Abstract
Introduction
Greater adiposity has been linked to an increased risk and/or poorer survival in a variety of cancers. We examined whether prediagnostic body weight 1–5 years prior to diagnosis is associated with survival in patients with high grade glioma.
Methods
The analysis was based on a series of patients with high-grade glioma (N=853) enrolled in a US-based multicenter case-control study. Subjects reported height and weight 1–5 years prior to interview and at age 21. BMI was categorized according to WHO criteria as underweight (BMI<18.5kg/m2), normal weight (BMI 18.5–24.9kg/m2), overweight (BMI 25–29.9kg/m2) and obese (BMI≥30kg/m2). Proportional hazards regression was used to estimate hazard ratios (HR) and 95% confidence intervals (CIs) for glioma-related death according to body mass index (BMI, kg/m2).
Results
Overall survival was reduced among patients underweight (median survival: 12.0 months) or obese (median: 13.6 months) when compared to patients of normal weight (median: 17.5 months) prior to glioma diagnosis (p=0.004). In a multivariate model controlling for other prognostic factors, an excess mortality was observed in patients reporting obese body weights 1–5 years prior to study interview when compared to patients with a normal BMI (HR=1.32; 95% CI:1.04–1.68). Consistent patterns of association with excess body weight were observed in men and women, and all findings were similar regardless of treatment for glioma. A lower than optimal body weight was associated with a nonsignificant excess mortality in multivariate analysis.
Conclusions
Premorbid obesity was significantly associated with a poor patient outcome independent of treatment and established prognostic factors. Excess body weight may be an adverse prognostic factor in glioma, a relationship observed across a spectrum of cancer types. The current findings linking prediagnostic body weight with mortality in high-grade glioma warrant further research.
Keywords: glioma, glioblastoma, BMI, survival, prediagnostic body weight
Introduction
Brain tumors are relatively rare tumors, with an estimated 23,910 men and women diagnosed with brain or nervous system cancers in 2012 and 13,700 cancer related deaths [1]. Despite advances in treatment, glioma remains one of the most aggressive human tumors with a median survival time of 12–15 months [2]. Factors associated with patient outcome include age at diagnosis, Karnofsky Performance Status (KPS) and treatment [2]. The influence of lifestyle-related factors on the outcome of glioma is poorly studied.
Obesity, an increasing public health concern in the US, is emerging as an important prognostic factor in cancer [3–6], with a higher BMI associated with an adverse outcome in breast [4], colon [5] and prostate cancer [6]. In contrast, the relationship of body fat mass to glioma outcome has not been determined. Two studies examined body weight in relation to survival in glioma [7, 8], one suggesting no significant association [7] and the other demonstrating higher death rates in patients with an excess body weight [8]. As body weight was measured after the diagnosis of glioma, weight gain from treatment (eg. steroids) and weight loss from disease progression may have affected inferences from these studies [9].
Using data from a recently completed US-based case-control study of glioma [10] in which information was collected from subjects on body weight throughout the lifespan, we examined whether body weight in the years immediately preceding glioma diagnosis is associated with survival in patients with high-grade tumors.
Subjects and Methods
Study Population
Subjects in the analysis were enrolled in a clinic-based case-control study examining risk factors for glioma [11]. All individuals were aged 25 or older and had a recent (within 3 months) primary diagnosis of high-grade glioma. Patients were identified in neurosurgery and neuro-oncology clinics in the Southeastern US including Vanderbilt University Medical Center (Nashville, TN); Moffitt Cancer Center (Tampa, FL); University of Alabama (Birmingham, AL); Emory University (Atlanta, GA), and Kentuckiana Cancer Institute (Louisville, KY) [11]. Subjects were enrolled between February 2005 and March 2012 and interviewed a median of 1.3 months following glioma diagnosis (interquartile range: 3 weeks-2.8 months). The study was approved by Investigational Review Committees at each participating center, and all participants provided written informed consent.
Data Collection
Demographic data and information on known and suspected glioma risk factors were collected through interviewer-administered questionnaires [11]. In the interview, subjects were asked to report their height and weight at age 21 and weight approximately 5-years before the interview (N=750) (or 1-year before interview in the study pilot phase (n=103)). Subjects also reported their lowest weight (excluding periods of brief illness) and highest weight (excluding pregnancy among women). Clinical prognostic data retrieved from medical records included patient age, gender, KPS, and treatment information. A total of 615 patients underwent full tumor resection, followed by concurrent radiation and temozolomide (TMZ) eg. the current accepted standard of care (SOC) for high grade glioma [2]. Among the remaining 238 patients not undergoing SOC, 192 (81%) had a biopsy only in combination with TMZ and/or radiation, 41 (17%) had full resection but no radiation and/or TMZ, and 5 (2%) could not be classified according to SOC treatment status due to missing information. Patients were followed for vital status and cause of death through the cancer registries at individual institutions referring cases to the study.
Statistical analysis
Overall survival (OS) was calculated as the number of months from diagnosis to date of death or last contact. BMI was categorized according to WHO criteria as underweight (BMI<18.5kg/m2), normal weight (BMI 18.5–24.9kg/m2), overweight (BMI 25–29.9kg/m2), obese (BMI≥30kg/m2) or morbidly obese (BMI≥40kg/m2) [5, 12]. Survival times within BMI categories were estimated using the Kaplan-Meier method and differences were tested using the log-rank test. Cox proportional hazards regression was used to estimate age-adjusted overall and gender-specific hazard ratios (HR) and 95% confidence intervals (CI) according to recent BMI. Multivariate models were adjusted for age, KPS, SOC treatment and, in an overall model, gender. We further examined whether BMI at age 21, lowest and highest adult body weight, and weight change from age 21 to prediagnostic body weight were associated with glioma survival. Associations were examined among all glioma patients (N=853) and, separately, among those treated with the current SOC (N=615). Statistical analysis was performed using SAS Version 9.3 (SAS Institute, Inc., Cary, NC). Kaplan-Meier curves were generated using R version 2.12.2. A P-value <0.05 was considered statistically significant, and all statistical tests were two-sided.
Results
Characteristics of the study population are shown in Table 1. The mean age at diagnosis was 57 years (range: 25–92). Males comprised 60% of the series and the majority of patients were Caucasian (95%). The most common histological diagnoses were glioblastoma multiforme (GBM; n=674, 79%) and anaplastic astrocytoma (AA; n=117, 14%). A total of 605 deaths were documented, all glioma-related, a median of 12.4 months following the diagnosis (range: 0.6 months 6.3 years). The 248 surviving patients were followed a median of 19 months (1.9 months 6.8 years). The mean BMI 1–5 years prior to diagnosis was 28.2±4.6kg/m2 in men and 26.7±6.0kg/m2 in women, with 1.5% (N=13) of subjects considered underweight and 28.3% (N=241) considered obese (Table 1).
Table 1.
Participant characteristics of 853 patients with primary glioma
| Number (%) | |
|---|---|
| Glioma Subtype | |
| High Grade Glioma, NOS | 4 (0.5%) |
| Grade III Mixed Oligodendroglioma | 17 (2.0%) |
| Grade III Pure Oligodendroglioma | 41 (4.8%) |
| Grade III Anaplastic astrocytoma | 117 (13.7%) |
| Grade IV Glioblastoma | 674 (79.0%) |
| Age at diagnosis, years | |
| Mean (SD) | 57 (13.3) |
| Range | 25 – 92 |
| Gender | |
| Male | 506 (59.3%) |
| Female | 347 (40.7%) |
| Race | |
| Caucasian | 804 (94.3%) |
| African American | 43 (5.0%) |
| Asian | 6 (0.7%) |
| Diabetes | |
| Yes | 114 (13.5%) |
| No | 728 (86.5%) |
| KPS | |
| >90 | 98 (13.7%) |
| 70 – 90 | 558 (77.9%) |
| <70 | 60 (8.4%) |
| Standard of Care Treatment | |
| Yes | 615 (72.1%) |
| No | 233 (27.3%) |
| Unknown | 5 (0.6%) |
| Body Mass Index Measures | |
| Recent BMI2, kg/m2, mean (SD) | 27.6 (5.3) |
| Categorical BMI | |
| Underweight (<18.5 kg/m2) | 13 (1.52%) |
| Normal (18.5–24.9 kg/m2) | 251 (29.43%) |
| Overweight (25.0–29.9 kg/m2) | 348 (40.80%) |
| Obese (30.0–39.9 kg/m2) | 216 (25.32%) |
| Morbidly Obese (≥40 kg/m2) | 25 (2.93%) |
| Lifetime Body Weight Measures, mean (SD) | |
| BMI @ age 21 | 22.6 (3.7) |
| Highest lifetime BMI | 30.5 (5.9) |
| Lowest lifetime BMI | 22.5 (3.6) |
| Weight change3 | 33.0 (29.8) |
Unequal Ns reflect missing data
Based on self-reported weight 1 year (N=103) or 5 years (N=750) prior to glioma diagnosis
Weight change in pounds from age 21 to recent pre-diagnostic weight
NOS = not otherwise specified
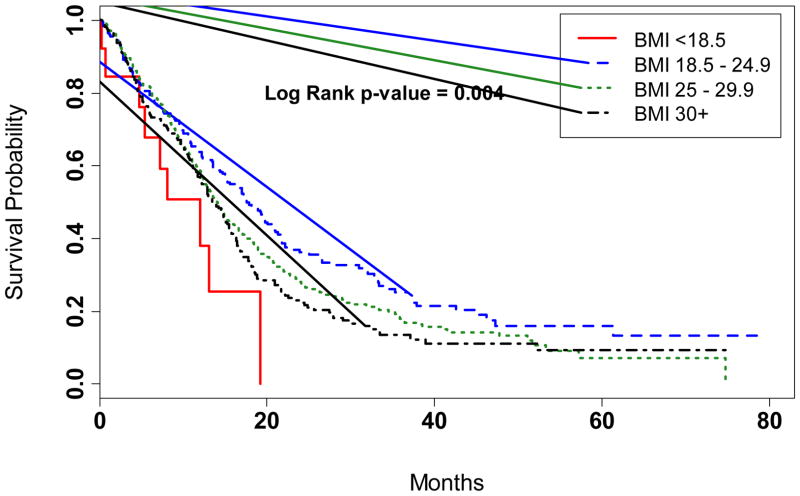
We evaluated glioma survival according to BMI defined as underweight, normal weight, overweight, and obese (Table 2; Figure). Overall survival was reduced among patients underweight (median survival: 12.0 months) or obese (median: 13.6 months) when compared to patients of normal weight (median: 17.5 months) prior to glioma diagnosis (p=0.004). Patients underweight 1–5 years prior to diagnosis had a significantly poorer outcome when compared to patients of normal weight (HR=2.02; 95% CI:1.03–3.96) adjusting for age and gender. The association for underweight was attenuated in models adjusting for KPS and treatment (HR=1.28; 95% CI:0.60–2.77; Table 2).
Table 2.
Glioma survival in relation to recent pre-diagnostic body weight
| BMI (Kg/m2)* | Age-Adjusted Models** | Multivariable Models† | ||||
|---|---|---|---|---|---|---|
| Number | Deaths | AHR (95% CI) | Number | Deaths | AHR (95% CI) | |
| Overall | ||||||
| <18.5 | 13 | 9 | 2.02 (1.03, 3.96) | 11 | 7 | 1.28 (0.60, 2.77) |
| 18.5– 24.9 | 251 | 164 | referent | 206 | 136 | referent |
| 25–29.9 | 348 | 251 | 1.04 (0.85, 1.27) | 300 | 221 | 1.16 (0.93, 1.45) |
| ≥30 | 241 | 181 | 1.24 (1.00, 1.54) | 195 | 143 | 1.32 (1.04, 1.68) |
| Males | ||||||
| <18.5 | 5 | 4 | 2.48 (0.90, 6.84) | 5 | 4 | 2.03 (0.73, 5.68) |
| 18.5–24.9 | 99 | 69 | referent | 83 | 59 | referent |
| 25–29.9 | 243 | 182 | 1.10 (0.84, 1.46) | 212 | 162 | 1.30 (0.96, 1.77) |
| ≥30 | 159 | 122 | 1.30 (0.97, 1.75) | 128 | 96 | 1.45 (1.04, 2.03) |
| Females | ||||||
| <18.5 | 8 | 5 | 1.70 (0.69, 4.20) | 6 | 3 | 1.06 (0.32, 3.50) |
| 18.5–24.9 | 152 | 95 | referent | 123 | 77 | referent |
| 25–29.9 | 105 | 69 | 0.96 (0.70, 1.31) | 88 | 59 | 1.01 (0.71, 1.42) |
| ≥30 | 82 | 59 | 1.25 (0.91, 1.74) | 67 | 47 | 1.29 (0.89, 1.87) |
BMI (body mass index) 1 year (N=103) or 5 years (N=750) prior to glioma diagnosis
Hazard ratio (HR) and 95% confidence interval (CI) adjusted for age and in overall model, gender
Hazard ratio (HR) and 95% confidence interval (CI) adjusted for age, KPS, treatment, and in overall model, gender.
Abbreviations: BMI (body mass index); AHR (Adjusted Hazards Ratio); CI (Confidence Interval). Unequal cell counts comparing age-adjusted and multivariate models reflect missing data.
Figure.
Kaplan-Meier curves showing proportion surviving after glioma diagnosis according to categories of body weight 1 to 5 years prior to glioma diagnosis.
A premorbid BMI of ≥30 kg/m2 was associated with an elevated mortality when compared to a normal BMI (AHR=1.24; 95% CI:1.00–1.54) adjusting for age and gender, whereas no excess mortality was observed in patients defined as overweight (AHR=1.04; 95% CI:0.85–1.27). Associations were strengthened with further adjustment for KPS and treatment status for the overweight (AHR=1.16; 95% CI:0.93–1.45) and obese (AHR=1.32; 95% CI:1.04–1.68; Table 2) categories of weight. All associations were comparable in men and women (Table 2). Furthermore, we observed positive associations for obesity after restricting analysis to patients undergoing treatment with the current standard of care, overall (multivariate AHR=1.23; 95% CI: 0.92–1.64), and in men (AHR=1.20; 95% CI: 0.81–1.78) and women (AHR=1.32; 95% CI: 0.85–2.06) separately, though results were imprecise. Similar results were observed after excluding subjects enrolled during the pilot phase that reported weight 1-year as opposed to 5-years before the diagnosis of glioma (data not shown). Morbid obesity (BMI≥40 kg/m2) was not significantly associated with patient outcome (AHR=1.30; 95% CI: 0.77–2.19); however, this result was based on few exposed subjects (a total of 25 subjects reported a recent BMI of ≥40 kg/m2).
In further analyses we considered whether glioma mortality rates varied according to early adult BMI or weight change in adult life. We observed no mortality association with BMI at age 21, with highest or lowest adult BMI, or with a weight change from age 21 to prediagnostic weight (not shown).
History of diabetes was not associated with an elevated risk of dying from glioma (AHR=1.08; 95% CI:0.83–1.40) adjusting for age, gender, KPS and SOC treatment. Results for diabetes were unchanged after further adjustment for BMI 1–5 years prior to interview (AHR=1.02; 95% CI:0.78–1.34). Associations of BMI with glioma outcome were not materially changed after adjustment for diabetes in multivariate adjusted models: after adjustment for diabetes and other covariates, hazard ratios were elevated for overweight (AHR=1.15; 95% CI: 0.92–1.44), obese (AHR=1.31; 95% CI: 1.02–1.68) and underweight (AHR=1.30; 95% CI: 0.60–2.81) status when compared to a normal body weight 1–5 year prior to diagnosis.
Discussion
In this multi-institutional study encompassing 853 persons with high-grade glioma, those that were obese 1–5 years prior to diagnosis had significantly elevated mortality from glioma when compared to persons with a normal body weight. These associations were demonstrated in males and females, and were maintained after restricting analyses to patients receiving current standard of care for high grade glioma. These findings, to our knowledge, are the first to link prediagnostic body weight with mortality in the setting of high-grade glioma.
Our findings are similar to those of Chambless and colleagues in a study of 171 glioma patients in which an increasing BMI (continuous) measured after glioma diagnosis was associated with elevated death rates (AHR=1.06; p=0.003) [8]. However, results conflict with those in a study by Jones et al. [7] involving 1,259 patients treated 1991–2008 in which no association was observed between body weight and glioma survival. Both previous studies [7, 8] considered body weight measured after glioma diagnosis results were subject to the effects of treatment including steroid-associated weight gain, and also body weight changes ensuing from the progression of disease. ‘Recent’ body weight examined in the current study provided a proxy for body weight prior to the initiation of treatment and before the onset of progressive disease, a period which is biologically and clinically most salient when considering body weight as a prognostic factor for patient outcome. In the current analysis, obesity remained a predictor of poor outcome after controlling for diabetes, similar to one previous report [8]; diabetes was not independently associated with survival in the present study. While obesity was significantly associated with a poor outcome, we could demonstrate no significant association with morbid levels of obesity (BMI≥40 kg/m2) though these results were imprecise due to the small number of subjects reporting this condition (N=25). Taken together, results suggest that excess body weight is an adverse prognostic factor in glioma, a relationship now observed in a spectrum of cancer types [3–6].
Obesity is a complex condition associated with hyperglycemia, insulin resistance, and elevated release of fatty acids [13]. Glucose is actively taken up by cancer cells as a form of energy [14, 15] a common feature of cancer cells, including glioma [16–18]. Providing mechanistic support for the current findings, hyperglycemia has consistently been associated with poor outcome in patients with glioma [8, 19–21]. Furthermore, there is in vitro data suggesting a role of glucose metabolism in the growth and invasiveness of glioma [22, 23]. These results are consistent with findings from our study and that of Chambless et al [8] in supporting an adverse influence of obesity, a condition associated with elevated glucose levels. Thus, uncontrolled hyperglycemia could plausibly promote tumor growth and aggressiveness; however, more research is needed to confirm this hypothesis. Suboptimal chemotherapy due to body size dosing thresholds may also explain, in part, the observed association between obesity and a poor patient outcome[24].
In the current data, persons underweight prior to diagnosis had higher death rates (nonsignificant in multivariate models) when compared to normal weight subjects. Shorter survival times have been reported previously among underweight cancer patients [5]. In the present study, all underweight subjects 1–5 years prior to diagnosis also reported a BMI <20kg/m2 at the age of 21, suggesting that the excess mortality observed in underweight patients was not secondary to glioma-associated weight loss. Patients chronically underweight may have diminished stamina or tolerance for aggressive therapy contributing to the reduced survival in these patients. (Chemotherapy dosing information was not available for the 92% of patients that underwent treatment with temozolomide.)
Strengths of the current study include the relatively large sample size of a rare tumor such as glioma, pathologic confirmation of all cases, and the limited potential influence of survival bias given exceptionally rapid enrollment of glioma cases in the case-control on which the study is based. Moreover, all patients were diagnosed with glioma in the modern era and the majority was treated with the currently recognized standard of care for high grade glioma, thus avoiding confounding by variable treatment regimens received by subjects included in the analysis. However, the study also had limitations. All body dimensions were based on self-report and results subject to misclassification and bias to the null. As subjects were affected with neural tumors that may have impacted memory, the current results may have underestimated the association of obesity with patient outcome. Weight gain or loss after the index date (1 or 5 years prior to the study interview) would have further attenuated associations. In addition, information missing on covariate information (KPS, extent of tumor resection) reduced power of multivariate analyses to detect association between premorbid body weight and patient outcome.
In summary, in this large well-characterized patient series, premorbid obesity was significantly associated with a poor patient outcome, independent of treatment and established prognostic factors. While the biologic mechanism is unknown, these data are consistent with previous literature suggesting that glucose homeostasis plays a role in the progression and outcome of glioma. The current finding that body weight contributes to glioma outcome warrants further research.
Acknowledgments
The authors wish to acknowledge the study participants and their families. We further wish to thank the clinicians and research staffs at participating medical centers for their contributions. In addition, we acknowledge Dr. Renato V. LaRocca at the Kentuckianna Cancer Institute in Louisville, KY and Dr. Sajeel A. Chowdhary at Florida Hospital Cancer Institute in Orlando, FL, as well as Harold Colbassani, MD; Dean Gobo, MD; and Christopher Mickler, DO at Morton Plant Mease Healthcare and Baycare Health System in Clearwater, FL for their efforts recruiting subjects to the study. The project was supported by the National Institutes of Health (R01CA116174) and institutional funding provided by the Moffitt Cancer Center (Tampa, FL) and the Vanderbilt-Ingram Comprehensive Cancer Center (Nashville, TN).
Footnotes
Potential conflicts of interest
The authors declare they have no conflicts of interest.
References
- 1.American Cancer Societiy Facts and Figures 2012. ACS; Atlanta: 2012. [Google Scholar]
- 2.Stupp R, Hegi ME, Gilbert MR, Chakravarti A. Chemoradiotherapy in malignant glioma: standard of care and future directions. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2007;25:4127–4136. doi: 10.1200/JCO.2007.11.8554. [DOI] [PubMed] [Google Scholar]
- 3.Demark-Wahnefried W, Platz EA, Ligibel JA, Blair CK, Courneya KS, Meyerhardt JA, Ganz PA, Rock CL, Schmitz KH, Wadden T, Philip EJ, Wolfe B, Gapstur SM, Ballard-Barbash R, McTiernan A, Minasian L, Nebeling L, Goodwin PJ. The role of obesity in cancer survival and recurrence. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2012;21:1244–1259. doi: 10.1158/1055-9965.EPI-12-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sinicrope FA, Dannenberg AJ. Obesity and breast cancer prognosis: weight of the evidence. J Clin Oncol. 2010;29:4–7. doi: 10.1200/JCO.2010.32.1752. doi:JCO.2010.32.175210.1200/JCO.2010.32.1752. [DOI] [PubMed] [Google Scholar]
- 5.Sinicrope FA, Foster NR, Sargent DJ, O’Connell MJ, Rankin C. Obesity is an independent prognostic variable in colon cancer survivors. Clin Cancer Res. 2010;16:1884–1893. doi: 10.1158/1078-0432.CCR-09-2636. doi:1078-0432.CCR-09-263610.1158/1078-0432.CCR-09-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsing AW, Sakoda LC, Chua S., Jr Obesity, metabolic syndrome, and prostate cancer. The American journal of clinical nutrition. 2007;86:s843–857. doi: 10.1093/ajcn/86.3.843S. [DOI] [PubMed] [Google Scholar]
- 7.Jones LW, Ali-Osman F, Lipp E, Marcello JE, McCarthy B, McCoy L, Rice T, Wrensch M, Il’yasova D. Association between body mass index and mortality in patients with glioblastoma mutliforme. Cancer Causes Control. 2010;21:2195–2201. doi: 10.1007/s10552-010-9639-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chambless LB, Parker SL, Hassam-Malani L, McGirt MJ, Thompson RC. Type 2 diabetes mellitus and obesity are independent risk factors for poor outcome in patients with high-grade glioma. Journal of neuro-oncology. 2012;106:383–389. doi: 10.1007/s11060-011-0676-4. [DOI] [PubMed] [Google Scholar]
- 9.Flegal KM, Graubard BI, Williamson DF, Cooper RS. Reverse causation and illness-related weight loss in observational studies of body weight and mortality. American journal of epidemiology. 2011;173:1–9. doi: 10.1093/aje/kwq341. [DOI] [PubMed] [Google Scholar]
- 10.Little RB, Madden MH, Thompson RC, Olson JJ, Larocca RV, Pan E, Browning JE, Egan KM, Nabors LB. Anthropometric factors in relation to risk of glioma. Cancer Causes Control. 2013 doi: 10.1007/s10552-013-0178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Egan KM, Thompson RC, Nabors LB, Olson JJ, Brat DJ, Larocca RV, Brem S, Moots PL, Madden MH, Browning JE, Ann Chen Y. Cancer susceptibility variants and the risk of adult glioma in a US case-control study. J Neurooncol. 2011 doi: 10.1007/s11060-010-0506-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO. World Health Organization Technical Report Series. WHO; Geneva: 1995. Physical status: the use and interpretation of anthropometry; pp. 1–47. [PubMed] [Google Scholar]
- 13.van Kruijsdijk RC, van der Wall E, Visseren FL. Obesity and cancer: the role of dysfunctional adipose tissue. Cancer Epidemiol Biomarkers Prev. 2009;18:2569–2578. doi: 10.1158/1055-9965.EPI-09-0372. doi:1055-9965.EPI-09-037210.1158/1055-9965.EPI-09-0372. [DOI] [PubMed] [Google Scholar]
- 14.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 15.Bensinger SJ, Christofk HR. New aspects of the Warburg effect in cancer cell biology. Seminars in cell & developmental biology. 2012;23:352–361. doi: 10.1016/j.semcdb.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 16.Jelluma N, Yang X, Stokoe D, Evan GI, Dansen TB, Haas-Kogan DA. Glucose withdrawal induces oxidative stress followed by apoptosis in glioblastoma cells but not in normal human astrocytes. Mol Cancer Res. 2006;4:319–330. doi: 10.1158/1541-7786.MCR-05-0061. [DOI] [PubMed] [Google Scholar]
- 17.Oudard S, Arvelo F, Miccoli L, Apiou F, Dutrillaux AM, Poisson M, Dutrillaux B, Poupon MF. High glycolysis in gliomas despite low hexokinase transcription and activity correlated to chromosome 10 loss. Br J Cancer. 1996;74:839–845. doi: 10.1038/bjc.1996.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seyfried TN, Kiebish MA, Marsh J, Shelton LM, Huysentruyt LC, Mukherjee P. Metabolic management of brain cancer. Biochim Biophys Acta. 2011;1807:577–594. doi: 10.1016/j.bbabio.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 19.Derr RL, Ye X, Islas MU, Desideri S, Saudek CD, Grossman SA. Association between hyperglycemia and survival in patients with newly diagnosed glioblastoma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27:1082–1086. doi: 10.1200/JCO.2008.19.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGirt MJ, Chaichana KL, Gathinji M, Attenello F, Than K, Ruiz AJ, Olivi A, Quinones-Hinojosa A. Persistent outpatient hyperglycemia is independently associated with decreased survival after primary resection of malignant brain astrocytomas. Neurosurgery. 2008;63:286–291. doi: 10.1227/01.NEU.0000315282.61035.48. discussion 291. [DOI] [PubMed] [Google Scholar]
- 21.Chaichana KL, McGirt MJ, Woodworth GF, Datoo G, Tamargo RJ, Weingart J, Olivi A, Brem H, Quinones-Hinojosa A. Persistent outpatient hyperglycemia is independently associated with survival, recurrence and malignant degeneration following surgery for hemispheric low grade gliomas. Neurol Res. 2010;32:442–448. doi: 10.1179/174313209X431101. [DOI] [PubMed] [Google Scholar]
- 22.Sato A, Sunayama J, Okada M, Watanabe E, Seino S, Shibuya K, Suzuki K, Narita Y, Shibui S, Kayama T, Kitanaka C. Glioma-initiating cell elimination by metformin activation of FOXO3 via AMPK. Stem cells translational medicine. 2012;1:811–824. doi: 10.5966/sctm.2012-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramao A, Gimenez M, Laure HJ, Izumi C, Vida RC, Oba-Shinjo S, Marie SK, Rosa JC. Changes in the expression of proteins associated with aerobic glycolysis and cell migration are involved in tumorigenic ability of two glioma cell lines. Proteome science. 2012;10:53. doi: 10.1186/1477-5956-10-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wildiers H, Reiser M. Relative dose intensity of chemotherapy and its impact on outcomes in patients with early breast cancer or aggressive lymphoma. Critical reviews in oncology/hematology. 2011;77:221–240. doi: 10.1016/j.critrevonc.2010.02.002. [DOI] [PubMed] [Google Scholar]