Abstract
The most prominent mechanism proposed for death of dopaminergic neurons in Parkinson’s disease (PD) is elevated generation of reactive oxygen/nitrogen species (ROS/RNS). Recent studies suggest that ROS produced during PD pathogenesis may contribute to cytotoxicity in cell culture models of PD. We hypothesized that inhibition of ROS production would prevent PD symptoms in the LRRK2R1441G transgenic (tg) mouse model of PD. These mice overexpress a mutant form of leucine-rich repeat kinase 2 (LRRK2) and are reported to develop PD-like symptoms at approximately 10 months of age. Despite similar expression of the transgene, our colony did not recapitulate the same type of motor dysfunction originally reported. However, tests of motor coordination (pole test, Rotor-Rod) revealed a significant defect in LRRK2R1441G mice by 16 months of age. LRRK2R1441G tg mice, or wild type littermates, were given diapocynin (200 mg/kg, a proposed NADPH oxidase inhibitor) three times per week by oral gavage starting at 12 weeks of age. Decreased performance on the pole test and Rotor-Rod in the LRRK2R1441G mice was prevented with diapocynin treatment. No loss in open field movement or rearing was found. As expected, tyrosine hydroxylase staining was similar in both the substantia nigra and striatum in all treatment groups. Together these data demonstrate that diapocynin is a viable agent for protection of neurobehavioral function.
Keywords: Neurodegeneration, Parkinson’s disease, neurobehavioral analysis
Introduction
In the last decade, Parkinson’s disease patients with genetic mutations predisposing them to the disease have been identified [19, 24]. This finding catalyzed the development of multiple transgenic animal models for studying PD progression [8]. One gene, leucine-rich repeat kinase 2 (LRRK2) has been the subject of recent research, as there have been several mutations identified that lead to PD [16, 21]. Of these, mutation of arginine to glycine at position 1441 has been extensively studied. In general, the R1441G mutation is not thought to affect LRRK2 kinase activity [14]; however, it is located in the GTPase domain, and may impact protein function in that way. Mice overexpressing human LRRK2R1441G were recently described [18]. These mice were reported to have no observable phenotype at 3 months, but had gross motor deficiencies by 10–12 months. Motor deficits were reversible with L-dopa treatment, but occurred in the absence of changes in total dopaminergic cell number as assessed by tyrosine hydroxylase (TH) staining.
In this study, we employed the LRRK2R1441G tg mice as a Parkinson’s disease model to study the impact of neuroinflammation on the progression of PD-like symptoms. Neuroinflammation is a well-known contributor to PD pathogenesis. Microglia isolated from brains of adult LRRK2R1441G mice have higher pro-inflammatory cytokine secretion [12], while LRRK2 knockdown impairs transcription of pro-inflammatory genes in response to LPS [17]. We thus hypothesized that the LRRK2R1441G mice would have chronic neuroinflammation similar to the human pathology.
Despite solid data supporting a neuroinflammatory mechanism in PD, therapeutic strategies to limit inflammation have yielded mixed results [10]. Indeed, prior non-steroidal anti-inflammatory drug (NSAID) use was reported to increase PD risk [6]. In the present study, LRRK2R1441G or wild type littermates were treated with the anti-inflammatory compound diapocynin. Apocynin and its derivatives are reported to prevent translocation of p47phox from the cytosol to the membrane where NADPH oxidase (NOX2) is located, thus preventing assembly of the active enzyme [27]. NOX2 is the particular isoform which is known to be expressed in microglia, and produces superoxide radical anion (O2·−) when activated [4]. Using a targeted strategy against NOX2 may thus be more efficacious in treating PD-specific neurobehavioral dysfunction.
Materials and Methods
Mice
A colony of LRRK2R1441G (FVB/N-Tg(LRRK2*R1441G)135Cjli/J) and wild type litter mates was established from commercially available breeders [18]. Male mice were used for all experiments. Mice were housed on a 12 h light/dark cycle with ad libitum access to food and water. All experiments were performed in accordance with the Guide for Care and use of Laboratory Animals and approved by the Medical College of Wisconsin Institutional Animal Care and Use Committee. Genotype was determined using tail biopsy DNA amplified using the following primers: forward 5′-TGA TTC TCG TTG GCA CAC AT-3′ reverse 5′-GCC AAA GCA TCA GAT TCC TC-3′ (Figure 1A). Genotype was confirmed after 16 months using Sanger sequencing of a 261bp PCR product amplified from genomic DNA using the following primers: forward 5′-TGC CAC CTA AAA GGG TGA AG-3′ reverse 5′-CTT TGC GTT GCT TCT CAT CA-3′ (Figure 1B). Sequence analysis was performed using Sequencher 4.10.1 (Gene Codes Corporation; Ann Arbor, MI). Relative (LRRK2*R1441G)135Cjli transgene copy number was analyzed alongside samples from The Jackson Laboratory colony. Quantitative PCR was performed on genomic DNA isolated from tails of hemizygous, homozygous, and wild type mice using an ABI 7500 (Life Technologies; Grand Island, NY), (Figure 1C). Beginning at 12 weeks of age, and continuing for the duration of the experiment, mice were given 200 mg/kg diapocynin or saline, 3x/wk, via oral gavage. A similar treatment schedule and dosage was reported to yield detectable levels of diapocynin in brains of mice with compound identity confirmed by mass spectrometry [11, 29].
Figure 1. LRRK2R1441G tg mice genotyping.
Wild type FVB and LRRK2R1441G tg genotype was confirmed using PCR (A) and Sanger sequencing (B). Transgene copy number was compared to animals with known genotype to confirm hemizygous expression of LRRK2R1441G (C). Samples from each mouse were run in triplicate. Data shown are means ± s.d. n≥2 mice per group as indicated.
Diapocynin synthesis
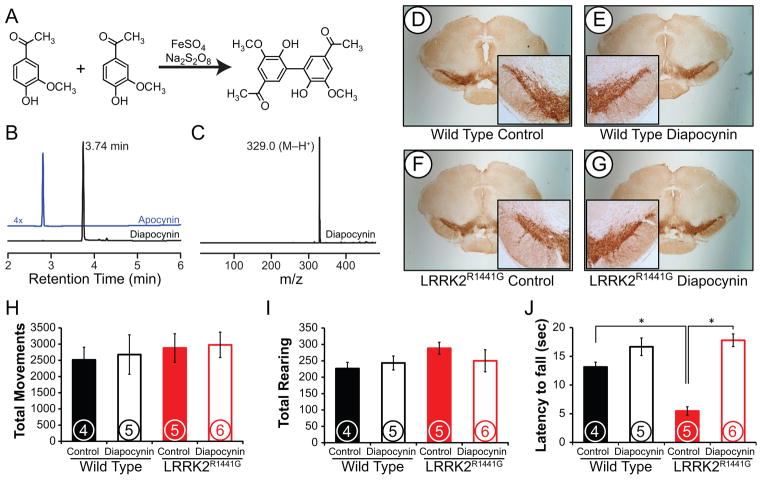
Diapocynin (5,5′-dehydrodiacetovanillone, Figure 3A) was synthesized as previously described [9, 30]. Briefly, to a solution of 1.0 g of apocynin (6 mmol) in 200 ml of hot water, 75 mg of ferrous sulfate heptahydrate (0.3 mmol) and 714 mg (3.0 mmol) sodium persulfate were added and stirred for 30 min. The precipitated product was filtered and washed with cold water. The product was dissolved in 4 N sodium hydroxide (50 ml), filtered, and the brown solution was acidified with 4 N hydrochloric acid to pH 3.0. The precipitate was filtered, and the process was repeated twice more to yield 0.6 g (60%) of a white solid. Purity was confirmed by HPLC analysis on a Kinetex C18 column (Phenomenex, 100 mm × 4.6 mm, 2.6 μm) equilibrated with 0.1% TFA in water containing 10% acetonitrile. Diapocynin was eluted using a 10%–100% acetonitrile gradient in the mobile phase over 7 min, followed by 100% acetonitrile containing 0.1% TFA for an additional 2.5 min. Flow rate was 1.5 mL/min. Diapocynin eluted at 3.7 min (Figure 3B). Negative ion mode mass spectrometry (MS) confirmed a single product with an m/z of 329.0 (M−H+), as expected (Figure 3C). This preparation of diapocynin inhibited superoxide production by retinoic acid-differentiated HL-60 cells stimulated with 1 μM phorbol-12-myristate-13-acetate (PMA) with an apparent IC50 of ca. 0.3 mM, as measured using HPLC-based monitoring of the conversion of hydroethidine into 2-hydroxyethidium [31].
Figure 3. Diapocynin corrects loss of motor coordination without affecting gross motor function.
Diapocynin was synthesized through the reaction of two apocynin monomers in the presence of ferrous sulfate and sodium persulfate (A). Purity of the isolated compound was confirmed by HPLC (B) and MS analysis (C). An apocynin standard was run in parallel to confirm purity of the diapocynin solution. At 16 mo, brains were formaldehyde fixed, sliced, and slices containing the nigral region were stained for TH (D-G). Images shown are representative with 4x magnification in insets. Total movement (H) and rearing (I) were assessed over 20 min in an open field using a photobeam system. Latency to fall from a Rotor-Rod apparatus was also measured (J). Data are the means ± s.d. *, p≤0.05 as determined by one-way ANOVA with Bonferroni post-hoc. Encircled numbers represent the number of animals in each group.
Open field measurements
Ambulatory function was monitored using a 40 × 40 cm open field photobeam system (San Diego Instruments; San Diego, CA). Each mouse was placed in the open field apparatus and tracked for 20 min. Beam breaks were counted for total movement measurements. A second level of photobeams was used to concomitantly track rearing. Time-resolved data were used to determine the amount of time that each mouse paused at a particular coordinate. A heatmap of location within the open field apparatus was generated using crosstab analysis of each dataset in Microsoft Access 2010. Data were normalized to percentage of time spent at each coordinate for each mouse, and then averaged.
Motor coordination behavioral analysis
Coordination was assessed using the pole test and Rotor-Rod. Mice were placed face up on a 1 cm diameter ringstand pole, and must turn and descend to the base. On the Rotor-Rod (San Diego Instruments; San Diego, CA), mice were placed on a horizontal bar (3.175 cm diameter) which rotates. The speed of rotation was increased from 0 to 8 rpm in 10 sec, from 8 to 10 rpm in the next 5 sec, maintained at 10 rpm for 5 sec, and then increased from 10 to 11 rpm over the next 5 sec.
Gait analysis
Using different colored non-toxic paints, front and back paws of each mouse were painted, and the mice were allowed to run through a 7.5 × 61 cm channel. Footfalls were assigned, and the interstep distance was calculated. Stride count and interstep distance for each paw was averaged to yield the respective values for each mouse.
Immunohistochemistry
Immunohistochemical staining for TH and the microglial marker Iba-1 (ionized calcium binding adaptor molecule 1) were performed essentially as described [11]. Briefly, mice were deeply anesthetized, and perfused with 4% paraformaldehyde. Brains were removed, fixed in 4% paraformaldehyde for 24 h, and then post-fixed in 30% sucrose for 48 h. Fixed brains were cut into 30 μm sections with a cryostat, mounted, and stained with anti-tyrosine hydroxylase (Calbiochem; Billerica, MA) or Iba-1 (Abcam; Cambridge, MA) antibodies. DAB-stained sections were imaged at multiple magnifications.
Statistical analysis
Motor function data were acquired using a San Diego Instruments Photobeam Activity System (PAS) Open Field apparatus or Rotor-Rod (San Diego, CA). Data were collected and analyzed using Microsoft Excel 2010 or Origin 8.5 (OriginLab, Northampton, MA). One-way ANOVA was used for significance tests unless otherwise specified in the legend. p≤0.05 was considered statistically significant.
Results
LRRK2R1441G mice do not have deficient gross motor ambulatory function
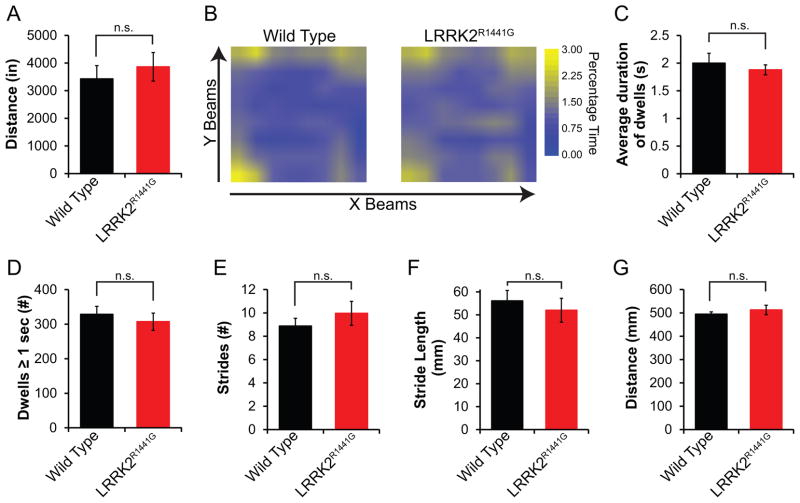
Total ambulatory function was measured in an open field apparatus as described when mice were 16 months of age. During the 20 min tracking period, LRRK2R1441G mice covered the same distance as wild type mice (Figure 2A). Beam coordinate pairs were used to determine the relative time each mouse spent at a point in the apparatus. As expected, wild type mice spent significant time exploring the corners of the open field apparatus. This behavior was not notably different in LRRK2R1441G tg mice (Figure 2B). LRRK2R1441G tg mice also did not differ from wild type in the number of dwells (defined as a stationary period ≥ 1 sec) or the duration of dwells (Figure 2C and D). Gait analysis was performed to ascertain differences in step behavior. LRRK2R1441G tg and wild type mice had similar stride number and length (Figure 2E and F). All mice covered the same distance during this measurement (Figure 2G).
Figure 2. Open field movement is not decreased in LRRK2R1441G tg mice.
From open field photobeam tracking experiments, the total distance travelled was calculated (A). Location in the open field apparatus was tracked as percentage time occupying each coordinate for each animal. Averages were then plotted as a heat map (B). The average duration of each dwell (C) and total number of dwells ≥ 1 s (D) were calculated. Gait analysis was used to monitor stride count (E) and length (F) over a constant distance (G). Data shown are means ± s.d. Significance was assessed by two-tailed t-test, and p values are shown for each measurement. n≥4 mice per group.
LRRK2R1441G tg mice have deficient motor coordination which is reversed by diapocynin
After 16 months of treatment with diapocynin, mice were sacrificed and brain sections were stained for TH to indicate presence or absence of dopaminergic neurons. TH staining in the substantia nigra (Figure 3D–G) did not differ between wild type and LRRK2R1441G tg mice as expected based on previous findings [18]. Long term treatment with diapocynin did not alter TH expression in either genotype. Diapocynin did not alter total open field movement in either genotype as expected based on the findings discussed in the above section (Figure 3H). Rearing in the open field apparatus was also measured, and showed no difference with diapocynin treatment in either genotype (Figure 3I).
Early symptoms of PD include decreases in motor coordination. Because we saw no changes in gross motor function, we next examined changes in two indices of motor coordination: the pole test and the Rotor-Rod. In the pole test (Video Still and Supplemental Video 1), LRRK2R1441G tg mice performed markedly poorer than wild type mice. This is evident in both an increased time to turn and time to descend the pole. LRRK2R1441G tg mice were also noted to have slower engagement of their tail on the pole, and frequently dragged their hind paws while descending. With diapocynin treatment, LRRK2R1441G tg mice showed improvement on the pole test, matching the speed of wild type mice.
Mice were also assessed for their ability to balance on a moving Rotor-Rod apparatus. As shown in Figure 3J, LRRK2R1441G tg mice had a markedly decreased latency to fall, indicating an inability to maintain balance on the rod. While long-term diapocynin treatment did not impact the function of wild type animals, the performance of LRRK2R1441G mice returned to normal with this treatment.
Discussion
LRRK2R1441G BAC tg mice have been reported to have dramatic deficiencies in gross motor function which are L-dopa responsive [18]. Within our colony, LRRK2R1441G tg mice never exhibited a loss of gross motor function, but did exhibit other PD-like symptoms suggestive of early stages of the disease. The lack of a motor function phenotype is consistent with more recent findings [2]. In this study, we found that mice at 16 months of age had normal open field behavior similar to wild type FVB mice, but exhibited loss of behavior associated with coordinated motor movement (e.g., balance). One possible explanation for this difference is that the original mice were bred onto an FVB/N strain from Taconic [The Jackson Laboratory, 1], whereas the commercially available strain, from which our colony is bred, are on an FVB/N sourced from The Jackson Laboratory. It is unclear what differences exist between these strains, and what effect this could have on motor function. Interestingly, LRRK2R1441G tg mice did not exhibit preference for the wall vs. middle of the enclosure, which would indicate a reliance on the wall for balance (Figure 2B). However, it is likely that this experiment may not be as sensitive as the Rotor-Rod and pole tests in delineating a balance deficit.
Activation of microglia is documented in PD patients and animal models [20, 25], though whether this activation is causative for disease progression remains controversial [13]. Concomitant with microglial activation, NADPH oxidase activity is also increased in PD brains [28]. NOX2, the specific isoform of NADPH oxidase found in microglia, may then be a major source of reactive oxygen species (ROS) during PD progression along with deregulated mitochondria [3]. Thus, preventing this ROS by inhibition of NOX2 is attractive therapeutically. Recent work from our lab and others has demonstrated therapeutic efficacy of the putative NADPH oxidase inhibitors apocynin and diapocynin against the progression of neurodegeneration in various models [11, 26]. Intriguingly, the potential for NOX1 involvement in PD was recently described, and apocynin effectively prevented neuronal death, though the mechanism by which this protection occurred remains unclear [5]. Notably, apocynin did not improve behavioral changes in a transgenic mouse model of Alzheimer’s disease, despite protection from oxidative protein damage [7].
In this study, we examined microglial activation by quantifying Iba-1 staining in the substantia nigra. Though gross motor function was not significantly impacted in LRRK2R1441G tg mice, we expected an increase in microglial activation due to the reversal of coordinated movement defects by diapocynin. Based on the absence of Iba-1 staining in LRRK2R1441G tg mice (data not shown), microglial activation in the substantia nigra does not appear to be a contributing factor in the motor deficits on Rotor-Rod and pole tests. Future studies are required to determine if there is inflammation occurring in other basal ganglia areas of the brain that may impact motor coordination. Alternatively, these mice may represent an early stage of the disease where ROS from activated microglia are not yet produced, or are produced at undetectable levels.
Despite the protective effect of diapocynin on early PD-like symptoms in the LRRK2R1441G mice, the mode of action of diapocynin in these mice is unknown. The high concentration of diapocynin required to inhibit hydroethidine oxidation to 2-hydroxyethidium (as a specific marker of O2·− production) suggests that direct inhibition of NOX2 is unlikely. Recently, a potential antioxidant capacity was ascribed to apocynin due to peroxidase-dependent hydrogen peroxide scavenging [15, 22]. Diapocynin is expected to participate in a similar reaction. In fact, it inhibits Amplex Red-derived fluorescence in the presence of H2O2 and peroxidase, but does not interfere with other non-peroxidase dependent detection methods (e.g. hydroethidine oxidation; data not shown). Furthermore, apocynin was recently shown to be a relatively poor direct radical scavenger [22]. Together, these data suggest that diapocynin is not working in this model directly as an antioxidant.
The future of PD patient care includes coupling an understanding of non-motor symptoms to new biomarkers to enable early disease detection [23]. Early detection of PD symptoms will allow for better patient care, specifically with regards to influencing the extended course of the disease. In this respect, future studies utilizing these mice will examine the early non-motor symptoms of PD including hyposmia. Based on our findings, LRRK2R1441G tg mice may offer better potential for the study of early PD symptoms than the gross motor defects expected later in PD progression.
Supplementary Material
LRRK2R1141G tg mice have difficulty turning to face downwards in the pole test, descend the pole more slowly, lack tail engagement with the pole, and drag their hind feet when descending. The video still shows representative images from the supplemental video taken 10 s after each mouse was placed on the pole. The LRRK2R1441G control treated mouse is the only one to not have turned to descend the pole by this time.
Highlights.
LRRK2R1441G mice lose coordinated movement without gross motor deficits.
Diapocynin prevents deficits in motor coordination in LRRK2R1441G mice.
LRRK2R1441G mice may be useful for modeling non-motor PD symptoms.
Acknowledgments
This study was funded by NIH grants NS039958 (to B.K.) and NS074443 (to A.K.), and by the Henry R. and Angeline E. Quadracci Chair Endowment (B.K.), and the Eugene and Linda Lloyd Chair Endowment (A.K.). The authors also thank Michael Sasner, Ph.D. and Melissa Osborne at The Jackson Lab for their helpful discussions, Sanger sequencing, and DNA copy number analysis expertise. Mass spectrometry was performed at the MCW Cancer Center Bioenergetics Shared Resource supported by Advancing a Healthier Wisconsin.
Abbreviations
- LRRK2
leucine-rich repeat kinase 2
- NADPH
nicotinamide adenine dinucleotide phosphate (reduced form)
- NOX2
NADPH oxidase isoform 2
- NSAID
non-steroidal anti-inflammatory drug
- PD
Parkinson’s disease
- PMA
phorbol-12-myristate-13-acetate
- ROS
reactive oxygen species
- RNS
reactive nitrogen species
- TH
tyrosine hydroxylase
Footnotes
The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1. [Accessed on: 10/30/2012];The Jackson Lab LRRK2R1441G Strain Development Information. http://jaxmice.jax.org/strain/009604.html.
- 2.Baptista M, Dave K, De Silva S, Sasner M, Gorodinsky A, Beck M, Quang C, Gamber K, Reith A, Lyon J, Roulois A, Aziz Y, Das S, Eberling J, Dufour A, Facheris M, Fiske B, Sheth N, Sherer T, Urkowitz A, Frasier M. Program No. 854.822. 2012 Neuroscience Meeting Planner. New Orleans, LA: Society for Neuroscience; 2012. MJFF Animal Models 2: Generation, characterization, and distribution of LRRK2 animal models for Parkinson’s research. Online. [Google Scholar]
- 3.Beal MF. Bioenergetic approaches for neuroprotection in Parkinson’s disease. Ann Neurol. 2003;53(Suppl 3):S39–47. doi: 10.1002/ana.10479. discussion S47-38. [DOI] [PubMed] [Google Scholar]
- 4.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 5.Cristovao AC, Choi DH, Baltazar G, Beal MF, Kim YS. The role of NADPH oxidase 1-derived reactive oxygen species in paraquat-mediated dopaminergic cell death. Antioxid Redox Signal. 2009;11:2105–2118. doi: 10.1089/ars.2009.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Driver JA, Logroscino G, Lu L, Gaziano JM, Kurth T. Use of non-steroidal anti-inflammatory drugs and risk of Parkinson’s disease: nested case-control study. BMJ. 2011;342:d198. doi: 10.1136/bmj.d198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dumont M, Stack C, Elipenhali C, Calingasan NY, Wille E, Beal MF. Apocynin administration does not improve behavioral and neuropathological deficits in a transgenic mouse model of Alzheimer’s disease. Neurosci Lett. 2011;492:150–154. doi: 10.1016/j.neulet.2011.01.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duty S, Jenner P. Animal models of Parkinson’s disease: a source of novel treatments and clues to the cause of the disease. Br J Pharmacol. 2011;164:1357–1391. doi: 10.1111/j.1476-5381.2011.01426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elbs K, Lerch H. Über Dehydrodivanillin. J Prakt Chem. 1916;93:1–9. [Google Scholar]
- 10.Gagne JJ, Power MC. Anti-inflammatory drugs and risk of Parkinson disease: a meta-analysis. Neurology. 2010;74:995–1002. doi: 10.1212/WNL.0b013e3181d5a4a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghosh A, Kanthasamy A, Joseph J, Anantharam V, Srivastava P, Dranka BP, Kalyanaraman B, Kanthasamy AG. Anti-inflammatory and neuroprotective effects of an orally active apocynin derivative in pre-clinical models of Parkinson’s disease. J Neuroinflammation. 2012;9:241–256. doi: 10.1186/1742-2094-9-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gillardon F, Schmid R, Draheim H. Parkinson’s disease-linked leucine-rich repeat kinase 2(R1441G) mutation increases proinflammatory cytokine release from activated primary microglial cells and resultant neurotoxicity. Neuroscience. 2012;208:41–48. doi: 10.1016/j.neuroscience.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Graeber MB, Streit WJ. Microglia: biology and pathology. Acta Neuropathol. 2010;119:89–105. doi: 10.1007/s00401-009-0622-0. [DOI] [PubMed] [Google Scholar]
- 14.Greggio E, Cookson MR. Leucine-rich repeat kinase 2 mutations and Parkinson’s disease: three questions. ASN Neuro. 2009;1 doi: 10.1042/AN20090007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heumuller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schroder K, Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension. 2008;51:211–217. doi: 10.1161/HYPERTENSIONAHA.107.100214. [DOI] [PubMed] [Google Scholar]
- 16.Khan NL, Jain S, Lynch JM, Pavese N, Abou-Sleiman P, Holton JL, Healy DG, Gilks WP, Sweeney MG, Ganguly M, Gibbons V, Gandhi S, Vaughan J, Eunson LH, Katzenschlager R, Gayton J, Lennox G, Revesz T, Nicholl D, Bhatia KP, Quinn N, Brooks D, Lees AJ, Davis MB, Piccini P, Singleton AB, Wood NW. Mutations in the gene LRRK2 encoding dardarin (PARK8) cause familial Parkinson’s disease: clinical, pathological, olfactory and functional imaging and genetic data. Brain. 2005;128:2786–2796. doi: 10.1093/brain/awh667. [DOI] [PubMed] [Google Scholar]
- 17.Kim B, Yang MS, Choi D, Kim JH, Kim HS, Seol W, Choi S, Jou I, Kim EY, Joe EH. Impaired inflammatory responses in murine Lrrk2-knockdown brain microglia. PLoS One. 2012;7:e34693. doi: 10.1371/journal.pone.0034693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y, Liu W, Oo TF, Wang L, Tang Y, Jackson-Lewis V, Zhou C, Geghman K, Bogdanov M, Przedborski S, Beal MF, Burke RE, Li C. Mutant LRRK2(R1441G) BAC transgenic mice recapitulate cardinal features of Parkinson’s disease. Nat Neurosci. 2009;12:826–828. doi: 10.1038/nn.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin I, Dawson VL, Dawson TM. The impact of genetic research on our understanding of Parkinson’s disease. Prog Brain Res. 2010;183:21–41. doi: 10.1016/S0079-6123(10)83002-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGeer PL, McGeer EG. Glial reactions in Parkinson’s disease. Mov Disord. 2008;23:474–483. doi: 10.1002/mds.21751. [DOI] [PubMed] [Google Scholar]
- 21.Paisan-Ruiz C, Jain S, Evans EW, Gilks WP, Simon J, van der Brug M, Lopez de Munain A, Aparicio S, Gil AM, Khan N, Johnson J, Martinez JR, Nicholl D, Carrera IM, Pena AS, de Silva R, Lees A, Marti-Masso JF, Perez-Tur J, Wood NW, Singleton AB. Cloning of the gene containing mutations that cause PARK8-linked Parkinson’s disease. Neuron. 2004;44:595–600. doi: 10.1016/j.neuron.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 22.Petronio MS, Zeraik ML, Fonseca LM, Ximenes VF. Apocynin: chemical and biophysical properties of a NADPH oxidase inhibitor. Molecules. 2013;18:2821–2839. doi: 10.3390/molecules18032821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schapira AH, Agid Y, Barone P, Jenner P, Lemke MR, Poewe W, Rascol O, Reichmann H, Tolosa E. Perspectives on recent advances in the understanding and treatment of Parkinson’s disease. Eur J Neurol. 2009;16:1090–1099. doi: 10.1111/j.1468-1331.2009.02793.x. [DOI] [PubMed] [Google Scholar]
- 24.Schapira AH, Jenner P. Etiology and pathogenesis of Parkinson’s disease. Mov Disord. 2011;26:1049–1055. doi: 10.1002/mds.23732. [DOI] [PubMed] [Google Scholar]
- 25.Sherer TB, Betarbet R, Kim JH, Greenamyre JT. Selective microglial activation in the rat rotenone model of Parkinson’s disease. Neurosci Lett. 2003;341:87–90. doi: 10.1016/s0304-3940(03)00172-1. [DOI] [PubMed] [Google Scholar]
- 26.Simonyi A, Serfozo P, Lehmidi TM, Cui J, Gu Z, Lubahn DB, Sun AY, Sun GY. The neuroprotective effects of apocynin. Front Biosci (Elite Ed) 2012;4:2183–2193. doi: 10.2741/535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stefanska J, Pawliczak R. Apocynin: molecular aptitudes. Mediators Inflamm. 2008;2008:106507. doi: 10.1155/2008/106507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Surace MJ, Block ML. Targeting microglia-mediated neurotoxicity: the potential of NOX2 inhibitors. Cell Mol Life Sci. 2012;69:2409–2427. doi: 10.1007/s00018-012-1015-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trumbull KA, McAllister D, Gandelman MM, Fung WY, Lew T, Brennan L, Lopez N, Morre J, Kalyanaraman B, Beckman JS. Diapocynin and apocynin administration fails to significantly extend survival in G93A SOD1 ALS mice. Neurobiol Dis. 2012;45:137–144. doi: 10.1016/j.nbd.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Q, Smith RE, Luchtefeld R, Sun AY, Simonyi A, Luo R, Sun GY. Bioavailability of apocynin through its conversion to glycoconjugate but not to diapocynin. Phytomedicine. 2008;15:496–503. doi: 10.1016/j.phymed.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zielonka J, Zielonka M, Sikora A, Adamus J, Joseph J, Hardy M, Ouari O, Dranka BP, Kalyanaraman B. Global Profiling of Reactive Oxygen and Nitrogen Species in Biological Systems: high-throughput real-time analyses. J Biol Chem. 2012;287:2984–2995. doi: 10.1074/jbc.M111.309062. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
LRRK2R1141G tg mice have difficulty turning to face downwards in the pole test, descend the pole more slowly, lack tail engagement with the pole, and drag their hind feet when descending. The video still shows representative images from the supplemental video taken 10 s after each mouse was placed on the pole. The LRRK2R1441G control treated mouse is the only one to not have turned to descend the pole by this time.