Abstract
The Hodgkin’s Lymphoma Committee of the Lymphoma Study Association (LYSA) gathered in 2012 to prepare guidelines on the management of transplant-eligible patients with relapsing or refractory Hodgkin’s lymphoma. The working group is made up of a multidisciplinary panel of experts with a significant background in Hodgkin’s lymphoma. Each member of the panel of experts provided an interpretation of the evidence and a systematic approach to obtain consensus was used. Grades of recommendation were not required since levels of evidence are mainly based on phase II trials or standard practice. Data arising from randomized trials are emphasized. The final version was endorsed by the scientific council of the LYSA. The expert panel recommends a risk-adapted strategy (conventional treatment, or single/double transplantation and/or radiotherapy) based on three risk factors at progression (primary refractory disease, remission duration < 1 year, stage III/IV), and an early evaluation of salvage chemosensitivity, including 18fluorodeoxy glucose-positron emission tomography interpreted according to the Deauville scoring system. Most relapsed or refractory Hodgkin’s lymphoma patients chemosensitive to salvage should receive high-dose therapy and autologous stem-cell transplantation as standard. Efforts should be made to increase the proportion of chemosensitive patients by alternating non-cross-resistant chemotherapy lines or exploring the role of novel drugs.
Introduction
Hodgkin’s lymphoma (HL) has become a curable malignancy for most patients. However, treatment failure occurs in approximately 10% of limited-stage disease.1 In advanced-stage disease, up to 10% of patients will not reach complete remission (CR) and 20–30% of responders eventually relapse after treatment.2 High-dose therapy (HDT) followed by autologous stem cell transplantation (ASCT) has been clearly identified as a reference treatment in relapsing patients by two large randomized controlled studies which show improved freedom from treatment survival in the ASCT group compared to standard chemotherapy, and from registry studies which also show advantage for HDT in matched patients.3–7 Non-randomized analyses also show that HDT is a reasonable option, maybe the best available, for patients with primary refractory HL.8–11 Despite this evidence, many questions remain regarding issues such as definition of subgroups with different risk, type and number of salvage chemotherapies, use of metabolic imaging, place of double ASCT, the need to consider allogeneic transplantation in selected patients, and the role of radiotherapy. The objective of these guidelines is to provide hematologists with concise and clinically sound guidance on the management of these challenging situations. This work will be restricted to refractory or relapsing HL patients who are fit enough (no age limit) to be eligible for HDT.
Diagnosis and staging of relapsing and refractory HL
The expert panel reached a consensus on the recommendation that a repeat biopsy to confirm the presence of HL is mandatory for all patients relapsing 12 months or more after end of primary treatment in order to exclude alternative diagnoses. For patients with suspected relapse occurring up to 12 months after end of first-line, a new biopsy is also highly recommended taking into account risks inherent to an invasive procedure. For patients with apparent primary refractory disease, histological confirmation of HL is only recommended if progression is suspected within new sites of disease. Biopsy may not be mandatory in patients with clear radiological progression in sites of primary disease during treatment. Unless contraindicated, a whole-body computed tomography (CT) scan with contrast dye injection is recommended, as well as a bone marrow (BM) biopsy to fully assess disease extension. Baseline 18fluorodeoxy glucose (FDG)-positron emission tomography (PET) is also recommended for further comparison.
Prognostic factors and definition of risk groups
Prognostic factors
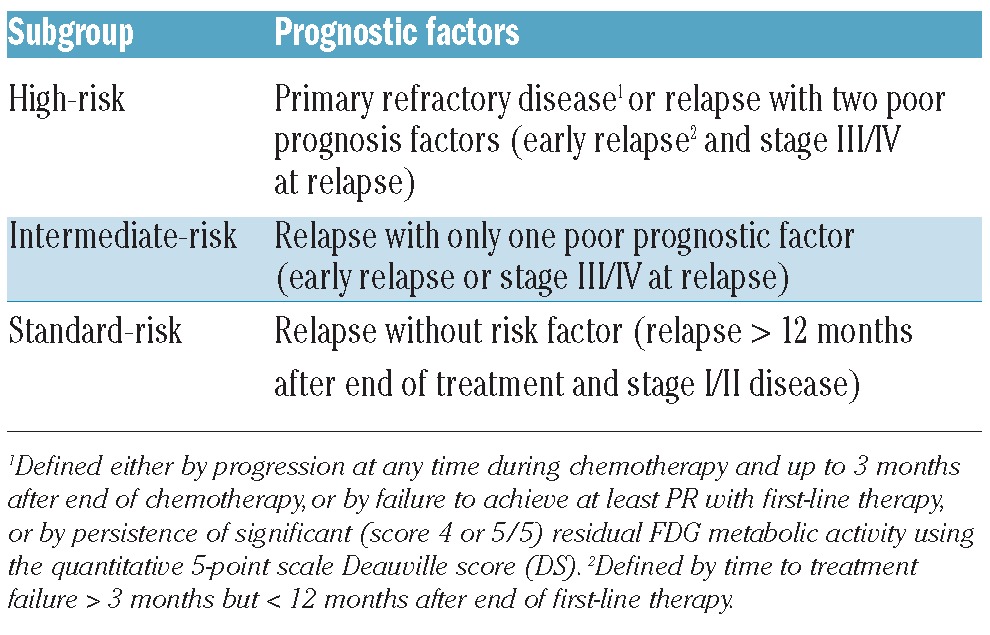
Key pre-salvage treatment risk factors that were almost consistently found to independently prognosticate outcome in relapsing and refractory HL are primary refractoriness, short time to HL relapse, and advanced clinical stage at relapse.2,12,13 The expert panel recommends using these robust prognostic factors to stratify patients into three risk groups (Table 1). There have been inconsistent reports of other predictive risk factors, sometimes with independent value, such as anemia, poor performance status (PS), presence of B symptoms, extranodal relapse, relapse in previous radiation field, age, or bulky disease, but the latter were not retained by the expert panel to serve in the risk group definition.2,14,15 Primary refractoriness is defined either by progression at any time during chemotherapy or radiotherapy (RT) and up to three months after the end of treatment, and/or by persistence of a PET positive residual mass, using the quantitative 5-point scale Deauville score (DS) for PET interpretation. Using these criteria, a positive FDG-PET (i.e. DS 4 or 5) after 3–4 cycles of ABVD for supra-diaphragmatic HL, and after four cycles of BEACOPPesc or ABVD for advanced HL, is considered as primary refractory disease if correlated with enlarged lymph node on CT scanning. CT scan was shown to be less accurate for the prediction of outcome than PET after two and four cycles of induction, as well as at end-of-treatment evaluation.16 However, given the risk of false positivity of PET, it is the consensus of the expert panel that a correlation between CT scan and metabolic responses remains mandatory in this definition of chemoresistance. In patients with metabolically active mass of uncertain significance, a biopsy is highly advised to prove refractoriness. Early relapse is defined by time to treatment failure more than three months but less than 12 months after end of first-line therapy. Time to relapse is a strong adverse factor since primary refractory patients (with response duration ≤ 3 months) have lower response rates and shorter OS as compared to patients with response lasting more than three months but less than 12 months.17 This is why these two factors are considered separately. The panel’s consensus is that additional risk factors, not retained for the stratification of patients into risk groups, may still have an impact on salvage strategy, such as a relapse in a previously irradiated site or a bulky relapse.
Table 1.
Classification of patients with relapsed and refractory HL in three risk groups: LYSA recommendations.
Risk groups
There is no standard prognostic model used to stratify patients in the published literature, and there is no uniform risk-group definition. Kuruvilla et al. combine the three major risk factors retained by the LYSA together with poor PS to test risk-stratified approaches. The German Hodgkin Study Group (GHSG) also incorporates low hemoglobin level (< 10.5 g/dL in females, < 12 g/dL in males) into their prognostic score.18 The lymphoma group of the Memorial Sloan-Kettering Cancer Center (MSKCC) uses three risk factors (remission duration < 1 year, extranodal disease, B symptoms) to stratify patients into favorable (0 or 1 risk factor), unfavorable (2 risk factors), and very high-risk (3 risk factors) groups.19
The expert panel recommends separating patients with relapsing or refractory HL into risk groups using the three prognostic factors (Table 1). The high-risk group encompasses patients with primary refractory disease and patients who relapse with two risk factors (early relapse and stage III/IV at relapse). The intermediate-risk group comprises patients who relapse with only one risk factor (either early relapse or stage III/IV at relapse). The standard-risk group includes patients who relapse but with no risk factor.
Salvage chemotherapy regimens before ASCT
No study has compared effectiveness of salvage regimens in refractory or relapsed HL. The expert panel recommends tailoring the choice of chemotherapy on an individual basis taking into account the initial therapy given, the risk of adding cumulative non-hematologic toxicity, and the possibility of harvesting stem cells (SC). Cardiac and pulmonary function should be evaluated prior to treatment. If indicated, reproductive counseling should be proposed prior to treatment. The objective of salvage chemotherapy is to produce a response, indicating that the tumor remains chemosensitive, which has a major impact on post-ASCT outcome. As will be discussed below (Restaging evaluation), the consensus of the expert panel is that achievement of FDG-PET negativity defines chemosensitivity and should be the goal of salvage chemotherapy.
Second-line regimens
The more commonly used salvage chemotherapy regimens have been recently summarized.2,20 For methodological reasons, it is impossible to compare response rate among these regimens. It is the recommendation of the LYSA expert panel to use a platinum-based regimen such as DHAP (dexamethasone, high-dose cytarabine-cisplatin) or ICE (ifosfamide-carboplatin-etoposide) for patients previously treated with ABVD or BEACOPP (especially if mediastinal radiotherapy has been delivered), given the risk of cardiac toxicity if the cumulative dose of doxorubicin has already reached 300–400 mg/m2. DHAP is usually given at 3–4 week intervals. However, the expert panel emphasizes that time-intensified DHAP, with a recycling time of 15 days, has been shown to be effective and well tolerated, while maintaining the possibility of harvesting stem cells.21 The expert panel recommends withholding chemotherapy until recovery to at least 0.8 × 109/L neutrophils and at least 80 × 109/L platelets, but this should be adapted to individual situations whenever appropriate. DHAP-like regimens with an alternate platinum compound, such as DHAOx (dexamethasone, high-dose cytarabine-oxaliplatin) or DHAC (dexamethasone, high-dose cytarabine-carboplatin) might be a preferred option in patients at risk for renal insufficiency and/or when a further allogeneic SCT is scheduled. The recommended dose for oxaliplatin is 130 mg/m2. The recommended area-under-the-curve (AUC) for carboplatin is 5 (with a maximum total dose of 800 mg). GDP (gemcitabine-cisplatin-dexamethasone) is also a potential option, with dose of gemcitabine set at 1,000 mg/m2 (Day 1) and dose of cisplatin fractionated at 33 mg/m2/day during three days. Alternatively, cisplatin could be given at 75 mg/m2 at Day 1 after gemcitabine.22,23 The ICE chemotherapy is also a widely used regimen retained by the LYSA experts as a potential option in refractory and relapsing HL.19 As for DHAP, a 2-week interval between cycles of ICE can be planned, although its administration is frequently delayed beyond two weeks because of scheduling difficulties, patient preference, or thrombocytopenia. IVOx (ifosfamide-etoposide-oxaliplatin) is also a potential outpatient option with good response rate, no cardiac toxicity, and without compromising stem cell mobilization.24 Although not commonly used by the LYSA experts, IGEV has demonstrated activity as a second-line therapy, with a low toxicity profile and mobilizing potential.25 There was no consensus among the expert panel regarding the use of more aggressive conventional regimens, such as mini-BEAM or dexa-BEAM. It is felt that these display significant toxic mortality, although they are still used by several as a bridge to transplantation.4,26 Dose-intensive sequential chemotherapy does not improve prognosis as compared to standard DHAP-based salvage, and is thus not recommended.27 The expert panel also does not recommend escalated BEACOPP as second-line therapy. As previously discussed, BEACOPP in relapsing patients carries a risk of exceeding critical cumulative dose of anthracyclins, and also displays significant hematologic toxicity with potential impairment of SC mobilization.28 Still, retrospective data indicate that BEACOPP intensification may effectively rescue refractory and relapsed HL, and might also improve prognosis in patients with a positive interim-PET after two cycles of ABVD.29,30 Overall, it is recommended to give 2–3 cycles of salvage regimen before evaluating response. Taking into account the risk/benefit ratio, a fourth cycle could be given to maintain response if the tranplantation has to be delayed.
Third-line regimens
In patients failing after two cycles of a second-line therapy, the expert panel recommends a third-line therapy with two or three cycles of chemotherapy containing non-cross-resistant drugs, in order to obtain tumor reduction and achieve chemosensitivity. ICE, IVOx, GVD (gemcitabine-vinorelbine-liposomal doxorubicin), or IGEV (ifosfamide-gemcitabine-vinorelbine) are recommended for patients failing to DHAP.25 DHAP or related protocols (DHAC, DHAOx, GPD), GVD or IGEV are recommended for patients failing second-line ICE. There was no consensus on the use of mini-BEAM in patients who are non-responders to platinum-containing regimen. Some use it routinely because approximately 50% of such patients may proceed to ASCT.2
Stem cell collection
Efficacy of peripheral blood stem cell (PBSC) mobilization after salvage chemotherapy has been recently reviewed by Kuruvilla et al.2 There is no standardized schedule of mobilization in this setting, and timing may depend on patient and treatment factors. Given the potential risk of PBSC mobilization failure, some experts recommend considering mobilization after the first cycle of salvage chemotherapy, unless there was bone marrow involvement at relapse. The expert panel agrees that for patients who have not been overwhelmingly pre-treated, and who display chemosensitivity, PBSC after second, third or even fourth cycle of salvage therapy is also acceptable.
Radiotherapy
Radiotherapy (RT) has been widely used as an adjunct to transplantation in refractory and relapsing HL. RT can be delivered either prior to, during, or after the conditioning regimen, and various modes of RT have been used in this setting. Total body irradiation (TBI), total lymphoid irradiation (TLI), or subtotal lymphoid irradiation (STLI) have been incorporated into the transplant conditioning regimen (although TBI has been now abandoned by certain groups because of toxicity).31 For instance, Moskowitz et al. routinely apply TLI and STLI before HDT for patients with no prior radiation therapy and nodal relapses.19,32 RT to more limited fields, such as involved-field RT (IFRT), has also been used, typically in patients with residual radiographic disease or bulky sites at relapse. Overall, retrospective studies tend to indicate a potential benefit in disease-specific survival if the patient has received IFRT. However, this is highly controversial because of several types of bias such as uneven distribution of patients between those who receive IFRT and those who do not, inadequate sizing of the studies, inconsistent dosing and scheduling of RT, types of selection bias, and frequent lack of study of RT-induced secondary cancers.33 Interestingly, thoracic RT before HDT and ASCT has been associated with a high post-transplant mortality rate, especially in patients who received thoracic RT within 50 days prior to HDT, or when the target volume included large volume of lung.34 Radiation to field encompassing the spinal cord also poses a risk of radiation myelitis, especially when the conditioning regimen contains busulfan that readily crosses the blood-brain barrier.35
Based on these data, the expert panel considers that the decision to include RT in the salvage strategy should be made individually taking into account prior irradiation, disease localization, and response to salvage chemotherapy. RT, which can be part of the strategy because HL remains a radiosensitive disease, should always be integrated in a combined modality treatment with chemotherapy, even for localized relapses. Indeed, the proportion of patients achieving long-term disease control after RT alone is too low.36 The expert panel recognizes two potential indications of RT (in addition to palliation of incurable HL). Firstly, TBI can be included in the conditioning regimen prior to ASCT for patients at high risk of relapse and who have not been previously irradiated (see below). Secondly, limited-field RT could be applied to patients with disease not achieving a metabolic CR prior to HDT, and which is amenable to RT. In this situation, the expert panel recommends using RT after transplantation to minimize the risk of pulmonary toxicity, although in highly selected patients at high risk for disease progression RT could be considered prior to transplantation on selected nodal sites. The recommended field is IFRT, individually adapted to target organs. The possibility of extending the field to portals involved at first presentation (if the patient has not been irradiated before), or to limit the field to that involved at relapse should be discussed on an individual basis. Recommended dose is at least 30 Gy with the potential of an additional 6–10 Gy, especially if the disease was not in metabolic CR prior to HDT.
Allogeneic stem-cell transplantation
The role of allogeneic SCT in HL is still a subject of controversy. Compared to ASCT, allogeneic SCT adds the potential of adoptive immunotherapy against HL via a graft-versus-Hodgkin’s lymphoma (GVHL) effect. Demonstrated responses to donor lymphocyte infusion (DLI) and a trend toward a lower relapse rate after allogeneic SCT than after ASCT, especially when chronic graft-versus-host disease (GVHD) occurs, are direct and indirect arguments for a GVHL effect.37–39 Because of the negative aspect of treatment-related mortality (TRM) of myeloablative conditioning regimens, reduced intensity conditioning (RIC) regimens have emerged as a potential option with acceptable TRM and long-term response.40,41 Importantly, several studies have shown that chemosensitivity at transplantation remains a major predictor of outcome of RIC allogeneic SCT (RIC-allo).38,41,42
The expert panel acknowledges that RIC-allo may have a role in HL. For patients with primary refractory disease, and for those relapsing after first-line standard-dose chemotherapy, RIC-allo without previous ASCT is not recommended. In this setting, RIC-allo could be considered but should be used exclusively in a tandem strategy after an ASCT and provided that the patient is chemosensitive to salvage and does not display disease progression between the two transplants.43 In spite of this, no consensus was reached on clear indications for using such a strategy. The expert panel agreed that failure to collect enough autologous CD34-positive cells to support a second ASCT or marrow invasion by HL is a potential indication. Some experts suggested that this strategy should also be proposed to patients accumulating poor-risk factors, as well as in patients needing more than one line of salvage chemotherapy before displaying chemosensitivity, in agreement with the recommendations of Mendler and Friedberg.20 In contrast, other LYSA experts emphasize that this latter group of patients are at high risk for rapid progression before any GVL effect can occur, and that they should then be oriented toward a second ASCT rather than RIC-allo. Benefit to risk ratio of RIC-allo and donor availability should also be weighted in the decision.
The expert panel also addressed the issue of patients relapsing after ASCT. RIC-allo could be considered in the latter provided that they display chemosensitivity to salvage regimens.40,44 It has been noted that a proportion of them could, as an alternative, undergo a second ASCT in case of very late relapse (> 5 years has been proposed) after the first ASCT using stem cells available from the initial procedure.45
For allogeneic transplantation, the expert panel recommends using a genoidentical donor, or a 10/10 HLA matched unrelated donor. There is no standardized RIC in this setting. The expert panel recommends using the ‘FluBu’ regimen with intravenous (i.v.) fludarabine at a total dosage of 120–180 mg/m2 over five days (max. 200 mg/m2) and busulfan (i.v.) at 0.8 mg/kg/day during four days.46
Restaging evaluation during and after salvage chemotherapy
Definition of chemosensitivity
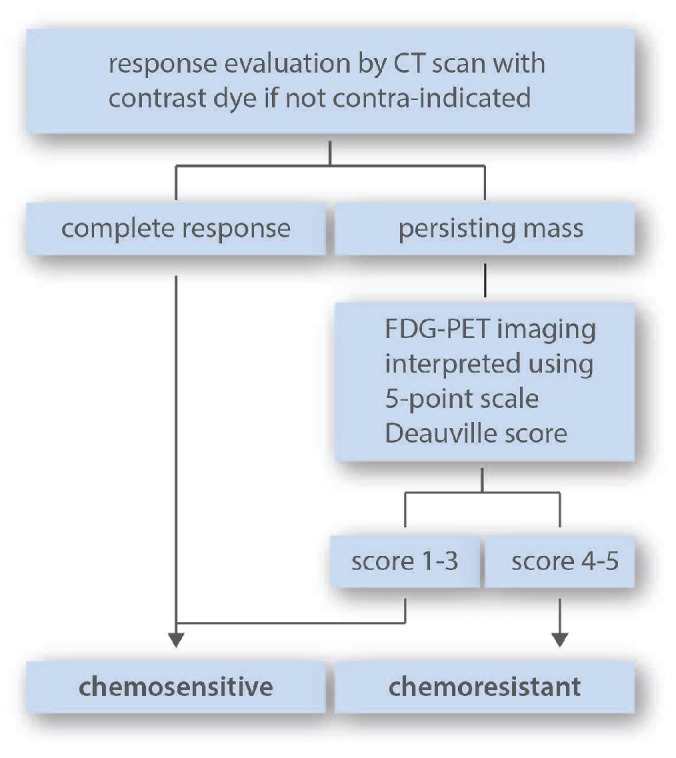
The objective of restaging is to identify patients who are chemosensitive for salvage therapy and hence eligible for transplantation (Figure 1). Functional imaging displays a strong prognostic value in this situation that overshadows classical risk factors.47–49 For instance, patients in partial remission (PR) with CT imaging have similar outcome to CR patients if they have negative functional imaging.50 More recently, Moskowitz et al. have also observed that metabolic imaging was the only factor significant for event-free survival (EFS) and OS, and clearly identified poor-risk patients since EFS for PET-negative patients was over 80% versus 28.6% for patients with a positive scan.47 In their program, it was prospectively scheduled to turn to a different potentially non-cross-resistant chemotherapy regimen in patients remaining PET-positive after the first salvage in another attempt to induce PET negativity. Their strategy resulted in post-transplant outcome for patients having received one (ICE) or two salvage chemotherapy (ICE followed by GVD) that was indistinguishable provided that pre-transplant FDG-PET was negative.32 The Houston team showed independent adverse effect (Hazard ratio 3.1) of positive PET at transplant in 180 poor-risk relapsing or refractory HL patients.51 Devillier et al. also recently reported in 111 patients with relapsed or refractory HL that only PET status significantly influenced OS in multivariate analysis, regardless of disease risk at relapse.52 On the other hand, the same studies emphasize that a substantial proportion of patients with positive PET before HDT will remain free of subsequent relapse, indicating that some patients could be cured despite a hypermetabolic residual mass after salvage therapy, but also that interpretation criteria could probably be improved to maximize FDG-PET positive predictive value in this setting.53,54
Figure 1.
Definition of response to salvage chemotherapy: recommendations by the LySA HL committee.
Based on these data, the expert panel agrees that patients can be qualified as chemosensitive to salvage if: a) PET is negative even in case of residual mass by CT scanning; and/or b) if CT scan does not show any residual mass. Patients should be considered as chemoresistant in case of progressive disease by CT scanning and/or a positive PET scan after either one or two salvage chemotherapies. In this definition of chemosensitivity, a correlation between anatomic and metabolic responses is mandatory. For assessment of PET response, the expert panel recommends using the 5-point DS with patients scoring 1–3 quoted as negative and those scoring 4–5 as positive (Table 2).55 In other words, a lesion showing residual activity higher than the liver background should be scored as positive. The 5-point scale, with a cut off between scores 3 and 4 as the threshold for a positive scan has been confirmed by the International Validation Studies (IVS) as a prognostic tool for interim evaluation of response in HL.56 The expert panel emphasizes that FDG-PET positivity alone, without residual mass by CT scanning, is not a sufficient criteria to initiate a salvage second-line therapy in HL, to switch a patient to a non-cross-resistant third-line regimen, or to rescue a patient for HDT. On the other hand, since many patients have residual mass at the end of salvage chemotherapy, response determination by CT scanning alone is not sufficient.
Table 2.
Deauville 5-point scoring system.

Timing of CT and FDG-PET evaluation
The expert panel advises restaging by CT, with contrast dye injection if not contraindicated, and FDG-PET after two cycles of salvage chemotherapy. This timing for metabolic evaluation during salvage has not been extensively validated. However, Moskowitz et al. have unequivocally shown a much better EFS for FDG-PET negative patients after two cycles of ICE, as compared to those who remain FDG-PET positive.32 Restaging should be further repeated before intensification if the patient has received more than one additional cycle of the same salvage chemotherapy, or if a third-line salvage regimen has been given (again after two cycles). Restaging by at least CT (FDG-PET at the discretion of the attending physician) is also advised prior to second transplantation to exclude progression. Repeat imaging by injected CT (if FDG-PET was negative prior to transplantation) should be performed three months after transplantation. Restaging should also include BM biopsy if involved by HL before salvage therapy.
Therapeutic guidelines according to risk group
The objective of a risk-adapted strategy is to avoid over-treatment of standard-risk patients, and to selectively increase treatment intensity for the poor risk patients. The expert panel recommends a treatment strategy in relapsed and refractory HL adapted to initial risk factors and to results of interim evaluation of response using anatomic and metabolic criteria.
High-risk group
This group includes patients with refractory HL, and relapses with two risk factors as defined in Table 1 (Figure 2). Several reports indicate that dose-increased strategy, including double transplantation, is a valuable option in patients with high-risk relapsing/refractory HL, taking into account that definition of high-risk status varies among the studies. In 2008, the Groupe d’ Etude des Lymphomes de l’Adulte (GELA) and the Société Française de Greffe de Moelle (SFGM) proposed a risk-adapted strategy in relapsed HL based on the separation of patients into three prognostic groups. Tandem ASCT results suggested a benefit for poor-risk patients (defined by primary refractoriness or > two of the following risk factors: time to relapse < 12 months, stage III/IV at relapse, and relapse within previously irradiated site at > 30 Gy), compared with previous reports with single ASCT. Some patients were converted in PR or CR by the second transplant, and overall outcome for PR, CR or CRu (only defined by CT) patients did not differ significantly if the patient had received double transplantation.31,57,58 Fung et al. also suggested that in primary progressive and poor-risk recurrent HL a tandem ASCT program was effective and compared favorably with the conventional single transplant.59 With the same goal of improving survival in patients with multiple risk factors, Moskowitz et al. conducted a study of risk-adapted salvage treatment. In this program, all patients were treated with ICE-based protocols and were offered, if proven chemosensitive, ASCT with whenever possible TLI (1800 cGy) in the conditioning regimen. However, dose-intensity of ICE and conditioning regimen were tailored to the presence of risk factors including an initial remission duration of less than one year, active B symptoms, and extranodal disease. In particular, high-risk patients were scheduled to receive tandem transplantation (either two ASCT or one ASCT followed by allogeneic SCT). Their comparison with historical data showed that their risk-adapted approach eliminated the difference in prognosis between the three different subgroups, primarily by improving the outcome for the less favorable patients.60 The strategy can be tailored not only according to initial risk factors, but also on the results of interim PET evaluation. This is exemplified in the study by Moskowitz et al. in which PET-positive patients receive an additional salvage line in order to achieve PET negativity, and in the report by Devillier et al. in which tandem transplantation is performed in PET-positive patients. In both reports, PET-guided dose-intensification appeared to reverse the poor prognosis endorsed by PET positivity.32,52 Interestingly, Devillier et al. also suggested that a significant benefit could be gained from using tandem transplantation even in PET-negative patients. Tandem transplantation has been performed in a subset of patients at other institutions.15
Figure 2.
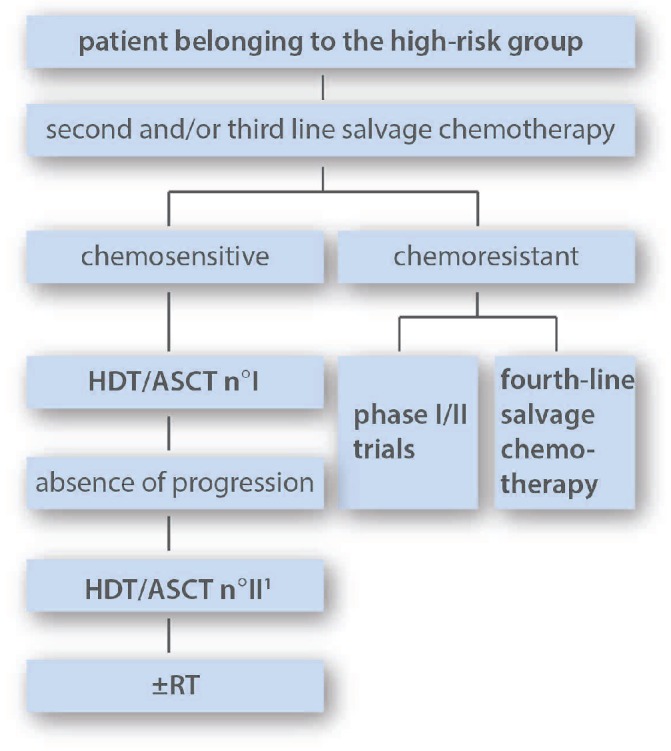
The suggested strategy for patients belonging to this group is tandem transplantation if chemosensitive to salvage therapy. Consolidation RT could be considered after transplantation in selected indications. 1 ASCT is standardly recommended as second transplant. In selected patients, RIC-allo could be considered as second transplant procedure. Decision between tandem ASCT or a single ASCT followed by RIC-allo should be made individually taking into account risk factors, the benefit to risk ratio and donor availability (see text for more informations). HTD: high-dose therapy; ASCT: autologous stem cell transplantation; RIC-alloSCT: reduced intensity conditioning allogeneic transplantation; RT: radiotherapy.
Based on the above-mentioned data, the opinion of the expert committee is that outcome of high-risk patients is unacceptably low and that these patients should be targeted with more intensive approaches, i.e. tandem transplantation, provided that they display chemosensitivity after salvage chemotherapy. Various conditioning regimens have been reported. The expert panel recommends BEAM as the first conditioning regimen, and 45–90 days later, a second conditioning regimen with TAM (TBI 12 Gy-cytarabine-melphalan) for previously unirradiated patients. For patients who have received prior dose-limiting radiation, recommendation for the second conditioning regimen is BAM (replacement of TBI by busulfan). Details of BAM and TAM dosages are presented in the paper by Morschhauser et al.57 As discussed above, the second ASCT could be replaced by an RIC-allo in selected patients. In patients from the high-risk group who are chemoresistant to the second-line treatment, a third-line regimen should be given as an attempt to induce chemosensitivity. Chemoresistant patients after two salvage lines are not ideal candidates either for ASCT or for RIC-allo and alternative strategies should be considered, including a fourth-line treatment (which could, however, carry the risk of further toxicity without tumoral benefit) or new drugs.
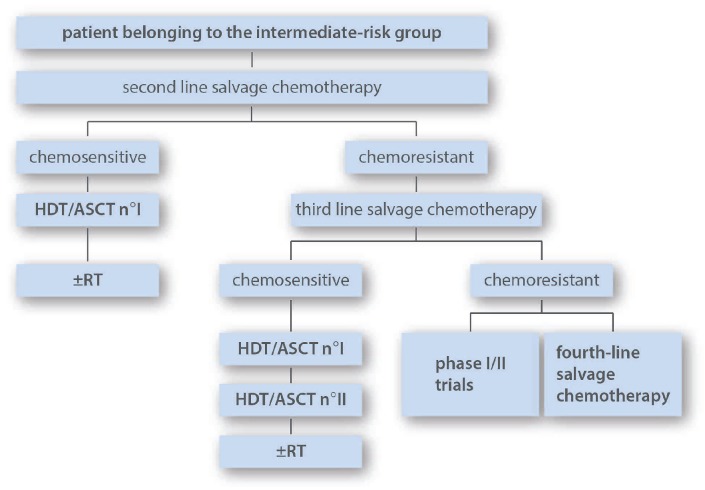
Intermediate-risk group
This group includes patients with relapsing HL and one of the two poor prognostic factors as depicted on Table 1 (Figure 3). The study by Morschhauser et al. shows that single ASCT in patients belonging to the intermediate-risk group (although with slightly different risk definition because relapse in a previously irradiated site was also considered as a risk factor) results in a 5-year freedom from second treatment failure (FF2F) and OS rates of 73% and 85% (median follow up 51 months), respectively.57
Figure 3.
The suggested strategy for patients belonging to this group is single ASCT in patients chemosensitive to salvage therapy. For patients who achieve chemosensitivity only after ≥ third-line salvage chemotherapy, tandem ASCT could be considered. Consolidation RT could be considered after transplantation in selected indications. HTD: high-dose therapy; ASCT: autologous stem cell transplantation; RT: radiotherapy.
The consensus of the expert panel is that single ASCT following HDT with BEAM in chemosensitive patients from the intermediate-group is the recommended treatment. There is no clear indication that TBI-containing regimens are superior to BEAM in this setting.31 However, IFRT could still be indicated after HDT in selected patients. For intermediate-risk patients who are chemoresistant to a second-line regimen, a third-line regimen should be attempted in order to obtain chemosensitivity and thus indication for consolidation HDT. In this latter subset of patients who achieve chemosensitivity only after a third-line regimen, a strategy comparable to that of the high-risk group (double transplant) could be considered on an individual basis, since the need to give additional salvage has been identified as prognostic marker for poor OS.44 Patients from the intermediate-risk group who do not achieve chemosensitivity are not ideal candidates for HDT and alternative strategies should be considered.
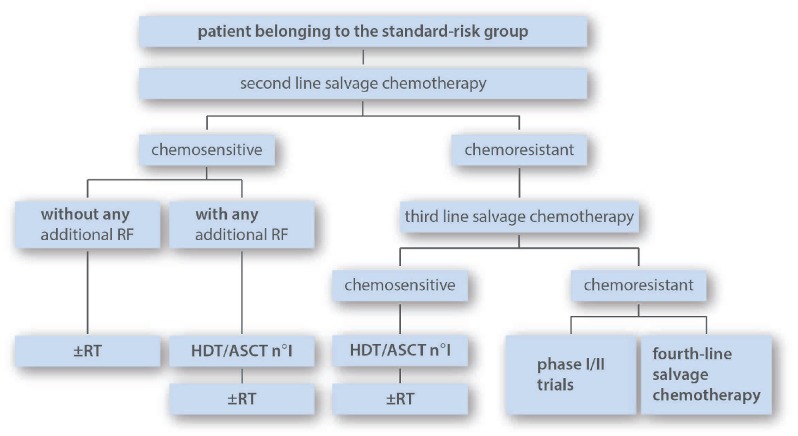
Standard-risk group
This group includes patients relapsing without any of the two stratifying adverse prognostic factors retained by the expert panel, in other terms patients with localized relapse occurring 12 months or more after the end of first-line treatment (Table 1, Figure 4). A proportion of these patients could be considered for combined modality treatment with conventional-dose chemotherapy followed by IFRT, provided that relapse is non-bulky, not in a previously irradiated site, and chemosensitive to second-line treatment. Indeed, it has been shown that conventional-dose chemotherapy, which has no curative potential by itself in the high- and intermediate-risk groups, can produce durable results in standard-risk patients.37,61 However, some authors reserve this non-ASCT-based strategy only for very late relapse (> 5 years for Kuruvilla et al.; > 3 years for Brusamolino et al.).2,37 For patients belonging to the standard-risk group with any additional risk factors (bulky relapse, relapse in irradiated site, B symptoms), the expert panel recommends the strategy proposed for the intermediate-risk group, consisting of BEAM and ASCT after having obtained chemosensitivity by second-line chemotherapy. For any patient from the standard-risk group with chemoresistance to second-line treatment, the expert panel recommends a double transplant strategy provided that the patient eventually responds to third-line regimen. As in the other groups, consolidation with RT after ASCT should be evaluated on an individual basis.
Figure 4.
The suggested strategy for patients belonging to this group is single ASCT in patients chemosensitive to salvage therapy. For selected chemosensitive patients with no additional RF such as relapse in a previously irradiated site, B symptoms or bulky relapse, a non-ASCT strategy could be considered with only RT in consolidation after salvage chemotherapy. For patients who have undergone ASCT, consolidation RT could be considered in selected indications. RF: risk factors; HTD: high-dose therapy; ASCT: autologous stem cell transplantation; RT: radiotherapy.
DHAP- or ICE-derived protocols are acceptable salvage regimens in this subgroup, as in the intermediate- and high-risk subgroups. However, for selected standard-risk patients who have received less than 250 mg/m2 doxorubicin as first-line therapy, 4–6 cycles of escalated BEACOPP before RT (if treated conventionally), or 2–3 cycles of IVA50 (ifosfamide-etoposide-doxorubicin at 50 mg/m2) before HDT are also potential options.
Management of chemoresistant patients and new agents
Unequivocally, the best results from ASCT are seen in patients in complete anatomic and/or metabolic response after salvage. This is why the panel of experts advises reserving HDT and transplantation for patients displaying clear chemosensitivity by stringent criteria (CT scan and metabolic imaging using DS). Still, given the lack of reliable curative alternative options, there is no consensus that HDT should be totally abandoned in chemoresistant patients. Indeed, with double, or even single transplantation, a proportion of them can be converted into remission and enjoy long-term DFS and even apparent cure. Connors et al. emphasize that among PET-positive patients immediately before HDT and ASCT, 7–56% will remain free of subsequent relapse, indicating that the positive predictive value of PET greatly depends on the PET positivity criteria used, and that “it would not be completely prudent to abandon HDT in these patients”.53 Morschauser et al. also showed a 45% 5-year OS estimate in chemoresistant high-risk patients on the basis of CT assessment if ASCT2 was completed, and also clearly showed the conversion rate after HDT since among 55 patients who experienced cytoreduction failure (SD + PD) 30 responded to ASCT1 and 17 patients achieved CR/CRu after ASCT2.57 Moskowitz et al. have recently shown that approximately one-third of patients with abnormal pre-ASCT functional imaging (FDG-PET or Gallium) are cured with ASCT, indicating, therefore, that ASCT may remain a valuable option even in these patients.47 Devillier et al. reported a 5-year PFS of 43% in PET-positive patients.52 Colpo et al. also state that an attempt should be made to achieve FDG-PET negativity before ASCT as part of routine procedure, but that they do also proceed to ASCT in certain FDG-PET-positive patients.14 Finally, Stiff et al. did not find chemoresistance at transplantation to be an adverse prognostic risk factor when using dose-augmented TBI-based preparative regimens. However, in their study, metabolic imaging was not available, implying that a proportion of resistant patients might actually be in remission.62 Overall, these data show that a proportion of chemoresistant patients can be salvaged by the transplantation itself, and that experts still recommend this strategy.
Several biological response modifiers, such as monoclonal antibodies, HDAC inhibitors, PI3K/Akt/mTOR inhibitors, lenalidomide, or proteasome inhibitors, are currently under development in HL (reviewed by Younes,63 Jona and Younes,64 and Moskowitz65). In the future, concommitant or sequential combinations of chemo-radiotherapy with these targeted agents could improve response rate prior to transplantation or play a role in maintenance post-ASCT intervention, potentially lessening the need to perform the second transplantation. Among the latter drugs, brentuximab vedotin, an antibody-drug conjugate composed of an anti-CD30 antibody conjugated to the microtubule-disrupting agent, monomethyl auristatin E, has demonstrated a high overall and CR rate in patients with relapsed and refractory HL after ASCT.66,67 The same drug also displayed encouraging activity in patients relapsing after allogeneic SCT.68 Finally, brentuximab vedotin was also given before RIC-alloSCT and did not appear to adversely affect engraftment, GVHD rate, or survival, and might thus play a role in improving pre-transplantation disease control.69,70 Brentuximab vedotin received FDA and EMEA approval in 2011 and 2012, respectively, for use as salvage therapy in HL following failure of HDT/ASCT or at least two prior therapies if patients are not candidates for HDT/ASCT. This means that the drug can be used for patients refractory to standard-dose salvage regimens, although there are no clinical results in this indication.71 The up-dated NCCN guidelines actually include brentuximab vedotin as an option for patients with progressive disease after HDT/ASCT or at least two prior chemotherapies for all patients regardless of their eligibility for HDT/ASCT.72 Given its activity, brentuximab vedotin could replace a chemotherapy regimen early in the strategy of salvage therapy, but this possibility requires further evaluation. HDAC inhibitors, such as panobinostat, have shown promising and durable activity in advanced HL patients who relapsed or were refractory to ASCT, and are good candidates for evaluation in combination with chemotherapy.73 Bendamustine is also an option with activity in heavily pre-treated patients with HL.65 Targeting surface proteins on the malignant Hodgkin’s and Reed-Sternberg (HRS) cells can also be achieved with rituximab when these cells express CD20 (15–30% of classical HL). In addition, HRS stem cells and supportive B-cell environments also express CD20. Remissions, mostly partial, in nodal and spleen sites, and alleviation of systemic symptoms, have been seen after single agent rituximab in patients with advanced classical HL irrespective of CD20 expression on HRS cells.74 The combination of rituximab with gemcitabine monotherapy or with GIFOX (gemcitabine-ifosfasmide-oxaliplatin) has also produced responses in recurrent or refractory classical HL that may indicate a role for rituximab in this setting.75–77
Based on these data, the expert panel recommends making every effort to obtain FDG-PET negativity prior to ASCT by turning to different potentially non-cross-resistant regimens and by including patients in phase I and II protocols with novel therapies if they have not achieved chemosensitivity after a second- or third-line salvage chemotherapy regimen. However, it is acknowledged that a proportion of chemoresistant patients could benefit from the transplant.
Conclusions
Second-line chemotherapy followed by HDT and ASCT is the standard treatment for patients with relapsing and refractory HL. With this strategy, the cure rate can be estimated at 50–60%.15 With the aim of improving these results, the expert panel of the HL committee of the LYSA recommends a strategy including: 1) dose-adaptation according to pre-transplant patient’s characteristics; 2) dynamic adjustment of salvage chemotherapy according to results of interim response evaluation; and 3) as far as possible, restriction of HDT to patients who are demonstrated as chemosensitive. Three major prognostic factors at relapse (refractoriness, short disease-free interval and disseminated disease) that allow stratification of patients into three meaningful risk groups have been retained by the panel. Patients with high-risk disease should be oriented to tandem transplantation provided that they display chemosensitivity and no progression between the two transplants. Patients with intermediate-risk disease or standard-risk with any additional risk factors can be treated with single transplantation. In some selected standard-risk patients with chemosensitivity and no additional risk factors, a strategy without HDT could be applied. Screening for response to salvage treatment is central to this program and should be performed with CT scanning and FDG-PET interpreted with criteria adapted for interim response analysis. Based on PET-guided evaluation, every effort should be made to increase the proportion of chemosensitive patients, by alternating non-cross-resistant chemotherapy lines or exploring the role of novel drugs. However, the expert panel warns of the care needed to avoid over-use of more than two salvage lines, because of concerns regarding the selection of highly chemoresistant lymphoma clones. These latter patients should rather be oriented towards targeted therapies and made eligible for HDT if they eventually respond. The consensus of the panel is that maintenance therapy after transplantation can not be routinely proposed at the present time. The expert panel also emphasizes that new drug development in HL is particularly important not only to improve prognosis of patients who fail first-line standard-dose chemotherapy, but also to decrease short- and long-term toxic effects that are correlated with the burden of current chemo-radiotherapy.
Appendix: Members of the HL committee of the LYSA
Physicians: M. André, CHU UCL Mont-Godinne Dinant, Yvoir, Belgique; M. Bernard, Centre Hospitalier Universitaire, Rennes; C. Besson, Centre Hospitalier Universitaire, Kremlin-Bicêtre; C. Borel, Centre Hospitalier Universitaire, Toulouse; S. Bologna, Centre Hospitalier Universitaire, Brabois Nancy; P. Brice, Centre Hospitalier Universitaire Saint-Louis, Paris; P. Carde, Institut Oncologie Hartmann, Neuilly sur Seine; O. Casasnovas, Centre Hospitalier Universitaire, Dijon; B. Deau, Centre Hospitalier Universitaire Cochin, Paris; C. Fermé, Institut de Cancérologie Gustave Roussy, Villejuif; P. Feugier, Centre Hospitalier Universitaire, Brabois Nancy; L. Fornecker, Centre Hospitalier Universitaire, Strasbourg; J. Gabarre, Centre Hospitalier Universitaire Pitié-Salpêtrière, Paris; I. Gaillard, Centre Hospitalier Universitaire Henri-Mondor, Créteil; T. Gastinne, Centre Hospitalier Universitaire, Nantes; H. Ghesquière, Centre Léon Bérard, Lyon; N. Milpied, Centre Hospitalier Universitaire, Bordeaux; L. Molina, Centre Hospitalier Universitaire, Grenoble; F. Morschhauser, Centre Hospitalier Universitaire, Lille; P. Quittet, Centre Hospitalier Universitaire, Montpellier; O. Reman, Centre Hospitalier Universitaire, Caen; V. Ribrag, Institut de Cancérologie Gustave Roussy, Villejuif; C. Sebban, Centre Léon Bérard, Lyon; D. Sénécal, Centre Hospitalier Universitaire, Chambery; C. Soussain, Institut Curie, Saint-Cloud; A. Stamatoullas, Centre Henri Becquerel, Rouen; M. Touati, Centre Hospitalier Universitaire, Limoges, France; E. Van Den Neste, Cliniques Universitaires UCL Saint-Luc, Belgique. Nuclear medicine: V. Edeline, Institut Curie, Saint-Cloud. Radiotherapy: L. Feuvret, Centre Hospitalier Universitaire, Pitié Salpêtrière, Paris; T. Girinsky, Institut de Cancérologie Gustave Roussy, Villejuif; C. Charra-Brunaud, Centre Alexis Vautrin, Nancy; C. Hennequin, Centre Hospitalier Universitaire Saint-Louis, Paris; K. Peignaux, Centre Georges-François Leclerc, Dijon; L. Quero, Centre Hospitalier Universitaire, Saint-Louis, Paris. Pathology: D. Damotte, Hôpital Georges Pompidou, Paris; P. Dartigues, Institut de Cancérologie Gustave Roussy, Villejuif; M. Parrens, Centre Hospitalier Universitaire, Bordeaux; A. Traverse-Glehen, Centre Hospitalier Lyon Sud, Lyon, France.
Footnotes
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Meyer RM, Hoppe RT. Point/counterpoint: early-stage Hodgkin lymphoma and the role of radiation therapy. Blood. 2012;120(23):4488–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuruvilla J, Keating A, Crump M. How I treat relapsed and refractory Hodgkin lymphoma. Blood. 2011;117(16):4208–17 [DOI] [PubMed] [Google Scholar]
- 3.Linch DC, Winfield D, Goldstone AH, Moir D, Hancock B, McMillan A, et al. Dose intensification with autologous bone-marrow transplantation in relapsed and resistant Hodgkin’s disease: results of a BNLI randomised trial. Lancet. 1993;341(8852): 1051–4 [DOI] [PubMed] [Google Scholar]
- 4.Schmitz N, Pfistner B, Sextro M, Sieber M, Carella AM, Haenel M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin’s disease: a randomised trial. Lancet. 2002; 359(9323):2065–71 [DOI] [PubMed] [Google Scholar]
- 5.Lazarus HM, Rowlings PA, Zhang MJ, Vose JM, Armitage JO, Bierman PJ, et al. Autotransplants for Hodgkin’s disease in patients never achieving remission: a report from the Autologous Blood and Marrow Transplant Registry. J Clin Oncol. 1999; 17(2):534–45 [DOI] [PubMed] [Google Scholar]
- 6.Yuen AR, Rosenberg SA, Hoppe RT, Halpern JD, Horning SJ. Comparison between conventional salvage therapy and high-dose therapy with autografting for recurrent or refractory Hodgkin’s disease. Blood. 1997;89(3):814–22 [PubMed] [Google Scholar]
- 7.Carella AM, Congiu AM, Gaozza E, Mazza P, Ricci P, Visani G, et al. High-dose chemotherapy with autologous bone marrow transplantation in 50 advanced resistant Hodgkin’s disease patients: an Italian study group report. J Clin Oncol. 1988;6(9): 1411–6 [DOI] [PubMed] [Google Scholar]
- 8.Josting A, Rueffer U, Franklin J, Sieber M, Diehl V, Engert A. Prognostic factors and treatment outcome in primary progressive Hodgkin lymphoma: a report from the German Hodgkin Lymphoma Study Group. Blood. 2000;96(4):1280–6 [PubMed] [Google Scholar]
- 9.Andre M, Henry-Amar M, Pico JL, Brice P, Blaise D, Kuentz M, et al. Comparison of high-dose therapy and autologous stem-cell transplantation with conventional therapy for Hodgkin’s disease induction failure: a case-control study. Societe Francaise de Greffe de Moelle. J Clin Oncol. 1999;17(1): 222–9 [DOI] [PubMed] [Google Scholar]
- 10.Ferme C, Mounier N, Divine M, Brice P, Stamatoullas A, Reman O, et al. Intensive salvage therapy with high-dose chemotherapy for patients with advanced Hodgkin’s disease in relapse or failure after initial chemotherapy: results of the Groupe d’Etudes des Lymphomes de l’Adulte H89 Trial. J Clin Oncol. 2002;20(2):467–75 [DOI] [PubMed] [Google Scholar]
- 11.Morabito F, Stelitano C, Luminari S, Mammi C, Marcheselli L, Callea V, et al. The role of high-dose therapy and autologous stem cell transplantation in patients with primary refractory Hodgkin’s lymphoma: a report from the Gruppo Italiano per lo Studio dei Linfomi (GISL). Bone Marrow Transplant. 2006;37(3):283–8 [DOI] [PubMed] [Google Scholar]
- 12.Josting P, Bierman PJ. Relapsed and refractory Hodgkin lymphoma. Engert A, Horning SJ. (eds), Hodgkin Lymphoma. 2011: 203–43 [Google Scholar]
- 13.Brice P, Bastion Y, Divine M, Nedellec G, Ferrant A, Gabarre J, et al. Analysis of prognostic factors after the first relapse of Hodgkin’s disease in 187 patients. Cancer. 1996;78(6):1293–9 [DOI] [PubMed] [Google Scholar]
- 14.Colpo A, Hochberg E, Chen YB. Current status of autologous stem cell transplantation in relapsed and refractory Hodgkin’s lymphoma. The Oncologist. 2012;17(1):80–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith SD, Moskowitz CH, Dean R, Pohlman B, Sobecks R, Copelan E, et al. Autologous stem cell transplant for early relapsed/refractory Hodgkin lymphoma: results from two transplant centres. Br J Haematol. 2011;153(3):358–63 [DOI] [PubMed] [Google Scholar]
- 16.Hutchings M, Loft A, Hansen M, Pedersen LM, Buhl T, Jurlander J, et al. FDG-PET after two cycles of chemotherapy predicts treatment failure and progression-free survival in Hodgkin lymphoma. Blood. 2006;107(1):52–9 [DOI] [PubMed] [Google Scholar]
- 17.Pfreundschuh MG, Rueffer U, Lathan B, Schmitz N, Brosteanu O, Hasenclever D, et al. Dexa-BEAM in patients with Hodgkin’s disease refractory to multidrug chemotherapy regimens: a trial of the German Hodgkin’s Disease Study Group. J Clin Oncol. 1994;12(3):580–6 [DOI] [PubMed] [Google Scholar]
- 18.Josting A, Franklin J, May M, Koch P, Beykirch MK, Heinz J, et al. New prognostic score based on treatment outcome of patients with relapsed Hodgkin’s lymphoma registered in the database of the German Hodgkin’s lymphoma study group. J Clin Oncol. 2002;20(1):221–30 [DOI] [PubMed] [Google Scholar]
- 19.Moskowitz CH, Nimer SD, Zelenetz AD, Trippett T, Hedrick EE, Filippa DA, et al. A 2-step comprehensive high-dose chemoradiotherapy second-line program for relapsed and refractory Hodgkin disease: analysis by intent to treat and development of a prognostic model. Blood. 2001;97(3): 616–23 [DOI] [PubMed] [Google Scholar]
- 20.Mendler JH, Friedberg JW. Salvage therapy in Hodgkin’s lymphoma. The Oncologist. 2009;14(4):425–32 [DOI] [PubMed] [Google Scholar]
- 21.Josting A, Rudolph C, Reiser M, Mapara M, Sieber M, Kirchner HH, et al. Time-intensified dexamethasone/cisplatin/cytarabine: an effective salvage therapy with low toxicity in patients with relapsed and refractory Hodgkin’s disease. Ann Oncol. 2002;13(10): 1628–35 [DOI] [PubMed] [Google Scholar]
- 22.Kuruvilla J, Nagy T, Pintilie M, Tsang R, Keating A, Crump M. Similar response rates and superior early progression-free survival with gemcitabine, dexamethasone, and cisplatin salvage therapy compared with carmustine, etoposide, cytarabine, and melphalan salvage therapy prior to autologous stem cell transplantation for recurrent or refractory Hodgkin lymphoma. Cancer. 2006;106(2):353–60 [DOI] [PubMed] [Google Scholar]
- 23.Baetz T, Belch A, Couban S, Imrie K, Yau J, Myers R, et al. Gemcitabine, dexamethasone and cisplatin is an active and non-toxic chemotherapy regimen in relapsed or refractory Hodgkin’s disease: a phase II study by the National Cancer Institute of Canada Clinical Trials Group. Ann Oncol. 2003;14(12):1762–7 [DOI] [PubMed] [Google Scholar]
- 24.Sibon D, Ertault M, Al Nawakil C, de Bazelaire C, Franchi P, Briere J, et al. Combined ifosfamide, etoposide and oxalipatin chemotherapy, a low-toxicity regimen for first-relapsed or refractory Hodgkin lymphoma after ABVD/EBVP: a prospective monocentre study on 34 patients. Br J Haematol. 2011;153(2):191–8 [DOI] [PubMed] [Google Scholar]
- 25.Santoro A, Magagnoli M, Spina M, Pinotti G, Siracusano L, Michieli M, et al. Ifosfamide, gemcitabine, and vinorelbine: a new induction regimen for refractory and relapsed Hodgkin’s lymphoma. Haematologica. 2007;92(1):35–41 [DOI] [PubMed] [Google Scholar]
- 26.Moore S, Kayani I, Peggs K, Qian W, Lowry L, Thomson K, et al. Mini-BEAM is effective as a bridge to transplantation in patients with refractory or relapsed Hodgkin lymphoma who have failed to respond to previous lines of salvage chemotherapy but not in patients with salvage-refractory DLBCL. Br J Haematol. 2012;157(5):543–52 [DOI] [PubMed] [Google Scholar]
- 27.Josting A, Muller H, Borchmann P, Baars JW, Metzner B, Dohner H, et al. Dose intensity of chemotherapy in patients with relapsed Hodgkin’s lymphoma. J Clin Oncol. 2010;28(34):5074–80 [DOI] [PubMed] [Google Scholar]
- 28.Eichenauer DA, Engert A. Is there a role for BEACOPP (bleomycin, etoposide, adriamycin, cyclophosphamide, vincristine, procarbazine, prednisone) in relapsed Hodgkin lymphoma? Leuk Lymphoma. 2009;50(11):1733–4 [DOI] [PubMed] [Google Scholar]
- 29.Gallamini A, Patti C, Viviani S, Rossi A, Fiore F, Di Raimondo F, et al. Early chemotherapy intensification with BEACOPP in advanced-stage Hodgkin lymphoma patients with a interim-PET positive after two ABVD courses. Br J Haematol. 2011;152(5):551–60 [DOI] [PubMed] [Google Scholar]
- 30.Cavalieri E, Matturro A, Annechini G, De Angelis F, Frattarelli N, Gentilini F, et al. Efficacy of the BEACOPP regimen in refractory and relapsed Hodgkin lymphoma. Leuk Lymphoma. 2009;50(11):1803–8 [DOI] [PubMed] [Google Scholar]
- 31.Brice P. Managing relapsed and refractory Hodgkin lymphoma. B J Haematol. 2008;141(1):3–13 [DOI] [PubMed] [Google Scholar]
- 32.Moskowitz CH, Matasar MJ, Zelenetz AD, Nimer SD, Gerecitano J, Hamlin P, et al. Normalization of pre-ASCT, FDG-PET imaging with second-line, non-cross-resistant, chemotherapy programs improves event-free survival in patients with Hodgkin lymphoma. Blood. 2012;119(7): 1665–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Biswas T, Culakova E, Friedberg JW, Kelly JL, Dhakal S, Liesveld J, et al. Involved field radiation therapy following high dose chemotherapy and autologous stem cell transplant benefits local control and survival in refractory or recurrent Hodgkin lymphoma. Radiother Oncol. 2012;103(3): 367–72 [DOI] [PubMed] [Google Scholar]
- 34.Tsang RW, Gospodarowicz MK, Sutcliffe SB, Crump M, Keating A. Thoracic radiation therapy before autologous bone marrow transplantation in relapsed or refractory Hodgkin’s disease. PMH Lymphoma Group, and the Toronto Autologous BMT Group. Eur J Cancer. 1999;35(1):73–8 [DOI] [PubMed] [Google Scholar]
- 35.Kahn S, Flowers C, Xu Z, Esiashvili N. Does the addition of involved field radiotherapy to high-dose chemotherapy and stem cell transplantation improve outcomes for patients with relapsed/refractory Hodgkin lymphoma? Int J Radiat Oncol Biol Phys. 2011;81(1):175–80 [DOI] [PubMed] [Google Scholar]
- 36.Josting A, Nogova L, Franklin J, Glossmann JP, Eich HT, Sieber M, et al. Salvage radiotherapy in patients with relapsed and refractory Hodgkin’s lymphoma: a retrospective analysis from the German Hodgkin Lymphoma Study Group. J Clin Oncol. 2005;23(7):1522–9 [DOI] [PubMed] [Google Scholar]
- 37.Brusamolino E, Bacigalupo A, Barosi G, Biti G, Gobbi PG, Levis A, et al. Classical Hodgkin’s lymphoma in adults: guidelines of the Italian Society of Hematology, the Italian Society of Experimental Hematology, and the Italian Group for Bone Marrow Transplantation on initial work-up, management, and follow-up. Haematologica. 2009;94(4):550–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corradini P, Sarina B, Farina L. Allogeneic transplantation for Hodgkin’s lymphoma. Br J Haematol. 2011;152(3):261–72 [DOI] [PubMed] [Google Scholar]
- 39.Peggs KS, Kayani I, Edwards N, Kottaridis P, Goldstone AH, Linch DC, et al. Donor lymphocyte infusions modulate relapse risk in mixed chimeras and induce durable salvage in relapsed patients after T-cell-depleted allogeneic transplantation for Hodgkin’s lymphoma. J Clin Oncol. 2011;29(8):971–8 [DOI] [PubMed] [Google Scholar]
- 40.Sureda A, Robinson S, Canals C, Carella AM, Boogaerts MA, Caballero D, et al. Reduced-intensity conditioning compared with conventional allogeneic stem-cell transplantation in relapsed or refractory Hodgkin’s lymphoma: an analysis from the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2008;26(3): 455–62 [DOI] [PubMed] [Google Scholar]
- 41.Robinson SP, Sureda A, Canals C, Russell N, Caballero D, Bacigalupo A, et al. Reduced intensity conditioning allogeneic stem cell transplantation for Hodgkin’s lymphoma: identification of prognostic factors predicting outcome. Haematologica. 2009;94(2):230–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sureda A, Canals C, Arranz R, Caballero D, Ribera JM, Brune M, et al. Allogeneic stem cell transplantation after reduced intensity conditioning in patients with relapsed or refractory Hodgkin’s lymphoma. Results of the HDR-ALLO study - a prospective clinical trial by the Grupo Espanol de Linfomas/Trasplante de Medula Osea (GEL/TAMO) and the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. Haematologica. 2012;97(2):310–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carella AM, Cavaliere M, Lerma E, Ferrara R, Tedeschi L, Romanelli A, et al. Autografting followed by nonmyeloablative immunosuppressive chemotherapy and allogeneic peripheral-blood hematopoietic stem-cell transplantation as treatment of resistant Hodgkin’s disease and non-Hodgkin’s lymphoma. J Clin Oncol. 2000;18(23):3918–24 [DOI] [PubMed] [Google Scholar]
- 44.Crump M. Management of Hodgkin lymphoma in relapse after autologous stem cell transplant. Hematology Am Soc Hematol Educ Program. 2008:326–33 [DOI] [PubMed] [Google Scholar]
- 45.Smith SM, van Besien K, Carreras J, Bashey A, Cairo MS, Freytes CO, et al. Second autologous stem cell transplantation for relapsed lymphoma after a prior autologous transplant. Biol Blood Marrow Transplant. 2008;14(8):904–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Armand P, Kim HT, Ho VT, Cutler CS, Koreth J, Antin JH, et al. Allogeneic transplantation with reduced-intensity conditioning for Hodgkin and non-Hodgkin lymphoma: importance of histology for outcome. Biol Blood Marrow Transplant. 2008;14(4):418–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moskowitz AJ, Yahalom J, Kewalramani T, Maragulia JC, Vanak JM, Zelenetz AD, et al. Pretransplantation functional imaging predicts outcome following autologous stem cell transplantation for relapsed and refractory Hodgkin lymphoma. Blood. 2010;116(23):4934–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Filmont JE, Gisselbrecht C, Cuenca X, Deville L, Ertault M, Brice P, et al. The impact of pre- and post-transplantation positron emission tomography using 18-fluorodeoxyglucose on poor-prognosis lymphoma patients undergoing autologous stem cell transplantation. Cancer. 2007;110 (6):1361–9 [DOI] [PubMed] [Google Scholar]
- 49.Cocorocchio E, Peccatori F, Vanazzi A, Piperno G, Calabrese L, Botteri E, et al. High-dose chemotherapy in relapsed or refractory Hodgkin lymphoma patients: a reappraisal of prognostic factors. Hematol Oncol. 2013;31(1):34–40 [DOI] [PubMed] [Google Scholar]
- 50.Jabbour E, Hosing C, Ayers G, Nunez R, Anderlini P, Pro B, et al. Pretransplant positive positron emission tomography/gallium scans predict poor outcome in patients with recurrent/refractory Hodgkin lymphoma. Cancer. 2007;109(12):2481–9 [DOI] [PubMed] [Google Scholar]
- 51.Nieto Y, Popat U, Anderlini P, Valdez B, Andersson B, Liu P, et al. Autologous Stem Cell Transplantation for Refractory or Poor-Risk Relapsed Hodgkin Lymphoma: Effect of the Specific High-Dose Chemotherapy Regimen on Outcome. Biol Blood Marrow Transplant. 2013;19(3):410–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Devillier R, Coso D, Castagna L, Brenot Rossi I, Anastasia A, Chiti A, et al. Positron emission tomography response at the time of autologous stem cell transplantation predicts outcome of patients with relapsed and/or refractory Hodgkin’s lymphoma responding to prior salvage therapy. Haematologica. 2012;97(7):1073–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Connors JM. Positron emission tomography in the management of Hodgkin lymphoma. Hematology / the Education Program of the American Society of Hematology American Society of Hematology Education Program 2011:317–22 [DOI] [PubMed] [Google Scholar]
- 54.Hutchings M. How does PET/CT help in selecting therapy for patients with Hodgkin lymphoma? Hematology Am Soc Hematol Educ Program. 2012;2012:322–7 [DOI] [PubMed] [Google Scholar]
- 55.Meignan M, Gallamini A, Meignan M, Gallamini A, Haioun C. Report on the First International Workshop on Interim-PET-Scan in Lymphoma. Leuk Lymphoma. 2009;50(8):1257–60 [DOI] [PubMed] [Google Scholar]
- 56.Meignan M, Gallamini A, Itti E, Barrington S, Haioun C, Polliack A. Report on the Third International Workshop on Interim Positron Emission Tomography in Lymphoma held in Menton, France, 26–27 September 2011 and Menton 2011 consensus. Leuk Lymphoma. 2012;53(10);1876–81 [DOI] [PubMed] [Google Scholar]
- 57.Morschhauser F, Brice P, Ferme C, Divine M, Salles G, Bouabdallah R, et al. Risk-adapted salvage treatment with single or tandem autologous stem-cell transplantation for first relapse/refractory Hodgkin’s lymphoma: results of the prospective multicenter H96 trial by the GELA/SFGM study group. J Clin Oncol. 2008;26(36): 5980–7 [DOI] [PubMed] [Google Scholar]
- 58.Brice P, Divine M, Simon D, Coiffier B, Leblond V, Simon M, et al. Feasibility of tandem autologous stem-cell transplantation (ASCT) in induction failure or very unfavorable (UF) relapse from Hodgkin’s disease (HD). SFGM/GELA Study Group. Ann Oncol. 1999;10(12):1485–8 [DOI] [PubMed] [Google Scholar]
- 59.Fung HC, Stiff P, Schriber J, Toor A, Smith E, Rodriguez T, et al. Tandem autologous stem cell transplantation for patients with primary refractory or poor risk recurrent Hodgkin lymphoma. Biol Blood Marrow Transplant. 2007;13(5):594–600 [DOI] [PubMed] [Google Scholar]
- 60.Moskowitz CH, Yahalom J, Zelenetz AD, Zhang Z, Filippa D, Teruya-Feldstein J, et al. High-dose chemo-radiotherapy for relapsed or refractory Hodgkin lymphoma and the significance of pre-transplant functional imaging. Br J Haematol. 2010;148(6): 890–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bonfante V, Santoro A, Viviani S, Devizzi L, Balzarotti M, Soncini F, et al. Outcome of patients with Hodgkin’s disease failing after primary MOPP-ABVD. J Clin Oncol. 1997;15(2):528–34 [DOI] [PubMed] [Google Scholar]
- 62.Stiff PJ, Unger JM, Forman SJ, McCall AR, LeBlanc M, Nademanee AP, et al. The value of augmented preparative regimens combined with an autologous bone marrow transplant for the management of relapsed or refractory Hodgkin disease: a Southwest Oncology Group phase II trial. Biol Blood Marrow Transplant. 2003;9(8):529–39 [DOI] [PubMed] [Google Scholar]
- 63.Younes A. Novel treatment strategies for patients with relapsed classical Hodgkin lymphoma. Hematology Am Soc Hematol Educ Program. 2009:507–19 [DOI] [PubMed] [Google Scholar]
- 64.Jona A, Younes A. Novel treatment strategies for patients with relapsed classical Hodgkin lymphoma. Blood Rev. 2010;24 (6):233–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moskowitz AJ. Novel agents in Hodgkin lymphoma. Curr Oncol Rep. 2012;14(5): 419–23 [DOI] [PubMed] [Google Scholar]
- 66.Younes A, Gopal AK, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J Clin Oncol. 2012;30(18):2183–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rothe A, Sasse S, Goergen H, Eichenauer DA, Lohri A, Jager U, et al. Brentuximab vedotin for relapsed or refractory CD30+ hematologic malignancies: the German Hodgkin Study Group experience. Blood. 2012;120(7):1470–2 [DOI] [PubMed] [Google Scholar]
- 68.Gopal AK, Ramchandren R, O’Connor OA, Berryman RB, Advani RH, Chen R, et al. Safety and efficacy of brentuximab vedotin for Hodgkin lymphoma recurring after allogeneic stem cell transplantation. Blood. 2012;120(3):560–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen R, Palmer JM, Thomas SH, Tsai NC, Farol L, Nademanee A, et al. Brentuximab vedotin enables successful reduced-intensity allogeneic hematopoietic cell transplantation in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2012;119 (26):6379–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gibb A, Jones C, Bloor A, Kulkarni S, Illidge T, Linton K, et al. Brentuximab vedotin in refractory CD30+ lymphomas: a bridge to allogeneic transplantation in approximately one quarter of patients treated on a Named Patient Programme at a single UK Centre. Haematologica. 2013;98(4):611–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goyal SD, Bartlett NL. Where does brentuximab vedotin fit into the management of patients with Hodgkin lymphoma? Curr Hematol Malig Rep. 2012;7(3):179–85 [DOI] [PubMed] [Google Scholar]
- 72.Hoppe RT, Advani RH, Ai WZ, Ambinder RF, Aoun P, Bello CM, et al. Hodgkin lymphoma, version 2.2012 featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2012;10(5):589–97 [DOI] [PubMed] [Google Scholar]
- 73.Younes A, Sureda A, Ben-Yehuda D, Zinzani PL, Ong TC, Prince HM, et al. Panobinostat in patients with relapsed/refractory Hodgkin’s lymphoma after autologous stem-cell transplantation: results of a phase II study. J Clin Oncol. 2012;30(18):2197–203 [DOI] [PubMed] [Google Scholar]
- 74.Younes A, Romaguera J, Hagemeister F, McLaughlin P, Rodriguez MA, Fiumara P, et al. A pilot study of rituximab in patients with recurrent, classic Hodgkin disease. Cancer. 2003;98(2):310–4 [DOI] [PubMed] [Google Scholar]
- 75.Oki Y, Pro B, Fayad LE, Romaguera J, Samaniego F, Hagemeister F, et al. Phase 2 study of gemcitabine in combination with rituximab in patients with recurrent or refractory Hodgkin lymphoma. Cancer. 2008;112(4):831–6 [DOI] [PubMed] [Google Scholar]
- 76.Oki Y, Younes A. Does rituximab have a place in treating classic hodgkin lymphoma? Curr Hematol Malig Rep. 2010; 5(3):135–9 [DOI] [PubMed] [Google Scholar]
- 77.Corazzelli G, Frigeri F, Marcacci G, Capobianco G, Arcamone M, Becchimanzi C, et al. Rituximab plus gemcitabine, ifosfamide, oxaliplatin (R-GIFOX) as salvage therapy for recurrent Hodgkin lymphoma. J Clin Oncol. 2009;27(15s). [Google Scholar]