Abstract
Fifty-five years after publication of the first hematopoietic stem cell transplantation this technique has become an accepted treatment option for defined hematologic and non-hematologic disorders. There is considerable interest in understanding differences in its use and trends on a global level and the macro-economic factors associated with these differences. Data on the numbers of hematopoietic stem cell transplants performed in the 3-year period 2006–2008 were obtained from Worldwide Network for Blood and Marrow Transplantation member registries and from transplant centers in countries without registries. Population and macro-economic data were collected from the World Bank and from the International Monetary Fund. Transplant rates were analyzed by indication, donor type, country, and World Health Organization regional offices areas and related to selected health care indicators using single and multiple linear regression analyses. Data from a total of 146,808 patients were reported by 1,411 teams from 72 countries over five continents. The annual number of transplants increased worldwide with the highest relative increase in the Asia Pacific region. Transplant rates increased preferentially in high income countries (P=0.02), not in low or medium income countries. Allogeneic transplants increased for myelodysplasia, chronic lymphocytic leukemia, acute leukemias, and non-malignant diseases but decreased for chronic myelogenous leukemia. Autologous transplants increased for autoimmune and lymphoproliferative diseases but decreased for leukemias and solid tumors. Transplant rates (P<0.01), donor type (P<0.01) aand disease indications (P<0.01) differed significantly between countries and regions. Transplant rates were associated with Gross National Income/capita (P<0.01) but showed a wide variation of explanatory content by donor type, disease indication and World Health Organization region. Hematopoietic stem cell transplantation activity is increasing worldwide. The preferential increase in high income countries, the widening gap between low and high income countries and the significant regional differences suggest that different strategies are required in individual countries to foster hematopoietic stem cell transplantation as an efficient and cost-effective treatment modality.
Introduction
Quantitative differences in rates of hematopoietic stem cell transplantation (HSCT) have been well described in the recent past: more patients are transplanted in countries with a higher national income. HSCT requires a specific infrastructure, depends on a network of specialists and remains associated with significant morbidity and mortality; it is a prime example of costly, specialized medicine. Broader use of HSCT has therefore long been limited to high income countries.1,2 This has changed over the last decade, for several reasons. Transplantation of autologous or allogeneic bone marrow, peripheral blood or cord blood stem cells has become the treatment of choice for many patients with defined severe congenital or acquired disorders of the hematopoietic system. Registers of unrelated donors have expanded to include more than 20 million human leukocyte antigen (HLA)-typed volunteer donors worldwide and increased the likelihood of finding a suitable matched donor. Results have improved, including those for elderly patients and for those with co-morbid conditions. As a consequence, novel indications are being explored and transplant numbers have increased worldwide.3–9
Furthermore, the World Health Organization (WHO) has recognized transplantation as an important global task. Transplantation of cells, tissues and organs has extended the lifespan of hundreds of thousands of patients worldwide and enhanced their quality of life; it has become standard of care for many patients with single organ failure and should no longer be restricted to affluent countries or individuals. The guiding principles of the WHO declare regulation of transplantation on a national level as a governmental responsibility. Regulation includes harmonized data collection on use and outcome as an essential tool to improve results and to achieve efficient and cost-effective use of resources.10,11 Information on use and trends is therefore a prime prerequisite for any health care agency. The Worldwide Network for Blood and Marrow Transplantation (WBMT), an umbrella organization of HSCT and a non-governmental organization recognized by WHO, has taken on the task of facilitating HSCT. It previously identified availability of resources, governmental support and access of patients to the therapy as key factors associated with quantitative differences in transplant rates.12 It now presents an in-depth assessment of factors associated with qualitative differences in use and trends on a global level.
Design and Methods
Study design
This retrospective survey followed the principles of the WBMT through data collection by its network of international or regional member organizations.12 The main outcome measures were the assessment of transplant rates by indication and donor type for each country, the changes over the 3-year period from 2006 to 2008 and their associations with defined macro-economic factors.
Data collection and validation
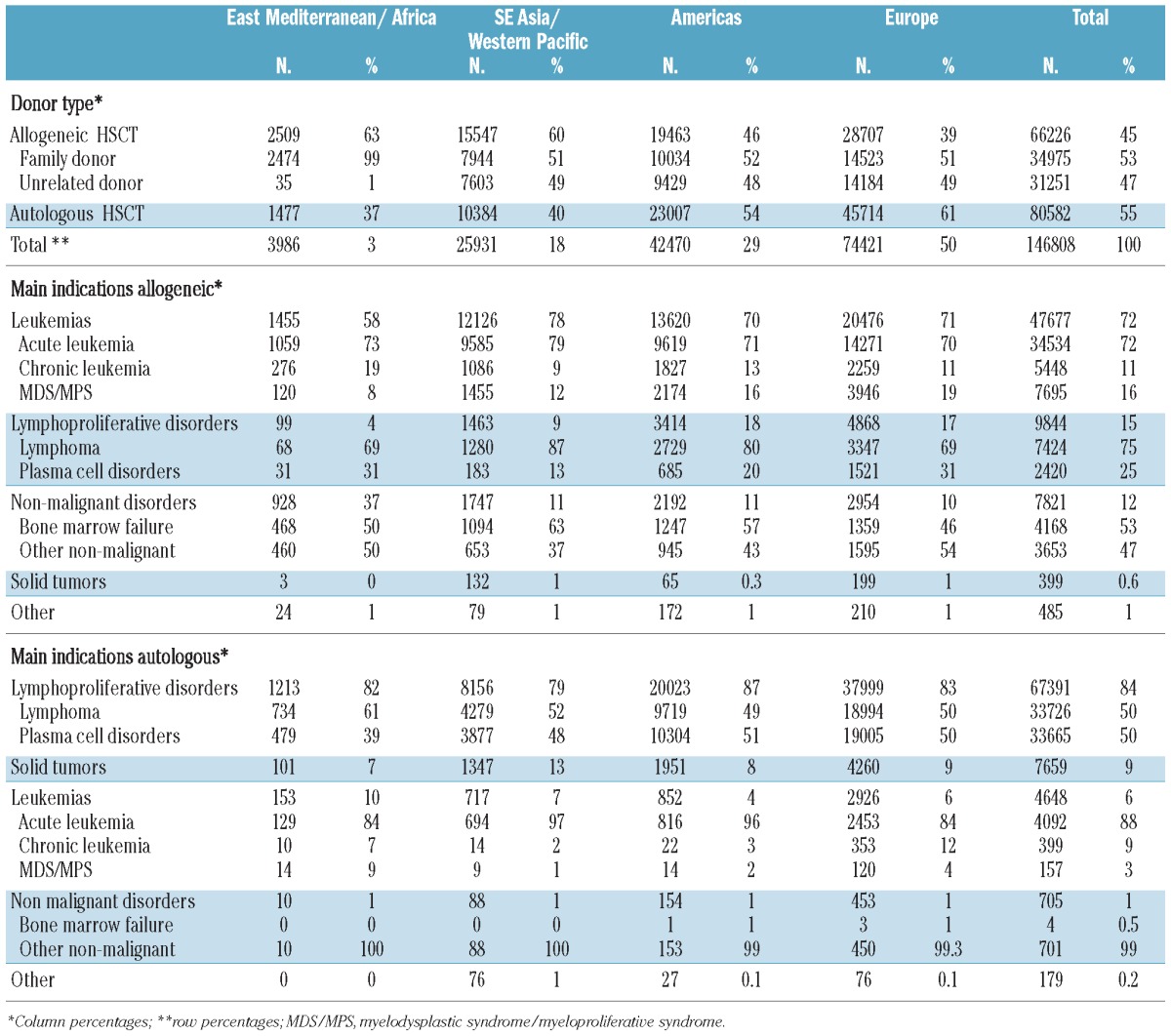
Data were obtained from 1,411 teams in 72 countries over five continents on the numbers of HSCT performed in the years 2006, 2007 and 2008 by indication and donor type (Table 1). Data were reported via the mandatory worldwide compatible reporting system of initial transplant data (ABMTRR, CBMTG, and CIBMTR) or by a separate survey data form (APBMT, EBMT, EMBMT, and SBTMO).9,13–16
Table 1.
Population description of patients with HSCT by WHO regional offices area from 2006 to 2008.
Data were pooled, validated through confirmation by the reporting team, which received a computer printout of the entered data, by selective comparison with MED-A data sets in the EBMT ProMISE data system or by cross-checking with National Registries. Double reporting was excluded. Onsite visits of selected teams are part of the quality control program within CIBMTR and EBMT teams.
Definitions
Transplant rates
Transplant rates were computed as the number of patients treated with a first HSCT per 10 million inhabitants.2 Patients with a re-transplant or a second or third HSCT were not included.
Population data and data on Gross National Income (GNI)/capita, health care expenditures/capita, governmental health care expenditure, and World Bank Category (by GNI/capita) were obtained from the World Bank (www.worldbank.org) and from the International Monetary Fund (www.imf.org).
World Health Organization regional offices areas
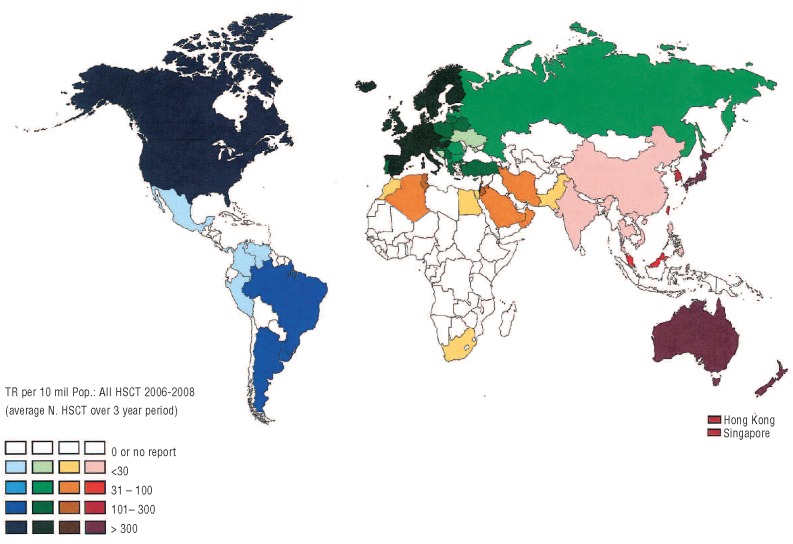
The allocation of individual countries to a region followed the WHO regional offices classification (www.who.int/about/regions/en/) and the previously reported restriction to four regions:11 (i) the Americas; (ii) Asia; (iii) Eastern-Mediterranean and Africa; and (iv) Europe (Figure 1).
Figure 1.
Transplant rates for the total number of HSCT in participating countries by WHO regional offices area for the years 2006–2008. Regions are colored by WHO regional offices area code (see text). Shades of colors reflect transplant rates (numbers of HSCT, allogeneic and autologous combined, by 10 million inhabitants).
Statistical analysis
The association of macro-economic factors with HSCT rates and the changes from 2006 to 2008 were estimated by single and multiple linear regression analyses using the least squares method. The significance of relationships was measured using τ statistics; a level of 5% was considered statistically significant. The goodness of fit was calculated using the coefficient of determination (R2), the square of Pearson’s correlation coefficient. For single and multiple regression analyses, the dependent variables were transformed to be closer to an underlying linear model. For the multiple regression analyses, all factors were assessed for their multicollinearity.
The t test was used to evaluate significant differences between the WHO regions. All statistical analyses were performed with EViews version 5.1 (Quantitative Micro Software, Irvine, CA, USA).
Results
Numbers of hematopoietic stem cell transplants for the years 2006–2008, indications, donor type and stem cell source
During the 3-year period considered, 146,808 patients underwent a first HSCT (45% allogeneic and 55% autologous) (Table 1). The analysis showed substantial heterogeneity in indication and donor type by WHO region. The main indications were lymphoproliferative disorders (53%) and leukemias (36%), followed by solid tumors (5%) and non-malignant disorders and others (6%). There was, however, a distinctly different pattern for allogeneic and autologous HSCT. The main indications for allogeneic HSCT were leukemias (72%), lymphoproliferative disorders (15%) and non-malignant disorders (12%), while the main indications for autologous HSCT were lymphoproliferative disorders (84%), solid tumors (9%) and non-malignant disorders (1%) (Table 1).
Information on stem cell source was available for a total of 142,822 patients. Peripheral blood was used predominantly in related and unrelated HSCT (64%) and in autologous HSCT (98%). Bone marrow remained an important source for allogeneic HSCT (26%), specifically for non-malignant disorders (56%); its use was minimal for autologous HSCT (2%). Allogeneic HSCT (in patients for whom information on stem cell source was available) were performed from family donors in 51% of cases (43% matched, 7% mismatched/haploidentical, 0.5% twins and 0.43% cord blood) and from unrelated donors in 49%. Of the 49% unrelated HSCT, 54% were obtained from peripheral blood, 27% from bone marrow and 19% from cord blood.
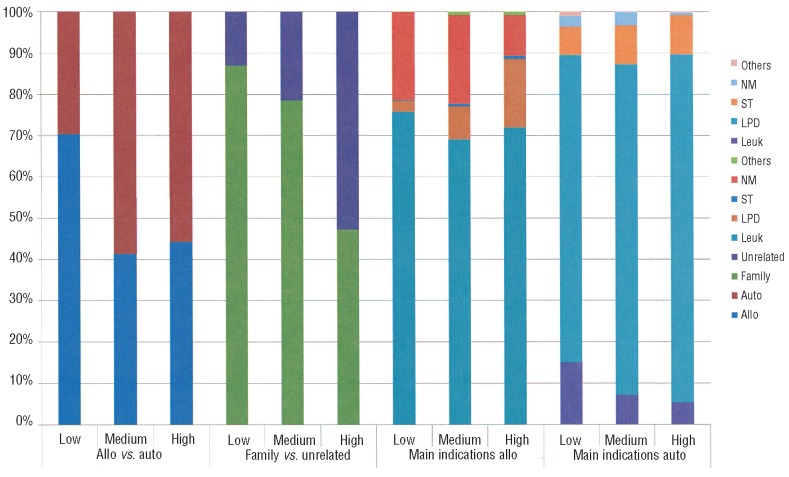
The highest number of HSCT was reported from Europe (51% of which 39% allogeneic HSCT) followed by the Americas (29%; 46% allogeneic HSCT), Asia (18%; 60% allogeneic HSCT) and Eastern Mediterranean/Africa (3%; 63% allogeneic HSCT) as shown in Table 1. The distribution was asymmetric concerning the proportion of autologous and allogeneic HSCT with the pattern in America and Europe being significantly different from that in Asia and Eastern Mediterranean/Africa (P<0.05) and concerning the repartition of main indications with a higher proportion of non-malignant indications in the Eastern Mediterranean/Africa region (P<0.01) and a higher proportion of acute leukemia in Asia (P<0.01). This asymmetric distribution was primarily influenced by the World Bank category of the participating countries (Figure 2). Low income countries preferentially used allogeneic HSCT compared to autologous HSCT, low and middle income countries preferentially used family donors compared to unrelated donors and showed a higher proportion of non-malignant indications.
Figure 2.
Indications and donor types of 146,808 HSCT by World Bank category in the years 2006–2008. The figure reflects the relative proportions of allogeneic (blue) or autologous (red) HSCT (left three columns), of allogeneic donor type [family donor (green) or unrelated donor (blue)] (central left three columns), main indications for allogeneic HSCT (central right three columns), and main indications for autologous HSCT (right three columns; for color code see figure) by low, middle of high income according to World Bank category. For definitions see the Design and Methods section. NM: non-malignant disorders; ST: solid tumors; LPD: lymphoproliferative disorders; Leuk: leukemia.
Transplant rates
Over the 3-year period studied, the average absolute number of HSCT in the participating countries ranged from 1 (Philippines) to 11,228 (USA) (Figure 1). The transplant rate ranged from 0.1 to 732 per 10 million inhabitants (median 119) for total HSCT, from 0 to 397 (median 49) for allogeneic HSCT and from 0 to 412 (median 81) for autologous HSCT. There were no autologous or allogeneic transplants in countries with fewer than 300,000 inhabitants or with a GNI/capita below $US 690; there were no unrelated donor transplants in countries with a GNI/capita below $US 850.
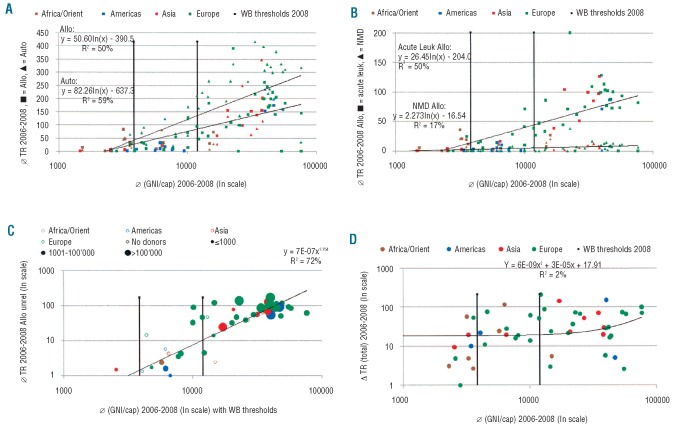
Transplant rates were significantly associated with common health care indicators, lnGNI/capita (R2 = 61%) (Figure 3), health care expenditure/capita (R2 = 64%) or governmental health care expenditure/capita (R2 = 63%) (data not shown). These associations were similar for 2006, 2007 and 2008. They differed significantly for donor types, indications and by the WHO regions.
Figure 3.
Transplant rates and Gross National Income per capita (GNI/cap). (A) Transplant rates for allogeneic and autologous HSCT by WHO regional offices area, donor type and GNI/cap. Symbols reflect transplant rates (TR; numbers of HSCT by 10 million inhabitants) in participating countries and the respective lnGNI/cap. Colors indicate WHO region (see Figure 1); squares indicate allogeneic HSCT, triangles autologous HSCT. Vertical lines separate countries by World Bank (WB) category. (B) Transplant rates for allogeneic HSCT for acute leukemia and non-malignant disorders by WHO regional offices areas and GNI/cap. Symbols reflect transplant rates (TR; numbers of HSCT by 10 million inhabitants) in participating countries and the respective lnGNI/cap. Colors indicate WHO regional offices areas (see Figure 1); squares indicate acute leukemia, triangles non-malignant disorders. Vertical lines separate countries by World Bank category. (C) Unrelated donor transplant rates by WHO regional offices areas, GNI/cap and presence of an unrelated donor registry. Symbols represent transplant rates; open symbols indicate absence of an unrelated donor registry, full symbols the presence of such a registry and size of symbols numbers of its registered donors. Colors indicate WHO region (see Figure 1). Only countries with unrelated donor HSCT are included. (D) Change in transplant rates (all transplants) from 2006 to 2008 by GNI/cap and WHO regional offices areas. Symbols represent increase or decrease in transplant rates (TR) from 2006 to 2008; colors indicate WHO regional offices areas (see figure 1).
The association was stronger and with a greater explanatory content for autologous HSCT (R2 = 55%) than for allogeneic HSCT (R2 = 49%) as shown for lnGNI/capita (Figure 3A). Explanatory content was higher for unrelated donor than for family donor HSCT. It was highest for acute leukemia (R2 = 49%), lower for non-malignant disorders (R2 =15%) (Figure 3B) and non-existent for non-malignant disorders with HSCT from family donors (R2 = 4%).
Unrelated donor transplant rates were also associated with lnGNI/capita (R2 = 48%), with the presence of an unrelated donor registry in the respective country (R2=30%) and the number of donors in the respective donor registry ( R2=15%). The combined effect of these three factors in a multiple regression reached even R2 =59%. If only countries performing unrelated donor transplants were included in the analysis, the explanatory content reached R2 =72% (Figure 3C). Unrelated cord blood transplant rates were weakly associated with lnGNI/capita (R2 = 24%) and with the presence of a cord blood bank in the respective country (R2=10%). The 264 family donor cord blood transplants were minimally associated (lnGNI/capita: R2 =5%). The three factors lnGNI/capita, presence of an unrelated donor registry and the number of donors in the respective donor registry also exerted a combined effect on total transplant rates (R2=63%; all regions combined) but to a different extent in the different regions. Associations with lnGNI/capita were strongest in the Americas (R2=94%), followed by Asia (R2=67%), Europe (R2=57%) and the Eastern Mediterranean/Africa region (R2=25%).
Trends from 2006 to 2008
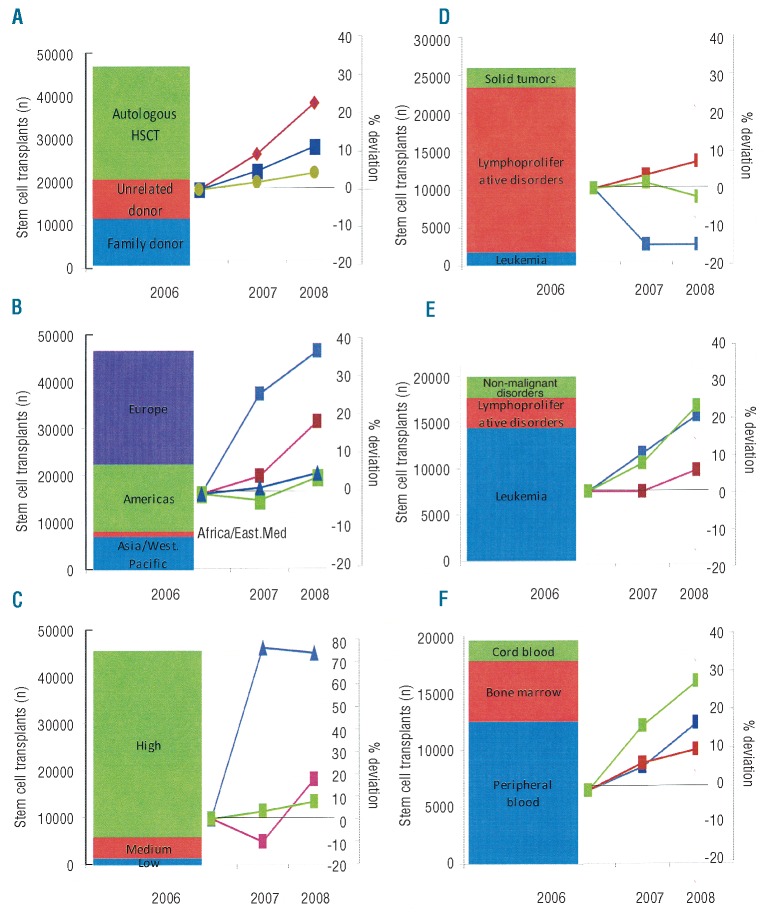
The numbers of HSCT increased from 46,563 in 2006 to 51,536 in 2008 (+10%). The increase in reporting teams from 1,327 in 2006 to 1,407 in 2008 (+6%) was one reason, but even more was the increase of the median number of transplants/year (+26.3%) performed at each center [38 (range 3–180), to 46 (3–421) and 48 (1–389) in 2006, 2007 and 2008, respectively]. Changes differed between regions as well as for main indications, donor types and stem cell sources (Figure 4).
Figure 4.
Total HSCT in 2006 and relative increase or decrease (in %) in 2007 and 2008 according to (A) donor type, (B) WHO region, (C) World Bank Category (high, medium and low income by GNI/capita), (D) autologous transplant indication, (E) allogeneic transplant indication and (F) allogeneic stem cell source
The relative increase was greater for related and unrelated allogeneic HSCT (+17%) than for autologous HSCT (+ 5%; Figure 4A). The greatest increase in absolute and relative numbers was observed in the Asia/Western Pacific region (+39%; Figure 4B) for both allogeneic (+50%) and autologous (+22%) HSCT, followed by Europe (+6% overall; allogeneic +10%, autologous +3%), the Americas (+4% overall; allogeneic +9%, autologous +1%, and the Eastern Mediterranean/Africa (+19) for allogeneic (+11%) and autologous (+34%) HSCT. The relative increase in HSCT numbers was higher in low income countries (Figure 4C) but not in absolute numbers or in transplant rates (see below). The increase in HSCT numbers was predominantly accounted for by unrelated donor HSCT for patients with leukemia in America and Europe, and by family donor HSCT for patients with non-malignant disorders in Asia and the Eastern Mediterranean/Africa.
The numbers of autologous HSCT increased for lymphoproliferative disorders (+8%) and decreased for leukemia (−15%) and solid tumors (−2%) as shown in Figure 4D. The numbers of allogeneic HSCT increased for leukemia (+20%) and non-malignant disorders (+26%; Figure 4E) with divergent trends for myelodysplasia (+26%), acute myeloid leukemia (+23%), acute lymphoblastic leukemia (+27%) and chronic lymphocytic lymphocytic (+24.6%), which all increased, compared to chronic myeloid leukemia (−17%), for which fewer allogeneic HSCT were performed. The numbers of allogeneic HSCT increased for bone marrow failure syndromes (+21%) and other non-malignant disorders (+27%). Changes in use of stem cell source are shown in Figure 4F with the highest relative but not absolute increase in cord blood HSCT. The relatively higher increase in transplant numbers in countries with lower incomes (R2 = 11%) did not translate into a greater increase in transplant rates. In contrast transplant rates were weakly but positively associated with lnGNI/capita (R2 = 3%) (Figure 3D). Linear trend analysis confirmed this with there being a positive and increasing linear trend (P=0.02, total HSCT) for the absolute number of HSCT in high income countries but none for the middle (P=0.57) and low (P=0.35) income countries. The trend was most clearly underpinned for unrelated donor HSCT for acute leukemia in high income countries (P=0.004). There was no association of increase or decrease in transplant rates with change in lnGNI/capita over time (R2 = 1%).
Discussion
This global analysis shows that availability of resources has quantitative and qualitative impacts on the use of HSCT. Transplant rates are higher in high income countries but the difference is not the same for all indications or all donor types. High income countries use autologous and allogeneic HSCT for more indications. They are more likely to use autologous than allogeneic HSCT and unrelated donors than family donors. Transplant rates for autologous HSCT are more likely to be influenced by GNI/capita, as illustrated by the higher explanatory content for autologous HSCT. In contrast, countries with limited resources preferentially restrict the use of HSCT to allogeneic transplants with stem cells from family donors for non-malignant indications or chronic leukemia. The previously described differences between the WHO regional offices areas11 might, therefore, reflect differences in resources rather than in opinions. It is comforting to observe the continued increase in transplant numbers in low income countries, but it remains a concern that transplant rates increased to a greater extent in high income than in middle or low income countries and the gap between the countries is widening.
Transplant rates were associated with GNI/capita for all indications and all donor types but with vast differences in explanatory content and impact. How can these findings be interpreted? A high explanatory content with a strong impact can be considered as a situation with increasing demand without saturation: more patients with acute leukemia will be transplanted in the coming years if the necessary resources, money and donors can be made available. A low explanatory content with a weak impact indicates a different situation. Transplant rates are no longer driven by a higher national income alone. Factors other than availability of resources must come into play. One factor could be related to different beliefs of the medical community on the value of a given therapy in different countries. However, the focus on transplantation from matched family donors for non-malignant disorders and chronic leukemia in lower income countries is suggestive of prioritization in a cost-effectiveness approach. HSCT might be less expensive and equally effective as lifelong treatment with supportive care or expensive drugs in selected patients. There is no need for intensive high cost pre-treatment as is the case for patients with acute leukemia and, the search for a matched family donor requires minimal resources.17–21
The economic aspects of HSCT with its patient-centered approach have traditionally concentrated on costs of the individual procedure for an individual patient.17,22–24 Studies on macro-economic aspects or on cost-effectiveness in individual countries have gained broader acceptance only recently.11,21,22,25 They were triggered in part by some rapid changes in the use of HSCT, such as for breast cancer or chronic myeloid leukemia18,26 and by the rising awareness of the disturbing gap between unlimited requests and limited resources in any health care system.27,28 Availability of resources, governmental support and access to therapy were identified as factors associated with use; availability of resources, evidence, external regulations and positive or negative expectations of transplant physicians as factors associated with diffusion.11,25 These previous findings and the observations in this report form an objective basis for recommendations or guidelines by professional organizations. They point to the different requirements within high or low income countries, hence different cost-effectiveness considerations.20,21,26–28 Unrelated donor transplant rates were associated with GNI/capita, the presence of an unrelated donor registry and the number of registered donors. The association is likely reciprocal; high income countries perform more HSCT in general and are more likely to invest in an unrelated donor registry. Competent authorities will have to balance the advantages and costs of establishing and maintaining a national donor registry with its own local HLA-haplotype distribution with alternative strategies.24,29,30 The even representation of unrelated HSCT in high income countries documents the functioning worldwide exchange of graft material.
Some caveats remain. Data for this survey were collected for the years 2006 to 2008. Patterns might have changed since; differences in indications might reflect different disease prevalences or missing information. Some congenital non-malignant disorders such as immune deficiency syndromes or hemoglobinopathies are highly present in some countries and absent in others.31,32 Evidently, a few teams known to have performed HSCT did choose not to report.13 Data reporting is mandatory by law in some countries, limited to allogeneic HSCT in other countries and not required in other countries. The discrepancy between performed and reported HSCT might be higher for autologous HSCT than for allogeneic HSCT.9,14–16 There is, however, no indication for a systematic bias and more recent data from the European survey are consistent with a widening gap.13
This report gives no information on outcome. Such a report would require additional time and another framework. Outcome is influenced by many factors, including the disease, the pre-treatment, characteristics of the patients and donors, transplant techniques, the transplant team, its quality management system and the income of the country in which the transplants take place.3,5,33–36 Combined analyses on use and outcome are needed to ascertain that those patients with the highest need and the best likelihood of benefiting from a transplant procedure are selected within a given country. Transplant organizations and competent authorities worldwide are currently challenged to implement the WHO guiding principles. The present data provide a platform to begin with. They indicate that one size will not fit all. Regulatory aspects and recommendations on therapy should not only be transparent and consistent but should also be well targeted according to specific cost-effectiveness considerations and needs in the individual countries.36,37
Acknowledgments
The cooperation of all participating teams, countries and organizations with their staff (see Online Supplementary Appendix) is greatly appreciated. Specific thanks to the following: ABMTRR, APBMT: Minako Iida MD, Ph.D., Aichi Medical School, CBMTG, CIBMTR: Kathy Sobocinski M.S. and Xiaobo Zhong, M.S., Medical College of Wisconsin, EBMT: Co-ordination offices in Barcelona, Paris and London and the Austrian Registry (ASCTR), the Czech BMT Registry, the French Registry (SFGM), the German Registry (DRST), the Italian Registry (GITMO), the Dutch Registry (HOVON), the Spanish BMT Registry (GETH), the Swiss Registry (SBST, the Turkish BMT Registry and the British Registry (BSBMT), EMBMT,SBTMO.
Footnotes
The online version of this article has a Supplementary Appendix.
Funding
Funding was solely to support the study; no individual payment was made to any of the persons involved in the study. The activity survey office is in part supported by the Swiss National Research Foundation NFP 63.
CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA76518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U01HL069294 from NHLBI and NCI; a contract HHSH234200637015C with Health Resources and Services Administration (HRSA/DHHS); two grants N00014-06-1-0704 and N00014-08-1-0058 from the Office of Naval Research; and grants from AABB; Aetna; American Society for Blood and Marrow Transplantation; Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Astellas Pharma US, Inc.; Baxter International, Inc.; Bayer HealthCare Pharmaceuticals; Be the Match Foundation; Biogen IDEC; BioMarin Pharmaceutical, Inc.; Biovitrum AB; Blood Center of Wisconsin; Blue Cross and Blue Shield Association; Bone Marrow Foundation; Canadian Blood and Marrow Transplant Group; CaridianBCT; Celgene Corporation; CellGenix, GmbH; Centers for Disease Control and Prevention; Children’s Leukemia Research Association; ClinImmune Labs; CTI Clinical Trial and Consulting Services; Cubist Pharmaceuticals; Cylex Inc.; CytoTherm; DOR BioPharma, Inc.; Dynal Biotech, an Invitrogen Company; Eisai, Inc.; Enzon Pharmaceuticals, Inc.; EBMT; Gamida Cell, Ltd.; GE Healthcare; Genentech, Inc.; Genzyme Corporation; Histogenetics, Inc.; HKS Medical Information Systems; Hospira, Inc.; Infectious Diseases Society of America; Kiadis Pharma; Kirin Brewery Co., Ltd.; The Leukemia & Lymphoma Society; Merck & Company; The Medical College of Wisconsin; MGI Pharma, Inc.; Michigan Community Blood Centers; Millennium Pharmaceuticals, Inc.; Miller Pharmacal Group; Milliman USA, Inc.; Miltenyi Biotec, Inc.; National Marrow Donor Program; Nature Publishing Group; New York Blood Center; Novartis Oncology; Oncology Nursing Society; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; Pall Life Sciences; Pfizer Inc; Saladax Biomedical, Inc.; Schering Corporation; Society for Healthcare Epidemiology of America; StemCyte, Inc.; StemSoft Software, Inc.; Sysmex America, Inc.; Teva Pharmaceutical Industries; THERAKOS, Inc.; Thermogenesis Corporation; Vidacare Corporation; Vion Pharmaceuticals, Inc.; ViraCor Laboratories; ViroPharma, Inc.; and Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institutes of Health, the Department of the Navy, the Department of Defense, or any other agency of the U.S. Government.
EBMT is supported by grants from the corporate members: Amgen Europe, Gilead Sciences UK, Miltenyl Biotec GmbH, Merck Sharp and Dohme, Celgene International SARL, Astellas, Fresenius Biotech GmbH, CaridianBCT Europe NV, Therakos, Cephalon, Gentium SpA, Genzyme, Pierre Fabre Médicament, Alexion Europe – Pfizer, Exem Consulting, Chugai sanofiaventis, Novartis, Hospira, MacoPharma and Millennium
Support from corporate members was solely for keeping the infrastructure of the organizations. The funding organizations and sponsors of the organizations had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; and preparation, review, or approval of the manuscript.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Horowitz MM. Uses and growth of hematopoietic cell transplantation. In “Thomas’ hematopoietic cell transplantation” (Appelbaum FR, Forman SJ, Negrin RS, Blume KG, eds) p 15–21; 2009. Wiley-Blackwell, Oxford [Google Scholar]
- 2.Gratwohl A, Passweg J, Baldomero H, Horisberger B, Urbano-Ispizua A; Accreditation Committee of the European Group for Blood and Marrow Transplantation (EBMT) Economics, health care systems and utilization of haematopoietic stem cell transplants in Europe. Br J Haematol. 2002;117(2):451–68 [DOI] [PubMed] [Google Scholar]
- 3.Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354(17): 1813–26 [DOI] [PubMed] [Google Scholar]
- 4.Appelbaum FR. Hematopoietic-cell transplantation at 50. N Engl J Med. 2007;357(15): 1472–5 [DOI] [PubMed] [Google Scholar]
- 5.Gooley TA, Chien JW, Pergam SA, Hingorani S, Sorror ML, Boeckh M, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363 (22):2091–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halter J, Schüpbach WM, Casali C, Elhasid R, Fay K, Hammans S, et al. Allogeneic hematopoietic SCT as treatment option for patients with mitochondrial neurogastrointestinal encephalomyopathy (MNGIE): a consensus conference proposal for a standardized approach. Bone Marrow Transplant. 2011;46:(3)330–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wagner JE, Ishida-Yamamoto A, McGrath JA, Hordinsky M, Keene DR, Woodley DT, et al. Bone marrow transplantation for recessive dystrophic epidermolysis bullosa. N Engl J Med. 2010;363(14):629–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sorror ML, Sandmaier BM, Storer BE, Franke GN, Laport GG, Chauncey TR, et al. Long-term outcomes among older patients following nonmyeloablative conditioning and allogeneic hematopoietic cell transplantation for advanced hematologic malignancies. JAMA. 2011;306(17):1874–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pasquini MC, Wang Z, Horowitz MM, Hordinsky M, Keene DR, Woodley DT, et al. 2010 report from the Center for International Blood and Marrow Transplant Research (CIBMTR): current uses and outcomes of hematopoietic cell transplants for blood and bone marrow disorders. Clin Transpl. 2010:87–105 [PubMed] [Google Scholar]
- 10.World Health Organization WHO guiding principles on human cell, tissue and organ transplantation. Transplantation. 2010;90(3): 229–33 [DOI] [PubMed] [Google Scholar]
- 11.Sixty-Third World Health Assembly, World Health Organization WHO guiding principles on human cell, tissue and organ transplantation. Cell Tissue Bank. 2010;11(4): 413–9 [DOI] [PubMed] [Google Scholar]
- 12.Gratwohl A, Baldomero H, Aljurf M, Pasquini MC, Bouzas LF, Yoshimi A, et al. Hematopoietic stem cell transplantation: a global perspective. JAMA 2010;303(16): 1617–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baldomero H, Gratwohl M, Gratwohl A, Tichelli A, Niederwieser D, Madrigal A, et al. ; European Group for Blood and Marrow Transplantation EBMT The EBMT activity survey 2009: trends over the past 5 years. Bone Marrow Transplant. 2011;46(4):485–501 [DOI] [PubMed] [Google Scholar]
- 14.Yoshimi A, Suzuki R, Atsuta Y, Iida M, Lu DP, Tong W, et al. Hematopoietic SCT activity in Asia: a report from the Asia-Pacific Blood and Marrow Transplantation Group. Bone Marrow Transplant. 2010;45(12):1682–91 [DOI] [PubMed] [Google Scholar]
- 15.Mohamed SY, Fadhil I, Hamladji RM, Hamidieh AA, Fahmy O, Ladeb S, et al. Hematopoietic stem cell transplantation in the Eastern Mediterranean Region (EMRO) 2008–2009: report on behalf of the Eastern Mediterranean Bone Marrow Transplantation (EMBMT) Group. Hematol Oncol Stem Cell Ther. 2011;4(2):81–93 [DOI] [PubMed] [Google Scholar]
- 16.Moore AS, Shaw PJ, Hallahan AR, Carter TL, Kilo T, Nivison-Smith I, et al. Haemopoietic stem cell transplantation for children in Australia and New Zealand, 1998–2006: a report on behalf of the Australasian Bone Marrow Transplant Recipient Registry and the Australian and New Zealand Children’s Haematology Oncology Group. Med J Aust. 2009;190 (3):121–5 [DOI] [PubMed] [Google Scholar]
- 17.Leelahavarong P, Chaikledkaew U, Hongeng S, Kasemsup V, Lubell Y, Teerawattananon Y. A cost-utility and budget impact analysis of allogeneic hematopoietic stem cell transplantation for severe thalassemic patients in Thailand. BMC Health Serv Res. 2010;10: 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gratwohl A, Baldomero H, Schwendener A, Gratwohl M, Urbano-Ispizua A, Frauendorfer K. Hematopoietic stem cell transplants for chronic myeloid leukemia in Europe--impact of cost considerations. Leukemia. 2007;21(3):383–6 [DOI] [PubMed] [Google Scholar]
- 19.Saussele S, Lauseker M, Gratwohl A, Beelen DW, Bunjes D, Schwerdtfeger R, et al. Allogeneic hematopoietic stem cell transplantation (allo SCT) for chronic myeloid leukemia in the imatinib era: evaluation of its impact within a subgroup of the randomized German CML Study IV. Blood. 2010;115 (10):1880–5 [DOI] [PubMed] [Google Scholar]
- 20.Gajewski JL, Robinson P. Do affluent societies have the only options for the best therapy? Leukaemia. 2007;21(3):387–8 [DOI] [PubMed] [Google Scholar]
- 21.Ruiz-Argüelles GJ. Whither the bone marrow transplant? Hematology. 2010;15(1):1–3 [DOI] [PubMed] [Google Scholar]
- 22.Tan SS, Uyl-de Groot CA, Huijgens PC, Fibbe WE. Stem cell transplantation in Europe: trends and prospects. Eur J Cancer. 2007;43(16):2359–65 [DOI] [PubMed] [Google Scholar]
- 23.Ashfaq K, Yahaya I, Hyde C, Andronis L, Barton P, Bayliss S, et al. Clinical effectiveness and cost-effectiveness of stem cell transplantation in the management of acute leukaemia: a systematic review. Health Technol Assess. 2010;14(54):1–141 [DOI] [PubMed] [Google Scholar]
- 24.Costa V, McGregor M, Laneuville P, Brophy JM. The cost-effectiveness of stem cell transplantations from unrelated donors in adult patients with acute leukemia. Value Health. 2007;10(4):247–55 [DOI] [PubMed] [Google Scholar]
- 25.Gratwohl A, Schwendener A, Baldomero H, Gratwohl M, Apperley J, Niederwieser D, et al. Changes in the use of hematopoietic stem cell transplantation: a model for diffusion of medical technology. Haematologica. 2010;95 (4):637–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berry DA, Ueno NT, Johnson MM, Lei X, Caputo J, Rodenhuis S, et al. High-dose chemotherapy with autologous stem-cell support as adjuvant therapy in breast cancer: overview of 15 randomized trials. J Clin Oncol. 2011;29(24):3214–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sinha SR, Barry M. Health technologies and innovation in the global health arena. New Engl J Med. 2011;365(9):779–82 [DOI] [PubMed] [Google Scholar]
- 28.Majhail NS, Omondi NA, Denzen E, Murphy EA, Rizzo JD. Access to hematopoietic cell transplantation in the United States. Biol Blood Marrow Transplant. 2010;16(8):1070–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaw BE, Arguello R, Garcia-Sepulveda CA, Madrigal JA. The impact of HLA genotyping on survival following unrelated donor haematopoietic stem cell transplantation. Br J Haematol. 2010;150(3):251–8 [DOI] [PubMed] [Google Scholar]
- 30.Confer D, Robinett P. The US National Marrow Donor Program role in unrelated donor hematopoietic cell transplantation. Bone Marrow Transplant. 2008;42 (Suppl 1):S3–S5 [DOI] [PubMed] [Google Scholar]
- 31.Wang WC. Sickle cell anemia and other sickling syndromes. In: Wintrobe’s Clinical Hematology (Greer JP, Foerster J, Rodgers GM, Paraskevas F, Glader B, Arber DA, Means RT, eds). p 1038–1082Wolters Kluwer, Lippincott Williams & Wilkins, Philadelphia, Baltimore, New York, London: 2009 [Google Scholar]
- 32.Borgna-Pignatti C, Galanello R. Thalassemias and related disorders. In: Wintrobe’s Clinical Hematology (Greer JP, Foerster J, Rodgers GM, Paraskevas F, Glader B, Arber DA, Means RT, eds). p 1083–1131 Wolters Kluwer, Lippincott Williams & Wilkins, Philadelphia, Baltimore, New York, London: 2009 [Google Scholar]
- 33.Gratwohl A, Brand R, Niederwieser D, Baldomero H, Chabannon C, Cornelissen J, et al. Introduction of a quality management system and outcome after hematopoietic stem-cell transplantation. J Clin Oncol. 2011;29(15):1980–6 [DOI] [PubMed] [Google Scholar]
- 34.Gyurkocza B, Rezvani A, Storb RF. Allogeneic hematopoietic cell transplantation: the state of the art. Expert Rev Hematol. 2010;3(3):285–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gratwohl A. The EBMT risk score. Bone Marrow Transplant. 2012;47(6):749–56 [DOI] [PubMed] [Google Scholar]
- 36.Giebel S, Labopin M, Ehninger G, Beelen D, Blaise D, Ganser A, et al. Association of Human Development Index with rates and outcomes of hematopoietic stem cell transplantation for patients with acute leukemia. Blood. 2010;116(1):122–8 [DOI] [PubMed] [Google Scholar]
- 37.Brawley O, Byers T, Chen A, Pignone M, Ransohoff D, Schenk M, et al. New American Cancer Society process for creating trustworthy cancer screening guidelines. JAMA 2011; 306(22):2495–9 [DOI] [PubMed] [Google Scholar]