Abstract
Rationale, Aims and Objectives
Decades of work on health disparities have culminated in identification of three contributors to variability in diagnosis and management of disease: 1) patient attributes, 2) physician characteristics, and 3) organizational. Understanding the relative influence of different contributors to variability in diagnosis and management of diabetes is important to improving quality and reducing disparities. This study was designed to examine the influence of patient, provider, and organizational factors on the diagnosis and management of a major chronic disease: diabetes.
Method
A factorial experiment using video vignettes was conducted among n=192 primary care physicians. Physicians were interviewed after viewing vignettes of (1) a “patient” with symptoms strongly suggestive of diabetes and (2) an already diagnosed diabetes “patient” with emerging peripheral neuropathy.
Results
60.9 percent of physicians identified diabetes as the correct diagnosis, with significant variations depending on the patients’ race/ethnicity. Many physicians offered competing diagnoses with high levels of certainty. For the “patient” with emerging peripheral neuropathy, 42.2 percent of physicians would do all essential components of a foot examination, while 21.9 percent would do none.
Conclusions
That half of all diabetes in the US remains undiagnosed is unsurprising given only 60.9 percent of physicians would diagnose it when the condition is strongly suggested, and nearly one quarter suspecting diabetes would not order tests necessary to confirm it. The diagnosis of diabetes is significantly influenced by a patient’s race/ethnicity and clinical management (specifically for foot neuropathy), is influenced by patient SES, physician gender, and access to clinical guidelines.
Keywords: Diabetes, clinical decision making, racial/ethnic disparities, factorial experiment, diagnosis/management, primary care
INTRODUCTION
The worldwide prevalence of diabetes mellitus was estimated at 285 million people in 2010, with an expected increase to 439 million by 2030.1 The World Health Organization has declared it “the health hazard of the 21st century”,2 and according to the National Insitute of Diabetes and Digestive and Kidney Diseases (NIDDK), “In both human and economic terms, it [diabetes] is one of our nation’s most costly diseases”.3
Rapid aging of the population and increasing obesity4 are producing an epidemic of diabetes in the United States (US).5 Some 21 million Americans (approximately 7 percent of the population) have diabetes, with a further 41 million considered pre-diabetic and likely to develop the disease.6 Associated financial costs are producing a worrisome fiscal crisis for federal and state governments: diabetes is estimated to cost $174 billion each year and contributes to 231,404 deaths annually.7 It is the major cause of adult blindness, kidney disease and lower limb amputations. Diabetes and its complications consume 27.6 percent of the US Medicare budget.8 While this paper focuses on diabetes in the US specifically, the negative impact of diabetes on health and economic resources are recognized as an urgent international problem.2, 9 Diabetes appears subject to the “rule of halves”—only about half of all diabetics are actually diagnosed, of these only about half are treated, and of these only about half are managed appropriately.10, 11 Since early symptoms of diabetes are often mild it is termed “a silent killer” and patients may have the disease for 7 to 10 years before initial diagnosis.12, 13 As an often insidious condition diabetes can be challenging for clinicians. Randomized trials have demonstrated the effectiveness and cost efficiency of both lifestyle and pharmacologic interventions for primary and secondary prevention.14-16 Strict glycemic control and management of blood pressure and lipids are known to reduce the incidence of microvascular (retinopathy, neuropathy and nephropathy) and macrovascular (myocardial infarction and stroke) end stage complications and death.17-19
Most diabetes is initially diagnosed and managed by primary care physicians. What occurs at the level of primary care is of linchpin importance: a) it shapes the trajectory of a patient’s disease and eventual outcomes; and b) it is the upstream source of up to 90 percent of every health care dollar spent.20, 21 There is reported variability in: a) the prevalence of diabetes (by race/ethnicity, age, gender, geographic location, and socio-economic status (SES)); and b) in the healthcare associated with its diagnosis and management.22 Clinical guidelines have been developed to improve treatment of diabetes and hopefully reduce variations in its diagnosis and the quality of care, but there are persistent gaps between these guidelines and clinical management of the condition.23
Decades of work on health disparities in the US culminated in the Institute of Medicine’s (IOM) report Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care24 which recommended future studies of the decision making behavior of physicians as an important contributor to variations in both reported disease rates and healthcare for conditions such as diabetes. Decision making research has evolved from an initial interest in: a) Patient attributes and behavior (race/ethnicity, age, gender, and help-seeking); to b) Provider Characteristics (age/clinical experience, gender, specialty, personality). Changes in the organization and financing of US health care have generated interest in: c) Organizational influences (geographic location, type of ownership, practice culture, use of clinical guidelines, and the effects of different reimbursement schemes like pay-for-performance).25, 26 Interest in these different influences and their potential for interventions is summarized in, “Eliminating Healthcare Disparities in America: Beyond the IOM Report”.27 Understanding the relative influence of these different contributors to variability in the diagnosis and management of diabetes is important to improving quality and reducing disparities.26, 28 Understanding precisely where variations are initially generated (for instance, at the patient, provider or organizational/system level) has important implications for the targeting of future educational, clinical and policy-level interventions.
This study was designed to produce estimates of the relative influence of patient, provider, and organizational factors on physician decision making with respect to the diagnosis and management of type 2 diabetes mellitus (T2DM).The goals of this paper are to:
describe variability in the initial diagnosis of diabetes and the contribution of patient, physician and organizational factors to any variability; and
explain how the variability in the proposed management of the already diagnosed case of diabetes is influenced by patient, provider, and organizational factors.
METHODS
Primary care physicians were randomized to view two video vignettes, each representing two different clinical scenarios. The first “patient” with undiagnosed diabetes presented clear indicators of type 2 diabetes mellitus (T2DM). The set of symptoms for T2DM, some of which may overlap with Type 1 diabetes, were distinguishable based on several factors. First, the case was developed based on three of the four key symptoms of marked hyperglycemia noted by the American Diabetes Association (polyuria –particularly at night, polydipsia, (excessive thirst), weight loss with polyphagia) and included moderate obesity and general fatigue.29 Second, the patient was of an age range atypical for Type 1 diabetes, but very typical for T2DM. Third, recognizing diabetes may be ‘silent epidemic’, the task was not to gain an absolute diabetes diagnosis, but to trigger the physician subject to consider T2DM as a diagnostic possibility, and do the required test to ascertain whether it was a case. The physicians’ task was assessing initial diagnostic possibilities. The second “patient” with already diagnosed diabetes reported symptoms suggestive of an emerging foot neuropathy, including “burning in the feet which sometimes goes up the ankle” with a burning sensation that “comes and goes.” The patient was also moderately overweight. The physician’s task was clinical management. Scripts for each case were developed with input from 6 internationally respected clinicians with expertise in either primary care currently caring for patients with diabetes, or experts in diabetes management and clinical guidelines30-32 on appropriate tests, to ensure sufficient signs and symptoms were included to strongly suggest diabetes and to confirm the realism of the presentation.
Clinical validity was established through a process involving both primary care physicians and clinical diabetes experts. First, we conducted an extensive background literature search, review of international guidelines, and involvement of clinical experts in diabetes and primary care. Triangulating these three components, we established the ‘text book’ signs and symptoms which must exist to strongly suggest a case of undiagnosed diabetes (Vignette 1) and emerging foot neuropathy (Vignette 2). Second, we conducted audio recorded role play sessions with practicing primary care physicians to capture the nuances of how patients typically present with symptoms for these two scenarios. Because patients seldom present as textbook cases and to increase the clinical authenticity of the scenario, we included other minor clinical distractions such as the patient’s concern over heart disease, a single high blood pressure reading, and excessive caffeine use (in consultation with the clinical experts) which did not interfere with the important study-related symptoms. Finally, a master script was developed and filmed with diabetes and primary care clinicians present at the filming to ensure that the actor presentations were both clinically accurate and typical of how a patient would present during a clinic visit. A balanced factorial experiment permits estimation of un-confounded main effects and interactions of any of the patient and provider influences.33, 34 Selected organizational influences were examined through covariate adjustment.
Physicians were randomized to view one version of the clinical vignettes (varying only the “patient’s” age, race/ethnicity, gender, and SES while presentation of symptoms were exactly the same). Eligible physicians were stratified according to gender and level of clinical experience and purposively recruited until each cell was complete. For the estimation of main effects, a total sample of 192 physicians gives 80 percent power to detect an absolute difference in means of 20.4 percent for two groups and an absolute difference of 24 percent for three groups. For two-way interactions it provides 80 percent power to detect an effect size of .204 (for factors with two levels each) - .225 (for one factor with two levels and one factor with three levels). The effect size is a ratio of the variability of the hypothesized means divided by the variability of the observations. For two means with a difference Δ, standard deviation of subjects s, the effect size is Δ/2s.
Immediately after viewing the first (undiagnosed) case, physicians were asked to identify “the most likely condition” and to list additional candidate diagnoses they were considering. Responses for diagnostic possibilities were open-ended, responses were recorded during the interview based on a predetermined code list. The prespecified codes were determined based on diabetes guidelines, extensive consultation with clinical colleagues, and pilot testing of the instrument. . For each differential diagnostic possibility participants were asked to assign a number indicating their level of certainty on a scale of 0-100, with 0 indicating no certainty and 100 indicating complete certainty. They were also asked a series of structured interview questions regarding how they would confirm their diagnosis and treat the patient in terms of asking for additional information, performing physical examinations, ordering tests, prescribing medications, giving lifestyle advice, and referring to other physicians.. A list of codes were developed for each question based on clinical expertise and guidelines. Respondents were also asked open ended questions, and responses were coded by the interview according to the predetermined list of possible codes. Alternatives not in the code list were entered as text and later coded. This allowed for open ended responses by the clinician (i.e., not predetermined answer categories) yet the interviewer could record and code the responses in real time (i.e., not retrospectively). Following the second (diagnosed) case, physicians were again asked a series of interview questions regarding their management of the “patient” with already diagnosed diabetes and an emerging complication. The same coding processes were instituted for the second scenario.
Experimental Stimuli (Scenarios)
Professional actors and actresses were recruited and trained (under physician supervision) to portray a “patient” presenting to a primary care doctor with the two clinical scenarios. Twenty-four versions of the scenario were filmed, systematically varying the “patient’s” age (35 vs. 65 years in order to preserve orthogonality and ensure adequate separation between younger and older patients), race/ethnicity (white vs. black vs. Hispanic), gender, and SES (a janitor vs. a lawyer). Filmed scenarios permit nonverbal indicators to be embedded without drawing undue attention to them; in this study, the “patients” were moderately overweight and from different race/ethnic and gender groups.35 Each video simulated an initial interview with a primary care physician and was of 5-7 minutes in duration, reflecting the average length of “face time” with a primary care physician.36
Experimental Subjects (Physicians)
The purposive sample was selected from the American Medical Association (AMA) masterfile of doctors practicing in New Jersey, New York and Pennsylvania to equally fill four design cells (gender by level of experience). Eligible physicians were identified in the AMA file as: (a) be internists, family practitioners, or general practitioners (listed and confirmed as their primary specialty); (b) have ≤12 years clinical experience (graduated between1993-99) or ≥22 years experience (graduated between 1969-83); (c) be trained at an accredited medical school in the US; and (d) be currently providing clinical care at least half time. Telephone calls were conducted to identify eligible subjects and an in-person interview was scheduled. A modest stipend ($100) was provided to acknowledge participation. The study protocols were submitted for formal review of relevant ethics procedures and approved by the New England Research Institutes’ (NERI) Institutional Review Board. Written informed consent was obtained for all study subjects.
Dependent Variables
Undiagnosed Vignette
Diagnostic accuracy and diagnostic certainty are focal dependent variables in these analyses. The presence of a diabetes diagnosis with the full differential diagnosis and sufficient diagnostic certainty are necessary to trigger clinical actions in terms of diagnostic testing and subsequent treatment.
Diagnosed Vignette
The Clinical guidelines developed by the American Diabetes Association (ADA) were used as an operational “gold standard” against which the quality of decision making could be assessed.30 These guidelines were selected as the appropriate guidelines at the time the data were being collected and were consistent with internationally developed guidelines (particularly the United Kingdom and Germany where similar interviews were conducted, and approaches to health care differ from those in the US). The ADA guidelines, an experienced consulting diabetologist, and a primary care clinical consultant to the project concurred that three examinations should be performed when a patient with diabetes complained of foot ailments.30 The three key examinations are: a) visual inspection for ulcers – the most common foot injuries leading to lower extremity amputation; b) vibration and/or monofilament exam – tests the degree of sensation in the feet; and c) palpation of foot pulses – absent foot pulses indicate peripheral vascular disease.
Following both vignettes we included additional measures of clinical actions that are less frequently addressed in other studies but provide more detailed information about clinical actions, including information-seeking, physical exams, advice-giving, time to follow-up and referrals.
Statistical Analysis
Analysis of variance was used to test the main effects and two-way interactions of the design variables (patient gender, race/ethnicity, age, and SES; physician gender and level of experience) on a range of diagnostic and treatment decisions. The balanced factorial design allows the unconfounded estimation of all main effects and two-way interactions using analysis of variance. Because the experiment was replicated, we used a pure error term with 192 degrees of freedom to test all effects using analysis of variance.33, 34 Due to the challenges of multiple testing, we emphasize consistency across results and focus on identifying general patterns.
RESULTS
1. The Case of Undiagnosed Diabetes
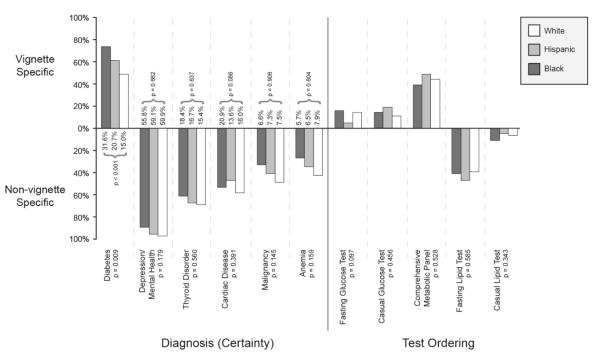
Major results are summarized in Figure 2. 60.9 percent of physicians provided a diagnosis of diabetes: 73.4 percent diagnosed diabetes when the “patient” was black, 60.9 percent when Hispanic, and 48.4 percent when white (p=.009). This statistically significant race/ethnic variation in the diagnosis of diabetes is corroborated by the race/ethnic disparities in physician diagnosed diabetes reported elsewhere (National Health and Nutrition Examination Survey). Physician subjects were asked to assign a certainty level to their initial diagnosis (from 0=complete uncertainty to 100=complete certainty). Physicians were significantly more certain (p<0.001) of their diagnosis when the patient was Black (31.6) compared with when they were Hispanic (20.7) or white (15.0). The diagnosis of diabetes also varied by gender (65.6% for male patients and 56.3% for females, Table 1). The mean level of certainty also varied significantly (p=0.033) by gender (26.0 for male and 18.8 for females respectively). The proportion of patients diagnosed with diabetes and the certainty of this diagnosis did not vary significantly by patient age or SES.
Figure 2. Variation in the diagnosis of diabetes and other conditions and in test ordering depending on the race/ethnicity of patients presenting with identical symptoms.
This Figure depicts: (1) the proportion of physicians correctly diagnosing diabetes by a “patient’s” race/ethnicity (bars above the horizontal line), and those incorrectly diagnosing other conditions (below the horizontal line); (2) the average level of certainty attached to each diagnosis; and (3) the test-ordering physicians would propose (appropriate tests above the line and inappropriate tests below the line).
Table 1.
Diagnoses, certainty of a diagnosis, and glucose test ordering for patients presenting with symptoms suggestive of diabetes*
| Patient Gender | Patient Age | Patient Race/Ethnicity | Patient SES | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Female N=96 |
Male N=96 |
p value | 35 N=96 |
65 N=96 |
p value | Black N=64 |
Hispanic N=64 |
White N=64 |
p value | Lower | Upper | p value | |
| Diagnoses / Certainty | |||||||||||||
| Diabetes (%) | 56.3 | 65.6 | 0.153 | 62.5 | 59.4 | 0.632 | 73.4 | 60.9 | 48.4 | 0.009 | 64.6 | 57.3 | 0.265 |
| Certainty (0-100) | 18.8 | 26.0 | 0.033 | 23.8 | 21.0 | 0.405 | 31.6 | 20.7 | 15.0 | <0.001 | 24.4 | 20.4 | 0.241 |
| Mental Health (%) | 94.8 | 92.7 | 0.565 | 94.8 | 92.7 | 0.565 | 89.1 | 95.3 | 96.9 | 0.179 | 92.7 | 94.8 | 0.565 |
| Certainty (0-100) | 61.3 | 55.3 | 0.131 | 59.1 | 57.5 | 0.680 | 55.8 | 59.1 | 59.9 | 0.662 | 56.7 | 59.9 | 0.409 |
| Thyroid (%) | 74.0 | 57.3 | 0.009 | 64.6 | 66.7 | 0.740 | 60.9 | 67.2 | 68.8 | 0.560 | 67.7 | 63.5 | 0.507 |
| Certainty (0-100) | 20.5 | 13.1 | 0.005 | 15.1 | 18.5 | 0.196 | 18.4 | 16.7 | 15.4 | 0.637 | 17.1 | 16.5 | 0.809 |
| Cardiac Disease (%) | 44.8 | 60.4 | 0.018 | 42.7 | 62.5 | 0.003 | 53.1 | 46.9 | 57.8 | 0.391 | 47.9 | 57.3 | 0.153 |
| Certainty (0-100) | 12.9 | 20.8 | 0.004 | 13.8 | 20.0 | 0.025 | 20.9 | 13.6 | 16.0 | 0.086 | 15.7 | 18.0 | 0.387 |
| Cancer (%) | 37.5 | 43.8 | 0.333 | 24.0 | 57.3 | <0.001 | 32.8 | 40.6 | 48.4 | 0.145 | 44.8 | 36.5 | 0.198 |
| Certainty (0-100) | 6.8 | 7.6 | 0.660 | 3.1 | 11.2 | <0.001 | 6.6 | 7.3 | 7.5 | 0.906 | 8.5 | 5.8 | 0.120 |
| Anemia (%) | 40.6 | 28.1 | 0.061 | 33.3 | 35.4 | 0.753 | 26.6 | 34.4 | 42.2 | 0.159 | 35.4 | 33.3 | 0.753 |
| Certainty (0-100) | 8.8 | 4.6 | 0.025 | 4.8 | 8.6 | 0.041 | 5.7 | 6.5 | 7.9 | 0.604 | 6.9 | 6.4 | 0.788 |
| Glucose Tests | |||||||||||||
| Fasting glucose (%) | 10.4 | 12.5 | 0.638 | 15.6 | 7.3 | 0.062 | 15.6 | 4.7 | 14.1 | 0.097 | 9.4 | 13.5 | 0.348 |
| Casual glucose (%) | 10.4 | 18.8 | 0.106 | 13.5 | 15.6 | 0.684 | 14.1 | 18.8 | 10.9 | 0.456 | 12.5 | 16.7 | 0.416 |
| CMP †(%) | 43.8 | 43.8 | 1.000 | 39.6 | 47.9 | 0.220 | 39.1 | 48.4 | 43.8 | 0.528 | 47.9 | 39.6 | 0.220 |
| Any glucose (%) | 64.6 | 75.0 | 0.099 | 68.8 | 70.8 | 0.740 | 68.8 | 71.9 | 68.8 | 0.895 | 69.8 | 69.8 | 1.000 |
Numbers are the percent giving the diagnosis, the average certainty on a scale of 0 to 100, and a p value for a test of the null hypotheses of no difference by the patient or physician characteristic
Complete metabolic panel
Competing diagnoses were offered even though sufficient signs and symptoms suggestive of these conditions were not embedded in the vignette: 93.8 percent would assign a diagnosis of mental health/depression, with an average certainty of 58.3; 65.6 percent would diagnose thyroid disease (more common for women (74.0 percent) compared to men (57.3 percent), p=.009), with an average certainty of 16.8 (20.5 for women and 13.1 for men, p=.005); 52.6 percent would give a cardiac diagnosis (more often for men than women (60.4 percent) versus (44.8 percent), p=.018) with an average certainty of 16.9 (20.8 for men and 12.9 for women, p=.004). A cancer diagnosis was more common with older patients (p<.0001) (See Table 1). The level of certainty (58.3) attached to a mental health diagnosis (sufficient symptoms of which were not embedded in the case presentation) exceeds the certainty level associated with the diagnosis of diabetes (the signs and symptoms of which were embedded).
Regarding the influence of physician characteristics (Table 2), there were no significant differences in diabetes diagnoses or mental health/depression diagnosis by physician gender or experience.
Table 2.
Diagnoses, certainty of a diagnosis, and glucose test ordering by physician characteristics for patients presenting with symptoms suggestive of diabetes*
| Physician Gender | Physician Level of Experience | |||||
|---|---|---|---|---|---|---|
| Female N=96 |
Male N=96 |
p value | Less N=96 |
More N=96 |
p value | |
|
Diagnoses (%)
Certainty (0-100) |
||||||
|
| ||||||
| Diabetes | 65.6 | 56.3 | 0.153 | 55.2 | 66.7 | 0.081 |
| Certainty | 24.9 | 19.9 | 0.144 | 22.0 | 22.8 | 0.798 |
| Mental Health | 94.8 | 92.7 | 0.565 | 96.9 | 90.6 | 0.087 |
| Certainty | 58.9 | 57.6 | 0.740 | 58.6 | 58.0 | 0.892 |
| Thyroid | 60.4 | 70.8 | 0.099 | 62.5 | 68.8 | 0.320 |
| Certainty | 17.6 | 16.0 | 0.525 | 17.3 | 16.3 | 0.717 |
| Cardiac Disease | 56.3 | 49.0 | 0.265 | 47.9 | 57.3 | 0.153 |
| Certainty | 17.9 | 15.8 | 0.457 | 15.6 | 18.1 | 0.366 |
| Cancer | 40.6 | 40.6 | 1.000 | 42.7 | 38.5 | 0.518 |
| Certainty | 8.2 | 6.1 | 0.236 | 7.6 | 6.7 | 0.617 |
| Anemia | 28.1 | 40.6 | 0.061 | 30.2 | 38.5 | 0.209 |
| Certainty | 6.1 | 7.3 | 0.533 | 6.0 | 7.3 | 0.474 |
|
| ||||||
|
Test Ordering
| ||||||
| Fasting glucose (%) | 14.6 | 8.3 | 0.161 | 13.5 | 9.4 | 0.348 |
| Casual glucose (%) | 13.5 | 15.6 | 0.684 | 9.4 | 19.8 | 0.044 |
| CMP † (%) | 41.7 | 45.8 | 0.539 | 47.9 | 39.6 | 0.220 |
| Any glucose (%) | 69.8 | 69.8 | 1.000 | 70.8 | 68.8 | 0.740 |
Numbers are the percent giving the diagnosis, the average certainty on a scale of 0 to 100, and a p value for a test of the null hypotheses of no difference by the patient or physician characteristic
Complete metabolic panel
Physicians requesting a confirmatory test for diabetes did so, not by specifically requesting a fasting glucose test, but rather by requesting a battery of tests (complete metabolic panel). Only 11.5 percent would specifically request a fasting glucose test, with an additional 14.6 percent ordering a casual glucose test, and 43.8 percent a complete metabolic panel, which includes a glucose test (for a total of 69.8 percent). Test ordering did not vary by the other patient attributes considered (age, race/ethnicity, and SES). Even when a physician assigned an initial diagnosis of diabetes, only 16.2 percent would request the test considered necessary for its confirmation (fasting glucose). When encountering the “patient” with symptoms strongly suggestive of diabetes, 49.5 percent of doctors would order some type of cholesterol tests. Black male “patients” were more likely to get a non-fasting lipid test (18.8 percent compared to Hispanics, whites or Black females, 5.0 percent, p=.032).
2. The Case of Already Diagnosed Diabetes (with emerging foot neuropathy)
Since the second “patient” presented with already diagnosed diabetes and suggested peripheral neuropathy we focus first on foot-related actions the physicians would be likely to initiate and then turn to other components recommended for a regular follow-up with a diabetic patient. As noted above, ADA guidelines identified three essential components of a foot examination for diabetes: a) a monofilament/vibration test for loss of sensation; b) a check of foot pulses for peripheral vascular disease; and c) a check for ulcers.30 Given the emphasis on an emerging foot neuropathy it is not surprising that 94.3 percent of the physicians would do some type of foot examination, but only 42.2 percent would do all three essential components of a foot examination (vibration/monofilament, foot pulses and a check for ulcers), and 21.9 percent would do none of them.
There were consistent differences in whether all three components of a thorough foot exam would be conducted depending on attributes of the patients. We wish to avoid overinflating the importance of isolated statistically significant influences on decisions and highlight the overall consistency of results. With respect to the influence of patient gender, men were slightly more likely than women to receive all essential components of a foot examination: monofilament/vibration (67.7 percent vs 58.3 percent, non-significant (ns)); foot pulses (72.9 percent vs 63.5 percent, ns); ulcer checking (53.1 percent vs 41.7 percent, ns). On average, men would get 1.9 of these essential examination components compared with 1.6 for women (ns). Men were more likely to be asked appropriate questions concerning their medical history (p<0.05 for adherence with diet, medications, exercise routine and cardiovascular disease). Men were also more likely to be advised to do foot self-exams (p= .015). Women were more likely to get a thyroid stimulating test (p=.002). Older patients were more likely than younger patients to receive essential components of a foot examination: monofilament/vibration (66.7 percent vs 59.4 percent, ns); foot pulses (74.0 percent vs 62.5 percent, ns); ulcer checks (51.0 percent vs 43.8 percent, ns). On average, older patients would receive 1.9 of these exams compared to 1.7 for the younger counterparts (ns). Socioeconomic status (SES) also influenced receipt of essential components of a foot exam. Upper SES patients were more likely to receive a monofilament/vibration test (64.6 percent vs 61.5 for lower SES, ns); foot pulses (75.0 percent vs 61.5 percent, p=.05); ulcer checks (55.2 percent vs 39.6 percent, p=.038). Overall, upper SES patients would likely receive 1.9 of these essential exams compared with 1.6 for lower SES patients (ns). Upper SES patients were slightly more likely to be asked questions about their medical history (p<.05 for history of eye disease) and were more frequently referred to an ophthalmologist (p=.024). Sixteen actions suggested in the guidelines were examined: upper SES patients were more likely to receive 15 of them. A patient’s race/ethnicity also appeared to have some isolated effects, with whites more likely to be asked about previous neuropathy than Blacks and Hispanics (p=.044). Hispanic patients were the least likely to receive advice concerning exercise (p=.036) (Table 3). The importance of these findings is that men, older patients and higher SES patients are consistently more likely to be asked questions or examined for foot neuropathy. Consequently, women, younger and lower SES patients with similar signs and symptoms may be diagnosed later for foot neuropathy, with greater consequences for long term outcomes.
Table 3.
Components of a comprehensive diabetic examination for diabetic patients presenting with symptoms suggestive of emerging foot neuropathy*
| Patient Gender | Patient Age | Patient Socioeconomic Status | Patient Race/Ethnicity | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Female N=96 |
Male N=96 |
p value | 35 N=96 |
65 N=96 |
p value | Janitor N=96 |
Lawyer N=96 |
p value | Black N=64 |
Hispanic N=64 |
White N=64 |
p value | |
| Medical History | |||||||||||||
| Adherence with diet | 36.5 | 54.2 | 0.015 | 41.7 | 49.0 | 0.310 | 41.7 | 49.0 | 0.310 | 46.9 | 40.6 | 48.4 | 0.641 |
| Adherence with meds | 41.7 | 58.3 | 0.020 | 51.0 | 49.0 | 0.769 | 47.9 | 52.1 | 0.557 | 51.6 | 48.4 | 50.0 | 0.937 |
| Exercise habits | 26.0 | 40.6 | 0.042 | 31.3 | 35.4 | 0.557 | 32.3 | 34.4 | 0.769 | 34.4 | 26.6 | 39.1 | 0.349 |
| Previous neuropathy | 14.6 | 16.7 | 0.696 | 15.6 | 15.6 | 1.000 | 11.5 | 19.8 | 0.120 | 9.4 | 12.5 | 25.0 | 0.044 |
| Eye disease | 30.2 | 28.1 | 0.740 | 34.4 | 24.0 | 0.099 | 22.9 | 35.4 | 0.048 | 25.0 | 28.1 | 34.4 | 0.462 |
| Examinations | |||||||||||||
| Blood pressure | 54.2 | 65.6 | 0.127 | 58.3 | 61.5 | 0.675 | 57.3 | 62.5 | 0.486 | 60.9 | 60.9 | 57.8 | 0.925 |
| Fundoscopic | 13.5 | 17.7 | 0.435 | 14.6 | 16.7 | 0.696 | 9.4 | 21.9 | 0.021 | 15.6 | 18.8 | 12.5 | 0.632 |
| Laboratory Tests | |||||||||||||
| HbA1c | 68.8 | 76.0 | 0.277 | 69.8 | 75.0 | 0.437 | 74.0 | 70.8 | 0.640 | 65.6 | 76.6 | 75.0 | 0.354 |
| Fasting lipid | 63.5 | 62.5 | 0.884 | 59.4 | 66.7 | 0.310 | 60.4 | 65.6 | 0.468 | 64.1 | 64.1 | 60.9 | 0.918 |
| Liver function | 41.7 | 42.7 | 0.873 | 41.7 | 42.7 | 0.873 | 34.4 | 50.0 | 0.018 | 43.8 | 34.4 | 48.4 | 0.204 |
| Microalbuminuria | 68.8 | 74.0 | 0.400 | 70.8 | 71.9 | 0.866 | 67.7 | 75.0 | 0.240 | 73.4 | 64.1 | 76.6 | 0.231 |
| TSH † | 34.4 | 14.6 | 0.002 | 21.9 | 27.1 | 0.413 | 24.0 | 25.0 | 0.870 | 18.8 | 31.3 | 23.4 | 0.271 |
| Referrals | |||||||||||||
| Ophthalmologist | 33.3 | 32.3 | 0.879 | 32.3 | 33.3 | 0.879 | 25.0 | 40.6 | 0.024 | 34.4 | 32.8 | 31.3 | 0.933 |
| Advice | |||||||||||||
| Exercise | 66.7 | 69.8 | 0.579 | 72.9 | 63.5 | 0.098 | 68.8 | 67.7 | 0.853 | 73.4 | 57.8 | 73.4 | 0.036 |
| Foot self exams | 20.8 | 36.5 | 0.015 | 31.3 | 26.0 | 0.413 | 25.0 | 32.3 | 0.253 | 26.6 | 31.3 | 28.1 | 0.828 |
| Diet | 71.9 | 74.0 | 0.732 | 76.0 | 69.8 | 0.306 | 69.8 | 76.0 | 0.306 | 76.6 | 75.0 | 67.2 | 0.405 |
Numbers are the percents and a p value for a test of the null hypotheses of no difference by the patient or physician characteristic.
Thyroid stimulating hormone
Characteristics of the primary care physicians (specifically gender and years of experience) also influenced the actions likely to be taken with the diabetic “patient” in the video. Again, the overall consistency of results is our primary interest (See Table 4). Female physicians were slightly more likely than their male counterparts to undertake two of the three essential components: monofilament/vibration (65.6 percent vs. 60.4 percent, ns); foot pulses (67.7 percent vs 68.8 percent ( ns); ulcers (54.2 percent vs 40.6 percent, ns). On average, female physicians would likely undertake 1.9 of the essential components, compared with 1.7 for their male counterparts, ns. Female physicians were also more likely to refer the “patient” to an ophthalmologist (p=.024) and to give advice about exercise (p=.044).
Table 4.
Components of a comprehensive diabetic examination by physician characteristics for diabetic patients presenting with symptoms suggestive of emerging foot neuropathy*
| Physician Gender | Physician Level of Experience | |||||
|---|---|---|---|---|---|---|
| Female N=96 |
Male N=96 |
p value | Less N=96 |
More N=96 |
p value | |
| Medical History | ||||||
| Adherence with diet | 49.0 | 41.7 | 0.310 | 49.0 | 41.7 | 0.310 |
| Adherence with meds | 55.2 | 44.8 | 0.144 | 49.0 | 51.0 | 0.769 |
| Exercise habits | 34.4 | 32.3 | 0.769 | 31.3 | 35.4 | 0.557 |
| Previous neuropathy | 15.6 | 15.6 | 1.000 | 11.5 | 19.8 | 0.120 |
| Eye disease | 35.4 | 22.9 | 0.048 | 30.2 | 28.1 | 0.740 |
| Examinations | ||||||
| Blood pressure | 61.5 | 58.3 | 0.675 | 55.2 | 64.6 | 0.211 |
| Fundoscopic | 18.8 | 12.5 | 0.242 | 16.7 | 14.6 | 0.696 |
| Laboratory Tests | ||||||
| HbA1c | 74.0 | 70.8 | 0.640 | 78.1 | 66.7 | 0.089 |
| Fasting lipid | 69.8 | 56.3 | 0.061 | 61.5 | 64.6 | 0.663 |
| Liver function | 49.0 | 35.4 | 0.040 | 51.0 | 33.3 | 0.008 |
| Microalbuminuria | 77.1 | 65.6 | 0.066 | 78.1 | 64.6 | 0.030 |
| TSH † | 27.1 | 21.9 | 0.413 | 27.1 | 21.9 | 0.413 |
| Referrals | ||||||
| Ophthalmologist | 40.6 | 25.0 | 0.024 | 33.3 | 32.3 | 0.879 |
| Advice | ||||||
| Exercise | 74.0 | 62.5 | 0.044 | 69.8 | 66.7 | 0.579 |
| Foot self exams | 28.1 | 29.2 | 0.870 | 32.3 | 25.0 | 0.253 |
| Diet | 75.0 | 70.8 | 0.494 | 79.2 | 66.7 | 0.042 |
Numbers are the percents and a p value for a test of the null hypotheses of no difference by the patient or physician characteristic.
Thyroid stimulating hormone
The case presented includes a patient-reported HbA1c level of 6.9 (which is considered borderline acceptable). 72.4 percent of the doctors reported they would repeat the HbA1c. 63.0 percent of physicians would be likely to request a fasting lipid test. The four patient characteristics included in the experiment had no influence on test ordering for the second case presented. Physician gender also appeared to have no effect of testing. Physician experience appeared to have some isolated effects -- those with less experience being more likely to request some of the tests: liver function (p=.008), microalbuminuria (p=.030) and to give diet and nutritional advice (p=.042)
3. Organizational/System-level Influences
Our experiment provided an opportunity to examine (as covariates) the additional influence of selected organizational factors on the diagnosis and management of diabetes: practice size (solo vs 2-10 and >10 physicians), for-profit vs not-for-profit status, type of ownership (physician, hospital and other), perceived sense of clinical and/or administrative autonomy, access to clinical guidelines, and whether electronic medical records are used. Elsewhere we have reported on the apparent influence of organizational factors on physician decision making including the influence of clinical guidelines, practice culture, and the overall importance of organizational factors.26, 37 Briefly, organizational factors appeared to have little influence on the outcomes of interest for the undiagnosed case (the diagnosis selected, level of certainty, range of competing diagnoses, test-ordering, etc). However, organizational factors, particularly practice culture and having access to clinical guidelines, influenced whether essential components of a foot examination would be conducted. For example, comparing those with access to guidelines to those without, 66.7 percent vs 47.2 percent would do a vibration/monofilament test (p=.031); 51.3 percent vs 30.6 percent would check for ulcers (p=.048); 70.5 percent vs 58.3 percent would check foot pulses (ns); and the overall number of tests conducted also differed (1.9 vs 1.4: p=.017).
DISCUSSION
The public health challenge of undiagnosed diabetes in the US is reflected in our results: only 60.9 percent of primary care physicians identified diabetes as the principal diagnosis. This finding is important in that signs and symptoms strongly suggestive of T2DM (as determined by guidelines and clinical expertise) were provided and were indicators for which a primary care physician should conduct confirmatory testing. There were statistically significant variations in the diagnosis of diabetes depending on the patients’ race/ethnicity. While these variations mirror the widely reported race/ethnic variations in diabetes in the US, the signs and symptoms presented in this experiment were exactly the same, regardless of other patient factors The level of certainty attached to a diagnosis of diabetes was markedly higher when the patient was Black (31.6 per cent), compared with 20.7 per cent when Hispanic and 15 per cent when the “patient” was white.
Competing diagnoses were offered by many physicians (e.g., depression, thyroid disease, cardiac disease, cancer and anemia). We recognize that some signs and symptoms specific to these conditions were embedded in the scenarios, but we took precautions not to include sufficient symptoms for a competing condition, while ensuring that sufficient key symptoms for T2DM were included. Moreover, the levels of physician certainty associated with these competing diagnoses sometimes exceeded the certainty level associated with the diagnosis of diabetes itself, reinforcing the concern that T2DM is under-recognized, and when recognized was not confirmed (the rule of halves). A high proportion (23.9%) of the physicians diagnosing diabetes would not request the test necessary for its confirmation (fasting glucose or A1c). Physician characteristics had little influence on the diagnosis of diabetes and test ordering was not influenced by the other patient attributes studied (age, gender, race/ethnicity and SES).
With respect to the second “patient” with already diagnosed diabetes and an emerging peripheral neuropathy, only 42.2 per cent of the physicians would do all three essential components of a foot examination (monofilament test for loss of sensation, a check of foot pulses for peripheral vascular disease and check for ulcers as recommended by both primary care and diabetes experts, and the guidelines), while 21.9 percent would do none of these. Patient attributes appeared to consistently influence whether a foot examination would be conducted: men more often than women, older more often than younger patients, and upper more often than lower socioeconomic status patients. Regarding physician characteristics, it is reassuring that physician gender and level of experience appeared to have no influence. The most significant organizational factors related to practice culture and having access to clinical guidelines. This appeared to significantly increase the likelihood that a foot examination would be conducted by physicians encountering the diagnosed case of diabetes.
Regarding the strengths and limitations of this study: First, focusing on the relative contributions of patient attributes, physician characteristics and organizational influences within a single study represents a new direction for research on clinical decision making.38 Second, earlier research has tended to focus on technical aspects of care whereas this study also includes processes of care (e.g. question asking, life style recommendations, patient education, referrals and follow-ups). Third, the study shows, yet again, rigorous experimentation is feasible in health services research, producing un-confounded estimates in a cost efficient manner. Fourth, use of clinically authentic video vignettes is an improvement over both written scenarios and standardized patients.35
Every study requires some tradeoff between internal and external validity. While experiments may produce excellent internal validity, external validity can be threatened. Four precautionary steps were taken to enhance external validity. To ensure clinical authenticity, physicians provided expertise during script development and were present during filming. Subjects viewed the scenarios in their offices and in the context of a typical practice day. They were instructed to view the “patient” as one of their own patients and respond as they normally would in everyday clinical practice. They were also specifically asked how typical the “patient” viewed on the videotape was compared with patients in their everyday practice (93.2% (for the undiagnosed diabetes vignette) and 84.9% (for the diagnosed case with foot neuropathy) considered them very or reasonably typical).
Ideally, a carefully selected random sample of physicians may produce results that are more generalizable. However, our methodology (experimentation) necessitated purposive sampling (which gives greater emphasis to obtaining sufficient numbers to fill cells and preserve orthogonality). A possible additional limitation is that this study includes physicians by race/ethnicity according to their prevalence in the states studied, thus making it difficult to have sufficient power to address differences by this factor.
Widely reported race/ethnic differences (among other socio-demographic variations) in diabetes are accepted as real and commonly attributed to either: a) genetics, family background or bio-physiologic influences; and/or b) the influence of social and behavioral risk factors (geographic location, diet and obesity). A third contributor to race/ethnic disparities in diabetes and healthcare may be the social patterning that results from variable decision making at the level of primary care. Despite encountering “patients” with identical signs and symptoms, physicians are significantly more likely to diagnose diabetes in black or Hispanic compared with white patients, and to be more certain of their diagnosis. If a disease is presupposed to be more or less common among particular groups a physician is more or less likely to request a test that is likely to confirm it. Without the recommended testing a diagnosis obviously cannot be confirmed, nor can a potentially damaging neuropathy be avoided. Our findings have an important implication: to what extent are race/ethnic disparities in diabetes and its associated healthcare the result of the continued reinforcement of expected differences?
Variations in diabetes decision making according to race and ethnicity are commonly justified as attending to base rates (apriori probabilities)—prevalence rates for major diseases like diabetes and coronary heart disease are thought to vary by race/ethnicity and gender. This appeal to base rates is referred to as “Bayesian” decision making, or “statistical discrimination”.39 Elsewhere we have shown that diabetes is actually varies by SES26, 40 and that physicians’ perceived base rates are often factually wrong, and that their decisions are not consistent with them anyway.41 If physicians are attending to presenting signs and symptoms as the basis for decisions, then findings should have been more consistent with study physicians reporting similar rates of diagnosis with similar certainty levels in the patient scenarios they viewed, irrespective of the race/ethnicity, gender, age or SES differences among the patients. Who the patient is, especially their race/ethnicity, appears to be more important in decision making than what the patient actually has. Without diminishing the importance of health care for minority patients, findings from this study indicate that diabetes diagnoses may be missed by placing higher emphasis (consciously or subconsciously) on base rates rather than on symptom presentation. Attending to this possibility, in combination with other approaches, may help reduce the widely reported and worrisome disparities in the delivery of US health care.
Figure 1. The same actor presenting as either a low or high SES “patient” with signs and symptoms of diabetes.
Note: The “patients” differed only slightly in appearance. Males presented with collar and tie (upper SES) or plaid shirt and jacket (lower SES). Females presented with either blazer with broach and makeup (high SES) or sweatshit and no makeup (lower SES).
ACKNOWLEDGEMENTS
We acknowledge helpful advice from Drs. Richard Grant, Allan Gorall, John Stoeckle and James Meigs (Massachusetts General Hospital), Dr. Werner Schernbaum (University of Dusseldorf, Germany), Dr. Osama Hamdy (Joslin Diabetes Center) Dr. Carol Link and Mr. Eric Gerstenberger (New England Research Institutes).
REFERENCES
- 1.International Diabetes Federation . Diabetes and Impaired Glucose Tolerance. International Diabetes Federation; Brussels, Belgium: 2009. [Google Scholar]
- 2.King H, Aubert RE, Herman WH. Global burden of diabetes, 1995-2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998;21(9):1414–31. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- 3.Diabetes Research Working Group [cited June, 2012]; [last accessed 26 November 2012];Conquering Diabetes: A Strategic Plan for the 21st Century. 1999 Available from: http://www2.niddk.nih.gov/AboutNIDDK/ReportsAndStrategicPlanning/Conquering_Diabetes_1999.htm.
- 4.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999-2000. Journal of the American Medical Association. 2002;288(14):1723–7. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 5.Steinbrook R. Facing the diabetes epidemic--mandatory reporting of glycosylated hemoglobin values in New York City. New England Journal of Medicine. 2006;354(6):545–8. doi: 10.1056/NEJMp068008. [DOI] [PubMed] [Google Scholar]
- 6.US Department of Health & Human Services [cited June, 2012]; [last accessed 26 November 2012];Diabetes Redefined. 2004 Available from: http://www.foh.dhhs.gov/NYCU/prediabetes.asp.
- 7.American Diabetes Association [cited June, 2011]; [last accessed 26 November 2012];Diabetes Statistics. 2011 Available from: http://www.diabetes.org/diabetes-basics/diabetes-statistics/
- 8.Centers for Disease Control and Prevention [cited April, 2005]; [last accessed 26 November 2012];National Diabetes Fact Sheet. 2005 Available from: http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2005.pdf.
- 9.Fradkin JE. Confronting the urgent challenge of diabetes: an overview. Health Affairs (Millwood) 2012;31(1):12–9. doi: 10.1377/hlthaff.2011.1150. [DOI] [PubMed] [Google Scholar]
- 10.Dunstan DW, Zimmet PZ, Welborn TA, et al. The rising prevalence of diabetes and impaired glucose tolerance: the Australian Diabetes, Obesity and Lifestyle Study. Diabetes Care. 2002;25(5):829–34. doi: 10.2337/diacare.25.5.829. [DOI] [PubMed] [Google Scholar]
- 11.McKinlay J, Marceau L. US public health and the 21st century: diabetes mellitus. The Lancet. 2000;356(9231):757–61. doi: 10.1016/S0140-6736(00)02641-6. [DOI] [PubMed] [Google Scholar]
- 12.Harris MI. Undiagnosed NIDDM: clinical and public health issues. Diabetes Care. 1993;16(4):642–52. doi: 10.2337/diacare.16.4.642. [DOI] [PubMed] [Google Scholar]
- 13.Harris MI, Flegal KM, Cowie CC, Eberhardt MS, Goldstein DE, Little RR, Wiedmeyer HM, Byrd-Holt DD. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The Third National Health and Nutrition Examination Survey, 1988-1994. Diabetes Care. 1998;21(4):518–24. doi: 10.2337/diacare.21.4.518. [DOI] [PubMed] [Google Scholar]
- 14.Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. The Lancet. 2002;359(9323):2072–7. doi: 10.1016/S0140-6736(02)08905-5. [DOI] [PubMed] [Google Scholar]
- 15.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. New England Journal of Medicine. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. New England Journal of Medicine. 2001;344(18):1343–50. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 17.Harris MI, National Health and Nutrition Examination Survey Frequency of blood glucose monitoring in relation to glycemic control in patients with type 2 diabetes. Diabetes Care. 2001;24(6):979–82. doi: 10.2337/diacare.24.6.979. [DOI] [PubMed] [Google Scholar]
- 18.UK Prospective Diabetes Study (UKPDS) Group Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. The Lancet. 1998;352(9131):854–65. [PubMed] [Google Scholar]
- 19.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. The Lancet. 1998;352(9131):837–53. [PubMed] [Google Scholar]
- 20.Eisenberg JM. Physician utilization: the state of research about physicians’ practice patterns. Medical Care. 2002;40(11):1016–35. doi: 10.1097/01.MLR.0000032181.98320.8D. [DOI] [PubMed] [Google Scholar]
- 21.Sager A, Socolar D. Health Costs Absorb One-Quarter of Economic Growth, 2000-2005. BU School of Public Health. 2005. Report No.: Data Brief No. 5.
- 22.Peek ME, Cargill A, Huang ES. Diabetes health disparities: a systematic review of health care interventions. Medical Care Research and Review. 2007;64(Suppl 5):101S–56S. doi: 10.1177/1077558707305409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saaddine JB, Engelgau MM, Beckles GL, Gregg EW, Thompson TJ, Narayan KM. A diabetes report card for the United States: quality of care in the 1990s. Annals of Internal Medicine. 2002;136(8):565–74. doi: 10.7326/0003-4819-136-8-200204160-00005. [DOI] [PubMed] [Google Scholar]
- 24.Institute of Medicine (IOM) Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. The National Academies Press; Washington, DC: 2003. [PubMed] [Google Scholar]
- 25.Keating NL, Landrum MB, Landon BE, Ayanian JZ, Borbas C, Wolf R, Guadagnoli E. The influence of physicians’ practice management strategies and financial arrangements on quality of care among patients with diabetes. Medical Care. 2004;42(9):829–39. doi: 10.1097/01.mlr.0000135829.73795.a7. [DOI] [PubMed] [Google Scholar]
- 26.Marceau L, McKinlay J, Shackelton R, Link C. The relative contribution of patient, provider and organizational influences to the appropriate diagnosis and management of diabetes mellitus. Journal of Evaluation in Clinical Practice. 2011;17:1122–8. doi: 10.1111/j.1365-2753.2010.01489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams RA, editor. Eliminating Healthcare Disparities in America: Beyond the IOM Report. Humana Press; Totowa, NJ: 2007. [Google Scholar]
- 28.Arday DR, Fleming BB, Keller DK, Pendergrass PW, Vaughn RJ, Turpin JM, Nicewander DA. Variation in diabetes care among states: do patient characteristics matter? Diabetes Care. 2002;25(12):2230–7. doi: 10.2337/diacare.25.12.2230. [DOI] [PubMed] [Google Scholar]
- 29.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2008;31(Suppl 1):S55–60. doi: 10.2337/dc08-S055. [DOI] [PubMed] [Google Scholar]
- 30.American Diabetes Association Standards of medical care in diabetes--2008. Diabetes Care. 2008;31(Suppl 1):S12–54. doi: 10.2337/dc08-S012. [DOI] [PubMed] [Google Scholar]
- 31.Agency for Healthcare Research and Quality [cited March 17, 2008];National Guideline Clearinghouse. Available from: http://www.guidelines.gov.
- 32.Ryden L, Standl E, Bartnik M, et al. Guidelines on diabetes, pre-diabetes, and cardiovascular diseases: executive summary. The Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC) and of the European Association for the Study of Diabetes (EASD) European Heart Journal. 2007;28(1):88–136. doi: 10.1093/eurheartj/ehl260. [DOI] [PubMed] [Google Scholar]
- 33.Cochran WG, Cox GM. Experimental Designs. Wiley; New York: 1992. [Google Scholar]
- 34.Montgomery DC. Design and Analysis of Experiments. Wiley; New York: 2001. [Google Scholar]
- 35.Marceau L, McKinlay J, Piccolo R. Clinical vignettes in health services research: advantages and limitations of different formats. AcademyHealth; Orlando, FL: Jun 24, 2012. 2012. [Google Scholar]
- 36.Konrad TR, Link CL, Shackelton RJ, Marceau LD, von dem Knesebeck O, Siegrist J, Arber S, Adams A, McKinlay JB. It’s about time: physicians’ perceptions of time constraints in primary care medical practice in three national healthcare systems. Medical Care. 2010;48(2):95–100. doi: 10.1097/MLR.0b013e3181c12e6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shackelton RJ, Marceau LD, Link CL, McKinlay JB. The intended and unintended consequences of clinical guidelines. Journal of Evaluation in Clinical Practice. 2009;15(6):1035–42. doi: 10.1111/j.1365-2753.2009.01201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lutfey K, Link C, Marceau L, McKinlay JB. Social Factors. In: Kattan MW, editor. Encyclopedia of Medical Decision Making. Sage Publications; Thousand Oaks, CA: 2009. pp. 1046–54. [Google Scholar]
- 39.McGuire TG, Ayanian JZ, Ford DE, Henke RE, Rost KM, Zaslavsky AM. Testing for statistical discrimination by race/ethnicity in panel data for depression treatment in primary care. Health Services Research. 2008;43(2):531–51. doi: 10.1111/j.1475-6773.2007.00770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McKinlay JB, Marceau LD, Piccolo RJ. Do doctors contribute to the social patterning of disease? The case of race/ethnic disparities in diabetes mellitus. Medical Care Research and Review. 2012;69(2):176–93. doi: 10.1177/1077558711429010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maserejian NN, Lutfey KE, McKinlay JB. Do Physicians Attend to Base Rates? Prevalence Data and Statistical Discrimination in the Diagnosis of Coronary Heart Disease. Health Services Research. 2009;44(6):1933–49. doi: 10.1111/j.1475-6773.2009.01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]