Abstract
Clinical trial results indicate that brentuximab vedotin brings considerable promise for the treatment of patients with relapsed or refractory Hodgkin’s lymphoma. A retrospective multicenter study was conducted on 65 heavily pretreated patients who underwent therapy through a Named Patient Program in Italy (non trial-setting). The primary study endpoint was the objective response rate; secondary endpoints were safety, overall survival and progression-free survival. The best overall response rate (70.7%), including 21.5% complete responses, was observed at the first restaging after the third cycle of treatment. After a median follow up of 13.2 months, the overall survival rate at 20 months was 73.8% while the progression-free survival rate at 20 months was 24.2%. Globally nine patients are in continuous complete response with a median follow up of 14 months (range, 10–19 months). Four patients proceeded to autotransplantation and nine to allotransplantation. The most frequent extra-hematologic toxicity was peripheral neuropathy, observed in 21.5% of cases (9 patients with grade 1/2 and 5 patients with grade 3/4); neurological toxicity led to discontinuation of treatment in three patients and to dose reduction in four. In general the treatment was well tolerated and toxicities, both hematologic and extra-hematologic, were manageable. This report indicates and confirms that brentuximab vedotin as a single agent is effective and safe also when used in standard, everyday clinical practice outside a clinical trial. Best overall responses were recorded after three or four cycles and showed that brentuximab vedotin provides an effective bridge to further therapeutic interventions.
Introduction
Based primarily on clinical advances optimizing systemic cytotoxic chemotherapy over the last several decades, patients with newly diagnosed Hodgkin’s lymphoma (HL) have an excellent prognosis after frontline therapy, with a 5-year progression-free survival rate of 75–80%.1,2 In addition, the standard approach for relapsed HL patients, salvage chemotherapy followed by autologous stem cell transplantation (ASCT), leads to long-term disease-free survival for 40–50% of patients.3–5 However, curing HL patients who have refractory disease after salvage chemotherapy, who relapse after ASCT, or those who are not candidates for ASCT, remains a clinical challenge due to limited effective treatments; such patients currently have a median overall survival of 2–3 years. Although there are several promising agents currently under investigation in HL,6–10 novel therapies that are well tolerated with minimal long-term complications are needed for these patients.
Brentuximab vedotin (BV) is a novel antibody-drug conjugate targeting CD30 linked to a payload comprising a potent synthetic antitubulin chemotherapeutic agent, monomethyl auristatin E. Cell surface expression of CD30 is a characteristic of malignant Hodgkin Reed-Sternberg cells. A multicenter phase II trial of BV in patients with HL recurring after ASCT demonstrated an overall response rate of 75% and a complete response rate of 34% with a median duration of 20.5 months.11 After accelerated approval by the American Food and Drug Administration, eligible patients in Italy were granted early access to BV through a Named Patient Program (NPP). Herein, we report this Italian multicenter experience with BV in patients with heavily pretreated relapsed and/or refractory HL.
Design and Methods
An observational multicenter retrospective study was conducted to analyze outcome and toxicity data of patients managed with BV in a non-trial setting. The study was approved by our institutional board (Azienda Ospedaliera di Bologna, Policlinico S.Orsola-Malpighi, coordinating center) and by all involved ethical committees and registered in the Italian Registry of Observational Studies (AIFA, id551). All patients gave written informed consent to their participation in the study in accordance with the Declaration of Helsinki. A shared database was used after the approval of all the authors and variables were strictly defined to avoid bias in reporting data.
From December 2010 to August 2011, a total of nine Italian centers utilized BV according to the NPP in 65 patients with refractory or relapsed HL. All patients had histologically confirmed CD30+ disease and all patients had relapsed after prior ASCT or relapsed after at least two lines of chemotherapy if not ASCT candidates because of insufficient stem cell collection or chemorefractory disease. Participants had an Eastern Cooperative Oncology Group (ECOG) performance status ≤2, and normal organ function including peripheral blood counts within the normal range. All patients underwent baseline assessments including physical examination, routine hematology and biochemistry tests as well as positron emission tomography (PET)/computed tomography (CT) studies prior to therapy.
Patients received a 30-min infusion of BV at the dose of 1.8 mg/kg of body weight every 3 weeks (for a maximum of 16 cycles) without any routine premedication prior to the first dose. However, patients who experienced an infusion-related reaction subsequently received premedication consisting of acetamino-phen (500 mg orally) and chlorphenamine (10 mg intravenously) or according to institutional standards.
The primary endpoint of the study was the objective response rate; secondary endpoints were safety, overall survival and progression-free survival. In the absence of specific indications, response was assessed by PET/CT scans after cycle 3 and 8 (PET3, PET8) and at treatment discontinuation, as reported in the pivotal study,11 using the International Working Group revised response criteria for malignant lymphoma.12
Safety and tolerability were evaluated by recording the incidence, severity, and type of any adverse event according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) version 3.0. Gastrointestinal side effects were treated according to institutional guidelines and granulocyte colony-stimulating factors were utilized as secondary pro-phylaxis of complications of neutropenia. In cases of grade ≥3 toxicity, it was recommended that the dose of BV was reduced to 1.2 mg/kg in the subsequent cycles.
Overall survival was defined as the time from initiation of therapy to death from any cause and was censored at the date of the last available follow up. Progression-free survival was measured from initiation of therapy to progression, relapse, or death from any cause and was censored at the date of the last available follow up.
Demographics and patients’ characteristics were summarized by descriptive statistics. Survival functions were estimated by using the Kaplan-Meier13 method and were compared using the log-rank test. Statistical analyses were performed with Stata 11 (StataCorp LP, TX, USA) and P values less than 0.05 were considered statistically significant.
Results
The characteristics of the 65 patients are summarized in Table 1. The median age at diagnosis was 27.5 years (range, 12–66 years); there were 34 males and 31 females. Fifty patients (77%) had a baseline ECOG performance status of 0 or 1 and 15 (23%) had an ECOG status of 2. Twenty-nine patients (44.5%) had systemic symptoms at baseline.
Table 1.
Demographics and baseline characteristics of the patients (n=65).
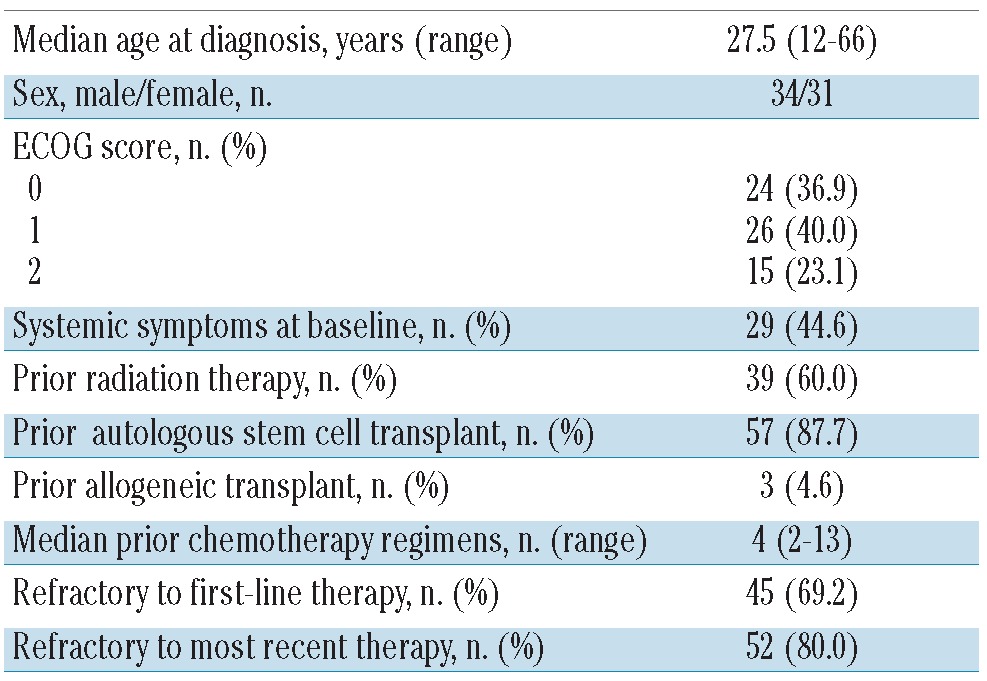
The median number of prior cancer-related systemic regimens was four (range, 2–13) including high-dose chemotherapy and ASCT or allogeneic stem cell transplant. Thirty-nine patients (60%) had received prior radiation therapy. For each patient the status after both frontline therapy and the most recent therapy was recorded. Forty-five patients (69%) had disease that was refractory to frontline therapy and 52 patients (80%) had disease that was refractory to the last therapy before BV. ASCT had failed in 57 patients (87.6%) and, in addition, in three (4.6%) of these patients, allogeneic transplantation had also failed. The remaining eight of the 65 patients had not undergone ASCT because it had not been possible to collect stem cells.
Safety
All patients received at least three doses of BV and were included in the safety analysis; patients received a median of eight cycles of BV (range, 3–16). In general, the treatment was well tolerated and the toxicity profile was very similar to that previously published. A dose reduction (to 1.2 mg/kg) because of grade 3/4 toxicity was necessary in only four patients (all for peripheral neuropathy). Globally, neurological toxicity was observed in 14 (21.5%) patients: peripheral sensory neuropathy grade 1/2 was documented in nine patients and grade 3/4 was reported in five patients (one of these patients also had a grade 4 peripheral motor neuropathy). Resolution or improvement of peripheral neuropathy was observed in 90% of patients with a median time to resolution or improvement of 12 weeks. Three patients had to stop treatment because of peripheral neuropathy. The other grade 3/4 adverse events were neutropenia (n=3), thrombocytopenia (n=3), diabetes (n=1), and aspergillosis of lung and liver (n=1); the relationship of the aspergillosis to BV is unclear.
Effectiveness
The best responses were seen at the PET3 evaluation with 14 (21.5%) complete responses, 32 (49.2%) partial responses, 11 cases of stable disease and 8 of progressive disease, for best overall response rate at this time-point of 70.7%.
Immediately after the PET3 evaluation, six patients discontinued the BV treatment: two patients died [one patient due to progressive disease and one patient with a complete response due to second neoplasia (acute myeloid leukemia)], two patients with stable disease underwent allogeneic transplantation, one patient with progressive disease was shifted to bendamustine therapy, and another patient with progressive disease was lost to follow-up.
Before the second interim evaluation scheduled after eight cycles (PET8), another 15 patients discontinued BV treatment: nine patients because of progressive disease, two patients because of adverse events (one for diabetes and one for infection), two patients proceeded to ASCT and two patients proceeded to allogeneic transplant. Thus, 44 patients completed eight cycles of BV and underwent the PET8 evaluation: ten (22.7%) patients were in complete response and ten (22.7%) in partial response, for an overall response rate of 45.4%.
Figure 1 summarizes the outcome of the whole study population at the PET3 and PET8 evaluations. From this point only 15 patients have continued BV treatment and only two patients have completed the scheduled 16 cycles.
Figure 1.
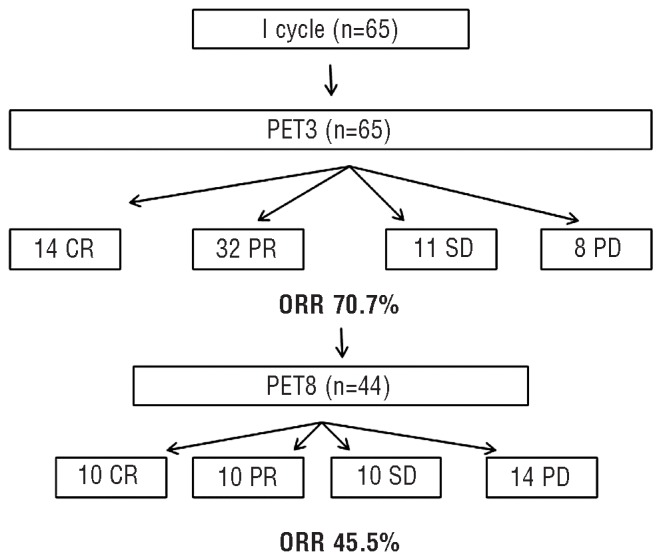
Algorithm of the responses at the PET3 and PET8 evaluations. CR: complete response; PR: partial response; SD: stable disease; PD: progressive disease; ORR: overall response rate.
All in all, 14 patients obtained a complete response within the first three cycles of BV therapy. Table 2 provides a summary of the demographics, disease characteristics, and prior treatments for these 14 patients who obtained a complete response. All patients underwent ABVD therapy in frontline treatment. The median number of BV cycles was six; in detail, three patients received three cycles, two patients five cycles, four patients six cycles, one patient eight cycles, two patients nine cycles, one patient ten cycles, and one patient all 16 cycles. Two out of the 14 patients had not been pretreated with ASCT and one patient had received both autologous and allogeneic transplants before treatment with BV. Among these 14 patients, four died: one of a second neoplasia (acute myeloid leukemia after 4 months), one of post-transplant lymphoproliferative disease, and two of lymphoma progression (one after ASCT and one after an allogeneic transplant). The outcome of remaining ten patients with a complete response at PET3 is reported in Figure 2.
Table 2.
Characteristics of all 14 patients who obtained a complete response.
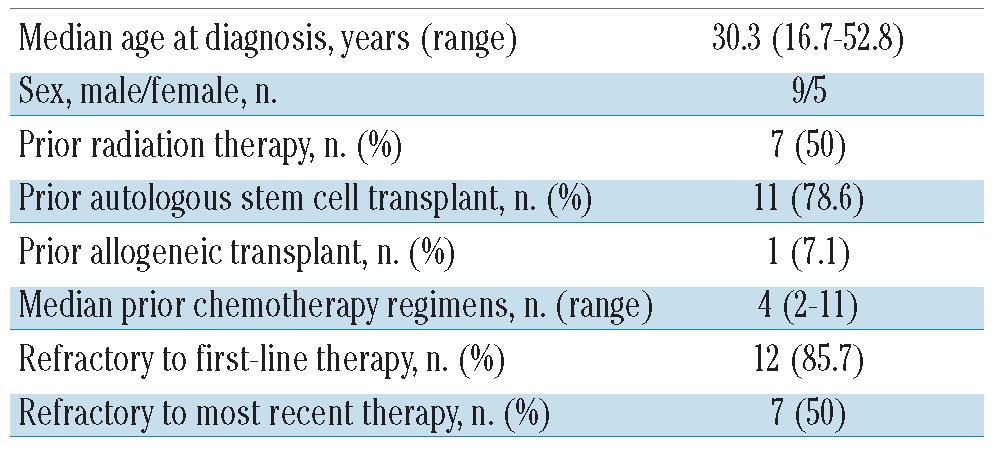
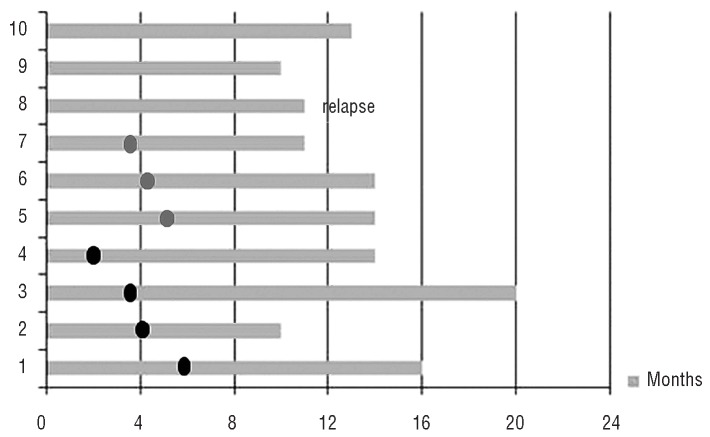
Figure 2.
Duration of complete response in alive patients. Black circles, allogeneic transplant; gray circles, autologous stem cell transplant.
Four out of ten patients alive proceeded to allogeneic transplantation and all of them are in continuous complete response after 10, 15, 16, and 20 months. Three patients underwent ASCT and they too are in continuous complete response after 11, 14, and 14 months. One patient had a relapse after 11 months (he had further treatment with bendamustine obtaining a partial response and then underwent allogeneic transplantation). The last two patients are in continuous complete response after 10 and 13 months without any transplant consolidation. The specific characteristics of these last two patients were that the former was refractory to the last prior treatment (ASCT) and globally received five prior chemotherapy regimens, while the latter had a relapse at the last treatment prior to BV (allogeneic transplant) and had received 11 prior chemotherapy regimens. These two patients received 9 and 16 cycles, respectively; both reported peripheral sensory neuropathy (grade 2/3 and grade 3, respectively), probably related to the drug, leading to dose reduction of BV.
The final response of the whole sample was as follows: 14 complete responses (21.5%), 5 partial responses (7.7%), 6 cases of stable disease and 40 cases of progressive disease. Globally, with a median follow up of 13.2 months, 49 out of the 65 patients were alive at the last available follow up. The overall survival rate at 20 months was 73.8% and the median overall survival has not been reached yet (Figure 3A). The global progression-free survival rate at 19.4 months was 24.2%, the median was achieved at 6.8 months (Figure 3B). The estimated progression-free survival rate as a function of the achieved response did not show statistically significant differences (P=0.087) between patients with a complete response (62.5%), partial response (80.0%) or stable disease (83.3%) (Figure 4).
Figure 3.
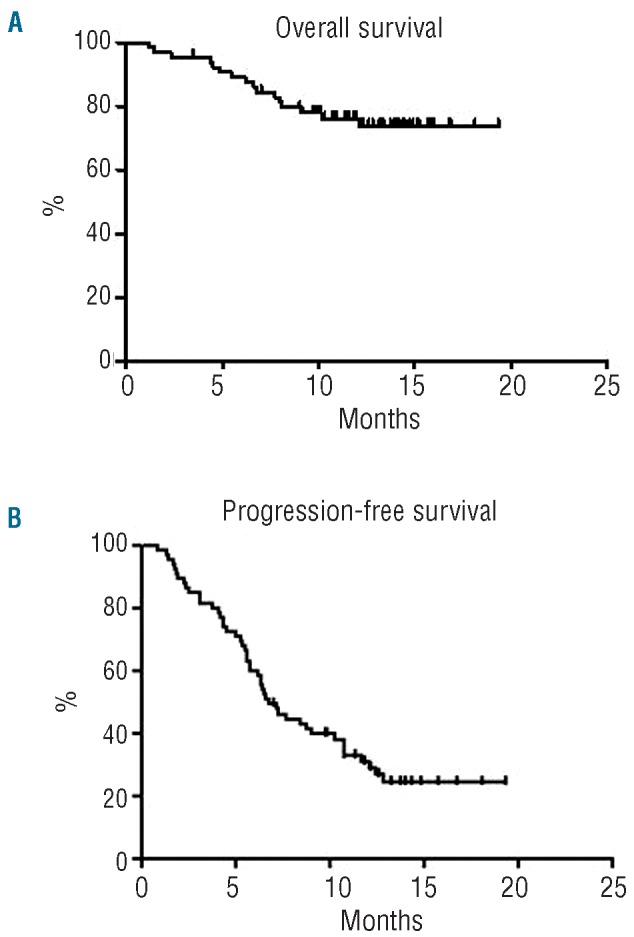
(A) Overall survival and (B) progression-free survival.
Figure 4.
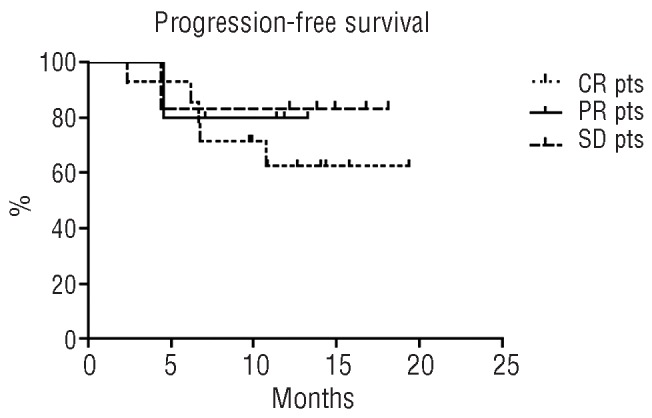
Progression-free survival of patients divided according to response. CR: complete response; PR: partial response; SD: stable disease; pts, patients.
Of the subset of eight patients who had not undergone ASCT prior to BV treatment, three are in continuous complete response after consolidation with ASCT after 11, 14, and 14 months. Of the subset of three patients who had had a prior allogeneic transplant, one is in continuous complete response after 13 months. Finally, a subset of nine patients underwent allogeneic transplantation following BV therapy, after a median of six BV cycles (range, 3–11 cycles); five of them were in complete response, one in partial response, two had stable disease, and one had progressive disease. After the transplant, five patients are in complete response (four patients maintained the complete response previously obtained with BV, one patient converted from a partial to a complete response, another patient died after 7 months from transplant-related toxicity, namely post-transplant lymphoproliferative disorder), two patients are in partial response and one patient has yet to be evaluated after the transplant.
Discussion
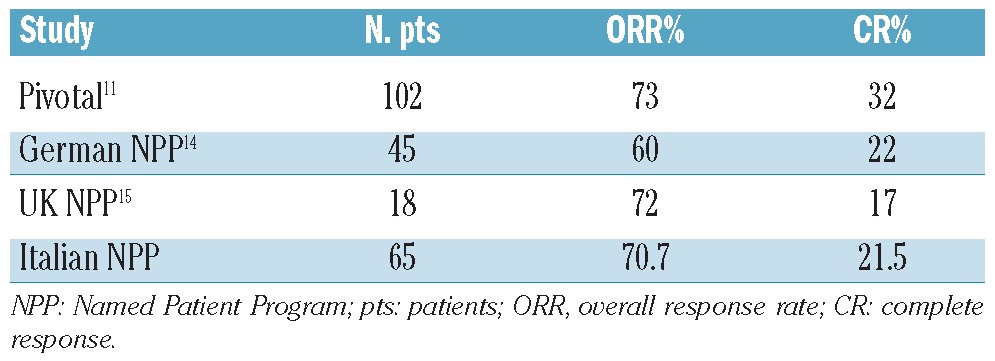
These data on BV in patients treated within the NPP indicate that this drug is highly effective and very well tolerated also in standard everyday clinical practice, i.e. outside the clinical trial setting, in relapsed/refractory HL.14–16 With regards to toxicity, peripheral neuropathy was the most common side effect, although it was less frequent than in the pivotal phase II study.11 In terms of effectiveness, this report confirms the trend of complete response and overall response rates. In fact, comparing our study with the pivotal study, the German and UK NPP experiences, the overall and complete response rates are the same (Table 3).11,14,15
Table 3.
Results of the pivotal study and NPP experiences.
In addition, there is a further demonstration of the real role of BV as a therapeutic “bridge”, inducing a rapid response, to ASCT or allogeneic transplant and, on the other hand, of the potential role of this drug to induce a long-lasting continuous complete response without any consolidation, albeit in a small subset of refractory HL patients. The major result of our study indicates that best responses were observed after three or four cycles, so consolidation should be considered early and scheduled after the first evaluation indicating response.
In conclusion, our data represent the largest NPP report on BV as a single agent in the real-life treatment of relapsed/refractory HL patients; further investigations could be designed with a larger population (for example, a global European NPP report) and longer follow up to assess long-term survival and unknown side effects.
Footnotes
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Canellos GP, Anderson JR, Propert KJ, Nissen N, Cooper MR, Henderson ES, et al. Chemotherapy of advanced Hodgkin’s disease with MOPP, ABVD, or MOPP alternating with ABVD. N Engl J Med. 1992;327 (21):1478–84 [DOI] [PubMed] [Google Scholar]
- 2.Diehl V, Franklin J, Pfreundschuh M, Lathan B, Paulus U, Hasenclever D, et al. Standard and increased-dose BEACOPP chemotherapy compared with COPP_ABVD for advanced Hodgkin’s disease. N Engl J Med. 2003;348:(7)2386–95 [DOI] [PubMed] [Google Scholar]
- 3.Linch DC, Winfield D, Goldstone AH, Moir D, Hancock B, McMillan A, et al. Dose intensification with autologous bone-marrow transplantation in relapsed and resistant Hodgkin’s disease: results of a BNLI randomised trial. Lancet. 1993;341(8852): 1051–4 [DOI] [PubMed] [Google Scholar]
- 4.Schmitz N, Pfistner B, Sextro M, Sieber M, Carella AM, Haenel M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haematopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin’s disease: a randomised trial. Lancet. 2002;359(9323):2065–71 [DOI] [PubMed] [Google Scholar]
- 5.Andre M, Henry-Amar M, Pico JL, Brice P, Blaise D, Kuentz M, et al. Comparison of high-dose therapy and autologous stem-cell transplantation with conventional therapy for Hodgkin’s disease induction failure: A case-control study. J Clin Oncol. 1999;17 (1):222–9 [DOI] [PubMed] [Google Scholar]
- 6.Fehniger TA, Larson S, Trinkaus K, Siegel MJ, Cashen AF, Blum KA, et al. A phase 2 multicenter study of lenalidomide in relapsed or refractory classical Hodgkin lymphoma. Blood. 2011;118(19):5119–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnston PB, Inwards DJ, Colgan JP, Laplant BR, Kabat BF, Habermann TM, et al. A phase II trial of the oral mTOR inhibitor everolimus in relapsed Hodgkin lymphoma. Am J Hematol. 2010;85(5):320–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Younes A, Sureda A, Ben-Yehuda D, Zinzani PL, Ong TC, Prince HM, et al. Panobinostat in patients with relapsed/refractory Hodgkin’s lymphoma after autologous stem-cell transplantation: results of a phase II study. J Clin Oncol. 2012;30(18):2197–03 [DOI] [PubMed] [Google Scholar]
- 9.Younes A, Bartlett NL, Leonard JP, Kennedy DA, Lynch CM, Sievers EL, et al. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med. 2010;363(19):1812–21 [DOI] [PubMed] [Google Scholar]
- 10.Fanale MA, Forero-Torres A, Rosenblatt JD, Advani RH, Franklin AR, Kennedy DA, et al. A phase I weekly dosing study of brentuximab vedotin in patients with relapsed/refractory CD30-positive hematologic malignancies. Clin Cancer Res. 2012;18(1):248–55 [DOI] [PubMed] [Google Scholar]
- 11.Younes A, Gopal AK, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J Clin Oncol. 2012;30(18):2183–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheson BD, Pfistner B, Juwed ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579–86 [DOI] [PubMed] [Google Scholar]
- 13.Kaplan ES, Meier P. Non-parametric estimation from incomplete observations. J Am Stat Assoc. 1958;58:457–81 [Google Scholar]
- 14.Rothe A, Sasse S, Goergen H, Eichenauer DA, Lohri A, Jäger U, et al. Brentuximab vedotin for relapsed or refractory CD30+ hematologic malignancies: the German Hodgkin Study Group experience. Blood. 2012;120(7):1470–2 [DOI] [PubMed] [Google Scholar]
- 15.Gibb A, Jones C, Bloor A, Kulkarni S, Illidge T, Linton K, et al. Brentuximab vedotin in refractory CD30+ lymphomas: a bridge to allogeneic transplantation in approximately one quarter of patients treated on a Named Patient Programme at a single UK Center. Haematologica. 2013;98(4)611–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen R, Palmer JM, Thomas SH, Tsai NC, Farol L, Nademanee A, et al. Brentuximab vedotin enables successful reduced-intensity allogeneic hematopoietic cell transplantation in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2012; 119(26):6379–81 [DOI] [PMC free article] [PubMed] [Google Scholar]