Abstract
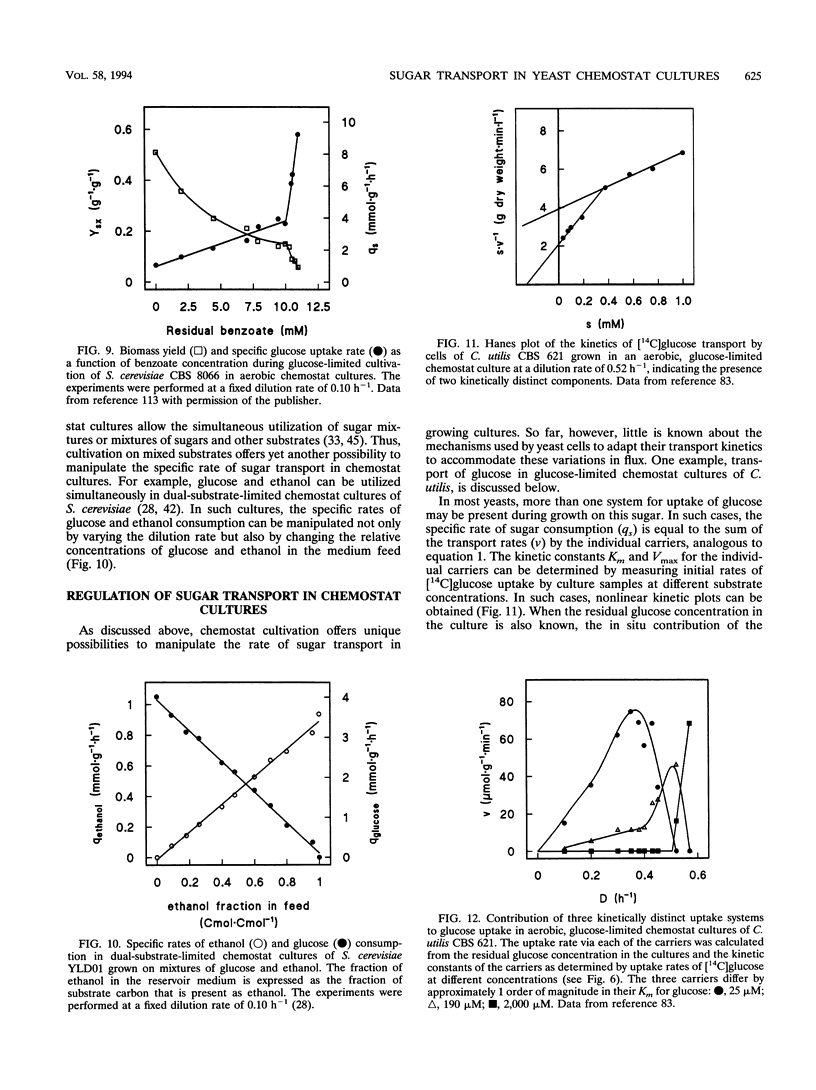
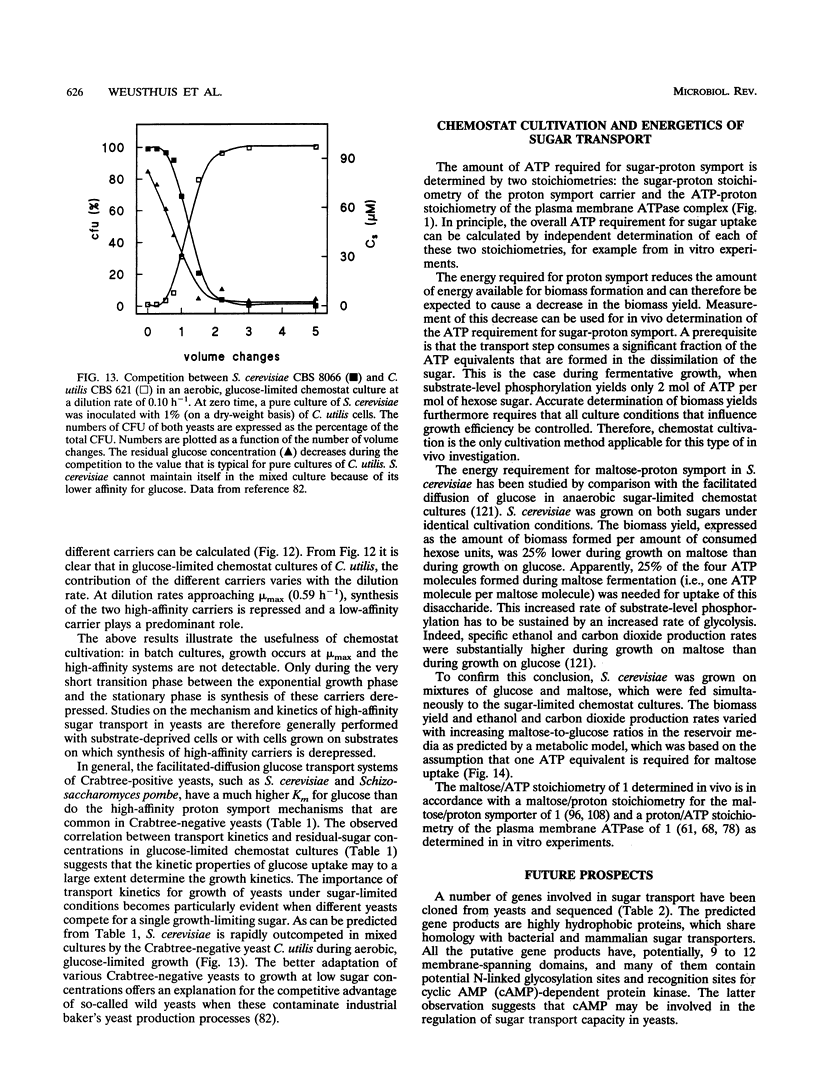
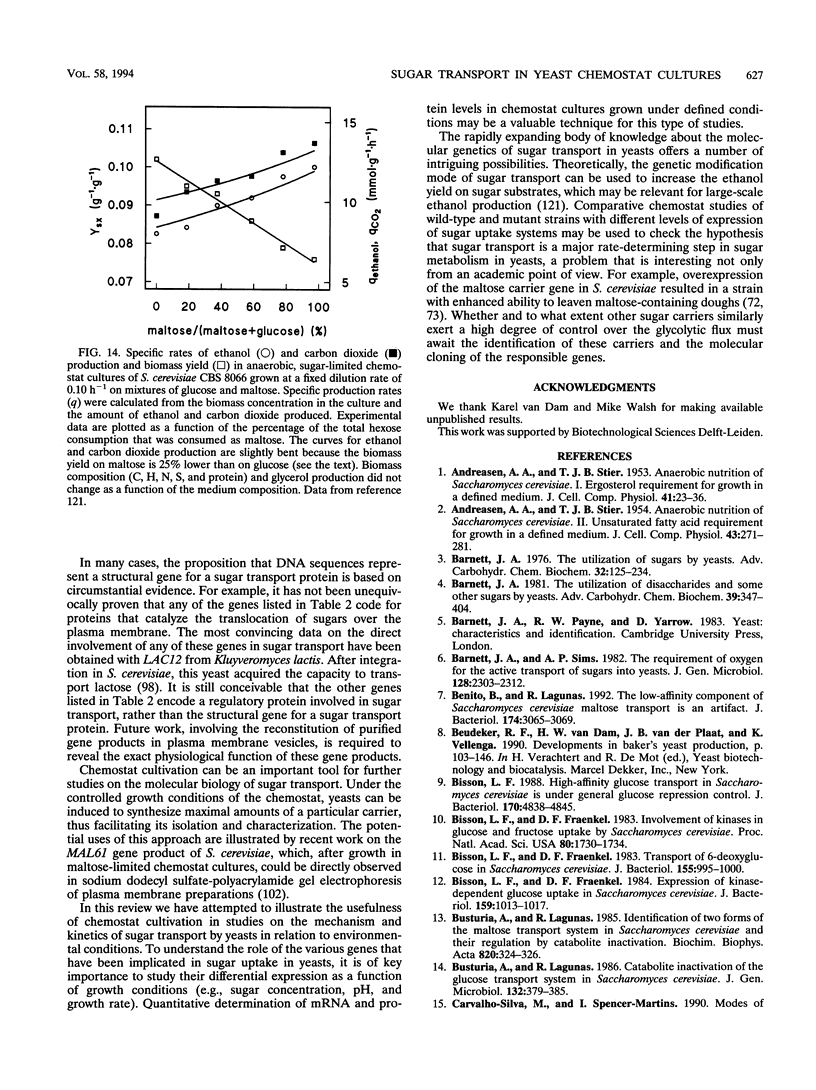
Chemostat cultivation enables investigations into the effects of individual environmental parameters on sugar transport in yeasts. Various means are available to manipulate the specific rate of sugar uptake (qs) in sugar-limited chemostat cultures. A straightforward way to manipulate qs is variation of the dilution rate, which, in substrate-limited chemostat cultures, is equal to the specific growth rate. Alternatively, qs can be varied independently of the growth rate by mixed-substrate cultivation or by variation of the biomass yield on sugar. The latter can be achieved, for example, by addition of nonmetabolizable weak acids to the growth medium or by variation of the oxygen supply. Such controlled manipulation of metabolic fluxes cannot be achieved in batch cultures, in which various parameters that are essential for the kinetics of sugar transport cannot be controlled. In sugar-limited chemostat cultures, yeasts adapt their sugar transport systems to cope with the low residual sugar concentrations, which are often in the micromolar range. Under the conditions, yeasts with high-affinity proton symport carriers have a competitive advantage over yeasts that transport sugars via facilitated-diffusion carriers. Chemostat cultivation offers unique possibilities to study the energetic consequences of sugar transport in growing cells. For example, anaerobic, sugar-limited chemostat cultivation has been used to quantify the energy requirement for maltose-proton symport in Saccharomyces cerevisiae. Controlled variation of growth conditions in chemostat cultures can be used to study the differential expression of genes involved in sugar transport and as such can make an important contribution to the ongoing studies on the molecular biology of sugar transport in yeasts.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDREASEN A. A., STIER T. J. B. Anaerobic nutrition of Saccharomyces cerevisiae. I. Ergosterol requirement for growth in a defined medium. J Cell Physiol. 1953 Feb;41(1):23–36. doi: 10.1002/jcp.1030410103. [DOI] [PubMed] [Google Scholar]
- ANDREASEN A. A., STIER T. J. Anaerobic nutrition of Saccharomyces cerevisiae. II. Unsaturated fatty acid requirement for growth in a defined medium. J Cell Physiol. 1954 Jun;43(3):271–281. doi: 10.1002/jcp.1030430303. [DOI] [PubMed] [Google Scholar]
- Barnett J. A. The utilization of sugars by yeasts. Adv Carbohydr Chem Biochem. 1976;32:125–234. doi: 10.1016/s0065-2318(08)60337-6. [DOI] [PubMed] [Google Scholar]
- Benito B., Lagunas R. The low-affinity component of Saccharomyces cerevisiae maltose transport is an artifact. J Bacteriol. 1992 May;174(9):3065–3069. doi: 10.1128/jb.174.9.3065-3069.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisson L. F., Fraenkel D. G. Expression of kinase-dependent glucose uptake in Saccharomyces cerevisiae. J Bacteriol. 1984 Sep;159(3):1013–1017. doi: 10.1128/jb.159.3.1013-1017.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisson L. F., Fraenkel D. G. Involvement of kinases in glucose and fructose uptake by Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1730–1734. doi: 10.1073/pnas.80.6.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisson L. F., Fraenkel D. G. Transport of 6-deoxyglucose in Saccharomyces cerevisiae. J Bacteriol. 1983 Sep;155(3):995–1000. doi: 10.1128/jb.155.3.995-1000.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisson L. F. High-affinity glucose transport in Saccharomyces cerevisiae is under general glucose repression control. J Bacteriol. 1988 Oct;170(10):4838–4845. doi: 10.1128/jb.170.10.4838-4845.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busturia A., Lagunas R. Catabolite inactivation of the glucose transport system in Saccharomyces cerevisiae. J Gen Microbiol. 1986 Feb;132(2):379–385. doi: 10.1099/00221287-132-2-379. [DOI] [PubMed] [Google Scholar]
- Celenza J. L., Marshall-Carlson L., Carlson M. The yeast SNF3 gene encodes a glucose transporter homologous to the mammalian protein. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2130–2134. doi: 10.1073/pnas.85.7.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y. D., Dickson R. C. Primary structure of the lactose permease gene from the yeast Kluyveromyces lactis. Presence of an unusual transcript structure. J Biol Chem. 1988 Nov 15;263(32):16696–16703. [PubMed] [Google Scholar]
- Charron M. J., Dubin R. A., Michels C. A. Structural and functional analysis of the MAL1 locus of Saccharomyces cerevisiae. Mol Cell Biol. 1986 Nov;6(11):3891–3899. doi: 10.1128/mcb.6.11.3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Q., Michels C. A. MAL11 and MAL61 encode the inducible high-affinity maltose transporter of Saccharomyces cerevisiae. J Bacteriol. 1991 Mar;173(5):1817–1820. doi: 10.1128/jb.173.5.1817-1820.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Q., Michels C. A. The maltose permease encoded by the MAL61 gene of Saccharomyces cerevisiae exhibits both sequence and structural homology to other sugar transporters. Genetics. 1989 Nov;123(3):477–484. doi: 10.1093/genetics/123.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton D., Walsh R. B., Fraenkel D. G. Functional studies of yeast glucokinase. J Bacteriol. 1993 Jun;175(11):3289–3294. doi: 10.1128/jb.175.11.3289-3294.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. D., Goldenthal M. J., Chow T., Buchferer B., Marmur J. Organization of the MAL loci of Saccharomyces. Physical identification and functional characterization of three genes at the MAL6 locus. Mol Gen Genet. 1985;200(1):1–8. doi: 10.1007/BF00383304. [DOI] [PubMed] [Google Scholar]
- DE LA FUENTE G., SOLS A. Transport of sugars in yeasts. II. Mechanisms of utilization of disaccharides and related glycosides. Biochim Biophys Acta. 1962 Jan 1;56:49–62. doi: 10.1016/0006-3002(62)90526-7. [DOI] [PubMed] [Google Scholar]
- De Bruijne A. W., Schuddemat J., Van den Broek P. J., Van Steveninck J. Regulation of sugar transport systems of Kluyveromyces marxianus: the role of carbohydrates and their catabolism. Biochim Biophys Acta. 1988 Apr 22;939(3):569–576. doi: 10.1016/0005-2736(88)90104-6. [DOI] [PubMed] [Google Scholar]
- DeJuan C., Lagunas R. Inactivation of the galactose transport system in Saccharomyces cerevisiae. FEBS Lett. 1986 Oct 27;207(2):258–261. doi: 10.1016/0014-5793(86)81500-9. [DOI] [PubMed] [Google Scholar]
- Dickson R. C., Barr K. Characterization of lactose transport in Kluyveromyces lactis. J Bacteriol. 1983 Jun;154(3):1245–1251. doi: 10.1128/jb.154.3.1245-1251.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egli T., Lendenmann U., Snozzi M. Kinetics of microbial growth with mixtures of carbon sources. Antonie Van Leeuwenhoek. 1993;63(3-4):289–298. doi: 10.1007/BF00871224. [DOI] [PubMed] [Google Scholar]
- Entian K. D. A defect in carbon catabolite repression associated with uncontrollable and excessive maltose uptake. Mol Gen Genet. 1980;179(1):169–175. doi: 10.1007/BF00268460. [DOI] [PubMed] [Google Scholar]
- Entian K. D., Loureiro-Dias M. C. Misregulation of maltose uptake in a glucose repression defective mutant of Saccharomyces cerevisiae leads to glucose poisoning. J Gen Microbiol. 1990 May;136(5):855–860. doi: 10.1099/00221287-136-5-855. [DOI] [PubMed] [Google Scholar]
- Franzusoff A. J., Cirillo V. P. Glucose transport activity in isolated plasma membrane vesicles from Saccharomyces cerevisiae. J Biol Chem. 1983 Mar 25;258(6):3608–3614. [PubMed] [Google Scholar]
- Franzusoff A., Cirillo V. P. Uptake and phosphorylation of 2-deoxy-D-glucose by wild-type and single-kinase strains of Saccharomyces cerevisiae. Biochim Biophys Acta. 1982 Jun 14;688(2):295–304. doi: 10.1016/0005-2736(82)90340-6. [DOI] [PubMed] [Google Scholar]
- Fuhrmann G. F., Völker B. Misuse of graphical analysis in nonlinear sugar transport kinetics by Eadie-Hofstee plots. Biochim Biophys Acta. 1993 Jan 18;1145(1):180–182. doi: 10.1016/0005-2736(93)90396-h. [DOI] [PubMed] [Google Scholar]
- Gasnier B. Characterization of low- and high-affinity glucose transports in the yeast Kluyveromyces marxianus. Biochim Biophys Acta. 1987 Oct 16;903(3):425–433. doi: 10.1016/0005-2736(87)90049-6. [DOI] [PubMed] [Google Scholar]
- Gonçalves T., Loureiro-Dias M. C. Aspects of glucose uptake in Saccharomyces cerevisiae. J Bacteriol. 1994 Mar;176(5):1511–1513. doi: 10.1128/jb.176.5.1511-1513.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Görts C. P. Effect of glucose on the activity and the kinetics of the maltose-uptake system and of alpha-glucosidase in Saccharomyces cerevisiae. Biochim Biophys Acta. 1969 Jul 30;184(2):299–305. doi: 10.1016/0304-4165(69)90032-4. [DOI] [PubMed] [Google Scholar]
- HERBERT D., ELSWORTH R., TELLING R. C. The continuous culture of bacteria; a theoretical and experimental study. J Gen Microbiol. 1956 Jul;14(3):601–622. doi: 10.1099/00221287-14-3-601. [DOI] [PubMed] [Google Scholar]
- Höfer M., Misra P. C. Evidence for a proton/sugar symport in the yeast Rhodotorula gracilis (glutinis). Biochem J. 1978 Apr 15;172(1):15–22. doi: 10.1042/bj1720015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalina V. Dynamics of microbial growth and metabolic activity and their control by aeration. Antonie Van Leeuwenhoek. 1993;63(3-4):353–373. doi: 10.1007/BF00871230. [DOI] [PubMed] [Google Scholar]
- Kaspar von Meyenburg H. Energetics of the budding cycle of Saccharomyces cerevisiae during glucose limited aerobic growth. Arch Mikrobiol. 1969;66(4):289–303. doi: 10.1007/BF00414585. [DOI] [PubMed] [Google Scholar]
- Ko C. H., Liang H., Gaber R. F. Roles of multiple glucose transporters in Saccharomyces cerevisiae. Mol Cell Biol. 1993 Jan;13(1):638–648. doi: 10.1128/mcb.13.1.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotyk A., Michaljanicová D., Veres K., Soukupová V. Transport of 4-deoxy- and 6-deoxy-D-glucose in baker's yeast. Folia Microbiol (Praha) 1975;20(6):496–503. doi: 10.1007/BF02891709. [DOI] [PubMed] [Google Scholar]
- Kruckeberg A. L., Bisson L. F. The HXT2 gene of Saccharomyces cerevisiae is required for high-affinity glucose transport. Mol Cell Biol. 1990 Nov;10(11):5903–5913. doi: 10.1128/mcb.10.11.5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Käppeli O. Regulation of carbon metabolism in Saccharomyces cerevisiae and related yeasts. Adv Microb Physiol. 1986;28:181–209. doi: 10.1016/s0065-2911(08)60239-8. [DOI] [PubMed] [Google Scholar]
- Lagunas R. Sugar transport in Saccharomyces cerevisiae. FEMS Microbiol Rev. 1993 Apr;10(3-4):229–242. doi: 10.1016/0378-1097(93)90598-v. [DOI] [PubMed] [Google Scholar]
- Lengeler J. W. Carbohydrate transport in bacteria under environmental conditions, a black box? Antonie Van Leeuwenhoek. 1993;63(3-4):275–288. doi: 10.1007/BF00871223. [DOI] [PubMed] [Google Scholar]
- Lewis D. A., Bisson L. F. The HXT1 gene product of Saccharomyces cerevisiae is a new member of the family of hexose transporters. Mol Cell Biol. 1991 Jul;11(7):3804–3813. doi: 10.1128/mcb.11.7.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loureiro-Dias M. C. Movements of protons coupled to glucose transport in yeasts. A comparative study among 248 yeast strains. Antonie Van Leeuwenhoek. 1988;54(4):331–343. doi: 10.1007/BF00393524. [DOI] [PubMed] [Google Scholar]
- Lucero P., Herweijer M., Lagunas R. Catabolite inactivation of the yeast maltose transporter is due to proteolysis. FEBS Lett. 1993 Oct 25;333(1-2):165–168. doi: 10.1016/0014-5793(93)80397-d. [DOI] [PubMed] [Google Scholar]
- Matern H., Holzer H. Catabolite inactivation of the galactose uptake system in yeast. J Biol Chem. 1977 Sep 25;252(18):6399–6402. [PubMed] [Google Scholar]
- Meredith S. A., Romano A. H. Uptake and phosphorylation of 2-deoxy-D-glucose by wild type and respiration-deficient bakers' yeast. Biochim Biophys Acta. 1977 May 26;497(3):745–759. doi: 10.1016/0304-4165(77)90295-1. [DOI] [PubMed] [Google Scholar]
- Needleman R. B., Kaback D. B., Dubin R. A., Perkins E. L., Rosenberg N. G., Sutherland K. A., Forrest D. B., Michels C. A. MAL6 of Saccharomyces: a complex genetic locus containing three genes required for maltose fermentation. Proc Natl Acad Sci U S A. 1984 May;81(9):2811–2815. doi: 10.1073/pnas.81.9.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neigeborn L., Carlson M. Genes affecting the regulation of SUC2 gene expression by glucose repression in Saccharomyces cerevisiae. Genetics. 1984 Dec;108(4):845–858. doi: 10.1093/genetics/108.4.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson N., Taiz L. The evolution of H+-ATPases. Trends Biochem Sci. 1989 Mar;14(3):113–116. doi: 10.1016/0968-0004(89)90134-5. [DOI] [PubMed] [Google Scholar]
- Nevado J., Navarro M. A., Heredia C. F. Transport of hexoses in yeast. Re-examination of the sugar phosphorylation hypothesis with a new experimental approach. Yeast. 1994 Jan;10(1):59–65. doi: 10.1002/yea.320100106. [DOI] [PubMed] [Google Scholar]
- Ongjoco R., Szkutnicka K., Cirillo V. P. Glucose transport in vesicles reconstituted from Saccharomyces cerevisiae membranes and liposomes. J Bacteriol. 1987 Jul;169(7):2926–2931. doi: 10.1128/jb.169.7.2926-2931.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POSTGATE J. R., HUNTER J. R. ACCELERATED DEATH OF AEROBACTER AEROGENES STARVED IN THE PRESENCE OF GROWTH-LIMITING SUBSTRATES. J Gen Microbiol. 1964 Mar;34:459–473. doi: 10.1099/00221287-34-3-459. [DOI] [PubMed] [Google Scholar]
- Paardekooper M., De Bruijne A. W., Van Steveninck J., Van den Broek P. J. Inhibition of transport systems in yeast by photodynamic treatment with toluidine blue. Biochim Biophys Acta. 1993 Sep 19;1151(2):143–148. doi: 10.1016/0005-2736(93)90097-j. [DOI] [PubMed] [Google Scholar]
- Peinado J. M., Loureiro-Dias M. C. Reversible loss of affinity induced by glucose in the maltose-H+ symport of Saccharomyces cerevisiae. Biochim Biophys Acta. 1986 Apr 14;856(2):189–192. doi: 10.1016/0005-2736(86)90027-1. [DOI] [PubMed] [Google Scholar]
- Perlin D. S., San Francisco M. J., Slayman C. W., Rosen B. P. H+/ATP stoichiometry of proton pumps from Neurospora crassa and Escherichia coli. Arch Biochem Biophys. 1986 Jul;248(1):53–61. doi: 10.1016/0003-9861(86)90400-5. [DOI] [PubMed] [Google Scholar]
- Postma E., Kuiper A., Tomasouw W. F., Scheffers W. A., van Dijken J. P. Competition for glucose between the yeasts Saccharomyces cerevisiae and Candida utilis. Appl Environ Microbiol. 1989 Dec;55(12):3214–3220. doi: 10.1128/aem.55.12.3214-3220.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma E., Scheffers W. A., van Dijken J. P. Kinetics of growth and glucose transport in glucose-limited chemostat cultures of Saccharomyces cerevisiae CBS 8066. Yeast. 1989 May-Jun;5(3):159–165. doi: 10.1002/yea.320050305. [DOI] [PubMed] [Google Scholar]
- Postma E., Van den Broek P. J. Continuous-culture study of the regulation of glucose and fructose transport in Kluyveromyces marxianus CBS 6556. J Bacteriol. 1990 Jun;172(6):2871–2876. doi: 10.1128/jb.172.6.2871-2876.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma E., Verduyn C., Kuiper A., Scheffers W. A., van Dijken J. P. Substrate-accelerated death of Saccharomyces cerevisiae CBS 8066 under maltose stress. Yeast. 1990 Mar-Apr;6(2):149–158. doi: 10.1002/yea.320060209. [DOI] [PubMed] [Google Scholar]
- Postma E., Verduyn C., Scheffers W. A., Van Dijken J. P. Enzymic analysis of the crabtree effect in glucose-limited chemostat cultures of Saccharomyces cerevisiae. Appl Environ Microbiol. 1989 Feb;55(2):468–477. doi: 10.1128/aem.55.2.468-477.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior C., Fukuhara H., Blaisonneau J., Wesolowski-Louvel M. Low-affinity glucose carrier gene LGT1 of Saccharomyces cerevisiae, a homologue of the Kluyveromyces lactis RAG1 gene. Yeast. 1993 Dec;9(12):1373–1377. doi: 10.1002/yea.320091211. [DOI] [PubMed] [Google Scholar]
- Ramos J., Szkutnicka K., Cirillo V. P. Characteristics of galactose transport in Saccharomyces cerevisiae cells and reconstituted lipid vesicles. J Bacteriol. 1989 Jun;171(6):3539–3544. doi: 10.1128/jb.171.6.3539-3544.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano A. H. Facilitated diffusion of 6-deoxy-D-glucose in bakers' yeast: evidence against phosphorylation-associated transport of glucose. J Bacteriol. 1982 Dec;152(3):1295–1297. doi: 10.1128/jb.152.3.1295-1297.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouwenhorst R. J., Visser L. E., Van Der Baan A. A., Scheffers W. A., Van Dijken J. P. Production, Distribution, and Kinetic Properties of Inulinase in Continuous Cultures of Kluyveromyces marxianus CBS 6556. Appl Environ Microbiol. 1988 May;54(5):1131–1137. doi: 10.1128/aem.54.5.1131-1137.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouwenhorst R. J., van der Baan A. A., Scheffers W. A., Van Dijken J. P. Production and localization of beta-fructosidase in asynchronous and synchronous chemostat cultures of yeasts. Appl Environ Microbiol. 1991 Feb;57(2):557–562. doi: 10.1128/aem.57.2.557-562.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos E., Rodriguez L., Elorza M. V., Sentandreu R. Uptake of sucrose by Saccharomyces cerevisiae. Arch Biochem Biophys. 1982 Jul;216(2):652–660. doi: 10.1016/0003-9861(82)90255-7. [DOI] [PubMed] [Google Scholar]
- Seaston A., Inkson C., Eddy A. A. The absorption of protons with specific amino acids and carbohydrates by yeast. Biochem J. 1973 Aug;134(4):1031–1043. doi: 10.1042/bj1341031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano R. Energy requirements for maltose transport in yeast. Eur J Biochem. 1977 Oct 17;80(1):97–102. doi: 10.1111/j.1432-1033.1977.tb11861.x. [DOI] [PubMed] [Google Scholar]
- Sreekrishna K., Dickson R. C. Construction of strains of Saccharomyces cerevisiae that grow on lactose. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7909–7913. doi: 10.1073/pnas.82.23.7909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szkutnicka K., Tschopp J. F., Andrews L., Cirillo V. P. Sequence and structure of the yeast galactose transporter. J Bacteriol. 1989 Aug;171(8):4486–4493. doi: 10.1128/jb.171.8.4486-4493.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkacz J. S., Lampen J. O. Surface distributon of invertase on growing Saccharomyces cells. J Bacteriol. 1973 Feb;113(2):1073–1075. doi: 10.1128/jb.113.2.1073-1075.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leeuwen C. C., Postma E., Van den Broek P. J., Van Steveninck J. Proton-motive force-driven D-galactose transport in plasma membrane vesicles from the yeast Kluyveromyces marxianus. J Biol Chem. 1991 Jul 5;266(19):12146–12151. [PubMed] [Google Scholar]
- Van Leeuwen C. C., Weusthuis R. A., Postma E., Van den Broek P. J., Van Dijken J. P. Maltose/proton co-transport in Saccharomyces cerevisiae. Comparative study with cells and plasma membrane vesicles. Biochem J. 1992 Jun 1;284(Pt 2):441–445. doi: 10.1042/bj2840441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Broek P. J., Van Leeuwen C. C., Weusthuis R. A., Postma E., Van Dijken J. P., Karssies R. H., Amons R. Identification of the maltose transport protein of Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1994 Apr 15;200(1):45–51. doi: 10.1006/bbrc.1994.1411. [DOI] [PubMed] [Google Scholar]
- Verduyn C., Postma E., Scheffers W. A., Van Dijken J. P. Effect of benzoic acid on metabolic fluxes in yeasts: a continuous-culture study on the regulation of respiration and alcoholic fermentation. Yeast. 1992 Jul;8(7):501–517. doi: 10.1002/yea.320080703. [DOI] [PubMed] [Google Scholar]
- Verduyn C., Postma E., Scheffers W. A., van Dijken J. P. Energetics of Saccharomyces cerevisiae in anaerobic glucose-limited chemostat cultures. J Gen Microbiol. 1990 Mar;136(3):405–412. doi: 10.1099/00221287-136-3-405. [DOI] [PubMed] [Google Scholar]
- Verduyn C., Stouthamer A. H., Scheffers W. A., van Dijken J. P. A theoretical evaluation of growth yields of yeasts. Antonie Van Leeuwenhoek. 1991 Jan;59(1):49–63. doi: 10.1007/BF00582119. [DOI] [PubMed] [Google Scholar]
- Walsh M. C., Smits H. P., Scholte M., van Dam K. Affinity of glucose transport in Saccharomyces cerevisiae is modulated during growth on glucose. J Bacteriol. 1994 Feb;176(4):953–958. doi: 10.1128/jb.176.4.953-958.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendell D. L., Bisson L. F. Physiological characterization of putative high-affinity glucose transport protein Hxt2 of Saccharomyces cerevisiae by use of anti-synthetic peptide antibodies. J Bacteriol. 1993 Dec;175(23):7689–7696. doi: 10.1128/jb.175.23.7689-7696.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weusthuis R. A., Adams H., Scheffers W. A., van Dijken J. P. Energetics and kinetics of maltose transport in Saccharomyces cerevisiae: a continuous culture study. Appl Environ Microbiol. 1993 Sep;59(9):3102–3109. doi: 10.1128/aem.59.9.3102-3109.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weusthuis R. A., Visser W., Pronk J. T., Scheffers W. A., van Dijken J. P. Effects of oxygen limitation on sugar metabolism in yeasts: a continuous-culture study of the Kluyver effect. Microbiology. 1994 Apr;140(Pt 4):703–715. doi: 10.1099/00221287-140-4-703. [DOI] [PubMed] [Google Scholar]
- Wésolowski-Louvel M., Goffrini P., Ferrero I., Fukuhara H. Glucose transport in the yeast Kluyveromyces lactis. I. Properties of an inducible low-affinity glucose transporter gene. Mol Gen Genet. 1992 May;233(1-2):89–96. doi: 10.1007/BF00587565. [DOI] [PubMed] [Google Scholar]
- Yao B., Sollitti P., Marmur J. Primary structure of the maltose-permease-encoding gene of Saccharomyces carlsbergensis. Gene. 1989 Jul 15;79(2):189–197. doi: 10.1016/0378-1119(89)90201-1. [DOI] [PubMed] [Google Scholar]
- de Hollander J. A. Kinetics of microbial product formation and its consequences for the optimization of fermentation processes. Antonie Van Leeuwenhoek. 1993;63(3-4):375–381. doi: 10.1007/BF00871231. [DOI] [PubMed] [Google Scholar]
- de Koning W., van Dam K. A method for the determination of changes of glycolytic metabolites in yeast on a subsecond time scale using extraction at neutral pH. Anal Biochem. 1992 Jul;204(1):118–123. doi: 10.1016/0003-2697(92)90149-2. [DOI] [PubMed] [Google Scholar]
- van Steveninck J. Transport-associated phosphorylation of 2-deoxy-D-glucose in yeast. Biochim Biophys Acta. 1968 Nov 5;163(3):386–394. doi: 10.1016/0005-2736(68)90123-5. [DOI] [PubMed] [Google Scholar]
- van Uden N. Transport-limited growth in the chemostat and its competitive inhibition; a theoretical treatment. Arch Mikrobiol. 1967;58(2):145–154. doi: 10.1007/BF00406675. [DOI] [PubMed] [Google Scholar]
- van Urk H., Postma E., Scheffers W. A., van Dijken J. P. Glucose transport in crabtree-positive and crabtree-negative yeasts. J Gen Microbiol. 1989 Sep;135(9):2399–2406. doi: 10.1099/00221287-135-9-2399. [DOI] [PubMed] [Google Scholar]
- van den Broek P. J., van Steveninck J. Kinetic analysis of simultaneously occurring proton-sorbose symport and passive sorbose transport in Saccharomyces fragilis. Biochim Biophys Acta. 1980 Nov 4;602(2):419–432. doi: 10.1016/0005-2736(80)90321-1. [DOI] [PubMed] [Google Scholar]



