Abstract
The alphaviruses are a genus of 26 enveloped viruses that cause disease in humans and domestic animals. Mosquitoes or other hematophagous arthropods serve as vectors for these viruses. The complete sequences of the +/- 11.7-kb plus-strand RNA genomes of eight alphaviruses have been determined, and partial sequences are known for several others; this has made possible evolutionary comparisons between different alphaviruses as well as comparisons of this group of viruses with other animal and plant viruses. Full-length cDNA clones from which infectious RNA can be recovered have been constructed for four alphaviruses; these clones have facilitated many molecular genetic studies as well as the development of these viruses as expression vectors. From these and studies involving biochemical approaches, many details of the replication cycle of the alphaviruses are known. The interactions of the viruses with host cells and host organisms have been exclusively studied, and the molecular basis of virulence and recovery from viral infection have been addressed in a large number of recent papers. The structure of the viruses has been determined to about 2.5 nm, making them the best-characterized enveloped virus to date. Because of the wealth of data that has appeared, these viruses represent a well-characterized system that tell us much about the evolution of RNA viruses, their replication, and their interactions with their hosts. This review summarizes our current knowledge of this group of viruses.
Full text
PDF




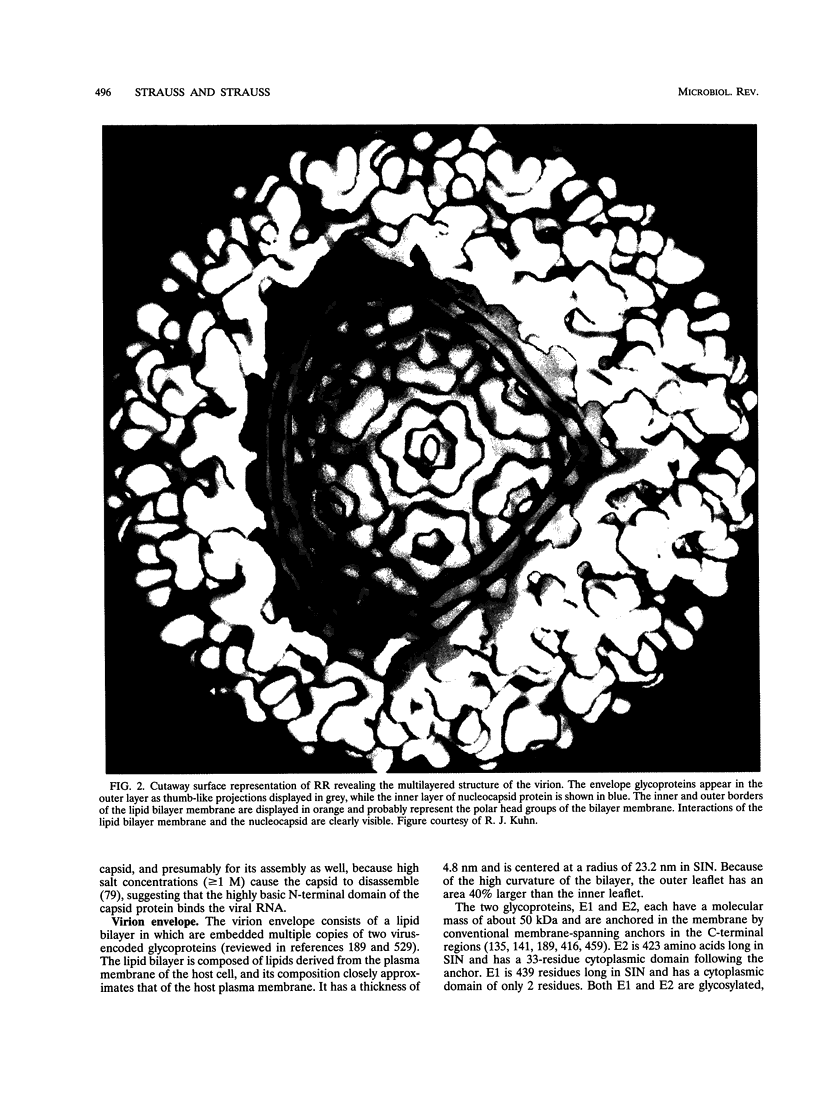
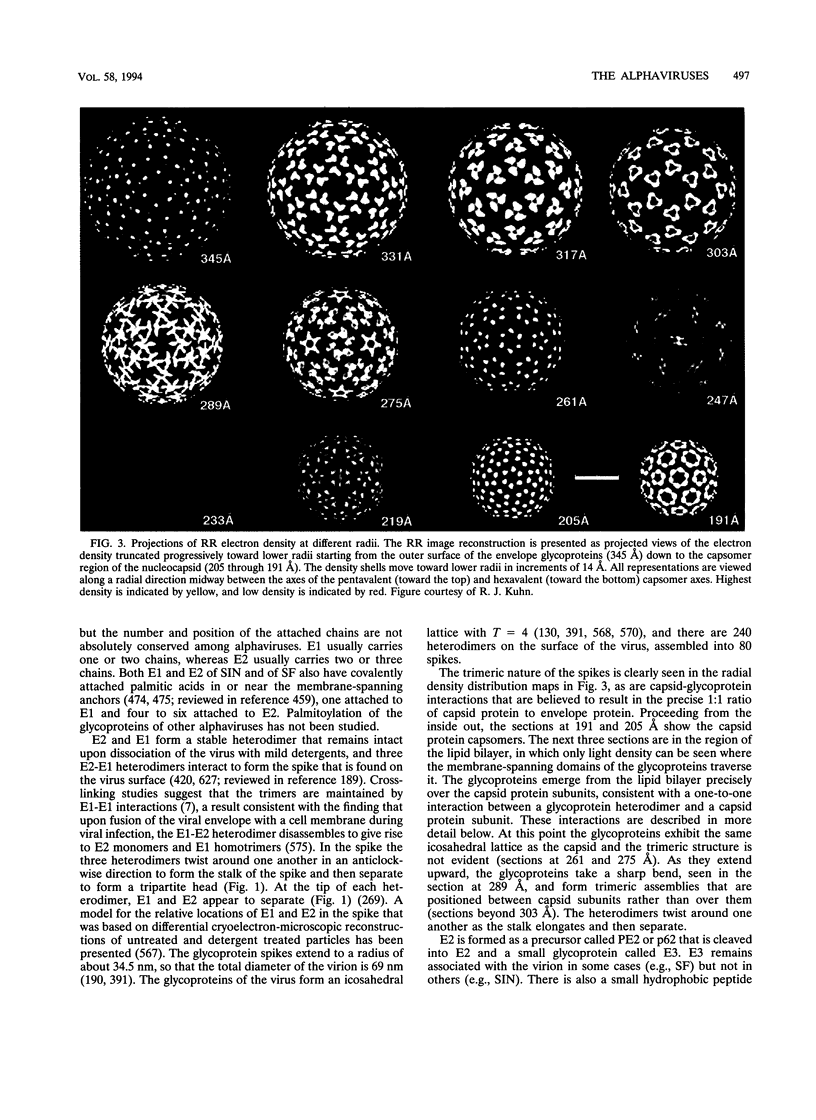
















































Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abell B. A., Brown D. T. Sindbis virus membrane fusion is mediated by reduction of glycoprotein disulfide bridges at the cell surface. J Virol. 1993 Sep;67(9):5496–5501. doi: 10.1128/jvi.67.9.5496-5501.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acheson N. H., Tamm I. Replication of Semliki Forest virus: an electron microscopic study. Virology. 1967 May;32(1):128–143. doi: 10.1016/0042-6822(67)90261-9. [DOI] [PubMed] [Google Scholar]
- Adams R. H., Brown D. T. BHK cells expressing Sindbis virus-induced homologous interference allow the translation of nonstructural genes of superinfecting virus. J Virol. 1985 May;54(2):351–357. doi: 10.1128/jvi.54.2.351-357.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlquist P., Strauss E. G., Rice C. M., Strauss J. H., Haseloff J., Zimmern D. Sindbis virus proteins nsP1 and nsP2 contain homology to nonstructural proteins from several RNA plant viruses. J Virol. 1985 Feb;53(2):536–542. doi: 10.1128/jvi.53.2.536-542.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alanen M., Wartiovaara J., Söderlund H. Sequences conserved in the defective interfering RNAs of Semliki Forest virus: an electron microscopic heteroduplex analysis. Hereditas. 1987;106(1):19–29. doi: 10.1111/j.1601-5223.1987.tb00232.x. [DOI] [PubMed] [Google Scholar]
- Aliperti G., Schlesinger M. J. Evidence for an autoprotease activity of sindbis virus capsid protein. Virology. 1978 Oct 15;90(2):366–369. doi: 10.1016/0042-6822(78)90321-5. [DOI] [PubMed] [Google Scholar]
- Anthony R. P., Brown D. T. Protein-protein interactions in an alphavirus membrane. J Virol. 1991 Mar;65(3):1187–1194. doi: 10.1128/jvi.65.3.1187-1194.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony R. P., Paredes A. M., Brown D. T. Disulfide bonds are essential for the stability of the Sindbis virus envelope. Virology. 1992 Sep;190(1):330–336. doi: 10.1016/0042-6822(92)91219-k. [DOI] [PubMed] [Google Scholar]
- Arias C., Bell J. R., Lenches E. M., Strauss E. G., Strauss J. H. Sequence analysis of two mutants of Sindbis virus defective in the intracellular transport of their glycoproteins. J Mol Biol. 1983 Jul 25;168(1):87–102. doi: 10.1016/s0022-2836(83)80324-6. [DOI] [PubMed] [Google Scholar]
- Atkins G. J. Establishment of persistent infection in BHK-21 cells by temperature-sensitive mutants of Sindbis virus. J Gen Virol. 1979 Oct;45(1):201–207. doi: 10.1099/0022-1317-45-1-201. [DOI] [PubMed] [Google Scholar]
- Atkins G. J., Sheahan B. J., Dimmock N. J. Semliki Forest virus infection of mice: a model for genetic and molecular analysis of viral pathogenicity. J Gen Virol. 1985 Mar;66(Pt 3):395–408. doi: 10.1099/0022-1317-66-3-395. [DOI] [PubMed] [Google Scholar]
- Atkins G. J. The effect of infection with Sindbis virus and its temperature-sensitive mutants on cellular protein and DNA synthesis. Virology. 1976 Jun;71(2):593–597. doi: 10.1016/0042-6822(76)90384-6. [DOI] [PubMed] [Google Scholar]
- Bachmair A., Finley D., Varshavsky A. In vivo half-life of a protein is a function of its amino-terminal residue. Science. 1986 Oct 10;234(4773):179–186. doi: 10.1126/science.3018930. [DOI] [PubMed] [Google Scholar]
- Bachmair A., Varshavsky A. The degradation signal in a short-lived protein. Cell. 1989 Mar 24;56(6):1019–1032. doi: 10.1016/0092-8674(89)90635-1. [DOI] [PubMed] [Google Scholar]
- Bailyes E. M., Shennan K. I., Seal A. J., Smeekens S. P., Steiner D. F., Hutton J. C., Docherty K. A member of the eukaryotic subtilisin family (PC3) has the enzymic properties of the type 1 proinsulin-converting endopeptidase. Biochem J. 1992 Jul 15;285(Pt 2):391–394. doi: 10.1042/bj2850391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baric R. S., Carlin L. J., Johnston R. E. Requirement for host transcription in the replication of Sindbis virus. J Virol. 1983 Jan;45(1):200–205. doi: 10.1128/jvi.45.1.200-205.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baric R. S., Trent D. W., Johnston R. E. A Sindbis virus variant with a cell-determined latent period. Virology. 1981 Apr 15;110(1):237–242. doi: 10.1016/0042-6822(81)90029-5. [DOI] [PubMed] [Google Scholar]
- Barr P. J. Mammalian subtilisins: the long-sought dibasic processing endoproteases. Cell. 1991 Jul 12;66(1):1–3. doi: 10.1016/0092-8674(91)90129-m. [DOI] [PubMed] [Google Scholar]
- Barrett A. D., Cubitt W. D., Dimmock N. J. Defective interfering particles of Semliki Forest virus are smaller than particles of standard virus. J Gen Virol. 1984 Dec;65(Pt 12):2265–2268. doi: 10.1099/0022-1317-65-12-2265. [DOI] [PubMed] [Google Scholar]
- Barth B. U., Suomalainen M., Liljeström P., Garoff H. Alphavirus assembly and entry: role of the cytoplasmic tail of the E1 spike subunit. J Virol. 1992 Dec;66(12):7560–7564. doi: 10.1128/jvi.66.12.7560-7564.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton D. J., Sawicki S. G., Sawicki D. L. Demonstration in vitro of temperature-sensitive elongation of RNA in Sindbis virus mutant ts6. J Virol. 1988 Oct;62(10):3597–3602. doi: 10.1128/jvi.62.10.3597-3602.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton D. J., Sawicki S. G., Sawicki D. L. Solubilization and immunoprecipitation of alphavirus replication complexes. J Virol. 1991 Mar;65(3):1496–1506. doi: 10.1128/jvi.65.3.1496-1506.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashford C. L., Alder G. M., Gray M. A., Micklem K. J., Taylor C. C., Turek P. J., Pasternak C. A. Oxonol dyes as monitors of membrane potential: the effect of viruses and toxins on the plasma membrane potential of animal cells in monolayer culture and in suspension. J Cell Physiol. 1985 Jun;123(3):326–336. doi: 10.1002/jcp.1041230306. [DOI] [PubMed] [Google Scholar]
- Beltzer J. P., Fiedler K., Fuhrer C., Geffen I., Handschin C., Wessels H. P., Spiess M. Charged residues are major determinants of the transmembrane orientation of a signal-anchor sequence. J Biol Chem. 1991 Jan 15;266(2):973–978. [PubMed] [Google Scholar]
- Berben-Bloemheuvel G., Kasperaitis M. A., van Heugten H., Thomas A. A., van Steeg H., Voorma H. O. Interaction of initiation factors with the cap structure of chimaeric mRNA containing the 5'-untranslated regions of Semliki Forest virus RNA is related to translational efficiency. Eur J Biochem. 1992 Sep 15;208(3):581–587. doi: 10.1111/j.1432-1033.1992.tb17222.x. [DOI] [PubMed] [Google Scholar]
- Berger M., Schmidt M. F. Identification of acyl donors and acceptor proteins for fatty acid acylation in BHK cells infected with Semliki Forest virus. EMBO J. 1984 Apr;3(4):713–719. doi: 10.1002/j.1460-2075.1984.tb01874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglund P., Sjöberg M., Garoff H., Atkins G. J., Sheahan B. J., Liljeström P. Semliki Forest virus expression system: production of conditionally infectious recombinant particles. Biotechnology (N Y) 1993 Aug;11(8):916–920. doi: 10.1038/nbt0893-916. [DOI] [PubMed] [Google Scholar]
- Birdwell C. R., Strauss E. G., Strauss J. H. Replication of Sindbis virus. III. An electron microscopic study of virus maturation using the surface replica technique. Virology. 1973 Dec;56(2):429–438. [PubMed] [Google Scholar]
- Birdwell C. R., Strauss J. H. Distribution of the receptor sites for Sindbis virus on the surface of chicken and BHK cells. J Virol. 1974 Sep;14(3):672–678. doi: 10.1128/jvi.14.3.672-678.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boege U., Wengler G., Wengler G., Wittmann-Liebold B. Primary structures of the core proteins of the alphaviruses Semliki Forest virus and Sindbis virus. Virology. 1981 Aug;113(1):293–303. doi: 10.1016/0042-6822(81)90156-2. [DOI] [PubMed] [Google Scholar]
- Boere W. A., Benaissa-Trouw B. J., Harmsen M., Kraaijeveld C. A., Snippe H. Neutralizing and non-neutralizing monoclonal antibodies to the E2 glycoprotein of Semliki Forest virus can protect mice from lethal encephalitis. J Gen Virol. 1983 Jun;64(Pt 6):1405–1408. doi: 10.1099/0022-1317-64-6-1405. [DOI] [PubMed] [Google Scholar]
- Boere W. A., Benaissa-Trouw B. J., Harmsen T., Erich T., Kraaijeveld C. A., Snippe H. Mechanisms of monoclonal antibody-mediated protection against virulent Semliki Forest virus. J Virol. 1985 May;54(2):546–551. doi: 10.1128/jvi.54.2.546-551.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boere W. A., Benaissa-Trouw B. J., Harmsen T., Erich T., Kraaijeveld C. A., Snippe H. The role of complement in monoclonal antibody-mediated protection against virulent Semliki Forest virus. Immunology. 1986 Aug;58(4):553–559. [PMC free article] [PubMed] [Google Scholar]
- Boere W. A., Harmsen T., Vinjé J., Benaissa-Trouw B. J., Kraaijeveld C. A., Snippe H. Identification of distinct antigenic determinants on Semliki Forest virus by using monoclonal antibodies with different antiviral activities. J Virol. 1984 Nov;52(2):575–582. doi: 10.1128/jvi.52.2.575-582.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggs W. M., Hahn C. S., Strauss E. G., Strauss J. H., Griffin D. E. Low pH-dependent Sindbis virus-induced fusion of BHK cells: differences between strains correlate with amino acid changes in the E1 glycoprotein. Virology. 1989 Apr;169(2):485–488. doi: 10.1016/0042-6822(89)90178-5. [DOI] [PubMed] [Google Scholar]
- Bonatti S., Blobel G. Absence of a cleavable signal sequence in Sindbis virus glycoprotein PE2. J Biol Chem. 1979 Dec 25;254(24):12261–12264. [PubMed] [Google Scholar]
- Bonatti S., Cancedda R., Blobel G. Membrane biogenesis. In vitro cleavage, core glycosylation, and integration into microsomal membranes of sindbis virus glycoproteins. J Cell Biol. 1979 Jan;80(1):219–224. doi: 10.1083/jcb.80.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonatti S., Migliaccio G., Blobel G., Walter P. Role of signal recognition particle in the membrane assembly of Sindbis viral glycoproteins. Eur J Biochem. 1984 May 2;140(3):499–502. doi: 10.1111/j.1432-1033.1984.tb08130.x. [DOI] [PubMed] [Google Scholar]
- Bonatti S., Migliaccio G., Simons K. Palmitylation of viral membrane glycoproteins takes place after exit from the endoplasmic reticulum. J Biol Chem. 1989 Jul 25;264(21):12590–12595. [PubMed] [Google Scholar]
- Both G. W., Sleigh M. J. Conservation and variation in the hemagglutinins of Hong Kong subtype influenza viruses during antigenic drift. J Virol. 1981 Sep;39(3):663–672. doi: 10.1128/jvi.39.3.663-672.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braakman I., Helenius J., Helenius A. Role of ATP and disulphide bonds during protein folding in the endoplasmic reticulum. Nature. 1992 Mar 19;356(6366):260–262. doi: 10.1038/356260a0. [DOI] [PubMed] [Google Scholar]
- Bredenbeek P. J., Frolov I., Rice C. M., Schlesinger S. Sindbis virus expression vectors: packaging of RNA replicons by using defective helper RNAs. J Virol. 1993 Nov;67(11):6439–6446. doi: 10.1128/jvi.67.11.6439-6446.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bron R., Wahlberg J. M., Garoff H., Wilschut J. Membrane fusion of Semliki Forest virus in a model system: correlation between fusion kinetics and structural changes in the envelope glycoprotein. EMBO J. 1993 Feb;12(2):693–701. doi: 10.1002/j.1460-2075.1993.tb05703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. T., Gliedman J. B. Morphological variants of Sindbis virus obtained from infected mosquito tissue culture cells. J Virol. 1973 Dec;12(6):1534–1539. doi: 10.1128/jvi.12.6.1534-1539.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. T., Smith J. F. Morphology of BHK-21 Cells Infected with Sindbis Virus Temperature-Sensitive Mutants in Complementation Groups D and E. J Virol. 1975 May;15(5):1262–1266. doi: 10.1128/jvi.15.5.1262-1266.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burness A. T., Pardoe I., Faragher S. G., Vrati S., Dalgarno L. Genetic stability of Ross River virus during epidemic spread in nonimmune humans. Virology. 1988 Dec;167(2):639–643. [PubMed] [Google Scholar]
- Buzan J. M., Schlesinger S. Expression of the nonstructural proteins of Sindbis virus in insect cells by a baculovirus vector. Virus Res. 1992 May;23(3):209–222. doi: 10.1016/0168-1702(92)90109-m. [DOI] [PubMed] [Google Scholar]
- CASALS J. ANTIGENIC VARIANTS OF EASTERN EQUINE ENCEPHALITIS VIRUS. J Exp Med. 1964 Apr 1;119:547–565. doi: 10.1084/jem.119.4.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calisher C. H., Karabatsos N., Lazuick J. S., Monath T. P., Wolff K. L. Reevaluation of the western equine encephalitis antigenic complex of alphaviruses (family Togaviridae) as determined by neutralization tests. Am J Trop Med Hyg. 1988 Mar;38(2):447–452. doi: 10.4269/ajtmh.1988.38.447. [DOI] [PubMed] [Google Scholar]
- Calisher C. H., Maness K. S., Lord R. D., Coleman P. H. Identification of two South American strains of eastern equine encephalomyelitis virus from migrant birds captured on the Mississippi delta. Am J Epidemiol. 1971 Aug;94(2):172–178. doi: 10.1093/oxfordjournals.aje.a121309. [DOI] [PubMed] [Google Scholar]
- Calzone F. J., Thézé N., Thiebaud P., Hill R. L., Britten R. J., Davidson E. H. Developmental appearance of factors that bind specifically to cis-regulatory sequences of a gene expressed in the sea urchin embryo. Genes Dev. 1988 Sep;2(9):1074–1088. doi: 10.1101/gad.2.9.1074. [DOI] [PubMed] [Google Scholar]
- Cancedda R., Bonatti S., Leone A. One extra oligosaccharide chain of the high-mannose class in the E2 protein of a Sindbis virus isolate. J Virol. 1981 Apr;38(1):8–14. doi: 10.1128/jvi.38.1.8-14.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco L., Otero M. J., Castrillo J. L. Modification of membrane permeability by animal viruses. Pharmacol Ther. 1989;40(2):171–212. doi: 10.1016/0163-7258(89)90096-x. [DOI] [PubMed] [Google Scholar]
- Carrasco L. The inhibition of cell functions after viral infection. A proposed general mechanism. FEBS Lett. 1977 Apr 1;76(1):11–15. doi: 10.1016/0014-5793(77)80110-5. [DOI] [PubMed] [Google Scholar]
- Cassell S., Edwards J., Brown D. T. Effects of lysosomotropic weak bases on infection of BHK-21 cells by Sindbis virus. J Virol. 1984 Dec;52(3):857–864. doi: 10.1128/jvi.52.3.857-864.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castronovo V., Claysmith A. P., Barker K. T., Cioce V., Krutzsch H. C., Sobel M. E. Biosynthesis of the 67 kDa high affinity laminin receptor. Biochem Biophys Res Commun. 1991 May 31;177(1):177–183. doi: 10.1016/0006-291x(91)91965-f. [DOI] [PubMed] [Google Scholar]
- Castronovo V., Taraboletti G., Sobel M. E. Functional domains of the 67-kDa laminin receptor precursor. J Biol Chem. 1991 Oct 25;266(30):20440–20446. [PubMed] [Google Scholar]
- Chanas A. C., Gould E. A., Clegg J. C., Varma M. G. Monoclonal antibodies to Sindbis virus glycoprotein E1 can neutralize, enhance infectivity, and independently inhibit haemagglutination or haemolysis. J Gen Virol. 1982 Jan;58(Pt 1):37–46. doi: 10.1099/0022-1317-58-1-37. [DOI] [PubMed] [Google Scholar]
- Chang G. J., Trent D. W. Nucleotide sequence of the genome region encoding the 26S mRNA of eastern equine encephalomyelitis virus and the deduced amino acid sequence of the viral structural proteins. J Gen Virol. 1987 Aug;68(Pt 8):2129–2142. doi: 10.1099/0022-1317-68-8-2129. [DOI] [PubMed] [Google Scholar]
- Chau V., Tobias J. W., Bachmair A., Marriott D., Ecker D. J., Gonda D. K., Varshavsky A. A multiubiquitin chain is confined to specific lysine in a targeted short-lived protein. Science. 1989 Mar 24;243(4898):1576–1583. doi: 10.1126/science.2538923. [DOI] [PubMed] [Google Scholar]
- Choi H. K., Tong L., Minor W., Dumas P., Boege U., Rossmann M. G., Wengler G. Structure of Sindbis virus core protein reveals a chymotrypsin-like serine proteinase and the organization of the virion. Nature. 1991 Nov 7;354(6348):37–43. doi: 10.1038/354037a0. [DOI] [PubMed] [Google Scholar]
- Clewley J. P., Kennedy S. I. Purification and polypeptide composition of Semliki Forest virus RNA polymerase. J Gen Virol. 1976 Sep;32(3):395–411. doi: 10.1099/0022-1317-32-3-395. [DOI] [PubMed] [Google Scholar]
- Condreay L. D., Brown D. T. Exclusion of superinfecting homologous virus by Sindbis virus-infected Aedes albopictus (mosquito) cells. J Virol. 1986 Apr;58(1):81–86. doi: 10.1128/jvi.58.1.81-86.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condreay L. D., Brown D. T. Suppression of RNA synthesis by a specific antiviral activity in Sindbis virus-infected Aedes albopictus cells. J Virol. 1988 Jan;62(1):346–348. doi: 10.1128/jvi.62.1.346-348.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs K. M., Brown D. T. Form-determining functions in Sindbis virus nucleocapsids: nucleosomelike organization of the nucleocapsid. J Virol. 1989 Feb;63(2):883–891. doi: 10.1128/jvi.63.2.883-891.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs K., Brown B., Brown D. T. Evidence for a change in capsid morphology during Sindbis virus envelopment. Virus Res. 1984;1(4):297–302. doi: 10.1016/0168-1702(84)90018-2. [DOI] [PubMed] [Google Scholar]
- Coombs K., Brown D. T. Topological organization of Sindbis virus capsid protein in isolated nucleocapsids. Virus Res. 1987 Apr;7(2):131–149. doi: 10.1016/0168-1702(87)90075-x. [DOI] [PubMed] [Google Scholar]
- Coombs K., Mann E., Edwards J., Brown D. T. Effects of chloroquine and cytochalasin B on the infection of cells by Sindbis virus and vesicular stomatitis virus. J Virol. 1981 Mar;37(3):1060–1065. doi: 10.1128/jvi.37.3.1060-1065.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross R. K., Gomatos P. J. Concomitant methylation and synthesis in vitro of Semliki Forest virus (SFV) ss RNAs by a fraction from infected cells. Virology. 1981 Oct 30;114(2):542–554. doi: 10.1016/0042-6822(81)90234-8. [DOI] [PubMed] [Google Scholar]
- Cross R. K. Identification of a unique guanine-7-methyltransferase in Semliki Forest virus (SFV) infected cell extracts. Virology. 1983 Oct 30;130(2):452–463. doi: 10.1016/0042-6822(83)90099-5. [DOI] [PubMed] [Google Scholar]
- Cutler D. F., Garoff H. Mutants of the membrane-binding region of Semliki Forest virus E2 protein. I. Cell surface transport and fusogenic activity. J Cell Biol. 1986 Mar;102(3):889–901. doi: 10.1083/jcb.102.3.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler D. F., Melancon P., Garoff H. Mutants of the membrane-binding region of Semliki Forest virus E2 protein. II. Topology and membrane binding. J Cell Biol. 1986 Mar;102(3):902–910. doi: 10.1083/jcb.102.3.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgarno L., Rice C. M., Strauss J. H. Ross River virus 26 s RNA: complete nucleotide sequence and deduced sequence of the encoded structural proteins. Virology. 1983 Aug;129(1):170–187. doi: 10.1016/0042-6822(83)90404-x. [DOI] [PubMed] [Google Scholar]
- Davey M. W., Dalgarno L. Semliki Forest virus replication in cultured Aedes albopictus cells: studies on the establishment of persistence. J Gen Virol. 1974 Sep;24(3):453–463. doi: 10.1099/0022-1317-24-3-453. [DOI] [PubMed] [Google Scholar]
- Davis N. L., Fuller F. J., Dougherty W. G., Olmsted R. A., Johnston R. E. A single nucleotide change in the E2 glycoprotein gene of Sindbis virus affects penetration rate in cell culture and virulence in neonatal mice. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6771–6775. doi: 10.1073/pnas.83.18.6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis N. L., Pence D. F., Meyer W. J., Schmaljohn A. L., Johnston R. E. Alternative forms of a strain-specific neutralizing antigenic site on the Sindbis virus E2 glycoprotein. Virology. 1987 Nov;161(1):101–108. doi: 10.1016/0042-6822(87)90175-9. [DOI] [PubMed] [Google Scholar]
- Davis N. L., Powell N., Greenwald G. F., Willis L. V., Johnson B. J., Smith J. F., Johnston R. E. Attenuating mutations in the E2 glycoprotein gene of Venezuelan equine encephalitis virus: construction of single and multiple mutants in a full-length cDNA clone. Virology. 1991 Jul;183(1):20–31. doi: 10.1016/0042-6822(91)90114-q. [DOI] [PubMed] [Google Scholar]
- Davis N. L., Willis L. V., Smith J. F., Johnston R. E. In vitro synthesis of infectious venezuelan equine encephalitis virus RNA from a cDNA clone: analysis of a viable deletion mutant. Virology. 1989 Jul;171(1):189–204. doi: 10.1016/0042-6822(89)90526-6. [DOI] [PubMed] [Google Scholar]
- Diamond D. C., Jameson B. A., Bonin J., Kohara M., Abe S., Itoh H., Komatsu T., Arita M., Kuge S., Nomoto A. Antigenic variation and resistance to neutralization in poliovirus type 1. Science. 1985 Sep 13;229(4718):1090–1093. doi: 10.1126/science.2412292. [DOI] [PubMed] [Google Scholar]
- Dickerman R. W., Martin M. S., Dipaola E. A. Studies of Venezuelan encephalitis in migrating birds in relation to possible transport of virus from South to Central America. Am J Trop Med Hyg. 1980 Mar;29(2):269–276. doi: 10.4269/ajtmh.1980.29.269. [DOI] [PubMed] [Google Scholar]
- Ding M. X., Schlesinger M. J. Evidence that Sindbis virus NSP2 is an autoprotease which processes the virus nonstructural polyprotein. Virology. 1989 Jul;171(1):280–284. doi: 10.1016/0042-6822(89)90539-4. [DOI] [PubMed] [Google Scholar]
- Docherty K., Rhodes C. J., Taylor N. A., Shennan K. I., Hutton J. C. Proinsulin endopeptidase substrate specificities defined by site-directed mutagenesis of proinsulin. J Biol Chem. 1989 Nov 5;264(31):18335–18339. [PubMed] [Google Scholar]
- Dominguez G., Wang C. Y., Frey T. K. Sequence of the genome RNA of rubella virus: evidence for genetic rearrangement during togavirus evolution. Virology. 1990 Jul;177(1):225–238. doi: 10.1016/0042-6822(90)90476-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doms R. W., Lamb R. A., Rose J. K., Helenius A. Folding and assembly of viral membrane proteins. Virology. 1993 Apr;193(2):545–562. doi: 10.1006/viro.1993.1164. [DOI] [PubMed] [Google Scholar]
- Dotti C. G., Kartenbeck J., Simons K. Polarized distribution of the viral glycoproteins of vesicular stomatitis, fowl plague and Semliki Forest viruses in hippocampal neurons in culture: a light and electron microscopy study. Brain Res. 1993 Apr 30;610(1):141–147. doi: 10.1016/0006-8993(93)91227-j. [DOI] [PubMed] [Google Scholar]
- Doxsey S. J., Brodsky F. M., Blank G. S., Helenius A. Inhibition of endocytosis by anti-clathrin antibodies. Cell. 1987 Jul 31;50(3):453–463. doi: 10.1016/0092-8674(87)90499-5. [DOI] [PubMed] [Google Scholar]
- Dubuisson J., Rice C. M. Sindbis virus attachment: isolation and characterization of mutants with impaired binding to vertebrate cells. J Virol. 1993 Jun;67(6):3363–3374. doi: 10.1128/jvi.67.6.3363-3374.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbin R. K., De Clercq E., Stollar V. SVLM21, a mutant of Sindbis virus able to grow in Aedes albopictus cells in the absence of methionine, shows increased sensitivity to S-adenosylhomocysteine hydrolase inhibitors such as neplanocin A. Virology. 1988 Mar;163(1):218–221. [PubMed] [Google Scholar]
- Durbin R. K., Stollar V. A mutant of sindbis virus with a host-dependent defect in maturation associated with hyperglycosylation of E2. Virology. 1984 Jun;135(2):331–344. doi: 10.1016/0042-6822(84)90190-9. [DOI] [PubMed] [Google Scholar]
- Durbin R. K., Stollar V. Sequence analysis of the E2 gene of a hyperglycosylated, host restricted mutant of Sindbis virus and estimation of mutation rate from frequency of revertants. Virology. 1986 Oct 15;154(1):135–143. doi: 10.1016/0042-6822(86)90436-8. [DOI] [PubMed] [Google Scholar]
- Durbin R. K., Stollar V. Sindbis virus mutants able to replicate in methionine-deprived Aedes albopictus cells. Virology. 1985 Jul 30;144(2):529–533. doi: 10.1016/0042-6822(85)90294-6. [DOI] [PubMed] [Google Scholar]
- Durbin R., Kane A., Stollar V. A mutant of Sindbis virus with altered plaque morphology and a decreased ratio of 26 S:49 S RNA synthesis in mosquito cells. Virology. 1991 Jul;183(1):306–312. doi: 10.1016/0042-6822(91)90143-y. [DOI] [PubMed] [Google Scholar]
- Edwards J., Brown D. T. Sindbis virus infection of a Chinese hamster ovary cell mutant defective in the acidification of endosomes. Virology. 1991 May;182(1):28–33. doi: 10.1016/0042-6822(91)90644-q. [DOI] [PubMed] [Google Scholar]
- Edwards J., Mann E., Brown D. T. Conformational changes in Sindbis virus envelope proteins accompanying exposure to low pH. J Virol. 1983 Mar;45(3):1090–1097. doi: 10.1128/jvi.45.3.1090-1097.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgizoli M., Dai Y., Kempf C., Koblet H., Michel M. R. Semliki Forest virus capsid protein acts as a pleiotropic regulator of host cellular protein synthesis. J Virol. 1989 Jul;63(7):2921–2928. doi: 10.1128/jvi.63.7.2921-2928.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erwin C., Brown D. T. Requirement of cell nucleus for Sindbis virus replication in cultured Aedes albopictus cells. J Virol. 1983 Feb;45(2):792–799. doi: 10.1128/jvi.45.2.792-799.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D. P., Sefton B. M. The entry into host cells of Sindbis virus, vesicular stomatitis virus and Sendai virus. Cell. 1978 Nov;15(3):985–992. doi: 10.1016/0092-8674(78)90282-9. [DOI] [PubMed] [Google Scholar]
- Faragher S. G., Dalgarno L. Regions of conservation and divergence in the 3' untranslated sequences of genomic RNA from Ross River virus isolates. J Mol Biol. 1986 Jul 20;190(2):141–148. doi: 10.1016/0022-2836(86)90287-1. [DOI] [PubMed] [Google Scholar]
- Faragher S. G., Marshall I. D., Dalgarno L. Ross River virus genetic variants in Australia and the Pacific Islands. Aust J Exp Biol Med Sci. 1985 Aug;63(Pt 4):473–488. doi: 10.1038/icb.1985.52. [DOI] [PubMed] [Google Scholar]
- Faragher S. G., Meek A. D., Rice C. M., Dalgarno L. Genome sequences of a mouse-avirulent and a mouse-virulent strain of Ross River virus. Virology. 1988 Apr;163(2):509–526. doi: 10.1016/0042-6822(88)90292-9. [DOI] [PubMed] [Google Scholar]
- Fazakerley J. K., Pathak S., Scallan M., Amor S., Dyson H. Replication of the A7(74) strain of Semliki Forest virus is restricted in neurons. Virology. 1993 Aug;195(2):627–637. doi: 10.1006/viro.1993.1414. [DOI] [PubMed] [Google Scholar]
- Feener E. P., Shen W. C., Ryser H. J. Cleavage of disulfide bonds in endocytosed macromolecules. A processing not associated with lysosomes or endosomes. J Biol Chem. 1990 Nov 5;265(31):18780–18785. [PubMed] [Google Scholar]
- Feng D. F., Doolittle R. F. Progressive sequence alignment as a prerequisite to correct phylogenetic trees. J Mol Evol. 1987;25(4):351–360. doi: 10.1007/BF02603120. [DOI] [PubMed] [Google Scholar]
- Feng Y. X., Copeland T. D., Oroszlan S., Rein A., Levin J. G. Identification of amino acids inserted during suppression of UAA and UGA termination codons at the gag-pol junction of Moloney murine leukemia virus. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8860–8863. doi: 10.1073/pnas.87.22.8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn D. C., Meyer W. J., Mackenzie J. M., Jr, Johnston R. E. A conformational change in Sindbis virus glycoproteins E1 and E2 is detected at the plasma membrane as a consequence of early virus-cell interaction. J Virol. 1990 Aug;64(8):3643–3653. doi: 10.1128/jvi.64.8.3643-3653.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn D. C., Olmsted R. A., Mackenzie J. M., Jr, Johnston R. E. Antibody-mediated activation of Sindbis virus. Virology. 1988 Sep;166(1):82–90. doi: 10.1016/0042-6822(88)90149-3. [DOI] [PubMed] [Google Scholar]
- Friedman R. M., Levin J. G., Grimley P. M., Berezesky I. K. Membrane-associated replication complex in arbovirus infection. J Virol. 1972 Sep;10(3):504–515. doi: 10.1128/jvi.10.3.504-515.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries E., Helenius A. Binding of Semliki Forest virus and its spike glycoproteins to cells. Eur J Biochem. 1979 Jun;97(1):213–220. doi: 10.1111/j.1432-1033.1979.tb13105.x. [DOI] [PubMed] [Google Scholar]
- Frolov I., Schlesinger S. Comparison of the effects of Sindbis virus and Sindbis virus replicons on host cell protein synthesis and cytopathogenicity in BHK cells. J Virol. 1994 Mar;68(3):1721–1727. doi: 10.1128/jvi.68.3.1721-1727.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froshauer S., Kartenbeck J., Helenius A. Alphavirus RNA replicase is located on the cytoplasmic surface of endosomes and lysosomes. J Cell Biol. 1988 Dec;107(6 Pt 1):2075–2086. doi: 10.1083/jcb.107.6.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulhorst C. F., Hardy J. L., Eldridge B. F., Presser S. B., Reeves W. C. Natural vertical transmission of western equine encephalomyelitis virus in mosquitoes. Science. 1994 Feb 4;263(5147):676–678. doi: 10.1126/science.8303276. [DOI] [PubMed] [Google Scholar]
- Fuller R. S., Sterne R. E., Thorner J. Enzymes required for yeast prohormone processing. Annu Rev Physiol. 1988;50:345–362. doi: 10.1146/annurev.ph.50.030188.002021. [DOI] [PubMed] [Google Scholar]
- Fuller S. D., Bravo R., Simons K. An enzymatic assay reveals that proteins destined for the apical or basolateral domains of an epithelial cell line share the same late Golgi compartments. EMBO J. 1985 Feb;4(2):297–307. doi: 10.1002/j.1460-2075.1985.tb03629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller S. D. The T=4 envelope of Sindbis virus is organized by interactions with a complementary T=3 capsid. Cell. 1987 Mar 27;48(6):923–934. doi: 10.1016/0092-8674(87)90701-x. [DOI] [PubMed] [Google Scholar]
- Gaedigk-Nitschko K., Ding M. X., Levy M. A., Schlesinger M. J. Site-directed mutations in the Sindbis virus 6K protein reveal sites for fatty acylation and the underacylated protein affects virus release and virion structure. Virology. 1990 Mar;175(1):282–291. doi: 10.1016/0042-6822(90)90210-i. [DOI] [PubMed] [Google Scholar]
- Gaedigk-Nitschko K., Schlesinger M. J. Site-directed mutations in Sindbis virus E2 glycoprotein's cytoplasmic domain and the 6K protein lead to similar defects in virus assembly and budding. Virology. 1991 Jul;183(1):206–214. doi: 10.1016/0042-6822(91)90133-v. [DOI] [PubMed] [Google Scholar]
- Gaedigk-Nitschko K., Schlesinger M. J. The Sindbis virus 6K protein can be detected in virions and is acylated with fatty acids. Virology. 1990 Mar;175(1):274–281. doi: 10.1016/0042-6822(90)90209-a. [DOI] [PubMed] [Google Scholar]
- Gahmberg C. G., Utermann G., Simons K. The membrane proteins of Semliki Forest virus have a hydrophobic part attached to the viral membrane. FEBS Lett. 1972 Dec 1;28(2):179–182. doi: 10.1016/0014-5793(72)80706-3. [DOI] [PubMed] [Google Scholar]
- Garavito R. M., Rossmann M. G., Argos P., Eventoff W. Convergence of active center geometries. Biochemistry. 1977 Nov 15;16(23):5065–5071. doi: 10.1021/bi00642a019. [DOI] [PubMed] [Google Scholar]
- Garoff H., Frischauf A. M., Simons K., Lehrach H., Delius H. Nucleotide sequence of cdna coding for Semliki Forest virus membrane glycoproteins. Nature. 1980 Nov 20;288(5788):236–241. doi: 10.1038/288236a0. [DOI] [PubMed] [Google Scholar]
- Garoff H., Frischauf A. M., Simons K., Lehrach H., Delius H. The capsid protein of Semliki Forest virus has clusters of basic amino acids and prolines in its amino-terminal region. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6376–6380. doi: 10.1073/pnas.77.11.6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garoff H., Huylebroeck D., Robinson A., Tillman U., Liljeström P. The signal sequence of the p62 protein of Semliki Forest virus is involved in initiation but not in completing chain translocation. J Cell Biol. 1990 Sep;111(3):867–876. doi: 10.1083/jcb.111.3.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garoff H., Kondor-Koch C., Pettersson R., Burke B. Expression of Semliki Forest virus proteins from cloned complementary DNA. II. The membrane-spanning glycoprotein E2 is transported to the cell surface without its normal cytoplasmic domain. J Cell Biol. 1983 Sep;97(3):652–658. doi: 10.1083/jcb.97.3.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garoff H., Simons K., Dobberstein B. Assembly of the Semliki Forest virus membrane glycoproteins in the membrane of the endoplasmic reticulum in vitro. J Mol Biol. 1978 Oct 5;124(4):587–600. doi: 10.1016/0022-2836(78)90173-0. [DOI] [PubMed] [Google Scholar]
- Garoff H., Simons K. Location of the spike glycoproteins in the Semliki Forest virus membrane. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3988–3992. doi: 10.1073/pnas.71.10.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garry R. F., Bishop J. M., Parker S., Westbrook K., Lewis G., Waite M. R. Na+ and K+ concentrations and the regulation of protein synthesis in Sindbis virus-infected chick cells. Virology. 1979 Jul 15;96(1):108–120. doi: 10.1016/0042-6822(79)90177-6. [DOI] [PubMed] [Google Scholar]
- Garry R. F., Bostick D. A. Induction of the stress response: alterations in membrane-associated transport systems and protein modification in heat shocked or Sindbis virus-infected cells. Virus Res. 1987 Sep;8(3):245–259. doi: 10.1016/0168-1702(87)90019-0. [DOI] [PubMed] [Google Scholar]
- Garry R. F. Sindbis virus-induced inhibition of protein synthesis is partially reversed by medium containing an elevated potassium concentration. J Gen Virol. 1994 Feb;75(Pt 2):411–415. doi: 10.1099/0022-1317-75-2-411. [DOI] [PubMed] [Google Scholar]
- Garry R. F., Westbrook K., Waite M. R. Differential effects of ouabain on host- and sindbis virus-specified protein synthesis. Virology. 1979 Nov;99(1):179–182. doi: 10.1016/0042-6822(79)90051-5. [DOI] [PubMed] [Google Scholar]
- Gaulton G. N., Greene M. I. Idiotypic mimicry of biological receptors. Annu Rev Immunol. 1986;4:253–280. doi: 10.1146/annurev.iy.04.040186.001345. [DOI] [PubMed] [Google Scholar]
- Geigenmüller-Gnirke U., Nitschko H., Schlesinger S. Deletion analysis of the capsid protein of Sindbis virus: identification of the RNA binding region. J Virol. 1993 Mar;67(3):1620–1626. doi: 10.1128/jvi.67.3.1620-1626.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geigenmüller-Gnirke U., Weiss B., Wright R., Schlesinger S. Complementation between Sindbis viral RNAs produces infectious particles with a bipartite genome. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3253–3257. doi: 10.1073/pnas.88.8.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidwitz S., Polo J. M., Davis N. L., Johnston R. E. Differences in virion stability among Sindbis virus pathogenesis mutants. Virus Res. 1988 May;10(2-3):225–239. doi: 10.1016/0168-1702(88)90018-4. [DOI] [PubMed] [Google Scholar]
- Glanville N., Ulmanen I. Biological activity of in vitro synthesised protein: binding of Semliki Forest virus capsid protein to the large ribosomal subunit. Biochem Biophys Res Commun. 1976 Jul 12;71(1):393–399. doi: 10.1016/0006-291x(76)90295-3. [DOI] [PubMed] [Google Scholar]
- Gomatos P. J., Käriäinen L., Keränen S., Ranki M., Sawicki D. L. Semliki Forest virus replication complex capable of synthesizing 42S and 26S nascent RNA chains. J Gen Virol. 1980 Jul;49(1):61–69. doi: 10.1099/0022-1317-49-1-61. [DOI] [PubMed] [Google Scholar]
- Gonda D. K., Bachmair A., Wünning I., Tobias J. W., Lane W. S., Varshavsky A. Universality and structure of the N-end rule. J Biol Chem. 1989 Oct 5;264(28):16700–16712. [PubMed] [Google Scholar]
- Gorbalenya A. E., Blinov V. M., Donchenko A. P., Koonin E. V. An NTP-binding motif is the most conserved sequence in a highly diverged monophyletic group of proteins involved in positive strand RNA viral replication. J Mol Evol. 1989 Mar;28(3):256–268. doi: 10.1007/BF02102483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya A. E., Donchenko A. P., Koonin E. V., Blinov V. M. N-terminal domains of putative helicases of flavi- and pestiviruses may be serine proteases. Nucleic Acids Res. 1989 May 25;17(10):3889–3897. doi: 10.1093/nar/17.10.3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya A. E., Koonin E. V., Donchenko A. P., Blinov V. M. A conserved NTP-motif in putative helicases. Nature. 1988 May 5;333(6168):22–22. doi: 10.1038/333022a0. [DOI] [PubMed] [Google Scholar]
- Gorbalenya A. E., Koonin E. V., Donchenko A. P., Blinov V. M. A novel superfamily of nucleoside triphosphate-binding motif containing proteins which are probably involved in duplex unwinding in DNA and RNA replication and recombination. FEBS Lett. 1988 Aug 1;235(1-2):16–24. doi: 10.1016/0014-5793(88)81226-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya A. E., Koonin E. V., Donchenko A. P., Blinov V. M. Two related superfamilies of putative helicases involved in replication, recombination, repair and expression of DNA and RNA genomes. Nucleic Acids Res. 1989 Jun 26;17(12):4713–4730. doi: 10.1093/nar/17.12.4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya A. E., Koonin E. V., Lai M. M. Putative papain-related thiol proteases of positive-strand RNA viruses. Identification of rubi- and aphthovirus proteases and delineation of a novel conserved domain associated with proteases of rubi-, alpha- and coronaviruses. FEBS Lett. 1991 Aug 19;288(1-2):201–205. doi: 10.1016/0014-5793(91)81034-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya A. E., Koonin E. V., Wolf Y. I. A new superfamily of putative NTP-binding domains encoded by genomes of small DNA and RNA viruses. FEBS Lett. 1990 Mar 12;262(1):145–148. doi: 10.1016/0014-5793(90)80175-i. [DOI] [PubMed] [Google Scholar]
- Grakoui A., Levis R., Raju R., Huang H. V., Rice C. M. A cis-acting mutation in the Sindbis virus junction region which affects subgenomic RNA synthesis. J Virol. 1989 Dec;63(12):5216–5227. doi: 10.1128/jvi.63.12.5216-5227.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray M. A., Austin S. A., Clemens M. J., Rodrigues L., Pasternak C. A. Protein synthesis in Semliki Forest virus-infected cells is not controlled by permeability changes. J Gen Virol. 1983 Dec;64(Pt 12):2631–2640. doi: 10.1099/0022-1317-64-12-2631. [DOI] [PubMed] [Google Scholar]
- Gray M. A., Micklem K. J., Brown F., Pasternak C. A. Effect of vesicular stomatitis virus and Semliki Forest Virus on uptake of nutrients and intracellular cation concentration. J Gen Virol. 1983 Jul;64(Pt 7):1449–1456. doi: 10.1099/0022-1317-64-7-1449. [DOI] [PubMed] [Google Scholar]
- Gray M. A., Micklem K. J., Pasternak C. A. Protein synthesis in cells infected with Semliki Forest virus is not controlled by intracellular cation changes. Eur J Biochem. 1983 Sep 15;135(2):299–302. doi: 10.1111/j.1432-1033.1983.tb07652.x. [DOI] [PubMed] [Google Scholar]
- Griffin D. E., Johnson R. T. Role of the immune response in recovery from Sindbis virus encephalitis in mice. J Immunol. 1977 Mar;118(3):1070–1075. [PubMed] [Google Scholar]
- Griffin D. E. Molecular pathogenesis of Sindbis virus encephalitis in experimental animals. Adv Virus Res. 1989;36:255–271. doi: 10.1016/s0065-3527(08)60587-4. [DOI] [PubMed] [Google Scholar]
- Griffiths G., Quinn P., Warren G. Dissection of the Golgi complex. I. Monensin inhibits the transport of viral membrane proteins from medial to trans Golgi cisternae in baby hamster kidney cells infected with Semliki Forest virus. J Cell Biol. 1983 Mar;96(3):835–850. doi: 10.1083/jcb.96.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimley P. M., Berezesky I. K., Friedman R. M. Cytoplasmic structures associated with an arbovirus infection: loci of viral ribonucleic acid synthesis. J Virol. 1968 Nov;2(11):1326–1338. doi: 10.1128/jvi.2.11.1326-1338.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimley P. M., Levin J. G., Berezesky I. K., Friedman R. M. Specific membranous structures associated with the replication of group A arboviruses. J Virol. 1972 Sep;10(3):492–503. doi: 10.1128/jvi.10.3.492-503.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosfeld H., Velan B., Leitner M., Cohen S., Lustig S., Lachmi B. E., Shafferman A. Semliki Forest virus E2 envelope epitopes induce a nonneutralizing humoral response which protects mice against lethal challenge. J Virol. 1989 Aug;63(8):3416–3422. doi: 10.1128/jvi.63.8.3416-3422.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosso L. E., Park P. W., Mecham R. P. Characterization of a putative clone for the 67-kilodalton elastin/laminin receptor suggests that it encodes a cytoplasmic protein rather than a cell surface receptor. Biochemistry. 1991 Apr 2;30(13):3346–3350. doi: 10.1021/bi00227a026. [DOI] [PubMed] [Google Scholar]
- Hahn C. S., Hahn Y. S., Braciale T. J., Rice C. M. Infectious Sindbis virus transient expression vectors for studying antigen processing and presentation. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2679–2683. doi: 10.1073/pnas.89.7.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn C. S., Lustig S., Strauss E. G., Strauss J. H. Western equine encephalitis virus is a recombinant virus. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5997–6001. doi: 10.1073/pnas.85.16.5997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn C. S., Rice C. M., Strauss E. G., Lenches E. M., Strauss J. H. Sindbis virus ts103 has a mutation in glycoprotein E2 that leads to defective assembly of virions. J Virol. 1989 Aug;63(8):3459–3465. doi: 10.1128/jvi.63.8.3459-3465.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn C. S., Strauss E. G., Strauss J. H. Sequence analysis of three Sindbis virus mutants temperature-sensitive in the capsid protein autoprotease. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4648–4652. doi: 10.1073/pnas.82.14.4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn C. S., Strauss J. H. Site-directed mutagenesis of the proposed catalytic amino acids of the Sindbis virus capsid protein autoprotease. J Virol. 1990 Jun;64(6):3069–3073. doi: 10.1128/jvi.64.6.3069-3073.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn Y. S., Grakoui A., Rice C. M., Strauss E. G., Strauss J. H. Mapping of RNA- temperature-sensitive mutants of Sindbis virus: complementation group F mutants have lesions in nsP4. J Virol. 1989 Mar;63(3):1194–1202. doi: 10.1128/jvi.63.3.1194-1202.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn Y. S., Hahn C. S., Braciale V. L., Braciale T. J., Rice C. M. CD8+ T cell recognition of an endogenously processed epitope is regulated primarily by residues within the epitope. J Exp Med. 1992 Nov 1;176(5):1335–1341. doi: 10.1084/jem.176.5.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn Y. S., Strauss E. G., Strauss J. H. Mapping of RNA- temperature-sensitive mutants of Sindbis virus: assignment of complementation groups A, B, and G to nonstructural proteins. J Virol. 1989 Jul;63(7):3142–3150. doi: 10.1128/jvi.63.7.3142-3150.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson R. P., Sulkin S. E., Beuscher E. L., Hammon W. M., McKinney R. W., Work T. H. Arbovirus infections of laboratory workers. Extent of problem emphasizes the need for more effective measures to reduce hazards. Science. 1967 Dec 8;158(3806):1283–1286. doi: 10.1126/science.158.3806.1283. [DOI] [PubMed] [Google Scholar]
- Hardy W. R., Hahn Y. S., de Groot R. J., Strauss E. G., Strauss J. H. Synthesis and processing of the nonstructural polyproteins of several temperature-sensitive mutants of Sindbis virus. Virology. 1990 Jul;177(1):199–208. doi: 10.1016/0042-6822(90)90473-5. [DOI] [PubMed] [Google Scholar]
- Hardy W. R., Strauss J. H. Processing the nonstructural polyproteins of Sindbis virus: study of the kinetics in vivo by using monospecific antibodies. J Virol. 1988 Mar;62(3):998–1007. doi: 10.1128/jvi.62.3.998-1007.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy W. R., Strauss J. H. Processing the nonstructural polyproteins of sindbis virus: nonstructural proteinase is in the C-terminal half of nsP2 and functions both in cis and in trans. J Virol. 1989 Nov;63(11):4653–4664. doi: 10.1128/jvi.63.11.4653-4664.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison S. C., Strong R. K., Schlesinger S., Schlesinger M. J. Crystallization of Sindbis virus and its nucleocapsid. J Mol Biol. 1992 Jul 5;226(1):277–280. doi: 10.1016/0022-2836(92)90141-6. [DOI] [PubMed] [Google Scholar]
- Haseloff J., Goelet P., Zimmern D., Ahlquist P., Dasgupta R., Kaesberg P. Striking similarities in amino acid sequence among nonstructural proteins encoded by RNA viruses that have dissimilar genomic organization. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4358–4362. doi: 10.1073/pnas.81.14.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K., Erdei S., Keränen S., Saraste J., Käriäinen L. Evidence for a separate signal sequence for the carboxy-terminal envelope glycoprotein E1 of Semliki forest virus. J Virol. 1981 Apr;38(1):34–40. doi: 10.1128/jvi.38.1.34-40.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidner H. W., McKnight K. L., Davis N. L., Johnston R. E. Lethality of PE2 incorporation into Sindbis virus can be suppressed by second-site mutations in E3 and E2. J Virol. 1994 Apr;68(4):2683–2692. doi: 10.1128/jvi.68.4.2683-2692.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A., Kartenbeck J., Simons K., Fries E. On the entry of Semliki forest virus into BHK-21 cells. J Cell Biol. 1980 Feb;84(2):404–420. doi: 10.1083/jcb.84.2.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A., Kartenbeck J. The effects of octylglucoside on the Semliki forest virus membrane. Evidence for a spike-protein--nucleocapsid interaction. Eur J Biochem. 1980 May;106(2):613–618. doi: 10.1111/j.1432-1033.1980.tb04609.x. [DOI] [PubMed] [Google Scholar]
- Helenius A., Kielian M., Wellsteed J., Mellman I., Rudnick G. Effects of monovalent cations on Semliki Forest virus entry into BHK-21 cells. J Biol Chem. 1985 May 10;260(9):5691–5697. [PubMed] [Google Scholar]
- Helenius A., Marsh M., White J. Inhibition of Semliki forest virus penetration by lysosomotropic weak bases. J Gen Virol. 1982 Jan;58(Pt 1):47–61. doi: 10.1099/0022-1317-58-1-47. [DOI] [PubMed] [Google Scholar]
- Helenius A., Morein B., Fries E., Simons K., Robinson P., Schirrmacher V., Terhorst C., Strominger J. L. Human (HLA-A and HLA-B) and murine (H-2K and H-2D) histocompatibility antigens are cell surface receptors for Semliki Forest virus. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3846–3850. doi: 10.1073/pnas.75.8.3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A. Semliki Forest virus penetration from endosomes: a morphological study. Biol Cell. 1984;51(2):181–185. doi: 10.1111/j.1768-322x.1984.tb00297.x. [DOI] [PubMed] [Google Scholar]
- Hertz J. M., Huang H. V. Utilization of heterologous alphavirus junction sequences as promoters by Sindbis virus. J Virol. 1992 Feb;66(2):857–864. doi: 10.1128/jvi.66.2.857-864.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higaki J. N., Gibson B. W., Craik C. S. Evolution of catalysis in the serine proteases. Cold Spring Harb Symp Quant Biol. 1987;52:615–621. doi: 10.1101/sqb.1987.052.01.070. [DOI] [PubMed] [Google Scholar]
- Hirsch R. L., Griffin D. E., Winkelstein J. A. Host modification of Sindbis virus sialic acid content influences alternative complement pathway activation and virus clearance. J Immunol. 1981 Nov;127(5):1740–1743. [PubMed] [Google Scholar]
- Hirsch R. L., Griffin D. E., Winkelstein J. A. Natural immunity to Sindbis virus is influenced by host tissue sialic acid content. Proc Natl Acad Sci U S A. 1983 Jan;80(2):548–550. doi: 10.1073/pnas.80.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch R. L., Griffin D. E., Winkelstein J. A. Role of complement in viral infections: participation of terminal complement components (C5 to C9) in recovery of mice from Sindbis virus infection. Infect Immun. 1980 Dec;30(3):899–901. doi: 10.1128/iai.30.3.899-901.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch R. L., Griffin D. E., Winkelstein J. A. The effect of complement depletion on the course of Sindbis virus infection in mice. J Immunol. 1978 Oct;121(4):1276–1278. [PubMed] [Google Scholar]
- Hirsch R. L., Griffin D. E., Winkelstein J. A. The role of complement in viral infections. II. the clearance of Sindbis virus from the bloodstream and central nervous system of mice depleted of complement. J Infect Dis. 1980 Feb;141(2):212–217. doi: 10.1093/infdis/141.2.212. [DOI] [PubMed] [Google Scholar]
- Hirsch R. L., Winkelstein J. A., Griffin D. E. The role of complement in viral infections. III. Activation of the classical and alternative complement pathways by Sindbis virus. J Immunol. 1980 May;124(5):2507–2510. [PubMed] [Google Scholar]
- Hodgman T. C. A new superfamily of replicative proteins. Nature. 1988 May 5;333(6168):22–23. doi: 10.1038/333022b0. [DOI] [PubMed] [Google Scholar]
- Hoekstra D., Kok J. W. Entry mechanisms of enveloped viruses. Implications for fusion of intracellular membranes. Biosci Rep. 1989 Jun;9(3):273–305. doi: 10.1007/BF01114682. [DOI] [PubMed] [Google Scholar]
- Holland J. J., De La Torre J. C., Steinhauer D. A. RNA virus populations as quasispecies. Curr Top Microbiol Immunol. 1992;176:1–20. doi: 10.1007/978-3-642-77011-1_1. [DOI] [PubMed] [Google Scholar]
- Holland J., Spindler K., Horodyski F., Grabau E., Nichol S., VandePol S. Rapid evolution of RNA genomes. Science. 1982 Mar 26;215(4540):1577–1585. doi: 10.1126/science.7041255. [DOI] [PubMed] [Google Scholar]
- Houk E. J., Arcus Y. M., Hardy J. L., Kramer L. D. Binding of western equine encephalomyelitis virus to brush border fragments isolated from mesenteronal epithelial cells of mosquitoes. Virus Res. 1990 Oct;17(2):105–117. doi: 10.1016/0168-1702(90)90072-j. [DOI] [PubMed] [Google Scholar]
- Hsieh P., Rosner M. R., Robbins P. W. Host-dependent variation of asparagine-linked oligosaccharides at individual glycosylation sites of Sindbis virus glycoproteins. J Biol Chem. 1983 Feb 25;258(4):2548–2554. [PubMed] [Google Scholar]
- Hsieh P., Rosner M. R., Robbins P. W. Selective cleavage by endo-beta-N-acetylglucosaminidase H at individual glycosylation sites of Sindbis virion envelope glycoproteins. J Biol Chem. 1983 Feb 25;258(4):2555–2561. [PubMed] [Google Scholar]
- HsuChen C. C., Dubin D. T. Di-and trimethylated congeners of 7-methylguanine in Sindbis virus mRNA. Nature. 1976 Nov 11;264(5582):190–191. doi: 10.1038/264190a0. [DOI] [PubMed] [Google Scholar]
- Huang H. V., Rice C. M., Xiong C., Schlesinger S. RNA viruses as gene expression vectors. Virus Genes. 1989 Sep;3(1):85–91. doi: 10.1007/BF00301989. [DOI] [PubMed] [Google Scholar]
- Hunt A. R., Johnson A. J., Roehrig J. T. Synthetic peptides of Venezuelan equine encephalomyelitis virus E2 glycoprotein. I. Immunogenic analysis and identification of a protective peptide. Virology. 1990 Dec;179(2):701–711. doi: 10.1016/0042-6822(90)90137-g. [DOI] [PubMed] [Google Scholar]
- Hunt A. R., Roehrig J. T. Biochemical and biological characteristics of epitopes on the E1 glycoprotein of western equine encephalitis virus. Virology. 1985 Apr 30;142(2):334–346. doi: 10.1016/0042-6822(85)90342-3. [DOI] [PubMed] [Google Scholar]
- Huth A., Rapoport T. A., Käriäinen L. Envelope proteins of Semliki Forest virus synthesized in Xenopus oocytes are transported to the cell surface. EMBO J. 1984 Apr;3(4):767–771. doi: 10.1002/j.1460-2075.1984.tb01882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova L., Schlesinger M. J. Site-directed mutations in the Sindbis virus E2 glycoprotein identify palmitoylation sites and affect virus budding. J Virol. 1993 May;67(5):2546–2551. doi: 10.1128/jvi.67.5.2546-2551.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A. C., Moench T. R., Griffin D. E., Johnson R. T. The pathogenesis of spinal cord involvement in the encephalomyelitis of mice caused by neuroadapted Sindbis virus infection. Lab Invest. 1987 Apr;56(4):418–423. [PubMed] [Google Scholar]
- Jackson A. C., Moench T. R., Trapp B. D., Griffin D. E. Basis of neurovirulence in Sindbis virus encephalomyelitis of mice. Lab Invest. 1988 May;58(5):503–509. [PubMed] [Google Scholar]
- Jain S. K., DeCandido S., Kielian M. Processing of the p62 envelope precursor protein of Semliki Forest virus. J Biol Chem. 1991 Mar 25;266(9):5756–5761. [PubMed] [Google Scholar]
- Jalanko A. Expression of Semliki Forest virus capsid protein from SV40 recombinant virus. FEBS Lett. 1985 Jul 1;186(1):59–64. doi: 10.1016/0014-5793(85)81339-9. [DOI] [PubMed] [Google Scholar]
- Jalanko A., Söderlund H. The repeated regions of Semliki Forest virus defective-inferfering RNA interferes with the encapsidation process of the standard virus. Virology. 1985 Mar;141(2):257–266. doi: 10.1016/0042-6822(85)90256-9. [DOI] [PubMed] [Google Scholar]
- Johnson B. J., Brubaker J. R., Roehrig J. T., Trent D. W. Variants of Venezuelan equine encephalitis virus that resist neutralization define a domain of the E2 glycoprotein. Virology. 1990 Aug;177(2):676–683. doi: 10.1016/0042-6822(90)90533-w. [DOI] [PubMed] [Google Scholar]
- Johnson B. J., Kinney R. M., Kost C. L., Trent D. W. Molecular determinants of alphavirus neurovirulence: nucleotide and deduced protein sequence changes during attenuation of Venezuelan equine encephalitis virus. J Gen Virol. 1986 Sep;67(Pt 9):1951–1960. doi: 10.1099/0022-1317-67-9-1951. [DOI] [PubMed] [Google Scholar]
- Johnson D. C., Schlesinger M. J., Elson E. L. Fluorescence photobleaching recovery measurements reveal differences in envelopment of Sindbis and vesicular stomatitis viruses. Cell. 1981 Feb;23(2):423–431. doi: 10.1016/0092-8674(81)90137-9. [DOI] [PubMed] [Google Scholar]
- Johnson D. C., Schlesinger M. J. Vesicular stomatitis virus and sindbis virus glycoprotein transport to the cell surface is inhibited by ionophores. Virology. 1980 Jun;103(2):407–424. doi: 10.1016/0042-6822(80)90200-7. [DOI] [PubMed] [Google Scholar]
- Johnson E. S., Gonda D. K., Varshavsky A. cis-trans recognition and subunit-specific degradation of short-lived proteins. Nature. 1990 Jul 19;346(6281):287–291. doi: 10.1038/346287a0. [DOI] [PubMed] [Google Scholar]
- Johnston R. E., Smith J. F. Selection for accelerated penetration in cell culture coselects for attenuated mutants of Venezuelan equine encephalitis virus. Virology. 1988 Feb;162(2):437–443. doi: 10.1016/0042-6822(88)90484-9. [DOI] [PubMed] [Google Scholar]
- Johnston R. E., Tovell D. R., Brown D. T., Faulkner P. Interfering passages of Sindbis virus: concomitant appearance of interference, morphological variants, and trucated viral RNA. J Virol. 1975 Oct;16(4):951–958. doi: 10.1128/jvi.16.4.951-958.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. E., Wan K., Bose H. R. Homologous interference induced by Sindbis virus. J Virol. 1974 Nov;14(5):1076–1082. doi: 10.1128/jvi.14.5.1076-1082.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. J., Scupham R. K., Pfeil J. A., Wan K., Sagik B. P., Bose H. R. Interaction of Sindbis virus glycoproteins during morphogenesis. J Virol. 1977 Feb;21(2):778–787. doi: 10.1128/jvi.21.2.778-787.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justman J., Klimjack M. R., Kielian M. Role of spike protein conformational changes in fusion of Semliki Forest virus. J Virol. 1993 Dec;67(12):7597–7607. doi: 10.1128/jvi.67.12.7597-7607.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kail M., Hollinshead M., Ansorge W., Pepperkok R., Frank R., Griffiths G., Vaux D. The cytoplasmic domain of alphavirus E2 glycoprotein contains a short linear recognition signal required for viral budding. EMBO J. 1991 Sep;10(9):2343–2351. doi: 10.1002/j.1460-2075.1991.tb07773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaluza G., Pauli G. The influence of intramolecular disulfide bonds on the structure and function of Semliki forest virus membrane glycoproteins. Virology. 1980 Apr 30;102(2):300–309. doi: 10.1016/0042-6822(80)90097-5. [DOI] [PubMed] [Google Scholar]
- Kamer G., Argos P. Primary structural comparison of RNA-dependent polymerases from plant, animal and bacterial viruses. Nucleic Acids Res. 1984 Sep 25;12(18):7269–7282. doi: 10.1093/nar/12.18.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempf C., Kohler U., Michel M. R., Koblet H. Semliki Forest virus-induced polykaryocyte formation is an ATP-dependent event. Arch Virol. 1987;95(1-2):111–122. doi: 10.1007/BF01311338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempf C., Michel M. R., Kohler U., Koblet H. A novel method for the detection of early events in cell-cell fusion of Semliki Forest virus infected cells growing in monolayer cultures. Arch Virol. 1987;95(3-4):283–289. doi: 10.1007/BF01310786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempf C., Michel M. R., Kohler U., Koblet H. Exposure of Semliki Forest virus-infected baby hamster kidney cells to low pH leads to a proton influx and a rapid depletion of intracellular ATP which in turn prevents cell-cell fusion. Brief report. Arch Virol. 1988;99(1-2):111–115. doi: 10.1007/BF01311028. [DOI] [PubMed] [Google Scholar]
- Kempf C., Michel M. R., Omar A., Jentsch P., Morell A. Semliki Forest virus induced cell-cell fusion at neutral extracellular pH. Biosci Rep. 1990 Aug;10(4):363–374. doi: 10.1007/BF01117236. [DOI] [PubMed] [Google Scholar]
- Kerr P. J., Fitzgerald S., Tregear G. W., Dalgarno L., Weir R. C. Characterization of a major neutralization domain of Ross river virus using anti-viral and anti-peptide antibodies. Virology. 1992 Mar;187(1):338–342. doi: 10.1016/0042-6822(92)90324-i. [DOI] [PubMed] [Google Scholar]
- Kerr P. J., Weir R. C., Dalgarno L. Ross River virus variants selected during passage in chick embryo fibroblasts: serological, genetic, and biological changes. Virology. 1993 Mar;193(1):446–449. doi: 10.1006/viro.1993.1143. [DOI] [PubMed] [Google Scholar]
- Keränen S., Käriäinen L. Functional defects of RNA-negative temperature-sensitive mutants of Sindbis and Semliki Forest viruses. J Virol. 1979 Oct;32(1):19–29. doi: 10.1128/jvi.32.1.19-29.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keränen S., Ruohonen L. Nonstructural proteins of Semliki Forest virus: synthesis, processing, and stability in infected cells. J Virol. 1983 Sep;47(3):505–515. doi: 10.1128/jvi.47.3.505-515.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielian M. C., Helenius A. Role of cholesterol in fusion of Semliki Forest virus with membranes. J Virol. 1984 Oct;52(1):281–283. doi: 10.1128/jvi.52.1.281-283.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielian M. C., Keränen S., Käriäinen L., Helenius A. Membrane fusion mutants of Semliki Forest virus. J Cell Biol. 1984 Jan;98(1):139–145. doi: 10.1083/jcb.98.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielian M. C., Marsh M., Helenius A. Kinetics of endosome acidification detected by mutant and wild-type Semliki Forest virus. EMBO J. 1986 Dec 1;5(12):3103–3109. doi: 10.1002/j.1460-2075.1986.tb04616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielian M., Helenius A. pH-induced alterations in the fusogenic spike protein of Semliki Forest virus. J Cell Biol. 1985 Dec;101(6):2284–2291. doi: 10.1083/jcb.101.6.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielian M., Jungerwirth S. Mechanisms of enveloped virus entry into cells. Mol Biol Med. 1990 Feb;7(1):17–31. [PubMed] [Google Scholar]
- Kielian M., Jungerwirth S., Sayad K. U., DeCandido S. Biosynthesis, maturation, and acid activation of the Semliki Forest virus fusion protein. J Virol. 1990 Oct;64(10):4614–4624. doi: 10.1128/jvi.64.10.4614-4624.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney R. M., Chang G. J., Tsuchiya K. R., Sneider J. M., Roehrig J. T., Woodward T. M., Trent D. W. Attenuation of Venezuelan equine encephalitis virus strain TC-83 is encoded by the 5'-noncoding region and the E2 envelope glycoprotein. J Virol. 1993 Mar;67(3):1269–1277. doi: 10.1128/jvi.67.3.1269-1277.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney R. M., Johnson B. J., Brown V. L., Trent D. W. Nucleotide sequence of the 26 S mRNA of the virulent Trinidad donkey strain of Venezuelan equine encephalitis virus and deduced sequence of the encoded structural proteins. Virology. 1986 Jul 30;152(2):400–413. doi: 10.1016/0042-6822(86)90142-x. [DOI] [PubMed] [Google Scholar]
- Kinney R. M., Johnson B. J., Welch J. B., Tsuchiya K. R., Trent D. W. The full-length nucleotide sequences of the virulent Trinidad donkey strain of Venezuelan equine encephalitis virus and its attenuated vaccine derivative, strain TC-83. Virology. 1989 May;170(1):19–30. doi: 10.1016/0042-6822(89)90347-4. [DOI] [PubMed] [Google Scholar]
- Kinney R. M., Tsuchiya K. R., Sneider J. M., Trent D. W. Genetic evidence that epizootic Venezuelan equine encephalitis (VEE) viruses may have evolved from enzootic VEE subtype I-D virus. Virology. 1992 Dec;191(2):569–580. doi: 10.1016/0042-6822(92)90232-e. [DOI] [PubMed] [Google Scholar]
- Kinney R. M., Tsuchiya K. R., Sneider J. M., Trent D. W. Molecular evidence for the origin of the widespread Venezuelan equine encephalitis epizootic of 1969 to 1972. J Gen Virol. 1992 Dec;73(Pt 12):3301–3305. doi: 10.1099/0022-1317-73-12-3301. [DOI] [PubMed] [Google Scholar]
- Koblet H., Kempf C., Kohler U., Omar A. Conformational changes at pH 6 on the cell surface of Semliki Forest virus-infected Aedes albopictus cells. Virology. 1985 May;143(1):334–336. doi: 10.1016/0042-6822(85)90120-5. [DOI] [PubMed] [Google Scholar]
- Kohara M., Omata T., Kameda A., Semler B. L., Itoh H., Wimmer E., Nomoto A. In vitro phenotypic markers of a poliovirus recombinant constructed from infectious cDNA clones of the neurovirulent Mahoney strain and the attenuated Sabin 1 strain. J Virol. 1985 Mar;53(3):786–792. doi: 10.1128/jvi.53.3.786-792.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondor-Koch C., Burke B., Garoff H. Expression of Semliki Forest virus proteins from cloned complementary DNA. I. The fusion activity of the spike glycoprotein. J Cell Biol. 1983 Sep;97(3):644–651. doi: 10.1083/jcb.97.3.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondor-Koch C., Riedel H., Söderberg K., Garoff H. Expression of the structural proteins of Semliki Forest virus from cloned cDNA microinjected into the nucleus of baby hamster kidney cells. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4525–4529. doi: 10.1073/pnas.79.15.4525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koonin E. V., Dolja V. V. Evolution and taxonomy of positive-strand RNA viruses: implications of comparative analysis of amino acid sequences. Crit Rev Biochem Mol Biol. 1993;28(5):375–430. doi: 10.3109/10409239309078440. [DOI] [PubMed] [Google Scholar]
- Koonin E. V. The phylogeny of RNA-dependent RNA polymerases of positive-strand RNA viruses. J Gen Virol. 1991 Sep;72(Pt 9):2197–2206. doi: 10.1099/0022-1317-72-9-2197. [DOI] [PubMed] [Google Scholar]
- Kowal K. J., Stollar V. Temperature-sensitive host-dependent mutants of Sindbis virus. Virology. 1981 Oct 15;114(1):140–148. doi: 10.1016/0042-6822(81)90260-9. [DOI] [PubMed] [Google Scholar]
- Kuhn R. J., Griffin D. E., Zhang H., Niesters H. G., Strauss J. H. Attenuation of Sindbis virus neurovirulence by using defined mutations in nontranslated regions of the genome RNA. J Virol. 1992 Dec;66(12):7121–7127. doi: 10.1128/jvi.66.12.7121-7127.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn R. J., Hong Z., Strauss J. H. Mutagenesis of the 3' nontranslated region of Sindbis virus RNA. J Virol. 1990 Apr;64(4):1465–1476. doi: 10.1128/jvi.64.4.1465-1476.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn R. J., Niesters H. G., Hong Z., Strauss J. H. Infectious RNA transcripts from Ross River virus cDNA clones and the construction and characterization of defined chimeras with Sindbis virus. Virology. 1991 Jun;182(2):430–441. doi: 10.1016/0042-6822(91)90584-x. [DOI] [PubMed] [Google Scholar]
- Kuismanen E., Saraste J. Low temperature-induced transport blocks as tools to manipulate membrane traffic. Methods Cell Biol. 1989;32:257–274. doi: 10.1016/s0091-679x(08)61174-7. [DOI] [PubMed] [Google Scholar]
- Käriäinen L., Pettersson R. F., Keränen S., Lehtovaara P., Söderlund H., Ukkonen P. Multiple structurally related defective-interfering RNAs formed during undiluted passages of Semliki forest virus. Virology. 1981 Sep;113(2):686–697. doi: 10.1016/0042-6822(81)90197-5. [DOI] [PubMed] [Google Scholar]
- Käriäinen L., Ranki M. Inhibition of cell functions by RNA-virus infections. Annu Rev Microbiol. 1984;38:91–109. doi: 10.1146/annurev.mi.38.100184.000515. [DOI] [PubMed] [Google Scholar]
- Lanzrein M., Käsermann N., Kempf C. Changes in membrane permeability during Semliki Forest virus induced cell fusion. Biosci Rep. 1992 Jun;12(3):221–236. doi: 10.1007/BF01121792. [DOI] [PubMed] [Google Scholar]
- Lanzrein M., Käsermann N., Weingart R., Kempf C. Early events of Semliki Forest virus-induced cell-cell fusion. Virology. 1993 Oct;196(2):541–547. doi: 10.1006/viro.1993.1509. [DOI] [PubMed] [Google Scholar]
- Lanzrein M., Weingart R., Kempf C. pH-dependent pore formation in Semliki forest virus-infected Aedes albopictus cells. Virology. 1993 Mar;193(1):296–302. doi: 10.1006/viro.1993.1125. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Air G. M., Webster R. G., Smith-Gill S. J. Epitopes on protein antigens: misconceptions and realities. Cell. 1990 May 18;61(4):553–556. doi: 10.1016/0092-8674(90)90464-p. [DOI] [PubMed] [Google Scholar]
- Leathers V., Tanguay R., Kobayashi M., Gallie D. R. A phylogenetically conserved sequence within viral 3' untranslated RNA pseudoknots regulates translation. Mol Cell Biol. 1993 Sep;13(9):5331–5347. doi: 10.1128/mcb.13.9.5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H., Brown D. T. Mutations in an exposed domain of Sindbis virus capsid protein result in the production of noninfectious virions and morphological variants. Virology. 1994 Jul;202(1):390–400. doi: 10.1006/viro.1994.1355. [DOI] [PubMed] [Google Scholar]
- Lehtovaara P., Söderlund H., Keränen S., Pettersson R. F., Käriäinen L. 18S defective interfering RNA of Semliki Forest virus contains a triplicated linear repeat. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5353–5357. doi: 10.1073/pnas.78.9.5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtovaara P., Söderlund H., Keränen S., Pettersson R. F., Käriäinen L. Extreme ends of the genome are conserved and rearranged in the defective interfering RNAs of Semliki Forest virus. J Mol Biol. 1982 Apr 25;156(4):731–748. doi: 10.1016/0022-2836(82)90139-5. [DOI] [PubMed] [Google Scholar]
- Lemm J. A., Durbin R. K., Stollar V., Rice C. M. Mutations which alter the level or structure of nsP4 can affect the efficiency of Sindbis virus replication in a host-dependent manner. J Virol. 1990 Jun;64(6):3001–3011. doi: 10.1128/jvi.64.6.3001-3011.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemm J. A., Rice C. M. Assembly of functional Sindbis virus RNA replication complexes: requirement for coexpression of P123 and P34. J Virol. 1993 Apr;67(4):1905–1915. doi: 10.1128/jvi.67.4.1905-1915.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemm J. A., Rice C. M. Roles of nonstructural polyproteins and cleavage products in regulating Sindbis virus RNA replication and transcription. J Virol. 1993 Apr;67(4):1916–1926. doi: 10.1128/jvi.67.4.1916-1926.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemm J. A., Rümenapf T., Strauss E. G., Strauss J. H., Rice C. M. Polypeptide requirements for assembly of functional Sindbis virus replication complexes: a model for the temporal regulation of minus- and plus-strand RNA synthesis. EMBO J. 1994 Jun 15;13(12):2925–2934. doi: 10.1002/j.1460-2075.1994.tb06587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner D. M., Deutsch J. M., Oster G. F. How does a virus bud? Biophys J. 1993 Jul;65(1):73–79. doi: 10.1016/S0006-3495(93)81071-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B., Griffin D. E. Molecular analysis of neurovirulent strains of Sindbis virus that evolve during persistent infection of scid mice. J Virol. 1993 Nov;67(11):6872–6875. doi: 10.1128/jvi.67.11.6872-6875.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B., Griffin D. E. Persistence of viral RNA in mouse brains after recovery from acute alphavirus encephalitis. J Virol. 1992 Nov;66(11):6429–6435. doi: 10.1128/jvi.66.11.6429-6435.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B., Hardwick J. M., Trapp B. D., Crawford T. O., Bollinger R. C., Griffin D. E. Antibody-mediated clearance of alphavirus infection from neurons. Science. 1991 Nov 8;254(5033):856–860. doi: 10.1126/science.1658936. [DOI] [PubMed] [Google Scholar]
- Levine B., Huang Q., Isaacs J. T., Reed J. C., Griffin D. E., Hardwick J. M. Conversion of lytic to persistent alphavirus infection by the bcl-2 cellular oncogene. Nature. 1993 Feb 25;361(6414):739–742. doi: 10.1038/361739a0. [DOI] [PubMed] [Google Scholar]
- Levinson R. S., Strauss J. H., Strauss E. G. Complete sequence of the genomic RNA of O'nyong-nyong virus and its use in the construction of alphavirus phylogenetic trees. Virology. 1990 Mar;175(1):110–123. doi: 10.1016/0042-6822(90)90191-s. [DOI] [PubMed] [Google Scholar]
- Levis R., Huang H., Schlesinger S. Engineered defective interfering RNAs of Sindbis virus express bacterial chloramphenicol acetyltransferase in avian cells. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4811–4815. doi: 10.1073/pnas.84.14.4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levis R., Schlesinger S., Huang H. V. Promoter for Sindbis virus RNA-dependent subgenomic RNA transcription. J Virol. 1990 Apr;64(4):1726–1733. doi: 10.1128/jvi.64.4.1726-1733.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levis R., Weiss B. G., Tsiang M., Huang H., Schlesinger S. Deletion mapping of Sindbis virus DI RNAs derived from cDNAs defines the sequences essential for replication and packaging. Cell. 1986 Jan 17;44(1):137–145. doi: 10.1016/0092-8674(86)90492-7. [DOI] [PubMed] [Google Scholar]
- Levy-Mintz P., Kielian M. Mutagenesis of the putative fusion domain of the Semliki Forest virus spike protein. J Virol. 1991 Aug;65(8):4292–4300. doi: 10.1128/jvi.65.8.4292-4300.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G. P., La Starza M. W., Hardy W. R., Strauss J. H., Rice C. M. Phosphorylation of Sindbis virus nsP3 in vivo and in vitro. Virology. 1990 Nov;179(1):416–427. doi: 10.1016/0042-6822(90)90310-n. [DOI] [PubMed] [Google Scholar]
- Li G. P., Prágai B. M., Rice C. M. Rescue of Sindbis virus-specific RNA replication and transcription by using a vaccinia virus recombinant. J Virol. 1991 Dec;65(12):6714–6723. doi: 10.1128/jvi.65.12.6714-6723.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G. P., Rice C. M. Mutagenesis of the in-frame opal termination codon preceding nsP4 of Sindbis virus: studies of translational readthrough and its effect on virus replication. J Virol. 1989 Mar;63(3):1326–1337. doi: 10.1128/jvi.63.3.1326-1337.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Rice C. M. The signal for translational readthrough of a UGA codon in Sindbis virus RNA involves a single cytidine residue immediately downstream of the termination codon. J Virol. 1993 Aug;67(8):5062–5067. doi: 10.1128/jvi.67.8.5062-5067.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljeström P., Garoff H. A new generation of animal cell expression vectors based on the Semliki Forest virus replicon. Biotechnology (N Y) 1991 Dec;9(12):1356–1361. doi: 10.1038/nbt1291-1356. [DOI] [PubMed] [Google Scholar]
- Liljeström P., Garoff H. Internally located cleavable signal sequences direct the formation of Semliki Forest virus membrane proteins from a polyprotein precursor. J Virol. 1991 Jan;65(1):147–154. doi: 10.1128/jvi.65.1.147-154.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljeström P., Lusa S., Huylebroeck D., Garoff H. In vitro mutagenesis of a full-length cDNA clone of Semliki Forest virus: the small 6,000-molecular-weight membrane protein modulates virus release. J Virol. 1991 Aug;65(8):4107–4113. doi: 10.1128/jvi.65.8.4107-4113.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist B. H., DiSalvo J., Rice C. M., Strauss J. H., Strauss E. G. Sindbis virus mutant ts20 of complementation group E contains a lesion in glycoprotein E2. Virology. 1986 May;151(1):10–20. doi: 10.1016/0042-6822(86)90099-1. [DOI] [PubMed] [Google Scholar]
- Lindsay M. D., Coelen R. J., Mackenzie J. S. Genetic heterogeneity among isolates of Ross River virus from different geographical regions. J Virol. 1993 Jun;67(6):3576–3585. doi: 10.1128/jvi.67.6.3576-3585.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N., Brown D. T. Phosphorylation and dephosphorylation events play critical roles in Sindbis virus maturation. Virology. 1993 Oct;196(2):703–711. doi: 10.1006/viro.1993.1527. [DOI] [PubMed] [Google Scholar]
- Liu N., Brown D. T. Transient translocation of the cytoplasmic (endo) domain of a type I membrane glycoprotein into cellular membranes. J Cell Biol. 1993 Feb;120(4):877–883. doi: 10.1083/jcb.120.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobigs M., Garoff H. Fusion function of the Semliki Forest virus spike is activated by proteolytic cleavage of the envelope glycoprotein precursor p62. J Virol. 1990 Mar;64(3):1233–1240. doi: 10.1128/jvi.64.3.1233-1240.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobigs M., Marshall I. D., Weir R. C., Dalgarno L. Genetic differentiation of Murray Valley encephalitis virus in Australia and Papua New Guinea. Aust J Exp Biol Med Sci. 1986 Dec;64(Pt 6):571–585. doi: 10.1038/icb.1986.61. [DOI] [PubMed] [Google Scholar]
- Lobigs M., Wahlberg J. M., Garoff H. Spike protein oligomerization control of Semliki Forest virus fusion. J Virol. 1990 Oct;64(10):5214–5218. doi: 10.1128/jvi.64.10.5214-5218.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobigs M., Weir R. C., Dalgarno L. Genetic analysis of Kunjin virus isolates using HaeIII and TaqI restriction digests of single-stranded cDNA to virion RNA. Aust J Exp Biol Med Sci. 1986 Apr;64(Pt 2):185–196. doi: 10.1038/icb.1986.20. [DOI] [PubMed] [Google Scholar]
- Lobigs M., Zhao H. X., Garoff H. Function of Semliki Forest virus E3 peptide in virus assembly: replacement of E3 with an artificial signal peptide abolishes spike heterodimerization and surface expression of E1. J Virol. 1990 Sep;64(9):4346–4355. doi: 10.1128/jvi.64.9.4346-4355.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London S. D., Schmaljohn A. L., Dalrymple J. M., Rice C. M. Infectious enveloped RNA virus antigenic chimeras. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):207–211. doi: 10.1073/pnas.89.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez S., Yao J. S., Kuhn R. J., Strauss E. G., Strauss J. H. Nucleocapsid-glycoprotein interactions required for assembly of alphaviruses. J Virol. 1994 Mar;68(3):1316–1323. doi: 10.1128/jvi.68.3.1316-1323.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord R. D., Calisher C. H. Further evidence of southward transport of arboviruses by migratory birds. Am J Epidemiol. 1970 Jul;92(1):73–78. doi: 10.1093/oxfordjournals.aje.a121181. [DOI] [PubMed] [Google Scholar]
- Luo T., Brown D. T. A 55-kDa protein induced in Aedes albopictus (mosquito) cells by antiviral protein. Virology. 1994 Apr;200(1):200–206. doi: 10.1006/viro.1994.1178. [DOI] [PubMed] [Google Scholar]
- Luo T., Brown D. T. Purification and characterization of a Sindbis virus-induced peptide which stimulates its own production and blocks virus RNA synthesis. Virology. 1993 May;194(1):44–49. doi: 10.1006/viro.1993.1233. [DOI] [PubMed] [Google Scholar]
- Lusa S., Garoff H., Liljeström P. Fate of the 6K membrane protein of Semliki Forest virus during virus assembly. Virology. 1991 Dec;185(2):843–846. doi: 10.1016/0042-6822(91)90556-q. [DOI] [PubMed] [Google Scholar]
- Lustig S., Jackson A. C., Hahn C. S., Griffin D. E., Strauss E. G., Strauss J. H. Molecular basis of Sindbis virus neurovirulence in mice. J Virol. 1988 Jul;62(7):2329–2336. doi: 10.1128/jvi.62.7.2329-2336.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maassen J. A., Terhorst C. Identification of a cell-surface protein involved in the binding site of Sindbis virus on human lymphoblastic cell lines using a heterobifunctional cross-linker. Eur J Biochem. 1981 Mar 16;115(1):153–158. doi: 10.1111/j.1432-1033.1981.tb06211.x. [DOI] [PubMed] [Google Scholar]
- Mabruk M. J., Flack A. M., Glasgow G. M., Smyth J. M., Folan J. C., Bannigan J. G., O'Sullivan M. A., Sheahan B. J., Atkins G. J. Teratogenicity of the Semliki Forest virus mutant ts22 for the foetal mouse: induction of skeletal and skin defects. J Gen Virol. 1988 Nov;69(Pt 11):2755–2762. doi: 10.1099/0022-1317-69-11-2755. [DOI] [PubMed] [Google Scholar]
- Mabruk M. J., Glasglow G. M., Flack A. M., Folan J. C., Bannigan J. G., Smyth J. M., O'Sullivan M. A., Sheahan B. J., Atkins G. J. Effect of infection with the ts22 mutant of Semliki Forest virus on development of the central nervous system in the fetal mouse. J Virol. 1989 Sep;63(9):4027–4033. doi: 10.1128/jvi.63.9.4027-4033.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinoski F., Stollar V. Inhibition of Sindbis virus replication in Aedes albopictus cells by virazole (ribavirin) and its reversal by actinomycin: a correction. Virology. 1980 Apr 30;102(2):473–476. doi: 10.1016/0042-6822(80)90117-8. [DOI] [PubMed] [Google Scholar]
- Mann E., Edwards J., Brown D. T. Polycaryocyte formation mediated by Sindbis virus glycoproteins. J Virol. 1983 Mar;45(3):1083–1089. doi: 10.1128/jvi.45.3.1083-1089.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt M. T., Phalen T., Kielian M. Cholesterol is required in the exit pathway of Semliki Forest virus. J Cell Biol. 1993 Oct;123(1):57–65. doi: 10.1083/jcb.123.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt T., Helenius A. Misfolding and aggregation of newly synthesized proteins in the endoplasmic reticulum. J Cell Biol. 1992 May;117(3):505–513. doi: 10.1083/jcb.117.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M., Bolzau E., Helenius A. Penetration of Semliki Forest virus from acidic prelysosomal vacuoles. Cell. 1983 Mar;32(3):931–940. doi: 10.1016/0092-8674(83)90078-8. [DOI] [PubMed] [Google Scholar]
- Marsh M., Helenius A. Adsorptive endocytosis of Semliki Forest virus. J Mol Biol. 1980 Sep 25;142(3):439–454. doi: 10.1016/0022-2836(80)90281-8. [DOI] [PubMed] [Google Scholar]
- Marsh M., Helenius A. Virus entry into animal cells. Adv Virus Res. 1989;36:107–151. doi: 10.1016/S0065-3527(08)60583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M. The entry of enveloped viruses into cells by endocytosis. Biochem J. 1984 Feb 15;218(1):1–10. doi: 10.1042/bj2180001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M., Wellsteed J., Kern H., Harms E., Helenius A. Monensin inhibits Semliki Forest virus penetration into culture cells. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5297–5301. doi: 10.1073/pnas.79.17.5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall I. D., Brown B. K., Keith K., Gard G. P., Thibos E. Variation in arbovirus infection rates in species of birds sampled in a serological survey during an encephalitis epidemic in the Murray Valley of South-eastern Australia, February 1974. Aust J Exp Biol Med Sci. 1982 Oct;60(Pt 5):471–478. doi: 10.1038/icb.1982.52. [DOI] [PubMed] [Google Scholar]
- Marshall I. D., Thibos E., Clarke K. Species composition of mosquitoes collected in the Murray Valley of South-eastern Australia during an epidemic of arboviral encephalitis. Aust J Exp Biol Med Sci. 1982 Oct;60(Pt 5):447–456. doi: 10.1038/icb.1982.50. [DOI] [PubMed] [Google Scholar]
- Marshall I. D., Woodroofe G. M., Hirsch S. Viruses recovered from mosquitoes and wildlife serum collected in the Murray Valley of South-eastern Australia, February 1974, during an epidemic of encephalitis. Aust J Exp Biol Med Sci. 1982 Oct;60(Pt 5):457–470. doi: 10.1038/icb.1982.51. [DOI] [PubMed] [Google Scholar]
- Martin E. M., Sonnabend J. A. Ribonucleic acid polymerase catalyzing synthesis of double-stranded arbovirus ribonucleic acid. J Virol. 1967 Feb;1(1):97–109. doi: 10.1128/jvi.1.1.97-109.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews J. H., Roehrig J. T. Determination of the protective epitopes on the glycoproteins of Venezuelan equine encephalomyelitis virus by passive transfer of monoclonal antibodies. J Immunol. 1982 Dec;129(6):2763–2767. [PubMed] [Google Scholar]
- Mathiot C. C., Grimaud G., Garry P., Bouquety J. C., Mada A., Daguisy A. M., Georges A. J. An outbreak of human Semliki Forest virus infections in Central African Republic. Am J Trop Med Hyg. 1990 Apr;42(4):386–393. doi: 10.4269/ajtmh.1990.42.386. [DOI] [PubMed] [Google Scholar]
- Matlin K. S., Simons K. Sorting of an apical plasma membrane glycoprotein occurs before it reaches the cell surface in cultured epithelial cells. J Cell Biol. 1984 Dec;99(6):2131–2139. doi: 10.1083/jcb.99.6.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matlin K. S. The sorting of proteins to the plasma membrane in epithelial cells. J Cell Biol. 1986 Dec;103(6 Pt 2):2565–2568. doi: 10.1083/jcb.103.6.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayne J. T., Bell J. R., Strauss E. G., Strauss J. H. Pattern of glycosylation of Sindbis virus envelope proteins synthesized in hamster and chicken cells. Virology. 1985 Apr 15;142(1):121–133. doi: 10.1016/0042-6822(85)90427-1. [DOI] [PubMed] [Google Scholar]
- McDowell W., Romero P. A., Datema R., Schwarz R. T. Glucose trimming and mannose trimming affect different phases of the maturation of Sindbis virus in infected BHK cells. Virology. 1987 Nov;161(1):37–44. doi: 10.1016/0042-6822(87)90168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek A. D., Faragher S. G., Weir R. C., Dalgarno L. Genetic and phenotypic studies on Ross River virus variants of enhanced virulence selected during mouse passage. Virology. 1989 Oct;172(2):399–407. doi: 10.1016/0042-6822(89)90182-7. [DOI] [PubMed] [Google Scholar]
- Mehta S., Pathak S., Webb H. E. Induction of membrane proliferation in mouse CNS by gold sodium thiomalate with reference to increased virulence of the avirulent Semliki Forest virus. Biosci Rep. 1990 Jun;10(3):271–279. doi: 10.1007/BF01117243. [DOI] [PubMed] [Google Scholar]
- Melancon P., Garoff H. Processing of the Semliki Forest virus structural polyprotein: role of the capsid protease. J Virol. 1987 May;61(5):1301–1309. doi: 10.1128/jvi.61.5.1301-1309.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melancon P., Garoff H. Reinitiation of translocation in the Semliki Forest virus structural polyprotein: identification of the signal for the E1 glycoprotein. EMBO J. 1986 Jul;5(7):1551–1560. doi: 10.1002/j.1460-2075.1986.tb04396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I., Simons K. The Golgi complex: in vitro veritas? Cell. 1992 Mar 6;68(5):829–840. doi: 10.1016/0092-8674(92)90027-A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza Q. P., Stanley J., Griffin D. E. Monoclonal antibodies to the E1 and E2 glycoproteins of Sindbis virus: definition of epitopes and efficiency of protection from fatal encephalitis. J Gen Virol. 1988 Dec;69(Pt 12):3015–3022. doi: 10.1099/0022-1317-69-12-3015. [DOI] [PubMed] [Google Scholar]
- Metsikkö K., Garoff H. Oligomers of the cytoplasmic domain of the p62/E2 membrane protein of Semliki Forest virus bind to the nucleocapsid in vitro. J Virol. 1990 Oct;64(10):4678–4683. doi: 10.1128/jvi.64.10.4678-4683.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer W. J., Gidwitz S., Ayers V. K., Schoepp R. J., Johnston R. E. Conformational alteration of Sindbis virion glycoproteins induced by heat, reducing agents, or low pH. J Virol. 1992 Jun;66(6):3504–3513. doi: 10.1128/jvi.66.6.3504-3513.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi S., Durbin R., Huang H. V., Rice C. M., Stollar V. Association of the Sindbis virus RNA methyltransferase activity with the nonstructural protein nsP1. Virology. 1989 Jun;170(2):385–391. doi: 10.1016/0042-6822(89)90429-7. [DOI] [PubMed] [Google Scholar]
- Mi S., Stollar V. Both amino acid changes in nsP1 of Sindbis virusLM21 contribute to and are required for efficient expression of the mutant phenotype. Virology. 1990 Oct;178(2):429–434. doi: 10.1016/0042-6822(90)90340-w. [DOI] [PubMed] [Google Scholar]
- Mi S., Stollar V. Expression of Sindbis virus nsP1 and methyltransferase activity in Escherichia coli. Virology. 1991 Sep;184(1):423–427. doi: 10.1016/0042-6822(91)90862-6. [DOI] [PubMed] [Google Scholar]
- Michel M. R., Elgizoli M., Dai Y., Jakob R., Koblet H., Arrigo A. P. Karyophilic properties of Semliki Forest virus nucleocapsid protein. J Virol. 1990 Oct;64(10):5123–5131. doi: 10.1128/jvi.64.10.5123-5131.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel M. R., Gomatos P. J. Semliki forest virus-specific RNAs synthesized in vitro by enzyme from infected BHK cells. J Virol. 1973 Jun;11(6):900–914. doi: 10.1128/jvi.11.6.900-914.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio G., Pascale M. C., Leone A., Bonatti S. Biosynthesis, membrane translocation, and surface expression of Sindbis virus E1 glycoprotein. Exp Cell Res. 1989 Nov;185(1):203–216. doi: 10.1016/0014-4827(89)90049-9. [DOI] [PubMed] [Google Scholar]
- Miller M. L., Brown D. T. The distribution of Sindbis virus proteins in mosquito cells as determined by immunofluorescence and immunoelectron microscopy. J Gen Virol. 1993 Feb;74(Pt 2):293–298. doi: 10.1099/0022-1317-74-2-293. [DOI] [PubMed] [Google Scholar]
- Mokhtarian F., Grob D., Griffin D. E. Role of the immune response in Sindbis virus-induced paralysis of SJL/J mice. J Immunol. 1989 Jul 15;143(2):633–637. [PubMed] [Google Scholar]
- Mokhtarian F., Swoveland P. Predisposition to EAE induction in resistant mice by prior infection with Semliki Forest virus. J Immunol. 1987 May 15;138(10):3264–3268. [PubMed] [Google Scholar]
- Monroe S. S., Ou J. H., Rice C. M., Schlesinger S., Strauss E. G., Strauss J. H. Sequence analysis of cDNA's derived from the RNA of Sindbis virions and of defective interfering particles. J Virol. 1982 Jan;41(1):153–162. doi: 10.1128/jvi.41.1.153-162.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe S. S., Schlesinger S. Common and distinct regions of defective-interfering RNAs of Sindbis virus. J Virol. 1984 Mar;49(3):865–872. doi: 10.1128/jvi.49.3.865-872.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe S. S., Schlesinger S. RNAs from two independently isolated defective interfering particles of Sindbis virus contain a cellular tRNA sequence at their 5' ends. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3279–3283. doi: 10.1073/pnas.80.11.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudge P. R., Lim R. S., Moore B., Radford A. F. Epidemic polyarthritis in South Australia 1979-1980. Med J Aust. 1980 Nov 29;2(11):626–627. doi: 10.5694/j.1326-5377.1980.tb77070.x. [DOI] [PubMed] [Google Scholar]
- Mulvey M., Brown D. T. Formation and rearrangement of disulfide bonds during maturation of the Sindbis virus E1 glycoprotein. J Virol. 1994 Feb;68(2):805–812. doi: 10.1128/jvi.68.2.805-812.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naim H. Y., Koblet H. The cleavage of p62, the precursor of E2 and E3, is an early and continuous event in Semliki Forest virus-infected Aedes albopictus cells. Arch Virol. 1990;110(3-4):221–237. doi: 10.1007/BF01311290. [DOI] [PubMed] [Google Scholar]
- Nakhasi H. L., Cao X. Q., Rouault T. A., Liu T. Y. Specific binding of host cell proteins to the 3'-terminal stem-loop structure of rubella virus negative-strand RNA. J Virol. 1991 Nov;65(11):5961–5967. doi: 10.1128/jvi.65.11.5961-5967.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhasi H. L., Rouault T. A., Haile D. J., Liu T. Y., Klausner R. D. Specific high-affinity binding of host cell proteins to the 3' region of rubella virus RNA. New Biol. 1990 Mar;2(3):255–264. [PubMed] [Google Scholar]
- Newton S. E., Dalgarno L. Antiviral activity released from Aedes albopictus cells persistently infected with Semliki forest virus. J Virol. 1983 Sep;47(3):652–655. doi: 10.1128/jvi.47.3.652-655.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niesters H. G., Strauss J. H. Defined mutations in the 5' nontranslated sequence of Sindbis virus RNA. J Virol. 1990 Sep;64(9):4162–4168. doi: 10.1128/jvi.64.9.4162-4168.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niesters H. G., Strauss J. H. Mutagenesis of the conserved 51-nucleotide region of Sindbis virus. J Virol. 1990 Apr;64(4):1639–1647. doi: 10.1128/jvi.64.4.1639-1647.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niklasson B., Espmark A., LeDuc J. W., Gargan T. P., Ennis W. A., Tesh R. B., Main A. J., Jr Association of a Sindbis-like virus with Ockelbo disease in Sweden. Am J Trop Med Hyg. 1984 Nov;33(6):1212–1217. doi: 10.4269/ajtmh.1984.33.1212. [DOI] [PubMed] [Google Scholar]
- Nitsch L., Tramontano D., Ambesi-Impiombato F. S., Quarto N., Bonatti S. Morphological and functional polarity of an epithelial thyroid cell line. Eur J Cell Biol. 1985 Jul;38(1):57–66. [PubMed] [Google Scholar]
- Nuss D. L., Oppermann H., Koch G. Selective blockage of initiation of host protein synthesis in RNA-virus-infected cells. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1258–1262. doi: 10.1073/pnas.72.4.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone M. B., Tishon A., Dutko F. J., Kennedy S. I., Holland J. J., Lampert P. W. Does the major histocompatibility complex serve as a specific receptor for Semliki Forest virus? J Virol. 1980 Apr;34(1):256–265. doi: 10.1128/jvi.34.1.256-265.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivo P. D., Frolov I., Schlesinger S. A cell line that expresses a reporter gene in response to infection by Sindbis virus: a prototype for detection of positive strand RNA viruses. Virology. 1994 Jan;198(1):381–384. doi: 10.1006/viro.1994.1046. [DOI] [PubMed] [Google Scholar]
- Olmsted R. A., Baric R. S., Sawyer B. A., Johnston R. E. Sindbis virus mutants selected for rapid growth in cell culture display attenuated virulence in animals. Science. 1984 Jul 27;225(4660):424–427. doi: 10.1126/science.6204381. [DOI] [PubMed] [Google Scholar]
- Olmsted R. A., Meyer W. J., Johnston R. E. Characterization of Sindbis virus epitopes important for penetration in cell culture and pathogenesis in animals. Virology. 1986 Jan 30;148(2):245–254. doi: 10.1016/0042-6822(86)90322-3. [DOI] [PubMed] [Google Scholar]
- Olson K., Trent D. W. Genetic and antigenic variations among geographical isolates of Sindbis virus. J Gen Virol. 1985 Apr;66(Pt 4):797–810. doi: 10.1099/0022-1317-66-4-797. [DOI] [PubMed] [Google Scholar]
- Omar A., Koblet H. Semliki Forest virus particles containing only the E1 envelope glycoprotein are infectious and can induce cell-cell fusion. Virology. 1988 Sep;166(1):17–23. doi: 10.1016/0042-6822(88)90141-9. [DOI] [PubMed] [Google Scholar]
- Omar A., Koblet H. The use of sulfite to study the mechanism of membrane fusion induced by E1 of Semliki Forest virus. Virology. 1989 Jan;168(1):177–179. doi: 10.1016/0042-6822(89)90418-2. [DOI] [PubMed] [Google Scholar]
- Orci L., Ravazzola M., Storch M. J., Anderson R. G., Vassalli J. D., Perrelet A. Proteolytic maturation of insulin is a post-Golgi event which occurs in acidifying clathrin-coated secretory vesicles. Cell. 1987 Jun 19;49(6):865–868. doi: 10.1016/0092-8674(87)90624-6. [DOI] [PubMed] [Google Scholar]
- Ou J. H., Rice C. M., Dalgarno L., Strauss E. G., Strauss J. H. Sequence studies of several alphavirus genomic RNAs in the region containing the start of the subgenomic RNA. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5235–5239. doi: 10.1073/pnas.79.17.5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou J. H., Strauss E. G., Strauss J. H. Comparative studies of the 3'-terminal sequences of several alpha virus RNAs. Virology. 1981 Mar;109(2):281–289. doi: 10.1016/0042-6822(81)90499-2. [DOI] [PubMed] [Google Scholar]
- Ou J. H., Strauss E. G., Strauss J. H. The 5'-terminal sequences of the genomic RNAs of several alphaviruses. J Mol Biol. 1983 Jul 25;168(1):1–15. doi: 10.1016/s0022-2836(83)80319-2. [DOI] [PubMed] [Google Scholar]
- Ou J. H., Trent D. W., Strauss J. H. The 3'-non-coding regions of alphavirus RNAs contain repeating sequences. J Mol Biol. 1982 Apr 25;156(4):719–730. doi: 10.1016/0022-2836(82)90138-3. [DOI] [PubMed] [Google Scholar]
- Pardigon N., Lenches E., Strauss J. H. Multiple binding sites for cellular proteins in the 3' end of Sindbis alphavirus minus-sense RNA. J Virol. 1993 Aug;67(8):5003–5011. doi: 10.1128/jvi.67.8.5003-5011.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardigon N., Strauss J. H. Cellular proteins bind to the 3' end of Sindbis virus minus-strand RNA. J Virol. 1992 Feb;66(2):1007–1015. doi: 10.1128/jvi.66.2.1007-1015.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes A. M., Brown D. T., Rothnagel R., Chiu W., Schoepp R. J., Johnston R. E., Prasad B. V. Three-dimensional structure of a membrane-containing virus. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):9095–9099. doi: 10.1073/pnas.90.19.9095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes A. M., Simon M. N., Brown D. T. The mass of the Sindbis virus nucleocapsid suggests it has T = 4 icosahedral symmetry. Virology. 1992 Mar;187(1):329–332. doi: 10.1016/0042-6822(92)90322-g. [DOI] [PubMed] [Google Scholar]
- Parry N., Fox G., Rowlands D., Brown F., Fry E., Acharya R., Logan D., Stuart D. Structural and serological evidence for a novel mechanism of antigenic variation in foot-and-mouth disease virus. Nature. 1990 Oct 11;347(6293):569–572. doi: 10.1038/347569a0. [DOI] [PubMed] [Google Scholar]
- Pavan A., Covelli E., Pascale M. C., Lucania G., Bonatti S., Pinto da Silva P., Torrisi M. R. Dynamics of transmembrane proteins during Sindbis virus budding. J Cell Sci. 1992 May;102(Pt 1):149–155. doi: 10.1242/jcs.102.1.149. [DOI] [PubMed] [Google Scholar]
- Pavan A., Lotti L. V., Torrisi M. R., Migliaccio G., Bonatti S. Regional distribution of Sindbis virus glycoproteins on the plasma membrane of infected baby hamster kidney cells. Exp Cell Res. 1987 Jan;168(1):53–62. doi: 10.1016/0014-4827(87)90415-0. [DOI] [PubMed] [Google Scholar]
- Pence D. F., Davis N. L., Johnston R. E. Antigenic and genetic characterization of Sindbis virus monoclonal antibody escape mutants which define a pathogenesis domain on glycoprotein E2. Virology. 1990 Mar;175(1):41–49. doi: 10.1016/0042-6822(90)90184-s. [DOI] [PubMed] [Google Scholar]
- Perez L., Guinea R., Carrasco L. Synthesis of Semliki Forest virus RNA requires continuous lipid synthesis. Virology. 1991 Jul;183(1):74–82. doi: 10.1016/0042-6822(91)90119-v. [DOI] [PubMed] [Google Scholar]
- Peränen J. Localization and phosphorylation of Semliki Forest virus non-structural protein nsP3 expressed in COS cells from a cloned cDNA. J Gen Virol. 1991 Jan;72(Pt 1):195–199. doi: 10.1099/0022-1317-72-1-195. [DOI] [PubMed] [Google Scholar]
- Peränen J., Rikkonen M., Liljeström P., Käriäinen L. Nuclear localization of Semliki Forest virus-specific nonstructural protein nsP2. J Virol. 1990 May;64(5):1888–1896. doi: 10.1128/jvi.64.5.1888-1896.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peränen J., Takkinen K., Kalkkinen N., Käriäinen L. Semliki Forest virus-specific non-structural protein nsP3 is a phosphoprotein. J Gen Virol. 1988 Sep;69(Pt 9):2165–2178. doi: 10.1099/0022-1317-69-9-2165. [DOI] [PubMed] [Google Scholar]
- Pettersson R. F. 5'-Terminal nucleotide sequence of Semliki forest virus 18S defective interfering RNA is heterogeneous and different from the genomic 42S RNA. Proc Natl Acad Sci U S A. 1981 Jan;78(1):115–119. doi: 10.1073/pnas.78.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phalen T., Kielian M. Cholesterol is required for infection by Semliki Forest virus. J Cell Biol. 1991 Feb;112(4):615–623. doi: 10.1083/jcb.112.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips D. A., Murray J. R., Aaskov J. G., Wiemers M. A. Clinical and subclinical Barmah Forest virus infection in Queensland. Med J Aust. 1990 May 7;152(9):463–466. doi: 10.5694/j.1326-5377.1990.tb125304.x. [DOI] [PubMed] [Google Scholar]
- Polo J. M., Davis N. L., Rice C. M., Huang H. V., Johnston R. E. Molecular analysis of Sindbis virus pathogenesis in neonatal mice by using virus recombinants constructed in vitro. J Virol. 1988 Jun;62(6):2124–2133. doi: 10.1128/jvi.62.6.2124-2133.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polo J. M., Johnston R. E. Attenuating mutations in glycoproteins E1 and E2 of Sindbis virus produce a highly attenuated strain when combined in vitro. J Virol. 1990 Sep;64(9):4438–4444. doi: 10.1128/jvi.64.9.4438-4444.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polo J. M., Johnston R. E. Mutational analysis of a virulence locus in the E2 glycoprotein gene of Sindbis virus. J Virol. 1991 Nov;65(11):6358–6361. doi: 10.1128/jvi.65.11.6358-6361.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presely J. F., Brown D. T. The proteolytic cleavage of PE2 to envelope glycoprotein E2 is not strictly required for the maturation of Sindbis virus. J Virol. 1989 May;63(5):1975–1980. doi: 10.1128/jvi.63.5.1975-1980.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez L., Irurzun A., Carrasco L. Activation of phospholipase activity during Semliki Forest virus infection. Virology. 1993 May;194(1):28–36. doi: 10.1006/viro.1993.1231. [DOI] [PubMed] [Google Scholar]
- Raju R., Huang H. V. Analysis of Sindbis virus promoter recognition in vivo, using novel vectors with two subgenomic mRNA promoters. J Virol. 1991 May;65(5):2501–2510. doi: 10.1128/jvi.65.5.2501-2510.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranki M., Käriäinen L. Solubilized RNA replication complex from Semliki Forest virus-infected cells. Virology. 1979 Oct 30;98(2):298–307. doi: 10.1016/0042-6822(79)90553-1. [DOI] [PubMed] [Google Scholar]
- Rao C. N., Castronovo V., Schmitt M. C., Wewer U. M., Claysmith A. P., Liotta L. A., Sobel M. E. Evidence for a precursor of the high-affinity metastasis-associated murine laminin receptor. Biochemistry. 1989 Sep 5;28(18):7476–7486. doi: 10.1021/bi00444a047. [DOI] [PubMed] [Google Scholar]
- Rennels M. B. Arthropod-borne virus infections of the central nervous system. Neurol Clin. 1984 May;2(2):241–254. [PubMed] [Google Scholar]
- Rentier-Delrue F., Young N. A. Genomic divergence among Sindbis virus strains. Virology. 1980 Oct 15;106(1):59–70. doi: 10.1016/0042-6822(80)90221-4. [DOI] [PubMed] [Google Scholar]
- Rice C. M., Bell J. R., Hunkapiller M. W., Strauss E. G., Strauss J. H. Isolation and characterization of the hydrophobic COOH-terminal domains of the sindbis virion glycoproteins. J Mol Biol. 1982 Jan 15;154(2):355–378. doi: 10.1016/0022-2836(82)90069-9. [DOI] [PubMed] [Google Scholar]
- Rice C. M., Franke C. A., Strauss J. H., Hruby D. E. Expression of Sindbis virus structural proteins via recombinant vaccinia virus: synthesis, processing, and incorporation into mature Sindbis virions. J Virol. 1985 Oct;56(1):227–239. doi: 10.1128/jvi.56.1.227-239.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice C. M., Levis R., Strauss J. H., Huang H. V. Production of infectious RNA transcripts from Sindbis virus cDNA clones: mapping of lethal mutations, rescue of a temperature-sensitive marker, and in vitro mutagenesis to generate defined mutants. J Virol. 1987 Dec;61(12):3809–3819. doi: 10.1128/jvi.61.12.3809-3819.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice C. M., Strauss J. H. Association of sindbis virion glycoproteins and their precursors. J Mol Biol. 1982 Jan 15;154(2):325–348. doi: 10.1016/0022-2836(82)90067-5. [DOI] [PubMed] [Google Scholar]
- Rice C. M., Strauss J. H. Nucleotide sequence of the 26S mRNA of Sindbis virus and deduced sequence of the encoded virus structural proteins. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2062–2066. doi: 10.1073/pnas.78.4.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rico-Hesse R., Roehrig J. T., Trent D. W., Dickerman R. W. Genetic variation of Venezuelan equine encephalitis virus strains of the ID variety in Colombia. Am J Trop Med Hyg. 1988 Jan;38(1):195–204. doi: 10.4269/ajtmh.1988.38.195. [DOI] [PubMed] [Google Scholar]
- Riedel B., Brown D. T. Novel antiviral activity found in the media of Sindbis virus-persistently infected mosquito (Aedes albopictus) cell cultures. J Virol. 1979 Jan;29(1):51–60. doi: 10.1128/jvi.29.1.51-60.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel H. Different membrane anchors allow the Semliki Forest virus spike subunit E2 to reach the cell surface. J Virol. 1985 Apr;54(1):224–228. doi: 10.1128/jvi.54.1.224-228.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikkonen M., Peränen J., Käriäinen L. Nuclear and nucleolar targeting signals of Semliki Forest virus nonstructural protein nsP2. Virology. 1992 Aug;189(2):462–473. doi: 10.1016/0042-6822(92)90570-f. [DOI] [PubMed] [Google Scholar]
- Robbins A. R., Peng S. S., Marshall J. L. Mutant Chinese hamster ovary cells pleiotropically defective in receptor-mediated endocytosis. J Cell Biol. 1983 Apr;96(4):1064–1071. doi: 10.1083/jcb.96.4.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehrig J. T., Day J. W., Kinney R. M. Antigenic analysis of the surface glycoproteins of a Venezuelan equine encephalomyelitis virus (TC-83) using monoclonal antibodies. Virology. 1982 Apr 30;118(2):269–278. doi: 10.1016/0042-6822(82)90346-4. [DOI] [PubMed] [Google Scholar]
- Roehrig J. T., Gorski D., Schlesinger M. J. Properties of monoclonal antibodies directed against the glycoproteins of Sindbis virus. J Gen Virol. 1982 Apr;59(Pt 2):421–425. doi: 10.1099/0022-1317-59-2-421. [DOI] [PubMed] [Google Scholar]
- Roehrig J. T., Hunt A. R., Chang G. J., Sheik B., Bolin R. A., Tsai T. F., Trent D. W. Identification of monoclonal antibodies capable of differentiating antigenic varieties of eastern equine encephalitis viruses. Am J Trop Med Hyg. 1990 Apr;42(4):394–398. doi: 10.4269/ajtmh.1990.42.394. [DOI] [PubMed] [Google Scholar]
- Roehrig J. T., Hunt A. R., Kinney R. M., Mathews J. H. In vitro mechanisms of monoclonal antibody neutralization of alphaviruses. Virology. 1988 Jul;165(1):66–73. doi: 10.1016/0042-6822(88)90659-9. [DOI] [PubMed] [Google Scholar]
- Roehrig J. T., Mathews J. H. The neutralization site on the E2 glycoprotein of Venezuelan equine encephalomyelitis (TC-83) virus is composed of multiple conformationally stable epitopes. Virology. 1985 Apr 30;142(2):347–356. doi: 10.1016/0042-6822(85)90343-5. [DOI] [PubMed] [Google Scholar]
- Roman L. M., Garoff H. Alteration of the cytoplasmic domain of the membrane-spanning glycoprotein p62 of Semliki Forest virus does not affect its polar distribution in established lines of Madin-Darby canine kidney cells. J Cell Biol. 1986 Dec;103(6 Pt 2):2607–2618. doi: 10.1083/jcb.103.6.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozanov M. N., Koonin E. V., Gorbalenya A. E. Conservation of the putative methyltransferase domain: a hallmark of the 'Sindbis-like' supergroup of positive-strand RNA viruses. J Gen Virol. 1992 Aug;73(Pt 8):2129–2134. doi: 10.1099/0022-1317-73-8-2129. [DOI] [PubMed] [Google Scholar]
- Russell D. L., Dalrymple J. M., Johnston R. E. Sindbis virus mutations which coordinately affect glycoprotein processing, penetration, and virulence in mice. J Virol. 1989 Apr;63(4):1619–1629. doi: 10.1128/jvi.63.4.1619-1629.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryser H. J., Mandel R., Ghani F. Cell surface sulfhydryls are required for the cytotoxicity of diphtheria toxin but not of ricin in Chinese hamster ovary cells. J Biol Chem. 1991 Oct 5;266(28):18439–18442. [PubMed] [Google Scholar]
- Rümenapf T., Strauss E. G., Strauss J. H. Subgenomic mRNA of Aura alphavirus is packaged into virions. J Virol. 1994 Jan;68(1):56–62. doi: 10.1128/jvi.68.1.56-62.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saborio J. L., Pong S. S., Koch G. Selective and reversible inhibition of initiation of protein synthesis in mammalian cells. J Mol Biol. 1974 May 15;85(2):195–211. doi: 10.1016/0022-2836(74)90360-x. [DOI] [PubMed] [Google Scholar]
- Salminen A., Wahlberg J. M., Lobigs M., Liljeström P., Garoff H. Membrane fusion process of Semliki Forest virus. II: Cleavage-dependent reorganization of the spike protein complex controls virus entry. J Cell Biol. 1992 Jan;116(2):349–357. doi: 10.1083/jcb.116.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders B. G., Wan K. M., Kline K., Garry R. F., Bose H. R., Jr Chicken fetal antigens: role as cell surface receptors for Sindbis virus hemagglutinin. Virology. 1980 Oct 15;106(1):183–186. doi: 10.1016/0042-6822(80)90240-8. [DOI] [PubMed] [Google Scholar]
- Saraste J., Hedman K. Intracellular vesicles involved in the transport of Semliki Forest virus membrane proteins to the cell surface. EMBO J. 1983;2(11):2001–2006. doi: 10.1002/j.1460-2075.1983.tb01691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste J., Kuismanen E. Pre- and post-Golgi vacuoles operate in the transport of Semliki Forest virus membrane glycoproteins to the cell surface. Cell. 1984 Sep;38(2):535–549. doi: 10.1016/0092-8674(84)90508-7. [DOI] [PubMed] [Google Scholar]
- Saraste J., von Bonsdorff C. H., Hashimoto K., Käriäinen L., Keränen S. Semliki forest virus mutants with temperature-sensitive transport defect of envelope proteins. Virology. 1980 Jan 30;100(2):229–245. doi: 10.1016/0042-6822(80)90516-4. [DOI] [PubMed] [Google Scholar]
- Sarver N., Stollar V. Sindbis virus-induced cytopathic effect in clones of Aedes albopictus (Singh) cells. Virology. 1977 Jul 15;80(2):390–400. doi: 10.1016/s0042-6822(77)80014-7. [DOI] [PubMed] [Google Scholar]
- Sawicki D. L., Sawicki S. G. A second nonstructural protein functions in the regulation of alphavirus negative-strand RNA synthesis. J Virol. 1993 Jun;67(6):3605–3610. doi: 10.1128/jvi.67.6.3605-3610.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawicki D. L., Sawicki S. G. Functional analysis of the A complementation group mutants of Sindbis HR virus. Virology. 1985 Jul 15;144(1):20–34. doi: 10.1016/0042-6822(85)90301-0. [DOI] [PubMed] [Google Scholar]
- Sawicki D. L., Sawicki S. G., Keränen S., Käriäinen L. Specific Sindbis virus-coded function for minus-strand RNA synthesis. J Virol. 1981 Aug;39(2):348–358. doi: 10.1128/jvi.39.2.348-358.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawicki D. L., Sawicki S. G. Short-lived minus-strand polymerase for Semliki Forest virus. J Virol. 1980 Apr;34(1):108–118. doi: 10.1128/jvi.34.1.108-118.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawicki D., Barkhimer D. B., Sawicki S. G., Rice C. M., Schlesinger S. Temperature sensitive shut-off of alphavirus minus strand RNA synthesis maps to a nonstructural protein, nsP4. Virology. 1990 Jan;174(1):43–52. doi: 10.1016/0042-6822(90)90052-s. [DOI] [PubMed] [Google Scholar]
- Sawicki S. G., Sawicki D. L., Käriäinen L., Keränen S. A Sindbis virus mutant temperature-sensitive in the regulation of minus-strand RNA synthesis. Virology. 1981 Nov;115(1):161–172. doi: 10.1016/0042-6822(81)90098-2. [DOI] [PubMed] [Google Scholar]
- Sawicki S. G., Sawicki D. L. The effect of loss of regulation of minus-strand RNA synthesis on Sindbis virus replication. Virology. 1986 Jun;151(2):339–349. doi: 10.1016/0042-6822(86)90054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawicki S. G., Sawicki D. L. The effect of overproduction of nonstructural proteins on alphavirus plus-strand and minus-strand RNA synthesis. Virology. 1986 Jul 30;152(2):507–512. doi: 10.1016/0042-6822(86)90157-1. [DOI] [PubMed] [Google Scholar]
- Scheidel L. M., Durbin R. K., Stollar V. SVLM21, a Sindbis virus mutant resistant to methionine deprivation, encodes an altered methyltransferase. Virology. 1989 Dec;173(2):408–414. doi: 10.1016/0042-6822(89)90553-9. [DOI] [PubMed] [Google Scholar]
- Scheidel L. M., Durbin R. K., Stollar V. Sindbis virus mutants resistant to mycophenolic acid and ribavirin. Virology. 1987 May;158(1):1–7. doi: 10.1016/0042-6822(87)90230-3. [DOI] [PubMed] [Google Scholar]
- Scheule R. K. Fusion of Sindbis virus with model membranes containing phosphatidylethanolamine: implications for protein-induced membrane fusion. Biochim Biophys Acta. 1987 May 29;899(2):185–195. doi: 10.1016/0005-2736(87)90399-3. [DOI] [PubMed] [Google Scholar]
- Schlegel A., Omar A., Jentsch P., Morell A., Kempf C. Semliki Forest virus envelope proteins function as proton channels. Biosci Rep. 1991 Oct;11(5):243–255. doi: 10.1007/BF01127500. [DOI] [PubMed] [Google Scholar]
- Schlesinger M. J., Cahill D. Verapamil and chlorpromazine inhibit the budding of Sindbis and vesicular stomatitis viruses from infected chicken embryo fibroblasts. Virology. 1989 Jan;168(1):187–190. doi: 10.1016/0042-6822(89)90421-2. [DOI] [PubMed] [Google Scholar]
- Schlesinger M. J., London S. D., Ryan C. An in-frame insertion into the Sindbis virus 6K gene leads to defective proteolytic processing of the virus glycoproteins, a trans-dominant negative inhibition of normal virus formation, and interference in virus shut off of host-cell protein synthesis. Virology. 1993 Mar;193(1):424–432. doi: 10.1006/viro.1993.1139. [DOI] [PubMed] [Google Scholar]
- Schlesinger S., Koyama A. H., Malfer C., Gee S. L., Schlesinger M. J. The effects of inhibitors of glucosidase I on the formation of Sindbis virus. Virus Res. 1985 Mar;2(2):139–149. doi: 10.1016/0168-1702(85)90244-8. [DOI] [PubMed] [Google Scholar]
- Schmaljohn A. L., Johnson E. D., Dalrymple J. M., Cole G. A. Non-neutralizing monoclonal antibodies can prevent lethal alphavirus encephalitis. Nature. 1982 May 6;297(5861):70–72. doi: 10.1038/297070a0. [DOI] [PubMed] [Google Scholar]
- Schmaljohn A. L., Kokubun K. M., Cole G. A. Protective monoclonal antibodies define maturational and pH-dependent antigenic changes in Sindbis virus E1 glycoprotein. Virology. 1983 Oct 15;130(1):144–154. doi: 10.1016/0042-6822(83)90124-1. [DOI] [PubMed] [Google Scholar]
- Schmid S. L., Fuchs R., Male P., Mellman I. Two distinct subpopulations of endosomes involved in membrane recycling and transport to lysosomes. Cell. 1988 Jan 15;52(1):73–83. doi: 10.1016/0092-8674(88)90532-6. [DOI] [PubMed] [Google Scholar]
- Schmid S., Fuchs R., Kielian M., Helenius A., Mellman I. Acidification of endosome subpopulations in wild-type Chinese hamster ovary cells and temperature-sensitive acidification-defective mutants. J Cell Biol. 1989 Apr;108(4):1291–1300. doi: 10.1083/jcb.108.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M. F. Acylation of viral spike glycoproteins: a feature of enveloped RNA viruses. Virology. 1982 Jan 15;116(1):327–338. doi: 10.1016/0042-6822(82)90424-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M. F., Bracha M., Schlesinger M. J. Evidence for covalent attachment of fatty acids to Sindbis virus glycoproteins. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1687–1691. doi: 10.1073/pnas.76.4.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M. F., Schlesinger M. J. Relation of fatty acid attachment to the translation and maturation of vesicular stomatitis and Sindbis virus membrane glycoproteins. J Biol Chem. 1980 Apr 25;255(8):3334–3339. [PubMed] [Google Scholar]
- Schmidt M., Schmidt M. F., Rott R. Chemical identification of cysteine as palmitoylation site in a transmembrane protein (Semliki Forest virus E1). J Biol Chem. 1988 Dec 15;263(35):18635–18639. [PubMed] [Google Scholar]
- Schoepp R. J., Johnston R. E. Directed mutagenesis of a Sindbis virus pathogenesis site. Virology. 1993 Mar;193(1):149–159. doi: 10.1006/viro.1993.1111. [DOI] [PubMed] [Google Scholar]
- Schoepp R. J., Johnston R. E. Sindbis virus pathogenesis: phenotypic reversion of an attenuated strain to virulence by second-site intragenic suppressor mutations. J Gen Virol. 1993 Aug;74(Pt 8):1691–1695. doi: 10.1099/0022-1317-74-8-1691. [DOI] [PubMed] [Google Scholar]
- Schärer C. G., Naim H. Y., Koblet H. Palmitoylation of Semliki Forest virus glycoproteins in insect cells (C6/36) occurs in an early compartment and is coupled to the cleavage of the precursor p62. Arch Virol. 1993;132(3-4):237–254. doi: 10.1007/BF01309536. [DOI] [PubMed] [Google Scholar]
- Scott T. W., Weaver S. C. Eastern equine encephalomyelitis virus: epidemiology and evolution of mosquito transmission. Adv Virus Res. 1989;37:277–328. doi: 10.1016/s0065-3527(08)60838-6. [DOI] [PubMed] [Google Scholar]
- Sefton B. M. Immediate glycosylation of Sindbis virus membrane proteins. Cell. 1977 Apr;10(4):659–668. doi: 10.1016/0092-8674(77)90099-x. [DOI] [PubMed] [Google Scholar]
- Shenk T. E., Koshelnyk K. A., Stollar V. Temperature-sensitive virus from Aedes albopictus cells chronically infected with Sindbis virus. J Virol. 1974 Feb;13(2):439–447. doi: 10.1128/jvi.13.2.439-447.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman L. A., Griffin D. E. Pathogenesis of encephalitis induced in newborn mice by virulent and avirulent strains of Sindbis virus. J Virol. 1990 May;64(5):2041–2046. doi: 10.1128/jvi.64.5.2041-2046.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirako Y., Niklasson B., Dalrymple J. M., Strauss E. G., Strauss J. H. Structure of the Ockelbo virus genome and its relationship to other Sindbis viruses. Virology. 1991 Jun;182(2):753–764. doi: 10.1016/0042-6822(91)90616-j. [DOI] [PubMed] [Google Scholar]
- Shirako Y., Strauss J. H. Cleavage between nsP1 and nsP2 initiates the processing pathway of Sindbis virus nonstructural polyprotein P123. Virology. 1990 Jul;177(1):54–64. doi: 10.1016/0042-6822(90)90459-5. [DOI] [PubMed] [Google Scholar]
- Shirako Y., Strauss J. H. Regulation of Sindbis virus RNA replication: uncleaved P123 and nsP4 function in minus-strand RNA synthesis, whereas cleaved products from P123 are required for efficient plus-strand RNA synthesis. J Virol. 1994 Mar;68(3):1874–1885. doi: 10.1128/jvi.68.3.1874-1885.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D. T., Strauss J. H. Translation of Sindbis virus 26 S RNA and 49 S RNA in lysates of rabbit reticulocytes. J Mol Biol. 1974 Jun 25;86(2):397–409. doi: 10.1016/0022-2836(74)90027-8. [DOI] [PubMed] [Google Scholar]
- Singh I., Helenius A. Role of ribosomes in Semliki Forest virus nucleocapsid uncoating. J Virol. 1992 Dec;66(12):7049–7058. doi: 10.1128/jvi.66.12.7049-7058.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeekens S. P., Montag A. G., Thomas G., Albiges-Rizo C., Carroll R., Benig M., Phillips L. A., Martin S., Ohagi S., Gardner P. Proinsulin processing by the subtilisin-related proprotein convertases furin, PC2, and PC3. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8822–8826. doi: 10.1073/pnas.89.18.8822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeekens S. P. Processing of protein precursors by a novel family of subtilisin-related mammalian endoproteases. Biotechnology (N Y) 1993 Feb;11(2):182–186. doi: 10.1038/nbt0293-182. [DOI] [PubMed] [Google Scholar]
- Smith A. L., Tignor G. H. Host cell receptors for two strains of Sindbis virus. Arch Virol. 1980;66(1):11–26. doi: 10.1007/BF01315041. [DOI] [PubMed] [Google Scholar]
- Smith D. B., Inglis S. C. The mutation rate and variability of eukaryotic viruses: an analytical review. J Gen Virol. 1987 Nov;68(Pt 11):2729–2740. doi: 10.1099/0022-1317-68-11-2729. [DOI] [PubMed] [Google Scholar]
- Sneider J. M., Kinney R. M., Tsuchiya K. R., Trent D. W. Molecular evidence that epizootic Venezuelan equine encephalitis (VEE) I-AB viruses are not evolutionary derivatives of enzootic VEE subtype I-E or II viruses. J Gen Virol. 1993 Mar;74(Pt 3):519–523. doi: 10.1099/0022-1317-74-3-519. [DOI] [PubMed] [Google Scholar]
- Sreevalsan T., Yin F. H. Sindbis virus-induced viral ribonucleic acid polymerase. J Virol. 1969 Jun;3(6):599–604. doi: 10.1128/jvi.3.6.599-604.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley J., Cooper S. J., Griffin D. E. Alphavirus neurovirulence: monoclonal antibodies discriminating wild-type from neuroadapted Sindbis virus. J Virol. 1985 Oct;56(1):110–119. doi: 10.1128/jvi.56.1.110-119.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley J., Cooper S. J., Griffin D. E. Monoclonal antibody cure and prophylaxis of lethal Sindbis virus encephalitis in mice. J Virol. 1986 Apr;58(1):107–115. doi: 10.1128/jvi.58.1.107-115.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stec D. S., Waddell A., Schmaljohn C. S., Cole G. A., Schmaljohn A. L. Antibody-selected variation and reversion in Sindbis virus neutralization epitopes. J Virol. 1986 Mar;57(3):715–720. doi: 10.1128/jvi.57.3.715-720.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegmann T., Delfino J. M., Richards F. M., Helenius A. The HA2 subunit of influenza hemagglutinin inserts into the target membrane prior to fusion. J Biol Chem. 1991 Sep 25;266(27):18404–18410. [PubMed] [Google Scholar]
- Stegmann T., Doms R. W., Helenius A. Protein-mediated membrane fusion. Annu Rev Biophys Biophys Chem. 1989;18:187–211. doi: 10.1146/annurev.bb.18.060189.001155. [DOI] [PubMed] [Google Scholar]
- Stegmann T., Schoen P., Bron R., Wey J., Bartoldus I., Ortiz A., Nieva J. L., Wilschut J. Evaluation of viral membrane fusion assays. Comparison of the octadecylrhodamine dequenching assay with the pyrene excimer assay. Biochemistry. 1993 Oct 26;32(42):11330–11337. doi: 10.1021/bi00093a009. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Smeekens S. P., Ohagi S., Chan S. J. The new enzymology of precursor processing endoproteases. J Biol Chem. 1992 Nov 25;267(33):23435–23438. [PubMed] [Google Scholar]
- Steinhauer D. A., Domingo E., Holland J. J. Lack of evidence for proofreading mechanisms associated with an RNA virus polymerase. Gene. 1992 Dec 15;122(2):281–288. doi: 10.1016/0378-1119(92)90216-c. [DOI] [PubMed] [Google Scholar]
- Steinhauer D. A., Holland J. J. Rapid evolution of RNA viruses. Annu Rev Microbiol. 1987;41:409–433. doi: 10.1146/annurev.mi.41.100187.002205. [DOI] [PubMed] [Google Scholar]
- Stollar V. Approaches to the study of vector specificity for arboviruses--model systems using cultured mosquito cells. Adv Virus Res. 1987;33:327–365. doi: 10.1016/s0065-3527(08)60322-x. [DOI] [PubMed] [Google Scholar]
- Strauss E. G., Birdwell C. R., Lenches E. M., Staples S. E., Strauss J. H. Mutants of Sindbis virus. II. Characterization of a maturation-defective mutant, ts103. Virology. 1977 Oct 1;82(1):122–149. doi: 10.1016/0042-6822(77)90038-1. [DOI] [PubMed] [Google Scholar]
- Strauss E. G., De Groot R. J., Levinson R., Strauss J. H. Identification of the active site residues in the nsP2 proteinase of Sindbis virus. Virology. 1992 Dec;191(2):932–940. doi: 10.1016/0042-6822(92)90268-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss E. G., Lenches E. M., Stamreich-Martin M. A. Growth and release of several alphaviruses in chick and BHK cells. J Gen Virol. 1980 Aug;49(2):297–307. doi: 10.1099/0022-1317-49-2-297. [DOI] [PubMed] [Google Scholar]
- Strauss E. G., Lenches E. M., Strauss J. H. Mutants of sindbis virus. I. Isolation and partial characterization of 89 new temperature-sensitive mutants. Virology. 1976 Oct 1;74(1):154–168. doi: 10.1016/0042-6822(76)90137-9. [DOI] [PubMed] [Google Scholar]
- Strauss E. G., Levinson R., Rice C. M., Dalrymple J., Strauss J. H. Nonstructural proteins nsP3 and nsP4 of Ross River and O'Nyong-nyong viruses: sequence and comparison with those of other alphaviruses. Virology. 1988 May;164(1):265–274. doi: 10.1016/0042-6822(88)90644-7. [DOI] [PubMed] [Google Scholar]
- Strauss E. G. Mutants of Sindbis virus. III. Host polypeptides present in purified HR and ts103 virus particles. J Virol. 1978 Nov;28(2):466–474. doi: 10.1128/jvi.28.2.466-474.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss E. G., Rice C. M., Strauss J. H. Complete nucleotide sequence of the genomic RNA of Sindbis virus. Virology. 1984 Feb;133(1):92–110. doi: 10.1016/0042-6822(84)90428-8. [DOI] [PubMed] [Google Scholar]
- Strauss E. G., Rice C. M., Strauss J. H. Sequence coding for the alphavirus nonstructural proteins is interrupted by an opal termination codon. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5271–5275. doi: 10.1073/pnas.80.17.5271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss E. G., Stec D. S., Schmaljohn A. L., Strauss J. H. Identification of antigenically important domains in the glycoproteins of Sindbis virus by analysis of antibody escape variants. J Virol. 1991 Sep;65(9):4654–4664. doi: 10.1128/jvi.65.9.4654-4664.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss E. G., Strauss J. H. Replication strategies of the single stranded RNA viruses of eukaryotes. Curr Top Microbiol Immunol. 1983;105:1–98. doi: 10.1007/978-3-642-69159-1_1. [DOI] [PubMed] [Google Scholar]
- Strauss J. H., Jr, Burge B. W., Darnell J. E. Sindbis virus infection of chick and hamster cells: synthesis of virus-specific proteins. Virology. 1969 Mar;37(3):367–376. doi: 10.1016/0042-6822(69)90220-7. [DOI] [PubMed] [Google Scholar]
- Strauss J. H., Strauss E. G. Evolution of RNA viruses. Annu Rev Microbiol. 1988;42:657–683. doi: 10.1146/annurev.mi.42.100188.003301. [DOI] [PubMed] [Google Scholar]
- Stubbs M. J., Miller A., Sizer P. J., Stephenson J. R., Crooks A. J. X-ray solution scattering of Sindbis virus. Changes in conformation induced at low pH. J Mol Biol. 1991 Sep 5;221(1):39–42. doi: 10.1016/0022-2836(91)80200-e. [DOI] [PubMed] [Google Scholar]
- Subak-Sharpe I., Dyson H., Fazakerley J. In vivo depletion of CD8+ T cells prevents lesions of demyelination in Semliki Forest virus infection. J Virol. 1993 Dec;67(12):7629–7633. doi: 10.1128/jvi.67.12.7629-7633.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suomalainen M., Garoff H. Alphavirus spike-nucleocapsid interaction and network antibodies. J Virol. 1992 Aug;66(8):5106–5109. doi: 10.1128/jvi.66.8.5106-5109.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suomalainen M., Liljeström P., Garoff H. Spike protein-nucleocapsid interactions drive the budding of alphaviruses. J Virol. 1992 Aug;66(8):4737–4747. doi: 10.1128/jvi.66.8.4737-4747.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symington J., Schlesinger M. J. Characterization of a Sinbis virus variant with altered host range. Arch Virol. 1978;58(2):127–136. doi: 10.1007/BF01315405. [DOI] [PubMed] [Google Scholar]
- Symington J., Schlesinger M. J. Isolation of a Sindbis virus variant by passage on mouse plasmacytoma cells. J Virol. 1975 Apr;15(4):1037–1041. doi: 10.1128/jvi.15.4.1037-1041.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderlund H., Käriänen L., Von Bonsdorff C. H., Weckström P. Properties of Semliki forest virus nucleocapsid. II. An irreversible contraction by acid pH. Virology. 1972 Mar;47(3):753–760. doi: 10.1016/0042-6822(72)90565-x. [DOI] [PubMed] [Google Scholar]
- Takkinen K. Complete nucleotide sequence of the nonstructural protein genes of Semliki Forest virus. Nucleic Acids Res. 1986 Jul 25;14(14):5667–5682. doi: 10.1093/nar/14.14.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takkinen K., Peränen J., Keränen S., Söderlund H., Käriäinen L. The Semliki-Forest-virus-specific nonstructural protein nsP4 is an autoproteinase. Eur J Biochem. 1990 Apr 20;189(1):33–38. doi: 10.1111/j.1432-1033.1990.tb15456.x. [DOI] [PubMed] [Google Scholar]
- Takkinen K., Peränen J., Käriäinen L. Proteolytic processing of Semliki Forest virus-specific non-structural polyprotein. J Gen Virol. 1991 Jul;72(Pt 7):1627–1633. doi: 10.1099/0022-1317-72-7-1627. [DOI] [PubMed] [Google Scholar]
- Tatem J., Stollar V. Effect of Sindbis virus infection on induction of heat shock proteins in Aedes albopictus cells. J Virol. 1989 Feb;63(2):992–996. doi: 10.1128/jvi.63.2.992-996.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theilmann D., Eaton B. T., Downe A. E. Properties of Sindbis virus variants from infected Culex tarsalis mosquitoes. J Gen Virol. 1984 May;65(Pt 5):945–953. doi: 10.1099/0022-1317-65-5-945. [DOI] [PubMed] [Google Scholar]
- Tobias J. W., Shrader T. E., Rocap G., Varshavsky A. The N-end rule in bacteria. Science. 1991 Nov 29;254(5036):1374–1377. doi: 10.1126/science.1962196. [DOI] [PubMed] [Google Scholar]
- Tong L., Wengler G., Rossmann M. G. Refined structure of Sindbis virus core protein and comparison with other chymotrypsin-like serine proteinase structures. J Mol Biol. 1993 Mar 5;230(1):228–247. doi: 10.1006/jmbi.1993.1139. [DOI] [PubMed] [Google Scholar]
- Tooze J., Hollinshead M., Frank R., Burke B. An antibody specific for an endoproteolytic cleavage site provides evidence that pro-opiomelanocortin is packaged into secretory granules in AtT20 cells before its cleavage. J Cell Biol. 1987 Jul;105(1):155–162. doi: 10.1083/jcb.105.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trent D. W., Grant J. A. A comparison of New World alphaviruses in the western equine encephalomyelitis complex by immunochemical and oligonucleotide fingerprint techniques. J Gen Virol. 1980 Apr;47(2):261–282. doi: 10.1099/0022-1317-47-2-261. [DOI] [PubMed] [Google Scholar]
- Tsiang M., Monroe S. S., Schlesinger S. Studies of defective interfering RNAs of Sindbis virus with and without tRNAAsp sequences at their 5' termini. J Virol. 1985 Apr;54(1):38–44. doi: 10.1128/jvi.54.1.38-44.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiang M., Weiss B. G., Schlesinger S. Effects of 5'-terminal modifications on the biological activity of defective interfering RNAs of Sindbis virus. J Virol. 1988 Jan;62(1):47–53. doi: 10.1128/jvi.62.1.47-53.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker P. C., Griffin D. E. Mechanism of altered Sindbis virus neurovirulence associated with a single-amino-acid change in the E2 Glycoprotein. J Virol. 1991 Mar;65(3):1551–1557. doi: 10.1128/jvi.65.3.1551-1557.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker P. C., Strauss E. G., Kuhn R. J., Strauss J. H., Griffin D. E. Viral determinants of age-dependent virulence of Sindbis virus for mice. J Virol. 1993 Aug;67(8):4605–4610. doi: 10.1128/jvi.67.8.4605-4610.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomi K., Kädäridäinen L., Söderlund H. Quantitation of Semlike Forest virus RNAs in infected cells using 32-P equilibrium labelling. Nucleic Acids Res. 1975 Apr;2(4):555–565. doi: 10.1093/nar/2.4.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubol S., Griffin D. E. Identification of a putative alphavirus receptor on mouse neural cells. J Virol. 1991 Dec;65(12):6913–6921. doi: 10.1128/jvi.65.12.6913-6921.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmanen I., Söderlund H., Käriäinen L. Role of protein synthesis in the assembly of Semliki forest virus nucleocapsid. Virology. 1979 Dec;99(2):265–276. doi: 10.1016/0042-6822(79)90006-0. [DOI] [PubMed] [Google Scholar]
- Ulmanen I., Söderlund H., Käriäinen L. Semliki Forest virus capsid protein associates with the 60S ribosomal subunit in infected cells. J Virol. 1976 Oct;20(1):203–210. doi: 10.1128/jvi.20.1.203-210.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulug E. T., Bose H. R., Jr Effect of tunicamycin on the development of the cytopathic effect in Sindbis virus-infected avian fibroblasts. Virology. 1985 Jun;143(2):546–557. doi: 10.1016/0042-6822(85)90393-9. [DOI] [PubMed] [Google Scholar]
- Ulug E. T., Garry R. F., Bose H. R., Jr The role of monovalent cation transport in Sindbis virus maturation and release. Virology. 1989 Sep;172(1):42–50. doi: 10.1016/0042-6822(89)90105-0. [DOI] [PubMed] [Google Scholar]
- Ulug E. T., Garry R. F., Waite M. R., Bose H. R., Jr Alterations in monovalent cation transport in Sindbis virus-infected chick cells. Virology. 1984 Jan 15;132(1):118–130. doi: 10.1016/0042-6822(84)90096-5. [DOI] [PubMed] [Google Scholar]
- Varshavsky A. The N-end rule. Cell. 1992 May 29;69(5):725–735. doi: 10.1016/0092-8674(92)90285-k. [DOI] [PubMed] [Google Scholar]
- Vaux D. J., Helenius A., Mellman I. Spike--nucleocapsid interaction in Semliki Forest virus reconstructed using network antibodies. Nature. 1988 Nov 3;336(6194):36–42. doi: 10.1038/336036a0. [DOI] [PubMed] [Google Scholar]
- Volchkov V. E., Volchkova V. A., Netesov S. V. Polnaia nukleotidnaia posledovatel'nost' genoma virusa vostochnogo éntsefalomielita loshadei. Mol Gen Mikrobiol Virusol. 1991 May;(5):8–15. [PubMed] [Google Scholar]
- Vrati S., Faragher S. G., Weir R. C., Dalgarno L. Ross River virus mutant with a deletion in the E2 gene: properties of the virion, virus-specific macromolecule synthesis, and attenuation of virulence for mice. Virology. 1986 Jun;151(2):222–232. doi: 10.1016/0042-6822(86)90044-9. [DOI] [PubMed] [Google Scholar]
- Vrati S., Fernon C. A., Dalgarno L., Weir R. C. Location of a major antigenic site involved in Ross River virus neutralization. Virology. 1988 Feb;162(2):346–353. doi: 10.1016/0042-6822(88)90474-6. [DOI] [PubMed] [Google Scholar]
- Vénien-Bryan C., Fuller S. D. The organization of the spike complex of Semliki Forest virus. J Mol Biol. 1994 Feb 18;236(2):572–583. doi: 10.1006/jmbi.1994.1166. [DOI] [PubMed] [Google Scholar]
- Wahlberg J. M., Boere W. A., Garoff H. The heterodimeric association between the membrane proteins of Semliki Forest virus changes its sensitivity to low pH during virus maturation. J Virol. 1989 Dec;63(12):4991–4997. doi: 10.1128/jvi.63.12.4991-4997.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlberg J. M., Bron R., Wilschut J., Garoff H. Membrane fusion of Semliki Forest virus involves homotrimers of the fusion protein. J Virol. 1992 Dec;66(12):7309–7318. doi: 10.1128/jvi.66.12.7309-7318.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlberg J. M., Garoff H. Membrane fusion process of Semliki Forest virus. I: Low pH-induced rearrangement in spike protein quaternary structure precedes virus penetration into cells. J Cell Biol. 1992 Jan;116(2):339–348. doi: 10.1083/jcb.116.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waite M. R., Brown D. T., Pfefferkorn E. R. Inhibition of Sindbis virus release by media of low ionic strength: an electron microscope study. J Virol. 1972 Sep;10(3):537–544. doi: 10.1128/jvi.10.3.537-544.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waite M. R., Pfefferkorn E. R. Inhibition of Sindbis virus production by media of low ionic strength: intracellular events and requirements for reversal. J Virol. 1970 Jan;5(1):60–71. doi: 10.1128/jvi.5.1.60-71.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K. S., Kuhn R. J., Strauss E. G., Ou S., Strauss J. H. High-affinity laminin receptor is a receptor for Sindbis virus in mammalian cells. J Virol. 1992 Aug;66(8):4992–5001. doi: 10.1128/jvi.66.8.4992-5001.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K. S., Schmaljohn A. L., Kuhn R. J., Strauss J. H. Antiidiotypic antibodies as probes for the Sindbis virus receptor. Virology. 1991 Apr;181(2):694–702. doi: 10.1016/0042-6822(91)90903-o. [DOI] [PubMed] [Google Scholar]
- Wang K. S., Strauss J. H. Use of a lambda gt11 expression library to localize a neutralizing antibody-binding site in glycoprotein E2 of Sindbis virus. J Virol. 1991 Dec;65(12):7037–7040. doi: 10.1128/jvi.65.12.7037-7040.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. F., Sawicki S. G., Sawicki D. L. Sindbis virus nsP1 functions in negative-strand RNA synthesis. J Virol. 1991 Feb;65(2):985–988. doi: 10.1128/jvi.65.2.985-988.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D. G., Moehring J. M., Moehring T. J. A mutant CHO-K1 strain with resistance to Pseudomonas exotoxin A and alphaviruses fails to cleave Sindbis virus glycoprotein PE2. J Virol. 1991 May;65(5):2332–2339. doi: 10.1128/jvi.65.5.2332-2339.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver S. C., Bellew L. A., Gousset L., Repik P. M., Scott T. W., Holland J. J. Diversity within natural populations of eastern equine encephalomyelitis virus. Virology. 1993 Aug;195(2):700–709. doi: 10.1006/viro.1993.1421. [DOI] [PubMed] [Google Scholar]
- Weaver S. C., Bellew L. A., Rico-Hesse R. Phylogenetic analysis of alphaviruses in the Venezuelan equine encephalitis complex and identification of the source of epizootic viruses. Virology. 1992 Nov;191(1):282–290. doi: 10.1016/0042-6822(92)90190-z. [DOI] [PubMed] [Google Scholar]
- Weaver S. C., Hagenbaugh A., Bellew L. A., Calisher C. H. Genetic characterization of an antigenic subtype of eastern equine encephalomyelitis virus. Arch Virol. 1992;127(1-4):305–314. doi: 10.1007/BF01309592. [DOI] [PubMed] [Google Scholar]
- Weaver S. C., Hagenbaugh A., Bellew L. A., Gousset L., Mallampalli V., Holland J. J., Scott T. W. Evolution of alphaviruses in the eastern equine encephalomyelitis complex. J Virol. 1994 Jan;68(1):158–169. doi: 10.1128/jvi.68.1.158-169.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver S. C., Hagenbaugh A., Bellew L. A., Netesov S. V., Volchkov V. E., Chang G. J., Clarke D. K., Gousset L., Scott T. W., Trent D. W. A comparison of the nucleotide sequences of eastern and western equine encephalomyelitis viruses with those of other alphaviruses and related RNA viruses. Virology. 1993 Nov;197(1):375–390. doi: 10.1006/viro.1993.1599. [DOI] [PubMed] [Google Scholar]
- Weaver S. C., Lorenz L. H., Scott T. W. Pathologic changes in the midgut of Culex tarsalis following infection with Western equine encephalomyelitis virus. Am J Trop Med Hyg. 1992 Nov;47(5):691–701. doi: 10.4269/ajtmh.1992.47.691. [DOI] [PubMed] [Google Scholar]
- Weaver S. C., Rico-Hesse R., Scott T. W. Genetic diversity and slow rates of evolution in New World alphaviruses. Curr Top Microbiol Immunol. 1992;176:99–117. doi: 10.1007/978-3-642-77011-1_7. [DOI] [PubMed] [Google Scholar]
- Weaver S. C., Scott T. W., Lorenz L. H., Lerdthusnee K., Romoser W. S. Togavirus-associated pathologic changes in the midgut of a natural mosquito vector. J Virol. 1988 Jun;62(6):2083–2090. doi: 10.1128/jvi.62.6.2083-2090.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver S. C., Scott T. W., Lorenz L. H., Repik P. M. Detection of eastern equine encephalomyelitis virus deposition in Culiseta melanura following ingestion of radiolabeled virus in blood meals. Am J Trop Med Hyg. 1991 Mar;44(3):250–259. doi: 10.4269/ajtmh.1991.44.250. [DOI] [PubMed] [Google Scholar]
- Weaver S. C., Scott T. W., Rico-Hesse R. Molecular evolution of eastern equine encephalomyelitis virus in North America. Virology. 1991 Jun;182(2):774–784. doi: 10.1016/0042-6822(91)90618-l. [DOI] [PubMed] [Google Scholar]
- Weiss B. G., Schlesinger S. Recombination between Sindbis virus RNAs. J Virol. 1991 Aug;65(8):4017–4025. doi: 10.1128/jvi.65.8.4017-4025.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B., Nitschko H., Ghattas I., Wright R., Schlesinger S. Evidence for specificity in the encapsidation of Sindbis virus RNAs. J Virol. 1989 Dec;63(12):5310–5318. doi: 10.1128/jvi.63.12.5310-5318.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B., Schlesinger S. Defective interfering particles of Sindbis virus do not interfere with the homologous virus obtained from persistently infected BHK cells but do interfere with Semliki Forest virus. J Virol. 1981 Feb;37(2):840–844. doi: 10.1128/jvi.37.2.840-844.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellink J., van Kammen A. Proteases involved in the processing of viral polyproteins. Brief review. Arch Virol. 1988;98(1-2):1–26. doi: 10.1007/BF01321002. [DOI] [PubMed] [Google Scholar]
- Wen D. Z., Schlesinger M. J. Regulated expression of Sindbis and vesicular stomatitis virus glycoproteins in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3639–3643. doi: 10.1073/pnas.83.11.3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wengler G., Boege U., Wengler G., Bischoff H., Wahn K. The core protein of the alphavirus Sindbis virus assembles into core-like nucleoproteins with the viral genome RNA and with other single-stranded nucleic acids in vitro. Virology. 1982 Apr 30;118(2):401–410. doi: 10.1016/0042-6822(82)90359-2. [DOI] [PubMed] [Google Scholar]
- Wengler G. Studies on the synthesis of viral RNA-polymerase-template complexes in BHK 21 cells infected with Semliki Forest virus. Virology. 1975 Jul;66(1):322–326. doi: 10.1016/0042-6822(75)90202-0. [DOI] [PubMed] [Google Scholar]
- Wengler G. The mode of assembly of alphavirus cores implies a mechanism for the disassembly of the cores in the early stages of infection. Brief review. Arch Virol. 1987;94(1-2):1–14. doi: 10.1007/BF01313721. [DOI] [PubMed] [Google Scholar]
- Wengler G., Wengler G., Boege U., Wahn K. Establishment and analysis of a system which allows assembly and disassembly of alphavirus core-like particles under physiological conditions in vitro. Virology. 1984 Jan 30;132(2):401–412. doi: 10.1016/0042-6822(84)90045-x. [DOI] [PubMed] [Google Scholar]
- Wengler G., Wengler G., Gross H. J. Terminal sequences of Sindbis virus-specific nucleic acids: identity in molecules synthesized in vertebrate and insect cells and characteristic properties of the replicative form RNA. Virology. 1982 Dec;123(2):273–283. doi: 10.1016/0042-6822(82)90261-6. [DOI] [PubMed] [Google Scholar]
- Wengler G., Wengler G., Gross H. S. Replicative form of Semliki Forest virus RNA contains an unpaired guanosine. Nature. 1979 Dec 13;282(5740):754–756. doi: 10.1038/282754a0. [DOI] [PubMed] [Google Scholar]
- Wengler G., Wengler G. Identification of a transfer of viral core protein to cellular ribosomes during the early stages of alphavirus infection. Virology. 1984 Apr 30;134(2):435–442. doi: 10.1016/0042-6822(84)90310-6. [DOI] [PubMed] [Google Scholar]
- Wengler G., Würkner D., Wengler G. Identification of a sequence element in the alphavirus core protein which mediates interaction of cores with ribosomes and the disassembly of cores. Virology. 1992 Dec;191(2):880–888. doi: 10.1016/0042-6822(92)90263-o. [DOI] [PubMed] [Google Scholar]
- Wewer U. M., Liotta L. A., Jaye M., Ricca G. A., Drohan W. N., Claysmith A. P., Rao C. N., Wirth P., Coligan J. E., Albrechtsen R. Altered levels of laminin receptor mRNA in various human carcinoma cells that have different abilities to bind laminin. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7137–7141. doi: 10.1073/pnas.83.19.7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wewer U. M., Taraboletti G., Sobel M. E., Albrechtsen R., Liotta L. A. Role of laminin receptor in tumor cell migration. Cancer Res. 1987 Nov 1;47(21):5691–5698. [PubMed] [Google Scholar]
- White J. M. Viral and cellular membrane fusion proteins. Annu Rev Physiol. 1990;52:675–697. doi: 10.1146/annurev.ph.52.030190.003331. [DOI] [PubMed] [Google Scholar]
- White J., Helenius A. pH-dependent fusion between the Semliki Forest virus membrane and liposomes. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3273–3277. doi: 10.1073/pnas.77.6.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J., Kartenbeck J., Helenius A. Fusion of Semliki forest virus with the plasma membrane can be induced by low pH. J Cell Biol. 1980 Oct;87(1):264–272. doi: 10.1083/jcb.87.1.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J., Matlin K., Helenius A. Cell fusion by Semliki Forest, influenza, and vesicular stomatitis viruses. J Cell Biol. 1981 Jun;89(3):674–679. doi: 10.1083/jcb.89.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems W. R., Kaluza G., Boschek C. B., Bauer H., Hager H., Schütz H. J., Feistner H. Semliki forest virus: cause of a fatal case of human encephalitis. Science. 1979 Mar 16;203(4385):1127–1129. doi: 10.1126/science.424742. [DOI] [PubMed] [Google Scholar]
- Wright H. T., Marston A. W., Goldstein D. J. A functional role for cysteine disulfides in the transmembrane transport of diphtheria toxin. J Biol Chem. 1984 Feb 10;259(3):1649–1654. [PubMed] [Google Scholar]
- Wust C. J., Crombie R., Brown A. Passive protection across subgroups of alphaviruses by hyperimmune non-cross-neutralizing anti-Sindbis serum. Proc Soc Exp Biol Med. 1987 Jan;184(1):56–63. doi: 10.3181/00379727-184-42446. [DOI] [PubMed] [Google Scholar]
- Wust C. J., Nicholas J. A., Fredin D., Dodd D. C., Brideau R. J., Levely M. E., Brown A. Monoclonal antibodies that cross-react with the E1 glycoprotein of different alphavirus serogroups: characterization including passive protection in vivo. Virus Res. 1989 Jun;13(2):101–112. doi: 10.1016/0168-1702(89)90009-9. [DOI] [PubMed] [Google Scholar]
- Xiong C., Levis R., Shen P., Schlesinger S., Rice C. M., Huang H. V. Sindbis virus: an efficient, broad host range vector for gene expression in animal cells. Science. 1989 Mar 3;243(4895):1188–1191. doi: 10.1126/science.2922607. [DOI] [PubMed] [Google Scholar]
- Yoshimasa Y., Seino S., Whittaker J., Kakehi T., Kosaki A., Kuzuya H., Imura H., Bell G. I., Steiner D. F. Insulin-resistant diabetes due to a point mutation that prevents insulin proreceptor processing. Science. 1988 May 6;240(4853):784–787. doi: 10.1126/science.3283938. [DOI] [PubMed] [Google Scholar]
- Zhao H., Garoff H. Role of cell surface spikes in alphavirus budding. J Virol. 1992 Dec;66(12):7089–7095. doi: 10.1128/jvi.66.12.7089-7095.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemiecki A., Garoff H., Simons K. Formation of the Semliki Forest virus membrane glycoprotein complexes in the infected cell. J Gen Virol. 1980 Sep;50(1):111–123. doi: 10.1099/0022-1317-50-1-111. [DOI] [PubMed] [Google Scholar]
- Ziemiecki A., Garofff H. Subunit composition of the membrane glycoprotein complex of Semliki Forest virus. J Mol Biol. 1978 Jul 5;122(3):259–269. doi: 10.1016/0022-2836(78)90189-4. [DOI] [PubMed] [Google Scholar]
- Zurzolo C., Polistina C., Saini M., Gentile R., Aloj L., Migliaccio G., Bonatti S., Nitsch L. Opposite polarity of virus budding and of viral envelope glycoprotein distribution in epithelial cells derived from different tissues. J Cell Biol. 1992 May;117(3):551–564. doi: 10.1083/jcb.117.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Curtis I., Simons K. Dissection of Semliki Forest virus glycoprotein delivery from the trans-Golgi network to the cell surface in permeabilized BHK cells. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8052–8056. doi: 10.1073/pnas.85.21.8052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot R. J., Rümenapf T., Kuhn R. J., Strauss E. G., Strauss J. H. Sindbis virus RNA polymerase is degraded by the N-end rule pathway. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):8967–8971. doi: 10.1073/pnas.88.20.8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duijn L. P., Kasperaitis M., Ameling C., Voorma H. O. Additional methylation at the N(2)-position of the cap of 26S Semliki Forest virus late mRNA and initiation of translation. Virus Res. 1986 Jul;5(1):61–66. doi: 10.1016/0168-1702(86)90065-1. [DOI] [PubMed] [Google Scholar]
- van Steeg H., Kasperaitis M., Voorma H. O., Benne R. Infection of neuroblastoma cells by Semliki Forest virus. The interference of viral capsid protein with the binding of host messenger RNAs into initiation complexes is the cause of the shut-off of host protein synthesis. Eur J Biochem. 1984 Feb 1;138(3):473–478. doi: 10.1111/j.1432-1033.1984.tb07940.x. [DOI] [PubMed] [Google Scholar]
- van Steeg H., Thomas A., Verbeek S., Kasperaitis M., Voorma H. O., Benne R. Shutoff of neuroblastoma cell protein synthesis by Semliki Forest virus: loss of ability of crude initiation factors to recognize early Semliki Forest virus and host mRNA's. J Virol. 1981 May;38(2):728–736. doi: 10.1128/jvi.38.2.728-736.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bonsdorff C. H., Harrison S. C. Hexagonal glycoprotein arrays from Sindbis virus membranes. J Virol. 1978 Nov;28(2):578–583. doi: 10.1128/jvi.28.2.578-583.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bonsdorff C. H., Harrison S. C. Sindbis virus glycoproteins form a regular icosahedral surface lattice. J Virol. 1975 Jul;16(1):141–145. doi: 10.1128/jvi.16.1.141-145.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]