Photosynthesis is the basis of plant growth, and it is argued that improving photosynthesis can contribute toward greater food security in the coming decades as world population increases.
Abstract
Photosynthesis is the basis of plant growth, and improving photosynthesis can contribute toward greater food security in the coming decades as world population increases. Multiple targets have been identified that could be manipulated to increase crop photosynthesis. The most important target is Rubisco because it catalyses both carboxylation and oxygenation reactions and the majority of responses of photosynthesis to light, CO2, and temperature are reflected in its kinetic properties. Oxygenase activity can be reduced either by concentrating CO2 around Rubisco or by modifying the kinetic properties of Rubisco. The C4 photosynthetic pathway is a CO2-concentrating mechanism that generally enables C4 plants to achieve greater efficiency in their use of light, nitrogen, and water than C3 plants. To capitalize on these advantages, attempts have been made to engineer the C4 pathway into C3 rice (Oryza sativa). A simpler approach is to transfer bicarbonate transporters from cyanobacteria into chloroplasts and prevent CO2 leakage. Recent technological breakthroughs now allow higher plant Rubisco to be engineered and assembled successfully in planta. Novel amino acid sequences can be introduced that have been impossible to reach via normal evolution, potentially enlarging the range of kinetic properties and breaking free from the constraints associated with covariation that have been observed between certain kinetic parameters. Capturing the promise of improved photosynthesis in greater yield potential will require continued efforts to improve carbon allocation within the plant as well as to maintain grain quality and resistance to disease and lodging.
Photosynthesis is the process plants use to capture energy from sunlight and convert it into biochemical energy, which is subsequently used to support nearly all life on Earth. Plant growth depends on photosynthesis, but it is simplistic to think that growth rate directly reflects photosynthetic rate. Continued growth requires the acquisition of water and nutrients in addition to light and CO2 and, in many cases, involves competition with neighboring plants. Biomass must be invested by the plant to acquire these resources, and respiration is necessary to maintain all the living cells in a plant. Photosynthetic rate is typically measured by enclosing part of a leaf in a chamber, but to understand growth, one needs to consider the daily integral of photosynthetic uptake by the whole plant or community and how it is allocated. Almost inevitably, changing photosynthesis in some way requires more resources. Consequently, in order to improve photosynthesis, one needs to consider the tradeoffs elsewhere in the system. The title, “Improving Photosynthesis,” could be interpreted in many ways. For this review, I am restricting the scope to focus on crop species growing under favorable conditions.
To support the forecast growth in human population, large increases in crop yields will be required (Reynolds et al., 2011; Ziska et al., 2012). Dramatic increases in yield were achieved by the Green Revolution through the introduction of dwarfing genes into the most important C3 cereal crops rice (Oryza sativa) and wheat (Triticum aestivum). This allowed greater use of fertilizer, particularly nitrogen, without the risk of lodging, where the canopy collapses under the weight of the grain, causing significant yield losses (Stapper and Fischer, 1990). It also meant that biomass allocation within the plant could be altered to increase grain mass at the expense of stem mass now that the plants were shorter. Retrospective comparisons of cultivars released over time, but grown concurrently under favorable conditions with weed, pest, and disease control and physical support to prevent lodging, reveal that while modern cultivars yield more grain, they have similar total aboveground biomass (Austin et al., 1980, 1989).
It is interesting to revisit the review by Gifford and Evans (1981): “over the course of evolution from the wild plant to modern cultivar, carbon partitioning was improved. Thus, as remaining scope for further improvement in carbon allocation must be small, it would be better to aim at increasing photosynthetic and growth rates. Alternatively, as partitioning is where flexibility has been manipulated in the past, it is better to aim for further increases in harvest index.” Just over 30 years have passed since this was published, and yield gains made by plant breeders have continued to come largely from increasing carbon allocation into grain (Fischer and Edmeades, 2010) and selecting for increased early vigor (Richards et al., 2010). By contrast, selection based on improving photosynthesis has yet to be achieved. Plants need leaves and roots to capture light, water, and nutrients for growth and stems to form the leaf canopy and support the flowers and grain, so further increases in harvest index may lead to a decrease in yield. Therefore, in order to increase yield potential further, it is necessary to increase total biomass. If light interception through the growing season is already fully exploited, then increasing biomass requires that photosynthesis be increased. It is the realization that further significant increases in yield potential will not be possible by continuing the current strategy that has turned attention toward improving photosynthesis. Recent technological developments now provide us with the means to engineer changes to photosynthesis that would not have been possible previously.
THE C3 AND C4 PHOTOSYNTHETIC PATHWAYS
There are few differences in photosynthetic properties between terrestrial plant species in comparison with the diversity in plant form that has evolved in order to exploit the range of ecosystems around the world. The most important difference occurs in the CO2 fixation pathway. All plants catalyze the fixation of CO2 into a stable three-carbon intermediate with a carboxylase enzyme called Rubisco. Rubisco is a bifunctional enzyme that also catalyzes a reaction with oxygen that diminishes the overall efficiency of photosynthesis. When oxygenic photosynthesis evolved, this was not a problem, because the atmosphere was rich in CO2 with little oxygen. However, over time, photosynthesis transformed the atmosphere to its present state, rich in oxygen with only a trace of CO2. Two strategies evolved to deal with the increasing oxygen-CO2 ratio. First, Rubisco kinetic properties changed to improve its ability to distinguish between CO2 and oxygen. Second, CO2-concentrating mechanisms evolved to allow Rubisco to operate in a CO2-rich space. The CO2-concentrating mechanism has evolved multiple times among terrestrial plant species but always involves phosphoenolpyruvate (PEP) carboxylase fixing bicarbonate into a four-carbon acid (Sage et al., 2012). This gives rise to the descriptive term C4 plants.
C4 plants have several advantages over C3 plants. First, by concentrating CO2, Rubisco carboxylation reactions are increased relative to oxygenation, which results in more CO2 being fixed per photon absorbed in C4 leaves than in C3 leaves (Ehleringer and Pearcy, 1983; Skillman, 2008). Second, raising the CO2 partial pressure around Rubisco means it operates at close to its maximum catalytic rate, so to achieve a given CO2 assimilation rate requires a smaller investment of protein into Rubisco. As the protein cost of the C4 cycle is considerably less than the saving in Rubisco, this results in C4 leaves having greater CO2 assimilation rates per unit of leaf nitrogen than C3 leaves. Third, PEP carboxylase utilizes bicarbonate formed by carbonic anhydrase rather than CO2. PEP carboxylase has a strong affinity for bicarbonate (27 µm for the C4 PEP carboxylase [Bauwe, 1986], which equates to an intercellular partial pressure of CO2 of 80 µbar [von Caemmerer, 2000]). Therefore, PEP carboxylase can achieve rates sufficient to satisfy the C4 pump at intercellular CO2 partial pressures much less than that found in C3 leaves. Typically, C4 leaves operate with a ratio of intercellular-to-ambient CO2 partial pressure of around 0.3 compared with C3 leaves, which operate around 0.7 under high irradiance (Wong et al., 1985). Consequently, C4 plants have greater transpiration efficiency, gaining more carbon per unit of water transpired than C3 plants. The combination of these three attributes means that C4 plants fix more carbon per unit of light, per unit of nitrogen, and per unit of water than C3 plants in many situations (Ghannoum et al., 2011). Why, then, have C4 plants not taken over the world? As the C4 pathway is virtually absent from woody plants, they are unable to displace forest biomes apart from resorting to fire (Osborne, 2011). With a few exceptions, C4 plants are also less competitive in colder climates.
TARGETS THAT HAVE BEEN IDENTIFIED TO IMPROVE PHOTOSYNTHESIS
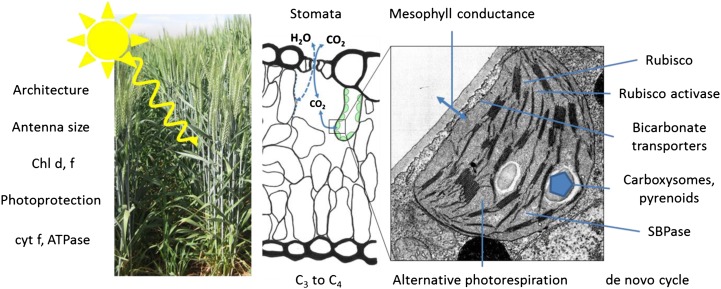
The concept of improving photosynthesis to raise crop yields has led to a number of recent reviews. Long et al. (2006) posed the question of whether improving photosynthesis could increase yields. The targets they identified (together with more recent reviews), in order of decreasing importance, were (1) improving Rubisco kinetic properties (Whitney et al., 2011a; Parry et al., 2013), (2) introduction of the C4 pathway into C3 crops (Gowik and Westhoff, 2011), (3) more rapid relaxation from photoprotection (Murchie and Niyogi, 2011), (4) increased activity of sedoheptulose bisphosphatase (Raines, 2011), and (5) improved canopy architecture. Others have proposed several additional targets, which are summarized in Figure 1. To improve light penetration into leaves and canopies and reduce light saturation, reducing photosystem antenna size has been suggested (Ort et al., 2011). Additional light could be captured by extending the waveband of sunlight available for photosynthesis by transferring cyanobacterial chlorophyll d and f into higher plant pigment-protein complexes (Chen and Blankenship, 2011) and is discussed in the last section of this review. Electron transport capacity could be increased by increasing cytochrome f content, and this may also require ATP synthase content to be increased (von Caemmerer and Evans, 2010). There are several ways to manipulate the process of CO2 uptake from the atmosphere. The classical approach has been to change stomatal conductance (Condon et al., 1990; Rebetzke et al., 2012). However, there is also scope to alter mesophyll conductance with the strategy dependent upon whether a CO2-concentrating mechanism is also being attempted. After many years of fundamental discovery, enough was known about carbon-concentrating mechanisms in cyanobacteria that Price et al. (2008) proposed that bicarbonate pumps should be engineered into the chloroplast envelope of C3 plants. To realize the full potential of these bicarbonate pumps, carboxysomes (Price et al., 2013; Zarzycki et al., 2013) or pyrenoids (Meyer and Griffiths, 2013) would need to be introduced into chloroplasts to enable CO2 to be concentrated around Rubisco. The tolerance of higher temperatures by photosynthesis could be increased by improving the thermal stability of Rubisco activase (Salvucci and Crafts-Brandner, 2004; Parry et al., 2011). Redesigning photorespiration has been attempted and could be refined (Peterhansel et al., 2008, 2013; Peterhansel and Maurino, 2011). The drastic option of starting again and designing a CO2-fixing pathway de novo from existing metabolic reactions has been explored (Bar-Even et al., 2012).
Figure 1.
Targets for improving photosynthesis. Processes associated with light capture from the canopy to the thylakoid membranes are shown on the left side, while conductances for CO2 diffusion are shown along the top. Chloroplast processes, dominated by Rubisco, other limiting enzymes, and CO2-concentrating mechanism components are shown on the right side. Complex traits where metabolic pathways are modified are shown along the bottom. SBPase, Sedoheptulose bisphosphatase.
The potential gains associated with each target differ and are not mutually exclusive. Greater benefits could be expected by combining them, but we currently lack quantitative assessment from crop models of the potential impact that would follow from manipulating these targets. Moreover, the technical difficulty of achieving each target varies widely. Until proof of concept can be demonstrated either in a model plant or the desired crop plant, prioritizing the choice of target is a gamble. There is merit in focusing effort into a few options when funding is limited, but it can also be argued that a diverse portfolio may achieve breakthroughs sooner. The main limitation on progress at present is raising sufficient funds to tackle these complex tasks. As Rowan Sage pointed out at the 2009 American Society of Plant Biologists Meeting (C3 to C4: Evaluating Strategies for Engineering C4 Photosynthesis into C3 Plants, July 18-22, Honolulu, HI), the amount of money required to significantly advance research toward these targets is less than a single next-generation jet fighter. We need to persuade governments that food security is a cheaper and more moral option than military investment.
Rice is a C3 cereal grown in warm climates where the C4 pathway should be superior. Using this logic, Sheehy et al. (2000) challenged the scientific community to consider the consequences of engineering the C4 pathway into rice. Following a second conference at the International Rice Research Institute (Sheehy et al., 2007b), the C4 rice consortium gained financial support from the Bill and Melinda Gates Foundation (http://c4rice.irri.org/). Objectives and progress can be found in Langdale (2011) and von Caemmerer et al. (2012). A complementary consortium to the C4 rice consortium subsequently formed to focus on increasing wheat yields by targeting photosynthesis and partitioning (Reynolds et al., 2009, 2011). This has yet to gain sufficient financial support to really progress. In December 2012, the Bill and Melinda Gates Foundation funded a second program “realizing increased photosynthetic efficiency,” with seven objectives: CO2-concentrating mechanism, photorespiratory bypass, transplanting better Rubisco, improving the photosynthetic carbon reduction cycle (e.g. sedoheptulose bisphosphatase), improving canopy light distribution, crop modeling, and plastid transformation.
DOES SOURCE OR SINK LIMIT PLANT GROWTH?
On paper, the proposition that increasing photosynthesis should lead to increases in yield seems straightforward. However, there is a perennial debate about whether plant growth is limited by the source (photosynthesis) or the sink (demand by new vegetative growth or developing grain). Given the length of time this argument has been running, one should conclude that both are important and need to be considered. This review focuses on photosynthesis, but sinks have been discussed elsewhere (Gifford and Evans, 1981; Foulkes et al., 2011; Reynolds et al., 2011). The justification that increasing photosynthesis will lead to increasing yield potential is based on two facts. One, C4 crops convert sunlight into biomass with a greater efficiency than C3 crops (Sheehy et al., 2007a). This is achieved by concentrating CO2 around Rubisco, which suppresses the oxygenase reaction. Two, enriching the atmosphere with CO2 leads to greater growth and yields of C3 plants (Kimball, 1983; Kimball et al., 2002; Long et al., 2004; Ainsworth and Long, 2005) due to increased rates of CO2 assimilation combined with a decrease in the rate of the oxygenase reaction of Rubisco and subsequent photorespiration. Both of these examples prove that photosynthesis can be increased by reducing flux through the oxygenase reaction. This increase translates into greater biomass and yield, allowing one to estimate the magnitude of potential gains for C3 plants. These two facts also explain why target 2, introducing the C4 pathway into a C3 crop, made the most persuasive case for financial support.
PHOTOSYNTHESIS IN THE CONTEXT OF THE CANOPY
Manipulating photosynthesis at the chloroplast or leaf level will only be beneficial if it confers an improvement at the level of the plant canopy. Once canopy closure occurs and plants are intercepting all available sunlight, the challenge is to convert that into biomass with the greatest efficiency. At low irradiance, this reflects the maximum photon yield of photosynthesis (mol CO2 fixed per mol PAR photon absorbed, where PAR is photosynthetically active radiation, defined as the waveband 400–700 nm on the basis of extensive research by McCree [1971]). At high irradiance, photosynthetic capacity limits the rate of CO2 assimilation and photon yield declines. The loss of efficiency at high irradiance by a single leaf can be reduced in a plant canopy by distributing light capture between leaves. This results in more leaf area operating with greater efficiency at low to intermediate irradiance and less leaf area operating with lower efficiency at high irradiance. To effectively utilize a higher leaf area per unit of ground area, leaves at the top of the canopy need to be held more erect. Leaf number, size, and angle can all be manipulated and interact with canopy height. To assess the impact and relative importance of changing any aspect requires comparisons with near isogenic lines, but ideotypes can be explored with canopy models (Song et al., 2013).
The gain in efficiency at the canopy level comes at a cost of producing and maintaining multiple leaves and the support structures required to display them. Alternatively, when viewed from a nitrogen perspective, rather than a cost perspective, it may be a necessary step enabling the plant to amass sufficient nitrogen to satisfy the demand set by grain protein at maturity (Sinclair and Sheehy, 1999). A late application of nitrogen fertilizer enables continued nitrogen uptake and incorporation into protein after flowering and has been shown to increase grain protein concentrations. However, at low rates of nitrogen fertilizer application, grain yield was reduced by splitting the fertilizer into two applications (Wuest and Cassman, 1992). Whether it is economic for the farmer to carry out a late application of nitrogen fertilizer depends on the price incentives for wheat quality (Angus and Fischer, 1991) and the climate and agronomic situation. Rising atmospheric CO2 concentrations are likely to increase grain yield but reduce grain protein concentration (Lam et al., 2012). Comparison between elite wheat cultivars reveals a tradeoff between yield and grain protein concentration (Barraclough et al., 2010). To achieve higher yield with a high grain protein concentration requires ever greater rates of nitrogen fertilizer. As this can be associated with negative environmental consequences, the nitrogen perspective raises a series of interacting issues that need to be managed.
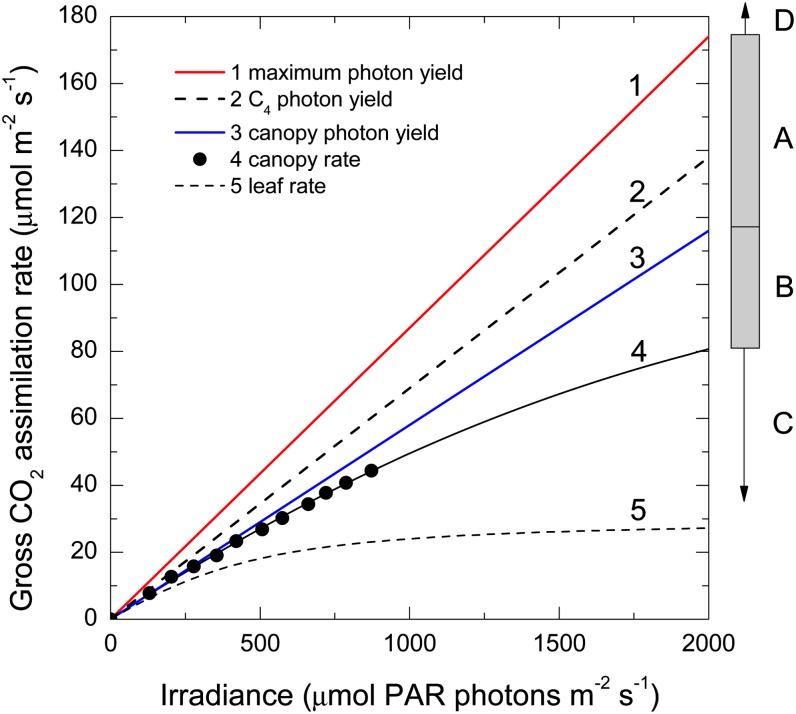
The response of CO2 assimilation rate to irradiance is shown for a wheat leaf and wheat crop canopy (Fig. 2). The upper bound (line 1) represents the maximum photon yield of C3 photosynthesis in the absence of photorespiration. This value is actually unclear, as not only do experimental values differ between studies (0.081 [Ehleringer and Pearcy, 1983], 0.106 [Björkman and Demmig, 1987], and 0.089 [Evans, 1987] mol CO2 per mol PAR photons absorbed), but the theoretical value is also uncertain. Uncertainty in the number of protons required to synthesize each ATP and in the cyclic electron transport pathways and electron flux though them prevents one being able to give definitive theoretical values (Kramer and Evans, 2011). Photon yield for C4 photosynthesis (line 2) is less than that for C3 photosynthesis in the absence of photorespiration, because the C4 cycle requires the equivalent of two ATPs for each CO2 it releases into the bundle sheath, and 20% to 30% of these CO2 leak back into the surrounding mesophyll without being captured by Rubisco. Although the estimation and interpretation of leakiness get complicated at low light (Henderson et al., 1992; Tazoe et al., 2008; Ubierna et al., 2011), maximum photon yield varies little between C4 species (Skillman, 2008). It is misleading to suggest that increased leakiness at low light reduces the photosynthetic efficiency of a C4 canopy as it grows and has more shaded leaves (Kromdijk et al., 2008). While leakiness decreases as irradiance increases, photon yield declines continuously with increasing irradiance, because other feedbacks are more important. In normal atmospheric conditions, the photon yield of CO2 assimilation in C3 photosynthesis is reduced because of competition by the oxygenase reaction (line 3). The equation describing this and more detailed discussion follow later.
Figure 2.
Canopy gross CO2 assimilation rate as a function of irradiance. Data measured with a wheat crop (Evans and Farquhar, 1991) are as follows: leaf area index, 7.1; leaf temperature, 22°C; ambient CO2 partial pressure, 340 µbar. Lines are as follows: 1, maximum photon yield for C3 plants in the absence of photorespiration (0.088 mol of CO2 per mol of PAR photons); 2, maximum photon yield for C4 plants (0.069); 3, photon yield for C3 plants in normal atmosphere (0.058); 4, response curve for gross canopy CO2 assimilation; 5, response curve for a single wheat leaf. Curve parameters are as follows: light-saturated gross CO2 assimilation rate, 135 or 30 µmol CO2 m−2 s−1 for the canopy and leaf, respectively; Θ (the convexity term), 0.7 using Equation 1a from Ögren and Evans (1993). On the right side, four regions for improvement are indicated: A, reducing photorespiration; B, increasing photosynthetic capacity; C, reducing losses due to non-steady-state conditions and sink limitations; D, increasing the PAR waveband.
The bars and arrows on the right side of Figure 2 represent areas where photosynthesis could be improved. Band A represents the loss associated with photorespiration in C3 plants and is widely accepted to be the best initial target to improve photosynthesis. Under ambient CO2 partial pressures, the CO2 assimilation rate of a single leaf (line 5) nears light saturation around 1,000 µmol PAR photons m−2 s−1, which is half of full sunlight. By contrast, the leaf canopy saturates much less (line 4), as in this example it has a 4.5-fold greater photosynthetic capacity per unit of ground area compared with the single leaf. Band B represents the gap between lines 3 and 4, where potential gains are available for improving photosynthesis through increasing photosynthetic capacity, efficiency, or improving canopy architecture. In order to quantify the gains, a canopy model calculating daily photosynthesis is required, which takes into account diurnal and seasonal variation in light and temperature. Such models exist with varying levels of complexity (DePury and Farquhar, 1997; Song et al., 2013), and there is a pressing need for them to be used to provide quantitative assessment of potential increases in biomass. This review avoids giving potential percentage gains because credible quantitative crop modeling is not yet available. Arrow C represents losses associated with the failure to achieve the potential steady-state rate. This can be due to inactivation of enzymes, photoprotection, stomatal closure, or sink limitations. Arrow D represents the potential to broaden the waveband capable of driving photosynthetic electron transport through the introduction of novel chlorophylls.
MODELING STEADY-STATE C3 PHOTOSYNTHESIS
Many of the characteristics of leaf photosynthesis can be represented in a model based on Rubisco biochemistry. The model of Farquhar et al. (1980) describes the rate of CO2 assimilation as being limited either by Rubisco and the supply of CO2 or the rate of regeneration of ribulose 1,5-bisphosphate (RuBP), the five-carbon substrate for Rubisco. The regeneration of RuBP consumes NADPH and ATP, which are produced by photosynthetic electron transport and photophosphorylation. The gross CO2 assimilation rate from a given rate of photosynthetic electron transport, J, depends on the balance between carboxylation and oxygenation reactions catalyzed by Rubisco (Farquhar and von Caemmerer, 1982):
where A is the rate of CO2 assimilation, R is the respiration rate excluding photorespiratory CO2 release, C is the partial pressure of CO2 within the chloroplasts, and Γ* is the CO2 compensation point in the absence of respiration given by:
where O is the partial pressure of oxygen, Vcmax and Vomax are the maximum rates of carboxylation and oxygenation, respectively, and Kc and Ko are the Michaelis-Menten constants for CO2 and oxygen, respectively. Γ* is inversely related to the specificity factor for Rubisco, Sc/o. This form of Equation 1 assumes that CO2 assimilation rate is limited by NADPH formation using linear electron transport, and its use is widespread (Sharkey et al., 2007). However, alternative forms can be used that assume that ATP formation is rate limiting (von Caemmerer and Farquhar, 1981). These depend on assumptions about the Q cycle, proton-to-ATP stoichiometry, and are complicated by the involvement of cyclic electron transport (von Caemmerer, 2000; Kramer and Evans, 2011).
Under conditions of high irradiance and low CO2 partial pressures, gross CO2 assimilation rate is given by:
and
where nR is the number of moles of Rubisco sites per unit of leaf area and kcat is the maximum catalytic turnover of carboxylase (mol CO2 mol−1 Rubisco sites s−1).
The C3 photosynthesis model of Farquhar et al. (1980) provides a quantitative framework for assessing the impact of changing kinetic parameters of Rubisco, the amount per unit of leaf area, the influence of CO2 partial pressure, light, and temperature, and RuBP regeneration rate, on the rate of CO2 assimilation.
RUBISCO KINETIC PARAMETERS
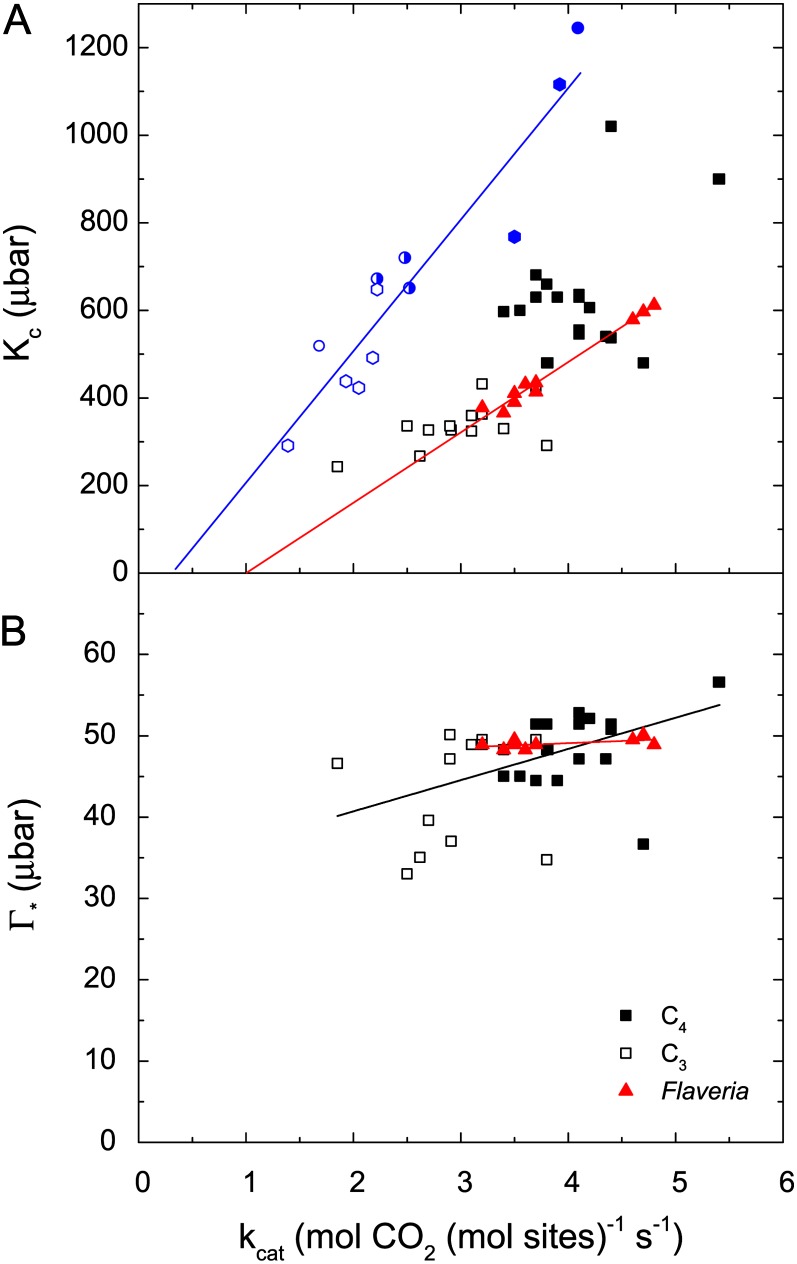
Despite the abundance of Rubisco and its importance in determining photosynthetic properties of a leaf, full characterization of Rubisco kinetic properties has been reported for only a few species. This reflects the difficulty in assaying both the carboxylase and oxygenase reactions. However, intriguing patterns are apparent. C3 plants contain Rubisco that has a higher affinity for CO2 (i.e. lower Kc) than C4 plants, which helps to minimize oxygenase reactions. By contrast, Rubisco in C4 plants exists in a high-CO2 environment, and selection pressure has favored an enzyme with a faster turnover rate (i.e. greater kcat). Possible improvements in Kc or kcat appear constrained by concomitant changes in the other parameter (Fig. 3). Rubisco from C3 species has lower Kc and kcat values than that from C4 species. When data from a single laboratory are considered in isolation, the covariation between Kc and kcat becomes more apparent. For example, Ishikawa et al. (2009) compared Rubisco properties from several cool- and warm-habitat C3 species against two C4 species (hollow and solid hexagons, respectively, in Fig. 3A). They also compared Rubisco from rice and sorghum (Sorghum bicolor) with that from rice transformed to produce a chimeric Rubisco with rice large subunits and sorghum small subunits (Ishikawa et al., 2011). Replacing the small subunits created a Rubisco with both Kc and kcat values shifted toward the C4 donor. When parameters for all Rubiscos from these two studies are compared together, the regression between Kc and kcat accounted for 87% of the variation. The kinetic properties of several transgenic Rubisco enzymes generated by Whitney et al. (2011b) provide a second example of covariation. The differences between the C3 and C4 forms of Rubisco from Flaveria spp. could be largely explained by a single amino acid change between Met-309 (C3 type with lower Kc and kcat) and Ile-309 (C4 type with higher Kc and kcat). In this case, the regression between Kc and kcat for the 10 Rubisco types that were characterized accounted for 97% of the variation (solid triangles, Fig. 3A). It is unclear whether the scatter in Figure 3A reflects uncertainty in assayed values or whether there truly is flexibility and independence in the relative changes between Kc and kcat that could be utilized.
Figure 3.
Comparison of Rubisco kinetic parameters from different species and transgenic constructs to examine whether parameters can vary independently. A, Kc versus kcat (25°C except for data from Ishikawa et al. [2009, 2011] shown in blue measured at 28°C). Due to the variability in assay results between studies, separate regressions are shown for subsets of species measured in blue (Kc = −97 + 303 kcat [r2 = 0.87]; Ishikawa et al., 2009, 2011) and in red (Kc = −156 + 160 kcat [r2 = 0.97]; Whitney et al., 2011b). B, Γ* (which is inversely related to the specificity factor; Eq. 2) versus kcat. The regression equations are Γ* = 33 + 3.8 kcat (r2 = 0.22; black line, all data) and Γ* = 47 + 0.53 kcat (r2 = 0.23; red line, Flaveria spp. data). Hollow and solid black squares represent C3 and C4 species, respectively, combined from Parry et al. (2011) and Whitney et al. (2011a); red solid triangles are from Whitney et al. (2011b); blue hollow and solid hexagons are C3 and C4 species, respectively, from Ishikawa et al. (2009); and blue circles are from Ishikawa et al. (2011) as follows: hollow circle, rice; solid circle, sorghum; half-solid circle, chimeric Rubisco with rice large subunits and sorghum small subunits.
The Sc/o (Eq. 2) has been determined for a diverse range of species from biochemical assays. Γ*, which is inversely related to Sc/o, is shown in relation to kcat (Fig. 3B), as this is the form in which the parameter usually appears in the C3 model of Farquhar et al. (1980). On average, Rubisco from C3 species has lower Γ* values than that from C4 species. Greater specificity reduces Γ* but is weakly related to slower kcat. However, for the transgenic enzymes based on C3 and C4 Flaveria spp. Rubisco, kcat varied but Γ* was unchanged (Whitney et al., 2011b). This suggests that it may be possible to reduce Γ* while maintaining a high kcat. A lower Γ* is beneficial because it reduces the flux through oxygenase, which increases photon yield. Increasing kcat reduces the cost of the enzyme, thereby requiring less investment in Rubisco (nR) to achieve a given carboxylase catalytic capacity, since Vcmax is the product of kcat and nR (Eq. 4).
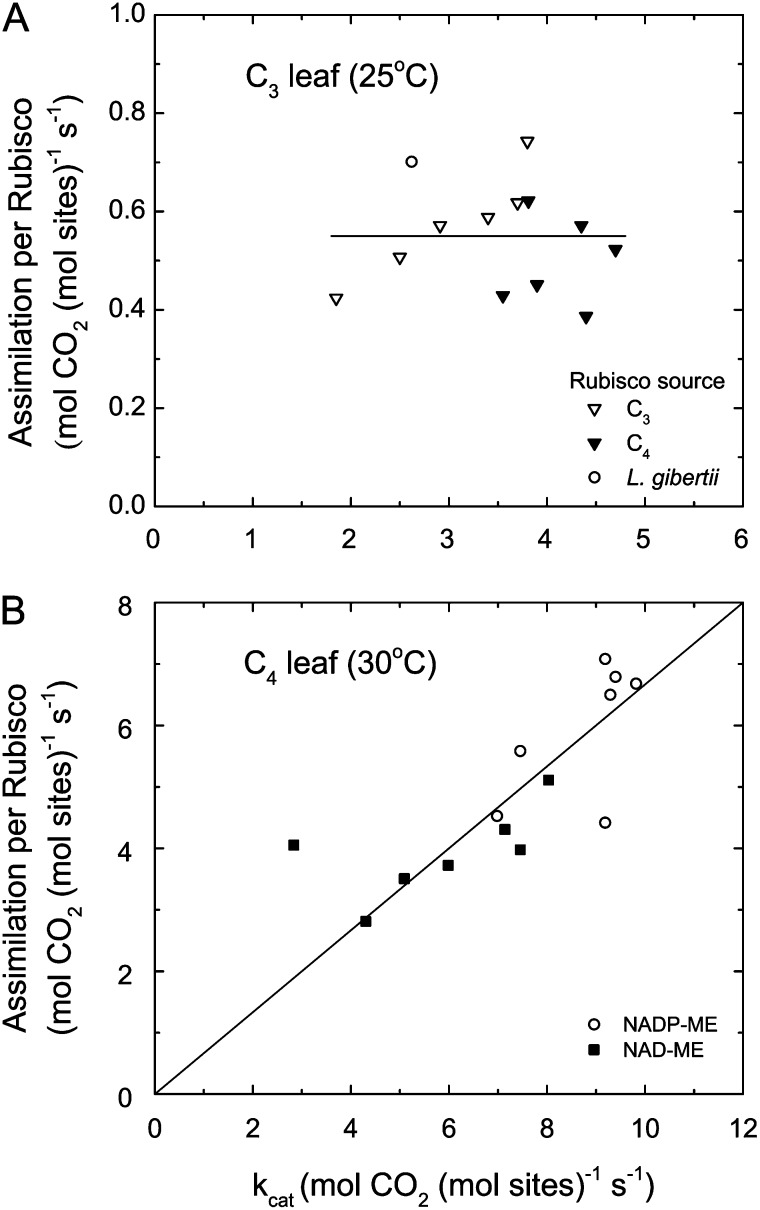
With a complete set of Rubisco kinetic parameters, it is possible to calculate potential gross CO2 assimilation rate when light is not limiting for a given amount of Rubisco, CO2, and oxygen partial pressures and temperature from Equation 3 (Fig. 4). The consequence of the apparent association between Kc and kcat shown in Figure 3A is that any benefit from increasing kcat is exactly canceled by poorer affinity, such that gross CO2 assimilation rate is independent of kcat. Engineering a C4 Rubisco into a C3 leaf would not change the CO2 assimilation rate under these conditions. While there are outliers such as Limonium gibertii (Galmes et al., 2005), it is unclear whether this represents a meaningful potential that could be captured, as it falls within the spread of values determined for wheat. By contrast, C4 plants show a clear benefit associated with having Rubisco with increased kcat (Ghannoum et al., 2005). When measured CO2 assimilation rates are expressed per unit of Rubisco for different C4 species, they vary in direct proportion to Rubisco kcat (Fig. 4B). Decarboxylation type appears to impose some constraint on how far Rubisco kinetic parameters changed from the C3 type. Species using NADP malic enzyme had greater kcat values than those using NAD malic enzyme. These two decarboxylation types differ in whether linear electron transport and oxygen evolution occur in the bundle sheath. By avoiding oxygen production in the bundle sheath, NADP malic enzyme species are able to capitalize on a faster Rubisco associated with a lower specificity factor without the risk of increasing oxygenase activity countering the gain.
Figure 4.
Relationships between CO2 assimilation rate per Rubisco site and Rubisco kcat for C3 and C4 species. A, Performance in a C3 leaf calculated using Rubisco kinetic parameters from Parry et al. (2011) at 25°C and assuming a partial pressure of CO2 in the chloroplast of 200 µbar (Eq. 3). B, Performance of C4 species recalculated from Ghannoum et al. (2005) at 30°C, differentiating between two decarboxylation subtypes. ME, Malic enzyme.
The current array of species for which complete Rubisco kinetic parameters exist is limited. Under high light, there appears to be no advantage in seeking a higher kcat, as this gain is completely offset by associated increases in the effective Kc in the presence of oxygen. Under low-light conditions, C3 photosynthesis benefits from Rubisco having a low Γ* value, as this reduces the loss associated with oxygenase activity. To gauge the usefulness of modifying Γ*, daily canopy photosynthesis needs to be simulated.
Despite the apparent lack of benefit to a C3 plant in substituting a C4 Rubisco type for a current C3 Rubisco, it is worthwhile widening the survey, because Rubisco plays such a pivotal role in determining the efficiency of leaf photosynthesis. To circumvent the difficulty in assaying Rubisco kinetic parameters, an alternative approach has been to analyze variation in DNA and amino acid sequences. Kapralov et al. (2012) applied a phylogenetic analysis of Rubisco DNA sequence to identify which amino acids were selected during the evolution of C4 photosynthesis in Amaranthaceae. Since this type of analysis relies on knowing that there are kinetic differences, it cannot be used to inform one of the properties of novel changes. The complexity of Rubisco transcription, modification, assembly, and catalysis challenges the use of rational design to engineer novel Rubisco with superior performance. Now that it is possible to generate novel Rubisco enzymes in tobacco (Nicotiana tabacum; Whitney and Sharwood, 2008; Whitney et al., 2009, 2011a) and rice (Ishikawa et al., 2011), once their kinetic properties have been measured, their potential for improving photosynthesis can be assessed. It is hoped that the currently known boundaries of Rubisco performance can then be expanded.
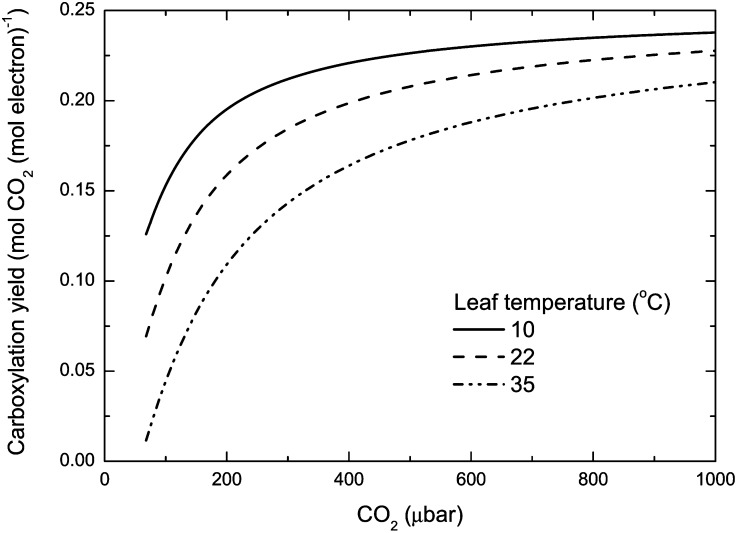
CARBOXYLATION YIELD
For most of each day, the majority of leaf area in a canopy operates under light limitation (DePury and Farquhar, 1997; Song et al., 2013). Under such conditions, it is appropriate to use Equation 1 to predict CO2 assimilation rates. The efficiency of photosynthesis is described by the balance between carboxylation and oxygenation reactions, which depends on Γ* and the partial pressure of CO2 inside the chloroplast, C (Fig. 5). For a given Γ*, carboxylation yield increases hyperbolically as C increases. C depends on the partial pressure of CO2 in the atmosphere, stomatal and mesophyll conductances, and CO2 assimilation rate. Human activity is increasing the atmospheric CO2 concentration through the exploitation of fossil fuel reserves and deforestation. The CO2 concentration has risen by over 70 μmol mol−1 in the last 50 years and passed 400 μmol mol−1 in May 2013 (http://www.esrl.noaa.gov/gmd/dv/iadv/). CO2 diffusion into leaves is restricted such that during photosynthesis, the CO2 partial pressure inside chloroplasts is less than in the surrounding atmosphere. Given the influence this has on carboxylation yield, manipulating stomatal and mesophyll conductance are obvious candidates for improving photosynthesis.
Figure 5.
Dependence of carboxylation yield on CO2 partial pressure and temperature. Carboxylation yield, ϕ (mol CO2 assimilated per mol electron in linear electron transport) is described by the function 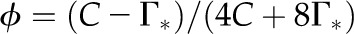 (Farquhar and von Caemmerer, 1982), where Γ* is the CO2 compensation point in the absence of day respiration (Eqs. 1 and 2). This equation assumes that NADPH regeneration limits photosynthesis. The temperature dependence of Γ* is taken from Brooks and Farquhar (1985).
(Farquhar and von Caemmerer, 1982), where Γ* is the CO2 compensation point in the absence of day respiration (Eqs. 1 and 2). This equation assumes that NADPH regeneration limits photosynthesis. The temperature dependence of Γ* is taken from Brooks and Farquhar (1985).
Stomatal conductance also affects the transpiration rate, so manipulating stomatal conductance would alter transpiration efficiency, the ratio of carbon gained to water transpired. Increasing stomatal conductance would be detrimental to productivity in dry environments but has been found to correlate with increased yields under well-watered conditions (Fischer et al., 1998). Consequently, efforts are being made to improve the monitoring of canopy temperatures, which provide a good proxy for comparing stomatal conductance between genotypes in field trials (Hackl et al., 2012; Maes and Steppe, 2012). This has been facilitated by developments in infrared cameras, which can capture multiple plots in a single image, enabling more reliable detection of canopy temperature differences between genotypes (Berni et al., 2009).
Mesophyll conductance depends on two leaf anatomical attributes, the surface area of mesophyll cells exposed to intercellular air space and the thickness of mesophyll cell walls (Evans et al., 1994, 2009; Tholen and Zhu, 2011; Tosens et al., 2012). In addition, the permeability of the plasma membrane and chloroplast envelope are also significant components. Evidence implicating a role for certain aquaporins as CO2 channels (Terashima and Ono, 2002) led to genetic engineering to manipulate HvPIP2;1 in rice (Hanba et al., 2004), NtAQP1 in tobacco (Flexas et al., 2006), and AtPIP1;2 in Arabidopsis (Arabidopsis thaliana; Uehlein et al., 2012). While reduction in aquaporin expression was associated with decreased mesophyll conductance, interpretation was not straightforward because of associated pleiotropic changes or technical challenges. For example, in rice, overexpression of HvPIP2;1 was accompanied by increased mesophyll cell wall thickness. Unfortunately, the measurement of membrane permeability to CO2 is difficult because it is at the limit of time resolution in stopped-flow assays. Interpretation is complicated when accounting for diffusion through unstirred layers and assumptions about whether CO2 or bicarbonate moves across the membrane. Although lipid bilayers are highly permeable to CO2, biological membranes are heavily populated by proteins, which greatly reduces the area available for CO2 diffusion through the lipids. Consequently, aquaporins may be necessary to facilitate CO2 diffusion across the plasma membrane and chloroplast envelope (Boron, 2010; Kaldenhoff, 2012). C4 leaves require a greater permeability per unit of mesophyll surface than C3 leaves, because C4 leaves have a smaller surface area of mesophyll cells exposed to intercellular air space per unit of leaf area and generally a greater rate of CO2 assimilation (Evans and von Caemmerer, 1996). Therefore, it is significant that comparative transcriptomics of C3 and C4 Cleome spp. revealed enhanced expression of the plasma membrane intrinsic protein AT2G45960 in the C4 Cleome gynandra (Bräutigam et al., 2011), as this could confer increased plasma membrane permeability to CO2 (Weber and von Caemmerer, 2010).
Membrane permeability to CO2 can be assessed by expressing aquaporins in systems such as Xenopus spp. oocytes. However, this may not properly reflect the situation in membranes within leaf mesophyll cells. Through the use of RNA interference and membrane permeability assays, Uehlein et al. (2008) demonstrated that aquaporins mainly affected the CO2 permeability of the chloroplast envelope. Although this result awaits independent confirmation, it is certainly encouraging for those attempting to engineer CO2-concentrating mechanisms into the chloroplast. Introducing functional cyanobacterial membrane transporters such as BicA and SbtA into chloroplast envelopes has been put forward as a simpler way to construct a CO2-concentrating mechanism (Price et al., 2008, 2011, 2013) than introducing the C4 cycle. In order for it to be really effective, it will be necessary to reduce CO2 leakage from the chloroplast. This could involve altering aquaporins to manipulate chloroplast envelope permeability or introducing other constraints such as carboxysomes to control the escape of CO2 by diffusion.
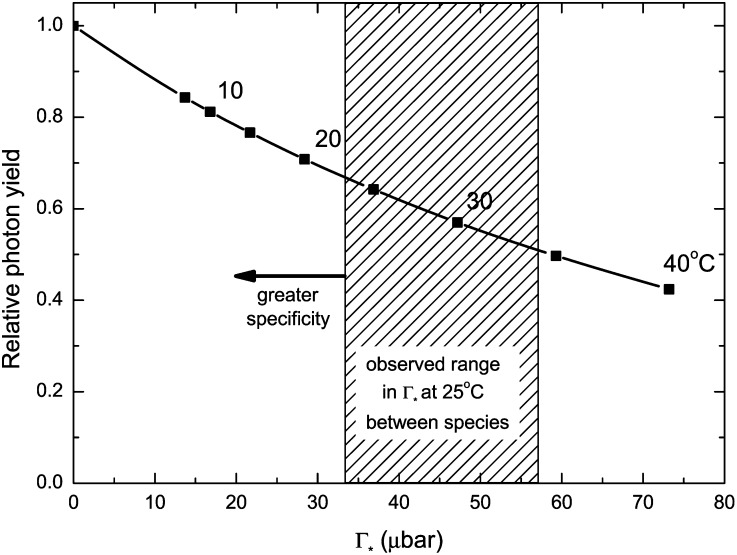
Carboxylation yield also varies with temperature as a consequence of the response of Γ* to temperature (Fig. 5). Γ* increases as temperature increases, reflecting the increase in oxygenation relative to carboxylation. This results in carboxylation yields decreasing as temperature increases. Another way of illustrating this is the relationship between relative carboxylation yield and Γ* (Fig. 6). The temperature response function from spinach (Spinacea oleracea; Brooks and Farquhar, 1985) was used to mark the leaf temperature points. There has been limited study of the temperature dependence of Γ* between species (Medlyn et al., 2002), so it is unknown how variable it might be or how much could be achieved by genetic engineering. However, many species have been assayed at 25°C, and the range in values is shown by the striped box in Figure 6. As Rubisco from C3 plants already tends to have the lowest Γ* value, the scope for reducing this further using existing germplasm seems limited. Genetic engineering could possibly extend the range in Γ*, because it can explore novel amino acid sequences that would be unlikely to evolve from the existing DNA sequence.
Figure 6.
Relative photon yield for a C3 leaf as a function of Γ*. Relative
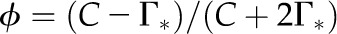 and a constant value for C are assumed (235 µbar). The temperature response function of Γ* was measured with spinach (Brooks and Farquhar, 1985), and the squares indicate 5°C increments. The striped area illustrates the range in Γ* that has been found for diverse terrestrial plants, including both C3 and C4 species (Kent and Tomany, 1995; Evans and Loreto, 2000; Galmes et al., 2005; Parry et al., 2011). To interconvert between Γ* and Sc/o, divide 3,961 by Γ* or Sc/o (valid for 25°C; von Caemmerer et al., 1994); that is, a value of 40 µbar for Γ* is equivalent to 99 for Sc/o.
and a constant value for C are assumed (235 µbar). The temperature response function of Γ* was measured with spinach (Brooks and Farquhar, 1985), and the squares indicate 5°C increments. The striped area illustrates the range in Γ* that has been found for diverse terrestrial plants, including both C3 and C4 species (Kent and Tomany, 1995; Evans and Loreto, 2000; Galmes et al., 2005; Parry et al., 2011). To interconvert between Γ* and Sc/o, divide 3,961 by Γ* or Sc/o (valid for 25°C; von Caemmerer et al., 1994); that is, a value of 40 µbar for Γ* is equivalent to 99 for Sc/o.
RAISING ELECTRON TRANSPORT RATE
For the parts of the canopy that are in bright light, photosynthesis is limited by Rubisco capacity. As atmospheric CO2 concentrations rise, the transition from electron transport limited to a Rubisco-limited rate is being shifted to higher irradiances, and it has been argued that plants could benefit from reallocating their resources away from Rubisco toward greater capacity for electron transport and RuBP regeneration. Electron transport capacity is strongly related to the cytochrome f content per unit of leaf area across diverse species (Evans, 1988; Evans and Seemann, 1989; Yamori et al., 2011a). A single relationship holds true regardless of whether cytochrome f content was varied by growth irradiance or nitrogen nutrition (Evans, 1996; Niinemets and Tenhunen, 1997). When translation of the Rieske Fe-S gene was reduced by an antisense construct, the reduction in photosynthetic electron transport capacity was directly related to cytochrome f content (Price et al., 1995; Yamori et al., 2011b). Armed with this knowledge, it is thought that an effective strategy to increase electron transport capacity is to increase the content of the cytochrome b/f complex (von Caemmerer and Evans, 2010). The cytochrome b/f complex is composed of three nucleus- and four chloroplast-encoded gene products. Therefore, the first challenge is to discover how to manipulate expression to increase the stable and functional amount of the cytochrome b/f complex. It is anticipated that there exists sufficient excess capacity in the other reactions associated with RuBP regeneration that initial increases in electron transport capacity require only the cytochrome b/f complex to be increased. The activity of the ATP synthase complex is normally down-regulated (Kramer et al., 2004). When ATP synthase content was reduced by antisense, it was possible to observe electron transport rates per ATP synthase twice that of the wild type (Yamori et al., 2011b; Rott et al., 2011), demonstrating that excess capacity exists at least for ATP synthase.
NON-STEADY-STATE CANOPY PHOTOSYNTHESIS
The power of the Farquhar et al. (1980) model of photosynthesis stems from the fact that so much of leaf photosynthetic properties are captured by Rubisco kinetics. Relatively few parameters are required in order to model how CO2 assimilation rate should vary with light, CO2, and temperature. However, a model cannot provide a meaningful explanation if a process outside the equations becomes important. In the real world, conditions are dynamic, with rapid fluctuations in light having the most dramatic effect on photosynthesis (Pearcy, 1990). In plant canopies, even lower leaves receive periods of bright light depending on the solar trajectory and the movement of leaves above by wind. Clouds also result in abrupt changes in irradiance, whereas overcast conditions enhance the proportion of diffuse light, which penetrates through the canopy in a different way than direct sunlight. Periods of low irradiance preceding high irradiance can result in low stomatal conductance, enzymes not being fully activated (Pons et al., 1992), and depleted metabolite pools, any of which reduces CO2 assimilation rate below the steady-state potential (Lawson et al., 2012). Conversely, periods of high irradiance can activate photoprotective mechanisms that reduce photon yields in subsequent periods of low irradiance (Zhu et al., 2004; Losciale et al., 2010; Murchie and Niyogi, 2011). The potential loss under fluctuating conditions is represented by arrow C in Figure 2.
To cope with fluctuating light, plants employ a range of strategies. Excess energy absorbed by the pigment-protein complexes can be dissipated as heat through a variety of mechanisms with different time constants (Murchie and Niyogi, 2011). The proton conductance through ATP synthases can vary, allowing independent control of nonphotochemical quenching from linear electron transport flux (Kramer et al., 2004). First, carotenoids are interconverted via the xanthophyll cycle to vary the capacity for nonphotochemical quenching (Demmig-Adams and Adams, 1992). Second, a component of PSII called PsbS acts as a sensor for rapid induction and relaxation of quenching (Li et al., 2004). Mutants lacking PsbS showed decreased fitness under fluctuating light conditions (Külheim et al., 2002). Another protein necessary for optimal growth under fluctuating light is STN7 kinase, which phosphorylates the major light-harvesting complex associated with PSII and regulates photosystem stoichiometry (Grieco et al., 2012). The mutant stn7 lacking the kinase could grow and survive under constant low or high irradiance, but it grew more slowly than the wild type under fluctuating light conditions. Intriguingly, the loss of light regulation of Rubisco activase activity by becoming insensitive to ADP enabled plants expressing the mutant enzyme to grow better than the wild type under fluctuating light conditions (Carmo-Silva and Salvucci, 2013).
Plants need flexible systems that respond rapidly to changes in light to provide protection against damage when light absorption exceeds the demand for NADPH and ATP regeneration. The speed with which these mechanisms relax when the leaf returns to low irradiance then influences the subsequent efficiency of photosynthesis. Predicting the optimal balance between protection and lost carbon gain when fluctuations in light are stochastic is a daunting challenge given that there are likely to be many systems that could be manipulated to achieve gains. Improving photosynthesis by manipulating the regulation of dynamic systems is likely to be far more challenging than improving efficiency in the steady state.
RADIATION USE EFFICIENCY: A TOP-DOWN APPROACH
The amount of sunlight varies with latitude, time of year, and weather. Plant growth reflects the amount of sunlight intercepted and the efficiency with which it is converted to biomass. Sequential destructive harvests combined with measurements of the cumulative sunlight intercepted by crops led Monteith (1977) to propose the concept of radiation use efficiency. For a given amount of light intercepted, the amount of biomass produced will depend on the daily integral of canopy photosynthesis (Fig. 2) and the efficiency of converting that fixed carbon into biomass that accounts for whole-plant respiration (Amthor, 2010; Zhu et al., 2010). Because the quantitative recovery of roots is time consuming and difficult, generally only aboveground biomass is measured. Consequently, variations in radiation use efficiency do not necessarily reflect variation in daily photosynthetic efficiency (Gower et al., 1999).
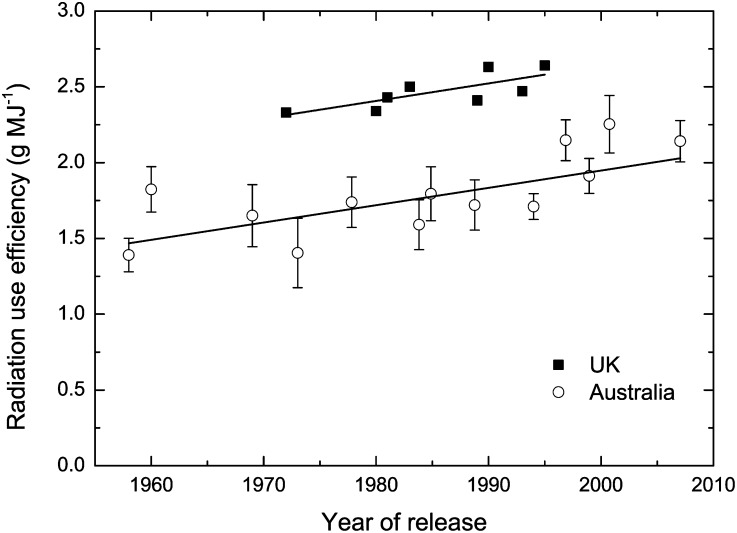
The radiation use efficiency of C4 crops has generally been found to exceed that of C3 crops (Monteith, 1978; Kiniry et al., 1989; Evans and von Caemmerer, 2000; Mitchell and Sheehy, 2000; Sheehy et al., 2007a). This is consistent with expectations based on the responses of canopy photosynthesis to irradiance. Not only do C4 plants have greater photon yields, they also have greater maximum photosynthetic rates, both of which increase daily gross CO2 assimilation per unit of intercepted light. Could measurements of radiation use efficiency thus provide a tool to enable selection for improved photosynthesis? Two studies have made comprehensive comparisons of radiation use efficiency for wheat cultivars released in the United Kingdom between 1972 and 1985 (Shearman et al., 2005) and in Australia between 1958 and 2007 (Sadras et al., 2012; Fig. 7). Aboveground biomass production was measured by destructive harvests at stem elongation and flowering. In both cases, there was a significant increase in radiation use efficiency with year of release. While the absolute values for radiation use efficiency differ between the two studies, remarkably, they both had the same slope of 0.011 g MJ−1 year−1. There is a risk in these types of experiments of underestimating the performance of older cultivars because of their increased susceptibility to disease, despite the use of fungicides in both experiments. However, the fact that both studies observed the same trend suggests that selection for yield has led to an improvement in photosynthesis. Detecting the underlying reason for this change at the leaf level is a challenge, because it would be very labor intensive. There are also few places capable of measuring photosynthesis in crop canopies growing in the field. To detect the gains made over a decade, one would need to be able to resolve a 5% difference in daily photosynthesis.
Figure 7.
Radiation use efficiency as a function of year of release for wheat cultivars in the United Kingdom (UK; Shearman et al., 2005) and Australia (Sadras et al., 2012). The slope of the regression in both cases was 0.011 g MJ−1 year−1. Radiation use efficiency, calculated using PAR, was determined for growth between the stages of stem elongation and flowering.
NEW OPPORTUNITIES
Field portable systems that measure photosynthesis require at least 20 min to determine a CO2 response curve that can be analyzed to derive estimates of Rubisco activity and electron transport capacity. This length of time severely limits the ability to survey variation in photosynthetic characters between elite cultivars or that may exist within germplasm collections. It is also difficult to extrapolate from single leaf photosynthesis measurements under optimal conditions to an integrated estimate for the canopy through time. Recently, a method using spectral reflectance measurements was shown to be useful for predicting several key attributes of leaves from two Populus species (Serbin et al., 2012). Ten reflectance spectra taking 5 s to scan from 350 to 2,500 nm were averaged from each leaf and related to parameters derived from a CO2 response curve measured at the growth temperature and subsequently leaf dry mass per unit of projected area and nitrogen concentration. Algorithms based on the reflectance spectra were able to predict nitrogen concentration, leaf dry mass per area, Vcmax, and rate of photosynthetic electron transport, all with r2 of at least 0.89 using 11 to 44 wavelengths. The combination of wavelengths differed for each character, so they were being detected independently. Remarkably, the increase in Vcmax with higher leaf temperatures in the afternoon was also predicted from reflectance spectra measured on leaves from field-grown trees. Reflectance spectra have also been used to predict leaf nitrogen concentration and leaf dry mass per area for wheat leaves (Ecarnot et al., 2013). Once this technique is established, then the speed with which reflectance can be measured should allow rapid germplasm exploration to identify variation in photosynthetic capacity per unit of leaf area or nitrogen, together with Rubisco activity and electron transport capacity.
BROADENING THE WAVEBAND FOR PHOTOSYNTHESIS
As mentioned earlier, PAR was defined between 400 and 700 nm based on the work of McCree (1971). The 400- to 700-nm waveband accounts for about 50% of the energy in sunlight. Below 400 nm, the effectiveness of UV light diminishes, probably due to the deployment of screening compounds to protect against damage to proteins and DNA. The level of screening compounds increases when leaves are grown in light containing UV. At the long wavelength end, the reaction center of PSI utilizes 700-nm photons. This coincides with the absorption edge of the pigment-protein complexes in terrestrial plants. Few photons with wavelengths beyond 700 nm are absorbed by leaves, as they are reflected or transmitted almost completely.
The discovery that an oxygenic cyanobacterium, Acaryochloris marina, substituted the bulk of its chlorophyll a with chlorophyll d meant that it was able to use 710-nm light for photochemistry (Miyashita et al., 1996). Subsequently, the even farther red-shifted chlorophyll f was discovered in cyanobacteria isolated from stromatoliths in western Australia, where it exists as a minor accessory pigment (Chen et al., 2010). These two novel chlorophylls offer the possibility of being able to extend the range of PAR toward 750 nm if they can be engineered into higher plants (Chen and Blankenship, 2011). The 700- to 750-nm waveband would increase photons available for photosynthesis from sunlight by up to 19%. One foreseeable hazard is that this could interfere with the role of phytochrome in plants, which senses the balance between red (660-nm) and far-red (730-nm) light. Phytochrome regulates many developmental processes, such as germination, greening, photoperiod sensing, perception of neighbors, and shade avoidance. The steep change in reflectance spectra due to chlorophyll is also widely used in remote sensing to quantify vegetation. However, if it is possible to engineer chlorophylls d and f into terrestrial plants, then tweaking phytochrome may only be a slight additional hurdle to deal with.
CONCLUSION
Evidence exists that increasing photosynthetic efficiency in crop plants can raise yield potential. Considerable research effort has established the conceptual framework from which it is possible to contemplate how photosynthesis might be improved, but it will require concomitant changes in the rest of the plant to fully realize the benefit. As many of the identified targets have already been reviewed by others, this review has focused on what I believe are the most promising options or targets that have received less coverage. Several international consortia are currently working toward improving photosynthesis. Grafting the more efficient C4 photosynthetic pathway onto cereals that produce the most desirable grains sought by humans (rice and wheat) is a bold venture. New technologies allowing novel Rubisco proteins to be assembled will enable their functional assessment and facilitate in pushing back the boundaries that currently constrain the suite of kinetic properties available. Genetic engineering continues to advance and improve our capability to manipulate targets such as the cytochrome b/f complex or introduce genes from other organisms like the bicarbonate transporters and red-shifted chlorophylls in cyanobacteria. Detailed crop modeling is required to provide quantitative estimates of the potential gains from changes to single and multiple targets. To tap into the diversity present in germplasm collections and discover useful photosynthetic traits, new screening techniques that are sufficiently rapid and practical must be developed. To develop these exciting opportunities, proper funding is required together with a greater acceptance by the public of the usefulness of genetic engineering.
Acknowledgments
My father, Lloyd T. Evans, made great contributions to this field during his career and undoubtedly has and continues to influence me greatly through the opportunities he provided and insights he conveyed. Many deliberate and inadvertent contributions have come from my colleagues at the Australian National University and overseas, but in particular I want to thank Susanne von Caemmerer.
Glossary
- PEP
phosphoenolpyruvate
- PAR
photosynthetically active radiation
- RuBP
ribulose 1,5-bisphosphate
- Γ*
CO2 compensation point
- Vcmax
maximum rate of carboxylation
- Kc
Michaelis-Menten constant for CO2
- Sc/o
specificity factor for Rubisco
- nR
number of moles of Rubisco sites per unit of leaf area
- kcat
maximum catalytic turnover of carboxylase
References
- Ainsworth EA, Long SP. (2005) What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol 165: 351–371 [DOI] [PubMed] [Google Scholar]
- Amthor JS. (2010) From sunlight to phytomass: on the potential efficiency of converting solar radiation to phyto-energy. New Phytol 188: 939–959 [DOI] [PubMed] [Google Scholar]
- Angus JF, Fischer RA. (1991) Grain and protein responses to nitrogen applied to wheat growing on a red earth. Aust J Agric Res 42: 735–746 [Google Scholar]
- Austin R, Bingham J, Blackwell R, Evans L, Ford M, Morgan C, Taylor M. (1980) Genetic improvements in winter wheat yields since 1900 and associated physiological changes. J Agric Sci 94: 675–689 [Google Scholar]
- Austin RB, Ford MA, Morgan CL. (1989) Genetic improvement in the yield of winter wheat: a further evaluation. J Agric Sci 112: 295–301 [Google Scholar]
- Bar-Even A, Noor E, Milo R. (2012) A survey of carbon fixation pathways through a quantitative lens. J Exp Bot 63: 2325–2342 [DOI] [PubMed] [Google Scholar]
- Barraclough PB, Howarth JR, Jones J, Lopez-Bellido R, Parmar S, Shepherd CE, Hawkesford MJ. (2010) Nitrogen efficiency of wheat: genotypic and environmental variation and prospects for improvement. Eur J Agron 33: 1–11 [Google Scholar]
- Bauwe H. (1986) An efficient method for the determination of Km values for HCO3− of phosphoenolpyruvate carboxylase. Planta 169: 356–360 [DOI] [PubMed] [Google Scholar]
- Berni JAJ, Zarco-Tejada PJ, Sepulcre-Canto G, Fereres E, Villalobos F. (2009) Mapping canopy conductance and CWSI in olive orchards using high resolution thermal remote sensing imagery. Remote Sens Environ 113: 2380–2388 [Google Scholar]
- Björkman O, Demmig B. (1987) Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77K among vascular plants of diverse origins. Planta 170: 489–504 [DOI] [PubMed] [Google Scholar]
- Boron WF. (2010) Sharpey-Schafer lecture: gas channels. Exp Physiol 95: 1107–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bräutigam A, Kajala K, Wullenweber J, Sommer M, Gagneul D, Weber KL, Carr KM, Gowik U, Mass J, Lercher MJ, et al. (2011) An mRNA blueprint for C4 photosynthesis derived from comparative transcriptomics of closely related C3 and C4 species. Plant Physiol 155: 142–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks A, Farquhar GD. (1985) Effect of temperature on the CO2/O2 specificity of ribulose-1,5-bisphosphate carboxylase/oxygenase and the rate of respiration in the light. Planta 165: 397–406 [DOI] [PubMed] [Google Scholar]
- Carmo-Silva AE, Salvucci ME. (2013) The regulatory properties of Rubisco activase differ among species and affect photosynthetic induction during light transitions. Plant Physiol 161: 1645–1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Blankenship RE. (2011) Expanding the solar spectrum used by photosynthesis. Trends Plant Sci 16: 427–431 [DOI] [PubMed] [Google Scholar]
- Chen M, Schliep M, Willows RD, Cai ZL, Neilan BA, Scheer H. (2010) A red-shifted chlorophyll. Science 329: 1318–1319 [DOI] [PubMed] [Google Scholar]
- Condon AG, Farquhar GD, Richards RA. (1990) Genotypic variation in carbon isotope discrimination and transpiration efficiency in wheat: leaf gas-exchange and whole plant studies. Aust J Plant Physiol 17: 9–22 [Google Scholar]
- Demmig-Adams B, Adams WW. (1992) Photoprotection and other responses of plants to high light stress. Annu Rev Plant Physiol Plant Mol Biol 43: 599–626 [Google Scholar]
- DePury DGG, Farquhar GD. (1997) Simple scaling of photosynthesis from leaves to canopies without the errors of big-leaf models. Plant Cell Environ 20: 537–557 [Google Scholar]
- Ecarnot M, Compan F, Roumet P. (2013) Assessing leaf nitrogen content and leaf mass per unit area of wheat in the field throughout plant cycle with a portable spectrometer. Field Crops Res 140: 44–50 [Google Scholar]
- Ehleringer J, Pearcy RW. (1983) Variation in quantum yield for CO2 uptake among C3 and C4 plants. Plant Physiol 73: 555–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JR. (1987) The dependence of quantum yield on wavelength and growth irradiance. Aust J Plant Physiol 14: 69–79 [Google Scholar]
- Evans JR. (1988) Acclimation by the thylakoid membranes to growth irradiance and the partitioning of nitrogen between soluble and thylakoid proteins. Aust J Plant Physiol 15: 93–106 [Google Scholar]
- Evans JR (1996) Developmental constraints on photosynthesis: effects of light and nutrition. In NR Baker, ed, Photosynthesis and the Environment. Kluwer, Dordrecht, The Netherlands, pp 281–304 [Google Scholar]
- Evans JR, Farquhar GD (1991) Modelling canopy photosynthesis from the biochemistry of the C3 chloroplast. In KJ Boote, RS Loomis, eds, Modelling Crop Photosynthesis: From Biochemistry to Canopy. Crop Science Society of America, Madison, WI, pp 1–15 [Google Scholar]
- Evans JR, Kaldenhoff R, Genty B, Terashima I. (2009) Resistances along the CO2 diffusion pathway inside leaves. J Exp Bot 60: 2235–2248 [DOI] [PubMed] [Google Scholar]
- Evans JR, Loreto F (2000) Acquisition and diffusion of CO2 in higher plant leaves. In RC Leegood, TD Sharkey, S von Caemmerer, eds, Photosynthesis: Physiology and Metabolism. Kluwer, Dordrecht, The Netherlands, pp 321–351 [Google Scholar]
- Evans JR, Seemann JR (1989) The allocation of protein nitrogen in the photosynthetic apparatus: costs, consequences, and control. In WR Briggs, ed, Photosynthesis. AR Liss, New York, pp 183–205 [Google Scholar]
- Evans JR, von Caemmerer S. (1996) Carbon dioxide diffusion inside leaves. Plant Physiol 110: 339–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JR, von Caemmerer S (2000) Would C4 rice produce more biomass than C3 rice? In JE Sheehy, PL Mitchell, B Hardy, eds, Redesigning Rice Photosynthesis to Increase Yield. Elsevier Science, Amsterdam, pp 53–71 [Google Scholar]
- Evans JR, von Caemmerer S, Setchell BA, Hudson GS. (1994) The relationship between CO2 transfer conductance and leaf anatomy in transgenic tobacco with a reduced content of Rubisco. Aust J Plant Physiol 21: 475–495 [Google Scholar]
- Farquhar GD, von Caemmerer S (1982) Modelling of photosynthetic responses to environmental conditions. In OL Lange, PS Nobel, CB Osmond, H Ziegler, eds, Physiological Plant Ecology. II. Water Relations and Carbon Assimilation Plant Physiol. New Series, Vol 12B. Springer Verlag, Berlin, pp 549–587 [Google Scholar]
- Farquhar GD, von Caemmerer S, Berry JA. (1980) A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149: 78–90 [DOI] [PubMed] [Google Scholar]
- Fischer RA, Edmeades GO. (2010) Breeding and cereal yield progress. Crop Sci 50: S85–S98 [Google Scholar]
- Fischer RA, Rees D, Sayre KD, Lu ZM, Condon AG, Saavedra AL. (1998) Wheat yield progress associated with higher stomatal conductance and photosynthetic rate, and cooler canopies. Crop Sci 38: 1467–1475 [Google Scholar]
- Flexas J, Ribas-Carbó M, Hanson DT, Bota J, Otto B, Cifre J, McDowell N, Medrano H, Kaldenhoff R. (2006) Tobacco aquaporin NtAQP1 is involved in mesophyll conductance to CO2 in vivo. Plant J 48: 427–439 [DOI] [PubMed] [Google Scholar]
- Foulkes MJ, Slafer GA, Davies WJ, Berry PM, Sylvester-Bradley R, Martre P, Calderini DF, Griffiths S, Reynolds MP. (2011) Raising yield potential of wheat. III. Optimizing partitioning to grain while maintaining lodging resistance. J Exp Bot 62: 469–486 [DOI] [PubMed] [Google Scholar]
- Galmes J, Flexas J, Keys AJ, Cifre J, Mitchell RAC, Madgwick PJ, Haslam RP, Medrano H, Parry MAJ. (2005) Rubisco specificity factor tends to be larger in plant species from drier habitats and in species with persistent leaves. Plant Cell Environ 28: 571–579 [Google Scholar]
- Ghannoum O, Evans JR, Chow WS, Andrews TJ, Conroy JP, von Caemmerer S. (2005) Faster Rubisco is the key to superior nitrogen-use efficiency in NADP-malic enzyme relative to NAD-malic enzyme C4 grasses. Plant Physiol 137: 638–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghannoum O, Evans JR, von Caemmerer S (2011) Nitrogen and water use efficiency of C4 plants. In AS Raghavendra, RF Sage, eds, C4 Photosynthesis and Related CO2 Concentrating Mechanisms. Springer, Dordrecht, The Netherlands, pp 129–146 [Google Scholar]
- Gifford RM, Evans L. (1981) Photosynthesis, carbon partitioning, and yield. Annu Rev Plant Physiol 32: 485–509 [Google Scholar]
- Gower ST, Kucharik CJ, Norman JM. (1999) Direct and indirect estimation of leaf area index, fAPAR, and net primary production of terrestrial ecosystems. Remote Sens Environ 70: 29–51 [Google Scholar]
- Gowik U, Westhoff P. (2011) The path from C3 to C4 photosynthesis. Plant Physiol 155: 56–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieco M, Tikkanen M, Paakkarinen V, Kangasjärvi S, Aro E-M. (2012) Steady-state phosphorylation of light-harvesting complex II proteins preserves photosystem I under fluctuating white light. Plant Physiol 160: 1896–1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackl H, Baresel JP, Mistele B, Hu Y, Schmidhalter U. (2012) A comparison of plant temperatures as measured by thermal imaging and infrared thermometry. J Agron Crop Sci 198: 415–429 [Google Scholar]
- Hanba YT, Shibasaka M, Hayashi Y, Hayakawa T, Kasamo K, Terashima I, Katsuhara M. (2004) Overexpression of the barley aquaporin HvPIP2;1 increases internal CO2 conductance and CO2 assimilation in the leaves of transgenic rice plants. Plant Cell Physiol 45: 521–529 [DOI] [PubMed] [Google Scholar]
- Henderson SA, von Caemmerer S, Farquhar GD. (1992) Short-term measurements of carbon isotope discrimination in several C4 species. Aust J Plant Physiol 19: 263–285 [Google Scholar]
- Ishikawa C, Hatanaka T, Misoo S, Fukayama H. (2009) Screening of high kcat Rubisco among Poaceae for improvement of photosynthetic CO2 assimilation in rice. Plant Prod Sci 12: 345–350 [Google Scholar]
- Ishikawa C, Hatanaka T, Misoo S, Miyake C, Fukayama H. (2011) Functional incorporation of sorghum small subunit increases the catalytic turnover rate of Rubisco in transgenic rice. Plant Physiol 156: 1603–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaldenhoff R. (2012) Mechanisms underlying CO2 diffusion in leaves. Curr Opin Plant Biol 15: 276–281 [DOI] [PubMed] [Google Scholar]
- Kapralov MV, Smith JAC, Filatov DA. (2012) Rubisco evolution in C4 eudicots: an analysis of Amaranthaceae sensu lato. PLoS ONE 7: e52974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent SS, Tomany MJ. (1995) The differential of the ribulose 1,5-bisphosphate carboxylase oxygenase specificity factor among higher-plants and the potential for biomass enhancement. Plant Physiol Biochem 33: 71–80 [Google Scholar]
- Kimball BA. (1983) Carbon dioxide and agricultural yield: an assemblage of 430 prior observations. Agron J 75: 779–788 [Google Scholar]
- Kimball BA, Zhu J, Cheng L, Kobayashi K, Bindi M. (2002) [Responses of agricultural crops of free-air CO2 enrichment]. Ying Yong Sheng Tai Xue Bao 13: 1323–1338 [PubMed] [Google Scholar]
- Kiniry JR, Jones CA, O’Toole JC, Blanchet R, Cabelguenne M, Spanel DA. (1989) Radiation-use efficiency in biomass accumulation prior to grain-filling for five grain-crop species. Field Crops Res 20: 51–64 [Google Scholar]
- Kramer DM, Avenson TJ, Edwards GE. (2004) Dynamic flexibility in the light reactions of photosynthesis governed by both electron and proton transfer reactions. Trends Plant Sci 9: 349–357 [DOI] [PubMed] [Google Scholar]
- Kramer DM, Evans JR. (2011) The importance of energy balance in improving photosynthetic productivity. Plant Physiol 155: 70–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kromdijk J, Schepers HE, Albanito F, Fitton N, Carroll F, Jones MB, Finnan J, Lanigan GJ, Griffiths H. (2008) Bundle sheath leakiness and light limitation during C4 leaf and canopy CO2 uptake. Plant Physiol 148: 2144–2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Külheim C, Agren J, Jansson S. (2002) Rapid regulation of light harvesting and plant fitness in the field. Science 297: 91–93 [DOI] [PubMed] [Google Scholar]
- Lam SK, Chen D, Norton R, Armstrong R, Mosier AR. (2012) Nitrogen dynamics in grain crop and legume pasture systems under elevated atmospheric carbon dioxide concentration: a meta-analysis. Glob Change Biol 18: 2853–2859 [DOI] [PubMed] [Google Scholar]
- Langdale JA. (2011) C4 cycles: past, present, and future research on C4 photosynthesis. Plant Cell 23: 3879–3892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson T, Kramer DM, Raines CA. (2012) Improving yield by exploiting mechanisms underlying natural variation of photosynthesis. Curr Opin Biotechnol 23: 215–220 [DOI] [PubMed] [Google Scholar]
- Li XP, Gilmore AM, Caffarri S, Bassi R, Golan T, Kramer D, Niyogi KK. (2004) Regulation of photosynthetic light harvesting involves intrathylakoid lumen pH sensing by the PsbS protein. J Biol Chem 279: 22866–22874 [DOI] [PubMed] [Google Scholar]
- Long SP, Ainsworth EA, Rogers A, Ort DR. (2004) Rising atmospheric carbon dioxide: plants FACE the future. Annu Rev Plant Biol 55: 591–628 [DOI] [PubMed] [Google Scholar]
- Long SP, Zhu X-G, Naidu SL, Ort DR. (2006) Can improvement in photosynthesis increase crop yields? Plant Cell Environ 29: 315–330 [DOI] [PubMed] [Google Scholar]
- Losciale P, Chow WS, Corelli Grappadelli L. (2010) Modulating the light environment with the peach ‘asymmetric orchard’: effects on gas exchange performances, photoprotection, and photoinhibition. J Exp Bot 61: 1177–1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes WH, Steppe K. (2012) Estimating evapotranspiration and drought stress with ground-based thermal remote sensing in agriculture: a review. J Exp Bot 63: 4671–4712 [DOI] [PubMed] [Google Scholar]
- McCree KJ. (1971) The action spectrum, absorptance and quantum yield of photosynthesis in crop plants. Agric Meteorol 9: 191–216 [Google Scholar]
- Medlyn BE, Dreyer E, Ellsworth D, Forstreuter M, Harley PC, Kirschbaum MUF, Le Roux X, Montpied P, Strassemeyer J, Walcroft A, et al. (2002) Temperature response of parameters of a biochemically based model of photosynthesis. II. A review of experimental data. Plant Cell Environ 25: 1167–1179 [Google Scholar]
- Meyer M, Griffiths H. (2013) Origins and diversity of eukaryotic CO2-concentrating mechanisms: lessons for the future. J Exp Bot 64: 769–786 [DOI] [PubMed] [Google Scholar]
- Mitchell PL, Sheehy JE (2000) Performance of a potential C4 rice: overview from quantum yield to grain yield. In JE Sheehy, PL Mitchell, B Hardy, eds, Redesigning Rice Photosynthesis to Increase Yield. Elsevier Science, Amsterdam, pp 145–163 [Google Scholar]
- Miyashita H, Ikemoto H, Kurano N, Adachi K, Chihara M, Miyachi S. (1996) Chlorophyll d as a major pigment. Nature 383: 402 [Google Scholar]
- Monteith JL. (1977) Climate and efficiency of crop production in Britain. Philos Trans R Soc Lond B Biol Sci 281: 277–294 [Google Scholar]
- Monteith JL. (1978) Reassessment of maximum growth rates for C3 and C4 crops. Exp Agric 14: 1–5 [Google Scholar]
- Murchie EH, Niyogi KK. (2011) Manipulation of photoprotection to improve plant photosynthesis. Plant Physiol 155: 86–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niinemets U, Tenhunen JD. (1997) A model separating leaf structural and physiological effects on carbon gain along light gradients for the shade-tolerant species Acer saccharum. Plant Cell Environ 20: 845–866 [Google Scholar]
- Ögren E, Evans JR. (1993) Photosynthetic light response curves. 1. The influence of CO2 partial pressure and leaf inversion. Planta 189: 182–190 [Google Scholar]
- Ort DR, Melis A. (2011) Optimizing antenna size to maximize photosynthetic efficiency. Plant Physiol 155: 79–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne CP (2011) The geologic history of C4 plants. In AS Raghavendra, RF Sage, eds, C4 Photosynthesis and Related CO2 Concentrating Mechanisms, Vol 32. Springer, Dordrecht, The Netherlands, pp 339–357 [Google Scholar]
- Parry MAJ, Andralojc PJ, Scales JC, Salvucci ME, Carmo-Silva AE, Alonso H, Whitney SM. (2013) Rubisco activity and regulation as targets for crop improvement. J Exp Bot 64: 717–730 [DOI] [PubMed] [Google Scholar]
- Parry MAJ, Reynolds M, Salvucci ME, Raines C, Andralojc PJ, Zhu X-G, Price GD, Condon AG, Furbank RT. (2011) Raising yield potential of wheat. II. Increasing photosynthetic capacity and efficiency. J Exp Bot 62: 453–467 [DOI] [PubMed] [Google Scholar]
- Pearcy RW. (1990) Sunflecks and photosynthesis in plant canopies. Annu Rev Plant Physiol Plant Mol Biol 41: 421–453 [Google Scholar]
- Peterhansel C, Blume C, Offermann S. (2013) Photorespiratory bypasses: how can they work? J Exp Bot 64: 709–715 [DOI] [PubMed] [Google Scholar]
- Peterhansel C, Maurino VG. (2011) Photorespiration redesigned. Plant Physiol 155: 49–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterhansel C, Niessen M, Kebeish RM. (2008) Metabolic engineering towards the enhancement of photosynthesis. Photochem Photobiol 84: 1317–1323 [DOI] [PubMed] [Google Scholar]
- Pons TL, Pearcy RW, Seemann JR. (1992) Photosynthesis in flashing light in soybean leaves grown in different conditions. 1. Photosynthetic induction state and regulation of ribulose-1,5-bisphosphate carboxylase activity. Plant Cell Environ 15: 569–576 [Google Scholar]
- Price GD, Badger MR, von Caemmerer S. (2011) The prospect of using cyanobacterial bicarbonate transporters to improve leaf photosynthesis in C3 crop plants. Plant Physiol 155: 20–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price GD, Badger MR, Woodger FJ, Long BM. (2008) Advances in understanding the cyanobacterial CO2-concentrating-mechanism (CCM): functional components, Ci transporters, diversity, genetic regulation and prospects for engineering into plants. J Exp Bot 59: 1441–1461 [DOI] [PubMed] [Google Scholar]
- Price GD, Pengelly JJL, Forster B, Du J, Whitney SM, von Caemmerer S, Badger MR, Howitt SM, Evans JR. (2013) The cyanobacterial CCM as a source of genes for improving photosynthetic CO2 fixation in crop species. J Exp Bot 64: 753–768 [DOI] [PubMed] [Google Scholar]
- Price GD, Yu JW, von Caemmerer S, Evans JR, Chow WS, Anderson JM, Hurry V, Badger MR. (1995) Chloroplast cytochrome b6/f and ATP synthase complexes in tobacco: transformation with antisense RNA against nuclear-encoded transcripts for the Rieske FeS and ATPδ polypeptides. Aust J Plant Physiol 22: 285–297 [Google Scholar]
- Raines CA. (2011) Increasing photosynthetic carbon assimilation in C3 plants to improve crop yield: current and future strategies. Plant Physiol 155: 36–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebetzke GJ, Rattey AR, Farquhar GD, Richards RA, Condon AG. (2012) Genomic regions for canopy temperature and their genetic association with stomatal conductance and grain yield in wheat. Funct Plant Biol 40: 14–33 [DOI] [PubMed] [Google Scholar]
- Reynolds M, Bonnett D, Chapman SC, Furbank RT, Manès Y, Mather DE, Parry MAJ. (2011) Raising yield potential of wheat. I. Overview of a consortium approach and breeding strategies. J Exp Bot 62: 439–452 [DOI] [PubMed] [Google Scholar]
- Reynolds M, Foulkes MJ, Slafer GA, Berry P, Parry MAJ, Snape JW, Angus WJ. (2009) Raising yield potential in wheat. J Exp Bot 60: 1899–1918 [DOI] [PubMed] [Google Scholar]
- Richards RA, Rebetzke GJ, Watt M, Condon AG, Spielmeyer W, Dolferus R. (2010) Breeding for improved water productivity in temperate cereals: phenotyping, quantitative trait loci, markers and the selection environment. Funct Plant Biol 37: 85–97 [Google Scholar]
- Rott M, Martins NF, Thiele W, Lein W, Bock R, Kramer DM, Schoettler MA. (2011) ATP synthase repression in tobacco restricts photosynthetic electron transport, CO2 assimilation, and plant growth by overacidification of the thylakoid lumen. Plant Cell 23: 304–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadras VO, Lawson C, Montoro A. (2012) Photosynthetic traits in Australian wheat varieties released between 1958 and 2007. Field Crops Res 134: 19–29 [Google Scholar]
- Sage RF, Sage TL, Kocacinar F (2012) Photorespiration and the evolution of C4 photosynthesis. Annu Rev Plant Biol 63: 19–47 [DOI] [PubMed] [Google Scholar]
- Salvucci ME, Crafts-Brandner SJ. (2004) Mechanism for deactivation of Rubisco under moderate heat stress. Physiol Plant 122: 513–519 [Google Scholar]
- Serbin SP, Dillaway DN, Kruger EL, Townsend PA. (2012) Leaf optical properties reflect variation in photosynthetic metabolism and its sensitivity to temperature. J Exp Bot 63: 489–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD, Bernacchi CJ, Farquhar GD, Singsaas EL. (2007) Fitting photosynthetic carbon dioxide response curves for C3 leaves. Plant Cell Environ 30: 1035–1040 [DOI] [PubMed] [Google Scholar]
- Shearman VJ, Sylvester-Bradley R, Scott RK, Foulkes MJ. (2005) Physiological processes associated with wheat yield progress in the UK. Crop Sci 45: 175–185 [Google Scholar]
- Sheehy JE, Ferrer AB, Mitchell PL, Elmido-Mabilangan A, Pablico P, Dionora MJA (2007a) How the rice crop works and why it needs a new engine. In JE Sheehy, PL Mitchell, B Hardy, eds, Charting New Pathways to C4 Rice. International Rice Research Institute, Los Banos, Philippines, pp 3–26 [Google Scholar]
- Sheehy JE, Mitchell PL, Hardy B (2000) Redesigning Rice Photosynthesis to Increase Yield. Elsevier Science, Amsterdam [Google Scholar]
- Sheehy JE, Mitchell PL, Hardy B, editors (2007b) Charting New Pathways to C4 Rice. International Rice Research Institute, Los Banos, Philippines [Google Scholar]
- Sinclair TR, Sheehy JE. (1999) Erect leaves and photosynthesis in rice. Science 283: 1456–145710206873 [Google Scholar]
- Skillman JB. (2008) Quantum yield variation across the three pathways of photosynthesis: not yet out of the dark. J Exp Bot 59: 1647–1661 [DOI] [PubMed] [Google Scholar]
- Song Q, Zhang G, Zhu X-G. (2013) Optimal crop canopy architecture to maximise canopy photosynthetic CO2 uptake under elevated CO2: a theoretical study using a mechanistic model of canopy photosynthesis. Funct Plant Biol 40: 108–124 [DOI] [PubMed] [Google Scholar]
- Stapper M, Fischer RA. (1990) Genotype, sowing date and plant spacing influence on high-yielding irrigated wheat in southern New South Wales. 3. Potential yields and optimum flowering dates. Aust J Agric Res 41: 1043–1056 [Google Scholar]
- Tazoe Y, Hanba YT, Furumoto T, Noguchi K, Terashima I. (2008) Relationships between quantum yield for CO2 assimilation, activity of key enzymes and CO2 leakiness in Amaranthus cruentus, a C4 dicot, grown in high or low light. Plant Cell Physiol 49: 19–29 [DOI] [PubMed] [Google Scholar]
- Terashima I, Ono K. (2002) Effects of HgCl2 on CO2 dependence of leaf photosynthesis: evidence indicating involvement of aquaporins in CO2 diffusion across the plasma membrane. Plant Cell Physiol 43: 70–78 [DOI] [PubMed] [Google Scholar]
- Tholen D, Zhu X-G. (2011) The mechanistic basis of internal conductance: a theoretical analysis of mesophyll cell photosynthesis and CO2 diffusion. Plant Physiol 156: 90–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosens T, Niinemets Ü, Westoby M, Wright IJ. (2012) Anatomical basis of variation in mesophyll resistance in eastern Australian sclerophylls: news of a long and winding path. J Exp Bot 63: 5105–5119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubierna N, Sun W, Cousins AB. (2011) The efficiency of C4 photosynthesis under low light conditions: assumptions and calculations with CO2 isotope discrimination. J Exp Bot 62: 3119–3134 [DOI] [PubMed] [Google Scholar]
- Uehlein N, Otto B, Hanson DT, Fischer M, McDowell N, Kaldenhoff R. (2008) Function of Nicotiana tabacum aquaporins as chloroplast gas pores challenges the concept of membrane CO2 permeability. Plant Cell 20: 648–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehlein N, Sperling H, Heckwolf M, Kaldenhoff R. (2012) The Arabidopsis aquaporin PIP1;2 rules cellular CO2 uptake. Plant Cell Environ 35: 1077–1083 [DOI] [PubMed] [Google Scholar]
- von Caemmerer S (2000) Biochemical Models of Leaf Photosynthesis, Vol 2. CSIRO Publishing, Collingwood, Australia [Google Scholar]
- von Caemmerer S, Evans JR. (2010) Enhancing C3 photosynthesis. Plant Physiol 154: 589–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Caemmerer S, Evans JR, Hudson GS, Andrews TJ. (1994) The kinetics of ribulose-1,5-bisphosphate carboxylase/oxygenase in vivo inferred from measurements of photosynthesis in leaves of transgenic tobacco. Planta 195: 88–97 [Google Scholar]
- von Caemmerer S, Farquhar GD. (1981) Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153: 376–387 [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Quick WP, Furbank RT. (2012) The development of C4 rice: current progress and future challenges. Science 336: 1671–1672 [DOI] [PubMed] [Google Scholar]
- Weber APM, von Caemmerer S. (2010) Plastid transport and metabolism of C3 and C4 plants: comparative analysis and possible biotechnological exploitation. Curr Opin Plant Biol 13: 257–265 [DOI] [PubMed] [Google Scholar]
- Whitney SM, Houtz RL, Alonso H. (2011a) Advancing our understanding and capacity to engineer nature’s CO2-sequestering enzyme, Rubisco. Plant Physiol 155: 27–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney SM, Kane HJ, Houtz RL, Sharwood RE. (2009) Rubisco oligomers composed of linked small and large subunits assemble in tobacco plastids and have higher affinities for CO2 and O2. Plant Physiol 149: 1887–1895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney SM, Sharwood RE. (2008) Construction of a tobacco master line to improve Rubisco engineering in chloroplasts. J Exp Bot 59: 1909–1921 [DOI] [PubMed] [Google Scholar]
- Whitney SM, Sharwood RE, Orr D, White SJ, Alonso H, Galmés J. (2011b) Isoleucine 309 acts as a C4 catalytic switch that increases ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) carboxylation rate in Flaveria. Proc Natl Acad Sci USA 108: 14688–14693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SC, Cowan IR, Farquhar GD. (1985) Leaf conductance in relation to rate of CO2 assimilation. I. Influence of nitrogen nutrition, phosphorus nutrition, photon flux density, and ambient partial pressure of CO2 during ontogeny. Plant Physiol 78: 821–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuest SB, Cassman KG. (1992) Fertilizer-nitrogen use efficiency of irrigated wheat. I. Uptake efficiency of preplant versus late-season applications. Agron J 84: 682–688 [Google Scholar]
- Yamori W, Nagai T, Makino A. (2011a) The rate-limiting step for CO2 assimilation at different temperatures is influenced by the leaf nitrogen content in several C3 crop species. Plant Cell Environ 34: 764–777 [DOI] [PubMed] [Google Scholar]
- Yamori W, Takahashi S, Makino A, Price GD, Badger MR, von Caemmerer S. (2011b) The roles of ATP synthase and the cytochrome b6/f complexes in limiting chloroplast electron transport and determining photosynthetic capacity. Plant Physiol 155: 956–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarzycki J, Axen SD, Kinney JN, Kerfeld CA. (2013) Cyanobacterial-based approaches to improving photosynthesis in plants. J Exp Bot 64: 787–798 [DOI] [PubMed] [Google Scholar]
- Zhu X-G, Long SP, Ort DR. (2010) Improving photosynthetic efficiency for greater yield. Annu Rev Plant Biol 61: 235–261 [DOI] [PubMed] [Google Scholar]
- Zhu XG, Ort DR, Whitmarsh J, Long SP. (2004) The slow reversibility of photosystem II thermal energy dissipation on transfer from high to low light may cause large losses in carbon gain by crop canopies: a theoretical analysis. J Exp Bot 55: 1167–1175 [DOI] [PubMed] [Google Scholar]
- Ziska LH, Bunce JA, Shimono H, Gealy DR, Baker JT, Newton PCD, Reynolds MP, Jagadish KSV, Zhu C, Howden M, et al. (2012) Food security and climate change: on the potential to adapt global crop production by active selection to rising atmospheric carbon dioxide. Proc Biol Sci 279: 4097–4105 [DOI] [PMC free article] [PubMed] [Google Scholar]