Plants must coordinate exclusion and internal detoxification to reduce aluminum toxicity effectively.
Abstract
Whether aluminum toxicity is an apoplastic or symplastic phenomenon is still a matter of debate. Here, we found that three auxin overproducing mutants, yucca, the recessive mutant superroot2, and superroot1 had increased aluminum sensitivity, while a transfer DNA insertion mutant, xyloglucan endotransglucosylase/hydrolases15 (xth15), showed enhanced aluminum resistance, accompanied by low endogenous indole-3-acetic acid levels, implying that auxin may be involved in plant responses to aluminum stress. We used yucca and xth15 mutants for further study. The two mutants accumulated similar total aluminum in roots and had significantly reduced cell wall aluminum and increased symplastic aluminum content relative to the wild-type ecotype Columbia, indicating that altered aluminum levels in the symplast or cell wall cannot fully explain the differential aluminum resistance of these two mutants. The expression of Al sensitive1 (ALS1), a gene that functions in aluminum redistribution between the cytoplasm and vacuole and contributes to symplastic aluminum detoxification, was less abundant in yucca and more abundant in xth15 than the wild type, consistent with possible ALS1 function conferring altered aluminum sensitivity in the two mutants. Consistent with the idea that xth15 can tolerate more symplastic aluminum because of possible ALS1 targeting to the vacuole, morin staining of yucca root tip sections showed more aluminum accumulation in the cytosol than in the wild type, and xth15 showed reduced morin staining of cytosolic aluminum, even though yucca and xth15 had similar overall symplastic aluminum content. Exogenous application of an active auxin analog, naphthylacetic acid, to the wild type mimicked the aluminum sensitivity and distribution phenotypes of yucca, verifying that auxin may regulate aluminum distribution in cells. Together, these data demonstrate that auxin negatively regulates aluminum tolerance through altering ALS1 expression and aluminum distribution within plant cells, and plants must coordinate exclusion and internal detoxification to reduce aluminum toxicity effectively.
Aluminum (Al) toxicity is a major growth-limiting factor for crop production on acid soils worldwide (Foy, 1988; Kochian, 1995), which occupy approximately 50% of the world’s potential arable land (von Uexküll and Mutert, 1995). Ionic aluminum inhibits root elongation as well as water and nutrient uptake and results in significant loss of crop productivity (Kochian et al., 1995). Despite the increasing evidence of the functional and structural damage resulting from aluminum toxicity, the mechanism underlying aluminum-induced root growth inhibition remains unclear.
Aluminum-resistant plants have developed two mechanisms to cope with aluminum toxicity. One is based on the exclusion of aluminum from the root symplasm, whereas the other relies on the ability to tolerate symplastic aluminum (Taylor, 1991; Kochian et al., 2004). The well-documented exclusion mechanism is to prevent aluminum from entering root cells by secretion of organic acid anions by the root apex, resulting in the formation of stable nonphytotoxic chelates with aluminum (Kochian, 1995; Ryan et al., 2001; Ma and Furukawa, 2003). The internal detoxification of aluminum is primarily based on the storage of aluminum in the vacuole as aluminum-oxalate (Ma et al., 1997a; Shen et al., 2002) or aluminum-citrate (Ma et al., 1997b) complexes, thus changing the distribution of aluminum within cells (Ma, 2000; Ma and Hiradate, 2000). Recently, some transporters involved in the aluminum distribution within cells have been identified. In Arabidopsis (Arabidopsis thaliana), the tonoplast-localized ATP-binding cassette (ABC) transporter Al sensitive1 (ALS1), which is involved in Al tolerance in Arabidopsis (Larsen et al., 2007), while ALS3 is responsible for movement of aluminum away from sensitive tissues for sequestration in more tolerant tissues (Larsen et al., 2005, 2007). Huang et al. (2012) identified a rice (Oryza sativa) tonoplast-localized aluminum transporter, encoded by OsALS1, which is responsible for sequestration of aluminum into vacuoles and thus contributes to the internal detoxification of aluminum in rice. In addition, some plants have been found to harbor multiple strategies for aluminum detoxification. Buckwheat (Fagopyrum esculentum), for example, can secrete oxalate to detoxify external aluminum and accumulate large amounts of aluminum within vacuoles by forming aluminum-oxalate complexes in a molar ratio of 1:3 (Ma et al., 1997c). Rice, which is the most aluminum-resistant cereal crop, employs multiple strategies to achieve high aluminum resistance. The rice aluminum stress-responsive transcriptional factor, ART1, regulates expression of 31 downstream genes (Yamaji et al., 2009). In addition to gene-based aluminum tolerance strategies, some external factors can also affect aluminum resistance, such as external phytohormone application. For example, exogenous application of cytokinin can alleviate aluminum-induced inhibition of lateral root growth in aluminum-sensitive soybean (Glycine max; Pan et al., 2001). Several studies have demonstrated that aluminum may interact with auxin-signaling pathways, leading to alterations of auxin accumulation and distribution in roots (Kollmeier et al., 2000; Doncheva et al., 2005; Shen et al., 2008). A recent study indicated that Al3+ induced alteration of auxin distribution in roots, leading to arrest of root elongation, while naphthylphthalamic acid (an auxin polar transport inhibitor) applied exogenously substantially alleviated the aluminum-induced inhibition of root elongation (Sun et al., 2010). However, these previous studies only present circumstantial evidence on the disruption of the accumulation and polar transportation of auxin, which may be a primary cause of the aluminum-induced inhibition of root growth. The main mechanism of how auxin contributes to aluminum sensitivity remains unclear.
Cell walls are not only a critical site for aluminum storage in plants, but also serve as the first barrier to cellular aluminum uptake. For example, 85% to 90% of the total aluminum accumulated by barley (Hordeum vulgare) roots is tightly bound to the cell walls (Clarkson, 1967), and almost 90% of the cellular aluminum is associated with the cell walls of cultured tobacco (Nicotiana tabacum) cells (Chang et al., 1999). Accumulating evidence demonstrates that the cell wall plays important roles in the manifestation and perception of aluminum toxicity (Horst et al., 2010). The cell wall is very complex in structure and composition. The binding of aluminum changes cell wall structure, makes the wall more rigid, and reduces mechanical extensibility and cell expansion (Tabuchi and Matsumoto, 2001; Ma et al., 2004). An aluminum-sensitive rice cultivar accumulates higher aluminum in the cell wall than an aluminum-resistant cultivar (Yang et al., 2008). Higher pectin content is partially attributed to higher aluminum accumulation in the cell wall (Eticha et al., 2005a; Liu et al., 2008; Yang et al., 2011a). Furthermore, aluminum stress also results in an increase of not only pectin, but also hemicellulose content in wheat (Triticum aestivum; Tabuchi and Matsumoto, 2001), triticale (× Triticosecale Wittmack; Liu et al., 2008), and rice (Yang et al., 2008). Recently, Yang et al. (2011b) reported that hemicellulose, not pectin, is the major cell wall component that binds aluminum in Arabidopsis. Aluminum inhibits xyloglucan endotransglucosylase action, an enzyme that may cut and rejoin xyloglucan chains leading to cell wall loosening (Fry et al., 1992; Nishitani and Tominaga, 1992; Thompson and Fry, 2001), and down-regulates the expression of xyloglucan endotransglucosylase/hydrolases (XTH14), XTH15, and XTH31. Further study showed that Arabidopsis with XTH31 knocked out has lower xyloglucan content and cell wall aluminum-binding capacity and higher aluminum resistance (Zhu et al., 2012). Aluminum-induced secretion of organic acid anions decreases aluminum retention in the cell wall (Zheng et al., 2004), and, as a consequence, aluminum content in the root apex is decreased, which renders plants more aluminum resistant. Together, these data support the view that higher aluminum retention in the cell wall results in greater aluminum sensitivity. However, an important question remains open; if the total aluminum content in the roots remains constant, but the aluminum retention in the cell wall is decreased, will plants be more aluminum sensitive or more aluminum resistant?
In this study, we used two Arabidopsis mutants that accumulate similar aluminum levels in the roots, but one mutant, yucca, is aluminum sensitive, whereas the other, xth15, is aluminum resistant. We explored the underlying mechanisms leading to this difference in aluminum sensitivity. Although both mutants accumulate similar aluminum levels in cell wall, xth15 may sequester more aluminum into vacuoles than yucca. These data suggest the importance of coordination between external and internal detoxification mechanisms in Arabidopsis.
RESULTS
To investigate the effect of auxin on aluminum sensitivity, we used three high endogenous auxin mutants, the recessive mutants superroot1 (sur1-3; Boerjan et al., 1995) and sur2 (Delarue et al., 1998; Barlier et al., 2000) and the dominant activation-tagged yucca1, which overexpresses the flavin monooxygenase-like YUCCA proposed to be involved in Trp-dependent indole-3-acetic acid (IAA) biosynthesis (Zhao et al., 2001).
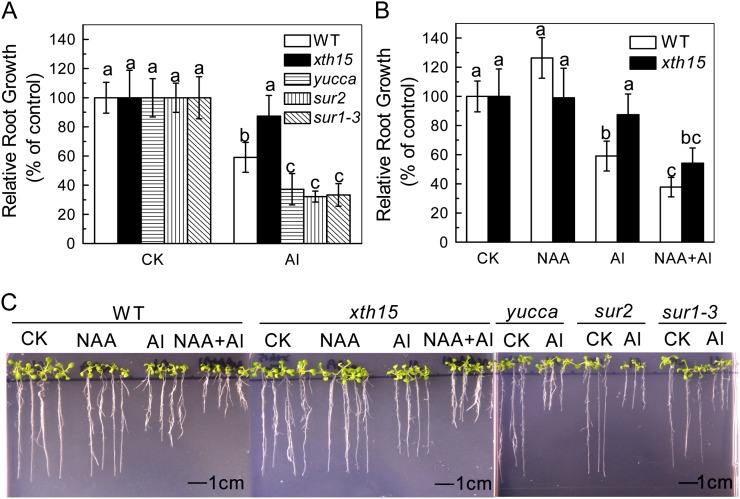
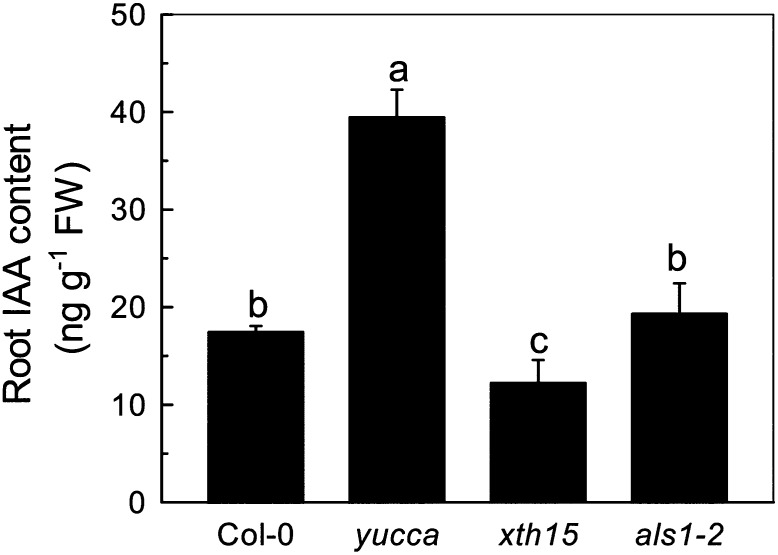
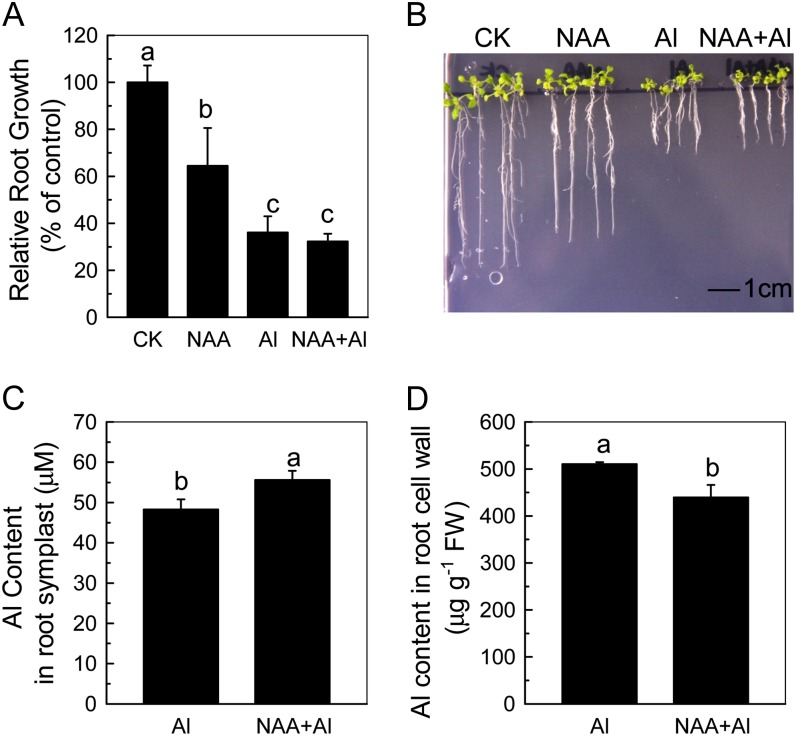
Inhibition of root elongation is the most typical symptom of aluminum toxicity in plants. When Arabidopsis seedlings were treated with 50 µm aluminum for 24 h, root elongation was inhibited by 63% in an auxin overproducing mutant, yucca, while 41% in the wild type (Fig. 1A). In longer-term (7-d) experiments, root growth was also more inhibited by exogenous aluminum in yucca than in the wild type (Fig. 1C). To confirm that the aluminum-sensitive phenotypes observed in yucca are caused by the overproduction of auxin, we analyzed the aluminum sensitivity of the other two high endogenous auxin mutants, sur2 and sur1-3, and found that they showed similar aluminum sensitivity as yucca (Fig. 1, A and C). As the increased endogenous auxin levels had similar effects on aluminum resistance, we used yucca as plants with “high levels of endogenous auxin” for the following experiments. Wild-type plants treated with naphthylacetic acid (NAA; an active auxin) also are more aluminum sensitive (Fig. 1, B and C), confirming that the aluminum sensitivity of yucca is likely due to auxin overaccumulation. We also measured the solution pH when 0.05 µm NAA was added to the nonbuffered aluminum solution and found that there was no decline of pH. Furthermore, when the solution was buffered at pH 4.5 with MES, the relative root elongation showed no significant difference with the nonbuffered solution (Fig. 1B; Supplemental Fig. S1). Moreover, if NAA increases the activity of aluminum, the inhibition of the root elongation should also be more profound in NAA plus aluminum treatment than aluminum treatment alone, but there was almost no difference of aluminum sensitivity in yucca between aluminum and NAA plus aluminum treatment (Supplemental Fig. S1). Therefore, the effect of NAA plus aluminum on root growth was caused by the synergetic action of aluminum and NAA. Furthermore, Figure 1A shows that xth15, mutants with a transfer DNA insertion in the XTH15 locus, treated with 50 µm aluminum for 24 h showed only 13% root elongation inhibition compared with wild-type root inhibition of 41% (Fig. 1A). Similarly, xth15 seedlings grown for 7 d on agar medium containing 50 µm Al3+ had longer roots than similarly treated wild-type seedlings (Fig. 1C), and the root growth of xth15 was also inhibited by NAA plus aluminum (Fig. 1, B and C). Interestingly, the IAA levels in xth15 roots were also lower than the wild type (Fig. 2) and correlated with enhanced aluminum resistance (Fig. 1). All these results demonstrate that higher levels of endogenous or exogenous auxin correlate with increased Arabidopsis sensitivity to aluminum.
Figure 1.
The effect of aluminum and NAA on root growth of Arabidopsis. A, One-centimeter-long seedlings were grown on 0.5 mm CaCl2 medium containing 0 (CK) or 50 µm aluminum for 24 h. Data are means ± sd (n = 10). Columns with different letters are significantly different at P < 0.05. B, One-centimeter-long seedlings were grown on 0.5 mm CaCl2 medium containing 0 or 50 µm aluminum in the presence or absence of NAA. Root elongation was measured before and after treatment for 24 h. Data are means ± sd (n = 10). Columns with different letters are significantly different at P < 0.05. C, The effect of aluminum and NAA on the root growth of Arabidopsis. One-centimeter-long seedlings were grown on nutrient plates containing 0 or 50 µm aluminum for 7 d. All the experiments were done at pH 4.5. Pictures were taken using a digital camera.
Figure 2.
Accumulation of IAA in Col-0, yucca, xth15, and als1-2 without aluminum treatment. Data are means ± sd (n = 4). Columns with different letters are significantly different at P < 0.05.
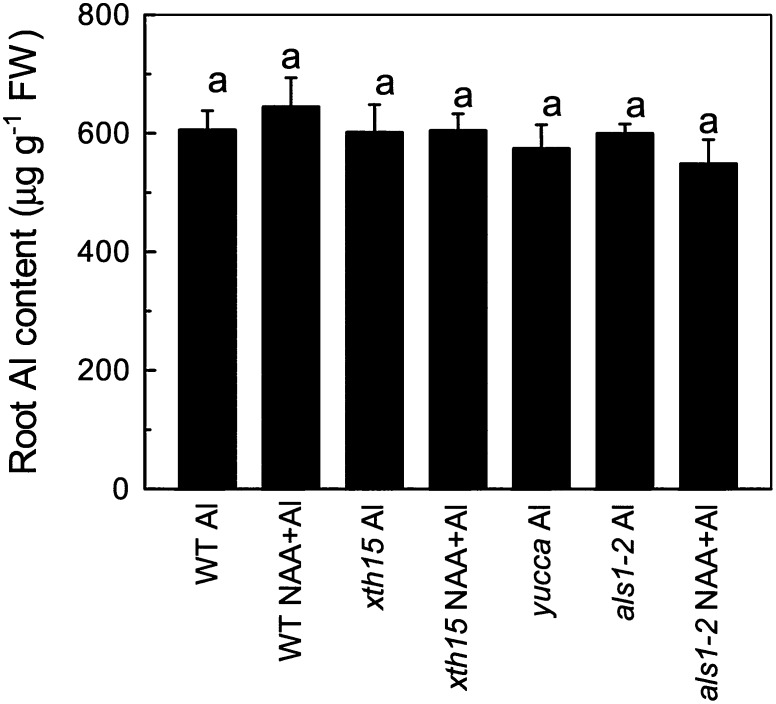
Aluminum content is a critical index to indicate whether exclusion or internal detoxification mechanism underlies aluminum resistance. To distinguish between these mechanisms in the altered aluminum sensitivity of yucca and xth15 mutants, we determined aluminum content in roots of seedlings exposed to 50 µm aluminum for 24 h. Surprisingly, the wild type, yucca, xth15, and the wild type and xth15 treated with NAA all accumulated similar levels of aluminum (Fig. 3). These results suggest that elevated auxin or loss of XTH15 function does not affect overall aluminum accumulation levels but may cause alterations in aluminum sensitivity by changing the distribution of aluminum within root cells.
Figure 3.
The aluminum content in the plant roots. Six-week-old plants were treated with 0.5 mm CaCl2 solution containing 50 µm aluminum or 50 µm aluminum combined with 0.05 µm NAA for treatment with auxin applied exogenously. The pH was adjusted to 4.5. Data are means ± sd (n = 4). Columns with different letters are significantly different at P < 0.05.
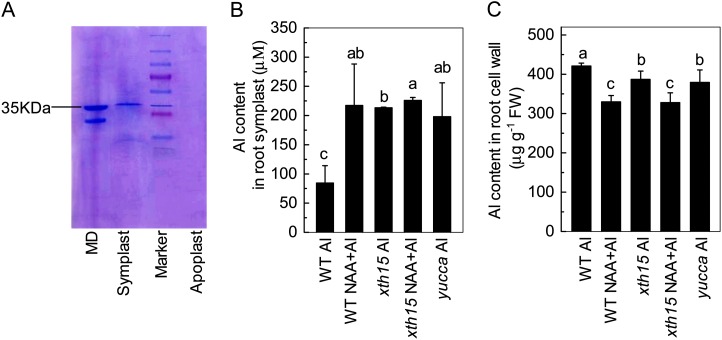
To address cellular distribution, we first measured aluminum content in symplast and root cell walls according to Xia et al. (2010). The purity of the apoplastic solution was reported by the absence of detectable malic dehydrogenase (about 35 kD; Fig. 4A). Aluminum accumulation in the symplast of the untreated wild type was lower than that in yucca, xth15, and the wild type treated with exogenous NAA (Fig. 4B), whereas the aluminum content in the cell wall was higher in the wild type than in the wild type treated with NAA or in xth15 or yucca (Fig. 4C). Although the reduction in cell wall aluminum content of xth15 relative to the wild type may correlate with the enhanced aluminum resistance of xth15, the reduction in cell wall aluminum of yucca and the NAA-treated wild type is unexpected, given the enhanced aluminum sensitivity of yucca and the NAA-treated wild type and xth15. These data prompted us to investigate whether differential subcellular localization of symplastic aluminum may impact the aluminum sensitivity phenotypes of yucca, xth15, and the wild type treated with exogenous NAA.
Figure 4.
Purity of apoplastic solution (A), aluminum content in symplast (B), and cell wall (C). Six-week-old plants were grown on 0.5 mm CaCl2 media containing 50 µm aluminum or 50 µm aluminum combined with 0.05 µm NAA for 24 h. The pH was adjusted to 4.5. Data are means ± sd (n = 4). Columns with different letters are significantly different at P < 0.05. MD, Malic dehydrogenase; Marker, protein standards. [See online article for color version of this figure.]
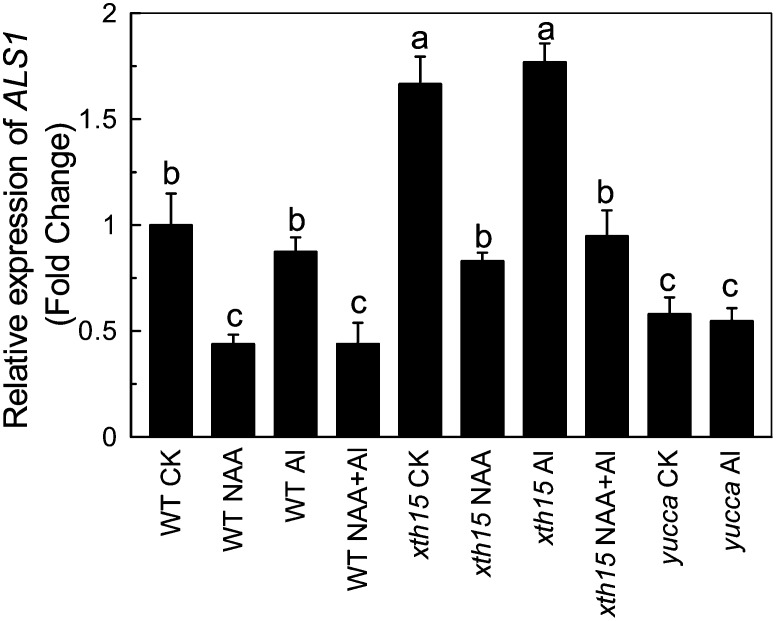
ALS1 is reported to be a root tip tonoplast transporter and responsible for aluminum redistribution between the cytoplasm and vacuole (Larsen et al., 2007). To determine whether differential expression abundance of ALS1, predicted to correlate with function levels, could underlie the differential sensitivity of yucca and xth15, we monitored ALS1 expression in roots with and without aluminum stress. Although ALS1 expression was not aluminum inducible (Fig. 5), in accordance with Larsen et al. (2007), root ALS1 expression was about 50% lower in yucca and the wild type treated with exogenous NAA than in the wild type (Fig. 5) but was about 50% higher than the wild type in xth15 under control conditions (Fig. 5). Therefore, relative ALS1 expression correlated with resistance in these two mutants, suggesting that high auxin levels may impair ALS1-dependent aluminum detoxification, whereas loss of XTH15, which also resulted in a low auxin level, may enhance this subcellular detoxifying mechanism. Furthermore, we found that als1-2 sensitivity to aluminum was not exacerbated by NAA treatment (Fig. 6, A and B), although NAA treatment of als1-2 led to lower cell wall aluminum content (Fig. 6, C and D), consistent with the idea that auxin enhances aluminum toxicity by down-regulating the expression of ALS1.
Figure 5.
Effect of aluminum on ALS1 expression. Six-week-old plants were treated with 0.5 mm CaCl2 solution containing 0 or 50 µm aluminum in the presence or absence of 0.05 µm NAA for 24 h. The pH was adjusted to 4.5. Total RNAs were extracted from roots and subjected to reverse transcription followed by real-time PCR. Expression levels without aluminum treatment of the wild type were normalized to the expression level of tubulin under control conditions (minus aluminum) and were assigned an expression level of 1. Data are means of three independent biological replicates. Columns with different letters are significantly different at P < 0.05.
Figure 6.
The effect of aluminum and NAA on the root growth and aluminum content in root symplast and cell wall of als1-2. A, One-centimeter-long seedlings were grown on 0.5 mm CaCl2 medium containing 0 or 50 µm aluminum in the presence or absence of NAA for 24 h. The pH was adjusted to 4.5. Data are means ± sd (n = 10). Columns with different letters are significantly different at P < 0.05. B, The effect of aluminum and NAA on the root growth of Arabidopsis. One-centimeter-long seedlings were grown on nutrient plates containing 0 or 50 µm aluminum for 7 d. The pH was adjusted to 4.5. Pictures were taken using a digital camera. Aluminum content in symplast (C) and cell wall (D). Six-week-old plants were grown on 0.5 mm CaCl2 solution containing 50 µm aluminum or 50 µm aluminum combined with 0.05 µM NAA for 24 h. The pH was adjusted to 4.5. Data are means ± sd (n = 4). Columns with different letters are significantly different at P < 0.05.
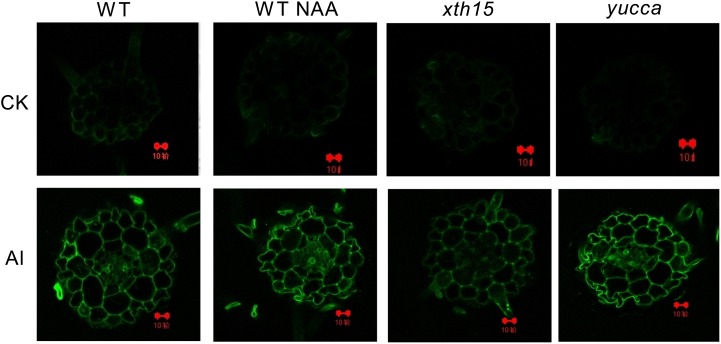
To gain further evidence for possible vacuole compartmentalization of aluminum, we localized the aluminum that enters cells with morin staining. Morin can detect aluminum in the cytosol but not cell wall-bound aluminum or vacuole-compartmentalized aluminum (Eticha et al., 2005b; Huang et al., 2012). The lack of morin staining in vacuole may be attributed to two reasons according to Huang et al. (2012): (1) morin is not permeable to the tonoplast, and (2) vacuolar aluminum is chelated by organic reagents, such as malic and citric acids, and morin cannot detect complexed aluminum forms, similar to cell wall-bound aluminum (Eticha et al., 2005b). Therefore, strong aluminum-dependent green fluorescence represents aluminum present in the cytosol and nucleus. The green fluorescence of morin was only faintly detected when seedlings were treated with 0.5 mm CaCl2 in the absence of aluminum, whereas after exposure to 0.5 mm CaCl2 combined with 50 μm aluminum, yucca and the NAA-treated wild type displayed stronger aluminum-dependent green fluorescence than the wild type in root cells (Fig. 7), and xth15 displayed relatively weaker morin staining (Fig. 7). These results are consistent with the conclusion that elevated auxin results in more aluminum accumulation in the cytosol, whereas loss of XTH15 function, with a lower level of endogenous auxin, accumulates less aluminum in the cytosol and therefore may target more aluminum to the vacuole.
Figure 7.
Subcellular distribution of aluminum stained with morin (green). About 1-cm-long seedlings were exposed to 0.5 mm CaCl2 solution with or without 50 µm aluminum in the presence or absence of NAA for 12 h. The pH was adjusted to 4.5. Roots were transversely sectioned at 5 and 10 mm from the apex for morin staining and fluorescence observation. Bar = 10 µm.
DISCUSSION
To survive in an aluminum-toxic environment, aluminum-resistant plant species adopt strategies to either restrict aluminum uptake (exclusion of aluminum from the root symplasm) or cope with internalized aluminum (tolerate symplastic aluminum; Taylor, 1991; Kochian et al., 2004). The typical exclusion mechanism is generally associated with lower aluminum content in the roots or fixation of aluminum in the apoplast. However, in this study, although there is no significant difference in the total aluminum content among the roots of yucca, xth15, the NAA-treated wild type, and the non-NAA-treated wild type, there are large differences in aluminum resistance. To elucidate the possible mechanisms leading to the differential aluminum sensitivity, we found that although less aluminum retention in the cell wall may contribute to enhanced aluminum resistance, when similar amounts of aluminum are present in the roots, more exclusion of aluminum from the more susceptible sites such as cytoplasm and nucleus may also be fundamental for plant resistance to aluminum. This work provides solid evidence for the importance of cooperation between aluminum exclusion and internal detoxification in plants.
Increasing evidence has shown that binding of aluminum in cell wall appears to be closely related to aluminum sensitivity, as fixation of aluminum in the cell wall will affect the proper functioning of cell wall (Horst et al., 2010). For example, Horst (1995) reported that aluminum bound to cell wall components increases wall rigidity, affects cell wall loosening, and thus ultimately inhibits root elongation. Ma et al. (2004) demonstrated that aluminum decreases cell wall viscosity and elasticity, thus reducing cell wall extensibility, and, as a consequence, cell elongation is inhibited. Therefore, higher aluminum sensitivity is correlated with more aluminum accumulation in the cell wall, as demonstrated in maize (Zea mays) suspension cells (Schmohl and Horst, 2000) and intact root apices (Eticha et al., 2005a), rice (Yang et al., 2008), triticale (Liu et al., 2008), and rice bean (Vigna umbellata; Zhou et al., 2012). In Arabidopsis, there is also a large difference in aluminum resistance among different ecotypes (Hoekenga et al., 2006). Although there is no report on the difference of aluminum content in roots among the different ecotypes, several studies demonstrated that aluminum-resistant mutants (Larsen et al., 1998) or transgenic lines (Ezaki et al., 2007) usually accumulate significantly less aluminum in the roots as compared with the wild type. Recently, we identified another Arabidopsis mutant xth31 that is highly aluminum resistant. xth31 accumulates significantly less aluminum in cell walls due to less xyloglucan content, as xyloglucan is responsible for aluminum binding in the hemicellulose of cell wall (Zhu et al., 2012). However, in some Arabidopsis mutants, aluminum sensitivity is not related to aluminum content. For example, als7 and als4 roots both accumulate less aluminum than the wild type after exposure to aluminum-containing solutions, yet root growth of the mutants is significantly inhibited (Larsen et al., 1996). Nezames et al. (2012) found that the increase in root growth seen for aluminum tolerant 2 (alt2-1) als3-1 compared with als3-1 and ecotype Columbia (Col-0) under aluminum stress is not related to the root aluminum accumulation because alt2-1 als3-1 roots accumulate wild-type levels of aluminum. Here, we screened a number cell wall-associated mutants for aluminum sensitivity and found a mutant, xth15, that displayed enhanced aluminum resistance. In this mutant, transfer DNA is inserted into the first exon of XTH15 (110 bp downstream of the translation initiator ATG codon; Supplemental Fig. S2A). XTH15 transcripts were not detected in the homozygous line (Supplemental Fig. S2B), indicating that the xth15 allele is likely a null mutation. XTH15 is likely to function in seedling roots because strong root staining is found in transgenics harboring a GUS reporter gene driven by the XTH15 5′ potential regulatory region (Becnel et al., 2006). We examined the two different mutants, the auxin-overproducing mutant, yucca, and the cell wall mutant, xth15, which showed large differences in aluminum sensitivity despite having similar root aluminum content. Furthermore, both mutants had moderate reductions of aluminum retention in the cell wall (Fig. 4C), suggesting that exclusion of aluminum from the cell wall is not necessarily sufficient to confer elevated aluminum resistance. Therefore, other mechanisms must exist that contribute to differential aluminum sensitivity.
A fundamental mechanism of internal detoxification is sequestration of aluminum into the vacuoles. Ma et al. (1997a, 1997c) identified forms of aluminum-organic acids compounds in the cell sap of hydrangea (Hydrangea macrophylla) and buckwheat leaves and proposed that these compounds might be sequestered in vacuoles. Later, Shen et al. (2002) demonstrated that most aluminum and oxalate in the protoplasts of buckwheat leaves is present in the vacuoles. These reports provide physiological evidence to demonstrate the compartmentalization of aluminum into the vacuole in the forms of aluminum-organic acids and the contribution of compartmentalization to internal aluminum detoxification. With the studies of various mutants altered in aluminum sensitivity, molecular mechanisms underlying resistance can be uncovered. Larsen et al. (2005) identified ALS3, which encodes a transporter that is localized to the phloem, leaf hydathodes, and root epidermis and is predicted to transport aluminum away from sensitive tissues for sequestration in more tolerant tissues. als3 displays severe aluminum inhibition of root growth. In addition, ALS1, which encodes a transporter localized to the root tip and the vasculature, has been implicated in aluminum sequestration to more tolerant tissues (Larsen et al., 2007). Recently, Huang et al. (2012) identified that OsALS1, which is expressed ubiquitously in rice, with the encoded protein localizing to the tonoplast, is responsible for sequestration of aluminum into the vacuoles and is required for internal detoxification of aluminum in rice. Although both ALS1 and ALS3 have critical roles in aluminum resistance, ALS3 is a plasma membrane transporter that moves aluminum away from the root tip and is not involved in redistribution between the cytoplasm and vacuole. We found, however, that ALS1 expression was lower in yucca and the NAA-treated wild type but higher in xth15 (Fig. 5), thus correlating with aluminum resistance. Moreover, the expression of ALS1 was also down-regulated when xth15 was treated with NAA (Fig. 5). However, there was lower IAA content in xth15 roots, suggesting that auxin accumulation may affect ALS1 expression and altered ALS1 expression may affect the efficacy of internal detoxifying mechanisms of aluminum in plants, which is in accordance with the no effect of NAA in the als1-2 mutant (Fig. 6). However, it is interesting that the NAA applied exogenously had no effect on the wild type and xth15 growth, while it inhibited the als1-2 growth (Fig. 1 and 6); this may be attributed to differential sensitivity to NAA, and als1-2 might have a lower suitable NAA level to promote the root growth. Furthermore, the morin staining reported more aluminum present in the cytosol in yucca and less cytosolic aluminum in xth15 than the wild type (Fig. 7). Therefore, when similar amounts of aluminum are present in the symplasm, there may be enhanced aluminum redistribution into the vacuoles facilitated by enhanced expression of ALS1, which may lead to more aluminum resistance. These results lead to the next question of how might auxin regulate the expression of ALS1. As there are no auxin response elements TGTCTC in the 2 kb-promoter regions of ALS1 genes (Hagen and Guilfoyle, 2002), the effect may be indirect, needing further investigation.
In this study, we also demonstrated that auxin is a negative factor in plant aluminum resistance, as both endogenous auxin overproduction, as in the yucca mutant, and exogenous application of NAA resulted in higher aluminum sensitivity in Arabidopsis, whereas xth15 with low levels of endogenous auxin exhibited higher aluminum resistance (Fig. 1). We have used a series of auxin concentrations and found that with each incremental increase in the auxin concentration, ALS1 expression was progressively repressed, accompanied by increasing inhibition of root growth (Supplemental Fig. S3). Kollmeier et al. (2000) found that aluminum alters auxin accumulation and distribution in roots possibly due to effects on the auxin polar transport system, while application of exogenous IAA to the elongation zone significantly alleviated the aluminum-induced inhibition of root growth in maize. Recently, Sun et al. (2010) demonstrated that aluminum affects auxin distribution through aluminum-induced changes in ethylene production; however, application of an IAA polar transport inhibitor can partially alleviate the inhibition of root growth in Arabidopsis under aluminum stress. The role of auxin in aluminum resistance is therefore complex. Effects may be influenced by species-specific responses or experimental treatment conditions. However, previous studies reported a relationship between aluminum-induced inhibition of root elongation and the disruption of auxin accumulation or distribution; the underlying physiological and molecular mechanisms remained undefined. Here, we demonstrate that auxin may exacerbate aluminum sensitivity by modifying the expression of ALS1 and therefore aluminum redistribution, as seen in xth15, yucca, and the wild type supplied with exogenous NAA (Fig. 5; Supplemental Fig. S3). Therefore, auxin may have a role in altering aluminum distribution within cells.
In conclusion, our study focusing on the aluminum resistance of the wild type, yucca, and xth15 demonstrates that auxin negatively regulates aluminum tolerance through altering ALS1 expression and aluminum distribution within plant cells, providing evidence for the importance for plant coordination of apoplastic and symplastic detoxification of aluminum to withstand aluminum toxicity.
MATERIALS AND METHODS
Plant Material and Growth Conditions
The Col-0 of Arabidopsis (Arabidopsis thaliana) served as the wild type and the background for all mutants, including xth15, yucca, sur2, sur1-3, and the aluminum-sensitive mutant als1-2 used in this study. For short-term (24-h) treatments, 0.5 mm CaCl2 solution (pH 4.5) was used as control media, while for longer-duration (7-d) treatments, the nutrient solution (pH 4.5) was used as control media and 50 μm aluminum and 0.05 μm NAA were directly added for aluminum, NAA, or NAA plus aluminum treatments. Seeds were surface sterilized and germinated on an agar-solidified nutrient medium in petri dishes. The nutrient medium consisted of the macronutrients 6.0 mm KNO3, 4.0 mm Ca(NO3)2, 1 mm MgSO4, and 0.1 mm NH4H2PO4 and the micronutrients 50 μm Fe(III)-EDTA, 12.5 μm H3BO3, 1 μm MnSO4, 0.5 μm CuSO4, 1 μm ZnSO4, 0.1 μm H2MoO4, and 0.1 μm NiSO4 according to Murashige and Skoog salts (Murashige and Skoog, 1962). The final pH was adjusted to 4.5. The seeds were vernalized at 4°C for 2 d. Petri dishes were placed into a growth chamber, positioned vertically, and kept under controlled environmental conditions at 24°C, 140 µmol photons m–2 s–1, and a 16-h/8-h day/night rhythm.
For hydroponic culture, seedlings were first aseptically germinated on the above solid Murashige and Skoog medium. After 2 weeks, the young plantlets were placed on vermiculite for additional 3 weeks in an environmentally controlled growth chamber. Seedlings of similar rosette diameters were then transferred to the nutrient solution containing Murashige and Skoog salts for another week. Then the plants were subjected to the following treatments: control media (0.5 mm CaCl2, pH 4.5), aluminum (50 μm aluminum in the 0.5 mm CaCl2, pH 4.5), NAA (adding 0.05 μm NAA to the above-mentioned control media solution), and NAA plus aluminum (adding 0.05 μm NAA to the above-mentioned aluminum solution). After 24 h, the roots were excised for RNA extraction or for aluminum content analysis. When for aluminum content analysis, the seedlings were washed three times with deionized water, and the fresh weight was recorded.
Effect of Aluminum on Root Growth
Seedlings with root lengths of 1 cm were selected and transferred to petri dishes containing agar-solidified CaCl2 (0.5 mm) medium with different aluminum concentrations (0 and 50 µm total concentration of aluminum in the form of AlCl3.6H2O). Root length measurements were performed using a digital camera connected to a computer. Data were quantified and analyzed by Photoshop 7.0 (Adobe Systems). For long-duration experiments, seedlings with a root length of 1 cm were selected and then transferred to petri dishes containing agar-solidified nutrient solution medium with different aluminum concentrations (0 and 50 µm total concentration of aluminum).
Gene Expression Analysis
Total RNA was isolated using TRIzol (Invitrogen). Complementary DNA was prepared from 1 µg of total RNA using the PrimeScript RT reagent kit (Takara). For real-time reverse transcription-PCR analysis, 1 µL of 10-fold-diluted complementary DNA was used for the quantitative analysis of gene expression performed with SYBR Premix ExTaq (Takara), with the following pairs of gene-specific primers: ALS1, forward, 5′-GACCGTTGGAGCACTCACTTC-3′ and reverse, 5′-CAGGATTACCGACTGGACACT-3′ and for tubulin, forward, 5′-AAGTTCTGGGAAGTGGTT-3′ and reverse, 5′-CTCCCAATGAGTGACAAA-3′. Each complementary DNA sample was run in triplicate. Expression data were normalized with the expression level of tubulin gene. For the semiquantitative reverse transcription-PCR analysis, the primers used were as follows: for 18S, forward, 5′-ATGATAACTCGACGGATCGC-3′ and reverse, 5′-CTTGGATGTGGTAGCCGTTT-3′ and for XTH15, forward, 5′-CCGCTCGAGAAGAGAAGCAACTTCTTCGACGAGT-3′ and reverse, 5′-GCTCTAGAGACTCTGGACTTCTTGCATTCTGG-3′.
Aluminum Content Measurement
After treatment, the roots were excised after washing three times with 0.5 mm CaCl2 and then put in Ultrafree-MC Centrifugal Filter Units (Millipore) and centrifuged at 3,000g for 10 min at 4°C to remove apoplastic solution. The roots were then frozen at –80°C overnight. The root cell sap solution was obtained by thawing the samples at room temperature and then centrifuging at 20,600g for 10 min. The residual cell walls were washed with 70% (v/v) ethanol three times before being immersed in 0.5 mL of 2 n HCl for 36 h with occasional vortexing, according to Xia et al. (2010). For root aluminum content analysis, materials were digested with HNO3:HClO4 (4:1, v/v). The aluminum in the root, symplastic solution, and cell wall extracts was determined by inductively coupled plasma-atomic emission spectrometry (IRIS/AP optical emission spectrometer).
SDS-PAGE
The protein from the apoplastic and root cell sap fractions and malic dehydrogenase (Thermus flavus; Sigma, M7032) were heated at 100°C for 10 min in 2×SDS-loading buffer to denature the proteins. Twenty microliters of the protein samples were loaded in each well.
SDS-PAGE was conducted using a 12% (w/v) resolving gel and 4% (w/v) stacking gel. The resolving gel, to a total volume of 10 mL, consisted of 3.35 mL water (distilled), 4 mL 30% (w/w) acrylamide/bis-acrylamide stock, 2.5 mL 1.5 m Tris-HCl (pH 8.8), 0.1 mL 10% (w/v) SDS, 0.05 mL 10% (w/v) ammonium persulfate, and 0.005 mL N,N,N′,N′-tetramethylethylenediamine, and was then carefully poured into glass plate and overlay gel with distilled, deionized H2O to ensure a flat surface and to exclude air. After the gel has set more than half an hour, we got rid of the distilled, deionized H2O, mixed the 4% stacking gel (3.05 mL water [distilled], 0.65 mL 30% acrylamide/bis-acrylamide stock, 1.25 mL 0.5 m Tris-HCl [pH 6.8], 0.05 mL 10% (w/v) SDS, 0.025 mL 10% ammonium persulfate, and 0.005 mL N,N,N′,N′-tetramethylethylenediamine), poured it onto the top of the set resolving gel, and inserted the comb. The electrophoresis buffer consisted of 3.03 g L–1 Tris base, 18.77 g L–1 Gly, and 1 g L–1 SDS. The gel runs at 80 V for about 3 h. The gel was stained with 0.1% (w/v) Coomassie Brilliant Blue (R250) in methanol:water:acetic acid (45:45:10, v/v/v) for 2 to 3 h at room temperature with agitation and then destained in ethanol:water:acetic acid (45:45:10, v/v/v). Finally, pictures were taken using a digital camera.
Morin Staining
About 1-cm-long seedlings of the wild type (Col-0) and the yucca and xth15 mutants were exposed to 0.5 mm CaCl2 solution (pH 4.5) containing 50 µm aluminum for 12 h. Roots were stained in 0.01% morin for 30 min, then excised and embedded in 5% (w/v) agar. Root tips were transversely sectioned from the apex, and the green fluorescence signal was observed using a laser-scanning confocal microscope (LSM510, Zeiss).
IAA Measurement
For analysis of the IAA concentration in roots, the whole root (about 20 mg) was collected for each sample. Four replicates of the samples were purified after the addition of 250 picograms 13C6-IAA internal standard and analyzed by gas chromatography-selected reaction monitoring mass spectrometry as described (Ljung et al., 2005).
Statistical Analysis
Each experiment was repeated at least three times. Data were analyzed by one-way ANOVA procedure, and the means were compared by Duncan’s multiple range test. Different letters on the histograms indicate that the means were statistically different at P < 0.05 level.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. The effect of aluminum on root growth of Arabidopsis.
Supplemental Figure 2. Schematic structure of the xth15 mutant carrying a single copy of the transfer DNA insert in the first exon of the XTH15 gene.
Supplemental Figure 3. The effect of aluminum and NAA on root growth of Arabidopsis.
Acknowledgments
We thank Catherine Bellini (University of Nottingham) for the sur2 and sur1-3 seeds, Yunde Zhao (University of California, San Diego) for the yucca seeds, and two anonymous reviewers for their valuable comments to improve the quality of our work.
Glossary
- IAA
indole-3-acetic acid
- NAA
naphthylacetic acid
- Col-0
ecotype Columbia
References
- Barlier I, Kowalczyk M, Marchant A, Ljung K, Bhalerao R, Bennett M, Sandberg G, Bellini C. (2000) The SUR2 gene of Arabidopsis thaliana encodes the cytochrome P450 CYP83B1, a modulator of auxin homeostasis. Proc Natl Acad Sci USA 97: 14819–14824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becnel J, Natarajan M, Kipp A, Braam J. (2006) Developmental expression patterns of Arabidopsis XTH genes reported by transgenes and Genevestigator. Plant Mol Biol 61: 451–467 [DOI] [PubMed] [Google Scholar]
- Boerjan W, Cervera M-T, Delarue M, Beeckman T, Dewitte W, Bellini C, Caboche M, Van Onckelen H, Van Montagu M, Inzé D. (1995) Superroot, a recessive mutation in Arabidopsis, confers auxin overproduction. Plant Cell 7: 1405–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YC, Yamamoto Y, Matsumoto H. (1999) Accumulation of aluminium in the cell wall pectin in cultured tobacco (Nicotiana tabacum L.) cells treated with a combination of aluminium and iron. Plant Cell Environ 22: 1009–1017 [Google Scholar]
- Clarkson DT. (1967) Interactions between aluminum and phosphorus on root surfaces and cell wall material. Plant Soil 27: 347–356 [Google Scholar]
- Delarue M, Prinsen E, Onckelen HV, Caboche M, Bellini C. (1998) Sur2 mutations of Arabidopsis thaliana define a new locus involved in the control of auxin homeostasis. Plant J 14: 603–611 [DOI] [PubMed] [Google Scholar]
- Doncheva S, Amenós M, Poschenrieder C, Barceló J. (2005) Root cell patterning: a primary target for aluminium toxicity in maize. J Exp Bot 56: 1213–1220 [DOI] [PubMed] [Google Scholar]
- Eticha D, Stass A, Horst WJ. (2005a) Cell-wall pectin and its degree of methylation in the maize root-apex: significance for genotypic differences in aluminium resistance. Plant Cell Environ 28: 1410–1420 [Google Scholar]
- Eticha D, Stass A, Horst WJ. (2005b) Localization of aluminium in the maize root apex: can morin detect cell wall-bound aluminium? J Exp Bot 56: 1351–1357 [DOI] [PubMed] [Google Scholar]
- Ezaki B, Kiyohara H, Matsumoto H, Nakashima S. (2007) Overexpression of an auxilin-like gene (F9E10.5) can suppress Al uptake in roots of Arabidopsis. J Exp Bot 58: 497–506 [DOI] [PubMed] [Google Scholar]
- Foy CD. (1988) Plant adaptation to acid, aluminum-toxic soils. Commun Soil Sci Plant 19: 959–987 [Google Scholar]
- Fry SC, Smith RC, Renwick KF, Martin DJ, Hodge SK, Matthews KJ. (1992) Xyloglucan endotransglycosylase, a new wall-loosening enzyme activity from plants. Biochem J 282: 821–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen G, Guilfoyle T. (2002) Auxin-responsive gene expression: genes, promoters and regulatory factors. Plant Mol Biol 49: 373–385 [PubMed] [Google Scholar]
- Hoekenga OA, Maron LG, Piñeros MA, Cançado GMA, Shaff J, Kobayashi Y, Ryan PR, Dong B, Delhaize E, Sasaki T, et al. (2006) AtALMT1, which encodes a malate transporter, is identified as one of several genes critical for aluminum tolerance in Arabidopsis. Proc Natl Acad Sci USA 103: 9738–9743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horst WJ. (1995) The role of the apoplast in aluminium toxicity and resistance of higher plants: a review. Z Pflanzenernähr Bodenkd 158: 419–428 [Google Scholar]
- Horst WJ, Wang Y, Eticha D. (2010) The role of the root apoplast in aluminium-induced inhibition of root elongation and in aluminium resistance of plants: a review. Ann Bot (Lond) 106: 185–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CF, Yamaji N, Chen Z, Ma JF. (2012) A tonoplast-localized half-size ABC transporter is required for internal detoxification of aluminum in rice. Plant J 69: 857–867 [DOI] [PubMed] [Google Scholar]
- Kochian LV. (1995) Cellular mechanisms of aluminum toxicity and resistance in plants. Annu Rev Plant Physiol Plant Mol Biol 46: 237–260 [Google Scholar]
- Kochian LV, Hoekenga OA, Piñeros MA. (2004) How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency. Annu Rev Plant Biol 55: 459–493 [DOI] [PubMed] [Google Scholar]
- Kollmeier M, Felle HH, Horst WJ. (2000) Genotypical differences in aluminum resistance of maize are expressed in the distal part of the transition zone. Is reduced basipetal auxin flow involved in inhibition of root elongation by aluminum? Plant Physiol 122: 945–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen PB, Cancel J, Rounds M, Ochoa V. (2007) Arabidopsis ALS1 encodes a root tip and stele localized half type ABC transporter required for root growth in an aluminum toxic environment. Planta 225: 1447–1458 [DOI] [PubMed] [Google Scholar]
- Larsen PB, Degenhardt J, Tai CY, Stenzler LM, Howell SH, Kochian LV. (1998) Aluminum-resistant Arabidopsis mutants that exhibit altered patterns of aluminum accumulation and organic acid release from roots. Plant Physiol 117: 9–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen PB, Geisler MJ, Jones CA, Williams KM, Cancel JD. (2005) ALS3 encodes a phloem-localized ABC transporter-like protein that is required for aluminum tolerance in Arabidopsis. Plant J 41: 353–363 [DOI] [PubMed] [Google Scholar]
- Larsen PB, Tai CY, Kochian LV, Howell SH. (1996) Arabidopsis mutants with increased sensitivity to aluminum. Plant Physiol 110: 743–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Yang JL, He LS, Li YY, Zheng SJ. (2008) Effect of aluminum on cell wall, plasma membrane, antioxidants and root elongation in triticale. Biol Plant 52: 87–92 [Google Scholar]
- Ljung K, Hull AK, Celenza J, Yamada M, Estelle M, Normanly J, Sandberg G. (2005) Sites and regulation of auxin biosynthesis in Arabidopsis roots. Plant Cell 17: 1090–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JF. (2000) Role of organic acids in detoxification of aluminum in higher plants. Plant Cell Physiol 41: 383–390 [DOI] [PubMed] [Google Scholar]
- Ma JF, Furukawa J. (2003) Recent progress in the research of external Al detoxification in higher plants: a minireview. J Inorg Biochem 97: 46–51 [DOI] [PubMed] [Google Scholar]
- Ma JF, Hiradate S. (2000) Form of aluminium for uptake and translocation in buckwheat (Fagopyrum esculentum Moench). Planta 211: 355–360 [DOI] [PubMed] [Google Scholar]
- Ma JF, Hiradate S, Nomoto K, Iwashita T, Matsumoto H. (1997a) Internal detoxification mechanism of Al in hydrangea. Identification of Al form in the leaves. Plant Physiol 113: 1033–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JF, Hiradate S, Nomoto K, Iwashita T, Matsumoto H. (1997b) Internal detoxification mechanism of Al in hydrangea (identification of Al form in the leaves). Plant Physiol 113: 1033–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JF, Shen RF, Nagao S, Tanimoto E. (2004) Aluminum targets elongating cells by reducing cell wall extensibility in wheat roots. Plant Cell Physiol 45: 583–589 [DOI] [PubMed] [Google Scholar]
- Ma JF, Zheng SJ, Hiradate S, Matsumoto H. (1997c) Detoxifying aluminium with buckwheat. Nature 390: 569–5709403684 [Google Scholar]
- Murashige T, Skoog F. (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15: 473–496 [Google Scholar]
- Nezames CD, Sjogren CA, Barajas JF, Larsen PB. (2012) The Arabidopsis cell cycle checkpoint regulators TANMEI/ALT2 and ATR mediate the active process of aluminum-dependent root growth inhibition. Plant Cell 24: 608–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishitani K, Tominaga R. (1992) Endo-xyloglucan transferase, a novel class of glycosyltransferase that catalyzes transfer of a segment of xyloglucan molecule to another xyloglucan molecule. J Biol Chem 267: 21058–21064 [PubMed] [Google Scholar]
- Pan JW, Zhu MY, Chen H. (2001) Aluminum-induced cell death in root-tip cells of barley. Environ Exp Bot 46: 71–79 [DOI] [PubMed] [Google Scholar]
- Ryan PR, Delhaize E, Jones DL. (2001) Function and mechanism of organic anion exudation from plant roots. Annu Rev Plant Physiol Plant Mol Biol 52: 527–560 [DOI] [PubMed] [Google Scholar]
- Schmohl N, Horst WJ. (2000) Cell wall pectin content modulates aluminium sensitivity of Zea mays (L.) cells grown in suspension culture. Plant Cell Environ 23: 735–742 [Google Scholar]
- Shen H, Hou NY, Schlicht M, Wan YL, Baluska F. (2008) Aluminum toxicity targets PIN2 in Arabidopsis root apices: effects on PIN2 endocytosis vesicular recycling, and polar auxin transport. Chin Sci Bull 53: 2480–2487 [Google Scholar]
- Shen RF, Ma JF, Kyo M, Iwashita T. (2002) Compartmentation of aluminium in leaves of an Al-accumulator, Fagopyrum esculentum Moench. Planta 215: 394–398 [DOI] [PubMed] [Google Scholar]
- Sun P, Tian QY, Chen J, Zhang WH. (2010) Aluminium-induced inhibition of root elongation in Arabidopsis is mediated by ethylene and auxin. J Exp Bot 61: 347–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabuchi A, Matsumoto H. (2001) Changes in cell-wall properties of wheat (Triticum aestivum) roots during aluminum-induced growth inhibition. Physiol Plant 112: 353–358 [DOI] [PubMed] [Google Scholar]
- Taylor GJ. (1991) Current views of the aluminum stress response: the physiological basis of tolerance. Curr Top Plant Biochem Physiol 10: 57–93 [Google Scholar]
- Thompson JE, Fry SC. (2001) Restructuring of wall-bound xyloglucan by transglycosylation in living plant cells. Plant J 26: 23–34 [DOI] [PubMed] [Google Scholar]
- von Uexküll HR, Mutert E (1995) Global extent, development and economic impact of acid soils. In RA Date, NJ Grundon, GE Raymet, ME Probert, eds, Plant-Soil Interactions at Low pH: Principles and Management. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 5–19 [Google Scholar]
- Xia JX, Yamaji N, Kasai T, Ma JF. (2010) Plasma membrane-localized transporter for aluminum in rice. Proc Natl Acad Sci USA 107: 18381–18385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaji N, Huang CF, Nagao S, Yano M, Sato Y, Nagamura Y, Ma JF. (2009) A zinc finger transcription factor ART1 regulates multiple genes implicated in aluminum tolerance in rice. Plant Cell 21: 3339–3349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JL, Li YY, Zhang YJ, Zhang SS, Wu YR, Wu P, Zheng SJ. (2008) Cell wall polysaccharides are specifically involved in the exclusion of aluminum from the rice root apex. Plant Physiol 146: 602–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JL, Zhu XF, Peng YX, Zheng C, Li GX, Liu Y, Shi YZ, Zheng SJ. (2011b) Cell wall hemicellulose contributes significantly to aluminum adsorption and root growth in Arabidopsis. Plant Physiol 155: 1885–1892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JL, Zhu XF, Zheng C, Zhang YJ, Zheng SJ. (2011a) Genotypic differences in Al resistance and the role of cell-wall pectin in Al exclusion from the root apex in Fagopyrum tataricum. Ann Bot (Lond) 107: 371–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Christensen SK, Fankhauser C, Cashman JR, Cohen JD, Weigel D, Chory J. (2001) A role for flavin monooxygenase-like enzymes in auxin biosynthesis. Science 291: 306–309 [DOI] [PubMed] [Google Scholar]
- Zheng SJ, Lin XY, Yang JL, Liu Q, Tang CX. (2004) The kinetics of aluminum adsorption and desorptiion by root cell walls of an aluminum resistant wheat (Triticum aestivum L.) cultivar. Plant Soil 261: 85–90 [Google Scholar]
- Zhou Y, Xu XY, Chen LQ, Yang JL, Zheng SJ. (2012) Nitric oxide exacerbates Al-induced inhibition of root elongation in rice bean by affecting cell wall and plasma membrane properties. Phytochemistry 76: 46–51 [DOI] [PubMed] [Google Scholar]
- Zhu XF, Shi YZ, Lei GJ, Fry SC, Zhang BC, Zhou YH, Braam J, Jiang T, Xu XY, Mao CZ, et al. (2012) XTH31, encoding an in-vitro XEH/XET-active enzyme, controls Al sensitivity by modulating in-vivo XET action, cell wall xyloglucan content and Al binding capacity in Arabidopsis. Plant Cell 24: 4731–4747 [DOI] [PMC free article] [PubMed] [Google Scholar]