Abstract
Rituximab is an effective treatment for autoimmune cytopenias associated with chronic lymphocytic leukemia. Despite the incorporation of rituximab into fludarabine-based chemotherapy regimens, the incidence of autoimmune cytopenias has remained high. Inadequate rituximab exposure due to rapid antibody clearance may be a contributing factor. To test this hypothesis, we measured serum rituximab levels in patients treated with fludarabine and rituximab (375 mg/m2). All patients had undetectable rituximab trough levels by the end of cycle 1, and one-third had undetectable levels already on Day 6 of cycle 1. Although rituximab trough levels increased progressively with each cycle, only by cycle 4 did the median trough level exceed 10 ug/mL. The median half-life of rituximab during cycle 1 was 27 hours, compared to 199 hours during cycle 4 (P<0.0001). There was a significant inverse correlation between the rituximab half-life in cycle 1 and the degree of tumor burden (P=0.02). Two patients who were identified as having subclinical autoimmune hemolysis prior to therapy were given additional doses of rituximab during the initial cycles of therapy and did not develop clinically significant hemolysis. One patient who developed clinically significant hemolysis during therapy was given additional rituximab doses during cycles 3–5 and was able to successfully complete his treatment. In conclusion, rituximab is cleared so rapidly during the initial cycles of therapy for chronic lymphocytic leukemia that most patients have only transient serum levels. More frequent dosing of rituximab may be required to prevent autoimmune complications in at-risk patients (clinicaltrials.gov identifier:00001586).
Introduction
Autoimmune cytopenias (AIC) are common in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), and are often precipitated by the initiation of fludarabine-based chemotherapy.1,2 In particular, autoimmune hemolytic anemia and immune thrombocytopenia have been serious adverse events of fludarabine monotherapy, at times leading to fatal outcomes.2,3 Over the past decade, the chimeric anti-CD20 monoclonal IgG1 antibody, rituximab, has gained recognition both as an integral component of therapy for CLL4–6 and as a safe and effective therapy for corticosteroid-refractory CLL-associated AIC.3,7,8 Despite the demonstrated efficacy of rituximab in treating AIC, its combination with fludarabine-based chemotherapy has not consistently resulted in a decline in the incidence of treatment-associated AIC. Borthakur et al., for example, reported that the incidence of AIC in patients with CLL treated at MD Anderson Cancer Center with fludarabine, cyclophosphamide, and rituximab (FCR) was comparable to that of historical controls treated with chemotherapy only.9 Thus, while rituximab has demonstrated success as treatment for AIC, it seems to be less effective as a preventive agent.
We hypothesized that this apparent discrepancy may be related to inadequate rituximab exposure. In the pivotal study of weekly rituximab as monotherapy for low-grade non-Hodgkin’s lymphoma, rituximab trough levels were significantly lower in patients with SLL than in patients with other lymphoma subtypes.10 In part this may be due to the high tumor burden, the elevated lymphocyte count, and the presence of disease in the bone marrow in CLL/SLL patients, all factors that have been shown to reduce rituximab serum levels.11–13 These data call into question whether the current paradigm of dosing rituximab purely by body surface area and administration only once every 4-week cycle in chemoimmunotherapy regimens is optimal for CLL patients. Pharmacokinetic data on rituximab in CLL patients have been obtained mostly from patients dosed higher and more frequently than what is used in standard chemoimmunotherapy, or have been assessed in patients with relapsed/refractory disease.14–17 These studies show that high serum levels of rituximab can be reached in CLL patients after repeated dosing, especially as the tumor burden is successfully reduced, and eventually the infused rituximab displays similar pharmacokinetics as in patients with NHL. However, serum concentrations and half-life of rituximab in previously untreated patients with CLL undergoing first-line chemoimmunotherapy are insufficiently characterized, especially for the first treatment cycle. Here we report detailed pharmacokinetics of rituximab in treatment-naïve CLL patients undergoing chemoimmunotherapy with fludarabine and rituximab (FR).
Design and Methods
Patients and treatment
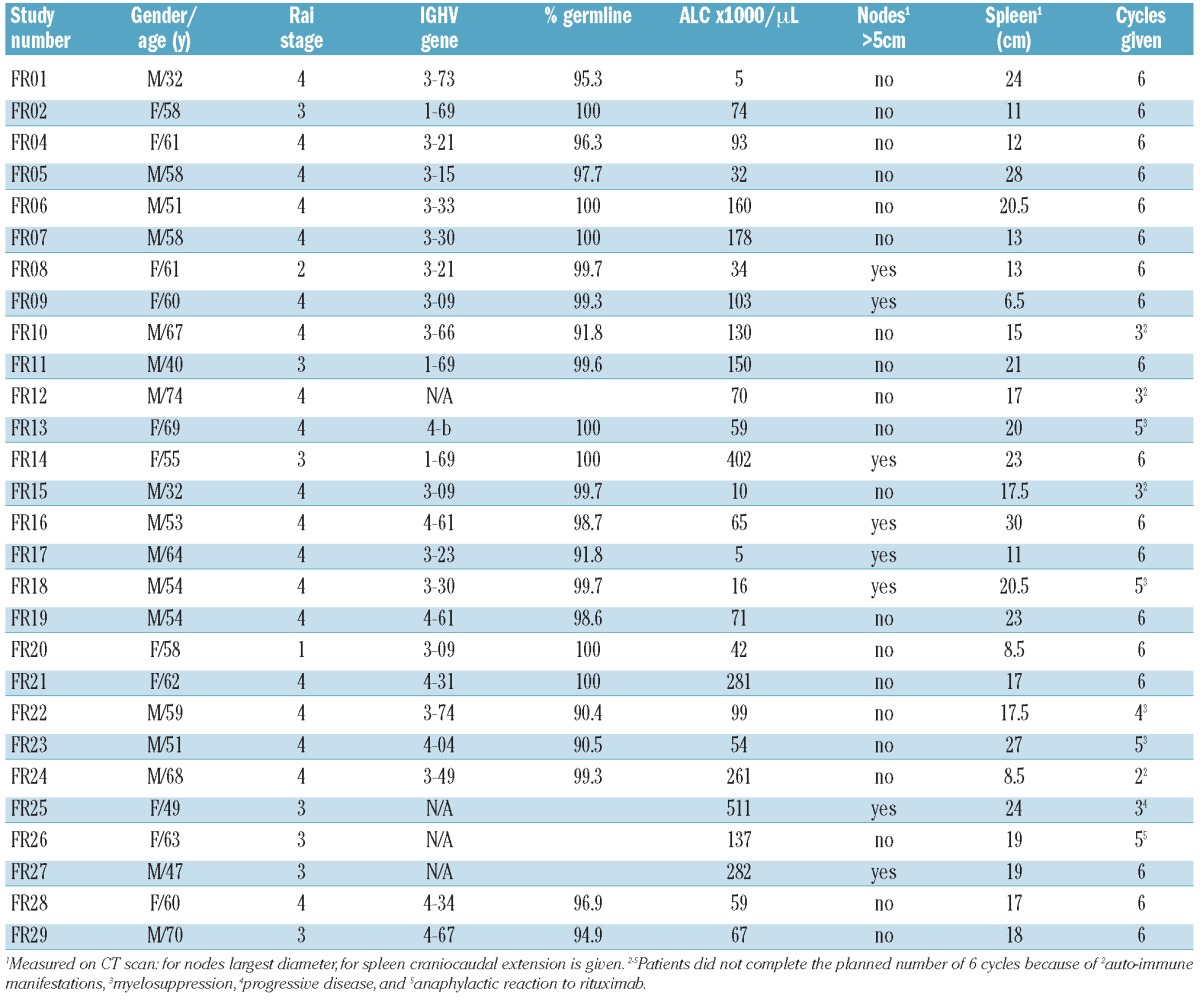
From August 2004 to December 2009, 28 previously untreated patients with symptomatic CLL fulfilling standard treatment criteria18 initiated chemoimmunotherapy with fludarabine (25 mg/m2/d ×5) and rituximab (375 mg/m2 in each cycle) repeated every four weeks for up to six cycles (NCI protocol 97-C-0178; registered in clinicaltrials.gov identifier:00001586). This study was approved by the institutional review board of the National Cancer Institute (NCI) and patients gave their written informed consent to take part in the study. Patients’ characteristics are listed in Table 1.
Table 1.
Patients’ characteristics.
Diagnosis of AIHA and pre-emptive rituximab administration
AIHA was diagnosed following criteria used by Borthakur,9 i.e. 2 g/dL or over drop in hemoglobin during therapy and either a positive DAT or at least two of the following: absolute reticulocyte count over 50 K/μL, elevated LDH, total bilirubin over 1.0 mg/dL, haptoglobin below 30 mg/dL. Prior to therapy, patients with hemoglobin of over 10g/dL and either a positive DAT or at least two of the aforementioned criteria were identified as having subclinical hemolysis, and were given additional doses of rituximab.
Rituximab serum levels and statistical analysis
Pre-and post-infusion rituximab serum levels in 17 patients during the first four treatment cycles were determined by flow cytometry, based on the binding of the monoclonal antibody HB43 (anti-human IgG, Fc-specific), to Raji cells reacted with standards and serum samples. Post-infusion levels were obtained at multiple time points up to 120 hours (h) after the start of rituximab. Trough levels were obtained immediately prior to initiation of the next cycle of therapy. Rituximab serum half-life was calculated for the first four cycles according to the formula:
where Ctime(1) was a post-infusion rituximab level obtained between 24 and 120 h after the start of rituximab and Ctime(2) was a rituximab level obtained at a subsequent time point during that cycle. Median values for cycle-specific rituximab levels and half-lives were compared across time using ANOVA. Where applicable, data are displayed in Whisker plots showing the median, the upper and lower quartile, and the minimum and maximum values.
To estimate differences in pharmacokinetics in relation to tumor burden, patients with at least one lymph node of more than 5 centimeters or an absolute lymphocyte count (ALC) over 100,000/μL were considered to have high tumor burden. Rituximab half-life in patients with and without high tumor burden was compared using two-tailed Student’s t-test.
Results and Discussion
Incidence of AIC
The overall incidence of AIC during treatment was 18%, quite comparable to the 29% incidence seen in therapy-naïve patients treated previously at our institution with single agent fludarabine. Five cases of AIC (AIHA in 3, autoimmune neutropenia in 1, amegakaryocytic thrombocytopenia in 1) developed during cycles 2–3 and led to discontinuation of fludarabine in 3 patients. One patient (FR23) was given additional doses of rituximab and successfully continued on FR.
Three patients (11%) had subclinical hemolysis prior to starting FR. One patient was started on FR and was able to complete six cycles of therapy but continued to have compensated hemolysis that progressed to overt AIHA six months after completion of treatment. Two patients with subclinical hemolysis were given additional doses of rituximab (375 mg/m2) during the first and second cycles of therapy in an attempt to prevent the development of clinically significant AIHA. Indeed, neither of these patients went on to develop clinical AIHA. One patient developed pure red cell aplasia prior to starting FR and was given additional doses of rituximab during the first two cycles with no response of the AIC and progression to amegakaryocytic thrombocytopenia.
Rituximab clearance and half-life
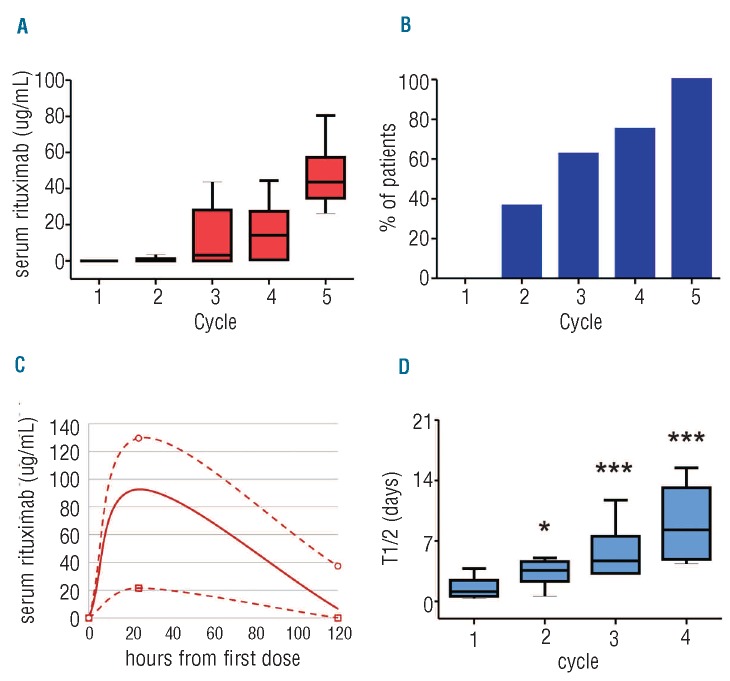
Rituximab was not detectable at the trough in the majority of first treatment cycles, with none of the 14 patients who received the standard dose of rituximab registering a detectable level at the end of cycle 1. By contrast, one of the 3 patients who received additional rituximab had a detectable level at the end of cycle 1, and another did so at the end of cycle 2. The median trough level in patients treated with the standard dose rituximab increased progressively with subsequent cycles (Figure 1A). However, only 63% of patients had detectable rituximab trough levels by the end of cycle 3 (Figure 1B). Indeed, the median rituximab level on Day 6 of cycle 1 was only 6.7 μg/mL and a full 35% of patients had no detectable rituximab in their serum at this early time point (Figure 1C).
Figure 1.
Rituximab pharmacokinetics in CLL. (A) Rituximab was administered every 4 weeks in combination with fludarabine. Rituximab trough levels during cycles 1–5 are depicted on Whisker plots (P<0.0001 by ANOVA). (B) Percentage of patients with detectable trough levels at the end of cycles 1–5 (P=0.0019 for time effect). (C) Minimum (open squares), median (solid line), and maximum (open circles) rituximab serum levels measured 24 and 120 hours after the first dose. Lines indicate an estimate of serum levels at time points in between actual measurements. (D) Median serum rituximab half-life during cycles 1–4. P values by t-test for comparison of the respective cycle to cycle 1 are *P<0.01 and ***P<0.0001.
Consistent with this rapid decrease in rituximab serum levels, we calculated a median rituximab half-life of 27 h in cycle 1 (range 9–91) which increased with each subsequent cycle: to 86, 113, and 199 h in cycles 2, 3 and 4, respectively (Figure 1D). These cycle-specific half-lives were remarkably shorter than the expected 8–10 day half-life of a chimeric IgG1 monoclonal antibody in a non-disease state;19 or the half-lives of rituximab measured in previous low-grade NHL studies.11
These short half-lives, coupled with the less frequent dosing of rituximab in CLL (once every four weeks) compared to other NHL subtypes (once every 1–3 weeks) result in a striking disparity in rituximab exposure between patients with CLL and other B-cell malignancies. Compared to their NHL counterparts, CLL patients spend a greater portion of their initial treatment cycles with little to no circulating rituximab, and reach steady-state therapeutic levels much later in the course of their treatment. This may explain why the inclusion of rituximab into fludarabine-based chemotherapeutic regimens has not consistently reduced the incidence of treatment-associated AIC in CLL. Accordingly, it stands to reason that increasing rituximab exposure early on in CLL treatment by means of repetitive dosing may prove more effective in preventing such complications. Although our sample size of at-risk patients treated with this approach was small, the fact that these patients achieved better-than-average rituximab serum levels and did not develop clinically significant AIC is encouraging and deserves further study.
Correlation of rituximab clearance and tumor burden
Previous clinical and pre-clinical studies have identified, on both inter- and intra-individual bases, an inverse correlation between tumor burden and rituximab levels.11,12 We confirmed this correlation and found it to be quite pronounced in our CLL patients. Within our cohort, median half-life during the first treatment cycle was 20 h in patients with high tumor burden compared to 58 h in patients without (P=0.02; Figure 2A). Likewise, within individual patients, absolute lymphocyte count and trough rituximab levels showed a striking inverse correlation across time (Figure 2B).
Figure 2.
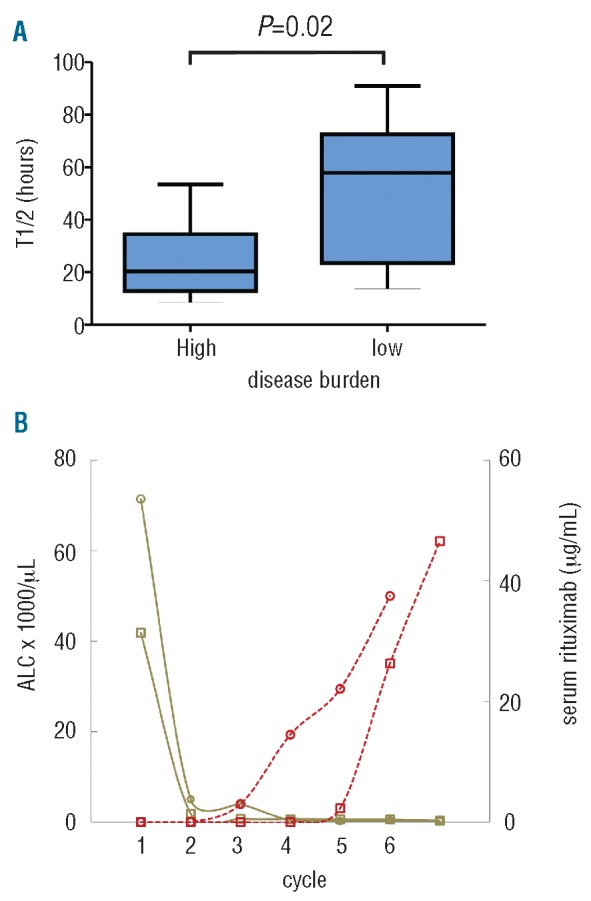
Tumor burden correlates inversely with rituximab serum concentration and half-life. (A) Median serum rituximab half-life in the first treatment cycle for patients with high (n=8) and low (n=9) tumor burden (P=0.02 by Student’s t-test). (B) ALC (solid lines) and rituximab trough levels (dotted lines) over time in 2 representative patients.
With regard to prevention of AIC, this inverse correlation between tumor burden and rituximab half-life strengthens the argument for rethinking our approach to rituximab dosing in CLL. As demonstrated recently by Barcellini et al., the presence of advanced disease in CLL is associated with an increased risk of developing AIC.20 Considering that patients with the most advanced disease (i.e. those with high tumor burden) spend the greatest percentage of a chemoimmunotherapy course with no detectable rituximab in their sera, it appears that the current standard practice of infrequent and static rituximab dosing may actually be contributing to the development of therapy-associated AIC by providing the least mitigation of risk for the highest-risk group of patients. While rituximab is now typically dosed at 500 mg/m2 once every four weeks, the dose for cycle 1 has remained at 375 mg/m2, and the moderate increase in subsequent doses is unlikely to significantly increase trough levels in cycle 2 or 3 given the short half-life of rituximab during these cycles.
In addition, there is an increasing body of data to suggest that administration of large bolus doses of anti-CD20 antibody exhausts effector mechanisms and promotes loss of CD20 from CLL cells21,22 while more frequent administration of rituximab better preserves effector functions and increases the anti-tumor activity.23 Specifically, binding of relatively small amounts of rituximab or ofatumumab to B cells (at levels considerably below saturation) is adequate to promote antibody-dependent cellular cytotoxicity, while higher (but still non-saturating) doses will mediate complement-dependent cytotoxicity.23–25 Large bolus doses that saturate the CD20 sites, however, have been shown to lead to rapid exhaustion of effector mechanisms as well as trogocytosis of antibody/CD20 complexes, also referred to as “shaving”, from opsonized cells that remain in the circulation.22,26 This phenomenon may actually accelerate the clearance of the infused rituximab, while rendering the “shaved” CLL cells resistant to further rituximab. Consequently, there is a scientific basis for arguing that lower, repeated doses of rituximab (with lower resultant peak values) may be superior to larger, less frequent doses, in terms of optimizing not only rituximab exposure but also rituximab efficacy.26
The inclusion of cyclophosphamide into the chemoimmunotherapeutic regimen may also be beneficial for patients at high risk for AIC, given the known therapeutic activity of this agent in autoimmune disease and the low incidence of AIC seen in patients treated with FCR in the CLL8 trial.4 It is worth noting, however, that the AIC incidence in FCR-treated patients reported by Borthakur et al. was substantially higher than in the CLL8 trial, although the reasons for this are unclear. Regardless of this, the inclusion of cyclophosphamide and/or more frequent dosing of rituximab may both be considered as preventive measures in high-risk patients.
We have not directly addressed the mechanism by which rituximab may ameliorate AIC in CLL. However, several lines of evidence suggest that immune complexes formed between rituximab and CD20 on B cells may serve as decoys to attract effector cells that express Fcγ, thus diverting the effector cells from mediating destruction of other cells opsonized by autoantibodies.27 Other mechanisms have also been proposed, but in all cases, it is clear that if the rituximab is rapidly cleared in the early infusion cycles, its potential effect on AIC will be minimized.
In conclusion, we find that rituximab is cleared rapidly from the circulation in previously untreated patients with CLL undergoing chemoimmunotherapy, with the most rapid clearance occurring in patients with high tumor burden. Our findings suggest that outcomes in CLL, to include autoimmune complications of therapy, could be improved by changing our approach to rituximab dosing to better reflect CLL-specific aspects of anti-tumor activity and pharmacokinetics.
Acknowledgments
The authors would like to thank our patients who participated in this trial, Therese White for protocol support and Delong Liu for statistical support. This research is in part supported by the intramural research program of NHLBI (AW) and NCI (WW) and a bench to bedside award (to AW, WW and RT).
Footnotes
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
Funding
This research was supported by the Intramural Research Program of the NIH, the National, Heart, Lung and Blood Institute, and the National Cancer Institute.
References
- 1.Di Raimondo F, Giustolisi R, Cacciola E, O'Brien S, Kantarjian H, Robertson LB, et al. Autoimmune hemolytic anemia in chronic lymphocytic leukemia patients treated with fludarabine. Leuk Lymphoma. 1993;11(1–2):63–8 [DOI] [PubMed] [Google Scholar]
- 2.Weiss RB, Freiman J, Kweder SL, Diehl LF, Byrd JC. Hemolytic anemia after fludarabine therapy for chronic lymphocytic leukemia. J Clin Oncol. 1998;16(5):1885–9 [DOI] [PubMed] [Google Scholar]
- 3.Hegde UP, Wilson WH, White T, Cheson BD. Rituximab treatment of refractory fludarabine-associated immune thrombocytopenia in chronic lymphocytic leukemia. Blood. 2002;100(6):2260–2 [PubMed] [Google Scholar]
- 4.Hallek M, Fischer K, Fingerle-Rowson G, Fink AM, Busch R, Mayer J, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376(9747):1164–74 [DOI] [PubMed] [Google Scholar]
- 5.Tam CS, O'Brien S, Wierda W, Kantarjian H, Wen S, Do KA, et al. Long-term results of the fludarabine, cyclophosphamide, and rituximab regimen as initial therapy of chronic lymphocytic leukemia. Blood. 2008;112 (4):975–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woyach JA, Ruppert AS, Heerema NA, Peterson BL, Gribben JG, Morrison VA, et al. Chemoimmunotherapy with fludarabine and rituximab produces extended overall survival and progression-free survival in chronic lymphocytic leukemia: long-term follow-up of CALGB study 9712. J Clin Oncol. 2011;29(10):1349–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D'Arena G, Laurenti L, Capalbo S, D'Arco AM, De Filippi R, Marcacci G, et al. Rituximab therapy for chronic lymphocytic leukemia-associated autoimmune hemolytic anemia. Am J Hematol. 2006;81(8):598–602 [DOI] [PubMed] [Google Scholar]
- 8.Kaufman M, Limaye SA, Driscoll N, Johnson C, Caramanica A, Lebowicz Y, et al. A combination of rituximab, cyclophosphamide and dexamethasone effectively treats immune cytopenias of chronic lymphocytic leukemia. Leuk Lymphoma. 2009;50(6):892–9 [DOI] [PubMed] [Google Scholar]
- 9.Borthakur G, O'Brien S, Wierda WG, Thomas DA, Cortes JE, Giles FJ, et al. Immune anaemias in patients with chronic lymphocytic leukaemia treated with fludarabine, cyclophosphamide and rituximab--incidence and predictors. Br J Haematol. 2007;136(6):800–5 [DOI] [PubMed] [Google Scholar]
- 10.McLaughlin P, Grillo-Lopez AJ, Link BK, Levy R, Czuczman MS, Williams ME, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16(8):2825–33 [DOI] [PubMed] [Google Scholar]
- 11.Berinstein NL, Grillo-Lopez AJ, White CA, Bence-Bruckler I, Maloney D, Czuczman M, et al. Association of serum Rituximab (IDEC-C2B8) concentration and anti-tumor response in the treatment of recurrent low-grade or follicular non-Hodgkin's lymphoma. Ann Oncol. 1998;9(9):995–1001 [DOI] [PubMed] [Google Scholar]
- 12.Dayde D, Ternant D, Ohresser M, Lerondel S, Pesnel S, Watier H, et al. Tumor burden influences exposure and response to rituximab: pharmacokinetic-pharmacodynamic modeling using a syngeneic bioluminescent murine model expressing human CD20. Blood. 2009;113(16):3765–72 [DOI] [PubMed] [Google Scholar]
- 13.Jager U, Fridrik M, Zeitlinger M, Heintel D, Hopfinger G, Burgstaller S, et al. Rituximab serum concentrations during immunochemotherapy of follicular lymphoma correlate with patient gender, bone marrow infiltration and clinical response. Haematologica. 2012;97(9):1431–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Byrd JC, Murphy T, Howard RS, Lucas MS, Goodrich A, Park K, et al. Rituximab using a thrice weekly dosing schedule in B-cell chronic lymphocytic leukemia and small lymphocytic lymphoma demonstrates clinical activity and acceptable toxicity. J Clin Oncol. 2001;19(8):2153–64 [DOI] [PubMed] [Google Scholar]
- 15.Castro JE, James DF, Sandoval-Sus JD, Jain S, Bole J, Rassenti L, et al. Rituximab in combination with high-dose methylprednisolone for the treatment of chronic lymphocytic leukemia. Leukemia. 2009;23(10):1779–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foon KA, Boyiadzis M, Land SR, Marks S, Raptis A, Pietragallo L, et al. Chemoimmunotherapy with low-dose fludarabine and cyclophosphamide and high dose rituximab in previously untreated patients with chronic lymphocytic leukemia. J Clin Oncol. 2009;27(4):498–503 [DOI] [PubMed] [Google Scholar]
- 17.Li J, Zhi J, Wenger M, Valente N, Dmoszynska A, Robak T, et al. Population Pharmacokinetics of Rituximab in Patients With Chronic Lymphocytic Leukemia. J Clin Pharmacol. 2012;52(12):1918–26 [DOI] [PubMed] [Google Scholar]
- 18.Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Dohner H, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111(12):5446–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tabrizi MA, Tseng CM, Roskos LK. Elimination mechanisms of therapeutic monoclonal antibodies. Drug Discov Today. 2006;11(1–2):81–8 [DOI] [PubMed] [Google Scholar]
- 20.Barcellini W, Capalbo S, Agostinelli RM, Mauro FR, Ambrosetti A, Calori R, et al. Relationship between autoimmune phenomena and disease stage and therapy in B-cell chronic lymphocytic leukemia. Haematologica. 2006;91(12):1689–92 [PubMed] [Google Scholar]
- 21.Beum PV, Kennedy AD, Williams ME, Lindorfer MA, Taylor RP. The shaving reaction: rituximab/CD20 complexes are removed from mantle cell lymphoma and chronic lymphocytic leukemia cells by THP-1 monocytes. J Immunol. 2006;176(4):2600–9 [DOI] [PubMed] [Google Scholar]
- 22.Beurskens FJ, Lindorfer MA, Farooqui M, Beum PV, Engelberts P, Mackus WJ, et al. Exhaustion of cytotoxic effector systems may limit monoclonal antibody-based immunotherapy in cancer patients. J Immunol. 2012;188(7):3532–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams ME, Densmore JJ, Pawluczkowycz AW, Beum PV, Kennedy AD, Lindorfer MA, et al. Thrice-weekly low-dose rituximab decreases CD20 loss via shaving and promotes enhanced targeting in chronic lymphocytic leukemia. J Immunol. 2006; 177(10):7435–43 [DOI] [PubMed] [Google Scholar]
- 24.Bleeker WK, Munk ME, Mackus WJ, van den Brakel JH, Pluyter M, Glennie MJ, et al. Estimation of dose requirements for sustained in vivo activity of a therapeutic human anti-CD20 antibody. Br J Haematol. 2008;140(3):303–12 [DOI] [PubMed] [Google Scholar]
- 25.Bologna L, Gotti E, Da Roit F, Intermesoli T, Rambaldi A, Introna M, et al. Ofatumumab is more efficient than rituximab in lysing B chronic lymphocytic leukemia cells in whole blood and in combination with chemotherapy. J Immunol. 2013;190(1):231–9 [DOI] [PubMed] [Google Scholar]
- 26.Lindorfer MA, Wiestner A, Zent CS, Taylor RP. Monoclonal antibody (mAb)-based cancer therapy: Is it time to reevaluate dosing strategies? Oncoimmunology. 2012;1(6): 959–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor RP, Lindorfer MA. Drug insight: the mechanism of action of rituximab in autoimmune disease--the immune complex decoy hypothesis. Nat Clin Pract Rheumatol. 2007;3(2):86–95 [DOI] [PubMed] [Google Scholar]