Abstract
This phase II study is the first prospective evaluation of bortezomib-dexamethasone as second-line therapy for relapsed/refractory multiple myeloma. A total of 163 patients were enrolled to receive four cycles of bortezomib-dexamethasone. Patients were investigator-assessed for response at cycle 5 Day 1, then treated as follows: responding patients received another four cycles of bortezomib-dexamethasone, while patients with stable disease were subsequently randomized to sequential treatment with a further four cycles of bortezomib-dexamethasone alone or with added cyclophosphamide or lenalidomide. The primary end point was response to sequential therapy; however, this could not be evaluated because investigator-assessed response rates to bortezomib-dexamethasone after four cycles were high, and an insufficient number of patients were randomized to sequential treatment per protocol. Among all 163 patients, validated best confirmed response rate was 66%, including 37% complete/very good partial responses; median response duration was 9.7 months. After a median follow up of 16.9 months, median time to progression and progression-free survival were 9.5 and 8.6 months, respectively; estimated 1-year overall survival was 81%. Median glomerular filtration rate improved from baseline during treatment. Among 58 patients with baseline glomerular filtration rate below 50 mL/min, 24 had renal responses. Grade 3/4 adverse events included: thrombocytopenia (17%), anemia (10%), constipation (6%), peripheral sensory neuropathy (5%), and polyneuropathy (5%). Overall, 57% of neuropathy events improved/resolved; median time to improvement was 2.1 months. These findings suggest bortezomib-dexamethasone represents an active, feasible second-line treatment option for patients with relapsed/refractory myeloma.
Introduction
Bortezomib (VELCADE®) plus dexamethasone has been shown to be effective and well tolerated in patients with multiple myeloma (MM). The regimen is active as induction therapy prior to autologous stem cell transplantation in the front-line setting.1–4 Bortezomib alone or in combination with dexamethasone has also demonstrated activity in multiple studies in the relapsed setting.5–13 However, no studies have prospectively assessed bortezomib-dexamethasone (from cycle 1) as second-line therapy in MM, although one phase II study has investigated the addition of dexamethasone for sub-optimal response to single-agent bortezomib in patients at first relapse or refractory to front-line therapy, demonstrating improved responses with the addition of dexamethasone.6,7
In patients who do not respond to bortezomib-dexamethasone, the addition of cyclophosphamide or lenalidomide (Revlimid®) may lead to improved efficacy. In front-line MM, bortezomib-dexamethasone plus cyclophosphamide (VDC),14–18 or plus lenalidomide (VDR),19,20 or plus cyclophosphamide and lenalidomide (VDCR)19,21 have demonstrated high response rates. These combinations have also been shown to be active in the relapsed setting.22,23 However, they may also be associated with increased toxicities compared with bortezomib-dexamethasone alone.
This study, therefore, set out to evaluate the efficacy and safety of bortezomib-dexamethasone, with the sequential addition of cyclophosphamide (VDC) or lenalidomide (VDR) for patients with stable disease (SD) after four cycles, in patients with relapsed or refractory MM following one prior line of therapy. The primary objective was to evaluate the response rate after randomization to sequential treatment with continued bortezomib-dexamethasone, VDC, or VDR among patients achieving SD after four cycles of bortezomib-dexamethasone. However, only a small number of patients were actually enrolled to this randomization part of the study with sequential therapy due to the substantial efficacy of the bortezomib-dexamethasone doublet. Consequently, the focus of this manuscript is to report the results of the first prospective study of bortezomib-dexamethasone as second-line therapy for MM alone or with added cyclophosphamide or lenalidomide after four cycles. Secondary study objectives consisted of other efficacy parameters, including time to response, duration of response, time to progression, progression-free survival, 1-year survival rate, and overall survival, as well as assessment of the safety profile.
In addition, change in renal function during therapy was evaluated as a secondary end point. Bortezomib-dexamethasone has recently been suggested as an appropriate regimen for patients with renal impairment, one of the major complications of MM,24 and this study represents one of very few prospective evaluations of bortezomib-based therapy in this setting.
Design and Methods
This randomized, open-label, parallel-group, phase II study was conducted at 49 sites in 10 European countries (France, Germany, Greece, Hungary, Lithuania, Poland, Serbia, Spain, Turkey, United Kingdom) from May 12, 2008 to Aug 2, 2011 (enrollment from May 5, 2008 to December 31, 2009), and is registered with ClinicalTrials.gov (NCT00908232) and EudraCT (2007-001462-33). Patients aged 18 years or older with measurable secretory MM who had relapsed/progressed following, or who were refractory to, one previous line of therapy were eligible. There were no eligibility restrictions based on renal function. Eligibility criteria are described in the Online Supplementary Appendix. All patients were initially to receive four 21-day cycles of bortezomib-dexamethasone: bortezomib 1.3 mg/m2 intravenous bolus on Days 1, 4, 8, and 11, and oral dexamethasone 20 mg on Days 1, 2, 4, 5, 8, 9, 11, and 12.
Subsequent treatment was dependent on patients’ investigator-assessed response at cycle 5, Day 1. Patients achieving at least partial response (PR) received a further four cycles of bortezomib-dexamethasone. Patients with SD were randomized (1:1:1), for cycles 5–8, to receive: a further four cycles of bortezomib-dexamethasone, four cycles of bortezomib-dexamethasone plus oral cyclophosphamide 500 mg on Days 1, 8, and 15 (VDC), or four cycles of bortezomib-dexamethasone plus oral lenalidomide 10 mg on Days 1–14 (VDR). Patients with progressive disease during/after the initial four cycles of bortezomib-dexamethasone discontinued study treatment.
All patients provided written informed consent. Review boards at all participating institutions approved the study, which was conducted according to the provisions of the Declaration of Helsinki, the International Conference on Harmonisation, and the Guidelines for Good Clinical Practice.
Response was assessed using the International Myeloma Working Group uniform response criteria25 and validated by an Independent Data Monitoring Committee. Time-to-event distributions were estimated using the Kaplan-Meier method. Adverse events (AEs) were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0. Bortezomib dose modifications for peripheral neuropathy (PN) AEs were recommended as per established guidelines.26
Renal function was defined by calculated glomerular filtration rate (GFR) using the Cockcroft-Gault formula27 and was divided into the following stages by GFR based on the US National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative:28 Stage I/II, 60 mL/min or over; Stage III, 30 to less than 60 mL/min; Stage IV, 15 to less than 30 mL/min; Stage V, less than 15 mL/min. GFR was assessed at baseline or screening, prior to treatment on Day 1 of each cycle, and at the visit at the end of treatment. Patients were assessed for renal function stage migration between baseline and best on-study GFR. In addition, patients were evaluated for renal response, based upon baseline and best on-study GFR, according to previously reported criteria.29 A renal complete response (CRrenal) was defined as an improvement in GFR from less than 15, 15 to less than 30, or 30 to less than 50 mL/min at baseline to 60 mL/min or over; a renal partial response (PRrenal) as an improvement from less than 15 to from 30 to less than 60 mL/min, with a more than 100% increase in GFR; and a renal minor response (MRrenal) as an improvement from less than 15 to from 15 to less than 30 mL/min or from 15 to less than 30 to from 30 to less than 60 mL/min, with a more than 50% increase in GFR.
Results
Patients
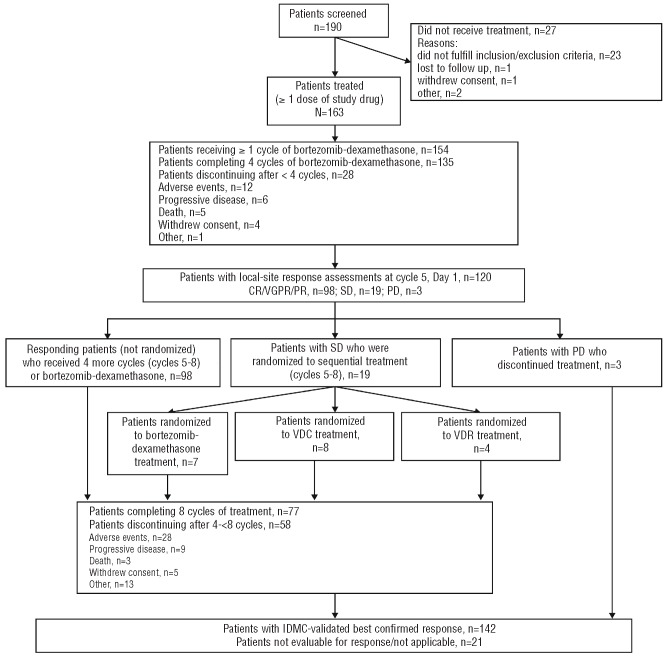
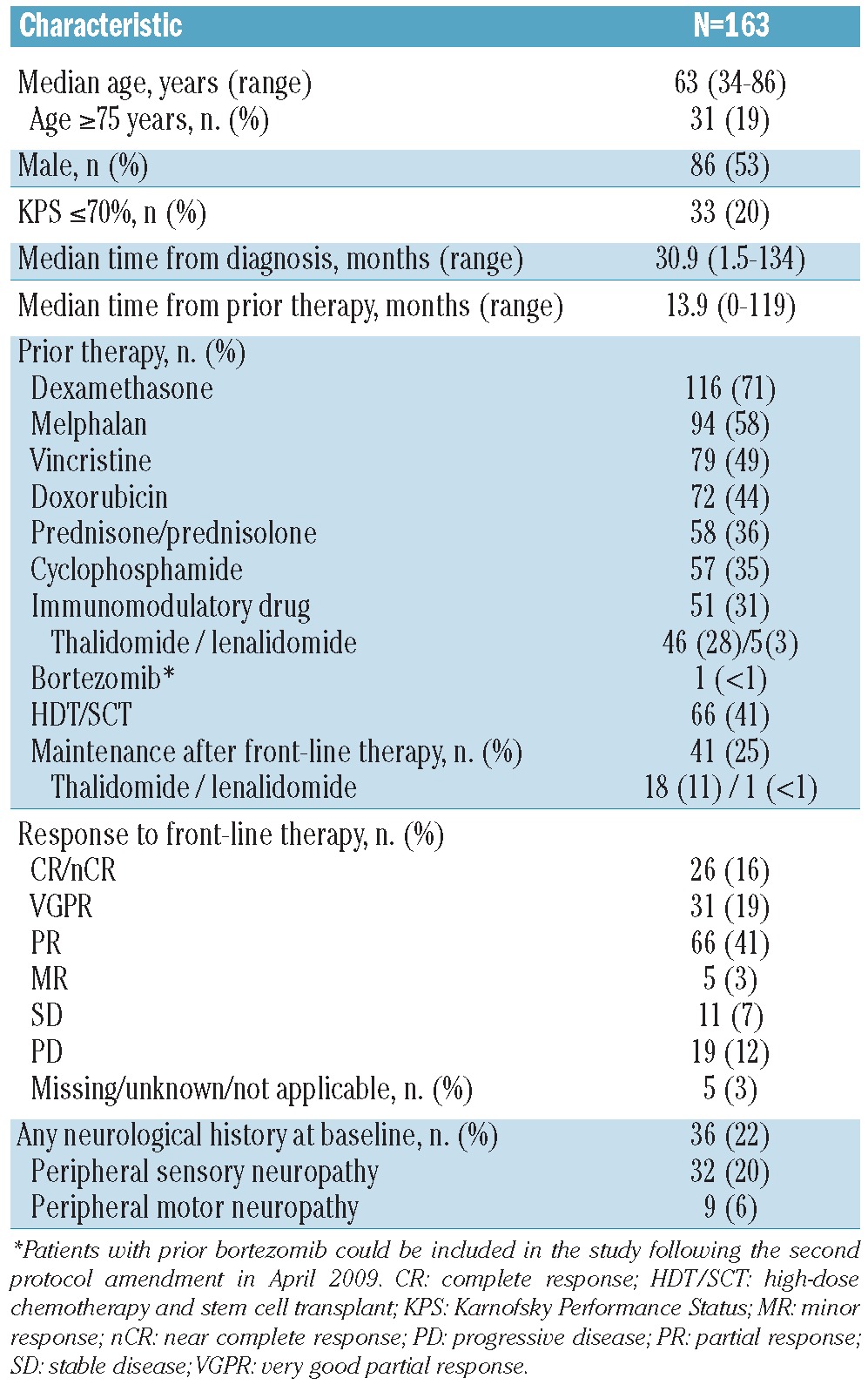
Patient flow through the study protocol is summarized in Figure 1. A total of 190 patients were screened, 27 did not receive any treatment (23 did not fulfill the inclusion and exclusion criteria, one was lost to follow up, one withdrew consent, and 2 discontinued for other reasons), leaving 163 in the modified intention-to-treat (mITT) population. Patients’ demographics and base-line characteristics for the mITT population are summarized in Table 1.
Figure 1.
CONSORT diagram of study design and patient flow through protocol.
Table 1.
Patients’ baseline characteristics (mITT population)
Of the 163 patients in the mITT population, 135 completed four cycles of bortezomib-dexamethasone, and 120 had local-site response assessments at cycle 5, Day 1 (Figure 1). Of these 120 patients, 19 were investigator-assessed as having SD following four cycles of bortezomib-dexamethasone, and were subsequently randomized to sequential treatment with bortezomib-dexamethasone (n=7), or add-on therapy with VDC (n=8) or VDR (n=4). Of the remaining patients who had cycle 5, Day 1 response assessments and were not randomized, 98 patients continued to receive a further four cycles of bortezomib-dexamethasone. Due to the efficacy of the bortezomib-dexamethasone combination in the first four cycles as per investigator assessment, not enough patients could be randomized to sequential therapy to allow us to evaluate the primary end point (evaluation of response rate following randomization to continued bortezomib-dexamethasone, VDC, or VDR, in patients achieving SD after four cycles). Thus, efficacy and safety data are reported for evaluable patients in the mITT population as a whole for treatment with bortezomib-dexamethasone ± cyclophosphamide or lenalidomide.
A total of 77 patients (47%) completed all 8 cycles of treatment; median number of cycles was 7 (range 1–8). The reasons for patients discontinuing treatment after less than 4 cycles and after 4 to less than 8 cycles are summarized in Figure 1.
Response to treatment
The overall best confirmed response to treatment for the 163 patients in the mITT population is shown in Table 2. The best overall response rate (ORR) was 66%, including 37% CR/VGPR (8% CR, 29% VGPR). Time to first response and time to best-confirmed response were analyzed using the Kaplan-Meier method (Figure 2). The median time to first response was 1.6 months (95% confidence interval [CI]: 1.4, 2.1), and the median time to best-confirmed response was 2.8 months (95%CI: 2.5, 3.5). Using the Kaplan-Meier method, the median duration of response was 9.7 months (95%CI: 8.1, 13.7).
Table 2.
Overall best confirmed response to treatment (Independent Data Monitoring Committee-validated) with bortezomib-dexamethasone (±cyclophosphamide or lenalidomide)
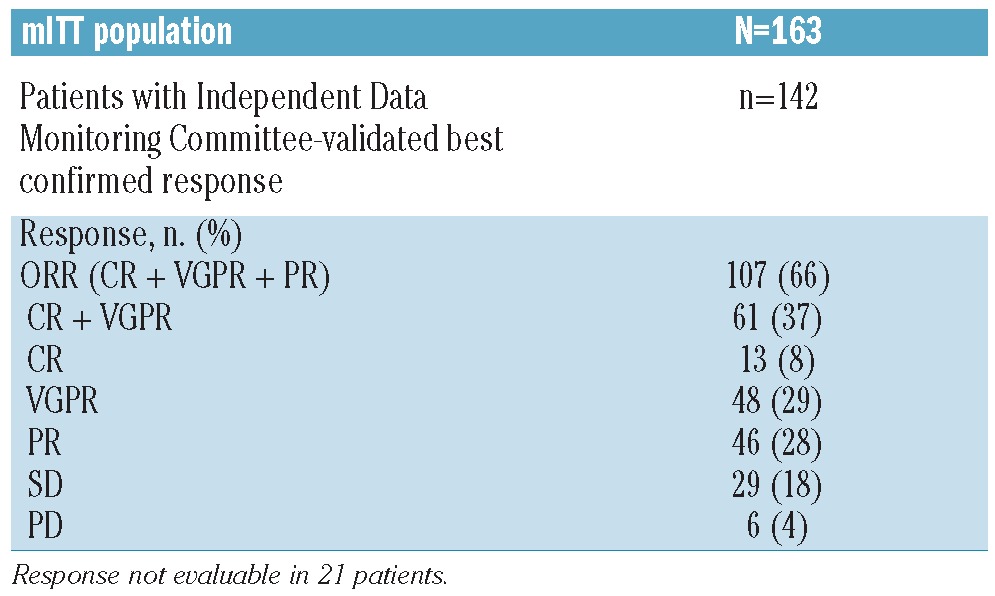
Figure 2.
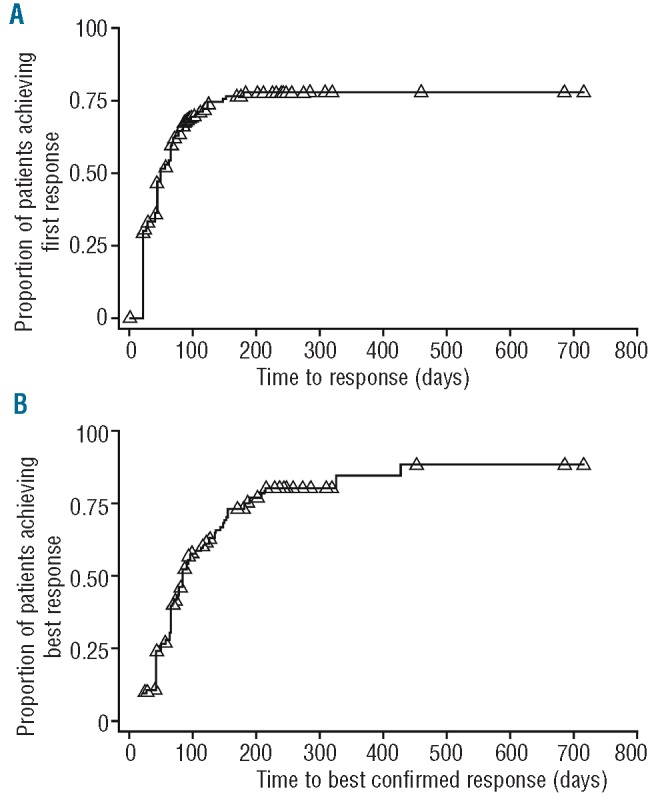
Kaplan Meier analyses of (A) time to first response (n=107) and (B) time to best confirmed response (n=107) in the mITT population (N=163)
Outcomes
Outcomes were analyzed in the mITT population (n=163). The median time to progression was 9.5 months (95%CI: 7.7, 13.4) (Figure 3A). Figure 3B shows the Kaplan-Meier estimate of the distribution of progression-free survival for the mITT population, and separately for the patients requiring randomization to bortezomib-dexamethasone/VDC/VDR (n=19), and those who were not randomized (n=144); median progression-free survival was 8.6 months (95%CI: 7.2, 11.9). After a median follow up of 16.9 months (range 0–34.2), 55 patients (34%) had died and the median overall survival had not been reached (95%CI: 32.3 months, not evaluable). The estimated 1-year overall survival rate was 81% (95%CI: 75, 87).
Figure 3.
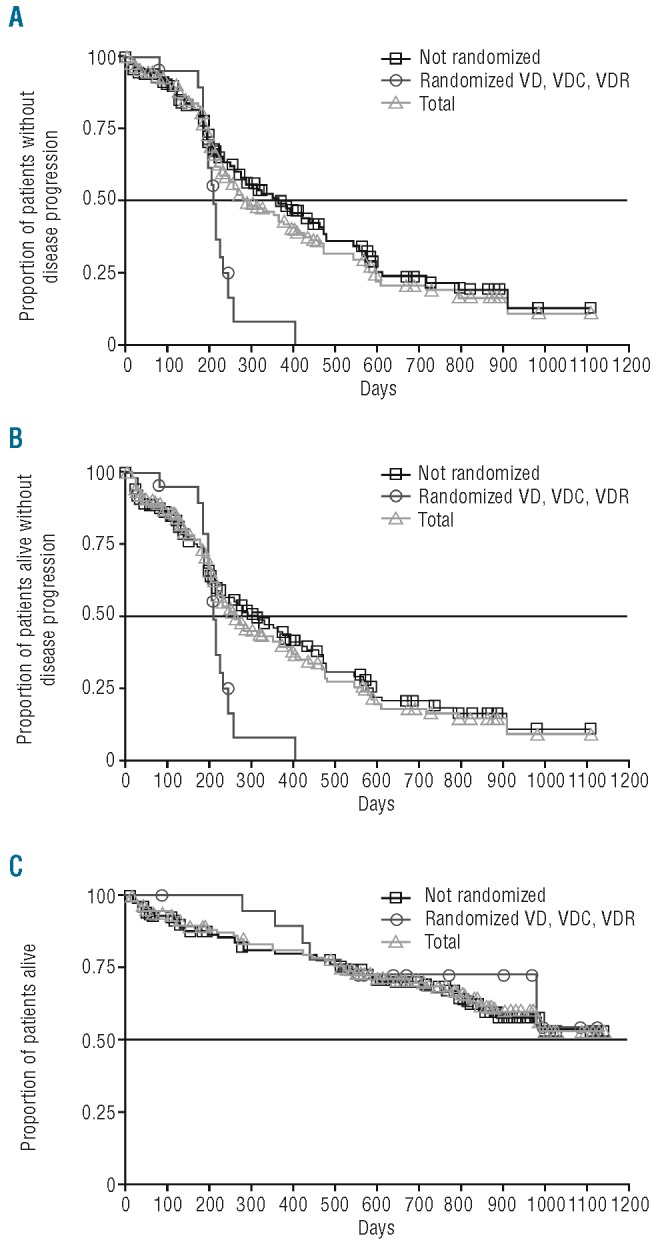
Kaplan Meier analysis of (A) time to progression (n=88) (B) progression-free survival (n=101) and (C) overall survival (n=55). Curves show data in all 163 patients in the mITT population, in patients who discontinued prior to completing, or did not require randomization after four cycles and received bortezomib-dexamethasone throughout their treatment (VD, n=144), and in patients who achieved SD after four cycles and were randomized to sequential therapy with bortezomib-dexamethasone (VD), VDC, or VDR (n=19).
Renal function improvement
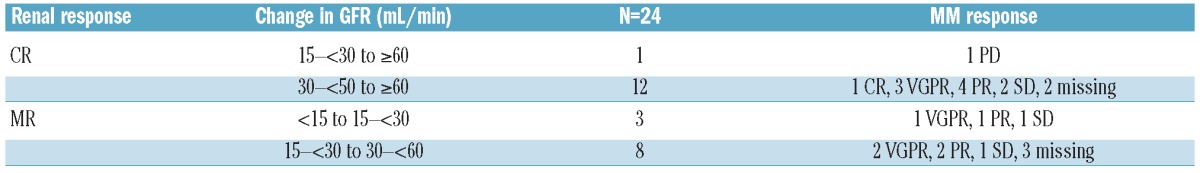
Baseline GFR was determined in all 163 patients as less than 15 mL/min (Stage V) in 5 (3%) patients, 15 to less than 30 mL/min (Stage IV) in 16 (10%) patients, 30 to less than 60 mL/min (Stage III) in 58 (36%) patients, and 60 mL/min or over (Stage I/II) in 83 (51%) patients. The median GFR among all patients with GFR assessments at each time point was examined, together with the median change in GFR from baseline/screening. Median GFR was 62.8 mL/min at baseline, and was 65.5, 68.2, 70.2, 74.4, 77.4, 77.6, and 80.2 mL/min after cycles 1, 2, 3, 4, 5, 6, and 7, respectively; the respective median GFR increases from baseline at these time points were 4.5, 5.9, 8.8, 8.8, 6.4, 9.5, and 9.6 mL/min. Patients with baseline GFR below 50 mL/min (n=58) were evaluated for their best GFR on study to determine how many had stage migrations in renal function. Table 3 summarizes the changes from baseline in renal function group in these patients according to whether they did or did not also meet the criteria for achieving a renal response.29 A total of 24 patients with stage migration from baseline GFR to best on-study GFR also achieved a renal response; CRrenal in 13 and MRrenal in 11 patients. Table 4 summarizes these renal responses and the associated MM responses achieved by these patients.
Table 3.
Changes in renal function group from baseline in patients with baseline GFR <50 mL/min according to whether they achieved a renal response or not.
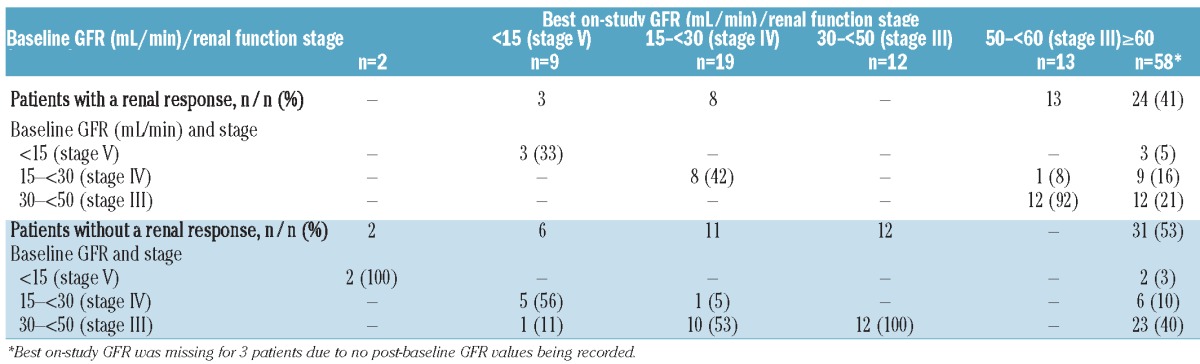
Table 4.
Patients with baseline GFR <50 mL/min who had a renal response29 and stage migration from baseline GFR to best on-study GFR.
Safety
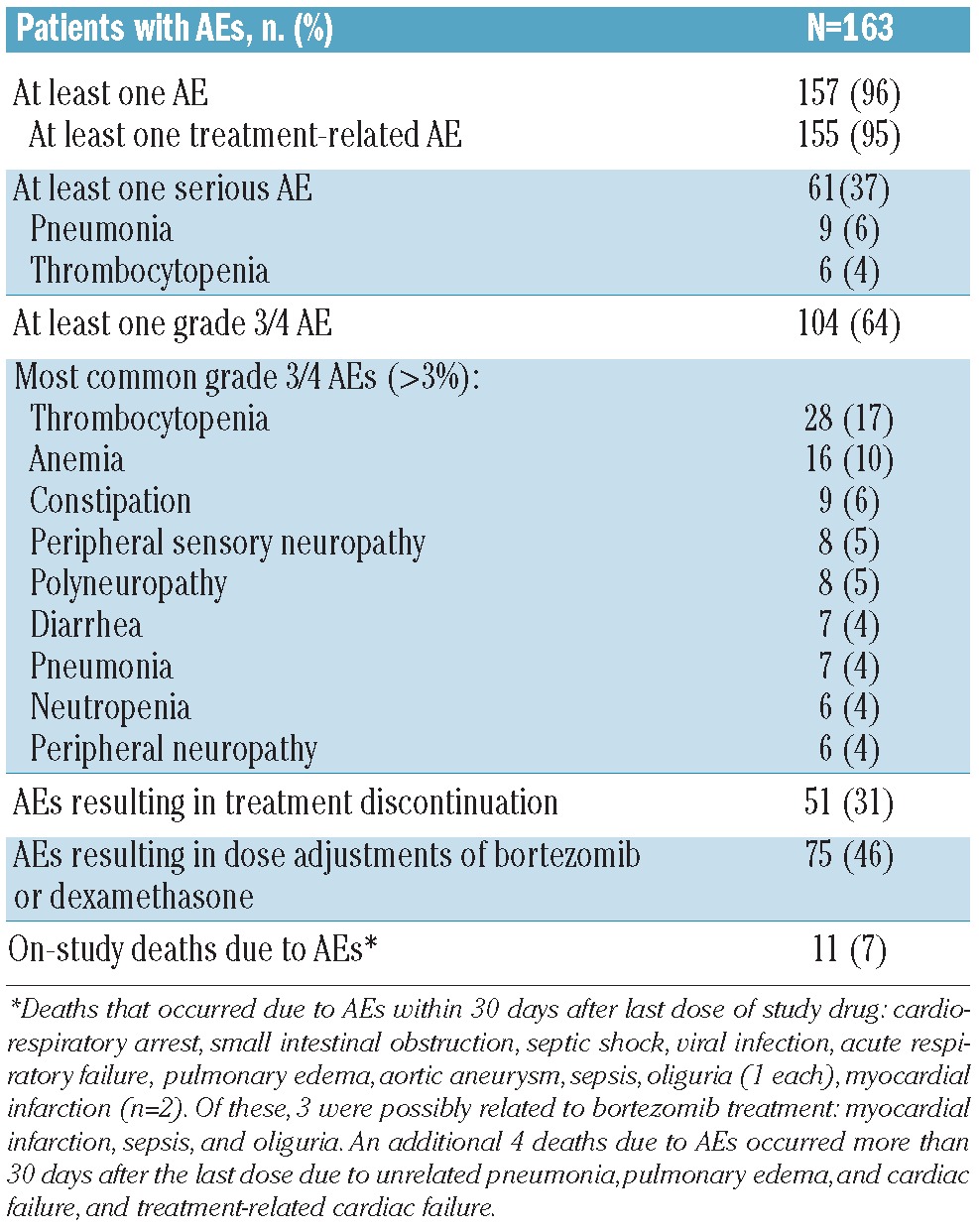
Among the mITT population (n=163), the median cumulative doses of bortezomib and dexamethasone during cycles 1–8 were 27.3 mg/m2 and 900 mg, respectively, overall. The respective median cumulative doses during cycles 1–4 were 18.4 mg/m2, and 600 mg and during cycles 5–8 were 14.6 mg/m2 and 560 mg. The overall safety profile of bortezomib-dexamethasone (± cyclophosphamide or lenalidomide) is summarized in Table 5. The most common AEs (any grade, ≥20%) were thrombocytopenia (37%), diarrhea (31%), constipation (31%), peripheral edema (26%), fatigue (23%), anemia (22%), peripheral sensory neuropathy (20%), and asthenia (20%). The most common grade 3/4 AEs are summarized in Table 5.
Table 5.
Safety profile of bortezomib-dexamethasone (±cyclophosphamide or lenalidomide after four cycles)
Peripheral neuropathy
The rates of PN AEs from within the Medical Dictionary for Regulatory Affairs (MedDRA) system organ class of ‘nervous system disorders’ were specifically assessed. PN AEs occurring in more than 10% of patients were peripheral sensory neuropathy (20%), polyneuropathy (18%), neuralgia (16%), paresthesia (14%), and peripheral neuropathy (13%). In addition, hypoesthesia occurred in 9%, and burning sensation, hyperesthesia, peripheral motor neuropathy, and peripheral sensorimotor neuropathy occurred in 1% of patients. Some patients experienced multiple PN AEs. Grade 3/4 PN AEs occurring in more than 3% of patients are shown in Table 5. In addition, grade 3/4 neuralgia was reported in 3% and grade 3/4 paresthesia and peripheral motor neuropathy occurred in 1% of patients.
Within these MedDRA preferred terms, 278 individual treatment-emergent PN events in 104 patients were recorded; due to multiple events being recorded simultaneously in a number of patients, these represented 185 discrete events. Among these events, 32 grade 3/4 PN AEs were recorded in 28 patients. Of the 185 PN events within the MedDRA preferred terms, 105 (57%) were reversible, with resolution in 82 (44%). The median time to any improvement was 2.1 months (95%CI: 1.2, 5.8), and median time to resolution was 7.9 months (95%CI: 5.8, 13.2). Among the 104 patients with treatment-emergent PN events, 23 (22.1%) had peripheral sensory neuropathy at baseline. A total of 29 PN AEs leading to bortezomib discontinuation were recorded in 25 patients, including polyneuropathy (n=7), peripheral neuropathy (n=6), neuralgia, peripheral sensory neuropathy (each n=5), and paresthesia (n=2). Bortezomib dose adjustments were required due to peripheral sensory neuropathy in 22 patients (13%), and neuralgia, peripheral neuropathy peripheral, and polyneuropathy in 10 patients (6%) each.
Discussion
The findings reported here, from the first prospective study of bortezomib-dexamethasone as second-line therapy in MM, demonstrate that the combination (with added cyclophosphamide or lenalidomide after four cycles in 12 of 163 patients) is an active and well tolerated regimen in this setting, resulting in an Independent Data Monitoring Committee-validated response rate of 66%, including 37% CR/VGPR. The activity is further highlighted by the fact that only 19 patients were investigator-assessed as having SD at cycle 5, Day 1 and thus, according to protocol, as requiring randomization to sequential therapy with continued bortezomib-dexamethasone, VDC, or VDR. Consequently, due to the high response rate at the cycle 5, Day 1 investigator assessment of response, the randomized sequential therapy phase of the study was not completed. The high response rates reported translated into noteworthy progression-free survival with bortezomib-dexamethasone (± cyclophosphamide/lenalidomide) in this setting, with a median progression-free survival of 8.6 months. It is also important to highlight that, although bortezomib-dexamethasone has been recommended as an appropriate treatment option for MM patients with renal impairment,24 this study represents one of the very few prospective evaluations of the impact of bortezomib-based therapy on renal function. Of particular interest, no patients were excluded because of their renal function status.
It is instructive to assess the efficacy data from our study of bortezomib-dexamethasone as initial therapy in second-line MM with those reported from other studies of bortezomib alone or with added dexamethasone in relapsed MM, albeit acknowledging that comparisons of data between studies should be undertaken with caution due to confounding factors such as differences in patients’ characteristics and the response criteria used. In the phase III APEX study of single-agent bortezomib versus dexamethasone30,31 in which patients could have received 1–3 prior lines of therapy, the ORR with bortezomib was 43%, including 15% CR/near-CR. The findings from our study, of an ORR of 66% and a CR/VGPR rate of 37%, suggest that addition of dexamethasone to therapy may have enhanced the response rate to bortezomib, although it should be acknowledged that only 40% of patients received bortezomib as second-line therapy in APEX.30,31 Similarly, our findings using dexamethasone from the start of therapy appear comparable or show improvement in the context of data from studies in which dexamethasone could be added following sub-optimal response to bortezomib alone. Results from the phase II CREST study,6,7 a phase IIIb open-access study,9 and the phase III MMY-3021 study of intravenous versus subcutaneous bortezomib,32 in which 33–56% of patients received added dexamethasone, showed ORRs of 50–52%, including 4–22% CR/near-CR and 25–33% CR/VGPR. Our data also appear similar to those reported in a single-center retrospective study of bortezomib alone or plus dexamethasone in 70 patients with relapsed/refractory MM, in which the ORR was 59%, including 7% CR, and 36% VGPR,11 and in a Japanese study of bortezomib-dexamethasone in 88 relapsed or refractory MM patients, in which the ORR was 67%,12 while in an observational study in China of different doses and schedules of bortezomib-dexamethasone in 168 patients with relapsed/refractory MM, ORRs of 72–79%, including 20–33% CR, were reported.13
As with these response rate data, the outcome data from our study, which included median time to progression and progression-free survival of 9.5 and 8.6 months, respectively, also support the benefit of adding dexamethasone to bortezomib therapy. For example, the median time to progression with single-agent bortezomib in APEX was 6.2 months,30,31 whereas in the phase III MMY-3021 study, in which 56% of patients received added dexamethasone, median time to progression and progression-free survival were 9.6 and 8.4 months with intravenous bortezomib and 9.7 and 9.3 months, respectively, in the subcutaneous arm.33
As highlighted, in our study, the high response rate to bortezomib-dexamethasone among patients assessed by investigators on cycle 5, Day 1 resulted in a limited need for the addition of a third drug to the regimen (n=19). In addition, the data for individual regimens in randomized patients were not evaluated due to the very small numbers of patients randomized to each group (continued bortezomib-dexamethasone n=7, VDC n=8, VDR n=4). Thus we could not address the question as to whether sequential VDC or VDR, in patients achieving SD following four cycles of bortezomib-dexamethasone, provides benefit compared with continued bortezomib-dexamethasone. It should be noted that time to progression and progression-free survival appeared to be poorer in the 19 patients randomized to sequential therapy with continued bortezomib-dexamethasone, VDC, or VDR than in those patients who were not randomized; this is likely due to the selection for randomization of patients achieving only stable disease after 4 cycles of bortezomib-dexamethasone, i.e. patients with disease that was possibly less sensitive to bortezomib-based therapy than that of the majority of those in the non-randomized population. Thus, no conclusions can be drawn from these data regarding the comparative efficacy of triplet or doublet therapy.
It is also important to note that, just as the use of dexamethasone from the start of bortezomib therapy may increase the response rate to treatment, the use of cyclophosphamide or lenalidomide from the start of bortezomib-dexamethasone treatment may further enhance response, as suggested by data from prospective studies of VDR22 and VDC,23 albeit with potentially greater toxicity than the doublet alone. Notably, the activity of lenalidomide and dexamethasone in combination in patients with relapsed MM has been demonstrated in two phase III trials, with pooled results showing an ORR of 61%, including 32% CR/VGPR, median progression-free survival of 11.1 months, and median overall survival of 38.0 months.34 In a subgroup analysis of patients receiving lenalidomide-dexamethasone as second-line therapy, ORR was 67%, including 40% CR/VGPR, median TTP and PFS were 17.1 and 14.1 months, respectively, and median OS was 42.0 months.35 In addition, it is important to note that although response rates were substantial after four cycles of bortezomib-dexamethasone, the additional four cycles of bortezomib-dexamethasone were important in achieving the response rates after 8 cycles versus adding a third agent.
The findings from our study indicate that the combination of bortezomib-dexamethasone (± cyclophosphamide, or lenalidomide, after four cycles) as second-line therapy appears generally well tolerated, with a manageable safety profile that reflects the previously established safety profile of bortezomib from phase II and III studies in relapsed MM.6,10,30 The good tolerability of the combination was evidenced by the relatively high median cumulative dose of bortezomib administered during the first four cycles of treatment (18.4 mg/m2, out of a planned total dose of 20.8 mg/m2), indicating a limited requirement for dose reductions.
Interestingly, our findings showed that PN was manageable, with high rates of reversibility; 57% of all PN events had improved or resolved by data cut off, including 44% that had resolved completely. These findings support data from phase II studies, APEX, and MMY-3021, in which PN improved or resolved in 62–71% of cases.32,36,37 In addition, the rates of specific PN AEs by MedDRA preferred terms appeared limited, with, for example, only 20% of patients reporting peripheral sensory neuropathy, including 5% at grade 3/4 severity. These rates appear similar or lower in the context of previous studies of bortezomib alone or with added dexamethasone, although comparisons between studies are confounded by the different MedDRA preferred terms included within the overall reported rate of PN, as well as by differences in patients’ baseline characteristics. Nevertheless, in APEX, the overall rate of PN (which included peripheral sensory neuropathy, peripheral motor neuropathy, and associated PN preferred terms, but which was primarily sensory neuropathy) was 37%, including 9% grade 3/4,37 while in the phase IIIb open-access study of bortezomib alone or with added dexamethasone, the rate of PN was 25%, with 6% grade 3/4.9 In the intravenous bortezomib arm of MMY-3021, the rate of peripheral sensory neuropathy was 49%, including 15% grade 3 or over.32 The reasons for the difference between these findings and our study are not clear, although the rate of previous exposure to neurotoxic agents in MMY-3021 (85%) and the rate of base-line grade 1 PN (28%) appear higher than in our study. It has been suggested that partnered dexamethasone dosing on the day of and day after bortezomib dosing, as used in our study, may reduce the inflammatory component of bortezomib-associated PN and thus reduce the severity.20,26,38 This may, therefore, have been a contributing factor to the limited rates of grade 3/4 specific PN AEs observed in our study. It is worth noting that in the context of limiting the rates of bortezomib-associated PN, the use of subcutaneous administration of bortezomib has been approved by the United States Food and Drug Administration and the European Medicines Agency based on the findings of the phase III MMY-3021 trial, in which rates of all-grade, grade 2 or over, and grade 3 or over PN were significantly lower with subcutaneous versus intravenous bortezomib.32 Therefore, it might be expected that the PN rates seen in our study could be substantially reduced with the use of subcutaneous administration of bortezomib.
As reported above, another important aspect of our study was the prospective evaluation of changes in renal function during bortezomib-dexamethasone treatment. This prospective evaluation contrasts with previous reports on the impact of bortezomib-based therapy in patients with renal impairment, which were primarily of a retrospective nature.24,39–45 A further difference was that the eligibility criteria for our study allowed patients with severe renal impairment to be included, which was not the case for many previous studies of MM patients with renal impairment. Our findings suggest that treatment with bortezomib-dexamethasone appeared feasible in patients with any-stage renal disease, supporting recent suggestions that this combination represents an appropriate treatment option for patients with renal impairment.24,46 Importantly, treatment resulted in some renal function improvement, with the median GFR improving versus baseline during the course of therapy, and a number of patients with baseline GFR below 50 mL/min demonstrating renal function stage migrations. Renal responses were seen in 24 of 58 patients with baseline GFR below 50 mL/min, including 13 CRrenal. Interestingly, 5 of these renal responses were seen in patients who achieved SD or progressive disease as their best MM response to therapy. This dissociation between MM response and renal response in some patients suggests that bortezomib-dexamethasone may have a renal-specific effect. These findings regarding reversibility of renal impairment with bortezomib-based therapy in patients with relapsed MM are supported by the results of previous retrospective analyses, as recently reviewed.24
In conclusion, the results presented here suggest that bortezomib-dexamethasone represents a feasible, active second-line treatment option for patients with relapsed MM, with investigator-assessed responses after four cycles suggesting a limited need for the addition of a third drug as per study protocol. This combination should be considered as one of the standard options for the second-line treatment of MM and may represent a useful backbone for more extensive combinations. Randomized studies in this setting would be required to determine the relative benefit of adding another agent, such as cyclophosphamide or lenalidomide, from the start of therapy.
Acknowledgments
The authors would like to thank the patients who participated in this study and their families. The authors would like to acknowledge the contributions of all study investigators. The authors also acknowledge the writing assistance of Helen Wilkinson of FireKite during the development of this manuscript, which was funded by Millennium: The Takeda Oncology Company, and Janssen Global Services.
Footnotes
The online version of this article has a Supplementary Appendix.
Funding
The funding for this study was provided by Janssen Global Services.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Corso A, Barbarano L, Mangiacavalli S, Spriano M, Alessandrino EP, Cafro AM, et al. Bortezomib plus dexamethasone can improve stem cell collection and overcome the need for additional chemotherapy before autologous transplant in patients with myeloma. Leuk Lymphoma. 2010;51 (2):236–42 [DOI] [PubMed] [Google Scholar]
- 2.Harousseau JL, Attal M, Leleu X, Troncy J, Pegourie B, Stoppa AM, et al. Bortezomib plus dexamethasone as induction treatment prior to autologous stem cell transplantation in patients with newly diagnosed multiple myeloma: results of an IFM phase II study. Haematologica. 2006;91(11):1498–505 [PubMed] [Google Scholar]
- 3.Harousseau JL, Attal M, Avet-Loiseau H, Marit G, Caillot D, Mohty M, et al. Bortezomib plus dexamethasone is superior to vincristine plus doxorubicin plus dexamethasone as induction treatment prior to autologous stem-cell transplantation in newly diagnosed multiple myeloma: results of the IFM 2005-01 phase III trial. J Clin Oncol. 2010;28(30):4621–9 [DOI] [PubMed] [Google Scholar]
- 4.Jagannath S, Durie BG, Wolf JL, Camacho ES, Irwin D, Lutzky J, et al. Extended follow-up of a phase 2 trial of bortezomib alone and in combination with dexamethasone for the frontline treatment of multiple myeloma. Br J Haematol. 2009;146(6):619–26 [DOI] [PubMed] [Google Scholar]
- 5.Freimann H, Calderoni A, Cornu P, Olie R. Daily practice use of Bortezomib in relapsed/refractory multiple myeloma. Safety/efficacy results of a compassionate use program in Switzerland. Swiss Med Wkly. 2007;137(21–22):317–22 [DOI] [PubMed] [Google Scholar]
- 6.Jagannath S, Barlogie B, Berenson J, Siegel D, Irwin D, Richardson PG, et al. A phase 2 study of two doses of bortezomib in relapsed or refractory myeloma. Br J Haematol. 2004;127(2):165–72 [DOI] [PubMed] [Google Scholar]
- 7.Jagannath S, Barlogie B, Berenson JR, Siegel DS, Irwin D, Richardson PG, et al. Updated survival analyses after prolonged follow-up of the phase 2, multicenter CREST study of bortezomib in relapsed or refractory multiple myeloma. Br J Haematol. 2008;143 (4):537–40 [DOI] [PubMed] [Google Scholar]
- 8.Kropff MH, Bisping G, Wenning D, Volpert S, Tchinda J, Berdel WE, et al. Bortezomib in combination with dexamethasone for relapsed multiple myeloma. Leuk Res. 2005;29(5):587–90 [DOI] [PubMed] [Google Scholar]
- 9.Mikhael JR, Belch AR, Prince HM, Lucio MN, Maiolino A, Corso A, et al. High response rate to bortezomib with or without dexamethasone in patients with relapsed or refractory multiple myeloma: results of a global phase 3b expanded access program. Br J Haematol. 2009;144(2):169–75 [DOI] [PubMed] [Google Scholar]
- 10.Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin D, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003; 348(26):2609–17 [DOI] [PubMed] [Google Scholar]
- 11.Corso A, Varettoni M, Mangiacavalli S, Zappasodi P, Pica GM, Algarotti A, et al. Bortezomib plus dexamethasone is highly effective in relapsed and refractory myeloma patients but responses are short-lived. Eur J Haematol. 2009;83(5):449–54 [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi T, Kuroda J, Shimura K, Akaogi T, Kawata E, Kiyota M, et al. Bortezomib plus dexamethasone for relapsed or treatment refractory multiple myeloma: the collaborative study at six institutes in Kyoto and Osaka. Int J Hematol. 2010;92(4):579–86 [DOI] [PubMed] [Google Scholar]
- 13.Yuan ZG, Jin J, Huang XJ, Li Y, Chen WM, Liu ZG, et al. Different dose combinations of bortezomib and dexamethasone in the treatment of relapsed or refractory myeloma: an open-label, observational, multicenter study in China. Chin Med J. 2011; 124(19):2969–74 [PubMed] [Google Scholar]
- 14.Einsele H, Liebisch P, Langer C, Kropff M, Wandt H, Jung W, et al. Velcade, intravenous cyclophosphamide and dexamethasone (VCD) induction for previously untreated multiple myeloma (German DSMM XIa trial). Blood. 2009;114(22):59a–60a [Abstract 131]. [Google Scholar]
- 15.Khan ML, Reeder CB, Kumar SK, Lacy MQ, Reece DE, Dispenzieri A, et al. A comparison of lenalidomide/dexamethasone versus cyclophosphamide/lenalidomide/dexamethasone versus cyclophosphamide/bortezomib/dexamethasone in newly diagnosed multiple myeloma. Br J Haematol. 2012;156(3):326–33 [DOI] [PubMed] [Google Scholar]
- 16.Kropff M, Liebisch P, Knop S, Weisel K, Wand H, Gann CN, et al. DSMM XI study: dose definition for intravenous cyclophosphamide in combination with bortezomib/dexamethasone for remission induction in patients with newly diagnosed myeloma. Ann Hematol. 2009;88(11):1125–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reeder CB, Reece DE, Kukreti V, Chen C, Trudel S, Hentz J, et al. Cyclophosphamide, bortezomib and dexamethasone induction for newly diagnosed multiple myeloma: high response rates in a phase II clinical trial. Leukemia. 2009;23(7):1337–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reeder CB, Reece DE, Kukreti V, Chen C, Trudel S, Laumann K, et al. Once- versus twice-weekly bortezomib induction therapy with CyBorD in newly diagnosed multiple myeloma. Blood. 2010;115(16):3416–7 [DOI] [PubMed] [Google Scholar]
- 19.Kumar S, Flinn I, Richardson PG, Hari P, Callander N, Noga SJ, et al. Randomized, multicenter, phase 2 study (EVOLUTION) of combinations of bortezomib, dexamethasone, cyclophosphamide, and lenalidomide in previously untreated multiple myeloma. Blood. 2012;119(19):4375–82 [DOI] [PubMed] [Google Scholar]
- 20.Richardson PG, Weller E, Lonial S, Jakubowiak AJ, Jagannath S, Raje NS, et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood. 2010;116(5):679–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar SK, Flinn I, Noga SJ, Hari P, Rifkin R, Callander N, et al. Bortezomib, dexamethasone, cyclophosphamide and lenalidomide combination for newly diagnosed multiple myeloma: phase 1 results from the multicenter EVOLUTION study. Leukemia. 2010;24(7):1350–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson KC, Jagannath S, Jakubowiak A, Lonial S, Raje N, Alsina M, et al. Lenalidomide, bortezomib, and dexamethasone in relapsed/refractory multiple myeloma (MM): Encouraging outcomes and tolerability in a phase II study. J Clin Oncol. 2009;27(34):5713–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kropff M, Bisping G, Schuck E, Liebisch P, Lang N, Hentrich M, et al. Bortezomib in combination with intermediate-dose dexamethasone and continuous low-dose oral cyclophosphamide for relapsed multiple myeloma. Br J Haematol. 2007;138(3):330–7 [DOI] [PubMed] [Google Scholar]
- 24.Chanan-Khan AA, San MJ, Jagannath S, Ludwig H, Dimopoulos AM. Novel therapeutic agents for the management of multiple myeloma patients with renal impairment. Clin Cancer Res. 2012;18(8):2145–63 [DOI] [PubMed] [Google Scholar]
- 25.Durie BG, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20(9):1467–73 [DOI] [PubMed] [Google Scholar]
- 26.Richardson PG, Delforge M, Beksac M, Wen P, Jongen JL, Sezer O, et al. Management of treatment-emergent peripheral neuropathy in multiple myeloma. Leukemia. 2012;26(4):595–608 [DOI] [PubMed] [Google Scholar]
- 27.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41 [DOI] [PubMed] [Google Scholar]
- 28.National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–266 [PubMed] [Google Scholar]
- 29.Ludwig H, Adam Z, Hajek R, Greil R, Tothova E, Keil F, et al. Light chain-induced acute renal failure can be reversed by bortezomib-doxorubicin-dexamethasone in multiple myeloma: results of a phase II study. J Clin Oncol. 2010;28(30):4635–41 [DOI] [PubMed] [Google Scholar]
- 30.Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352(24):2487–98 [DOI] [PubMed] [Google Scholar]
- 31.Richardson PG, Sonneveld P, Schuster M, Irwin D, Stadtmauer E, Facon T, et al. Extended follow-up of a phase 3 trial in relapsed multiple myeloma: final time-to-event results of the APEX trial. Blood. 2007;110(10):3557–60 [DOI] [PubMed] [Google Scholar]
- 32.Moreau P, Pylypenko H, Grosicki S, Karamanesht I, Leleu X, Grishunina M, et al. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomised, phase 3, non-inferiority study. Lancet Oncol. 2011;12(5):431–40 [DOI] [PubMed] [Google Scholar]
- 33.Arnulf B, Pylypenko H, Grosicki S, Karamanesht I, Leleu X, van de Velde H, et al. Updated survival analysis of a randomized phase III study of subcutaneous versus intravenous bortezomib in patients with relapsed multiple myeloma. Haematologica. 2012;97(12):1925–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dimopoulos MA, Chen C, Spencer A, Niesvizky R, Attal M, Stadtmauer EA, et al. Long-term follow-up on overall survival from the MM-009 and MM-010 phase III trials of lenalidomide plus dexamethasone in patients with relapsed or refractory multiple myeloma. Leukemia. 2009;23(11):2147–52 [DOI] [PubMed] [Google Scholar]
- 35.Stadtmauer EA, Weber DM, Niesvizky R, Belch A, Prince MH, San Miguel JF, et al. Lenalidomide in combination with dexamethasone at first relapse in comparison with its use as later salvage therapy in relapsed or refractory multiple myeloma. Eur J Haematol. 2009;82(6):426–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richardson PG, Briemberg H, Jagannath S, Wen PY, Barlogie B, Berenson J, et al. Frequency, characteristics, and reversibility of peripheral neuropathy during treatment of advanced multiple myeloma with bortezomib. J Clin Oncol. 2006;24(19):3113–20 [DOI] [PubMed] [Google Scholar]
- 37.Richardson PG, Sonneveld P, Schuster MW, Stadtmauer EA, Facon T, Harousseau JL, et al. Reversibility of symptomatic peripheral neuropathy with bortezomib in the phase III APEX trial in relapsed multiple myeloma: impact of a dose-modification guideline. Br J Haematol. 2009;144(6):895–903 [DOI] [PubMed] [Google Scholar]
- 38.Kumar S, Laubach JP, Sonneveld P, Mitsiades CS, Broijl A, Giove T, et al. Bortezomib (btz)-induced peripheral neuropathy (BiPN) in previously untreated MM: impact of dexamethasone (dex) schedule. Clin Lymphoma Myeloma Leuk. 2013;13(1 Suppl):S235 (abstract P-416). [Google Scholar]
- 39.Blade J, Sonneveld P, San Miguel JF, Sutherland HJ, Hajek R, Nagler A, et al. Pegylated liposomal doxorubicin plus bortezomib in relapsed or refractory multiple myeloma: efficacy and safety in patients with renal function impairment. Clin Lymphoma Myeloma. 2008;8(6):352–5 [DOI] [PubMed] [Google Scholar]
- 40.Chanan-Khan AA, Kaufman JL, Mehta J, Richardson PG, Miller KC, Lonial S, et al. Activity and safety of bortezomib in multiple myeloma patients with advanced renal failure: a multicenter retrospective study. Blood. 2007;109(6):2604–6 [DOI] [PubMed] [Google Scholar]
- 41.Dimopoulos MA, Roussou M, Gavriatopoulou M, Zagouri F, Migkou M, Matsouka C, et al. Reversibility of renal impairment in patients with multiple myeloma treated with bortezomib-based regimens: identification of predictive factors. Clin Lymphoma Myeloma. 2009;9(4):302–6 [DOI] [PubMed] [Google Scholar]
- 42.Dimopoulos MA, Richardson PG, Schlag R, Khuageva NK, Shpilberg O, Kastritis E, et al. VMP (Bortezomib, Melphalan, and Prednisone) is active and well tolerated in newly diagnosed patients with multiple myeloma with moderately impaired renal function, and results in reversal of renal impairment: cohort analysis of the phase III VISTA study. J Clin Oncol. 2009;27(36): 6086–93 [DOI] [PubMed] [Google Scholar]
- 43.Jagannath S, Barlogie B, Berenson JR, Singhal S, Alexanian R, Srkalovic G, et al. Bortezomib in recurrent and/or refractory multiple myeloma. Initial clinical experience in patients with impaired renal function. Cancer. 2005;103(6):1195–200 [DOI] [PubMed] [Google Scholar]
- 44.Morabito F, Gentile M, Ciolli S, Petrucci MT, Galimberti S, Mele G, et al. Safety and efficacy of bortezomib-based regimens for multiple myeloma patients with renal impairment: a retrospective study of Italian Myeloma Network GIMEMA. Eur J Haematol. 2010;84(3):223–8 [DOI] [PubMed] [Google Scholar]
- 45.San-Miguel JF, Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, et al. Efficacy and safety of bortezomib in patients with renal impairment: results from the APEX phase 3 study. Leukemia. 2008; 22(4):842–9 [DOI] [PubMed] [Google Scholar]
- 46.Dimopoulos MA, Terpos E, Chanan-Khan A, Leung N, Ludwig H, Jagannath S, et al. Renal impairment in patients with multiple myeloma: a consensus statement on behalf of the International Myeloma Working Group. J Clin Oncol. 2010;28(33):4976–84 [DOI] [PubMed] [Google Scholar]