Abstract
Chlamydiae are medically important bacteria responsible for a wide range of human infections and diseases. Repeated episodes of infection promote chronic inflammation associated with detrimental immune system-mediated pathologic changes. However, the true nature of chlamydial pathogenesis may encompass repeated infection superimposed upon persistent infection, which would allow for heightened immune reactivity. During the course of chlamydial infection, numerous host elaborated factors with inhibitory or modifying effects may cause alterations in the chlamydia-host cell relationship such that the organism is maintained in a nonproductive stage of growth. Abnormal or persistent chlamydiae have been recognized under a variety of cell culture systems. The numerous factors associated with altered growth suggest an innate flexibility in the developmental cycle of chlamydiae. This review evaluates in vitro studies of chlamydial persistence and correlates these model systems to features of natural chlamydial disease.
Full text
PDF
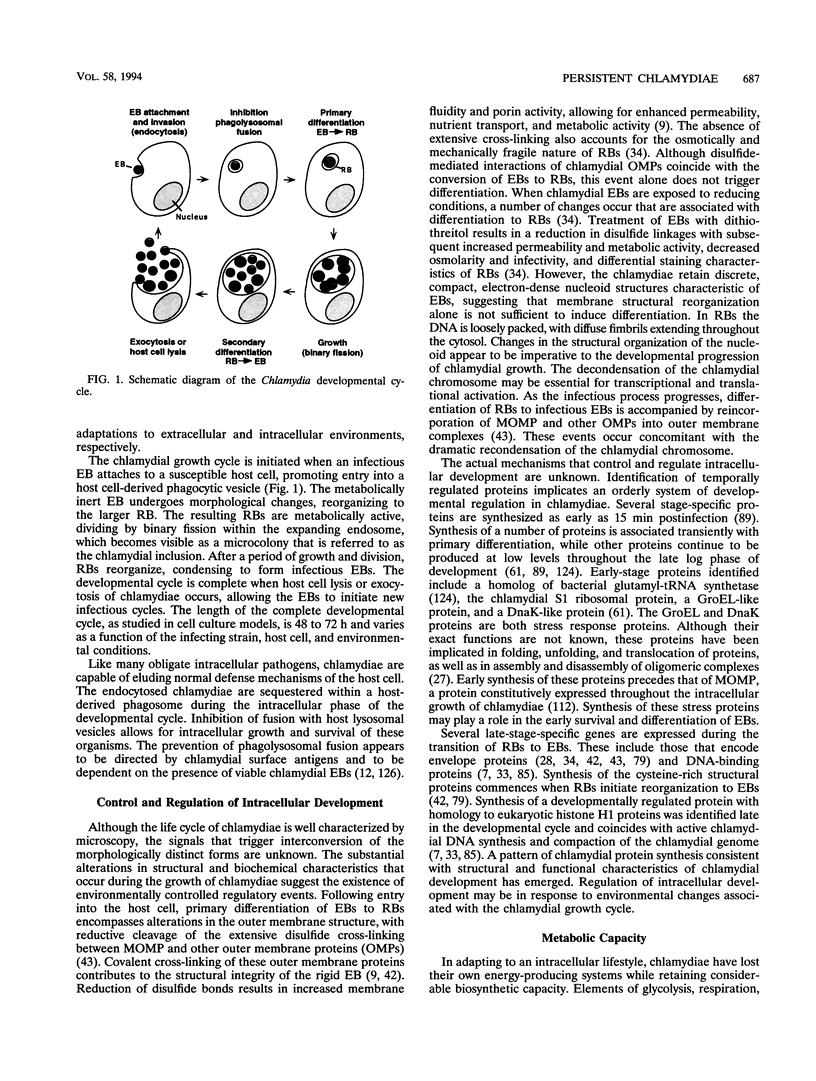









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan I., Hatch T. P., Pearce J. H. Influence of cysteine deprivation on chlamydial differentiation from reproductive to infective life-cycle forms. J Gen Microbiol. 1985 Dec;131(12):3171–3177. doi: 10.1099/00221287-131-12-3171. [DOI] [PubMed] [Google Scholar]
- Allan I., Pearce J. H. Amino acid requirements of strains of Chlamydia trachomatis and C. psittaci growing in McCoy cells: relationship with clinical syndrome and host origin. J Gen Microbiol. 1983 Jul;129(7):2001–2007. doi: 10.1099/00221287-129-7-2001. [DOI] [PubMed] [Google Scholar]
- BADER J. P., MORGAN H. R. Latent viral infection of cells in tissue culture. VI. Role of amino acids, glutamine, and glucose in psittacosis virus propagation in L cells. J Exp Med. 1958 Nov 1;108(5):617–630. doi: 10.1084/jem.108.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BADER J. P., MORGAN H. R. Latent viral infection of cells in tissue culture. VII. Role of water-soluble vitamins in psittacosis virus propagation in L cells. J Exp Med. 1961 Feb 1;113:271–281. doi: 10.1084/jem.113.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Amano K., Hackstadt T., Perry L., Caldwell H. D. Chlamydia trachomatis has penicillin-binding proteins but not detectable muramic acid. J Bacteriol. 1982 Jul;151(1):420–428. doi: 10.1128/jb.151.1.420-428.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry C. E., 3rd, Hayes S. F., Hackstadt T. Nucleoid condensation in Escherichia coli that express a chlamydial histone homolog. Science. 1992 Apr 17;256(5055):377–379. doi: 10.1126/science.256.5055.377. [DOI] [PubMed] [Google Scholar]
- Batteiger B. E., Fraiz J., Newhall W. J., Katz B. P., Jones R. B. Association of recurrent chlamydial infection with gonorrhea. J Infect Dis. 1989 Apr;159(4):661–669. doi: 10.1093/infdis/159.4.661. [DOI] [PubMed] [Google Scholar]
- Bavoil P., Ohlin A., Schachter J. Role of disulfide bonding in outer membrane structure and permeability in Chlamydia trachomatis. Infect Immun. 1984 May;44(2):479–485. doi: 10.1128/iai.44.2.479-485.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty W. L., Byrne G. I., Morrison R. P. Morphologic and antigenic characterization of interferon gamma-mediated persistent Chlamydia trachomatis infection in vitro. Proc Natl Acad Sci U S A. 1993 May 1;90(9):3998–4002. doi: 10.1073/pnas.90.9.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownridge E., Wyrick P. B. Interaction of Chlamydia psittaci reticulate bodies with mouse peritoneal macrophages. Infect Immun. 1979 Jun;24(3):697–700. doi: 10.1128/iai.24.3.697-700.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunham R. C., Maclean I. W., Binns B., Peeling R. W. Chlamydia trachomatis: its role in tubal infertility. J Infect Dis. 1985 Dec;152(6):1275–1282. doi: 10.1093/infdis/152.6.1275. [DOI] [PubMed] [Google Scholar]
- Byrne G. I., Faubion C. L. Inhibition of Chlamydia psittaci in oxidatively active thioglycolate-elicited macrophages: distinction between lymphokine-mediated oxygen-dependent and oxygen-independent macrophage activation. Infect Immun. 1983 May;40(2):464–471. doi: 10.1128/iai.40.2.464-471.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne G. I., Faubion C. L. Lymphokine-mediated microbistatic mechanisms restrict Chlamydia psittaci growth in macrophages. J Immunol. 1982 Jan;128(1):469–474. [PubMed] [Google Scholar]
- Byrne G. I., Krueger D. A. Lymphokine-mediated inhibition of Chlamydia replication in mouse fibroblasts is neutralized by anti-gamma interferon immunoglobulin. Infect Immun. 1983 Dec;42(3):1152–1158. doi: 10.1128/iai.42.3.1152-1158.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne G. I., Lehmann L. K., Landry G. J. Induction of tryptophan catabolism is the mechanism for gamma-interferon-mediated inhibition of intracellular Chlamydia psittaci replication in T24 cells. Infect Immun. 1986 Aug;53(2):347–351. doi: 10.1128/iai.53.2.347-351.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell L. A., Patton D. L., Moore D. E., Cappuccio A. L., Mueller B. A., Wang S. P. Detection of Chlamydia trachomatis deoxyribonucleic acid in women with tubal infertility. Fertil Steril. 1993 Jan;59(1):45–50. [PubMed] [Google Scholar]
- Campbell S., Richmond S. J., Haynes P., Gump D., Yates P., Allen T. D. An in vitro model of Chlamydia trachomatis infection in the regenerative phase of the human endometrial cycle. J Gen Microbiol. 1988 Jul;134(7):2077–2087. doi: 10.1099/00221287-134-7-2077. [DOI] [PubMed] [Google Scholar]
- Cates W., Jr, Wasserheit J. N. Genital chlamydial infections: epidemiology and reproductive sequelae. Am J Obstet Gynecol. 1991 Jun;164(6 Pt 2):1771–1781. doi: 10.1016/0002-9378(91)90559-a. [DOI] [PubMed] [Google Scholar]
- Cheema M. A., Schumacher H. R., Hudson A. P. RNA-directed molecular hybridization screening: evidence for inapparent chlamydial infection. Am J Med Sci. 1991 Nov;302(5):261–268. doi: 10.1097/00000441-199111000-00001. [DOI] [PubMed] [Google Scholar]
- Clark R. B., Schatzki P. F., Dalton H. P. Ultrastructural analysis of the effects of erythromycin on the morphology and developmental cycle of Chlamydia trachomatis HAR-13. Arch Microbiol. 1982 Dec 3;133(4):278–282. doi: 10.1007/BF00521290. [DOI] [PubMed] [Google Scholar]
- Clark R. B., Schatzki P. F., Dalton H. P. Ultrastructural effect of penicillin and cycloheximide on Chlamydia trachomatis strain HAR-13. Med Microbiol Immunol. 1982;171(3):151–159. doi: 10.1007/BF02123623. [DOI] [PubMed] [Google Scholar]
- Coles A. M., Reynolds D. J., Harper A., Devitt A., Pearce J. H. Low-nutrient induction of abnormal chlamydial development: a novel component of chlamydial pathogenesis? FEMS Microbiol Lett. 1993 Jan 15;106(2):193–200. doi: 10.1111/j.1574-6968.1993.tb05958.x. [DOI] [PubMed] [Google Scholar]
- Ellis R. J., van der Vies S. M. Molecular chaperones. Annu Rev Biochem. 1991;60:321–347. doi: 10.1146/annurev.bi.60.070191.001541. [DOI] [PubMed] [Google Scholar]
- Everett K. D., Hatch T. P. Sequence analysis and lipid modification of the cysteine-rich envelope proteins of Chlamydia psittaci 6BC. J Bacteriol. 1991 Jun;173(12):3821–3830. doi: 10.1128/jb.173.12.3821-3830.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALASSO G. J., MANIRE G. P. Effect of antiserum and antibiotics on persistent infection of HeLa cells with meningopneumonitis virus. J Immunol. 1961 Apr;86:382–385. [PubMed] [Google Scholar]
- Garrett A. J., Harrison M. J., Manire G. P. A search for the bacterial mucopeptide component, muramic acid, in Chlamydia. J Gen Microbiol. 1974 Jan;80(1):315–318. doi: 10.1099/00221287-80-1-315. [DOI] [PubMed] [Google Scholar]
- Grayston J. T., Wang S. New knowledge of chlamydiae and the diseases they cause. J Infect Dis. 1975 Jul;132(1):87–105. doi: 10.1093/infdis/132.1.87. [DOI] [PubMed] [Google Scholar]
- Hackstadt T., Baehr W., Ying Y. Chlamydia trachomatis developmentally regulated protein is homologous to eukaryotic histone H1. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3937–3941. doi: 10.1073/pnas.88.9.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackstadt T., Todd W. J., Caldwell H. D. Disulfide-mediated interactions of the chlamydial major outer membrane protein: role in the differentiation of chlamydiae? J Bacteriol. 1985 Jan;161(1):25–31. doi: 10.1128/jb.161.1.25-31.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschlag M. R. Activity of trimethoprim-sulfamethoxazole against Chlamydia trachomatis in vitro. Rev Infect Dis. 1982 Mar-Apr;4(2):500–505. doi: 10.1093/clinids/4.2.500. [DOI] [PubMed] [Google Scholar]
- Hammerschlag M. R., Vuletin J. C. Ultrastructural analysis of the effect of trimethoprim and sulphamethoxazole on the development of Chlamydia trachomatis in cell culture. J Antimicrob Chemother. 1985 Feb;15(2):209–217. doi: 10.1093/jac/15.2.209. [DOI] [PubMed] [Google Scholar]
- Hanna L., Dawson C. R., Briones O., Thygeson P., Jawetz E. Latency in human infections with TRIC agents. J Immunol. 1968 Jul;101(1):43–50. [PubMed] [Google Scholar]
- Hanna L., Merigan T. C., Jawetz E. Inhibition of TRIC agents by virus-induced interferon. Proc Soc Exp Biol Med. 1966 Jun;122(2):417–421. doi: 10.3181/00379727-122-31150. [DOI] [PubMed] [Google Scholar]
- Hatch T. P., Al-Hossainy E., Silverman J. A. Adenine nucleotide and lysine transport in Chlamydia psittaci. J Bacteriol. 1982 May;150(2):662–670. doi: 10.1128/jb.150.2.662-670.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch T. P., Allan I., Pearce J. H. Structural and polypeptide differences between envelopes of infective and reproductive life cycle forms of Chlamydia spp. J Bacteriol. 1984 Jan;157(1):13–20. doi: 10.1128/jb.157.1.13-20.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch T. P. Competition between Chlamydia psittaci and L cells for host isoleucine pools: a limiting factor in chlamydial multiplication. Infect Immun. 1975 Jul;12(1):211–220. doi: 10.1128/iai.12.1.211-220.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch T. P., Miceli M., Sublett J. E. Synthesis of disulfide-bonded outer membrane proteins during the developmental cycle of Chlamydia psittaci and Chlamydia trachomatis. J Bacteriol. 1986 Feb;165(2):379–385. doi: 10.1128/jb.165.2.379-385.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry-Suchet J., Catalan F., Loffredo V., Sanson M. J., Debache C., Pigeau F., Coppin R. Chlamydia trachomatis associated with chronic inflammation in abdominal specimens from women selected for tuboplasty. Fertil Steril. 1981 Nov;36(5):599–605. doi: 10.1016/s0015-0282(16)45857-7. [DOI] [PubMed] [Google Scholar]
- Henry-Suchet J., Utzmann C., De Brux J., Ardoin P., Catalan F. Microbiologic study of chronic inflammation associated with tubal factor infertility: role of Chlamydia trachomatis. Fertil Steril. 1987 Feb;47(2):274–277. doi: 10.1016/s0015-0282(16)50005-3. [DOI] [PubMed] [Google Scholar]
- Holland S. M., Hudson A. P., Bobo L., Whittum-Hudson J. A., Viscidi R. P., Quinn T. C., Taylor H. R. Demonstration of chlamydial RNA and DNA during a culture-negative state. Infect Immun. 1992 May;60(5):2040–2047. doi: 10.1128/iai.60.5.2040-2047.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooton T. M., Rogers M. E., Medina T. G., Kuwamura L. E., Ewers C., Roberts P. L., Stamm W. E. Ciprofloxacin compared with doxycycline for nongonococcal urethritis. Ineffectiveness against Chlamydia trachomatis due to relapsing infection. JAMA. 1990 Sep 19;264(11):1418–1421. [PubMed] [Google Scholar]
- Hudson A. P., McEntee C. M., Reacher M., Whittum-Hudson J. A., Taylor H. R. Inapparent ocular infection by Chlamydia trachomatis in experimental and human trachoma. Curr Eye Res. 1992 Mar;11(3):279–283. doi: 10.3109/02713689209001780. [DOI] [PubMed] [Google Scholar]
- Johnson F. W., Hobson D. The effect of penicillin on genital strains of Chlamydia trachomatis in tissue culture. J Antimicrob Chemother. 1977 Jan;3(1):49–56. doi: 10.1093/jac/3.1.49. [DOI] [PubMed] [Google Scholar]
- Jones R. B., Ardery B. R., Hui S. L., Cleary R. E. Correlation between serum antichlamydial antibodies and tubal factor as a cause of infertility. Fertil Steril. 1982 Nov;38(5):553–558. doi: 10.1016/s0015-0282(16)46634-3. [DOI] [PubMed] [Google Scholar]
- Jones R. B., Van der Pol B., Martin D. H., Shepard M. K. Partial characterization of Chlamydia trachomatis isolates resistant to multiple antibiotics. J Infect Dis. 1990 Dec;162(6):1309–1315. doi: 10.1093/infdis/162.6.1309. [DOI] [PubMed] [Google Scholar]
- Kahane S., Friedman M. G. Reversibility of heat shock in Chlamydia trachomatis. FEMS Microbiol Lett. 1992 Oct 1;76(1-2):25–30. doi: 10.1016/0378-1097(92)90358-u. [DOI] [PubMed] [Google Scholar]
- Kane J. L., Woodland R. M., Forsey T., Darougar S., Elder M. G. Evidence of chlamydial infection in infertile women with and without fallopian tube obstruction. Fertil Steril. 1984 Dec;42(6):843–848. doi: 10.1016/s0015-0282(16)48254-3. [DOI] [PubMed] [Google Scholar]
- Kaul R., Tao S., Wenman W. M. Cyclic AMP inhibits protein synthesis in Chlamydia trachomatis at a transcriptional level. Biochim Biophys Acta. 1990 Jun 12;1053(1):106–112. doi: 10.1016/0167-4889(90)90032-9. [DOI] [PubMed] [Google Scholar]
- Kaul R., Wenman W. M. Cyclic AMP inhibits developmental regulation of Chlamydia trachomatis. J Bacteriol. 1986 Nov;168(2):722–727. doi: 10.1128/jb.168.2.722-727.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazar J., Gillmore J. D., Gordon F. B. Effect of Interferon and Interferon Inducers on Infections with a Nonviral Intracellular Microorganism, Chlamydia trachomatis. Infect Immun. 1971 Jun;3(6):825–832. doi: 10.1128/iai.3.6.825-832.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiviat N. B., Wølner-Hanssen P., Peterson M., Wasserheit J., Stamm W. E., Eschenbach D. A., Paavonen J., Lingenfelter J., Bell T., Zabriskie V. Localization of Chlamydia trachomatis infection by direct immunofluorescence and culture in pelvic inflammatory disease. Am J Obstet Gynecol. 1986 Apr;154(4):865–873. doi: 10.1016/0002-9378(86)90473-4. [DOI] [PubMed] [Google Scholar]
- Kramer M. J., Gordon F. B. Ultrastructural analysis of the effects of penicillin and chlortetracycline on the development of a genital tract Chlamydia. Infect Immun. 1971 Feb;3(2):333–341. doi: 10.1128/iai.3.2.333-341.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H. S., Moulder J. W. Patterns of response to sulfadiazine, D-cycloserine and D-alanine in members of the psittacosis group. J Infect Dis. 1966 Jun;116(3):372–376. doi: 10.1093/infdis/116.3.372. [DOI] [PubMed] [Google Scholar]
- Lundemose A. G., Birkelund S., Larsen P. M., Fey S. J., Christiansen G. Characterization and identification of early proteins in Chlamydia trachomatis serovar L2 by two-dimensional gel electrophoresis. Infect Immun. 1990 Aug;58(8):2478–2486. doi: 10.1128/iai.58.8.2478-2486.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundemose A. G., Kay J. E., Pearce J. H. Chlamydia trachomatis Mip-like protein has peptidyl-prolyl cis/trans isomerase activity that is inhibited by FK506 and rapamycin and is implicated in initiation of chlamydial infection. Mol Microbiol. 1993 Mar;7(5):777–783. doi: 10.1111/j.1365-2958.1993.tb01168.x. [DOI] [PubMed] [Google Scholar]
- Lundemose A. G., Rouch D. A., Birkelund S., Christiansen G., Pearce J. H. Chlamydia trachomatis Mip-like protein. Mol Microbiol. 1992 Sep;6(17):2539–2548. doi: 10.1111/j.1365-2958.1992.tb01430.x. [DOI] [PubMed] [Google Scholar]
- MOULDER J. W., NOVOSEL D. L., OFFICER J. E. INHIBITION OF THE GROWTH OF AGENTS OF THE PSITTACOSIS GROUP BY D-CYCLOSERINE AND ITS SPECIFIC REVERSAL BY D-ALANINE. J Bacteriol. 1963 Mar;85:707–711. doi: 10.1128/jb.85.3.707-711.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabey D. C., Robertson J. N., Ward M. E. Detection of Chlamydia trachomatis by enzyme immunoassay in patients with trachoma. Lancet. 1987 Dec 26;2(8574):1491–1492. doi: 10.1016/s0140-6736(87)92623-7. [DOI] [PubMed] [Google Scholar]
- Matsumoto A., Manire G. P. Electron microscopic observations on the effects of penicillin on the morphology of Chlamydia psittaci. J Bacteriol. 1970 Jan;101(1):278–285. doi: 10.1128/jb.101.1.278-285.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merigan T. C., Hanna L. Characteristics of interferon induced in vitro and in vivo by a TRIC agent. Proc Soc Exp Biol Med. 1966 Jun;122(2):421–424. doi: 10.3181/00379727-122-31151. [DOI] [PubMed] [Google Scholar]
- Moore D. E., Spadoni L. R., Foy H. M., Wang S. P., Daling J. R., Kuo C. C., Grayston J. T., Eschenbach D. A. Increased frequency of serum antibodies to Chlamydia trachomatis in infertility due to distal tubal disease. Lancet. 1982 Sep 11;2(8298):574–577. doi: 10.1016/s0140-6736(82)90659-6. [DOI] [PubMed] [Google Scholar]
- Morrison R. P., Belland R. J., Lyng K., Caldwell H. D. Chlamydial disease pathogenesis. The 57-kD chlamydial hypersensitivity antigen is a stress response protein. J Exp Med. 1989 Oct 1;170(4):1271–1283. doi: 10.1084/jem.170.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison R. P., Lyng K., Caldwell H. D. Chlamydial disease pathogenesis. Ocular hypersensitivity elicited by a genus-specific 57-kD protein. J Exp Med. 1989 Mar 1;169(3):663–675. doi: 10.1084/jem.169.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder J. W., Levy N. J., Schulman L. P. Persistent infection of mouse fibroblasts (L cells) with Chlamydia psittaci: evidence for a cryptic chlamydial form. Infect Immun. 1980 Dec;30(3):874–883. doi: 10.1128/iai.30.3.874-883.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder J. W. Why is Chlamydia sensitive to penicillin in the absence of peptidoglycan? Infect Agents Dis. 1993 Apr;2(2):87–99. [PubMed] [Google Scholar]
- Newhall W. J., 5th Biosynthesis and disulfide cross-linking of outer membrane components during the growth cycle of Chlamydia trachomatis. Infect Immun. 1987 Jan;55(1):162–168. doi: 10.1128/iai.55.1.162-168.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OFFICER J. E., BROWN A. Serial changes in virus and cells in cultures chronically infected with psittacosis virus. Virology. 1961 May;14:88–99. doi: 10.1016/0042-6822(61)90136-2. [DOI] [PubMed] [Google Scholar]
- Oriel J. D. The carrier state: Chlamydia trachomatis. J Antimicrob Chemother. 1986 Jul;18 (Suppl A):67–71. doi: 10.1093/jac/18.supplement_a.67. [DOI] [PubMed] [Google Scholar]
- POLLARD M., SHARON N. Induction of prolonged latency in psittacosis-infected cells by aminopterin. Proc Soc Exp Biol Med. 1963 Jan;112:51–54. doi: 10.3181/00379727-112-27947. [DOI] [PubMed] [Google Scholar]
- Patton D. L., Moore D. E., Spadoni L. R., Soules M. R., Halbert S. A., Wang S. P. A comparison of the fallopian tube's response to overt and silent salpingitis. Obstet Gynecol. 1989 Apr;73(4):622–630. [PubMed] [Google Scholar]
- Perara E., Ganem D., Engel J. N. A developmentally regulated chlamydial gene with apparent homology to eukaryotic histone H1. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2125–2129. doi: 10.1073/pnas.89.6.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Martinez J. A., Storz J. Persistent infection of L cells with an ovine abortion strain of Chlamydia psittaci. Infect Immun. 1985 Nov;50(2):453–458. doi: 10.1128/iai.50.2.453-458.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferkorn E. R. Interferon gamma blocks the growth of Toxoplasma gondii in human fibroblasts by inducing the host cells to degrade tryptophan. Proc Natl Acad Sci U S A. 1984 Feb;81(3):908–912. doi: 10.1073/pnas.81.3.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips D. M., Swenson C. E., Schachter J. Ultrastructure of Chlamydia trachomatis infection of the mouse oviduct. J Ultrastruct Res. 1984 Sep;88(3):244–256. doi: 10.1016/s0022-5320(84)90122-9. [DOI] [PubMed] [Google Scholar]
- Plaunt M. R., Hatch T. P. Protein synthesis early in the developmental cycle of Chlamydia psittaci. Infect Immun. 1988 Dec;56(12):3021–3025. doi: 10.1128/iai.56.12.3021-3025.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M. U., Cheema M. A., Schumacher H. R., Hudson A. P. Molecular evidence for the presence of chlamydia in the synovium of patients with Reiter's syndrome. Arthritis Rheum. 1992 May;35(5):521–529. doi: 10.1002/art.1780350506. [DOI] [PubMed] [Google Scholar]
- Rapoza P. A., Tahija S. G., Carlin J. P., Miller S. L., Padilla M. L., Byrne G. I. Effect of interferon on a primary conjunctival epithelial cell model of trachoma. Invest Ophthalmol Vis Sci. 1991 Oct;32(11):2919–2923. [PubMed] [Google Scholar]
- Reeve P., Taverne J., Bushby S. R. Inhibition by pyrimidine analogues of the synthesis of folic acid by trachoma agents. J Hyg (Lond) 1968 Jun;66(2):295–306. [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz H. S., Gutter B., Becker Y. Studies on the developmental cycle of Chlamydia trachomatis: selective inhibition by hydroxyurea. J Bacteriol. 1973 Aug;115(2):682–690. doi: 10.1128/jb.115.2.682-690.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothermel C. D., Byrne G. I., Havell E. A. Effect of interferon on the growth of Chlamydia trachomatis in mouse fibroblasts (L cells). Infect Immun. 1983 Jan;39(1):362–370. doi: 10.1128/iai.39.1.362-370.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothermel C. D., Rubin B. Y., Jaffe E. A., Murray H. W. Oxygen-independent inhibition of intracellular Chlamydia psittaci growth by human monocytes and interferon-gamma-activated macrophages. J Immunol. 1986 Jul 15;137(2):689–692. [PubMed] [Google Scholar]
- Rothermel C. D., Rubin B. Y., Murray H. W. Gamma-interferon is the factor in lymphokine that activates human macrophages to inhibit intracellular Chlamydia psittaci replication. J Immunol. 1983 Nov;131(5):2542–2544. [PubMed] [Google Scholar]
- Sardinia L. M., Segal E., Ganem D. Developmental regulation of the cysteine-rich outer-membrane proteins of murine Chlamydia trachomatis. J Gen Microbiol. 1988 Apr;134(4):997–1004. doi: 10.1099/00221287-134-4-997. [DOI] [PubMed] [Google Scholar]
- Sarov I., Geron E., Shemer-Avni Y., Manor E., Zvillich M., Wallach D., Schmitz E., Holtman H. Implications for persistent chlamydial infections of phagocyte-microorganism interplay. Eur J Clin Microbiol Infect Dis. 1991 Feb;10(2):119–123. doi: 10.1007/BF01964423. [DOI] [PubMed] [Google Scholar]
- Schachter J. A Bedsonia isolated from a patient with clinical lymphogranuloma venereum. Am J Ophthalmol. 1967 May;63(5 Suppl):1049–1053. doi: 10.1016/0002-9394(67)94081-0. [DOI] [PubMed] [Google Scholar]
- Schachter J. Chlamydial infections (first of three parts). N Engl J Med. 1978 Feb 23;298(8):428–435. doi: 10.1056/NEJM197802232980805. [DOI] [PubMed] [Google Scholar]
- Schachter J., Moncada J., Dawson C. R., Sheppard J., Courtright P., Said M. E., Zaki S., Hafez S. F., Lorincz A. Nonculture methods for diagnosing chlamydial infection in patients with trachoma: a clue to the pathogenesis of the disease? J Infect Dis. 1988 Dec;158(6):1347–1352. doi: 10.1093/infdis/158.6.1347. [DOI] [PubMed] [Google Scholar]
- Sellors J. W., Mahony J. B., Chernesky M. A., Rath D. J. Tubal factor infertility: an association with prior chlamydial infection and asymptomatic salpingitis. Fertil Steril. 1988 Mar;49(3):451–457. doi: 10.1016/s0015-0282(16)59772-6. [DOI] [PubMed] [Google Scholar]
- Shainkin-Kestenbaum R., Winikoff Y., Kol R., Chaimovitz C., Sarov I. Inhibition of growth of Chlamydia trachomatis by the calcium antagonist verapamil. J Gen Microbiol. 1989 Jun;135(6):1619–1623. doi: 10.1099/00221287-135-6-1619. [DOI] [PubMed] [Google Scholar]
- Shemer-Avni Y., Wallach D., Sarov I. Reversion of the antichlamydial effect of tumor necrosis factor by tryptophan and antibodies to beta interferon. Infect Immun. 1989 Nov;57(11):3484–3490. doi: 10.1128/iai.57.11.3484-3490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemer Y., Sarov I. Inhibition of growth of Chlamydia trachomatis by human gamma interferon. Infect Immun. 1985 May;48(2):592–596. doi: 10.1128/iai.48.2.592-596.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard M. K., Jones R. B. Recovery of Chlamydia trachomatis from endometrial and fallopian tube biopsies in women with infertility of tubal origin. Fertil Steril. 1989 Aug;52(2):232–238. doi: 10.1016/s0015-0282(16)60847-6. [DOI] [PubMed] [Google Scholar]
- Soong Y. K., Kao S. M., Lee C. J., Lee P. S., Pao C. C. Endocervical chlamydial deoxyribonucleic acid in infertile women. Fertil Steril. 1990 Nov;54(5):815–818. [PubMed] [Google Scholar]
- Spears P., Storz J. Changes in the ultrastructure of Chlamydia psittaci produced by treatment of the host cell with DEAE-dextran and cycloheximide. J Ultrastruct Res. 1979 May;67(2):152–160. doi: 10.1016/s0022-5320(79)80004-0. [DOI] [PubMed] [Google Scholar]
- Stamm W. E. Azithromycin in the treatment of uncomplicated genital chlamydial infections. Am J Med. 1991 Sep 12;91(3A):19S–22S. doi: 10.1016/0002-9343(91)90396-f. [DOI] [PubMed] [Google Scholar]
- Stephens R. S., Wagar E. A., Edman U. Developmental regulation of tandem promoters for the major outer membrane protein gene of Chlamydia trachomatis. J Bacteriol. 1988 Feb;170(2):744–750. doi: 10.1128/jb.170.2.744-750.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H., Watkins N. G., Zhang Y. X., Caldwell H. D. Chlamydia trachomatis-host cell interactions: role of the chlamydial major outer membrane protein as an adhesin. Infect Immun. 1990 Apr;58(4):1017–1025. doi: 10.1128/iai.58.4.1017-1025.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura A., Matsumoto A., Higashi N. Purification and chemical composition of reticulate bodies of the meningopneumonitis organisms. J Bacteriol. 1967 Jun;93(6):2003–2008. doi: 10.1128/jb.93.6.2003-2008.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanami Y., Yamada Y. Miniature cell formation in Chlamydia psittaci. J Bacteriol. 1973 Apr;114(1):408–412. doi: 10.1128/jb.114.1.408-412.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor H. R., Rapoza P. A., West S., Johnson S., Munoz B., Katala S., Mmbaga B. B. The epidemiology of infection in trachoma. Invest Ophthalmol Vis Sci. 1989 Aug;30(8):1823–1833. [PubMed] [Google Scholar]
- Taylor H. R., Young E., MacDonald A. B., Schachter J., Prendergast R. A. Oral immunization against chlamydial eye infection. Invest Ophthalmol Vis Sci. 1987 Feb;28(2):249–258. [PubMed] [Google Scholar]
- Thejls H., Gnarpe J., Lundkvist O., Heimer G., Larsson G., Victor A. Diagnosis and prevalence of persistent chlamydia infection in infertile women: tissue culture, direct antigen detection, and serology. Fertil Steril. 1991 Feb;55(2):304–310. [PubMed] [Google Scholar]
- Tribby I. I., Friis R. R., Moulder J. W. Effect of chloramphenicol, rifampicin, and nalidixic acid on Chlamydia psittaci growing in L cells. J Infect Dis. 1973 Feb;127(2):155–163. doi: 10.1093/infdis/127.2.155. [DOI] [PubMed] [Google Scholar]
- WANG S., GRAYSTON J. T. Trachoma in the Taiwan monkey Macaca cyclopis. Ann N Y Acad Sci. 1962 Mar 5;98:177–187. doi: 10.1111/j.1749-6632.1962.tb30542.x. [DOI] [PubMed] [Google Scholar]
- Ward M. E., Salari H. Control mechanisms governing the infectivity of Chlamydia trachomatis for hela cells: modulation by cyclic nucleotides, prostaglandins and calcium. J Gen Microbiol. 1982 Mar;128(3):639–650. doi: 10.1099/00221287-128-3-639. [DOI] [PubMed] [Google Scholar]
- Ward M., Bailey R., Lesley A., Kajbaf M., Robertson J., Mabey D. Persisting inapparent chlamydial infection in a trachoma endemic community in The Gambia. Scand J Infect Dis Suppl. 1990;69:137–148. [PubMed] [Google Scholar]
- Wichlan D. G., Hatch T. P. Identification of an early-stage gene of Chlamydia psittaci 6BC. J Bacteriol. 1993 May;175(10):2936–2942. doi: 10.1128/jb.175.10.2936-2942.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workowski K. A., Lampe M. F., Wong K. G., Watts M. B., Stamm W. E. Long-term eradication of Chlamydia trachomatis genital infection after antimicrobial therapy. Evidence against persistent infection. JAMA. 1993 Nov 3;270(17):2071–2075. [PubMed] [Google Scholar]
- Wyrick P. B., Brownridge E. A., Ivins B. E. Interaction of Chlamydia psittaci with mouse peritoneal macrophages. Infect Immun. 1978 Mar;19(3):1061–1067. doi: 10.1128/iai.19.3.1061-1067.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. S., Kuo C. C., Chen W. J. Reactivation of Chlamydia trachomatis lung infection in mice by cortisone. Infect Immun. 1983 Feb;39(2):655–658. doi: 10.1128/iai.39.2.655-658.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeilstra-Ryalls J., Fayet O., Georgopoulos C. The universally conserved GroE (Hsp60) chaperonins. Annu Rev Microbiol. 1991;45:301–325. doi: 10.1146/annurev.mi.45.100191.001505. [DOI] [PubMed] [Google Scholar]
- Zhang Y. X., Stewart S., Joseph T., Taylor H. R., Caldwell H. D. Protective monoclonal antibodies recognize epitopes located on the major outer membrane protein of Chlamydia trachomatis. J Immunol. 1987 Jan 15;138(2):575–581. [PubMed] [Google Scholar]
- de la Maza L. M., Peterson E. M., Fennie C. W., Czarniecki C. W. The anti-chlamydial and anti-proliferative activities of recombinant murine interferon-gamma are not dependent on tryptophan concentrations. J Immunol. 1985 Dec;135(6):4198–4200. [PubMed] [Google Scholar]