Abstract
Aim
To assess the outcomes and incidence of postoperative complications of Ahmad Glaucoma Valve implant in eyes with complicated glaucoma performed in Kuwait.
Method
This is a retrospective study done at the Al-Bahar Eye Center in Kuwait. Charts of all patients who underwent Ahmad Glaucoma Valve implant at the Al-Bahar Ophthalmic Center in Kuwait between 2006 and 2009 were reviewed. Surgical success was defined as intraocular pressure less than 22 mmHg and greater than 5 mmHg without additional glaucoma surgery and without loss of light perception.
Results
A total of 33 eyes from 30 patients with complicated glaucoma not responsive to conventional medical and non-implant surgical treatment received Ahmad Glaucoma Valve implant. The success rate was 79% (26 cases). 20/26 (77%) cases of them required antiglaucoma medications. The most common complication was encapsulated bleb (27%) and transient postoperative hypotony was found in 19% of the cases.
Conclusion
Ahmad Glaucoma Valve implant appears to be effective and relatively safe for complicated glaucoma in Kuwait. The success rate is comparable with those reported in other studies.
Keywords: Glaucoma, Ahmad Glaucoma Valve, Hypotony
Introduction
Glaucoma drainage implants provide an alternative treatment in complicated and refractory glaucoma cases,1,2 that are resistant to medical therapy, laser treatment and glaucoma filtration surgery.
These implants have a small caliber silicone tube that drains aqueous from the anterior or posterior chamber to an extrascleral device that maintains a fibrous pseudo-cyst through which filtration can occur. Varieties of aqueous shunting devices have been developed, including open-tube and valved designs. Hypotony during the immediate postoperative period is a common complication associated with the open-tube implants.3 Although valve implants may not completely close after initial perfusion with fluid, they do function as flow-restricting devices.4 The Ahmad Glaucoma Valve (AGV) implant (New World Medical, Inc., Rancho Cucamonga, California) directs aqueous flow through the silicone tube and between two thin silicone elastomer membranes in a tapered chamber. The initial clinical experience with this implant indicated that hypotony and its attendant complications during the immediate postoperative period were less common than reported with other glaucoma drainage devices.5,6
Ahmad Glaucoma Valve was introduced in 1993, it has a built in venture valve which offers resistance to the aqueous outflow below about 7 mmHg helping to minimize postoperative hypotony.7 However, the valve may be a potential site for obstruction by inflammatory debris.8
Aim
To assess the outcomes and incidence of postoperative complications of AGV implant in eyes with complicated glaucoma performed at the Al-Bahar Ophthalmic Center – Kuwait between 2006 and 2009.
Patients and methods
This is a retrospective study approved by the Al-Bahar Eye Center Ethics Committee in Kuwait. This center serves as the only referral tertiary eye center in Kuwait so that patients attending this hospital are representative of various Kuwaiti tribes and districts. Charts of all patients who were treated with AGV at the Al-Bahar Ophthalmic Center in Kuwait between 2006 and 2009 were reviewed. Those patients were not responsive to medical treatment, laser treatment or previous glaucoma surgery.
After informed written consent was obtained, the AGV was implanted by one of three glaucoma subspecialty surgeons. Patients with neovascular glaucoma (NVG) were treated with PRP before the AGV was implemented when feasible. For aphakic patients, anterior vitrectomy was performed. The superotemporal quadrant was used for the implant fixation in all eyes. Human donor pericardium was used to cover the tube in all patients. No additional surgical procedures were done at the same time of implant fixation for any patient, post operative treatment consisted of topical steroids and antibiotics.
Intraocular pressure was measured with a Goldman applanation tonometer, a tonopen (Mentor O & O, Norwell, Massachusetts), or Perkins (Clement Clarke, Columbus, Ohio).
The tube of AGV was irrigated with balanced salt solution to open the valve mechanism. After conjunctival incision and separation of Tenon capsule from the sclera, the implant plate was secured 8–10 mm posterior to the corneal limbus with two interrupted 10-0 nylon. All the implants were inserted in the superotemporal quadrant. The tube was trimmed to extend from 1 to 3 mm beyond the posterior surgical limbus. A 23-gauge needle was used to enter the anterior chamber at the surgical limbus parallel to the iris plane, and the tube was inserted into the anterior chamber through the needle track. The tube was loosely secured to the sclera posterior to the limbus and then covered with pericardium which was secured with interrupted 10-0 nylon. The conjunctiva was closed with a 9-0 vicryl suture, and subconjunctival injections of antibiotic and corticosteroids were administered before the eye was patched. Postoperative treatment consisted of topical corticosteroids and antibiotics.
Surgical success was defined as intraocular pressure less than 22 mmHg and greater than 5 mmHg without additional glaucoma surgery and without loss of light perception. Postoperative use of antiglaucoma medications was not a criterion of success or failure. The definition of hypotony in this study was IOP of 5 mmHg or less in two consecutive visits.
The cumulative probability of success was analyzed by the Kaplan–Meier life-table analysis (Fig. 1).
Figure 1.
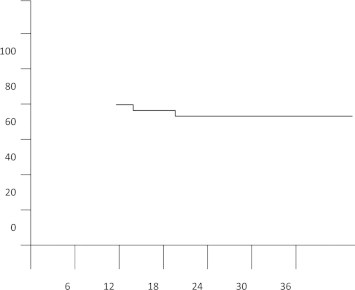
Cumulative probability of success following Ahmed Glaucoma Valve. Implantation was 79% at 1 year and 75% at 2 years (Kaplan–Meier life table analysis).
Results
A total of 33 eyes from 30 patients were included in the study. The mean age of the patients was 36.1 years (range 2–64 yeas); the mean follow up period was 21.8 ± 9.2 months (range 6–44 months). There were 19 females and 11 males (Table 1). The preoperative diagnosis was, 14 cases (42.4%) were Neovascular glaucoma with proliferative diabetic Retinopathy, 4 cases (12%) were Congenital glaucoma, 3 cases (9%) were glaucoma with penetrating keratoplasty, 3cases (9%) were Primary open angle glaucoma with previous surgery, 5 cases (15%) were chronic Angle closure glaucoma, 3 cases (9%) were Uveitic glaucoma, one case (3.1%) was ICE syndrome (Table 1) and 18 cases (54.5%) were pseudophakic at the time of surgery. Before AGV were implanted 20 cases (60.6%) had ocular procedures including cataract extraction, penetrating keratoplasty, trabeclectomy, trabeclotomy and cyclophotocoagulation, and pan-retinal laser photocoagulation. The mean pre-operative IOP was 37 ± 12.1 mmHg; postoperatively the mean IOP was decreased to 10 mmHg on one day, 18.7 mmHg after 3 months, and 18.0 mmHg after 1 year and to 19.5 mmHg after 2 years (Fig. 2). The mean number of antiglaucoma medication pre-operatively was 3.1 which decreases to 1.1 postoperatively.
Table 1.
Characteristics of patients and preoperative diagnosis.
| Number of patients | 30 | |
| Number of eyes | 33 | |
| Mean (range) age years | 36.1 range (2–64) | |
| Female:male | 19:11 | |
| Mean (SD) follow up (months) | 21.8 (9.2) | |
| Types of glaucoma | ||
| No. of cases |
% |
|
| Neovascular glaucoma | 14 | 42.4 |
| Congenital glaucoma | 4 | 12 |
| Glaucoma with PKP | 3 | 9.0 |
| POAG with previous surgery | 3 | 9.0 |
| Chronic – angle closure-glaucoma | 5 | 15 |
| Uveitic glaucoma | 3 | 9.0 |
| ICE Syndrome | 1 | 3.1 |
Figure 2.
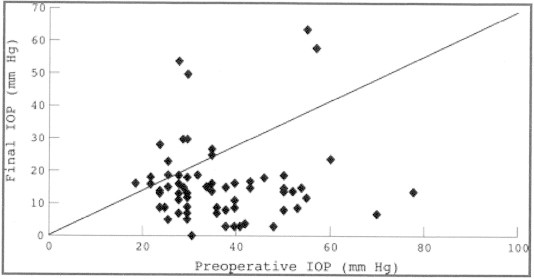
Scattergram of the preoperative versus the final intraocular pressure (IOP). The line of equivalence is also shown.
The success rate was 79% (26 cases). 20/26 (77%) cases of them required antiglaucoma medications and the remaining 6/26 cases (23%) did not.
The final best corrected visual acuity improved by one snellen line in only 3 cases (9%) and decreased by one line in 9 cases (27%), the remaining 21 cases did not show change in visual acuity (64%).
Postoperative complications occurred in 26 cases (78.8%). Some eyes had more than one complication (Table 2).
Table 2.
Postoperative complication.
| No. of cases | % | |
|---|---|---|
| Encapsulated bleb | 9 | 27 |
| Corneal edema | 5 | 15 |
| Corneal decompensation | 2 | 6 |
| Hyphema | 6 | 18 |
| Shallow Ac and hypotony | 6 | 18 |
| Choroidal detachment | 1 | 3 |
| Cataract | 2 | 6 |
| Iritis | 2 | 6 |
| Tube obstruction | 6 | 18 |
| Post operative leakage | 2 | 6 |
| Erosion of graft | 1 | 3 |
| Vitreous hemorrhage | 2 | 6 |
Postoperative complications encountered in this study were encapsulated bleb in 9 cases (27%), the mean time for development of encapsulated bleb was 4.1 months, corneal edema occurred in 5 cases (15%); two eyes of them developed corneal decompensation and needed Penetrating keratoplasty (one in the PKP group and the other was in the congenital glaucoma group) and Hyphema occurred in 6 cases (18%) especially within neovascular glaucoma group.
Shallow AC with hypotony occurred in 6 cases (18%) one of these cases developed choroidal detachment which resolved spontaneously without any surgical intervention, 6 cases (18%) developed tube obstruction, Cataract developed in 2 cases (6%), and iritis developed in 2 cases (6%). The postoperative leakage happened in 2 cases (6%) and this complication was secondary to short conjunctiva that needs further management. Tube erosion occurred after 7 months in one case (3%).
Two eyes (6%) developed vitreous hemorrhage, secondary to progression of proliferative diabetic retinopathy, so these cases were managed by vitrectomy and endolaser photocoagulation.
Discussion
Glaucoma drainage implants have been used successfully for the treatment of complicated glaucomas including NVG; aphakic and pseudophakic glaucoma; glaucomas with multiple filtering procedures; post penetrating keratoplasty glaucoma; pediatric glaucoma; and uveitic glaucoma.1,2 One of the common early postoperative complications is overdrainage resulting in shallow AC, hypotony, and choroidal detachment.3,9 There are various types of glaucoma drainage implants available in the market. Some of them have a set resistance mechanism to minimize over-drainage in the early postoperative period. Those without set resistance mechanism may require either a two staged procedure or ligature technique. The AGV implant employs a built in Venturi valve to provide resistance to aqueous flow.6
In our study, the AGV implant was able to decrease the mean (SD) preoperative IOP from 37.0 ± 12.1 mmHg to 18.4 ± 10.7 mmHg at 3 months and 18.0 ± 14.1 mmHg at 1 year postoperatively. The success rate achieved was 79%. Most case series reported success rates between 67% and 94%, depending on their criteria for success.3,11
The overall success rate in this study compares favorably with the success rates of other glaucoma drainage devices. The Baerveldt implant had a success rate of 72%,3 and the Molteno implant showed 58–95% success rate,10 all of the these glaucoma drainage implants divert aqueous from the eye to the tenon space posterior to the limbus, which may explain similar success rates with different implants.
Hypotony and complications of hypotony are common during the immediate postoperative period after nonvalved glaucoma drainage implants. The most common complications after the Baerveldt implant were hypotony (32% of eyes),3 in the current study, we found complications associated with hypotony in the immediate postoperative period less frequently (18%). No eyes in our study required surgical anterior chamber reformation.
Neovascular glaucoma was the most common preoperative diagnosis (42.4%) in our series and it also carries the highest failure rate in our study (54.5%). No other types of glaucoma were found to have associated with such a high failure rate in our series. It has been well shown that NVG is associated with a much higher risk of failure than other types of glaucoma.9–14
The most common medications used to lower postoperative IOP in our series were topical latanoprost, topical carbonic anhydrase inhibitor and Alpha agonist.
The hypertensive phase reported to be as high as 20–40% incidence in other implant series was not distinct in our series. Although the mean IOP at 3 months was slightly higher than that in 1 year, it might have been modified by the medications.11–15
Tube obstruction occurred in 6 cases (18%) it was due to blood in 3 cases, iris or cornea in 2 cases and the last case had presumed obstruction manifested by increases in intraocular pressure during the first postoperative week, with an apparently patent tube on slit lamp examination. The IOP returned to normal spontaneously or with digital pressure in all the cases. We did not observe any instances of tube occlusion by the posterior capsule15 or irreversible valve membrane adhesion16 which may be described in eyes with AGV implants.
Conclusion
The Ahmad Glaucoma Valve implant appears to be effective and relatively safe for complicated glaucoma in Kuwait. The success rate is comparable with those reported in other studies. Hypotony is not commonly encountered with this implant. Formation of encapsulated bleb is the most common postoperative complication.
Footnotes
Peer review under responsibility of Saudi Ophthalmological Society, King Saud University.
![]()
Contributor Information
Adel M. Aljazzaf, Email: adeljazzaf@gmail.com.
Sidky M.A. Abdelmoaty, Email: sidky71@hotmail.com.
Abdelmutalib H. Behbehani, Email: mutbe@hotmail.com.
Hadeel A. Aljazzaf, Email: adeljazzaf@gmail.com.
References
- 1.Krupin T., Kaufman P., Mnadell A. Filtering valve implant surgery for eyes with neovascular glaucoma. Am J Ophthalmol. 1980;89:338–343. doi: 10.1016/0002-9394(80)90002-1. [DOI] [PubMed] [Google Scholar]
- 2.Englert J.A., Freedman S.F., Cox T.A. The Ahmed valve glaucoma implant in refractory pediatric glaucoma. Am J Ophthalmol. 1999;127:34–42. doi: 10.1016/s0002-9394(98)00292-x. [DOI] [PubMed] [Google Scholar]
- 3.Siegner S.W., Netland P.A., Urban R.C. Clinical experience with the Baerveldt glaucoma drainage implant. Ophthalmology. 1995;102:1298–1307. doi: 10.1016/s0161-6420(95)30871-8. [DOI] [PubMed] [Google Scholar]
- 4.Francis B.A., Cortes A., Chen J., Alvarado J.A. Characteristics of glaucoma drainage implants during dynamic and steady state flow conditions. Ophthalmology. 1998;105:1708–1714. doi: 10.1016/S0161-6420(98)99042-X. [DOI] [PubMed] [Google Scholar]
- 5.Colman A.L., Hill R., Wilson M.R. Initial clinical experience with the Ahmad Glaucoma Valve implant. Am J Ophthalmol. 1995;120:23–31. doi: 10.1016/s0002-9394(14)73755-9. [DOI] [PubMed] [Google Scholar]
- 6.Lim K.S., Allan B.D.S., Lloyd A.W. Glaucoma drainage devices; past, present, and future. Br J Ophthalmol. 1998;82:1083–1089. doi: 10.1136/bjo.82.9.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jimmy S.M. Lai, Agnes S.Y. Poon, John K.H. Chua. Efficacy and safety of the Ahmed Glaucoma Valve implant in Chinese eyes with complicated glaucoma. Br J Ophthalmol. 2000;84(July):718–721. doi: 10.1136/bjo.84.7.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feldman R.M., El-Harazi S.M., Villanueva G. Valve membrane adhesion as a cause of Ahmed Glaucoma Valve failure. J Glaucoma. 1997;6:10–12. [PubMed] [Google Scholar]
- 9.Chihara E., Kubota H., Takanashi T. Outcome of White pump shunt surgery for neovascular glaucoma in Asians. Ophthalmol Surg. 1992;23:666. [PubMed] [Google Scholar]
- 10.Minckler D.S., Heuer D.K., Hasty B. Clinical experience with the single-plate Molteno implant in complicated glaucomas. Ophthalmology. 1998;95:1181–1188. doi: 10.1016/s0161-6420(88)33029-0. [DOI] [PubMed] [Google Scholar]
- 11.Huang M.C., Netland P.A., Coleman A.L. Intermediate-term clinical experience with the Ahmed Glaucoma Valve implant. Am J Ophthalmol. 1999;127:27–33. doi: 10.1016/s0002-9394(98)00394-8. [DOI] [PubMed] [Google Scholar]
- 12.Krupin T., Kaufman P., Mnadell A. Long-term results of valve implants in filtering surgery for eyes with neovascular glaucoma. Am J Ophthalmol. 1983;95:775. doi: 10.1016/0002-9394(83)90064-8. [DOI] [PubMed] [Google Scholar]
- 13.Lavin M.J., Franks W.A., Wormald R.P.L. Clinical risk factors for failure in glaucoma tube surgery. A comparison of three tube designs. Arch Ophthalmol. 1992;110:480–485. doi: 10.1001/archopht.1992.01080160058030. [DOI] [PubMed] [Google Scholar]
- 14.Fellenbaum P.S., Almeida A.R., Minckler D.S. Krupin disk implantation for complicated glaucoma. Ophthalmology. 1994;101:1178–1182. doi: 10.1016/s0161-6420(13)31724-2. [DOI] [PubMed] [Google Scholar]
- 15.Tessler Z., Jlchoded S., Rosenthal G. Nd: YAG laser for Ahmed tube shunt occlusion by the posterior capsule. Ophthalmic Surg Lasers. 1997;28:69–70. [PubMed] [Google Scholar]
- 16.Feldman R.M., El-Harazi S.M., Villanueva G. Valve membrane adhesion as a cause of Ahmed Glaucoma Valve failure. J Glaucoma. 1997;6:10–12. [PubMed] [Google Scholar]


