Pepper arginine decarboxylase, CaADC1, which interacts with Xanthomonas effector AvrBsT, induces increased polyamine and γ-aminobutyric acid levels, and triggers nitric oxide and reactive oxygen species bursts, ultimately leading to plant cell death and defense responses.
Abstract
The Xanthomonas campestris pv vesicatoria (Xcv) effector AvrBsT induces a hypersensitive cell death in pepper (Capsicum annuum). However, the molecular mechanisms underlying AvrBsT-triggered cell death are not fully understood. Here, we identified pepper arginine decarboxylase (CaADC1) as an AvrBsT-interacting protein, which is early and strongly induced in incompatible pepper-Xcv interactions. Bimolecular fluorescence complementation and coimmunoprecipitation assays showed that the CaADC1-AvrBsT complex was localized to the cytoplasm. Transient coexpression of CaADC1 with avrBsT in Nicotiana benthamiana leaves specifically enhanced AvrBsT-triggered cell death, accompanied by an accumulation of polyamines, nitric oxide (NO), and hydrogen peroxide (H2O2) bursts. Among the polyamines, spermine application strongly induced NO and H2O2 bursts, ultimately leading to cell death. CaADC1 silencing in pepper leaves significantly compromised NO and H2O2 accumulation and cell death induction, leading to the enhanced avirulent Xcv growth during infection. The levels of salicylic acid, polyamines, and γ-aminobutyric acid (GABA), and the expression of defense response genes during avirulent Xcv infection, were distinctly lower in CaADC1-silenced plants than those in the empty vector control plants. GABA application significantly inhibited avirulent Xcv growth in CaADC1-silenced leaves and the empty vector control plants. Together, these results suggest that CaADC1 may act as a key defense and cell death regulator via mediation of polyamine and GABA metabolism.
The activation of plant defense signaling induces massive transcriptional reprogramming, leading to the accumulation of pathogenesis-related (PR) proteins, reactive oxygen species (ROS), phytoalexins, and various defense-related metabolites (Asai et al., 2002; Garcia-Brugger et al., 2006; Berger et al., 2007). Polyamines (PAs), including putrescine, spermidine, and spermine, are positively charged small metabolites that are implicated in plant disease resistance and other physiological processes such as cell proliferation, differentiation, morphogenesis, flowering, senescence, seed dormancy, and germination (Walden et al., 1997; Martin-Tanguy, 2001; Walters, 2003a; Deeb et al., 2010). PAs have been shown to confer protection against abiotic stresses such as mineral nutrient deficiency, salt, drought, cold, and oxidative stress (Bouchereau et al., 1999; Kasinathan and Wingler, 2004).
In plants, PAs are synthesized from the amino acids Orn or Arg via decarboxylation (Walters, 2003a). Decarboxylation of Orn or Arg is catalyzed by ornithine decarboxylase (ODC; EC 4.1.1.17) or arginine decarboxylase (ADC; EC 4.1.1.19) and yields putrescine or agmatine, respectively (Walters, 2003b). Agmatine is hydrolyzed by agmatine deiminase (EC 3.5.3.12) to N-carbamoylputrescine, which in turn is hydrolyzed by N-carbamoylputrescine amidohydrolase (EC 3.5.1.53) to putrescine (Janowitz et al., 2003; Piotrowski et al., 2003). Spermidine is synthesized from putrescine via the addition of aminopropyl groups to putrescine by spermidine synthase (EC 2.5.1.16; Bagni and Tassoni, 2001; Walters, 2003b). Spermine is synthesized via the addition of aminopropyl groups to spermidine by spermine synthase (EC 2.5.1.22; Bagni and Tassoni, 2001; Walters, 2003b).
ODC and ADC act as rate-limiting factors in PA biosynthesis and play pivotal roles in PA metabolism (Bagni and Tassoni, 2001). Perturbation of endogenous PA levels by ODC and ADC affects various physiological processes in plants (Capell et al., 2004; Kasinathan and Wingler, 2004; Alcázar et al., 2005). Transgenic Arabidopsis overexpressing ADC displayed dwarf stature and exhibited delayed flowering (Alcázar et al., 2005). Transgenic rice (Oryza sativa) overexpressing ADC had higher tolerance to drought stress (Capell et al., 2004). Transgenic tobacco (Nicotiana tabacum) with increased ODC activity was more tolerant to salt stress, whereas Arabidopsis (Arabidopsis thaliana) mutants with reduced ADC activity were less tolerant to salt stress (Kumria and Rajam, 2002; Kasinathan and Wingler, 2004).
The disease resistance response and protection against abiotic stresses conferred by PAs are intimately related to a ROS burst (Takahashi et al., 2004; Moschou et al., 2008). ROS directly inhibit pathogen growth, stimulate cross-linking of the cell wall, and mediate signal transduction for the expression of defense- and stress-responsive genes (Lamb and Dixon, 1997; Skopelitis et al., 2006). The oxidation of PAs in the apoplast, which is a major source of the ROS burst, is mediated by NADPH oxidases or peroxidases in the plasma membrane or cell wall, respectively (Takahashi et al., 2004). Spermidine is oxidized by polyamine oxidase (EC 1.5.3.3) to diaminopropane, pyrroline, and hydrogen peroxide (H2O2). Polyamine oxidase also oxidizes spermine to diaminopropane, aminopropylpyrroline, and H2O2. Putrescine is converted by diamine oxidase (EC 1.4.3.22) to pyrroline, ammonia, and H2O2 (Bagni and Tassoni, 2001).
Pyrroline can be further processed to form γ-aminobutyric acid (GABA), a nonprotein amino acid that is best known as an inhibitory neurotransmitter in the mammalian central nervous system (Bhat et al., 2010) and as an intracellular signaling molecule in plant development and stress responses (Roberts, 2007). GABA is involved in nitrogen metabolism, protection against oxidative stress, osmoregulation, and defense against herbivorous pests (Bouché and Fromm, 2004). Arabidopsis ssadh mutants that are defective in GABA catabolism were hypersensitive to photodamage and heat, leading to cell death and the concomitant accumulation of high levels of H2O2 (Bouché et al., 2003). Plant GABA was proposed to mediate quorum-sensing in Agrobacterium tumefaciens, thereby affecting its virulence on plants (Chevrot et al., 2006). Plant-produced GABA is imported into A. tumefaciens, where it induces the lactonase AttM (BlcC), which degrades the quorum-sensing signal and attenuates bacterial virulence. Plant L-proline was demonstrated to antagonize GABA-induced quenching of quorum-sensing in A. tumefaciens (Haudecoeur et al., 2009). Both GABA and proline are taken up by a specific ABC transporter in concert with the periplasmic binding protein Atu2422. More recently, it has been reported that a bacterial small RNA controls uptake of a plant-generated signaling molecule GABA into bacteria (Wilms et al., 2011). The periplasmic binding protein, Atu2422, which is essential for the transportation of GABA into A. tumefaciens, is negatively regulated by a conserved small RNA AbcR1. GABA transaminase-deficient Pseudomonas syringae mutants showed significantly reduced virulence, suggesting that GABA has multiple effects on pathogen-plant interactions with increased disease resistance (Park et al., 2010).
The relationship of ROS to plant cell death and/or defense signaling has been extensively documented (Torres et al., 2006; Van Breusegem and Dat, 2006). The ROS burst and plant cell death are hallmarks of the hypersensitive response (HR) that results from pathogen recognition (Lamb and Dixon, 1997). The HR is characterized by rapid local cell death at the site of pathogen invasion, which often leads to systemic and broad-spectrum disease resistance termed systemic acquired resistance (Durrant and Dong, 2004). Xanthomonas campestris pv vesicatoria (Xcv) strain Bv5-4a harbors the type III effector protein AvrBsT and triggers hypersensitive cell death in pepper (Capsicum annuum) plants on infection (Kim et al., 2010). Expression of avrBsT in the Xcv strain Ds1 rendered the strain avirulent to pepper plants. Infection with Xcv Ds1 (avrBsT) expressing avrBsT triggered cell death in pepper leaves. The hypersensitive cell death response elicited by AvrBsT is reminiscent of the resistance (R) gene-mediated defense system in plants (Eitas and Dangl, 2010; Kim et al., 2010). However, the precise molecular mechanisms underlying AvrBsT recognition and cell death initiation remain to be elucidated. Identifying host proteins that interact with AvrBsT will provide a basis for understanding cell death and molecular defense mechanisms.
In this study, we identified pepper ADC (CaADC1) as an AvrBsT-interacting protein by using yeast two-hybrid screening. The interaction of CaADC1 and AvrBsT was visualized in the cytoplasm. Transient coexpression of CaADC1 and AvrBsT in Nicotiana benthamiana significantly enhanced AvrBsT-triggered cell death. CaADC1 expression induced the accumulation of PAs and triggered NO and H2O2 bursts in N. benthamiana leaves. In pepper, the expression of CaADC1 was rapidly and strongly induced by inoculation with Xcv Ds1 (avrBsT). CaADC1-silenced pepper plants that were susceptible to avirulent Xcv Ds1 (avrBsT) infection did not exhibit increases in NO and H2O2 levels or cell death responses. The level of salicylic acid (SA) and the expression of defense response genes also were compromised by CaADC1 silencing. PA levels were greatly reduced in CaADC1-silenced pepper leaves during Xcv infection. Notably, spermine levels were only compromised during avirulent Xcv Ds1 (avrBsT) infection. Xcv Ds1 (avrBsT) infection did not induce GABA accumulation in CaADC1-silenced plants, which suggests that the nonprotein amino acid GABA may contribute to R gene-mediated resistance against avirulent Xcv infection. Taken together, the results of this study suggest that CaADC1 expression induces increased PA and GABA levels and triggers NO and H2O2 bursts, ultimately leading to plant defense and cell death responses.
RESULTS
Identification of CaADC1 as an AvrBsT-Interacting Protein
AvrBsT is a type III effector protein of Xcv that elicits the hypersensitive cell death in pepper and N. benthamiana leaves (Orth et al., 2000; Escolar et al., 2001). To identify the molecular components that interact with AvrBsT, we used the yeast two-hybrid system (Fig. 1). AvrBsT was used as bait, and then we screened a pepper complementary DNA (cDNA) prey library that was generated from leaves undergoing the hypersensitive cell death. Approximately 35,000 transformants were screened, and 12 positive clones were isolated and sequenced. One of the major AvrBsT-interacting proteins encoded ADC (four clones; Supplemental Figs. S1 and S2). This clone was designated CaADC1 and used for further characterization. Other clones were also found to encode SGT1 (for suppressor of the G2 allele of skp1; three clones), HEAT SHOCK PROTEIN70 (three clones), and a putative aldehyde dehydrogenase (two clones; Kim, 2012).
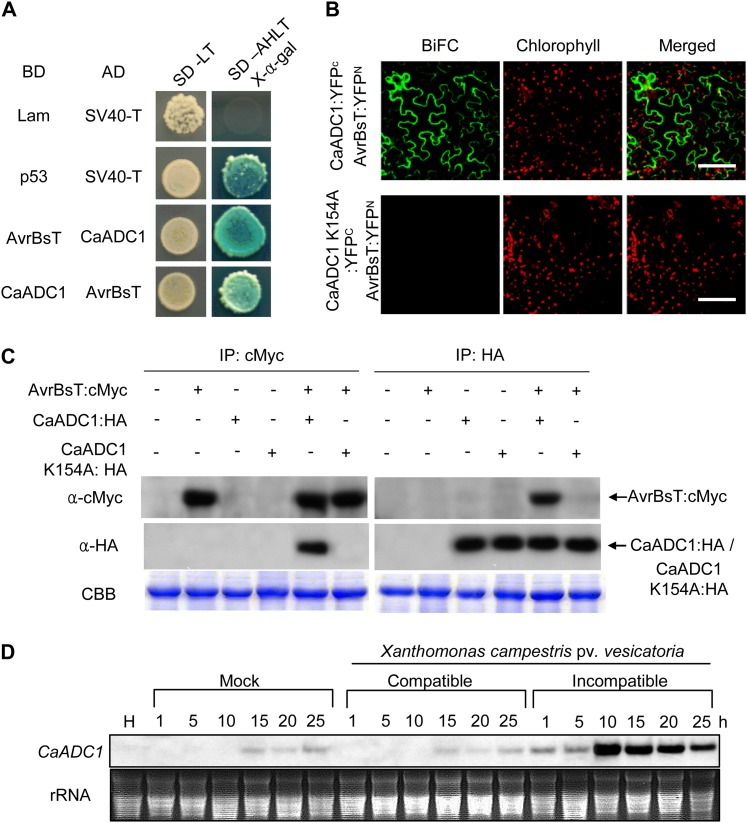
Figure 1.
Interactions of CaADC1 with AvrBsT and CaADC1 expression patterns. A, Yeast two-hybrid assay of CaADC1 and AvrBsT. CaADC1 and AvrBsT fused with activation (AD) or binding (BD) domains of GAL4 were cointroduced into Saccharomyces cerevisiae strain AH109, and reporter gene activation was monitored on synthetic dropout media (Ala [SD-A], His [SD-H], Leu [SD-L], or Trp [SD-T]) containing X-α-galactose. Lam and p53 combinations with SV40-T were used as negative and positive controls, respectively. B, BiFC analyses of the CaADC1 and AvrBsT interaction in planta. N. benthamiana leaves were infiltrated with a mixture of A. tumefaciens carrying 35S:CaADC1::YFPC, 35S:CaADC1 K154A:YFPC, and 35S:avrBsT::YFPN. YFP fluorescence in epidermal cells was observed by a confocal microscope at 40 h after agroinfiltration. CBB, Coomassie Brilliant Blue. Bars = 50 μm. C, Coimmunoprecipitation (IP) and immunoblotting of AvrBsT:cMyc and CaADC1:HA or CaADC1 K154A:HA proteins coexpressed in N. benthamiana leaves. D, RNA gel-blot analyses of CaADC1 expression in pepper leaves infected with virulent (Ds1) or avirulent (Bv5-4a) Xcv. The mock control was treated with 10 mm MgCl2. H, Healthy leaves. [See online article for color version of this figure.]
To verify the interaction between AvrBsT and CaADC1, we swapped vectors and generated a DNA-binding domain (BD) fused with CaADC1 and an activation domain (AD) fused with AvrBsT. We transformed these constructs into yeast along with positive and negative control vector pairs. AD-AvrBsT and BD-CaADC1 pairs, and vice versa, interacted with each other and grew on synthetic dropout adenine, His, Leu, Trp medium (Fig. 1A).
The AvrBsT and CaADC1 interaction was verified in planta by using bimolecular fluorescence complementation (BiFC) assays (Walter et al., 2004). CaADC1 and AvrBsT were fused to the N-terminal 155-amino acid domain of yellow fluorescent protein (YFP) in the pSPYNE vector and the C-terminal 84-amino acid domain of YFP, respectively. A. tumefaciens cells harboring the corresponding constructs were mixed and coinfiltrated into N. benthamiana leaves. Confocal microscopy of N. benthamiana epidermal cells shows that AvrBsT and CaADC1 interact in the cytoplasm (Fig. 1B).
The AvrBsT and CaADC1 or CaADC1 K154A interaction in planta was investigated by coimmunoprecipitation using a transient coexpression system in N. benthamiana (Fig. 1C). Total proteins extracted from N. benthamiana leaves were incubated with anti-cMyc agarose to immunoprecipitate cMyc-tagged AvrBsT. Immunoprecipitates were resolved by SDS-PAGE. Immunoblotting using anti-cMyc antibody detected AvrBsT in the samples expressing AvrBsT alone and coexpressing AvrBsT:cMyc, CaADC1:hemagglutinin (HA), and CaADC1 K154A:HA. When immunoblotted using anti-HA, CaADC1, but not CaADC1 K154A, was detected from the samples coexpressing AvrBsT:cMyc with CaADC1:HA or CaADC1 K154A:HA. Next, the total protein extracts were incubated with anti-HA agarose to immunoprecipitate HA-tagged CaADC1 or CaADC1 K154A. AvrBsT was immunodetected against anti-cMyc when coexpressed with CaADC1 but not with CaADC1 K154A or AvrBsT alone. Collectively, the coimmunoprecipitation assay revealed that AvrBsT forms a complex with CaADC1, but not with CaADC1 K154A, in planta.
Spatiotemporal Expression Profiles of CaADC1
RNA gel-blot analyses were used to investigate CaADC1 expression profiles in pepper plants. CaADC1 was constitutively expressed in stems, roots, flowers, and fruits but not in leaves (Supplemental Fig. S3A). Notably, CaADC1 was highly induced in pepper leaves during avirulent (incompatible) Xcv infection compared with the mock control or the virulent (compatible) Xcv infection (Fig. 1D). In the incompatible interactions, CaADC1 induction was detected 1 h after inoculation and reached a high level by 10 h after inoculation (Fig. 1D). Exogenous application of SA, methyl jasmonate, and ethylene also differentially induced CaADC1 transcription in pepper leaves (Supplemental Fig. S3, B–D), indicating that CaADC1 is involved in signal transduction pathways mediated by these plant hormones.
Transient Expression of CaADC1 Specifically Promotes an avrBsT-Triggered Cell Death Response
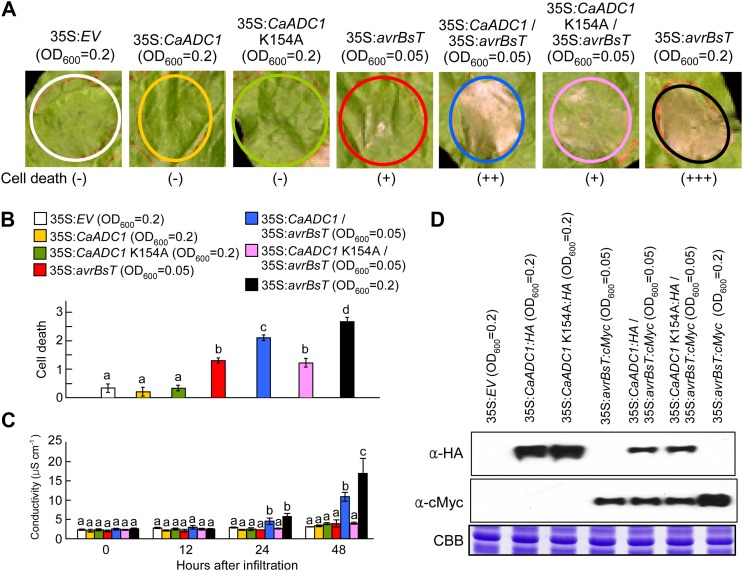
To define the role of CaADC1 in the AvrBsT-triggered cell death response, we used an A. tumefaciens-mediated transient expression system in N. benthamiana leaves. As CaADC1 expression was rapidly induced during the hypersensitive cell death response (Fig. 1D), we assumed that CaADC1 may function as a regulator of the cell death response. Because the activity of ADC requires the binding of pyridoxal phosphate at its Lys residue (Cohen et al., 1983), we performed site-directed mutagenesis to generate a CaADC1 K154A mutant that cannot bind pyridoxal phosphate. To determine whether CaADC1 expression regulates the cell death response, we coexpressed CaADC1 or CaADC1 K154A with avrBsT at the lower limit of the cell death-inducing A. tumefaciens titer (optical density at 600 nm [OD600] = 0.05). As shown in Figure 2A, transient expression of CaADC1 or CaADC1 K154A did not induce a cell death phenotype in N. benthamiana leaves. At OD600 = 0.05, transient expression of avrBsT also did not trigger a full cell death response. However, coexpression of avrBsT with CaADC1, but not CaADC1 K154A, at OD600 = 0.05 produced a severely necrotic cell death phenotype that was similar to avrBsT expression at OD600 = 0.2 (Fig. 2, A and B). The extent of cell death was quantified by measuring electrolyte leakage from N. benthamiana leaves that were transiently expressing CaADC1, CaADC1 K154A, avrBsT, CaADC1 K154A/avrBsT, or CaADC1/avrBsT. Consistent with the visual cell death phenotypes, CaADC1-expressing leaf tissues exhibited very low levels of electrolyte leakage that were similar to those of the empty vector control and low-level expression of avrBsT (Fig. 2C). At 24 to 48 h after agroinfiltration, coexpression of CaADC1 and avrBsT at the agrotiter of OD600 = 0.05 induced significantly higher electrolyte leakage than the expression of avrBsT alone at OD600 = 0.05. Coexpression of CaADC1 K154A and avrBsT at the agrotiter of OD600 = 0.05 showed a cell death phenotype and electrolyte leakage similar to that of expression of avrBsT alone at OD600 = 0.05. These results indicate that transient coexpression of the CaADC1 K154A mutant does not promote avrBsT-triggered cell death in N. benthamiana leaves. Immunoblot analyses confirmed that the epitope-tagged CaADC1, CaADC1 K154A, and AvrBsT proteins were transiently expressed in agroinfiltrated N. benthamiana leaves (Fig. 2D). For a specificity control, the mouse proapoptotic effector gene BAX, whose expression triggers cell death in plants (Lacomme and Santa Cruz, 1999; del Pozo et al., 2004), was coexpressed with CaADC1 or CaADC1 K154A at the lower limit of the cell death-inducing A. tumefaciens titer (OD600 = 0.05). CaADC1 or CaADC1 K154A coexpression did not promote a BAX-induced cell death response in N. benthamiana leaves (Supplemental Fig. S4, A and B). The avrBsT C222A mutant, which does not cause cell death in plants (Orth et al., 2000), was also coexpressed with CaADC1 or CaADC1 K154A. The CaADC1 or CaADC1 K154A coexpression in agroinfiltrated N. benthamiana leaves did not trigger cell death-inducing activity in AvrBsT C222A, although CaADC1 formed a complex with AvrBsT C222A (Supplemental Fig. S5, A and B). Collectively, these results indicate that CaADC1 coexpression specifically promotes the avrBsT-triggered cell death response.
Figure 2.
Transient expression of CaADC1 promotes AvrBsT-triggered cell death. A, Cell death phenotypes. N. benthamiana leaves were infiltrated with A. tumefaciens carrying the indicated constructs at different inoculum ratios and photographed 2 d later. B, Cell death levels were rated based on a 0 to 3 scale: 0, no cell death (less than 10%); 1, weak cell death (10%–30%); 2, partial cell death (30%–80%); and 3, full cell death (80%–100%). Values represent averages of 10 samples. C, Electrolyte leakage from leaf discs at different time points after infiltration with A. tumefaciens carrying the indicated constructs at different inoculum ratios. Data are means ± sd from three independent experiments. Different letters indicate statistically significant differences (lsd; P < 0.05). D, Immunoblot analyses of the expression of 35S:avrBsT:cMyc, 35S:CaADC1:HA, and 35S:CaADC1 K154A:HA. Protein loading is visualized by Coomassie Brilliant Blue (CBB) staining. [See online article for color version of this figure.]
Effects of Transient Expression of CaADC1 and avrBsT on PA Levels in N. benthamiana Leaves
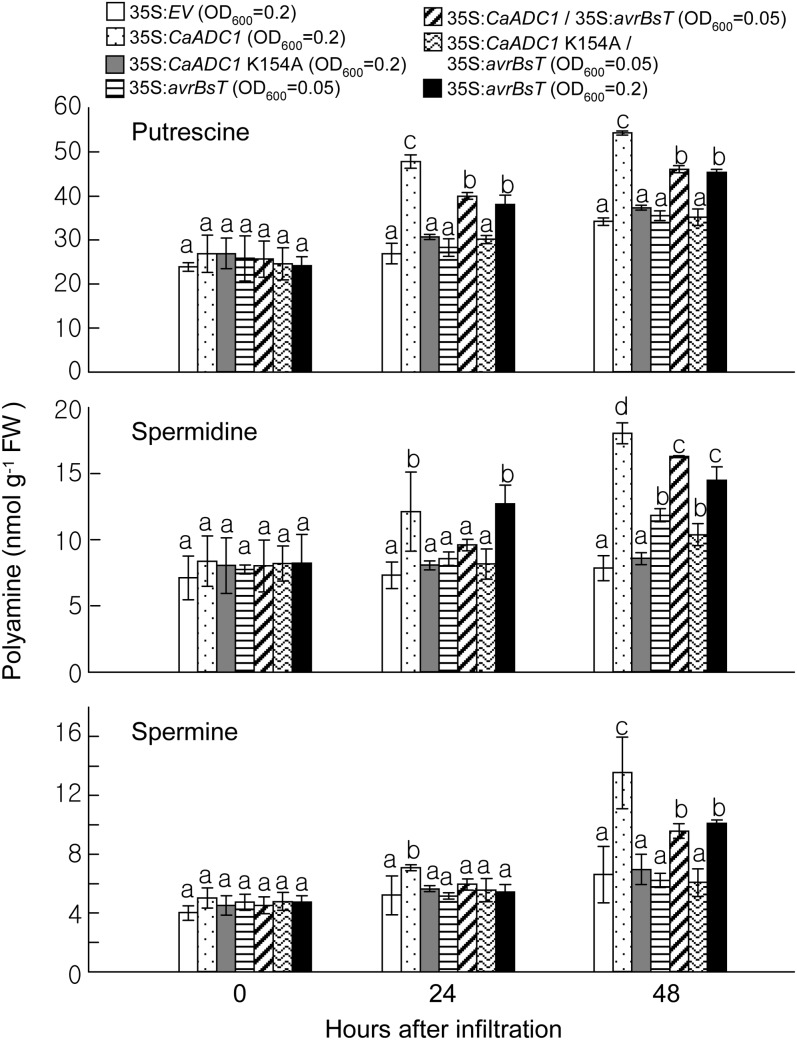
Decarboxylation of Arg by ADC leads to the sequential formation of PAs such as putrescine, spermidine, and spermine (Flores and Filner, 1985). To investigate whether the transient expression of CaADC1 and avrBsT affects PA metabolism, the levels of PAs were determined in N. benthamiana leaf tissues at 0, 24, and 48 h after A. tumefaciens-mediated transient expression of experimental constructs (Fig. 3). Transient expression of CaADC1 resulted in increased accumulation of the three PAs at 24 h after agroinfiltration. The PAs remained at higher levels compared with those of the other constructs at 48 h after agroinfiltration. However, the CaADC1 K154A mutant did not exhibit induced accumulation of PAs, which was similar to the results with empty vector controls. Putrescine and spermidine were highly induced at 24 h after transient expression of avrBsT (OD600 = 0.2). By 48 h after agroinfiltration, the levels of the three PAs were significantly increased compared with those in the empty vector controls. At OD600 = 0.05, transient expression of avrBsT did not trigger the induction of PAs at 24 h after infiltration. However, coexpression of avrBsT with CaADC1 at OD600 = 0.05 led to a significantly higher accumulation of putrescine at 24 h after agroinfiltration; by 48 h, all three PAs accumulated to levels that were similar to avrBsT expression at OD600 = 0.2. Coexpression of CaADC1 K154A and avrBsT at the agrotiter of OD600 = 0.05 induced the accumulation of all three PAs, similar to the expression of avrBsT alone at OD600 = 0.05. Together, these data indicate that transient expression of CaADC1, avrBsT, or CaADC1/avrBsT induces PA accumulation in N. benthamiana leaves.
Figure 3.
Effect of the transient expression of CaADC1, CaADC1 K154A, and avrBsT on PA levels in N. benthamiana leaves. Putrescine, spermidine, and spermine contents were determined in leaf tissues at 0, 24, and 48 h after A. tumefaciens-mediated transient expression of the indicated constructs. Data are means ± sd from three independent experiments. Different letters indicate statistically significant differences (lsd; P < 0.05). FW, Fresh weight.
Effects of the Transient Expression of CaADC1 and avrBsT on Amino Acid Levels in N. benthamiana Leaves
The catabolism of PAs produces GABA, which can be further metabolized to Glu or Ala (Bouché and Fromm, 2004). To analyze the effects of the transient expression of CaADC1 and avrBsT on Arg and related amino acid levels, amino acid contents were determined in N. benthamiana leaves 1 and 2 d after infiltration of A. tumefaciens carrying 35S:EV, 35S:CaADC1, or 35S:avrBsT at OD600 = 0.2 (Supplemental Fig. S6). Transient expression of ADC1 resulted in significantly lower levels of Arg and Ala, but not Glu or GABA, at 1 to 2 d after agroinfiltration. However, transient expression of avrBsT did not significantly alter levels of Glu, Arg, Ala, or GABA in N. benthamiana. These results indicate that transient expression of CaADC1 reduces the accumulation of Arg and Ala in N. benthamiana.
Effects of CaADC1 and avrBsT Transient Expression and PA Treatment on Nitric Oxide Production in N. benthamiana Leaves
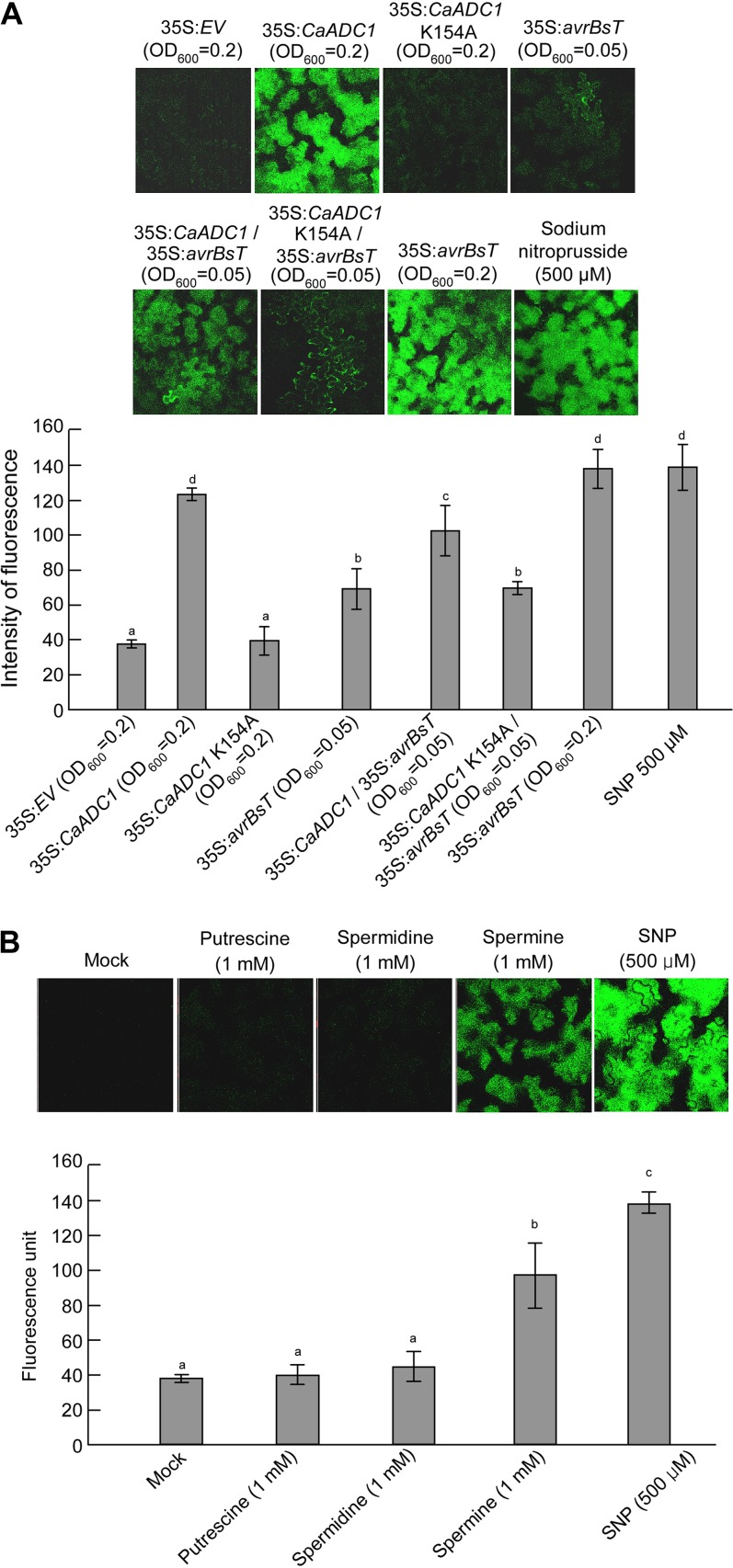
There is evidence that nitric oxide (NO) and ROS bursts are involved in HR in plants (Asai et al., 2008; Yun et al., 2011). To investigate whether CaADC1 and avrBsT transient expression and PA treatment induce the NO burst, NO was detected by 4,5-diaminofluorescein diacetate (DAF-2DA) staining of N. benthamiana leaves 24 h after infiltration with A. tumefaciens or PA (Fig. 4). Transient expression of CaADC1 or avrBsT at OD600 = 0.2 markedly induced the NO burst, similar to that induced by the NO generator sodium nitroprusside (SNP; Fig. 4A). However, expression of CaADC1 K154A did not induce the NO burst, similar to the result with the empty vector control. NO induction by avrBsT expression at OD600 = 0.05 was significantly lower than that induced by the OD600 = 0.2 high-titer infiltration (Fig. 4A). Coexpression of avrBsT with CaADC1, but not with CaADC1 K154A, at OD600 = 0.05 induced significantly higher accumulation of NO than avrBsT alone at OD600 = 0.05.
Figure 4.
Quantification of the NO burst in N. benthamiana leaves 24 h after infiltration with either PAs or A. tumefaciens harboring the indicated constructs. Leaves were stained with DAF-FM DA and visualized with confocal microscopy. Signal intensities were quantified by color histogram analysis. Data are means ± sd from three independent experiments. Different letters indicate statistically significant differences (lsd; P < 0.05). A, Effect of the transient expression of CaADC1, CaADC1 K154A, or avrBsT on NO production. B, Effect of PA treatment on NO production. SNP (500 μm) treatment was used as a positive control. [See online article for color version of this figure.]
To investigate whether PA application triggers the NO burst, putrescine, spermidine, and spermine were infiltrated into N. benthamiana leaves and NO accumulation was visualized 24 h after infiltration (Fig. 4B). As negative and positive controls, 10 mm MgCl2 and 500 μm SNP were infiltrated into leaves, respectively. Infiltration of putrescine and spermidine at 1 mm did not significantly induce NO production; however, application of 1 mm spermine induced significantly higher NO production in N. benthamiana leaves.
Effects of CaADC1 and avrBsT Transient Expression and PA Treatment on H2O2 Production in N. benthamiana Leaves
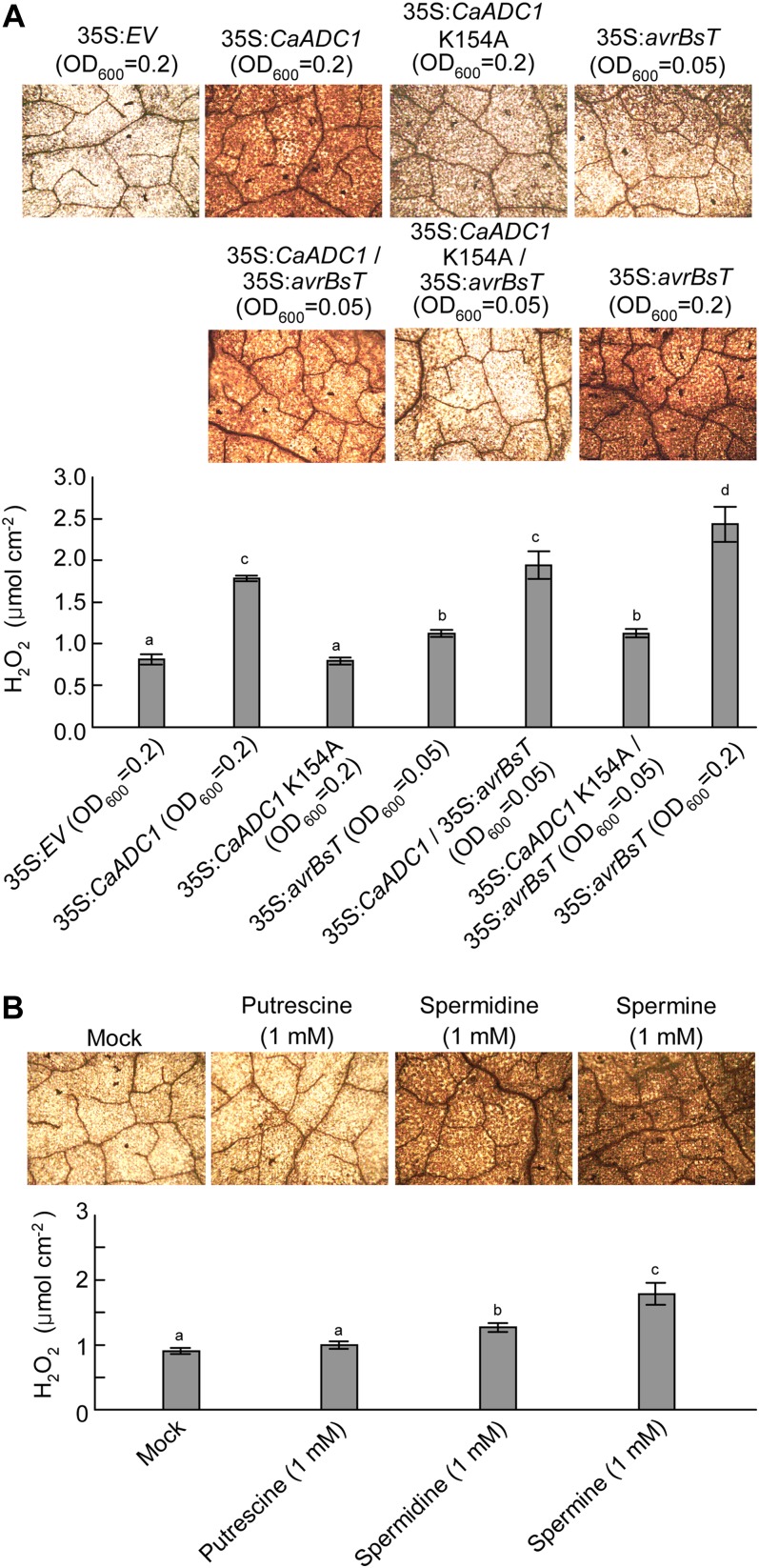
To investigate the effects of CaADC1 and avrBsT-transient expression and PA treatments on the ROS burst in N. benthamiana leaves, H2O2 accumulation was visualized by 3,3′-diaminobenzidine (DAB) staining, and H2O2 levels were quantified by using the xylenol orange assay (Gay et al., 1999) 24 h after infiltration with A. tumefaciens or PA (Fig. 5). The H2O2 burst was distinctly induced by the transient expression of CaADC1 or avrBsT at OD600 = 0.2 (Fig. 5A). However, expression of CaADC1 K154A did not induce the H2O2 burst, similar to the result with the empty vector control. At OD600 = 0.2, transient expression of avrBsT triggered higher H2O2 induction than did avrBsT expression at OD600 = 0.05. Coexpression of avrBsT with CaADC1 at OD600 = 0.05 led to a significantly higher accumulation of H2O2 than did avrBsT alone at OD600 = 0.05. Coexpression of CaADC1 K154A and avrBsT at the agrotiter of OD600 = 0.05 induced H2O2 accumulation, similar to the expression of avrBsT alone at OD600 = 0.05.
Figure 5.
Quantification of the H2O2 burst in N. benthamiana leaves 24 h after infiltration with either PAs or A. tumefaciens harboring the indicated constructs. Leaves were stained with DAB. H2O2 concentrations were quantified by xylenol orange analysis. Data are means ± sd from three independent experiments. Different letters indicate statistically significant differences (lsd; P < 0.05). A, Effect of the transient expression of CaADC1, CaADC1 K154A, or avrBsT on H2O2 production. B, Effect of PA treatment on H2O2 production. The mock control was treated with 10 mm MgCl2. [See online article for color version of this figure.]
To determine the effects of PAs on the H2O2 burst, putrescine, spermidine, and spermine at 1 mm were directly infiltrated into N. benthamiana leaves, and H2O2 was visualized 24 h after infiltration (Fig. 5B). Infiltration with 10 mm MgCl2 was used as a mock control. Infiltration of 1 mm spermine resulted in the highest H2O2 accumulation. Spermidine also triggered significantly higher H2O2 accumulation as compared with the mock treatment. However, putrescine at 1 mm did not significantly induce the H2O2 burst (Fig. 5B).
Spermine Triggers Cell Death in N. benthamiana Leaves
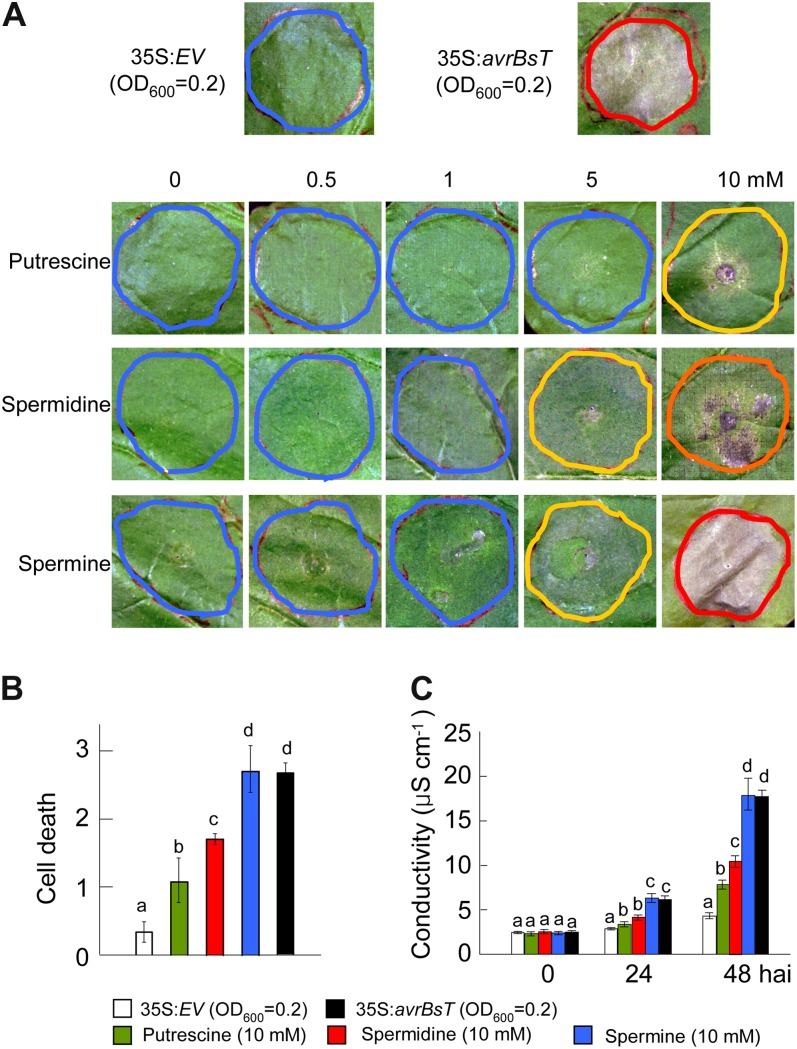
The ability of spermine to trigger NO and H2O2 bursts (Figs. 4 and 5) prompted us to investigate whether PAs such as spermine function as positive triggers of cell death in plants. We infiltrated putrescine, spermidine, and spermine into N. benthamiana leaves using increasing concentrations from 0.5 to 10 mm (Fig. 6). For comparison, A. tumefaciens carrying 35S:EV and 35S:avrBsT was infiltrated at OD600 = 0.2 as negative and positive controls of cell death, respectively. Two days after infiltration, the empty vector control did not show any cell death response, whereas avrBsT transient expression induced hypersensitive cell death in N. benthamiana leaves (Fig. 6A). Surprisingly, infiltration of spermine at 10 mm induced a full necrotic cell death response, similar to that observed with avrBsT transient expression (Fig. 6, A and B). Infiltration of 10 mm putrescine or spermidine also triggered intermediate levels of cell death. The extent of cell death was quantified by measuring electrolyte leakage from N. benthamiana leaves infiltrated with 10 mm PAs. Among the PAs tested, spermine triggered the highest electrolyte leakage, similar to that resulting from avrBsT transient expression at 48 h after infiltration (Fig. 6C). The electrolyte leakage by putrescine infiltration was lower than that induced by spermidine but higher than that of the empty vector control (Fig. 6C). Collectively, these results indicate that spermine functions as a positive trigger of cell death in plants.
Figure 6.
Effect of PA treatment on cell death response in N. benthamiana leaves. A, Cell death phenotypes in N. benthamiana leaves 2 d after infiltration with the indicated concentrations of putrescine, spermidine, or spermine. A. tumefaciens harboring 35S:EV or 35S:avrBsT was used as negative and positive controls, respectively. Red, orange, yellow, and blue circles indicate full, severe, partial, and no cell death, respectively. B, Quantification of cell death levels in leaves infiltrated with 10 mm PAs. Cell death levels were rated based on a 0 to 3 scale: 0, no cell death (less than 10%); 1, weak cell death (10%–30%); 2, partial cell death (30%–80%); and 3, full cell death (80%–100%). C, Electrolyte leakage from leaf discs at different time points after infiltration with 10 mm PAs. Data are means ± sd from three independent experiments. Different letters indicate statistically significant differences at different time points (Fisher’s lsd; P < 0.05). [See online article for color version of this figure.]
Spermine Promotes an avrBsT-Triggered Cell Death Response
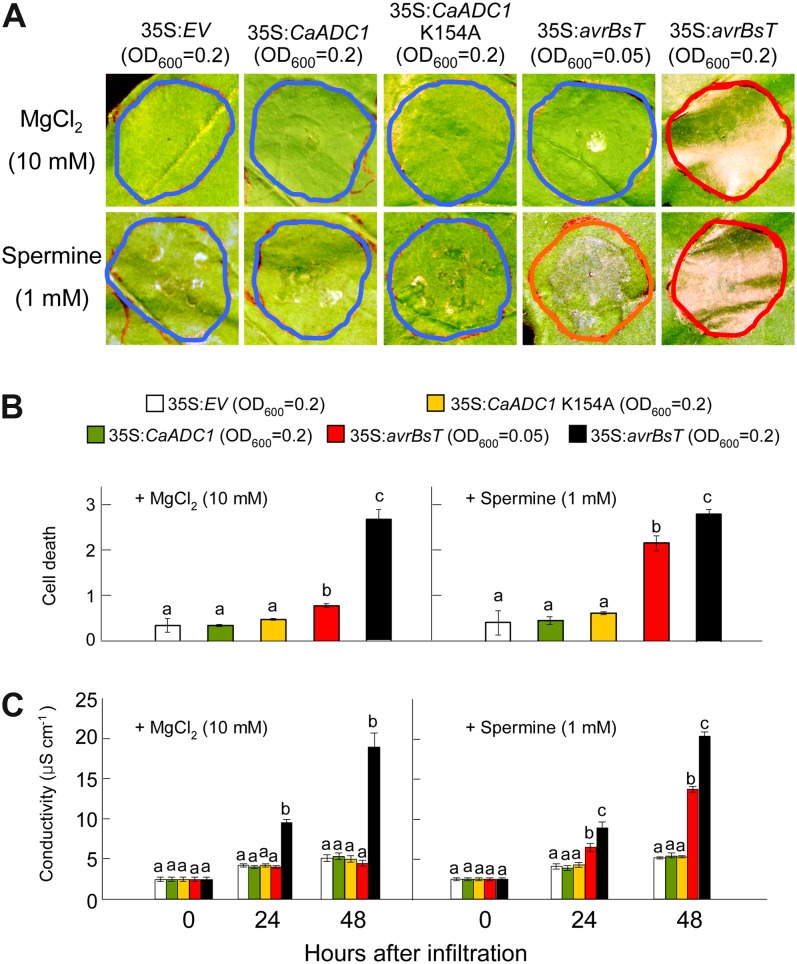
To define the role of spermine in the avrBsT-triggered cell death response, we infiltrated 1 mm spermine mixed with A. tumefaciens carrying 35S:EV, 35S:CaADC1, 35S:CaADC1 K154A, or 35S:avrBsT into N. benthamiana leaves (Fig. 7). The control was infiltration of 10 mm MgCl2 mixed with A. tumefaciens suspensions. Infiltration of CaADC1 or CaADC1 K154A did not trigger cell death in N. benthamiana with or without 1 mm spermine (Fig. 7). Transient expression of avrBsT at OD600 = 0.05 was not able to trigger the full cell death response. However, addition of 1 mm spermine to A. tumefaciens carrying 35S:avrBsT at OD600 = 0.05 produced a severely necrotic cell death phenotype (Fig. 7, A and B). The extent of cell death was quantified by measuring electrolyte leakage from N. benthamiana leaves transiently expressing CaADC1, CaADC1 K154A, or avrBsT with or without 1 mm spermine. Consistent with the visual cell death phenotypes, very low levels of electrolyte leakage were observed in leaf tissues transiently expressing CaADC1 or CaADC1 K154A, similar to that observed with the empty vector control. Addition of 1 mm spermine to A. tumefaciens carrying 35S:avrBsT at OD600 = 0.05 induced higher electrolyte leakage than the empty vector control at 24 to 48 h after infiltration (Fig. 7C). Transient expression of avrBsT at the agrotiter of OD600 = 0.2 induced high levels of cell death phenotype and electrolyte leakage with or without spermine. Collectively, these results indicate that spermine positively regulates the avrBsT-triggered cell death response.
Figure 7.
Spermine promotes AvrBsT-triggered cell death. A, Cell death phenotypes in N. benthamiana leaves 2 d after infiltration with A. tumefaciens carrying the indicated constructs and supplemented with 10 mm MgCl2 or 1 mm spermine. Red, orange, and blue circles indicate full, severe, and no cell death, respectively. B, Quantification of cell death levels. Cell death levels were rated based on a 0 to 3 scale: 0, no cell death (less than 10%); 1, weak cell death (10%–30%); 2, partial cell death (30%–80%); and 3, full cell death (80%–100%). C, Electrolyte leakage from leaf discs at different time points after infiltration with A. tumefaciens carrying the indicated constructs and supplemented with 10 mm MgCl2 or 1 mm spermine. Data are means ± sd from three independent experiments. Different letters indicate statistically significant differences at different time points (Fisher’s lsd; P < 0.05). [See online article for color version of this figure.]
CaADC1 Is Required for the avrBsT-Triggered Cell Death Response to Incompatible Xcv Infection
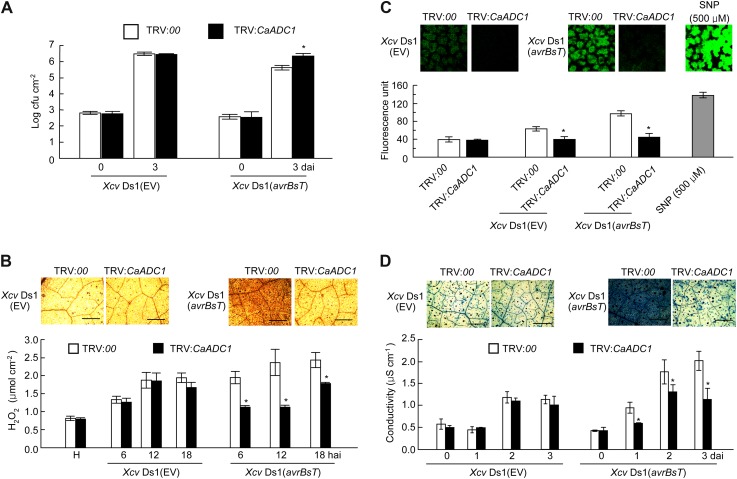
We used the tobacco rattle virus (TRV)-based virus-induced gene silencing (VIGS) to investigate CaADC1 loss of function in pepper plants (Liu et al., 2002). Leaves of empty vector (TRV:00) or silenced (TRV:CaADC1) pepper plants were inoculated with virulent (compatible) Ds1 (EV) or avirulent (incompatible) Ds1 (avrBsT) Xcv strains (107 and 108 colony-forming units [cfu] mL−1). Silencing of CaADC1 in pepper plants led to a highly susceptible response to incompatible Xcv infection (Supplemental Fig. S7). Inoculation with the avirulent Ds1 (avrBsT) Xcv strain (108 cfu mL−1) caused cell death in the empty vector control leaves. However, HR-like cell death was not observed in CaADC1-silenced leaves 2 d after inoculation. Reduced cell death in leaves was more clearly noticeable, as observed under UV light (Supplemental Fig. S7). Incompatible Xcv Ds1 (avrBsT) growth in CaADC1-silenced leaves was approximately 10-fold higher than that in empty vector control leaves 3 d after inoculation (Fig. 8A). By contrast, no significant differences in compatible Xcv Ds1 (EV) growth were found between the empty vector control and silenced plants during infection. These results indicate that CaADC1 is required for cell death-mediated resistance to Xcv infection.
Figure 8.
Enhanced susceptibility of CaADC1-silenced pepper plants to avirulent Xcv (avrBsT) infection. A, Bacterial growth in empty vector control and silenced leaves. Bacterial growth in leaves was measured at 0 and 3 d after inoculation with 5 × 104 cfu mL−1 Xcv Ds1 (EV) and Xcv Ds1 (avrBsT). B, DAB staining and H2O2 quantification at different time points after inoculation with 5 × 107 cfu mL−1 Xcv Ds1 (EV) and Xcv Ds1 (avrBsT). C, NO detection and relative intensity measurement by DAF-2DA staining. D, Trypan blue staining and electrolyte leakage measurement. Control (TRV:00) and CaADC1-silenced (TRV:CaADC1) leaves were inoculated with 5 × 107 cfu mL−1 Xcv Ds1 (EV) and Xcv Ds1 (avrBsT). Trypan blue staining was performed 24 h after infiltration, and electrolyte leakage was monitored at the indicated time points after infiltration. Data are means ± sd from three independent experiments. Asterisks indicate statistically significant differences (Student’s t test; P < 0.05). H, Healthy leaves. [See online article for color version of this figure.]
Cell Death, ROS, and NO Bursts Are Compromised in CaADC1-Silenced Pepper
To investigate whether CaADC1 regulates cell signaling pathways to induce early defense responses, we analyzed and compared ROS (H2O2) and NO accumulation and cell death response in empty vector control and CaADC1-silenced pepper leaves during Xcv infection (Fig. 8, B–D). H2O2 and NO production and cell death were visualized by DAB, DAF-2DA, and trypan blue staining, respectively. Significantly compromised H2O2 and NO accumulation and cell death response were observed in the CaADC1-silenced leaves inoculated with incompatible Xcv Ds1 (avrBsT). Xylenol orange assay, relative intensity of DAF-2DA signals, and ion conductivity measurements were used to quantify H2O2 and NO production and cell death, respectively. Silencing of CaADC1 significantly compromised the accumulation of H2O2 and NO and lowered ion leakage in pepper leaves during the incompatible Xcv (avrBsT) infection. NO accumulation was also significantly compromised by CaADC1 silencing during the compatible Xcv infection and remained at the basal level.
CaADC1 Expression Positively Regulates SA-Induced Defense Genes
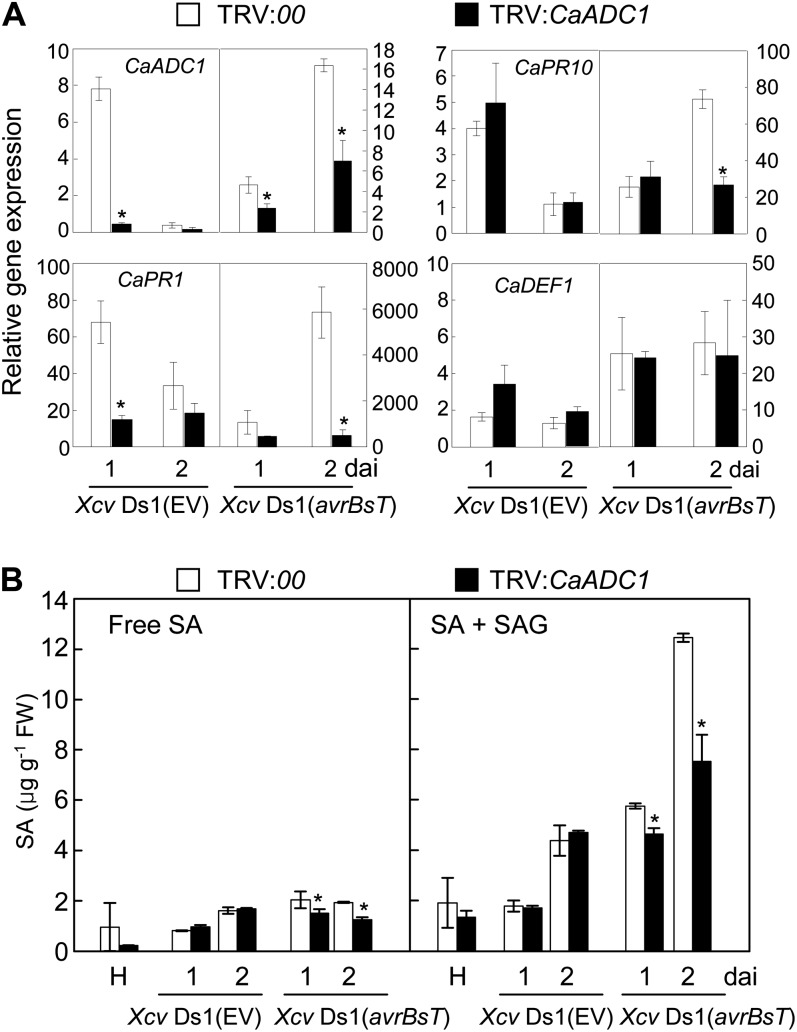
To determine whether CaADC1 silencing alters the expression of pepper defense-related genes at early infection stages, real-time reverse transcription (RT)-PCR analyses were performed using gene-specific primer pairs for CaADC1, CaPR1, CaPR10, and CaDEF1 (for defensin; Supplemental Table S1) at 1 and 2 d after inoculation with the compatible Xcv Ds1 (EV) and incompatible Xcv Ds1 (avrBsT) strains (Fig. 9A). CaADC1 silencing in pepper leaves significantly compromised induction of the SA-induced defense genes CaPR1 and CaPR10, but not the jasmonate-related gene CaDEF1, during the incompatible Xcv infection. By contrast, consistently compromised induction of these defense-related genes was not detected in CaADC1-silenced leaves that were infected by the compatible Xcv Ds1 (EV) strain. Collectively, these results suggest the involvement of CaADC1 in defense signaling mediated by SA during incompatible interactions of Xcv with pepper.
Figure 9.
CaADC1 silencing compromises SA-dependent defense gene expression and SA accumulation in pepper leaves infected with Xcv. A, Expression profiles of defense-related genes. B, Free and total SA levels in empty vector control (TRV:00) and CaADC1-silenced (TRV:CaADC1) pepper leaves infected by Xcv. Total RNA was isolated from empty vector control (TRV:00) and CaADC1-silenced (TRV:CaADC1) pepper leaves inoculated with 5 × 107 cfu mL−1 of the Xcv strains Ds1 (EV) and Ds1 (avrBsT). Quantitative real-time PCR was performed for CaADC1 and pepper PR genes: CaPR1, CaPR10, and CaDEF1. Relative expression levels at 1 and 2 d after inoculation (dai) are shown. Relative expression values are normalized to the expression of pepper 18S ribosomal RNA in each sample. SA contents were determined in empty vector control and silenced leaves at 1 and 2 d after inoculation with Xcv. Data are means ± sd from three independent experiments. Asterisks indicate significant differences as determined by Student’s t test (P < 0.05). FW, Fresh weight.
Accumulation of SA Is Compromised in CaADC1-Silenced Pepper during Incompatible Xcv Infection
To determine if CaADC1 silencing regulates SA signaling in the defense response to bacterial infection, we quantified the levels of free and total SA in empty vector control and CaADC1-silenced pepper leaves during Xcv infection (Fig. 9B). Accumulation of free and total SA (free SA plus Glc-conjugated SA) significantly declined in CaADC1-silenced leaves compared with empty vector control leaves at 1 and 2 d after incompatible Xcv infection. In contrast, CaADC1 silencing did not affect SA accumulation during compatible Xcv infection.
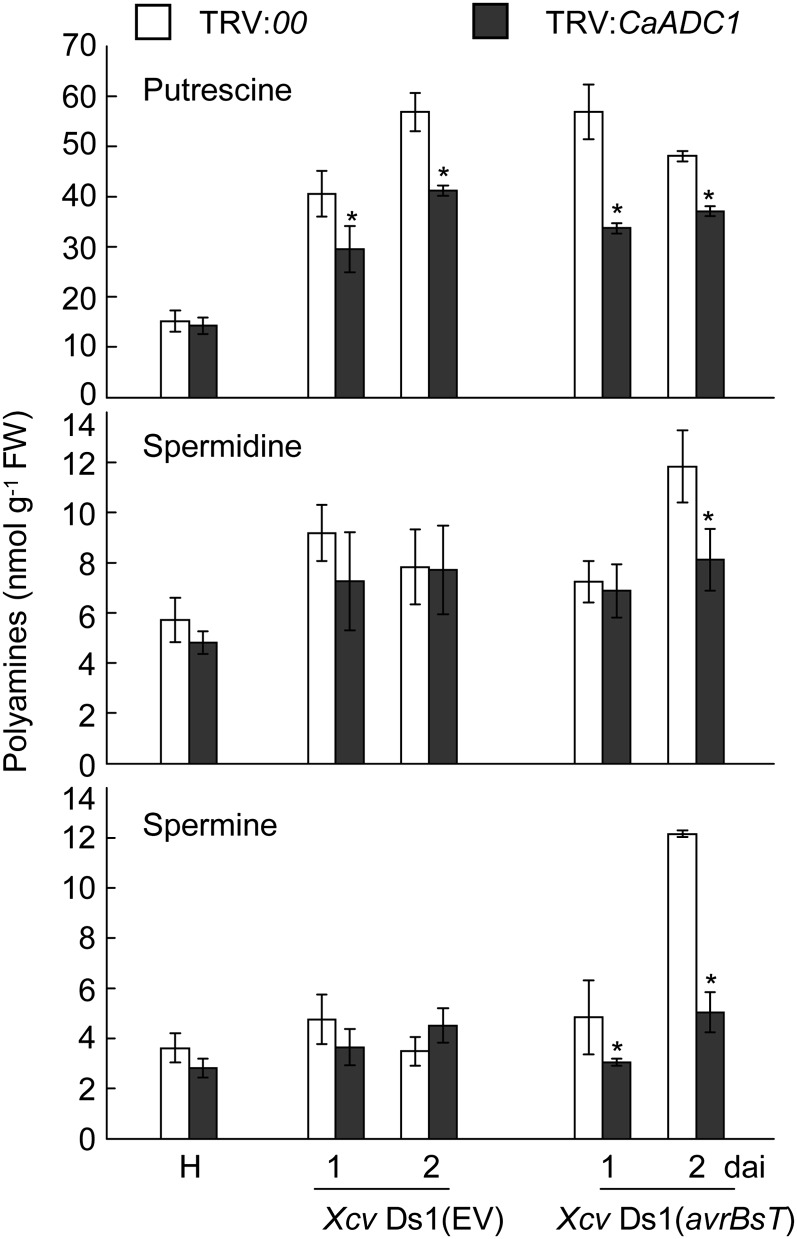
CaADC1 Silencing Compromises PA Accumulation in Pepper during Incompatible Xcv Infection
To investigate whether the silencing of CaADC1 affects PA metabolism, the levels of putrescine, spermidine, and spermine were determined in empty vector control and CaADC1-silenced pepper leaves at 1 and 2 d after Xcv inoculation (107 cfu mL−1; Fig. 10). In empty vector control leaves, putrescine and spermidine levels were greatly increased by both Xcv Ds1 (EV) and Xcv Ds1 (avrBsT) infection. Interestingly, the levels of spermine were only increased by avirulent Xcv Ds1 (avrBsT) infection. In contrast, CaADC1 silencing compromised the accumulation of putrescine, spermidine, and spermine in pepper leaves during avirulent Xcv Ds1 (avrBsT) infection. However, virulent Xcv Ds1 infection compromised putrescine accumulation in CaADC1-silenced pepper leaves but did not distinctly affect the contents of spermidine and spermine in empty vector control and ADC1-silenced pepper leaves.
Figure 10.
Levels of putrescine, spermidine, and spermine in leaves of empty vector control (TRV:00) and CaADC1-silenced (TRV:CaADC1) pepper plants infected with 107 cfu mL−1 of the virulent Ds1 (EV) and avirulent Ds1(avrBsT) strains of Xcv. Data are means ± sd from three independent experiments. Asterisks indicate statistically significant differences (Student’s t test; P < 0.05). FW, Fresh weight; H, healthy leaves.
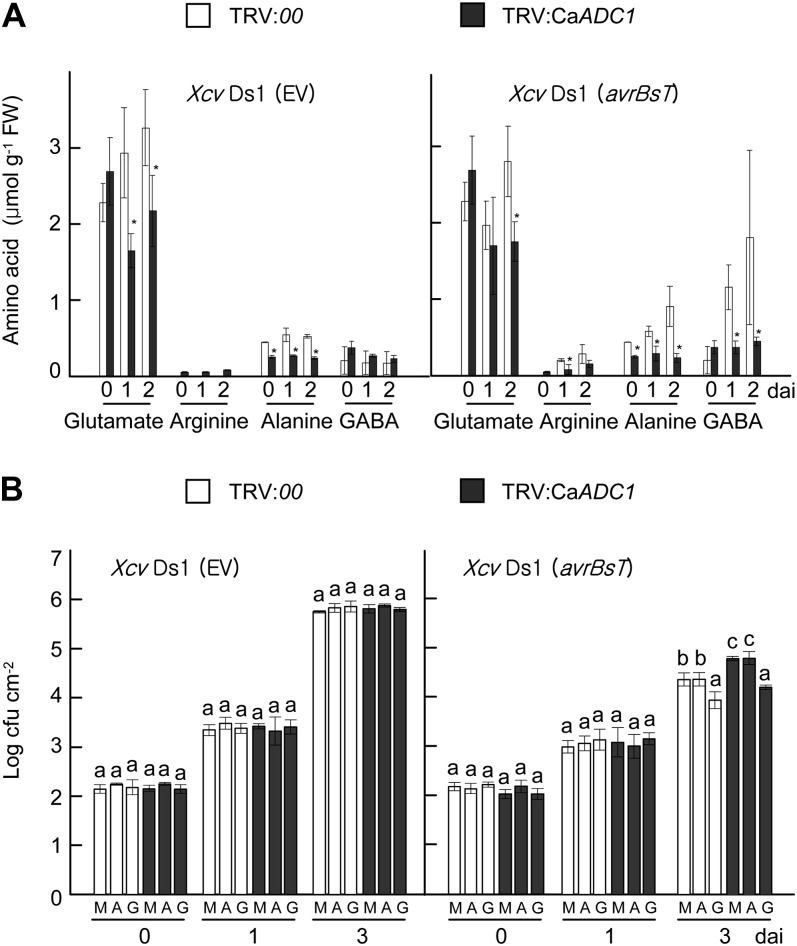
CaADC1 Silencing Compromises the Accumulation of Gln, Ala, and GABA in Pepper Leaves
To determine whether the silencing of CaADC1 affects levels of Arg and related amino acids, free amino acids levels of Gln, Arg, Ala, and GABA were compared in empty vector control (TRV:00) and CaADC1-silenced leaves during incompatible Xcv infection (Fig. 11A; Supplemental Fig. S8). There were no significant differences in the levels of Arg between empty vector control and CaADC1-silenced leaves. However, decreased Ala and Glu levels were detected in CaADC1-silenced leaves compared with empty vector control leaves during both Xcv Ds1 (EV) and Xcv Ds1 (avrBsT) infection. Notably, CaADC1 silencing significantly compromised GABA accumulation in pepper leaves during avirulent Xcv Ds1 (avrBsT) infection. Together, these results indicate that CaADC1 plays a crucial role in the accumulation of Gln, Ala, and GABA during Xcv Ds1 (avrBsT) infection.
Figure 11.
GABA suppresses Xcv growth in pepper leaves. A, Levels of amino acids in leaves of empty vector control (TRV:00) and CaADC1-silenced (TRV:CaADC1) pepper plants infected with 107 cfu mL−1 of the virulent Ds1 (EV) and avirulent Ds1 (avrBsT) strains of Xcv. Data are means ± sd from three independent experiments. Asterisks indicate statistically significant differences (Student’s t test; P < 0.05). B, Exogenous application of GABA suppresses the proliferation of avirulent Xcv Ds1 (avrBsT) in leaves of empty vector control and CaADC1-silenced plants. Leaves were treated with 10 mm MgCl2 (mock [M]), Ala (A), or GABA (G) 1 d before infiltration with 5 × 104 cfu mL−1 Xcv Ds1 (EV) and Ds1 (avrBsT). Data are means ± sd. Different letters indicate statistically significant differences (lsd; P < 0.05). FW, Fresh weight.
GABA Promotes Resistance against Xcv Ds1 (avrBsT) Infection
We investigated whether the application of exogenous Ala or GABA affects resistance against Xcv infection in pepper leaves. The addition of Ala or GABA to the culture medium did not have any positive or adverse effect on Xcv growth (Supplemental Fig. S9). Empty vector control and CaADC1-silenced pepper leaves were infiltrated with 10 mm MgCl2 (mock), 10 mm Ala, or 10 mm GABA 1 d prior to Xcv inoculation, and bacterial growth was monitored at 0, 1, and 3 d after Xcv inoculation (Fig. 11B). Treatment with Ala did not suppress the growth of compatible Xcv Ds1 (EV) and incompatible Ds1 (avrBsT) in empty vector control and CaADC1-silenced pepper leaves. However, GABA treatment of empty vector control and CaADC1-silenced pepper leaves significantly reduced avirulent Xcv Ds1 (avrBst) growth 3 d after inoculation (Fig. 11B). These results suggest that GABA enhances cell death-mediated resistance against Xcv at a late infection stage.
DISCUSSION
Pepper plants recognize the type III effector protein AvrBsT that is secreted by Xcv and respond by triggering the cell death response (Escolar et al., 2001; Kim et al., 2010). The involvement of phosphatidic acids in the AvrBsT-triggered cell death was revealed by the identification of SUPPRESSOR OF AVRBST-ELICITED RESISTANCE1, a conserved carboxylesterase of the Arabidopsis Pi-0 ecotype (Cunnac et al., 2007; Kirik and Mudgett, 2009). In pepper, AvrBsT was found to target a putative regulator of sugar metabolism, SNF1-RELATED KINASE1, which is required for AvrBs1-induced immunity (Szczesny et al., 2010). However, the cognate R proteins for AvrBsT have not yet been identified. To better understand the molecular mechanisms underlying AvrBsT-triggered cell death in pepper plants, we have identified CaADC1 as an AvrBsT-interacting protein.
AvrBsT has been proposed to localize at the cytoplasm and nucleus (Szczesny et al., 2010). The in vitro and in planta interactions between AvrBsT and CaADC1 were revealed using yeast two-hybrid, BiFC, and coimmunoprecipitation assays. These data suggest that the interaction of AvrBsT with CaADC1 occurs at the cytoplasm only when CaADC1 is in the active state. The inactive CaADC1 K154A mutant that cannot bind to the essential coenzyme, pyridoxal phosphate (Cohen et al., 1983), did not interact with AvrBsT, which could not ultimately enhance the AvrBst-triggered cell death response in N. benthamiana leaves. However, the cell death-inactive AvrBsT C222A mutant was able to bind to CaADC1. Interestingly, coexpression of the CaADC1 that was only able to bind to AvrBsT specifically enhanced AvrBsT-triggered cell death. Collectively, these results suggest that CaADC1 acts as a positive regulator of cell death signaling in plants.
Coexpression of AvrBsT with CaADC1 significantly enhanced the cell death phenotype and concurrently increased electrolyte leakage. This enhancement effect may be dependent on the ADC activity of CaADC1, because the CaADC1 K154A mutant did not undergo cell death triggered by AvrBsT. Moreover, the CaADC1 enhancement effect was specific to AvrBsT, as CaADC1 coexpression did not promote BAX-induced cell death. CaADC1 expression induced an overaccumulation of PAs, and the results demonstrate that spermine could induce NO and H2O2 bursts that lead to cell death. During the resistance response of barley (Hordeum vulgare) to powdery mildew (Blumeria graminis f. sp. hordei), ODC or ADC activities greatly increased and resulted in higher PA levels (Cowley and Walters, 2002). However, ODC genes are proposed to be absent in Arabidopsis (Hanfrey et al., 2001). This suggests a predominant role for ADC in PA metabolism, at least in Arabidopsis. Similarly, significantly higher spermine levels accumulated in tobacco (Nicotiana tabacum) plants due to increased ADC activity during Pseudomonas viridiflava infection, suggesting a beneficial role of ADC for the defense against the pathogen (Marina et al., 2008).
The PA catabolism mediated by NADPH oxidases or peroxidases is a major source of the ROS burst (Takahashi et al., 2004). ROS have widely been known to be crucial for various plant defense responses, such as cell wall strengthening, defense gene activation, and cell death and systemic acquired resistance induction (Lamb and Dixon, 1997; Torres et al., 2006; Van Breusegem and Dat, 2006). Exogenous application of PAs to Arabidopsis seedlings induced NO production (Wimalasekera et al., 2011). However, the processes in which the NO is produced in response to increased PA levels remain unclear (Yamasaki and Cohen, 2006). The nitrate reductase is only an enzyme required to produce NO (Rockel et al., 2002; Moreau et al., 2010). More recently, it has been demonstrated that PAs could increase nitrate reductase activity in wheat (Triticum aestivum; Rosales et al., 2012), suggesting an indirect role for PAs to generate NO. Whether an oxidative way is required to produce NO from the substrates, such as Arg, PA and hydroxylamines are actively being investigated (Besson-Bard et al., 2008; Moreau et al., 2010). NO has been shown to play essential roles in animal innate immune and inflammatory responses, and several downstream targets of NO, such as guanylate cyclase and mitogen-activated protein kinases, are shared by both animals and plants (Klessig et al., 2000). Recently, S-nitrosylation of NADPH oxidase was proposed to regulate cell death in plant immunity (Yun et al., 2011).
More importantly, the experimental evidence that the addition of 1 mm spermine to A. tumefaciens carrying 35S:avrBsT could promote AvrBsT-triggered cell death in inoculated leaves supports the hypothesis that CaADC1 expression induces the production of PAs such as spermine to trigger the cell death response in plants. The involvement of spermine in defense and cell death responses has been demonstrated in other plant-pathogen systems (Mitsuya et al., 2009; Gonzalez et al., 2011). In tobacco, treatment with spermine induced the activation of mitogen-activated protein kinases through mitochondrial dysfunction (Takahashi et al., 2003). A subset of hypersensitive response marker genes is proposed to be the downstream target of a spermine signal transduction pathway in plants (Takahashi et al., 2004). Interestingly, spermine was demonstrated to induce cell death in human embryonic cerebral cortical neurons and mouse melanoma cells (Averill-Bates et al., 2008; de Vera et al., 2008).
In contrast to the promotion of cell death by transient expression of CaADC1, avrBsT-triggered cell death was compromised in CaADC1-silenced pepper leaves, as shown by decreased electrolyte leakage. CaADC1 silencing in pepper leaves significantly compromised the induction of H2O2, SA, and SA-dependent CaPR1 and CaPR10 defense genes during avirulent Xcv infection. SA plays a pivotal role for plant inducible immunity (Du et al., 2009; Tsuda et al., 2009). Furthermore, CaADC1-silenced pepper leaves contained significantly lower levels of spermine and GABA. The signaling and metabolic changes that affect defense and cell death responses in CaADC1-silenced pepper leaves may be primarily due to the reduced expression of CaADC1. ADC functions as a rate-limiting factor that regulates PA levels in plants (Kasinathan and Wingler, 2004; Alcázar et al., 2005). PA catabolic processes directly produce H2O2 (Bagni and Tassoni, 2001), which is consistent with the reduced levels of H2O2 that were observed in CaADC1-silenced pepper leaves. It has been suggested that the H2O2 burst is linked to HR and the defense hormone SA accumulation (Durrant and Dong, 2004; Torres et al., 2006). More importantly, CaADC1 silencing significantly compromised avirulent Xcv Ds1 (avrBsT) proliferation in pepper leaves during infection. The increased Xcv Ds1 (avrBsT) growth in CaADC1-silenced pepper leaves supports the notion that CaADC1 interacts with AvrBsT and enhances AvrBsT-triggered cell death resistance, ultimately to the inhibition of Xcv Ds1 (avrBsT) proliferation in the incompatible interaction of pepper plants.
The AvrBsT-triggered cell death resistance conferred by CaADC1 expression may be, in part, related to GABA. During the avirulent Xcv Ds1 (avrBsT) infection, but not the Xcv Ds1 (EV) infection, CaADC1 silencing did not induce GABA accumulation compared with the empty vector control plants. As a result, CaADC1-silenced plants were more susceptible to avirulent Xcv Ds1 (avrBsT) but not to virulent Xcv Ds1 (EV). In addition, exogenous application of GABA significantly reduced avirulent Xcv Ds1 (avrBsT) growth in the empty vector control and CaADC1-silenced pepper leaves. Together, these results strongly suggest that the nonprotein amino acid GABA may contribute to AvrBsT-triggered cell death-like resistance against Xcv infection. As a remarkably versatile signaling molecule, GABA has been proposed to mediate communication between plants and other organisms, including bacterial and fungal pathogens, nematodes, and insect pests (Shelp et al., 2006). Accumulation of GABA in tobacco plants induces quenching of quorum-sensing in A. tumefaciens, thereby attenuating its virulence on plants (Chevrot et al., 2006). Mutations in GABA transaminase genes in plants or Pseudomonas syringae reduces bacterial virulence (Park et al., 2010), suggesting the role of GABA in plant defense response to pathogenic bacteria. There is evidence that GABA is linked to ROS generation and cell death in plants (Bouché et al., 2003). The GABA isomer, β-aminobutyric acid, has been shown to induce disease resistance in Arabidopsis against Alternaria brassicicola and Plectosphaerella cucumerina (Ton and Mauch-Mani, 2004). GABA is known to be accumulated in plants to counteract abiotic and biotic stresses (Choi et al., 2004; Lima et al., 2010). Like these previous findings, our data support the role of GABA as an active defense inducer in plants.
In summary, these data provide novel information regarding AvrBsT-triggered cell death in pepper plants that is positively regulated by CaADC1. We propose a working model based on these results in which CaADC1 plays a pivotal role in PA and GABA signaling in defense responses in plants (Supplemental Fig. S10). AvrBsT induces CaADC1 expression, interacts with CaADC1, and presumably activates CaADC1. Active CaADC1 catalyzes the synthesis of agmatine, which is further metabolized to produce putrescine (Bagni and Tassoni, 2001). Spermidine is synthesized from putrescine via the addition of aminopropyl groups to putrescine by spermidine synthase (Bagni and Tassoni, 2001; Walters, 2003b), followed by the production of H2O2 as a by-product. Spermine is synthesized via the addition of aminopropyl groups to spermidine by spermine synthase (Bagni and Tassoni, 2001), leading to the production of H2O2. In addition, spermine can directly trigger a NO burst. PAs also can be catabolized to pyrroline, which is further processed to form GABA (Flores and Filner, 1985). GABA is proposed to contribute to defense responses by affecting the ROS burst during pathogen infection (Bouché et al., 2003). The ROS and NO bursts induced by PAs may activate downstream signaling pathways, such as mitogen-activated protein kinase cascades (Wang et al., 2010). ROS are involved in SA signaling, which regulates cell death and defense responses in plants (Torres et al., 2006). In conclusion, these results suggest that CaADC1 may act as a key regulator of cell death and defense signaling and contribute to disease resistance by fine-tuning PA and GABA levels in pepper plants.
MATERIALS AND METHODS
Plant Materials and Pathogen Inoculation
Pepper (Capsicum annuum ‘Nockwang’) and Nicotiana benthamiana were grown in plastic pots (8 cm in diameter) containing a soil mix (loam soil:perlite:vermiculite, 3:1:1, v/v/v) at 28°C with long-day cycles (16 h of light and 8 h of dark) at a light intensity of 100 μmol photons m−2 s−1.
The Xanthomonas campestris pv vesicatoria virulent Ds1 (EV) and avirulent Ds1 (avrBsT) strains (Kim et al., 2010) were grown overnight in yeast nutrient medium (5 g L−1 yeast extract and 8 g L−1 nutrient broth), harvested, and then resuspended in 10 mm MgCl2 solution. Bacterial growth in leaves was monitored at 0 and 3 d after infiltration with Xcv (5 × 104 cfu mL−1) using a syringe (without needle).
Yeast Two-Hybrid Assays
The open reading frame of the Xcv type III effector protein gene avrBsT was cloned into the pGBKT7 (BamHI/HindIII sites) vector. A yeast prey library was generated from a pepper cDNA library by ligating cDNA inserts with the pGADT7 vector. The constructs were cotransformed into yeast strain AH109 and plated onto synthetic dropout His, Leu, Trp medium (Ito et al., 1983). Approximately 50,000 colonies grown on synthetic dropout His, Leu, Trp medium were transferred onto selection medium (synthetic dropout adenine, His, Leu, Trp medium). Plasmids were extracted from surviving yeast colonies and used to transform Escherichia coli. Colonies carrying the pGADT7 vector were selected on Luria-Bertani medium containing 100 mg L−1 ampicillin. Isolated plasmids were sequenced, and sequence homology was analyzed using GenBank BLAST tools.
BiFC Assay
The BiFC assay was conducted as described previously (Walter et al., 2004). To generate the BiFC constructs, cDNAs encoding AvrBsT and CaADC1 without termination codons were PCR amplified and subcloned into the binary vectors pSPYNE (XbaI/XhoI) and pSPYCE (XbaI/XhoI) vectors under the control of the cauliflower mosaic virus 35S promoter (for oligonucleotide sequences, see Supplemental Table S2). pSPYCE:CaADC1 K154A was generated using the QuickChange site-directed mutagenesis kit (Stratagene). AvrBsT and CaADC1 were coexpressed in Nicotiana benthamiana leaves by infiltrating Agrobacterium tumefaciens strain GV3101 carrying each construct (OD600 = 0.5). The association of AvrBsT and CaADC1 was visualized using a confocal laser scanning microscope (LSM 5 Exciter; Carl-Zeiss) operated with the LSM Imager at 40 h after transformation.
VIGS
TRV-based VIGS was used to generate CaADC1 knockdown pepper plants (Liu et al., 2002; Choi et al., 2007). The unconserved 3′ region of the CaADC1 untranslated region was PCR amplified using the primers listed in Supplemental Table S2. The resulting fragment was cloned into pCR2.1/TOPO, digested with EcoRI, and inserted into pTRV2 (EcoRI/EcoRI). The fully expanded cotyledons of pepper plants were coinfiltrated with A. tumefaciens strain GV3101 carrying the VIGS vectors pTRV1 and pTRV2 or pTRV2:CaADC1 (OD600 = 0.2). The efficacy of CaADC1 silencing in pepper plants was examined by RT-PCR after Xcv inoculation at the six-leaf stage.
RNA Gel-Blot and Real-Time RT-PCR Analyses
Total RNA was extracted from pepper plants using Trizol reagent (Invitrogen). RNA was resolved by agarose gel electrophoresis, transferred onto Hybond N+ membranes (GE Healthcare), and hybridized overnight with 32P-labeled CaADC1 cDNA.
For real-time RT-PCR, 2 μg of RNA were used in a RT reaction with Moloney murine leukemia virus reverse transcriptase (Enzynomics). Real-time PCR was performed using iQ SYBR Green Supermix and iCycler iQ (Bio-Rad). The 18S ribosomal RNA transcript level was used to normalize the transcript level of each gene. Relative expression levels were determined by comparing the values with that of the uninoculated control.
Coimmunoprecipitation and Immunoblot Analyses
For coimmunoprecipitation constructs, avrBsT and CaADC1 were PCR amplified using the primers listed in Supplemental Table S2. The fragments were digested with XbaI/XhoI and ethanol precipitated. Digested avrBsT and CaADC1 fragment was recombined into p35S:8xMyc and p35S:6xHA, respectively (Choi et al., 2012). A. tumefaciens strain GV3101 harboring the constructs was coinfiltrated into N. benthamiana leaves. Proteins were extracted from leaf samples using extraction buffer (50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 10 mm EDTA, 0.2% (v/v) Triton X-100, and 2× complete protease inhibitor cocktail [Roche]). The protein extracts were incubated with monoclonal anti-HA or anti-Myc agarose (Sigma-Aldrich) overnight. Beads were collected and washed with immunoprecipitation buffer (50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 10 mm EDTA, and 2× complete protease inhibitor cocktail [Roche]). Eluted proteins were analyzed by immunoblotting using monoclonal anti-HA-peroxidase antibody (Sigma-Aldrich) or polyclonal anti-cMyc-peroxidase antibody (Sigma-Aldrich). Immunodetection was performed using the ECL Prime Western Blotting Detection Reagent (GE Healthcare).
Ion Leakage Assay
Leaves of N. benthamiana or pepper plants were harvested at various time points after infiltration with A. tumefaciens or Xcv. Leaf discs (0.5 cm in diameter) were removed with a cork borer and washed in 10 mL of sterile double-distilled water for 30 min with gentle agitation. Washed leaf discs were transferred to 20 mL of sterile double-distilled water and incubated for 2 h at room temperature with gentle agitation. The conductivity of the leaf samples was measured using a Sension 7 conductivity meter (HACH). Experiments were carried out three times with similar results.
SA Measurement
SA levels in pepper leaves were determined as described previously (Choi and Hwang, 2011). Leaf tissues (0.5 g) were homogenized in liquid nitrogen and extracted in 1 mL of 90% (v/v) methanol. The leaf extracts were then centrifuged at 15,000g for 5 min. The resulting pellet was reextracted with 100% methanol. The supernatant fractions were then pooled and vacuum dried. The residue was resuspended in 1 mL of 5% (v/v) TCA. Organic extraction of free SA was performed following the addition of 1 mL of ethyl acetate:cyclopentane:isopropanol (50:50:1). The aqueous phase was then reextracted with ethyl acetate:cyclopentane:isopropanol, and the two organic phases were pooled and vacuum dried. The pellet was resuspended in 1 mL of 100% methanol. The suspension was centrifuged at 15,000g for 2 min and filtered through a 0.2-μm Acrodisc syringe filter (PALL). The aqueous phase containing the SA glucoside fraction was acidified with HCl to pH 1 and boiled for 30 min to release SA from any acid-labile conjugated forms. The released SA was extracted with ethyl acetate:cyclopentane:isopropanol, vacuum dried, and resuspended in 1 mL of 100% methanol. 3-Hydroxybenzoic acid (50 μg per leaf sample) was used as an internal standard. SA in the extracts was analyzed by reverse-phase HPLC in a Waters 515 system using a C18 column. SA in the eluates was detected by passage through a fluorescence detector (excitation at 305 nm and emission at 405 nm).
Histochemical Analyses
H2O2 production was visualized by placing healthy or inoculated leaves in 1 mg mL−1 DAB (Sigma-Aldrich) solution for 15 h (Thordal-Christensen et al., 1997). Chlorophyll was cleared from the stained leaves by boiling in 95% (v/v) ethanol. Cell death was monitored by trypan blue staining of healthy or inoculated leaves (Koch and Slusarenko, 1990). Leaves were stained with lactophenol-trypan blue solution (10 mL of lactic acid, 10 mL of glycerol, 10 g of phenol, and 10 mg of trypan blue, dissolved in 10 mL of distilled water) and destained in 2.5 g mL−1 chloral hydrate solution. The samples were photographed using a light microscope mounted with a digital camera (Olympus). Chlorosis, cell death, or phenolic compound accumulation in leaves was visualized using a hand-held UV lamp (UVP).
PA Measurement
PA accumulation was monitored using a gas chromatography-mass spectrometry method as described previously (Chen et al., 2009). Briefly, 100-mg leaf samples were homogenized and extracted with 1 mL of 10% (v/v) NaCl (pH 1) and centrifuged at 12,000g for 15 min. The resulting supernatants were extracted with 3 mL of diethyl ether by vortexing for 10 min and centrifuged at 12,000g for 20 min. Aliquots (0.5 mL) of the samples were adjusted to pH 10 with 5 m NaOH and mixed with 1 mL of diethyl ether containing 50 μL of ethyl chloroformate by shaking for 30 min at room temperature. The mixture was centrifuged at 12,000g for 5 min, and the ether layer containing the PA N-ethoxylcarbonyl derivatives was transferred to a separate glass vial and dried under a nitrogen stream. Ethyl acetate (100 μL) was added to the dried N-ethoxylcarbonyl PA derivatives, mixed with 200 μL of trifluoroacetic acid anhydride, and incubated at 75°C for 1 h. The mixture was dried completely, and the PA derivatives were reconstituted in 200 μL of ethyl acetate and analyzed by gas chromatography-mass spectrometry (7890A GC/5975C MSD; Agilent).
NO Measurement
NO accumulation was monitored using the NO-sensitive dye DAF-2DA (Sigma-Aldrich) as described previously (Asai et al., 2008). For NO detection, leaves were infiltrated with 200 mm sodium phosphate buffer (pH 7.4) supplemented with 12.5 μm DAF-2DA and then incubated for 1 h in the dark at room temperature. As a positive control, SNP (500 μm) was infiltrated into leaves with DAF-2DA. The fluorescent products of the reaction between DAF-2DA and NO were observed using a confocal laser scanning microscope with excitation at 470 nm. Emission images at 525 nm were captured using a constant acquisition time, and fluorescence intensity was determined via color histogram analysis.
H2O2 Measurement
H2O2 accumulation in leaves was quantified using the xylenol orange method (Gay et al., 1999; Choi and Hwang, 2011). Xylenol orange assay reagent was freshly prepared by adding 500 μL of reagent [25 mm FeSO4 and 25 mm (NH4)2SO4 in 2.5 m H2SO4] to 50 mL of 125 μm xylenol orange in 100 mm sorbitol. Eight leaf discs (0.5 cm2) were floated on 1 mL of distilled water in a microtube for 1 h, centrifuged for 1 min at 12,000g, and 100 μL of supernatant was immediately added to 1 mL of xylenol orange assay reagent. The mixture was incubated for 30 min at room temperature. A standard curve for H2O2 was generated from measurements obtained from a serial dilution of 100 nmol to 100 μmol of H2O2. H2O2 was quantified by measuring the A560 using a DU 650 spectrophotometer (Beckman).
Amino Acid Analysis
Amino acids were extracted from pepper leaves with 3% (v/v) 5-SA in 70% (v/v) ethanol as described previously (Hwang et al., 2011). Briefly, leaf extracts were centrifuged at 13,000g for 15 min. The pellet was reextracted, and supernatants were pooled. Detection and quantification of amino acids were performed using the PICO-Tag system (Waters; http://www.waters.com). Free amino acids were separated on a Nova-Pak C18 column (4 μm, 3.9 × 3,300 mm; Waters) at 46°C. The sample was injected and eluted with a linear gradient of 0% to 100% (v/v) acetonitrile. Fluorescence was monitored using an HP 1100 Series (Agilent; http://www.chem.agilent.com/) at 254 nm. l-nor-Leu (Sigma-Aldrich; http://www.sigmaaldrich.com/), an amino acid that is not commonly found in proteins, was used as an internal standard.
Sequence data from this article can be found in the GenBank/EMBL data libraries under the following accession numbers: Xcv avrBsT (GQ266402), pepper CaADC1 (KC160547), pepper CaPR1 (AF053343), pepper CaPR10 (AF244121), pepper CaDEF1 (AF442388), tobacco ADC (AAQ14851), pea (Pisum sativum) ADC (CAA85773), Arabidopsis ADC1 (NP_179243), and Arabidopsis ADC2 (NP_195197).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Nucleotide and deduced amino acid sequences of CaADC1 cDNA.
Supplemental Figure S2. Alignment of CaADC1 with other ADCs.
Supplemental Figure S3. RNA gel-blot analyses of CaADC1 expression in pepper plants.
Supplemental Figure S4. Coexpression of CaADC1 does not promote BAX-triggered cell death in N. benthamiana leaves.
Supplemental Figure S5. CaADC1 forms a complex with the AvrBsT C222A mutant but does not trigger cell death-inducing activity in AvrBsT C222A.
Supplemental Figure S6. Effects of transient expression of CaADC1 and AvrBsT on amino acid levels in N. benthamiana.
Supplemental Figure S7. Disease symptoms in empty vector control (TRV:00) and CaADC1-silenced (TRV:CaADC1) leaves infiltrated with Xcv Ds1 (EV) and Ds1 (avrBsT).
Supplemental Figure S8. Effects of CaADC1 silencing on amino acid levels of pepper leaves infected with Xcv.
Supplemental Figure S9. GABA and Ala do not inhibit Xcv growth in yeast nutrient media.
Supplemental Figure S10. Working model for the role of CaADC1 in signaling mediated by PAs and GABA in cell death and defense responses in plants.
Supplemental Table S1. Gene-specific primers for quantitative RT-PCR analyses.
Supplemental Table S2. Primers for the generation of gene constructs.
Glossary
- ROS
reactive oxygen species
- PA
polyamine
- ODC
ornithine decarboxylase
- ADC
arginine decarboxylase
- GABA
γ-aminobutyric acid
- HR
hypersensitive response
- Xcv
Xanthomonas campestris pv vesicatoria
- cDNA
complementary DNA
- BiFC
bimolecular fluorescence complementation
- YFP
yellow fluorescent protein
- HA
hemagglutinin
- SA
salicylic acid
- OD600
optical density at 600 nm
- NO
nitric oxide
- DAF-2DA
4,5-diaminofluorescein diacetate
- SNP
sodium nitroprusside
- DAB
3,3′-diaminobenzidine
- TRV
tobacco rattle virus
- VIGS
virus-induced gene silencing
- cfu
colony-forming units
- RT
reverse transcription
- H2O2
hydrogen peroxide
References
- Alcázar R, García-Martínez JL, Cuevas JC, Tiburcio AF, Altabella T. (2005) Overexpression of ADC2 in Arabidopsis induces dwarfism and late-flowering through GA deficiency. Plant J 43: 425–436 [DOI] [PubMed] [Google Scholar]
- Asai S, Ohta K, Yoshioka H. (2008) MAPK signaling regulates nitric oxide and NADPH oxidase-dependent oxidative bursts in Nicotiana benthamiana. Plant Cell 20: 1390–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, Gomez-Gomez L, Boller T, Ausubel FM, Sheen J. (2002) MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 415: 977–983 [DOI] [PubMed] [Google Scholar]
- Averill-Bates DA, Ke Q, Tanel A, Roy J, Fortier G, Agostinelli E. (2008) Mechanism of cell death induced by spermine and amine oxidase in mouse melanoma cells. Int J Oncol 32: 79–88 [PubMed] [Google Scholar]
- Bagni N, Tassoni A. (2001) Biosynthesis, oxidation and conjugation of aliphatic polyamines in higher plants. Amino Acids 20: 301–317 [DOI] [PubMed] [Google Scholar]
- Berger S, Sinha AK, Roitsch T. (2007) Plant physiology meets phytopathology: plant primary metabolism and plant-pathogen interactions. J Exp Bot 58: 4019–4026 [DOI] [PubMed] [Google Scholar]
- Besson-Bard A, Pugin A, Wendehenne D. (2008) New insights into nitric oxide signaling in plants. Annu Rev Plant Biol 59: 21–39 [DOI] [PubMed] [Google Scholar]
- Bhat R, Axtell R, Mitra A, Miranda M, Lock C, Tsien RW, Steinman L. (2010) Inhibitory role for GABA in autoimmune inflammation. Proc Natl Acad Sci USA 107: 2580–2585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouché N, Fait A, Bouchez D, Møller SG, Fromm H. (2003) Mitochondrial succinic-semialdehyde dehydrogenase of the gamma-aminobutyrate shunt is required to restrict levels of reactive oxygen intermediates in plants. Proc Natl Acad Sci USA 100: 6843–6848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouché N, Fromm H. (2004) GABA in plants: just a metabolite? Trends Plant Sci 9: 110–115 [DOI] [PubMed] [Google Scholar]
- Bouchereau A, Aziz A, Larher F, Martin-Tanguy J. (1999) Polyamines and environmental challenges: recent development. Plant Sci 140: 103–125 [Google Scholar]
- Capell T, Bassie L, Christou P. (2004) Modulation of the polyamine biosynthetic pathway in transgenic rice confers tolerance to drought stress. Proc Natl Acad Sci USA 101: 9909–9914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GG, Turecki G, Mamer OA. (2009) A quantitative GC-MS method for three major polyamines in postmortem brain cortex. J Mass Spectrom 44: 1203–1210 [DOI] [PubMed] [Google Scholar]
- Chevrot R, Rosen R, Haudecoeur E, Cirou A, Shelp BJ, Ron E, Faure D. (2006) GABA controls the level of quorum-sensing signal in Agrobacterium tumefaciens. Proc Natl Acad Sci USA 103: 7460–7464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HW, Kim YJ, Lee SC, Hong JK, Hwang BK. (2007) Hydrogen peroxide generation by the pepper extracellular peroxidase CaPO2 activates local and systemic cell death and defense response to bacterial pathogens. Plant Physiol 145: 890–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S, Hwang BK. (2011) Proteomics and functional analyses of pepper abscisic acid-responsive 1 (ABR1), which is involved in cell death and defense signaling. Plant Cell 23: 823–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S, Hwang IS, Hwang BK. (2012) Requirement of the cytosolic interaction between PATHOGENESIS-RELATED PROTEIN10 and LEUCINE-RICH REPEAT PROTEIN1 for cell death and defense signaling in pepper. Plant Cell 24: 1675–1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YH, Tapias EC, Kim HK, Lefeber AWM, Erkelens C, Verhoeven JTJ, Brzin J, Zel J, Verpoorte R. (2004) Metabolic discrimination of Catharanthus roseus leaves infected by phytoplasma using 1H-NMR spectroscopy and multivariate data analysis. Plant Physiol 135: 2398–2410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen E, Arad SM, Heimer YM, Mizrahi Y. (1983) Polyamine biosynthetic enzymes in Chlorella: characterization of ornithine and arginine decarboxylase. Plant Cell Physiol 24: 1003–1010 [Google Scholar]
- Cowley T, Walters DR. (2002) Polyamine metabolism in barley reacting hypersensitively to the powdery mildew fungus Blumeria graminis f.sp hordei. Plant Cell Environ 25: 461–468 [Google Scholar]
- Cunnac S, Wilson A, Nuwer J, Kirik A, Baranage G, Mudgett MB. (2007) A conserved carboxylesterase is a SUPPRESSOR OF AVRBST-ELICITED RESISTANCE in Arabidopsis. Plant Cell 19: 688–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeb F, van der Weele CM, Wolniak SM. (2010) Spermidine is a morphogenetic determinant for cell fate specification in the male gametophyte of the water fern Marsilea vestita. Plant Cell 22: 3678–3691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Pozo O, Pedley KF, Martin GB. (2004) MAPKKKalpha is a positive regulator of cell death associated with both plant immunity and disease. EMBO J 23: 3072–3082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vera N, Martínez E, Sanfeliu C. (2008) Spermine induces cell death in cultured human embryonic cerebral cortical neurons through N-methyl-D-aspartate receptor activation. J Neurosci Res 86: 861–872 [DOI] [PubMed] [Google Scholar]
- Du LQ, Ali GS, Simons KA, Hou JG, Yang TB, Reddy ASN, Poovaiah BW. (2009) Ca2+/calmodulin regulates salicylic-acid-mediated plant immunity. Nature 457: 1154–1158 [DOI] [PubMed] [Google Scholar]
- Durrant WE, Dong X. (2004) Systemic acquired resistance. Annu Rev Phytopathol 42: 185–209 [DOI] [PubMed] [Google Scholar]
- Eitas TK, Dangl JL. (2010) NB-LRR proteins: pairs, pieces, perception, partners, and pathways. Curr Opin Plant Biol 13: 472–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escolar L, Van Den Ackerveken G, Pieplow S, Rossier O, Bonas U. (2001) Type III secretion and in planta recognition of the Xanthomonas avirulence proteins AvrBs1 and AvrBsT. Mol Plant Pathol 2: 287–296 [DOI] [PubMed] [Google Scholar]
- Flores HE, Filner P. (1985) Polyamine catabolism in higher plants: characterization of pyrroline dehydrogenase. Plant Growth Regul 3: 277–291 [Google Scholar]
- Garcia-Brugger A, Lamotte O, Vandelle E, Bourque S, Lecourieux D, Poinssot B, Wendehenne D, Pugin A. (2006) Early signaling events induced by elicitors of plant defenses. Mol Plant Microbe Interact 19: 711–724 [DOI] [PubMed] [Google Scholar]
- Gay C, Collins J, Gebicki JM. (1999) Hydroperoxide assay with the ferric-xylenol orange complex. Anal Biochem 273: 149–155 [DOI] [PubMed] [Google Scholar]
- Gonzalez ME, Marco F, Minguet EG, Carrasco-Sorli P, Blázquez MA, Carbonell J, Ruiz OA, Pieckenstain FL. (2011) Perturbation of spermine synthase gene expression and transcript profiling provide new insights on the role of the tetraamine spermine in Arabidopsis defense against Pseudomonas viridiflava. Plant Physiol 156: 2266–2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanfrey C, Sommer S, Mayer MJ, Burtin D, Michael AJ. (2001) Arabidopsis polyamine biosynthesis: absence of ornithine decarboxylase and the mechanism of arginine decarboxylase activity. Plant J 27: 551–560 [DOI] [PubMed] [Google Scholar]
- Haudecoeur E, Planamente S, Cirou A, Tannieres M, Shelp BJ, Morera S, Faure D. (2009) Proline antagonizes GABA-induced quenching of quorum-sensing in Agrobacterium tumefaciens. Proc Natl Acad Sci USA 106: 14587–14592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang IS, An SH, Hwang BK. (2011) Pepper asparagine synthetase 1 (CaAS1) is required for plant nitrogen assimilation and defense responses to microbial pathogens. Plant J 67: 749–762 [DOI] [PubMed] [Google Scholar]
- Ito H, Fukuda Y, Murata K, Kimura A. (1983) Transformation of intact yeast cells treated with alkali cations. J Bacteriol 153: 163–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowitz T, Kneifel H, Piotrowski M. (2003) Identification and characterization of plant agmatine iminohydrolase, the last missing link in polyamine biosynthesis of plants. FEBS Lett 544: 258–261 [DOI] [PubMed] [Google Scholar]
- Kasinathan V, Wingler A. (2004) Effect of reduced arginine decarboxylase activity on salt tolerance and on polyamine formation during salt stress in Arabidopsis thaliana. Physiol Plant 121: 101–107 [DOI] [PubMed] [Google Scholar]
- Kim NH (2012) Molecular, immunocytological and metabolic studies of the AvrBsT, AvrBsT-SGT1-PIK1 complex, pepper arginine decarboxylase, heat shock protein 70, aldehyde dehydrogenase and protease inhibitor in plant cell death and defense responses to microbial pathogens. PhD thesis. Korea University, Seoul, Korea [Google Scholar]
- Kim NH, Choi HW, Hwang BK. (2010) Xanthomonas campestris pv. vesicatoria effector AvrBsT induces cell death in pepper, but suppresses defense responses in tomato. Mol Plant Microbe Interact 23: 1069–1082 [DOI] [PubMed] [Google Scholar]
- Kirik A, Mudgett MB. (2009) SOBER1 phospholipase activity suppresses phosphatidic acid accumulation and plant immunity in response to bacterial effector AvrBsT. Proc Natl Acad Sci USA 106: 20532–20537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klessig DF, Durner J, Noad R, Navarre DA, Wendehenne D, Kumar D, Zhou JM, Shah J, Zhang S, Kachroo P, et al. (2000) Nitric oxide and salicylic acid signaling in plant defense. Proc Natl Acad Sci USA 97: 8849–8855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch E, Slusarenko A. (1990) Arabidopsis is susceptible to infection by a downy mildew fungus. Plant Cell 2: 437–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumria R, Rajam MV. (2002) Ornithine decarboxylase transgene in tobacco affects polyamines, in vitro morphogenesis and response to salt stress. J Plant Physiol 159: 983–990 [Google Scholar]
- Lacomme C, Santa Cruz S. (1999) Bax-induced cell death in tobacco is similar to the hypersensitive response. Proc Natl Acad Sci USA 96: 7956–7961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb C, Dixon RA. (1997) The oxidative burst in plant disease resistance. Annu Rev Plant Physiol Plant Mol Biol 48: 251–275 [DOI] [PubMed] [Google Scholar]
- Lima MRM, Felgueiras ML, Graça G, Rodrigues JEA, Barros A, Gil AM, Dias ACP. (2010) NMR metabolomics of esca disease-affected Vitis vinifera cv. Alvarinho leaves. J Exp Bot 61: 4033–4042 [DOI] [PubMed] [Google Scholar]
- Liu Y, Schiff M, Dinesh-Kumar SP. (2002) Virus-induced gene silencing in tomato. Plant J 31: 777–786 [DOI] [PubMed] [Google Scholar]
- Marina M, Maiale SJ, Rossi FR, Romero MF, Rivas EI, Gárriz A, Ruiz OA, Pieckenstain FL. (2008) Apoplastic polyamine oxidation plays different roles in local responses of tobacco to infection by the necrotrophic fungus Sclerotinia sclerotiorum and the biotrophic bacterium Pseudomonas viridiflava. Plant Physiol 147: 2164–2178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Tanguy J. (2001) Metabolism and function of polyamines in plants: recent development (new approaches). Plant Growth Regul 34: 135–148 [Google Scholar]
- Mitsuya Y, Takahashi Y, Berberich T, Miyazaki A, Matsumura H, Takahashi H, Terauchi R, Kusano T. (2009) Spermine signaling plays a significant role in the defense response of Arabidopsis thaliana to cucumber mosaic virus. J Plant Physiol 166: 626–643 [DOI] [PubMed] [Google Scholar]
- Moreau M, Lindermayr C, Durner J, Klessig DF. (2010) NO synthesis and signaling in plants: where do we stand? Physiol Plant 138: 372–383 [DOI] [PubMed] [Google Scholar]
- Moschou PN, Paschalidis KA, Delis ID, Andriopoulou AH, Lagiotis GD, Yakoumakis DI, Roubelakis-Angelakis KA. (2008) Spermidine exodus and oxidation in the apoplast induced by abiotic stress is responsible for H2O2 signatures that direct tolerance responses in tobacco. Plant Cell 20: 1708–1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth K, Xu Z, Mudgett MB, Bao ZQ, Palmer LE, Bliska JB, Mangel WF, Staskawicz B, Dixon JE. (2000) Disruption of signaling by Yersinia effector YopJ, a ubiquitin-like protein protease. Science 290: 1594–1597 [DOI] [PubMed] [Google Scholar]
- Park DH, Mirabella R, Bronstein PA, Preston GM, Haring MA, Lim CK, Collmer A, Schuurink RC. (2010) Mutations in γ-aminobutyric acid (GABA) transaminase genes in plants or Pseudomonas syringae reduce bacterial virulence. Plant J 64: 318–330 [DOI] [PubMed] [Google Scholar]
- Piotrowski M, Janowitz T, Kneifel H. (2003) Plant C-N hydrolases and the identification of a plant N-carbamoylputrescine amidohydrolase involved in polyamine biosynthesis. J Biol Chem 278: 1708–1712 [DOI] [PubMed] [Google Scholar]
- Roberts MR. (2007) Does GABA act as a signal in plants? Plant Signal Behav 2: 408–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockel P, Strube F, Rockel A, Wildt J, Kaiser WM. (2002) Regulation of nitric oxide (NO) production by plant nitrate reductase in vivo and in vitro. J Exp Bot 53: 103–110 [PubMed] [Google Scholar]
- Rosales EP, Iannone MF, Groppa MD, Benavides MP. (2012) Polyamines modulate nitrate reductase activity in wheat leaves: involvement of nitric oxide. Amino Acids 42: 857–865 [DOI] [PubMed] [Google Scholar]
- Shelp BJ, Bown AW, Faure D. (2006) Extracellular gamma-aminobutyrate mediates communication between plants and other organisms. Plant Physiol 142: 1350–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skopelitis DS, Paranychianakis NV, Paschalidis KA, Pliakonis ED, Delis ID, Yakoumakis DI, Kouvarakis A, Papadakis AK, Stephanou EG, Roubelakis-Angelakis KA. (2006) Abiotic stress generates ROS that signal expression of anionic glutamate dehydrogenases to form glutamate for proline synthesis in tobacco and grapevine. Plant Cell 18: 2767–2781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczesny R, Büttner D, Escolar L, Schulze S, Seiferth A, Bonas U. (2010) Suppression of the AvrBs1-specific hypersensitive response by the YopJ effector homolog AvrBsT from Xanthomonas depends on a SNF1-related kinase. New Phytol 187: 1058–1074 [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Berberich T, Miyazaki A, Seo S, Ohashi Y, Kusano T. (2003) Spermine signalling in tobacco: activation of mitogen-activated protein kinases by spermine is mediated through mitochondrial dysfunction. Plant J 36: 820–829 [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Uehara Y, Berberich T, Ito A, Saitoh H, Miyazaki A, Terauchi R, Kusano T. (2004) A subset of hypersensitive response marker genes, including HSR203J, is the downstream target of a spermine signal transduction pathway in tobacco. Plant J 40: 586–595 [DOI] [PubMed] [Google Scholar]
- Thordal Christensen H, Zhang Z, Wei Y, Collinge DB. (1997) Subcellular localization of H2O2 in plants: H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J 11: 1187–1194 [Google Scholar]
- Ton J, Mauch-Mani B. (2004) β-Amino-butyric acid-induced resistance against necrotrophic pathogens is based on ABA-dependent priming for callose. Plant J 38: 119–130 [DOI] [PubMed] [Google Scholar]
- Torres MA, Jones JDG, Dangl JL. (2006) Reactive oxygen species signaling in response to pathogens. Plant Physiol 141: 373–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda K, Sato M, Stoddard T, Glazebrook J, Katagiri F. (2009) Network properties of robust immunity in plants. PLoS Genet 5: e1000772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Breusegem F, Dat JF. (2006) Reactive oxygen species in plant cell death. Plant Physiol 141: 384–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walden R, Cordeiro A, Tiburcio AF. (1997) Polyamines: small molecules triggering pathways in plant growth and development. Plant Physiol 113: 1009–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter M, Chaban C, Schütze K, Batistic O, Weckermann K, Näke C, Blazevic D, Grefen C, Schumacher K, Oecking C, et al. (2004) Visualization of protein interactions in living plant cells using bimolecular fluorescence complementation. Plant J 40: 428–438 [DOI] [PubMed] [Google Scholar]
- Walters DR. (2003a) Resistance to plant pathogens: possible roles for free polyamines and polyamine catabolism. New Phytol 159: 109–115 [DOI] [PubMed] [Google Scholar]
- Walters DR. (2003b) Polyamines and plant disease. Phytochemistry 64: 97–107 [DOI] [PubMed] [Google Scholar]
- Wang PC, Du YY, Li YA, Ren DT, Song CP. (2010) Hydrogen peroxide-mediated activation of MAP kinase 6 modulates nitric oxide biosynthesis and signal transduction in Arabidopsis. Plant Cell 22: 2981–2998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilms I, Voss B, Hess WR, Leichert LI, Narberhaus F. (2011) Small RNA-mediated control of the Agrobacterium tumefaciens GABA binding protein. Mol Microbiol 80: 492–506 [DOI] [PubMed] [Google Scholar]
- Wimalasekera R, Tebartz F, Scherer GF. (2011) Polyamines, polyamine oxidases and nitric oxide in development, abiotic and biotic stresses. Plant Sci 181: 593–603 [DOI] [PubMed] [Google Scholar]
- Yamasaki H, Cohen MF. (2006) NO signal at the crossroads: polyamine-induced nitric oxide synthesis in plants? Trends Plant Sci 11: 522–524 [DOI] [PubMed] [Google Scholar]
- Yun BW, Feechan A, Yin MH, Saidi NBB, Le Bihan T, Yu M, Moore JW, Kang JG, Kwon E, Spoel SH, et al. (2011) S-Nitrosylation of NADPH oxidase regulates cell death in plant immunity. Nature 478: 264–268 [DOI] [PubMed] [Google Scholar]