011-04-347484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Colmone A, et al. Leukemic Cells Create Bone Marrow Niches That Disrupt the Behavior of Normal Hematopoietic Progenitor Cells. Science. 2008;322:1861–1865. doi: 10.1126/science.1164390. [DOI] [PubMed] [Google Scholar]
- 76.Sperka T, et al. DNA damage checkpoints in stem cells, ageing and cancer. Nature Reviews Molecular Cell Biology. 2012;13:579–590. doi: 10.1038/nrm3420. [DOI] [PubMed] [Google Scholar]