Abstract
Since the Scheimpflug principle was first described over a century ago, there has been a great interest among ophthalmologists for the use of Scheimpflug camera in anterior segment imaging. Scheimpflug imaging has since advanced significantly and modern day instruments provide comprehensive imaging and topographic data of the anterior segment. In this article the clinical applications and limitations of Scheimpflug imaging in modern cataract surgery patients are discussed. This article reviews recent work on assessment of lens transparency for cataract grading and integrity, using preoperative lens density measurements to help predict phacoemulsification parameters, its utility in challenging situations like capsular bag distension syndrome and traumatic cataract and assessment of density of the posterior capsule for objectively quantifying posterior-capsule opacification.
Keywords: Scheimpflug imaging, Rotating Scheimpflug camera, Anterior segment imaging, Lens density, Posterior-capsule opacification
Introduction
Despite phenomenal advances in cataract surgery outcomes and efficiency over the last two decades, an objective reproducible system of classification has eluded the vision research community. Quantitative measurement of cataract is vital for investigating the possible risk factors of cataract formation, documenting progression of cataract in longitudinal studies, as well as for epidemiologic studies and clinical trials.1 The ideal lens grading system should be objective and reproducible. A standardized grading system is also imperative to accurately compare the severity of cataract visualized at past appointments, because in the modern healthcare system, the patient is often examined by a different ophthalmologist at each visit. This is imperative in longitudinal studies on nuclear cataract as well. Objective grading systems are desirable in nuclear cataract because it comprises the largest subgroup of cataract in clinical studies, and its grading is often challenging. Several clinical classifications have been used to evaluate cataract, including the lens opacities classification system (LOCS) III,2 the age-related eye disease study,3 and Laser slit-lamp evaluation,4 with the LOCS III being the most established.
However, the LOCS III and other systems are based on clinical measurements such as slit-lamp evaluation, lens photography, patient age, best corrected visual acuity (BCVA) and have a common limitation of the subjective nature of grading. They are influenced by the slit-lamp settings and level of training of the evaluator. This could lead to inconsistencies in the application of this system over time and between observers.
These concerns about the LOCS III system encouraged the development of objective methods based on Scheimpflug photography. The Scheimpflug principle, first introduced by Theodor Scheimpflug, a cartographer of the Austrian navy, describes an optical imaging condition, which allows documentation of an obliquely tilted object with the maximally possible depth of focus and minimal image distortion. This increased depth of field or plane of sharp focus in Scheimpflug imaging is achieved by changing the relative plane of film (sensor) to the camera lens. In a regular camera, the film plane and the lens plane are parallel to each other, resulting in a plane of focus that runs parallel to these. Slit-lamp photographs, on the other hand, provide an image with the best focus corresponding to only a part of the lens either the anterior or posterior capsule, while other parts of the image are not focused.5 Out of focus images cannot be accurately analyzed for lens density. In Fig. 1 the lens plane is inclined to the film plane resulting in change of focus plane along the line of intersection (Scheimpflug line). All objects on this Scheimpflug line will remain in focus.
Figure 1.
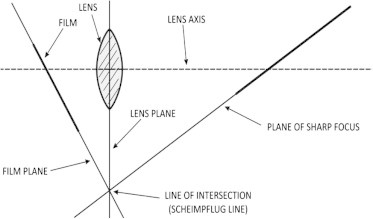
The Scheimpflug principle describing three planes must converge along a single line. These three planes are the film plane, the subject plane and lens plane. The lens plane is a flat surface drawn through the center of the lens and remaining perpendicular to the lens axis (a line straight through the lens). With a normal camera, when the subject is not parallel to the image plane, only a small region is in focus. This limitation is overcome with the Scheimpflug camera.
The Pentacam Scheimplfug camera (Oculus, Wetzlar, Germany) captures a sharply focused section of lens and can compute analysis of density at different points of the image in up to 100 radii (using the HR model) thus giving a nearly 3-D calculation of lens density. Scheimpflug photographs were initially described for imaging of cataracts by Brown6,7 and subsequently Hockwin et al.8 Several systems have since been developed and researched for grading lens opacities, including the Oxford Scheimpflug System,9 the Topcon SL-45 (Topcon, Tokyo, Japan),10 the Zeiss Scheimpflug video camera (Carl Zeiss Meditec, Dublin, CA),11 the Nidek EAS-1000 (Nidek, Japan).12
Scheimpflug images provide a section of the lens, evenly focused from the anterior to posterior capsule. The Scheimpflug images also allow for a truly continuous measure as compared with the LOCS III system, which has grading systems in steps, thereby permitting the detection of subtle amounts of cataract progression in a shorter period of time. The rotating Scheimpflug camera offers several distinct advantages over previous Scheimpflug systems,13–16 which were mostly designed to capture an image in a single meridian, thereby providing lens density values limited to that single section. By capturing up to 100 slices in a single 180° sweep around the central axis of the lens of the eye in approximately 2 s, the rotating Scheimpflug camera overcomes the limitation of capturing images in a single section, thereby providing a global 360° evaluation of lens density and avoiding the need to acquire multiple scans at different meridians. Scheimpflug densitometry of the human lens provides an analysis of the lens opacification or the loss of transparency or cataract and this calculation is based on reflectometry, since we measure the reflected light.
We recently evaluated the relationship of lens density calculated using Scheimpflug images system with the LOCS III grading and visual function as measured by logarithmic minimal angle resolution (logMAR) BCVA and photopic contrast sensitivity.17 The importance of contrast sensitivity compared with visual acuity in assessment of quality of vision has been reported since our surrounding environment contains greater stimuli, which are low contrast as compared to high contrast visual stimuli. We obtained standardized digital images of the lens according to the LOCS III protocol on the Topcon SL-D digital slit-lamp (Topcon, Tokyo, Japan) and Scheimpflug images were obtained using the rotating Scheimpflug camera. All images were obtained for one eye of each patient. The LOCS III nuclear opacity was graded by comparing the digital photograph of the lens to be graded with the standard color photographic transparencies of cortical cataract, nuclear opalescence (NO), nuclear color (NC) and posterior subcapsular cataract. For this study only the NO and NC gradings were used.
Analysis of Scheimpflug images
In our recent study we defined a region of interest on individual Scheimpflug Images which was analyzed using ImageJ Software18 Since certain lens regions are more representative of the opacification and change in severity of nuclear sclerosis (or lens density) than others, we defined a “region of interest” (ROI) that excluded the lens cortex, similar to previous work by Duncan et al.19 Eliminating information from the lens cortex is important in defining a common lens nuclear area and measuring the optical density in that area. A standard elliptical mask was defined to encompass as much of the lens nucleus as possible without including the cortex (Fig. 2). This area provided an integral sample for documentation and evaluation of nuclear density, and it has been reported to be capable of being used almost universally for all patients in the age range of 50–80 years.20 The average lens density was calculated by marking the edges of the lens. Fifty Scheimpflug images were captured and analyzed for each eye.
Figure 2.
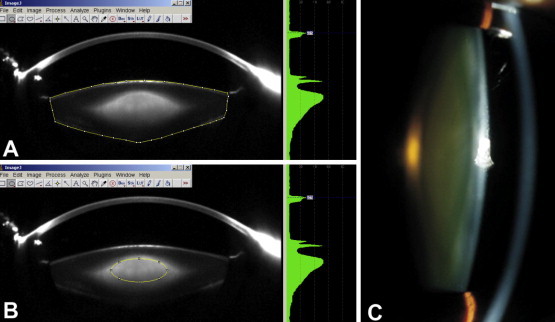
Lens density assessment with ImageJ. Scheimpflug image of the lens exported to ImageJ software for measuring the average lens density (A), nuclear lens density in the region of interest, marked by the elliptical mask (B), and a digital slit-lamp photograph of the same lens (C).
The reproducibility of lens density was evaluated using Scheimpflug images and ImageJ software based on five successive scans obtained by the same operator in the right eye in a subset of 30 patients. Bland-Altman plot showing (Fig. 3A) the repeatability of lens density measurements (average lens density and nuclear lens density measured in pixel intensity units) obtained using ImageJ software on 50 Scheimpflug images each, for a subset of 30 patients. Average lens density measurements demonstrated an intraclass correlation coefficient (ICC) of 0.983 (95% CI, 0.972–0.991). The coefficient of variation (CoV) was 3.92 ± 1.76% (range 0.55–7.32%). Nuclear lens density measurements demonstrated an ICC of 0.99 (95% CI, 0.982–0.998) and a CoV of 2.57 ± 0.74% (range, 0.32–4.21%). Similar results were also recently demonstrated by Kirkwood and associates.21
Figure 3.
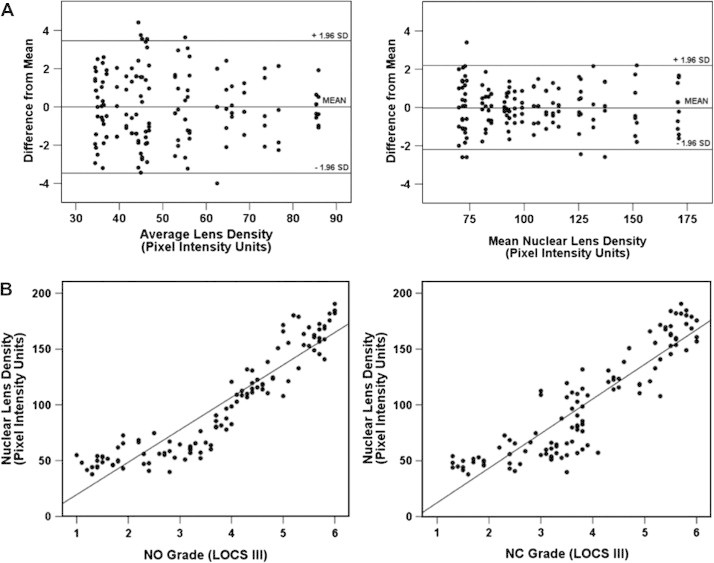
(A) Repeatability of lens density using Scheimpflug images. (B) Correlation of lens density using Scheimplfug images with LOCS III grading.
The average lens density correlated with NO grade (r = 0.774; p < 0.001), NC grade (r = 0.732; p < 0.001) (Fig. 3B), logMAR BCVA (r = 0.696; p < 0.001), CS at 3 CPD (r = 0.242; p = 0.011), at 6 CPD (r = 0.473; p < 0.001), 12 CPD (r = 0.497; p < 0.001), and 18 CPD (r = 0.480; p = 0.001). The pixel intensity for the nuclear lens density (ROI) correlated with NO grade (r = 0.859; p < 0.001; NC (r = 0.81; p < 0.001), logMAR BCVA (r = 0.760; p < 0.001), CS at 3 CPD (r = 0.299; p = 0.002), at 6 CPD (r = 0.548; p < 0.001), 12 CPD (r = 0.603; p < 0.001), and 18 CPD (r = 0.485; p < 0.001).
We observed that although both the average and nuclear lens density measurements significantly correlated with LOCS III grading, logMAR BCVA, and photopic contrast sensitivity, the correlation of nuclear lens density was significantly stronger. This confirms earlier reports highlighting the importance of this region and its strong correlation with visual function.22 The reproducibility of lens density for the nuclear region (CoV 2.57%) was higher than that for the average lens density (CoV 3.92%).
The correlation of contrast sensitivity with the nuclear lens density is significant because it suggests that the Scheimpflug grading system could provide a sensitive indicator of the quality of vision. Drews-Bankiewicz et al.23 had earlier demonstrated a similar correlation using a Scheimpflug slit-lamp video camera. Our data concerning the reproducibility of average lens density on Scheimpflug images showed an ICC of 0.983, which was marginally higher than the 0.95–0.97 reported for the LOCS III24 and similar to that reported using the anterior segment optical coherence tomography (ASOCT).25 Pei and associates also demonstrated similar results using the peak nuclear density value.26 The CoV for reproducibility of nuclear lens density measurements was 2.57% as compared with 4.55% using the ASOCT.25
Lens density values derived from semi-automated densitometry measurements of Scheimpflug photographs diminish grading variability and therefore permit better estimates of change. We demonstrated that the mean 360° lens density value obtained using a rotating Scheimpflug camera correlated well with the currently established LOCS III grading system, visual acuity, and photopic contrast sensitivity. The correlation obtained for the nuclear lens density was the highest and this was also found to be the most reproducible measurement, suggesting that this index could be a valuable tool in future clinical and research trials permitting the detection of changes in nuclear density over a short period of time.
Commercially available software on the Oculus Scheimpflug camera now incorporates the Pentacam Nucleus Staging (PNS) function that evaluates volume and optical density in three dimensions through a dilated pupil. The software uses the data from up to 100 Scheimpflug images to virtually reconstruct the lens, which it then evaluates using a series of 3-D shapes to assess the mean optical density of the sampled volumes. The density is subsequently compared against a developed nomogram and the cataract is assigned a grade from 0 to 5.
One of the limitations of Scheimpflug imaging is that the internal structure of the lens is observed through the preceding refractive surfaces, that is, the cornea and the anterior lens surface, and the refraction at these surfaces distorts the shape of the internal structure of the lens. Other limitations are imaging through a poorly dilated pupil interferes with the Scheimpflug camera’s sampling technology, eyes with pseudoexfoliation, and intraoperative floppy iris syndrome and, white cataracts which limit the Scheimpflug camera’s ability to accurately sample the central nucleus. Fig. 4 demonstrates two different grades of nuclear sclerosis using slit-lamp images on the left and Scheimpflug images on the right. Fig. 5 provides representative images of different types of cataract as seen on Scheimpflug images.
Figure 4.
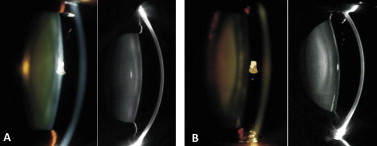
Comparison of slit-lamp and Scheimpflug images. This figure (A and B) demonstrates two different grades of nuclear sclerosis using slit-lamp images on the left and Scheimpflug images on the right.
Figure 5.
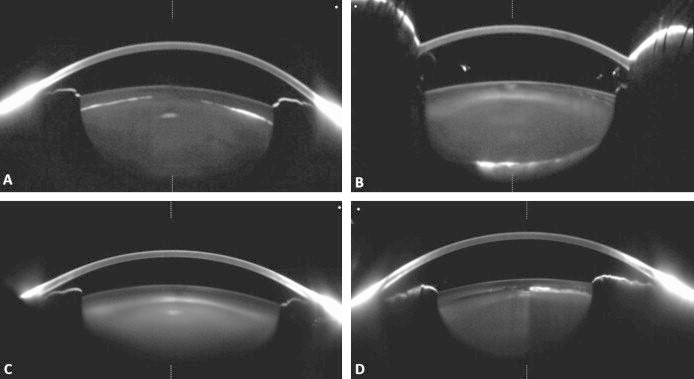
(A) Anterior cortical cataract – the anterior capsule is clearly delineated. The plane of the opacities is defined. The depth can be measured in microns. The density of the cataract is displayed by placing the cursor over the cataract. (B Posterior polar cataract. (C) Nuclear sclerosis. Note the uniform increase in the opalescence of the nucleus. A band of increased density is observed in the deep anterior cortex. The cortex anterior to it is clear. (D) Zonular cataract.
Representative images of different types of cataract
Utility of preoperative lens density in planning phacoemulsification
Objective lens density quantification could assist in predicting phacodynamics in cataract surgery. The efficiency of ultrasound (US) energy use in phacoemulsification systems is important. The nucleus and the epi-nucleus are the primary parts of a cataract that require US energy via phacoemulsification for removal. In the context of phaco efficiency, little phacoemulsification power is expended to remove the sub-capsular cortex of the cataract or posterior sub-capsular cataract, which can usually be extracted during the irrigation/aspiration phase of cataract removal. Lower levels of US power may help limit endothelial cell damage and result in clearer post operative corneas; therefore, it is helpful to plan phacoemulsification surgery with the lowest levels of US power needed based on cataract density.
Previous studies have shown that cataracts at a more advanced stage on the LOCS scale were found to require more energy and a longer effective phacoemulsification time. Effective phacoemulsification time (EPT), EPT = (Average phacoemulsification power × US Time)/100%. (This represents how long the phacoemulsification time would have been if 100% power, continuous mode had been utilized.)
Nixon15 recently demonstrated that when using PNS to grade cataracts, there was an overall reduction in needle time, BSS use and the phacoemulsification energy required to remove grade 1 cataracts as well as cataracts of grades 4 and 5. Both Nixon15 and Kim et al.14 also recently showed that Scheimpflug-measured lens nuclear density had a positive correlation with cumulated dissipated energy and torsional amplitude and time during phacoemulsification. They also demonstrated a linear relationship between the phacoemulsification energy and the cataract’s relative grade.
We analyzed 250 eyes of 250 patients undergoing cataract surgery. We found that nuclear lens density had a stronger correlation as compared to average lens density with effective phacoemulsification time (Z = −3.06, p = 0.002); UST (Z = −2.21, p = 0.03) and average phacoemulsification power (Z = −3.6, p = 0.0003) (Fig. 6). The stronger correlation of nuclear lens density could be explained by the assumption that the majority of phacoemulsification energy is expended in removing the nucleus. This suggests the utility of nuclear lens density in preoperative planning.
Figure 6.
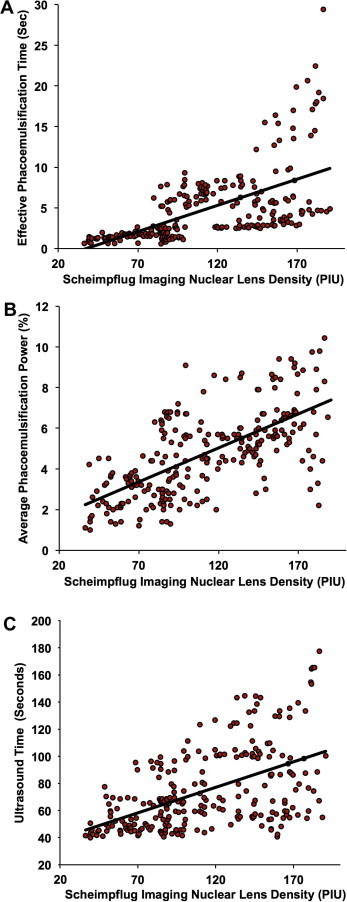
Scatter plots demonstrating correlation between nuclear lens density calculated using Scheimpflug images and phacoemulsification parameters: effective phacoemulsification time (r = 0.597, p < 0.001; top), average phacoemulsification power (r = 0.653, p < 0.001; middle) and ultrasound time (r = 0.521, p < 0.001; bottom).
Scheimpflug lens densitometry offers the ability to preoperatively assess and quantify an individual nuclear cataract. This could potentially allow for pre-programming the phacoemulsification parameters and tip design to optimize surgical efficiency, improve safety and predict the phacodynamics and facilitate the development of “customized cataract surgery”. Preoperatively customized phacoemulsification settings based on an objective measurement of cataract rather than making intraoperative adjustments could yield lower phacoemulsification time, lower needle time, and (potentially) better outcomes.
In addition, the significant correlation between EPT and cataract density allows this technique to be advanced as a possible standard for future comparisons in cataract surgery such as comparing longitudinal and transverse modes of phacoemulsification and other machine parameters.
Capsular bag distension syndrome
Capsular bag distention syndrome (CBDS) is an uncommon but well recognized complication after cataract surgery. It is associated with enlargement of the space between intraocular lens (IOL) implant and posterior capsule. It often produces anterior vaulting of IOL optic, anterior bowing of the iris, shallowing of anterior chamber, and a myopic shift. The fluid within the capsular space can turn turbid or cloudy, resulting in decreased vision. Scheimpflug imaging can document CBDS27 including the exact measurements of the distended posterior capsule, density or turbidity of the fluid, IOL position, and its relation to the capsular bag and iris to rule out pupillary block (Fig. 7). The decrease in distention of the posterior capsule post-capsulotomy could be measured. Scheimpflug imaging in such cases is quicker and more precise than ultrasound biomicroscopy and high-frequency ultrasonography. It is important to realize these imaging technologies this is not a substitute for a thorough and accurate slit-lamp examination. Screening pseudophakic eyes using Scheimpflug imaging could help to detect and quantify CBDS at an early stage and to document changes over a period of time and the post-capsulotomy status. In a series of 11 eyes with CBDS we found that the average amount of myopia induced due to CBDS was 0.75 D and the average hyperopic shift after capsulotomy was 0.64 D.
Figure 7.
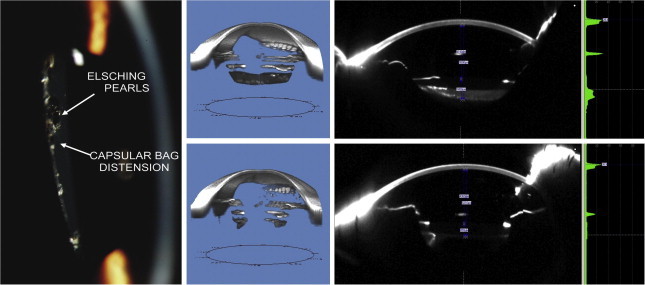
Slit-lamp photograph of capsular bag distension syndrome showing posterior bowing of the opacified posterior capsule with Elschnig pearls (left), Scheimpflug image based tomogram (top center) and Scheimpflug image (top right) showing capsular bag distension syndrome allowing for calculation of exact measurements of the distended posterior capsule, assessment of density or turbidity of the fluid, IOL position, and its relation to the capsular bag and iris to rule out pupillary block with pre-capsulotomy measurements. The post-capsulotomy tomogram (bottom center) and Scheimpflug image (bottom right) illustrate the change in measurements. Size of the distended capsular bag may help predict the refractive outcome after capsulotomy.
Posterior-capsule opacification
Posterior-capsule opacification (PCO) remains the most common postoperative cause of impaired visual acuity following cataract extraction. There is continued focus on several experimental and clinical trials, including studies of surgical techniques, IOL design, and drugs to reduce the incidence of PCO. An objective quantitative measurement of PCO is of paramount importance to assess the efficacy of such trials. Although several imaging systems have been reported, at present there is no consensus on an optimal quantification method for PCO analysis. The use of Scheimpflug imaging to quantify PCO was first reported in 1995 by Lasa et al.28 The earlier Scheimpflug systems could only capture images in one meridian at a time,28 and Hayashi et al.29,30 had analyzed data using single-slit Scheimpflug images in up to four meridians. Subsequently, several studies have been published using Scheimpflug images, and the results have been correlated with histologic findings.31 The rotating Scheimpflug camera has a distinct advance as it can capture images in multiple meridians in a single automated scan. We recently described32 a new system of measurement to objectively quantify PCO using ImageJ software (National Institutes of Health, Bethesda, MD) based analysis of Scheimpflug Tomograms which produced highly reproducible results that correlated with the results obtained from analysis of slit-lamp-based retro-illumination photographs using the POCOman system.
Scheimpflug image based tomogram images have a distinct advantage over the previous Scheimpflug camera (Anterior Eye Segment Analysis System EAS-1000; Nidek, Tokyo, Japan) on which PCO density was analyzed in the central 3-mm region. Because the tomogram is reconstructed from 50 Scheimpflug images, it covers almost the entire area of the posterior capsule instead of a single-slit beam meridian or the average density calculated from four meridians. The rotating Scheimpflug camera allows 50 images to be reconstructed into a single image. Before the availability of the Scheimpflug image based tomograms, there was no way to correlate the value of PCO obtained on Scheimpflug images with the slit-lamp images because the principles of the two photographic techniques are different. Tomograms allow the creation of a Scheimpflug-based PCO tomogram in the same plane as a slit-lamp retro-illumination image, and the two may be compared (Fig. 8) Given that the tomograms are easier and quicker to obtain, provide PCO pixel intensity in up to 50 meridians, have no observer bias, and allow for a more objective analysis than slit-lamp images, Scheimpflug image based tomograms have the potential to become an efficient grading system for PCO.
Figure 8.
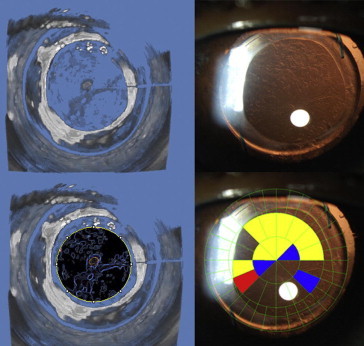
Assessment of posterior-capsule opacification (PCO) using Scheimpflug image based tomograms. Scheimpflug tomogram (which is a 3-D reconstruction from Scheimpflug images) (top left) demonstrating an opacified posterior capsule and the corresponding slit-lamp retro-illumination image (top right). ImageJ software (NIH, Bethesda, MD) was used to detect the density of PCO on the Scheimpflug tomogram (bottom left) which correlated with the PCO grades obtained using an established method of assessing PCO on slit-lamp images: POCOman software (bottom right).
Conclusion
In conclusion, Scheimpflug imaging is simple to perform, rapid, and has an easier learning curve in contrast with slit-lamp-based photographic lens grading systems. It provides lens densitometry measurements as an easy, quick, objective, repeatable assessment of cataract and is a step forward for precise grading and tracking lens changes over time. This technology has great potential in documenting progression of cataract in longitudinal studies, as well as for epidemiologic studies and clinical trials.
The versatility of Scheimpflug imaging in assessing posterior-capsule opacification, different types of cataract and its utility in challenging cataract cases such as those with preexisting posterior-capsule rupture and traumatic cataracts make it an indispensable tool for the modern day cataract surgeon.
References
- 1.Chylack L.T., Jr, Wolfe J.K., Singer D.M. The lens opacities classification system III. Arch Ophthalmol. 1993;111(6):831–836. doi: 10.1001/archopht.1993.01090060119035. [DOI] [PubMed] [Google Scholar]
- 2.Chylack L.T., Wolfe J.K., Singer D.M. The lens opacities classification system III. The longitudinal study of cataract study group. Arch Ophthalmol. 1993;111(6):831–836. doi: 10.1001/archopht.1993.01090060119035. [DOI] [PubMed] [Google Scholar]
- 3.The Age-Related Eye Disease Research Group. The age-related eye disease study (AREDS) system for classifying cataracts from photographs: AREDS report no. 4. Am J Ophthalmol 2001;131(2):167–75. [DOI] [PMC free article] [PubMed]
- 4.Hall N.F., Lempert P., Shier R.P., Zakir R., Phillips D. Grading nuclear cataract: reproducibility and validity of a new method. Br J Ophthalmol. 1999;83(10):1159–1163. doi: 10.1136/bjo.83.10.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.West S.K., Rosenthal F., Newland H.S., Taylor H.R. Use of photographic techniques to grade nuclear cataracts. Invest Ophthalmol Vis Sci. 1988;29(1):73–77. [PubMed] [Google Scholar]
- 6.Brown N. An advanced slit-image camera. Br J Ophthalmol. 1972;56(8):624–631. doi: 10.1136/bjo.56.8.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown N. Quantitative slit-image photography of the lens. Trans Ophthalmol Soc UK. 1972;92:303–307. [PubMed] [Google Scholar]
- 8.Hockwin O., Dragomirescu V., Laser H. Measurements of lens transparency or its disturbances by densitometric image analysis of Scheimpflug photographs. Graefes Arch Clin Exp Ophthalmol. 1982;219(6):255–262. doi: 10.1007/BF00231409. [DOI] [PubMed] [Google Scholar]
- 9.Brown N.A.P., Bron A.J., Sparrow J.M. Methods for evaluation of lens changes. Int Ophthalmol. 1988;12(4):227–235. doi: 10.1007/BF00133938. [DOI] [PubMed] [Google Scholar]
- 10.Kashima K., Trus B.L., Unser M., Edwards P.A., Datiles M.B. Aging studies on normal lens using the Scheimpflug slit-lamp camera. Invest Ophthalmol Vis Sci. 1993;34(1):263–269. [PubMed] [Google Scholar]
- 11.Magno B.V., Freidlin V., Datiles M.B. Reproducibility of the NEI Scheimpflug cataract imaging system. Invest Ophthalmol Vis Sci. 1994;35(7):3078–3084. [PubMed] [Google Scholar]
- 12.Foo K.P., Maclean H. Measured changes in cataract over six months: sensitivity of the Nidek EAS-1000. Ophthalmic Res. 1996;28(Suppl. 2):32–36. doi: 10.1159/000267954. [DOI] [PubMed] [Google Scholar]
- 13.Pei X., Bao Y., Chen Y., Li X. Correlation of lens density measured using the Pentacam Scheimpflug system with the lens opacities classification system III grading score and visual acuity in age-related nuclear cataract. Br J Ophthalmol. 2008;92:1471–1475. doi: 10.1136/bjo.2007.136978. [DOI] [PubMed] [Google Scholar]
- 14.Kim J.-S., Chung S.-H., Joo C.-K. Clinical application of a Scheimpflug system for lens density measurements in phacoemulsification. J Cataract Refract Surg. 2009;35(7):1204–1209. doi: 10.1016/j.jcrs.2009.02.032. [DOI] [PubMed] [Google Scholar]
- 15.Nixon D. Preoperative cataract grading by Scheimpflug imaging and effect on operative fluidics and phacoemulsification energy. J Cataract Refract Surg. 2010;36(2):242–246. doi: 10.1016/j.jcrs.2009.08.032. [DOI] [PubMed] [Google Scholar]
- 16.Kirkwood B.J., Hendicott P.L., Read S.A., Pesudovs K. Repeatability and validity of lens densitometry measured with Scheimpflug imaging. J Cataract Refract Surg. 2009;35(19545810):1210–1215. doi: 10.1016/j.jcrs.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 17.Grewal D.S., Brar G.S., Grewal S.P. Correlation of nuclear cataract lens density using Scheimpflug images with lens opacities classification system III and visual function. Ophthalmology. 2009;116(8):1436–1443. doi: 10.1016/j.ophtha.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 18.Abramoff M.D., Magalhaes P.J., Ram S.J. Image processing with ImageJ. Biophotonics Int. 2004;1(7):36–42. [Google Scholar]
- 19.Duncan D.D., Shukla O.B., West S.K., Schein O.D. New objective classification system for nuclear opacification. J Opt Soc Am A Opt Image Sci Vis. 1997;14(6):1197–1204. doi: 10.1364/josaa.14.001197. [DOI] [PubMed] [Google Scholar]
- 20.Qian W., Söderberg P.G., Chen E., Magnius K., Philipson B. 3 year simvastatin treatment and lens nuclear back scattering. Br J Ophthalmol. 2000;84(5):512–516. doi: 10.1136/bjo.84.5.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirkwood B.J., Hendicott P.L., Read S.A., Pesudovs K. Repeatability and validity of lens densitometry measured with Scheimpflug imaging. J Cataract Refract Surg. 2009;35(7):1210–1215. doi: 10.1016/j.jcrs.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 22.Qian W., Söderberg P.G., Chen E., Magnius K., Philipson B. A common lens nuclear area in Scheimpflug photographs. Eye. 1993;7(Pt. 6):799–804. doi: 10.1038/eye.1993.187. [DOI] [PubMed] [Google Scholar]
- 23.Drews-Bankiewicz M.A., Caruso R.C., Datiles M.B., Kaiser-Kupfer M.I. Contrast sensitivity in patients with nuclear cataracts. Arch Ophthalmol. 1992;110(7):953–959. doi: 10.1001/archopht.1992.01080190059029. [DOI] [PubMed] [Google Scholar]
- 24.Chylack L.T., Wolfe J.K., Friend J. Validation of methods for the assessment of cataract progression in the Roche European-American Anticataract Trial (REACT) Ophthalmic Epidemiol. 1995;2(2):59–75. doi: 10.3109/09286589509057085. [DOI] [PubMed] [Google Scholar]
- 25.Wong A.L., Leung C.K., Weinreb R.N. Quantitative assessment of lens opacities with anterior segment optical coherence tomography. Br J Ophthalmol. 2009;93(1):61–65. doi: 10.1136/bjo.2008.137653. [DOI] [PubMed] [Google Scholar]
- 26.Pei Xueting. Correlation of lens density measured using Pentacam Scheimpflug system with LOCS III grading score and visual acuity in age-related nuclear cataract. Br J Ophthalmol. 2008;92(11):bjo–136978. doi: 10.1136/bjo.2007.136978. [DOI] [PubMed] [Google Scholar]
- 27.Jain R., Grewal D., Gupta R., Grewal S.P. Scheimpflug imaging in late capsular bag distention syndrome after phacoemulsification. Am J Ophthalmol. 2006;142(6):1083–1085. doi: 10.1016/j.ajo.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 28.Lasa M.S., Datiles M.B., Magno B.V., Mahurkar A. Scheimpflug photography and postcataract surgery posterior capsule opacification. Ophthalmic Surg. 1995;26(2):110–113. [PubMed] [Google Scholar]
- 29.Hayashi H., Hayashi K., Nakao F., Hayashi F. Quantitative comparison of posterior capsule opacification after polymethylmethacrylate, silicone, and soft acrylic intraocular lens implantation. Arch Ophthalmol. 1998;116(12):1579–1582. doi: 10.1001/archopht.116.12.1579. [DOI] [PubMed] [Google Scholar]
- 30.Hayashi K., Hayashi H. Posterior capsule opacification after implantation of a hydrogel intraocular lens. Br J Ophthalmol. 2004;88(2):182–185. doi: 10.1136/bjo.2003.023580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saika S., Miyamoto T., Ishida I. Comparison of Scheimpflug images of posterior capsule opacification and histological findings in rabbits and humans. J Cataract Refract Surg. 2001;27(7):1088–1092. doi: 10.1016/s0886-3350(00)00860-9. [DOI] [PubMed] [Google Scholar]
- 32.Grewal D., Jain R., Brar G.S., Grewal S.P. Pentacam tomograms: a novel method for quantification of posterior capsule opacification. Invest Ophthalmol Vis Sci. 2008;49(5):2004–2008. doi: 10.1167/iovs.07-1056. [DOI] [PubMed] [Google Scholar]


