Abstract
The leucine-responsive regulatory protein (Lrp) regulates the expression of more than 40 genes and proteins in Escherichia coli. Among the operons that are positively regulated by Lrp are operons involved in amino acid biosynthesis (ilvIH, serA)), in the biosynthesis of pili (pap, fan, fim), and in the assimilation of ammonia (glnA, gltBD). Negatively regulated operons include operons involved in amino acid catabolism (sdaA, tdh) and peptide transport (opp) and the operon coding for Lrp itself (lrp). Detailed studies of a few members of the regulon have shown that Lrp can act directly to activate or repress transcription of target operons. A substantial fraction of operons regulated by Lrp are also regulated by leucine, and the effect of leucine on expression of these operons requires a functional Lrp protein. The patterns of regulation are surprising and interesting: in some cases activation or repression mediated by Lrp is antagonized by leucine, in other cases Lrp-mediated activation or repression is potentiated by leucine, and in still other cases leucine has no effect on Lrp-mediated regulation. Current research is just beginning to elucidate the detailed mechanisms by which Lrp can mediate such a broad spectrum of regulatory effects. Our view of the role of Lrp in metabolism may change as more members of the regulon are identified and their regulation characterized, but at this point Lrp seems to be important in regulating nitrogen metabolism and one-carbon metabolism, permitting adaptations to feast and to famine.
Full text
PDF




















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham J. M., Freitag C. S., Clements J. R., Eisenstein B. I. An invertible element of DNA controls phase variation of type 1 fimbriae of Escherichia coli. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5724–5727. doi: 10.1073/pnas.82.17.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhya S., Garges S. Positive control. J Biol Chem. 1990 Jul 5;265(19):10797–10800. [PubMed] [Google Scholar]
- Andersen J., Delihas N. micF RNA binds to the 5' end of ompF mRNA and to a protein from Escherichia coli. Biochemistry. 1990 Oct 2;29(39):9249–9256. doi: 10.1021/bi00491a020. [DOI] [PubMed] [Google Scholar]
- Andersen L., Kilstrup M., Neuhard J. Pyrimidine, purine and nitrogen control of cytosine deaminase synthesis in Escherichia coli K 12. Involvement of the glnLG and purR genes in the regulation of codA expression. Arch Microbiol. 1989;152(2):115–118. doi: 10.1007/BF00456087. [DOI] [PubMed] [Google Scholar]
- Anderson J. J., Oxender D. L. Escherichia coli transport mutants lacking binding protein and other components of the branched-chain amino acid transport systems. J Bacteriol. 1977 Apr;130(1):384–392. doi: 10.1128/jb.130.1.384-392.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. J., Quay S. C., Oxender D. L. Mapping of two loci affecting the regulation of branched-chain amino acid transport in Escherichia coli K-12. J Bacteriol. 1976 Apr;126(1):80–90. doi: 10.1128/jb.126.1.80-90.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews J. C., Blevins T. C., Short S. A. Regulation of peptide transport in Escherichia coli: induction of the trp-linked operon encoding the oligopeptide permease. J Bacteriol. 1986 Feb;165(2):428–433. doi: 10.1128/jb.165.2.428-433.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews J. C., Short S. A. opp-lac Operon fusions and transcriptional regulation of the Escherichia coli trp-linked oligopeptide permease. J Bacteriol. 1986 Feb;165(2):434–442. doi: 10.1128/jb.165.2.434-442.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson B. D., Levinthal M., Somerville R. L. Activation of a cryptic pathway for threonine metabolism via specific IS3-mediated alteration of promoter structure in Escherichia coli. J Bacteriol. 1989 Oct;171(10):5503–5511. doi: 10.1128/jb.171.10.5503-5511.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak Z., Gilvarg C. Triornithine-resistant strains of Escherichia coli. Isolation, definition, and genetic studies. J Biol Chem. 1974 Jan 10;249(1):143–148. [PubMed] [Google Scholar]
- Bender R. A., Macaluso A., Magasanik B. Glutamate dehydrogenase: genetic mapping and isolation of regulatory mutants of Klebsiella aerogenes. J Bacteriol. 1976 Jul;127(1):141–148. doi: 10.1128/jb.127.1.141-148.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilge S. S., Apostol J. M., Jr, Fullner K. J., Moseley S. L. Transcriptional organization of the F1845 fimbrial adhesin determinant of Escherichia coli. Mol Microbiol. 1993 Mar;7(6):993–1006. doi: 10.1111/j.1365-2958.1993.tb01191.x. [DOI] [PubMed] [Google Scholar]
- Bilge S. S., Clausen C. R., Lau W., Moseley S. L. Molecular characterization of a fimbrial adhesin, F1845, mediating diffuse adherence of diarrhea-associated Escherichia coli to HEp-2 cells. J Bacteriol. 1989 Aug;171(8):4281–4289. doi: 10.1128/jb.171.8.4281-4289.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomfield I. C., Calie P. J., Eberhardt K. J., McClain M. S., Eisenstein B. I. Lrp stimulates phase variation of type 1 fimbriation in Escherichia coli K-12. J Bacteriol. 1993 Jan;175(1):27–36. doi: 10.1128/jb.175.1.27-36.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomfield I. C., McClain M. S., Princ J. A., Calie P. J., Eisenstein B. I. Type 1 fimbriation and fimE mutants of Escherichia coli K-12. J Bacteriol. 1991 Sep;173(17):5298–5307. doi: 10.1128/jb.173.17.5298-5307.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blyn L. B., Braaten B. A., Low D. A. Regulation of pap pilin phase variation by a mechanism involving differential dam methylation states. EMBO J. 1990 Dec;9(12):4045–4054. doi: 10.1002/j.1460-2075.1990.tb07626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blyn L. B., Braaten B. A., White-Ziegler C. A., Rolfson D. H., Low D. A. Phase-variation of pyelonephritis-associated pili in Escherichia coli: evidence for transcriptional regulation. EMBO J. 1989 Feb;8(2):613–620. doi: 10.1002/j.1460-2075.1989.tb03416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braaten B. A., Blyn L. B., Skinner B. S., Low D. A. Evidence for a methylation-blocking factor (mbf) locus involved in pap pilus expression and phase variation in Escherichia coli. J Bacteriol. 1991 Mar;173(5):1789–1800. doi: 10.1128/jb.173.5.1789-1800.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braaten B. A., Nou X., Kaltenbach L. S., Low D. A. Methylation patterns in pap regulatory DNA control pyelonephritis-associated pili phase variation in E. coli. Cell. 1994 Feb 11;76(3):577–588. doi: 10.1016/0092-8674(94)90120-1. [DOI] [PubMed] [Google Scholar]
- Braaten B. A., Platko J. V., van der Woude M. W., Simons B. H., de Graaf F. K., Calvo J. M., Low D. A. Leucine-responsive regulatory protein controls the expression of both the pap and fan pili operons in Escherichia coli. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4250–4254. doi: 10.1073/pnas.89.10.4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenchley J. E., Baker C. A., Patil L. G. Regulation of the ammonia assimilatory enzymes in Salmonella typhimurium. J Bacteriol. 1975 Oct;124(1):182–189. doi: 10.1128/jb.124.1.182-189.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan R. G., Matthews B. W. The helix-turn-helix DNA binding motif. J Biol Chem. 1989 Feb 5;264(4):1903–1906. [PubMed] [Google Scholar]
- Bueno R., Pahel G., Magasanik B. Role of glnB and glnD gene products in regulation of the glnALG operon of Escherichia coli. J Bacteriol. 1985 Nov;164(2):816–822. doi: 10.1128/jb.164.2.816-822.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J. L., Kleckner N. E. coli oriC and the dnaA gene promoter are sequestered from dam methyltransferase following the passage of the chromosomal replication fork. Cell. 1990 Sep 7;62(5):967–979. doi: 10.1016/0092-8674(90)90271-f. [DOI] [PubMed] [Google Scholar]
- Castaño I., Flores N., Valle F., Covarrubias A. A., Bolivar F. gltF, a member of the gltBDF operon of Escherichia coli, is involved in nitrogen-regulated gene expression. Mol Microbiol. 1992 Sep;6(18):2733–2741. doi: 10.1111/j.1365-2958.1992.tb01450.x. [DOI] [PubMed] [Google Scholar]
- Clark R. L., Neidhardt F. C. Roles of the two lysyl-tRNA synthetases of Escherichia coli: analysis of nucleotide sequences and mutant behavior. J Bacteriol. 1990 Jun;172(6):3237–3243. doi: 10.1128/jb.172.6.3237-3243.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collado-Vides J., Magasanik B., Gralla J. D. Control site location and transcriptional regulation in Escherichia coli. Microbiol Rev. 1991 Sep;55(3):371–394. doi: 10.1128/mr.55.3.371-394.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ari R., Lin R. T., Newman E. B. The leucine-responsive regulatory protein: more than a regulator? Trends Biochem Sci. 1993 Jul;18(7):260–263. doi: 10.1016/0968-0004(93)90177-o. [DOI] [PubMed] [Google Scholar]
- De Felice M., Levinthal M. The acetohydroxy acid synthase III isoenzyme of Escherichia coli K-12: regulation of synthesis by leucine. Biochem Biophys Res Commun. 1977 Nov 7;79(1):82–87. doi: 10.1016/0006-291x(77)90063-8. [DOI] [PubMed] [Google Scholar]
- Delaney J. M., Georgopoulos C. Physical map locations of the trxB, htrD, cydC, and cydD genes of Escherichia coli. J Bacteriol. 1992 Jun;174(11):3824–3825. doi: 10.1128/jb.174.11.3824-3825.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dev I. K., Harvey R. J. Regulation of synthesis of serine hydroxymethyltransferase in chemostat cultures of Escherichia coli. J Biol Chem. 1984 Jul 10;259(13):8394–8401. [PubMed] [Google Scholar]
- Dodd I. B., Egan J. B. Systematic method for the detection of potential lambda Cro-like DNA-binding regions in proteins. J Mol Biol. 1987 Apr 5;194(3):557–564. doi: 10.1016/0022-2836(87)90681-4. [DOI] [PubMed] [Google Scholar]
- Dorman C. J., Higgins C. F. Fimbrial phase variation in Escherichia coli: dependence on integration host factor and homologies with other site-specific recombinases. J Bacteriol. 1987 Aug;169(8):3840–3843. doi: 10.1128/jb.169.8.3840-3843.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew H. R., Travers A. A. DNA bending and its relation to nucleosome positioning. J Mol Biol. 1985 Dec 20;186(4):773–790. doi: 10.1016/0022-2836(85)90396-1. [DOI] [PubMed] [Google Scholar]
- Eisenstein B. I. Phase variation of type 1 fimbriae in Escherichia coli is under transcriptional control. Science. 1981 Oct 16;214(4518):337–339. doi: 10.1126/science.6116279. [DOI] [PubMed] [Google Scholar]
- Eisenstein B. I., Sweet D. S., Vaughn V., Friedman D. I. Integration host factor is required for the DNA inversion that controls phase variation in Escherichia coli. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6506–6510. doi: 10.1073/pnas.84.18.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
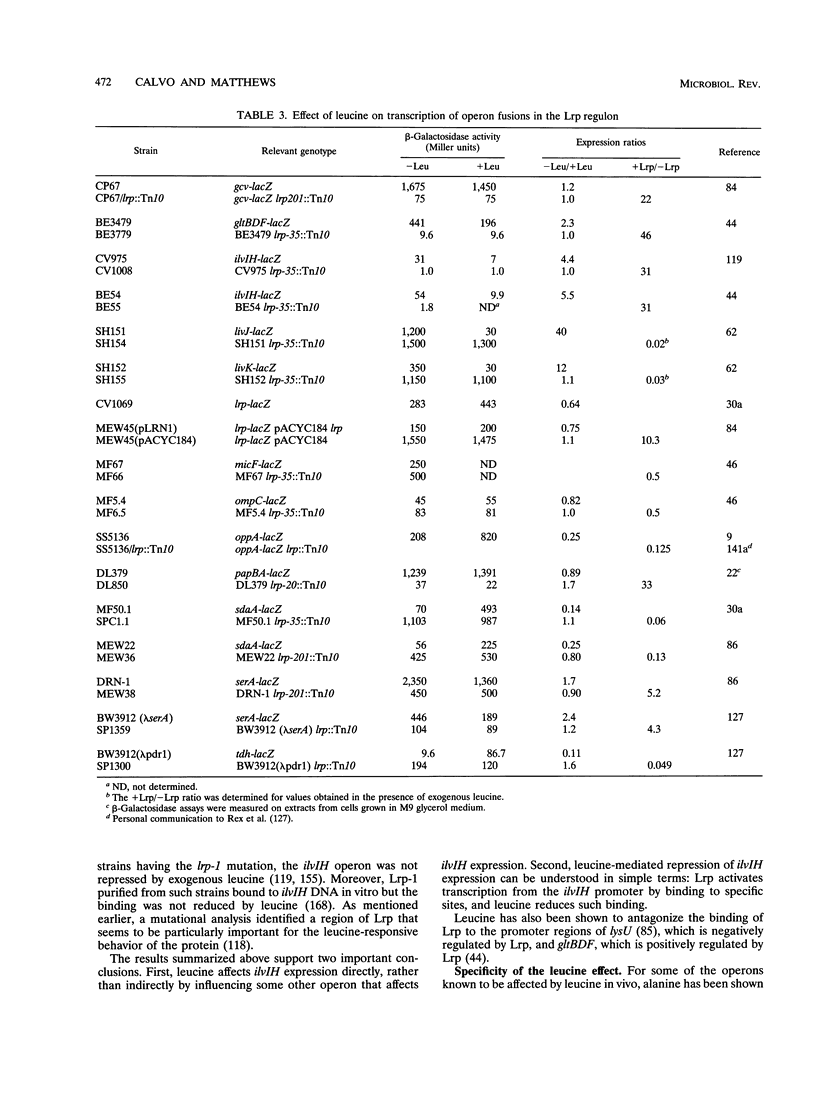
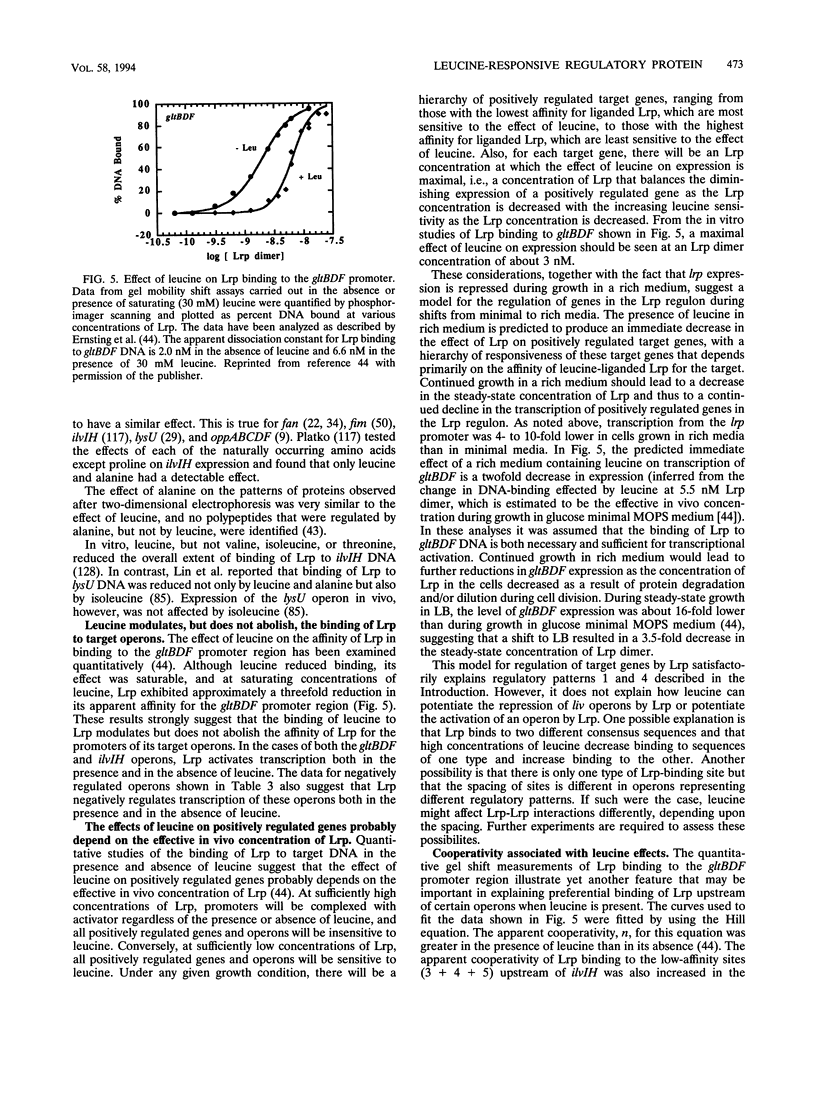
- Ernsting B. R., Atkinson M. R., Ninfa A. J., Matthews R. G. Characterization of the regulon controlled by the leucine-responsive regulatory protein in Escherichia coli. J Bacteriol. 1992 Feb;174(4):1109–1118. doi: 10.1128/jb.174.4.1109-1118.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernsting B. R., Denninger J. W., Blumenthal R. M., Matthews R. G. Regulation of the gltBDF operon of Escherichia coli: how is a leucine-insensitive operon regulated by the leucine-responsive regulatory protein? J Bacteriol. 1993 Nov;175(22):7160–7169. doi: 10.1128/jb.175.22.7160-7169.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J., Atkinson M. R., McCleary W., Stock J. B., Wanner B. L., Ninfa A. J. Role of phosphorylated metabolic intermediates in the regulation of glutamine synthetase synthesis in Escherichia coli. J Bacteriol. 1992 Oct;174(19):6061–6070. doi: 10.1128/jb.174.19.6061-6070.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser J., Newman E. B. Derivation of glycine from threonine in Escherichia coli K-12 mutants. J Bacteriol. 1975 Jun;122(3):810–817. doi: 10.1128/jb.122.3.810-817.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitag C. S., Abraham J. M., Clements J. R., Eisenstein B. I. Genetic analysis of the phase variation control of expression of type 1 fimbriae in Escherichia coli. J Bacteriol. 1985 May;162(2):668–675. doi: 10.1128/jb.162.2.668-675.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaastra W., de Graaf F. K. Host-specific fimbrial adhesins of noninvasive enterotoxigenic Escherichia coli strains. Microbiol Rev. 1982 Jun;46(2):129–161. doi: 10.1128/mr.46.2.129-161.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gally D. L., Bogan J. A., Eisenstein B. I., Blomfield I. C. Environmental regulation of the fim switch controlling type 1 fimbrial phase variation in Escherichia coli K-12: effects of temperature and media. J Bacteriol. 1993 Oct;175(19):6186–6193. doi: 10.1128/jb.175.19.6186-6193.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganoza M. C., Cunningham C., Green R. M. Isolation and point of action of a factor from Escherichia coli required to reconstruct translation. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1648–1652. doi: 10.1073/pnas.82.6.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazeau M., Delort F., Dessen P., Blanquet S., Plateau P. Escherichia coli leucine-responsive regulatory protein (Lrp) controls lysyl-tRNA synthetase expression. FEBS Lett. 1992 Apr 6;300(3):254–258. doi: 10.1016/0014-5793(92)80857-d. [DOI] [PubMed] [Google Scholar]
- Gemmill R. M., Wessler S. R., Keller E. B., Calvo J. M. leu operon of Salmonella typhimurium is controlled by an attenuation mechanism. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4941–4945. doi: 10.1073/pnas.76.10.4941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerolimatos B., Hanson R. L. Repression of Escherichia coli pyridine nucleotide transhydrogenase by leucine. J Bacteriol. 1978 May;134(2):394–400. doi: 10.1128/jb.134.2.394-400.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson M. M., Price M., Higgins C. F. Genetic characterization and molecular cloning of the tripeptide permease (tpp) genes of Salmonella typhimurium. J Bacteriol. 1984 Oct;160(1):122–130. doi: 10.1128/jb.160.1.122-130.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollop N., Tavori H., Barak Z. Acetohydroxy acid synthase is a target for leucine containing peptide toxicity in Escherichia coli. J Bacteriol. 1982 Jan;149(1):387–390. doi: 10.1128/jb.149.1.387-390.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gralla J. D. Transcriptional control--lessons from an E. coli promoter data base. Cell. 1991 Aug 9;66(3):415–418. doi: 10.1016/0092-8674(81)90001-5. [DOI] [PubMed] [Google Scholar]
- Greene R. C., Hunter J. S., Coch E. H. Properties of metK mutants of Escherichia coli K-12. J Bacteriol. 1973 Jul;115(1):57–67. doi: 10.1128/jb.115.1.57-67.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göransson M., Forsman K., Uhlin B. E. Functional and structural homology among regulatory cistrons of pili-adhesin determinants in Escherichia coli. Mol Gen Genet. 1988 Jun;212(3):412–417. doi: 10.1007/BF00330844. [DOI] [PubMed] [Google Scholar]
- Göransson M., Forsman P., Nilsson P., Uhlin B. E. Upstream activating sequences that are shared by two divergently transcribed operons mediate cAMP-CRP regulation of pilus-adhesin in Escherichia coli. Mol Microbiol. 1989 Nov;3(11):1557–1565. doi: 10.1111/j.1365-2958.1989.tb00141.x. [DOI] [PubMed] [Google Scholar]
- Hale W. B., van der Woude M. W., Low D. A. Analysis of nonmethylated GATC sites in the Escherichia coli chromosome and identification of sites that are differentially methylated in response to environmental stimuli. J Bacteriol. 1994 Jun;176(11):3438–3441. doi: 10.1128/jb.176.11.3438-3441.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hama H., Shimamoto T., Tsuda M., Tsuchiya T. Characterization of a novel L-serine transport system in Escherichia coli. J Bacteriol. 1988 May;170(5):2236–2239. doi: 10.1128/jb.170.5.2236-2239.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney S. A., Platko J. V., Oxender D. L., Calvo J. M. Lrp, a leucine-responsive protein, regulates branched-chain amino acid transport genes in Escherichia coli. J Bacteriol. 1992 Jan;174(1):108–115. doi: 10.1128/jb.174.1.108-115.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughn G. W., Squires C. H., De Felice M., Largo C. T., Calvo J. M. Unusual organization of the ilvIH promoter of Escherichia coli. J Bacteriol. 1985 Jul;163(1):186–198. doi: 10.1128/jb.163.1.186-198.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiles I. D., Gallagher M. P., Jamieson D. J., Higgins C. F. Molecular characterization of the oligopeptide permease of Salmonella typhimurium. J Mol Biol. 1987 May 5;195(1):125–142. doi: 10.1016/0022-2836(87)90332-9. [DOI] [PubMed] [Google Scholar]
- Hirshfield I. N., Bloch P. L., Van Bogelen R. A., Neidhardt F. C. Multiple forms of lysyl-transfer ribonucleic acid synthetase in Escherichia coli. J Bacteriol. 1981 Apr;146(1):345–351. doi: 10.1128/jb.146.1.345-351.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshfield I. N., Yeh F. M., Sawyer L. E. Metabolites influence control of lysine transfer ribonucleic acid synthetase formation in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1364–1367. doi: 10.1073/pnas.72.4.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochschild A., Ptashne M. Cooperative binding of lambda repressors to sites separated by integral turns of the DNA helix. Cell. 1986 Mar 14;44(5):681–687. doi: 10.1016/0092-8674(86)90833-0. [DOI] [PubMed] [Google Scholar]
- Huisman T. T., Bakker D., Klaasen P., de Graaf F. K. Leucine-responsive regulatory protein, IS1 insertions, and the negative regulator FaeA control the expression of the fae (K88) operon in Escherichia coli. Mol Microbiol. 1994 Feb;11(3):525–536. doi: 10.1111/j.1365-2958.1994.tb00333.x. [DOI] [PubMed] [Google Scholar]
- Ito K., Kawakami K., Nakamura Y. Multiple control of Escherichia coli lysyl-tRNA synthetase expression involves a transcriptional repressor and a translational enhancer element. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):302–306. doi: 10.1073/pnas.90.1.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson D. J., Higgins C. F. Anaerobic and leucine-dependent expression of a peptide transport gene in Salmonella typhimurium. J Bacteriol. 1984 Oct;160(1):131–136. doi: 10.1128/jb.160.1.131-136.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami K., Ito K., Nakamura Y. Differential regulation of two genes encoding lysyl-tRNA synthetases in Escherichia coli: lysU-constitutive mutations compensate for a lysS null mutation. Mol Microbiol. 1992 Jul;6(13):1739–1745. doi: 10.1111/j.1365-2958.1992.tb01346.x. [DOI] [PubMed] [Google Scholar]
- Kayahara T., Thelen P., Ogawa W., Inaba K., Tsuda M., Goldberg E. B., Tsuchiya T. Properties of recombinant cells capable of growing on serine without NhaB Na+/H+ antiporter in Escherichia coli. J Bacteriol. 1992 Nov;174(22):7482–7485. doi: 10.1128/jb.174.22.7482-7485.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Zwieb C., Wu C., Adhya S. Bending of DNA by gene-regulatory proteins: construction and use of a DNA bending vector. Gene. 1989 Dec 21;85(1):15–23. doi: 10.1016/0378-1119(89)90459-9. [DOI] [PubMed] [Google Scholar]
- Klemm P. Two regulatory fim genes, fimB and fimE, control the phase variation of type 1 fimbriae in Escherichia coli. EMBO J. 1986 Jun;5(6):1389–1393. doi: 10.1002/j.1460-2075.1986.tb04372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kölling R., Lother H. AsnC: an autogenously regulated activator of asparagine synthetase A transcription in Escherichia coli. J Bacteriol. 1985 Oct;164(1):310–315. doi: 10.1128/jb.164.1.310-315.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landick R., Oxender D. L. The complete nucleotide sequences of the Escherichia coli LIV-BP and LS-BP genes. Implications for the mechanism of high-affinity branched-chain amino acid transport. J Biol Chem. 1985 Jul 15;260(14):8257–8261. [PubMed] [Google Scholar]
- Lange R., Barth M., Hengge-Aronis R. Complex transcriptional control of the sigma s-dependent stationary-phase-induced and osmotically regulated osmY (csi-5) gene suggests novel roles for Lrp, cyclic AMP (cAMP) receptor protein-cAMP complex, and integration host factor in the stationary-phase response of Escherichia coli. J Bacteriol. 1993 Dec;175(24):7910–7917. doi: 10.1128/jb.175.24.7910-7917.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenny A. B., Margolin P. Locations of the opp and supX genes of Salmonella typhimurium and Escherichia coli. J Bacteriol. 1980 Aug;143(2):747–752. doi: 10.1128/jb.143.2.747-752.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinthal M., Lejeune P., Danchin A. The H-NS protein modulates the activation of the ilvIH operon of Escherichia coli K12 by Lrp, the leucine regulatory protein. Mol Gen Genet. 1994 Mar;242(6):736–743. doi: 10.1007/BF00283429. [DOI] [PubMed] [Google Scholar]
- Lewis L. K., Jenkins M. E., Mount D. W. Isolation of DNA damage-inducible promoters in Escherichia coli: regulation of polB (dinA), dinG, and dinH by LexA repressor. J Bacteriol. 1992 May;174(10):3377–3385. doi: 10.1128/jb.174.10.3377-3385.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R., D'Ari R., Newman E. B. Lambda placMu insertions in genes of the leucine regulon: extension of the regulon to genes not regulated by leucine. J Bacteriol. 1992 Mar;174(6):1948–1955. doi: 10.1128/jb.174.6.1948-1955.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R., Ernsting B., Hirshfield I. N., Matthews R. G., Neidhardt F. C., Clark R. L., Newman E. B. The lrp gene product regulates expression of lysU in Escherichia coli K-12. J Bacteriol. 1992 May;174(9):2779–2784. doi: 10.1128/jb.174.9.2779-2784.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg F., Lund B., Johansson L., Normark S. Localization of the receptor-binding protein adhesin at the tip of the bacterial pilus. Nature. 1987 Jul 2;328(6125):84–87. doi: 10.1038/328084a0. [DOI] [PubMed] [Google Scholar]
- Lobell R. B., Schleif R. F. DNA looping and unlooping by AraC protein. Science. 1990 Oct 26;250(4980):528–532. doi: 10.1126/science.2237403. [DOI] [PubMed] [Google Scholar]
- Lévêque F., Gazeau M., Fromant M., Blanquet S., Plateau P. Control of Escherichia coli lysyl-tRNA synthetase expression by anaerobiosis. J Bacteriol. 1991 Dec;173(24):7903–7910. doi: 10.1128/jb.173.24.7903-7910.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévêque F., Plateau P., Dessen P., Blanquet S. Homology of lysS and lysU, the two Escherichia coli genes encoding distinct lysyl-tRNA synthetase species. Nucleic Acids Res. 1990 Jan 25;18(2):305–312. doi: 10.1093/nar/18.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhusudhan K. T., Lorenz D., Sokatch J. R. The bkdR gene of Pseudomonas putida is required for expression of the bkd operon and encodes a protein related to Lrp of Escherichia coli. J Bacteriol. 1993 Jul;175(13):3934–3940. doi: 10.1128/jb.175.13.3934-3940.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallick U., Herrlich P. Regulation of synthesis of a major outer membrane protein: cyclic AMP represses Escherichia coli protein III synthesis. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5520–5523. doi: 10.1073/pnas.76.11.5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinus M. G., Morris N. R. Isolation of deoxyribonucleic acid methylase mutants of Escherichia coli K-12. J Bacteriol. 1973 Jun;114(3):1143–1150. doi: 10.1128/jb.114.3.1143-1150.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews R. G., Neidhardt F. C. Abnormal induction of heat shock proteins in an Escherichia coli mutant deficient in adenosylmethionine synthetase activity. J Bacteriol. 1988 Apr;170(4):1582–1588. doi: 10.1128/jb.170.4.1582-1588.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews R. G., Neidhardt F. C. Elevated serine catabolism is associated with the heat shock response in Escherichia coli. J Bacteriol. 1989 May;171(5):2619–2625. doi: 10.1128/jb.171.5.2619-2625.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain M. S., Blomfield I. C., Eisenstein B. I. Roles of fimB and fimE in site-specific DNA inversion associated with phase variation of type 1 fimbriae in Escherichia coli. J Bacteriol. 1991 Sep;173(17):5308–5314. doi: 10.1128/jb.173.17.5308-5314.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKitrick J. C., Pizer L. I. Regulation of phosphoglycerate dehydrogenase levels and effect on serine synthesis in Escherichia coli K-12. J Bacteriol. 1980 Jan;141(1):235–245. doi: 10.1128/jb.141.1.235-245.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooi F. R., van Buuren M., Koopman G., Roosendaal B., de Graaf F. K. K88ab gene of Escherichia coli encodes a fimbria-like protein distinct from the K88ab fimbrial adhesin. J Bacteriol. 1984 Aug;159(2):482–487. doi: 10.1128/jb.159.2.482-487.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon H. W. Colonization factor antigens of enterotoxigenic Escherichia coli in animals. Curr Top Microbiol Immunol. 1990;151:147–165. doi: 10.1007/978-3-642-74703-8_8. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., VanBogelen R. A., Lau E. T. Molecular cloning and expression of a gene that controls the high-temperature regulon of Escherichia coli. J Bacteriol. 1983 Feb;153(2):597–603. doi: 10.1128/jb.153.2.597-603.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman E. B., Ahmad D., Walker C. L-Serine deaminase activity is induced by exposure of Escherichia coli K-12 to DNA-damaging agents. J Bacteriol. 1982 Nov;152(2):702–705. doi: 10.1128/jb.152.2.702-705.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman E. B., D'Ari R., Lin R. T. The leucine-Lrp regulon in E. coli: a global response in search of a raison d'être. Cell. 1992 Feb 21;68(4):617–619. doi: 10.1016/0092-8674(92)90135-y. [DOI] [PubMed] [Google Scholar]
- Newman E. B., Kapoor V., Potter R. Role of L-threonine dehydrogenase in the catabolism of threonine and synthesis of glycine by Escherichia coli. J Bacteriol. 1976 Jun;126(3):1245–1249. doi: 10.1128/jb.126.3.1245-1249.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Rosenberg E. Y. Porin channels in Escherichia coli: studies with liposomes reconstituted from purified proteins. J Bacteriol. 1983 Jan;153(1):241–252. doi: 10.1128/jb.153.1.241-252.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nou X., Skinner B., Braaten B., Blyn L., Hirsch D., Low D. Regulation of pyelonephritis-associated pili phase-variation in Escherichia coli: binding of the PapI and the Lrp regulatory proteins is controlled by DNA methylation. Mol Microbiol. 1993 Feb;7(4):545–553. doi: 10.1111/j.1365-2958.1993.tb01145.x. [DOI] [PubMed] [Google Scholar]
- O'Day K., Lopilato J., Wright A. Physical locations of bglA and serA on the Escherichia coli K-12 chromosome. J Bacteriol. 1991 Mar;173(5):1571–1571. doi: 10.1128/jb.173.5.1571.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Halloran T. V., Frantz B., Shin M. K., Ralston D. M., Wright J. G. The MerR heavy metal receptor mediates positive activation in a topologically novel transcription complex. Cell. 1989 Jan 13;56(1):119–129. doi: 10.1016/0092-8674(89)90990-2. [DOI] [PubMed] [Google Scholar]
- Okamura-Ikeda K., Ohmura Y., Fujiwara K., Motokawa Y. Cloning and nucleotide sequence of the gcv operon encoding the Escherichia coli glycine-cleavage system. Eur J Biochem. 1993 Sep 1;216(2):539–548. doi: 10.1111/j.1432-1033.1993.tb18172.x. [DOI] [PubMed] [Google Scholar]
- PARDEE A. B., PRESTIDGE L. S. Induced formation of serine and threonine deaminases by Escherichia coli. J Bacteriol. 1955 Dec;70(6):667–674. doi: 10.1128/jb.70.6.667-674.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIZER L. I., POTOCHNY M. L. NUTRITIONAL AND REGULATORY ASPECTS OF SERINE METABOLISM IN ESCHERICHIA COLI. J Bacteriol. 1964 Sep;88:611–619. doi: 10.1128/jb.88.3.611-619.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahel G., Rothstein D. M., Magasanik B. Complex glnA-glnL-glnG operon of Escherichia coli. J Bacteriol. 1982 Apr;150(1):202–213. doi: 10.1128/jb.150.1.202-213.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahel G., Tyler B. A new glnA-linked regulatory gene for glutamine synthetase in Escherichia coli. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4544–4548. doi: 10.1073/pnas.76.9.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahel G., Zelenetz A. D., Tyler B. M. gltB gene and regulation of nitrogen metabolism by glutamine synthetase in Escherichia coli. J Bacteriol. 1978 Jan;133(1):139–148. doi: 10.1128/jb.133.1.139-148.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platko J. V., Calvo J. M. Mutations affecting the ability of Escherichia coli Lrp to bind DNA, activate transcription, or respond to leucine. J Bacteriol. 1993 Feb;175(4):1110–1117. doi: 10.1128/jb.175.4.1110-1117.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platko J. V., Willins D. A., Calvo J. M. The ilvIH operon of Escherichia coli is positively regulated. J Bacteriol. 1990 Aug;172(8):4563–4570. doi: 10.1128/jb.172.8.4563-4570.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quay S. C., Dick T. E., Oxender D. L. Role of transport systems in amino acid metabolism: leucine toxicity and the branched-chain amino acid transport systems. J Bacteriol. 1977 Mar;129(3):1257–1265. doi: 10.1128/jb.129.3.1257-1265.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmanian M., Claus D. R., Oxender D. L. Multiplicity of leucine transport systems in Escherichia coli K-12. J Bacteriol. 1973 Dec;116(3):1258–1266. doi: 10.1128/jb.116.3.1258-1266.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmanian M., Oxender D. L. Derepressed leucine transport activity in Escherichia coli. J Supramol Struct. 1972;1(1):55–59. doi: 10.1002/jss.400010108. [DOI] [PubMed] [Google Scholar]
- Ravnikar P. D., Somerville R. L. Genetic characterization of a highly efficient alternate pathway of serine biosynthesis in Escherichia coli. J Bacteriol. 1987 Jun;169(6):2611–2617. doi: 10.1128/jb.169.6.2611-2617.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravnikar P. D., Somerville R. L. Structural and functional analysis of a cloned segment of Escherichia coli DNA that specifies proteins of a C4 pathway of serine biosynthesis. J Bacteriol. 1987 Oct;169(10):4716–4721. doi: 10.1128/jb.169.10.4716-4721.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex J. H., Aronson B. D., Somerville R. L. The tdh and serA operons of Escherichia coli: mutational analysis of the regulatory elements of leucine-responsive genes. J Bacteriol. 1991 Oct;173(19):5944–5953. doi: 10.1128/jb.173.19.5944-5953.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricca E., Aker D. A., Calvo J. M. A protein that binds to the regulatory region of the Escherichia coli ilvIH operon. J Bacteriol. 1989 Mar;171(3):1658–1664. doi: 10.1128/jb.171.3.1658-1664.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricca E., Lago C. T., Sacco M., De Felice M. Absence of acetohydroxy acid synthase III in Salmonella typhimurium is due to early termination of translation within the ilvl gene. Mol Microbiol. 1991 Jul;5(7):1741–1743. doi: 10.1111/j.1365-2958.1991.tb01923.x. [DOI] [PubMed] [Google Scholar]
- Roosendaal E., Boots M., de Graaf F. K. Two novel genes, fanA and fanB, involved in the biogenesis of K99 fimbriae. Nucleic Acids Res. 1987 Aug 11;15(15):5973–5984. doi: 10.1093/nar/15.15.5973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russel M., Model P. Sequence of thioredoxin reductase from Escherichia coli. Relationship to other flavoprotein disulfide oxidoreductases. J Biol Chem. 1988 Jun 25;263(18):9015–9019. [PubMed] [Google Scholar]
- Sacco M., Ricca E., Marasco R., Paradiso R., De Felice M. A stereospecific alignment between the promoter and the cis-acting sequence is required for Lrp-dependent activation of ilvIH transcription in Escherichia coli. FEMS Microbiol Lett. 1993 Mar 1;107(2-3):331–336. doi: 10.1111/j.1574-6968.1993.tb06053.x. [DOI] [PubMed] [Google Scholar]
- Satishchandran C., Taylor J. C., Markham G. D. Isozymes of S-adenosylmethionine synthetase are encoded by tandemly duplicated genes in Escherichia coli. Mol Microbiol. 1993 Aug;9(4):835–846. doi: 10.1111/j.1365-2958.1993.tb01742.x. [DOI] [PubMed] [Google Scholar]
- Satishchandran C., Taylor J. C., Markham G. D. Novel Escherichia coli K-12 mutants impaired in S-adenosylmethionine synthesis. J Bacteriol. 1990 Aug;172(8):4489–4496. doi: 10.1128/jb.172.8.4489-4496.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saukkonen K. M., Nowicki B., Leinonen M. Role of type 1 and S fimbriae in the pathogenesis of Escherichia coli O18:K1 bacteremia and meningitis in the infant rat. Infect Immun. 1988 Apr;56(4):892–897. doi: 10.1128/iai.56.4.892-897.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmoll T., Morschhäuser J., Ott M., Ludwig B., van Die I., Hacker J. Complete genetic organization and functional aspects of the Escherichia coli S fimbrial adhesion determinant: nucleotide sequence of the genes sfa B, C, D, E, F. Microb Pathog. 1990 Nov;9(5):331–343. doi: 10.1016/0882-4010(90)90067-z. [DOI] [PubMed] [Google Scholar]
- Sella C., Weinstock O., Barak Z., Chipman D. M. Subunit association in acetohydroxy acid synthase isozyme III. J Bacteriol. 1993 Sep;175(17):5339–5343. doi: 10.1128/jb.175.17.5339-5343.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaibe E., Metzer E., Halpern Y. S. Control of utilization of L-arginine, L-ornithine, agmatine, and putrescine as nitrogen sources in Escherichia coli K-12. J Bacteriol. 1985 Sep;163(3):938–942. doi: 10.1128/jb.163.3.938-942.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaibe E., Metzer E., Halpern Y. S. Metabolic pathway for the utilization of L-arginine, L-ornithine, agmatine, and putrescine as nitrogen sources in Escherichia coli K-12. J Bacteriol. 1985 Sep;163(3):933–937. doi: 10.1128/jb.163.3.933-937.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. W., Stine W. B., Svitil A. L., Bakker A., Zyskind J. W. Escherichia coli cells lacking methylation-blocking factor (leucine-responsive regulatory protein) have precise timing of initiation of DNA replication in the cell cycle. J Bacteriol. 1992 May;174(9):3078–3082. doi: 10.1128/jb.174.9.3078-3082.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires C. H., De Felice M., Devereux J., Calvo J. M. Molecular structure of ilvIH and its evolutionary relationship to ilvG in Escherichia coli K12. Nucleic Acids Res. 1983 Aug 11;11(15):5299–5313. doi: 10.1093/nar/11.15.5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires C. H., De Felice M., Lago C. T., Calvo J. M. IlvHI locus of Salmonella typhimurium. J Bacteriol. 1983 Jun;154(3):1054–1063. doi: 10.1128/jb.154.3.1054-1063.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires C. H., De Felice M., Wessler S. R., Calvo J. M. Physical characterization of the ilvHI operon of Escherichia coli K-12. J Bacteriol. 1981 Sep;147(3):797–804. doi: 10.1128/jb.147.3.797-804.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiert J. G., Rolfes R. J., Zalkin H., Stauffer G. V. Regulation of the Escherichia coli glyA gene by the purR gene product. J Bacteriol. 1990 Jul;172(7):3799–3803. doi: 10.1128/jb.172.7.3799-3803.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern M. J., Higgins C. F., Ames G. F. Isolation and characterization of lac fusions to two nitrogen-regulated promoters. Mol Gen Genet. 1984;195(1-2):219–227. doi: 10.1007/BF00332750. [DOI] [PubMed] [Google Scholar]
- Stock J. B., Ninfa A. J., Stock A. M. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol Rev. 1989 Dec;53(4):450–490. doi: 10.1128/mr.53.4.450-490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H. S., Lang B. F., Newman E. B. L-serine degradation in Escherichia coli K-12: cloning and sequencing of the sdaA gene. J Bacteriol. 1989 Sep;171(9):5095–5102. doi: 10.1128/jb.171.9.5095-5102.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton A., Freundlich M. Regulation of cyclic AMP of the ilvB-encoded biosynthetic acetohydroxy acid synthase in Escherichia coli K-12. Mol Gen Genet. 1980 Apr;178(1):179–183. doi: 10.1007/BF00267227. [DOI] [PubMed] [Google Scholar]
- Tartaglia L. A., Gimeno C. J., Storz G., Ames B. N. Multidegenerate DNA recognition by the OxyR transcriptional regulator. J Biol Chem. 1992 Jan 25;267(3):2038–2045. [PubMed] [Google Scholar]
- Travers A. A. DNA conformation and protein binding. Annu Rev Biochem. 1989;58:427–452. doi: 10.1146/annurev.bi.58.070189.002235. [DOI] [PubMed] [Google Scholar]
- Tsai S. Y., Tsai M. J., O'Malley B. W. Cooperative binding of steroid hormone receptors contributes to transcriptional synergism at target enhancer elements. Cell. 1989 May 5;57(3):443–448. doi: 10.1016/0092-8674(89)90919-7. [DOI] [PubMed] [Google Scholar]
- Tuan L. R., D'Ari R., Newman E. B. The leucine regulon of Escherichia coli K-12: a mutation in rblA alters expression of L-leucine-dependent metabolic operons. J Bacteriol. 1990 Aug;172(8):4529–4535. doi: 10.1128/jb.172.8.4529-4535.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursini M. V., Arcari P., De Felice M. Acetohydroxy acid synthase isoenzymes of Escherichia coli K-12: a trans-acting regulatory locus of ilvHI gene expression. Mol Gen Genet. 1981;181(4):491–496. doi: 10.1007/BF00428741. [DOI] [PubMed] [Google Scholar]
- Wang Q., Calvo J. M. Lrp, a global regulatory protein of Escherichia coli, binds co-operatively to multiple sites and activates transcription of ilvIH. J Mol Biol. 1993 Jan 20;229(2):306–318. doi: 10.1006/jmbi.1993.1036. [DOI] [PubMed] [Google Scholar]
- Wang Q., Calvo J. M. Lrp, a major regulatory protein in Escherichia coli, bends DNA and can organize the assembly of a higher-order nucleoprotein structure. EMBO J. 1993 Jun;12(6):2495–2501. doi: 10.1002/j.1460-2075.1993.tb05904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Sacco M., Ricca E., Lago C. T., De Felice M., Calvo J. M. Organization of Lrp-binding sites upstream of ilvIH in Salmonella typhimurium. Mol Microbiol. 1993 Mar;7(6):883–891. doi: 10.1111/j.1365-2958.1993.tb01179.x. [DOI] [PubMed] [Google Scholar]
- Wang Q., Wu J., Friedberg D., Plakto J., Calvo J. M. Regulation of the Escherichia coli lrp gene. J Bacteriol. 1994 Apr;176(7):1831–1839. doi: 10.1128/jb.176.7.1831-1839.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner B. L., Kodaira R., Neidhardt F. C. Physiological regulation of a decontrolled lac operon. J Bacteriol. 1977 Apr;130(1):212–222. doi: 10.1128/jb.130.1.212-222.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weichart D., Lange R., Henneberg N., Hengge-Aronis R. Identification and characterization of stationary phase-inducible genes in Escherichia coli. Mol Microbiol. 1993 Oct;10(2):407–420. [PubMed] [Google Scholar]
- Wessler S. R., Calvo J. M. Control of leu operon expression in Escherichia coli by a transcription attenuation mechanism. J Mol Biol. 1981 Jul 15;149(4):579–597. doi: 10.1016/0022-2836(81)90348-x. [DOI] [PubMed] [Google Scholar]
- Willins D. A., Calvo J. M. In vitro transcription from the Escherichia coli ilvIH promoter. J Bacteriol. 1992 Dec;174(23):7648–7655. doi: 10.1128/jb.174.23.7648-7655.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willins D. A., Ryan C. W., Platko J. V., Calvo J. M. Characterization of Lrp, and Escherichia coli regulatory protein that mediates a global response to leucine. J Biol Chem. 1991 Jun 15;266(17):10768–10774. [PubMed] [Google Scholar]
- Wilson R. L., Stauffer L. T., Stauffer G. V. Roles of the GcvA and PurR proteins in negative regulation of the Escherichia coli glycine cleavage enzyme system. J Bacteriol. 1993 Aug;175(16):5129–5134. doi: 10.1128/jb.175.16.5129-5134.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R. L., Steiert P. S., Stauffer G. V. Positive regulation of the Escherichia coli glycine cleavage enzyme system. J Bacteriol. 1993 Feb;175(3):902–904. doi: 10.1128/jb.175.3.902-904.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim H. H., Villarejo M. osmY, a new hyperosmotically inducible gene, encodes a periplasmic protein in Escherichia coli. J Bacteriol. 1992 Jun;174(11):3637–3644. doi: 10.1128/jb.174.11.3637-3644.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaboura M., Halpern Y. S. Regulation of gamma-aminobutyric acid degradation in Escherichia coli by nitrogen metabolism enzymes. J Bacteriol. 1978 Feb;133(2):447–451. doi: 10.1128/jb.133.2.447-451.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieg J., Simon M. Analysis of the nucleotide sequence of an invertible controlling element. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4196–4200. doi: 10.1073/pnas.77.7.4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Felice M., Lago C. T., Squires C. H., Calvo J. M. Acetohydroxy acid synthase isoenzymes of Escherichia coli K12 and Salmonella typhimurium. Ann Microbiol (Paris) 1982 Mar-Apr;133(2):251–256. [PubMed] [Google Scholar]
- de Graaf F. K., Klaasen-Boor P., van Hees J. E. Biosynthesis of the K99 surface antigen is repressed by alanine. Infect Immun. 1980 Oct;30(1):125–128. doi: 10.1128/iai.30.1.125-128.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Woude M. W., Braaten B. A., Low D. A. Evidence for global regulatory control of pilus expression in Escherichia coli by Lrp and DNA methylation: model building based on analysis of pap. Mol Microbiol. 1992 Sep;6(17):2429–2435. doi: 10.1111/j.1365-2958.1992.tb01418.x. [DOI] [PubMed] [Google Scholar]
- van der Woude M. W., Low D. A. Leucine-responsive regulatory protein and deoxyadenosine methylase control the phase variation and expression of the sfa and daa pili operons in Escherichia coli. Mol Microbiol. 1994 Feb;11(4):605–618. doi: 10.1111/j.1365-2958.1994.tb00340.x. [DOI] [PubMed] [Google Scholar]


