Abstract
Functional magnetic resonance imaging (fMRI) studies have shown dysfunction in key areas associated with the thalamocortical circuit in patients with schizophrenia. This study examined the functional connectivity involving the frontal-thalamic circuitry during a spatial focusing-of-attention task in 18 unmedicated patients with schizophrenia and 38 healthy controls. Functional connectivity was analyzed by assigning seed regions (in the thalamic nuclei (mediodorsal nucleus (MDN), pulvinar, anterior nucleus (AN)), the dorsolateral prefrontal cortex (Brodmann areas 9 and 46), and the caudate), and correlating their respective activity with that in the non-seed regions voxel-wise. Functional connectivity analysis demonstrated that functional connectivity was significantly impaired in patients e.g., between the right pulvinar and regions such as the prefrontal and temporal cortices and the cerebellum. On the other hand, enhanced functional connectivity was found in patients e.g., between the AN and regions such as the prefrontal and temporal cortices. In addition, the patients had significantly lower task performance and less (but non-significant) brain activation than those of controls. These results revealed disturbed functional integration in schizophrenia, and suggested that the functional connectivity abnormalities in the thalamocortical circuitry, especially the frontal-thalamic circuitry, may underlie the attention deficits in schizophrenia patients. Further, this study suggested that functional connectivity analysis might be more sensitive than brain activation analysis in detecting the functional abnormalities in schizophrenia.
Keywords: Thalamocortical circuitry, Functional connectivity, Spatial attention
Introduction
Rather than a disorder of focal brain abnormalities, much evidence has suggested that schizophrenia may be characterized as a failure of functional integration in the brain (Friston 2002) or a disorder of dysconnectivity in a disturbed neural network (Andreasen et al 1997; Bullmore et al 1997; Friston 1998; Friston 2002; Friston and Frith 1995; Gold JM and DR. 1991; McGlashan and Hoffman 2000; McGuire and Frith 1996; Stephan et al 2006). Cognitive deficits in schizophrenia are associated with disruptions of defined information processing pathways in the brain encompassing such neural network in the frontal, temporal, and parietal cortices and involving the striatum, thalamus and cerebellum (Weinberger et al 2001).
Schizophrenia patients often suffer from attention deficits, which makes them hard to screen out irrelevant sensory input and their attention system is impaired in the allocation of controlled attentional processes to an important, task-relevant stimulus (Callaway and Naghdi 1982). Dysfunction within highly recursive circuits involving the frontal cortex, striatum and thalamus may help explain the attentional deficits associated with schizophrenia (Swerdlow and Koob 1987). Functional magnetic resonance imaging (fMRI) studies have revealed dysfunction in the frontal-striatal-thalamic circuitry in schizophrenia patients for sensorimotor gating during attention tasks (Hazlett et al 2008; Kumari et al 2007; Kumari et al 2003), and functional abnormalities in the thalamus and the dorsolateral prefrontal cortex (DLPFC) in schizophrenia during working memory tasks (Andrews et al 2006; Barch et al 2002). In addition, it has been reported that impairment in the prefrontal-thalamic-cerebellar circuitry may lead to deficits involving executive function in schizophrenia patients (Andreasen et al 1996).
There has been ample evidence for structural and functional disconnection in schizophrenia (Andreasen et al 1999; Friston 2005; Hoffman and McGlashan 2001). Magnetic resonance imaging (MRI) in first-episode schizophrenia patients has indicated that fiber pathways in the anterior limb of the internal capsule, which connect midline and anterior thalamic nuclei to the prefrontal cortex, are reduced in volume (Lang et al 2006). A number of diffusion tensor imaging (DTI) studies have reported white matter abnormalities indicative of abnormal structural connectivity in schizophrenia (Hulshoff Pol et al 2004; Kumra et al 2005; Szeszko et al 2005). More DTI clinical studies have shown a reduction in fractional anisotropy (FA) within the white matter fibers in schizophrenia between the prefrontal cortex and the thalamus (Kunimatsu et al 2008) and between the thalamus and the cerebellum (Magnotta et al 2008).
In neuroimaging, functional connectivity refers to the correlations between spatially remote neurophysiological events (Friston and Büchel 2004). Disrupted functional connectivity between temporal and frontal regions in schizophrenia patients has been reported by positron emission tomography (PET) and fMRI studies (Friston et al 1996; Lawrie et al 2002; Meyer-Lindenberg et al 2005). Abnormal Gamma synchrony in schizophrenia patients during sensory processing has been revealed by MEG/EEG (Lee et al 2003) and abnormal patterns of functional interactions in schizophrenia by EEG (Breakspear et al 2003). In addition, antipsychotic medication may alter cerebellar functional connectivity with the thalamic and prefrontal regions in schizophrenia patients (Stephan et al 2001).
The thalamocortical connectivity is of special interest in schizophrenia research because the thalamus plays an important role in information transmission and processing in the brain (Welsh et al 2008). The thalamus is like a hub in the neural network and thalamic nuclei such as the anterior nucleus (AN), the centromedian nucleus (CMN), the mediodorsal nucleus (MDN) and the pulvinar have strong reciprocal connections with cerebral cortex. The MDN has interconnections with the prefrontal cortex (PFC) and plays a crucial role in attention, planning, abstract thinking, multi-tasking and active memory (Buchsbaum et al 2006). The pulvinar has connections with the visual and somatosensory cortex, cingulate, posterior parietal and prefrontal cortex and is involved in attention (Shipp 2003). Thalamus nuclei have been implicated in the pathophysiology of schizophrenia (Clinton and Meador-Woodruff 2004). Thalamic dysfunction and thalamocortical dysconnectivity may lead to failure in information transmission and processing, which is attributed to cognitive deficits, especially attention deficit, in schizophrenia patients (Andreasen et al 1998; Jones 1997).
Hypofrontality has been commonly but inconsistently found in schizophrenia (Ebmeier et al 1995; Walter et al 2003). Accumulated evidence has implicated the DLPFC in the pathophysiology of schizophrenia and revealed the compromised dorsolateral prefrontal circuit in schizophrenia (Bunney and Bunney 2000). State-related abnormalities exist in the dorsolateral-prefrontal-to-superior/middle-temporal-gyrus connectivity in those at high risk with psychotic symptoms as reported in schizophrenia studies (Friston et al 1996; Frith et al 1995; Lawrie et al 2002).
Diminished thalamic-prefrontal connectivity may contribute to the cognitive deficits in early stages of schizophrenia (Lambe et al 2007). Thalamocortical connectivity has been studied in normal controls (Buchsbaum et al 2006; Stein et al 2000) and reduced thalamocortical connectivity (including functional connectivity between the MDN and PFC) was found in medicated schizophrenia patients in a resting-state low-frequency BOLD fluctuation study (Welsh et al 2008). Further, decreased connectivity between right medial prefrontal regions and contralateral cerebellum was found which reflected diminished prefrontal-thalamic-cerebellar network in subjects at high risk of schizophrenia (Whalley et al 2005). However, few fMRI studies investigated the functional connectivity in the thalamocortical circuitry during attention task in schizophrenia. In this study, we assessed functional thalamocortical connectivity in unmedicated schizophrenia patients with an fMRI spatial focusing-of-attention task in order to identify functional abnormalities in the thalamocortical circuitry in schizophrenia.
Methods
Subjects
Eighteen unmedicated schizophrenia patients (7 females, 11 males; age: 31 ± 10 years) who met DSM-IV schizophrenia criteria were recruited for this study. The patients were withdrawn from psychotropic and/or antidepressant medications for the study, and they remained free of psychotropic medications for 2 weeks and antidepressant medications for 6 weeks, and none of them had used depot medications. Patients with a history of bipolar (Type 1) disorder, schizoaffective disorder, substance dependence and organic mental syndrome were excluded. Thirty eight healthy subjects (16 females, 22 males; age: 31 ± 8 years) served as controls. All participants were screened for severe medical illness, neurological illness (such as head trauma, central nervous system (CNS) neurological disease and seizure disorder) and substance abuse (within the past 6 months of entry into the study). They also had a negative urine toxicology screen and females a negative pregnancy test on scan day. All subjects were without metallic implants and eligible for MRI scan. They provided written informed consent in accordance with the Mount Sinai School of Medicine and James J. Peters Institutional Review Board guidelines. There were no significant differences in age and gender between the patient and control group (Table 1).
Table 1.
Subject demographic characteristics
| Controls | Patients | t-test (p value) | Chi-square test (p value) | |
|---|---|---|---|---|
| Sample size | 38 | 18 | ||
| Age | 30.5 ± 8.1 | 31.7 ± 10.0 | 0.613 | |
| Sex (F/M) | 16/22 | 7/11 | 0.823 | > 0.75 |
| Education | 16.3 ± 2.0 (n=29) | 13.0 ± 2.2 (n=5*) | 0.002 | |
| Handedness (R/L/Mx) | 31/4/1 (n=36) | 13/2/2 (n=17) | 0.246 | > 0.75 |
F: Females; M: Males; L: Left; R: Right; Mx: Mixed;
Few patients had their education information available.
fMRI Data
The patients and controls were scanned on a head-dedicated Siemens Allegra 3T MRI scanner at MSMC. T1-weighted MP-RAGE (Magnetization Prepared Rapid Gradient Echo) imaging was acquired (208 slices with slice thickness=0.82 mm, matrix size=256×256×208, FOV=21 cm, TR=2500 ms, TE=4.38 ms, TI=1100 ms and an 8° flip angle FLASH acquisition) for high resolution structural images with good gray/white matter contrast.
Echoplanar images were acquired with a multi-slice 2D-EPI sequence (128×28 matrix, TR=2s, TE=40ms, flip angle = 90°, FOV= 23 cm, slice thickness = 5 mm, skip = 2.5 mm) yielding 14 slices. During the BOLD fMRI acquisition, the subjects performed a block-design visual spatial attention task. They viewed four types of stimuli positioned to the right or left of the central fixation point: (1) left hemifield-large letter display with flanking letters; (2) left hemifield-small letter display with flanking letters; (3) right hemifield-large letter display with flankers; (4) right hemifield-small letter display with flankers. For details of the paradigm, see (Buchsbaum et al 2006). Briefly, there were four runs in the paradigm, each lasted for 264 s. Each run began with a 24 s period of blank, the stimuli were presented at 2 s intervals (total stimulus block time = 24 s), and there was a rest interval of 24 s afterward. During the task, the subjects clicked on a mouse button (modified for use with MRI) each time he detected the large letter target, ignored the flanking letters, and pressed on the right button for a right-sided target and the left button for a left-sided target.
Image Processing and Data Analysis
SPM analysis was performed with FSL tools (Smith et al 2004). fMRI data were preprocessed with motion correction using MCFLIRT (Jenkinson et al 2002), non-brain removal using Brain Extraction Tool (Smith 2002), spatial smoothing with Gaussian profile filter (full-width-half-maximum FWHM=5 mm) and high-pass temporal filtering with Gaussian-weighted running line detrending (cutoff=70 s). fMRI images were co-registered to their structural MRI with a 7 degrees-of-freedom (DOF) linear transformation followed by alignment to the MNI brain template using a 12 DOF linear fit.
The responses to small letters surrounded by flankers were compared with those to the big isolated letters. Functional connectivity was analyzed by computing the Pearson correlations between the seed regions (the mediodorsal nucleus (MDN), pulvinar, anterior nucleus (AN), BA9, BA46 and the caudate) and non-seed regions voxel-wise. The seed regions in the DLPFC (BAs 9 and 46) and the caudate were used to detect abnormal functional connectivity in the frontal-striatal-thalamic circuitry in schizophrenia patients. The locations of the seed regions are listed in Table 2.
Table 2.
Seed regions and the corresponding coordinates
| MNI coordinates | |||
|---|---|---|---|
| Left | Right | ||
| Thalamus Nucleus | MDN | −6 −22 8 | 6 − 22 8 |
| Pulvinar | −12 −32 6 | 12 − 32 6 | |
| AN | −6 −8 2 | 6 − 8 2 | |
| DLPFC | BA 9 | −22 40 38 | 22 40 38 |
| BA 46 | −32 46 22 | 32 46 22 | |
| Caudate | −12 12 12 | 12 12 12 | |
MDN: mediodorsal nucleus; AN: anterior nucleus; DLPFC: dorsolateral prefrontal cortex
The connectivity difference between controls and patients at each voxel was examined by the following z-test statistic (Rosner 2006):
where the r is the correlation coefficient of controls or patients at each voxel, the denominator σ is the standard error of the difference between two independent transformed correlations:
and zr is the Fisher z transformation of a correlation coefficient:
Results
Behavioral Performance
Among the 18 patients taking the fMRI scan, 3 failed to respond to the stimuli using the mouse during the attention task. For the rest patients, their task performance (measured by the percentage of correct answers) was significantly lower (p<0.05) than that of controls (the mean performance of the patients is 79.8%, the mean performance of controls is 92.4%, t-value=4.77, df=97, p=0.00001).
Functional Activations
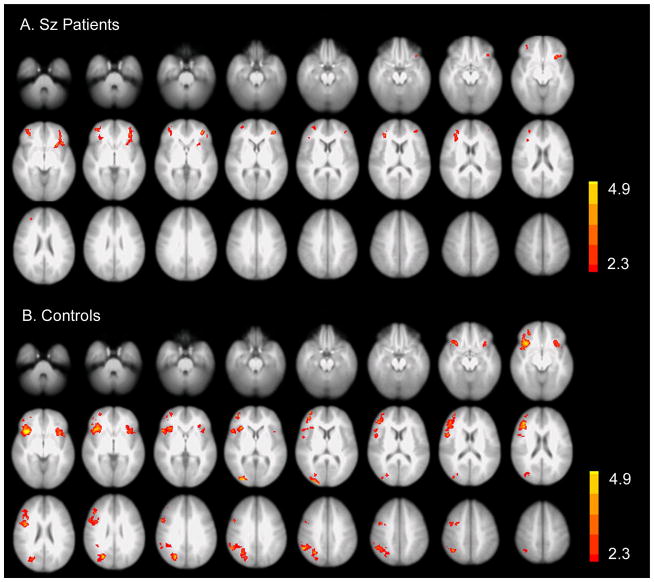
In response to the small – (subtracted by) big letter condition, the patients had much less brain activation in the PFC (especially the right PFC) and no activation in other regions such as the parietal cortex, compared with controls (Figure 1). However, the differences of functional activations between patients and controls were not significant.
Figure 1.
Brain activation of patients and controls in response to small – big letter condition (p<0.05, corrected). A. Activation in schizophrenia patients. B. Activation in controls. Controls have more activation in the right prefrontal and parietal regions than patients, but the difference was non-significant (p>0.05, corrected). Sz: schizophrenia.
Functional Connectivity
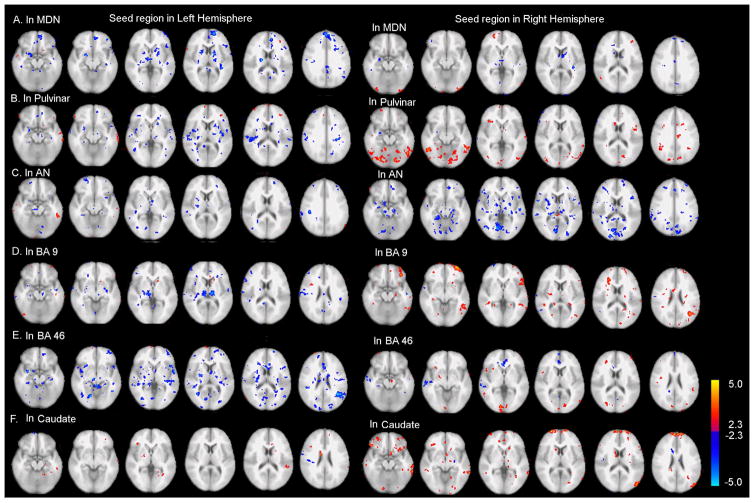
Compared with controls, functional connectivity was significantly reduced in patients between the right MDN and the PFC; between the right pulvinar and regions such as the PFC, temporal cortex and the cerebellum; between the right BA 9 and regions such as the PFC, temporal and parietal cortices; between the right BA 46 and regions such as the parietal cortex; between the bilateral caudates and regions such as the PFC and temporal cortex (Figure 2).
Figure 2.
Group difference of functional connectivity between patients and controls. Seed regions are in the thalamus nuclei (MDN, pulvinar, AN), DLPFC (BAs 9 and 46) and the caudate. Red clusters indicate more functional connectivity in controls than in patients; blue clusters indicate less functional connectivity in controls than in patients. The connectivity difference was displayed at p<0.025 (uncorrected) with a minimum of 10 voxels for each cluster. MDN: mediodorsal nucleus; Pulv: Pulvinar; AN: anterior nucleus; CMN: centromedian nucleus; DLPFC: dorsolateral prefrontal cortex.
In addition, enhanced functional connectivity was found in patients between the left MDN and PFC, between the AN and regions such as the PFC, between the left BA 9 and the thalamus, and between the left BA 46 and regions such as the temporal cortex (Figure 2).
Discussion
In this study, significantly lower behavioral performance, less (but non-significant) brain activity and significant functional connectivity abnormalities in the thalamocortical circuitry were found in schizophrenia patients during a spatial attention task. These results indicated attention deficits and disturbed functional integrity in schizophrenia. Some of these findings, e.g., reduced connectivity between the thalamus nuclei and the prefrontal cortex, are consistent with the findings of the resting-state low-frequency BOLD fluctuations study (Welsh et al 2008).
Abnormal Functional Connectivity in the Thalamocortical Circuitry in Schizophrenia
Among the thalamus nuclei that we used as seed regions in this study, we found the reduced functional connectivity (between the right MDN and PFC, between the right pulvinar and the PFC) and the enhanced functional connectivity (e.g., between the AN and PFC) in schizophrenia patients (Figure 2). In schizophrenia, the MDN is critical in the attentional “selective engagement” system that has an impact on semantic functions (Crosson 1999). The loss of correlation in temporal fluctuations in BOLD signal may arise from the degradation of the connection between the MDN and PFC which may lead to attention deficits in schizophrenia. Reduced functional connectivity between the right pulvinar and the PFC suggested that the information transmission through such connections may be impaired, which may lead to the visual, auditory and even language disturbances in schizophrenia patients. The above results are consistent with the findings from a FDG-PET study using a verbal learning task (Mitelman et al 2005). The AN, which receives effects from mammillary bodies and projects to the cingulate gyrus, is involved in modulating alertness, learning and memory (Aggleton and Brown 1999). Interconnected with the striatum and the cerebral cortex, the CMN is involved in attention and arousal (Powell and Cowan 1967). The reduced functional connectivity between the thalamic nuclei and the PFC suggests that the information transmission and filtering is affected not only for attention and arousal, but also for learning and memory in schizophrenia patients. On the other hand, the enhanced functional connectivity between the thalamic nuclei and the PFC may reflect the compensatory mechanism for prefrontal dysfunction due to increased demands on executive resources during the attention task (Schirmer et al 2009). Taken together, the abnormal thalamocortical connectivity found in the study reveals functionally altered connection in the thalamocortical circuitry in schizophrenia, which supports the concept that schizophrenia may involve faulty processing or filtering of sensory signals form input to the cortex via the thalamus (Andreasen et al 1996; Bunney and Bunney 2000; Carlsson 2006).
For seed regions other than the thalamus nuclei, we found the reduced functional connectivity (e.g., between the caudate and regions such as the PFC and temporal cortex) and enhanced functional connectivity (e.g., between the left BA 9 and the thalamus) (Figure 2) in patients. Some results of this study, e.g., reduced functional connectivity between the PFC and the thalamus in patients are consistent with the findings in a resting-state fMRI study (Zhou et al 2007). The abnormally reduced or enhanced functional connectivity to the DLPFC reveals improper functional integration in schizophrenia. Prefrontal-parietal disconnectivity in schizophrenia has been suggested by deficits in working memory processing (Kim et al 2003). The reduced functional connectivity between the bilateral caudates and PFC and between the right caudate and thalamus indicate the disconnection in frontal-striatal-thalamo-cortical loops in schizophrenia patients which may lead to dysfunction or dysregulation in sensorimotor gating during attention task (Hazlett et al 2008). Further, the reduced connectivity between the right BA 46 and cerebellum (via the thalamus) is seen in a report where decreased connectivity between the right medial PFC and contralateral cerebellum revealed diminished prefrontal-thalamic-cerebellar network in subjects at high risk of schizophrenia (Whalley et al 2005) and two other schizophrenia studies (Schlosser et al 2003; Stephan et al 2001). Dysfunction of fronto-cerebellar networks may result in abnormal synchrony of mental processing in schizophrenia (Andreasen et al 1999). On the other hand, the enhanced functional connectivity may reflect the compensatory mechanism for prefrontal dysfunction or an over-engagement of brain regions in response to the attention task.
Taken together, the altered functional connectivity found in this study especially the altered functional connectivity between the thalamic nuclei and PFC may reveal disrupted feedback regulation of sensory filtering at a thalamic level and diminished cortical processing of incoming information from the ascending arousal system (Lambe et al 2007).
Synaptic Plasticity and Disconnectivity in Schizophrenia
Altered brain connectivity that leads to the failure of functional integration in schizophrenia may result from structural changes of white matter fibers at the cellular level, or from functional changes in brain networks, i.e., abnormal control of synaptic plasticity at the synaptic level, or both (Stephan et al 2006). Functional integration is mediated by functional connections between neuronal systems in the brain and the pattern of connectivity is a function of epigenetic activity and experience-dependent plasticity (Friston 2002). Impairment of synaptic plasticity has a large impact on long-range connections in the developing brain because functional coupling is a function of experience-dependent synaptic plasticity (Zhang and Poo 2001) and the strength of functional coupling between two neurons determines whether their connection could survive developmental pruning (Hua and Smith 2004). Dysconnectivity due to impaired experience-dependent synaptic plasticity supports that schizophrenia is explained by the interactions between genes and environment (Sullivan et al 2003).
The abnormalities in functional connectivity in schizophrenia may be a result of abnormal synaptic plasticity: long-term potentiation (LTP) induces growth of dendrites and upregulation of dendritic spines (Monfils et al 2004), while blocking LTP and inducing long-term depression (LTD) decreases dendritic length and spine density (Monfils and Teskey 2004). Pharmacological studies show that impairments of synaptic plasticity induced symptoms of schizophrenia-like cognitive deficits in healthy volunteers (Kapur 2003), psychotic symptoms and cognitive deficits induced by N-methyl-D-aspartate (NMDA) antagonists are similar to those of schizophrenia (Domino et al 2004) and abnormal event-related potential (ERPs) of sensory learning in schizophrenia patients can be mimicked by pharmacological NMDA blockage in healthy controls (Kreitschmann-Andermahr et al 2001). Further, Harrison and Weinberger (2005) identified the seven candidate genes for schizophrenia and six of them are related to glutamanergic synapses. They concluded that these candidate genes predispose to the central pathological processes: an alteration in synaptic plasticity (Harrison and Weinberger 2005). These results suggested that abnormal synaptic plasticity or abnormal synaptic regulation may be the main cause of schizophrenia.
The abnormalities in the frontal-striatal-thalamic functional connectivity may reflect the dysregulation of glutamatergic projections from the thalamus to the prefrontal cortex proposed as a component for the NMDA receptor hypofunction in schizophrenia (Clinton and Meador-Woodruff 2004; Olney and Farber 1995; Stone et al 2007). Postmortem histopathologic studies demonstrated reduced spine densities and smaller dendritic arbors on the pyramidal cells of the prefrontal cortex in schizophrenia (Black et al 2004; Garey et al 1998; Glantz and Lewis 2000). In addition, reduced densities of thalamocortical projection neurons were reported in the bilateral anteroventral thalamus nucleus in schizophrenic subjects (Danos et al 1998), which might suggest that impairments at both the synaptic and cellular level may contribute to the thalamocortical disconnectivity in schizophrenia. Further, pharmacological studies indicated that antipsychotic and hycocretin drugs excite midline-intralaminar thalamic neurons (Bayer et al 2002; Cohen et al 1998; Deutch et al 1995), hypocretin excites thalamocortical terminals in PFC (Lambe and Aghajanian 2003; Lambe et al 2005), and nicotine excites nicotinic acetylcholine receptors on thalamocortical terminals which increases glutamate release onto prefrontal layer V payramidal neurons (Lambe et al 2005) and enhances attention. On the other hand, stress and activation of glucocorticoid pathways may lead to apical dendritic atrophy and the associated loss of excitatory synapses, which exacerbates symptoms of schizophrenia (Jones and Fernyhough 2007).
Methodological Issues
In contrast to functional connectivity assessed during tasks, a number of connectivity studies examined functional correlations in healthy subjects (Lowe et al 1998; Xiong et al 1999) and schizophrenia patients (Welsh et al 2008; Zhou et al 2007) at rest. During the resting state, there is spontaneous firing of neurons which is followed by BOLD signal changes (Golanov et al 1994). Functional connectivity analysis during the resting state reveals a default mode neural network in the rest brain (Greicius et al 2003).
Later connectivity studies used conventional task activation methods and examined regional time courses over periods of task and baseline conditions and there are some concerns on whether functional connectivity measured during task can provide any more information than standard activation maps due to task related co-activity (Whalley et al 2005). To address these issues, several approaches have been undertaken: e.g., exclude baseline conditions and examining correlations during active periods only (Just et al 2004); or, remove effects of task activation from the data (Arfanakis et al 2000).
Further, Greicius et al. demonstrated that the connectivity patterns obtained during a visual processing task (a checkerboard paradigm) are virtually identical to those obtained during rest which confirmed that the default mode neural network during rest is not disrupted by low-level processing tasks (Greicius et al 2003). However, this may not hold true for high-level processing tasks such as the spatial focus-of-attention task in this study, which may help explain that the similarities and differences between the results of thalamocortical connectivity obtained from a resting-state fMRI study (Welsh et al 2008) and this one.
In addition, aside from the seed-region based voxel-wise correlation approach, there are other methods in functional connectivity analysis such as principal component analysis (PCA), eigenimage analysis and independent component analysis (ICA) (Buchel and Friston 1997; Friston et al 1997; Friston et al 1993; McKeown et al 1998). However, such multivariate methods involve issues such as data dimension reduction and have vulnerability to noise. In addition to functional connectivity, effective connectivity, i.e., the influence of a neural system exerts over another (Friston and Büchel 2004), and effective connectivity analysis deserve further investigation.
Limitations
Due to the difficulty in recruiting unmedicated patients, only 18 patients were finally recruited. The relatively smaller sample size of patients compared with that of controls (18 vs. 38) is a limitation of this research. The larger sample size of controls may influence the group difference, and a higher threshold could be applied to the group difference to adjust such effect. In addition, limited knowledge of prior treatment of the patients in this study may prevent us from understanding their brain function and behavior. Further study with more balanced sample sizes of patients and controls, better knowledge of prior treatment of the patients, and a higher threshold on group difference could be explored in the future.
Another limitation of functional connectivity analysis is that since the results are Pearson correlation values, the direction of a functional connectivity between two ROIs is not clear. The effective connectivity analysis is often based on causal models and could be explored in our future studies.
In summary, we found significantly lower behavioral performance, less (but non-significant) brain activity and functional connectivity abnormalities in unmedicated schizophrenia patients during a spatial attention task. The functional connectivity abnormalities (in the frontal-striatal-thalamic circuitry) may reflect the neurophysiological basis of the cognitive deficits in schizophrenia. Further, this study suggested that functional connectivity analysis might be more sensitive than brain activation analysis in detecting the functional abnormalities in schizophrenia.
Acknowledgments
We are grateful to the Department of Radiology at the Mt. Sinai Medical Center for their collaboration in image acquisition. We are also grateful to Chelain Goodman for running subjects and acquiring the data. This work was partly supported by NIH grant MH-60023, and the Natural Science Foundation of China (Grant No. 81071211).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggleton JP, Brown MW. Episodic memory, amnesia, and the hippocampal-anterior thalamic axis. Behav Brain Sci. 1999;22:425–444. discussion 444–489. [PubMed] [Google Scholar]
- Andreasen N, O’Leary D, Cizadlo T, Arndt S, Rezai K, Boles Ponto L, et al. Schizophrenia and cognitive dymetria: A positron-emission tomography study of dysfunctional prefrontal-thalamic-cerebellar circuitry. Proc Natl Acad Sci USA. 1996;93:9985–9990. doi: 10.1073/pnas.93.18.9985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen N, O’Leary D, Flaum M, Nopoulos P, Watkins G, Boles-Ponto L, et al. Hypofrontality in schizophrenia: distributed dysfunctional circuits in neuroleptic-naive patients. The Lancet. 1997;349:1730–1734. doi: 10.1016/s0140-6736(96)08258-x. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Nopoulos P, O’Leary DS, Miller DD, Wassink T, Flaum M. Defining the phenotype of schizophrenia: cognitive dysmetria and its neural mechanisms. Biol Psychiatry. 1999;46:908–920. doi: 10.1016/s0006-3223(99)00152-3. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Paradiso S, O’Leary DS. “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull. 1998;24:203–218. doi: 10.1093/oxfordjournals.schbul.a033321. [DOI] [PubMed] [Google Scholar]
- Andrews J, Wang L, Csernansky JG, Gado MH, Barch DM. Abnormalities of thalamic activation and cognition in schizophrenia. Am J Psychiatry. 2006;163:463–469. doi: 10.1176/appi.ajp.163.3.463. [DOI] [PubMed] [Google Scholar]
- Arfanakis K, Cordes D, Haughton VM, Moritz CH, Quigley MA, Meyerand ME. Combining independent component analysis and correlation analysis to probe interregional connectivity in fMRI task activation datasets. Magn Reson Imaging. 2000;18:921–930. doi: 10.1016/s0730-725x(00)00190-9. [DOI] [PubMed] [Google Scholar]
- Barch DM, Csernansky JG, Conturo T, Snyder AZ. Working and long-term memory deficits in schizophrenia: is there a common prefrontal mechanism? Journal of abnormal psychology. 2002;111:478–494. doi: 10.1037//0021-843x.111.3.478. [DOI] [PubMed] [Google Scholar]
- Bayer L, Eggermann E, Saint-Mleux B, Machard D, Jones BE, Muhlethaler M, et al. Selective action of orexin (hypocretin) on nonspecific thalamocortical projection neurons. The Journal of Neuroscience. 2002;22:7835–7839. doi: 10.1523/JNEUROSCI.22-18-07835.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JE, Kodish IM, Grossman AW, Klintsova AY, Orlovskaya D, Vostrikov V, et al. Pathology of layer V pyramidal neurons in the prefrontal cortex of patients with schizophrenia. The American journal of psychiatry. 2004;161:742–744. doi: 10.1176/appi.ajp.161.4.742. [DOI] [PubMed] [Google Scholar]
- Breakspear M, Terry JR, Friston KJ, Harris AW, Williams LM, Brown K, et al. A disturbance of nonlinear interdependence in scalp EEG of subjects with first episode schizophrenia. Neuroimage. 2003;20:466–478. doi: 10.1016/s1053-8119(03)00332-x. [DOI] [PubMed] [Google Scholar]
- Buchel C, Friston KJ. Modulation of connectivity in visual pathways by attention: cortical interactions evaluated with structural equation modelling and fMRI. Cerebral cortex (New York, NY. 1997;7:768–778. doi: 10.1093/cercor/7.8.768. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Buchsbaum BR, Chokron S, Tang C, Wei TC, Byne W. Thalamocortical circuits: fMRI assessment of the pulvinar and medial dorsal nucleus in normal volunteers. Neuroscience Letters. 2006;404:282–287. doi: 10.1016/j.neulet.2006.05.063. [DOI] [PubMed] [Google Scholar]
- Bullmore ET, Frangou S, Murray RM. The dysplastic net hypothesis: an integration of developmental and dysconnectivity theories of schizophrenia. Schizophr Res. 1997;28:143–156. doi: 10.1016/s0920-9964(97)00114-x. [DOI] [PubMed] [Google Scholar]
- Bunney WE, Bunney BG. Evidence for a compromised dorsolateral prefrontal cortical parallel circuit in schizophrenia. Brain Res Brain Res Rev. 2000;31:138–146. doi: 10.1016/s0165-0173(99)00031-4. [DOI] [PubMed] [Google Scholar]
- Callaway E, Naghdi S. An information processing model for schizophrenia. Arch Gen Psychiatry. 1982;39:339–347. doi: 10.1001/archpsyc.1982.04290030069012. [DOI] [PubMed] [Google Scholar]
- Carlsson A. The neurochemical circuitry of schizophrenia. Pharmacopsychiatry. 2006;39(Suppl 1):10–14. doi: 10.1055/s-2006-931483. [DOI] [PubMed] [Google Scholar]
- Clinton SM, Meador-Woodruff JH. Thalamic dysfunction in schizophrenia: neurochemical, neuropathological, and in vivo imaging abnormalities. Schizophr Res. 2004;69:237–253. doi: 10.1016/j.schres.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Cohen BM, Wan W, Froimowitz MP, Ennulat DJ, Cherkerzian S, Konieczna H. Activation of midline thalamic nuclei by antipsychotic drugs. Psychopharmacology (Berl) 1998;135:37–43. doi: 10.1007/s002130050483. [DOI] [PubMed] [Google Scholar]
- Crosson B. Subcortical mechanisms in language: lexical-semantic mechanisms and the thalamus [In Process Citation] Brain Cogn. 1999;40:414–438. doi: 10.1006/brcg.1999.1088. [DOI] [PubMed] [Google Scholar]
- Danos P, Bauman B, Bernstein H, Franz M, Stauch R, Northoff G, et al. Schizophrenia and anteroventral thalamic nucleus: selective decrease of parvalbumin-immunoreactive thalamocortical projection neurons. Psychiatry Res Neuroimaging. 1998;82:1–10. doi: 10.1016/s0925-4927(97)00071-1. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Ongur D, Duman RS. Antipsychotic drugs induce Fos protein in the thalamic paraventricular nucleus: a novel locus of antipsychotic drug action. Neuroscience. 1995;66:337–346. doi: 10.1016/0306-4522(94)00571-l. [DOI] [PubMed] [Google Scholar]
- Domino EF, Mirzoyan D, Tsukada H. N-methyl-D-aspartate antagonists as drug models of schizophrenia: a surprising link to tobacco smoking. Progress in neuro-psychopharmacology & biological psychiatry. 2004;28:801–811. doi: 10.1016/j.pnpbp.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Ebmeier KP, Lawrie SM, Blackwood DH, Johnstone EC, Goodwin GM. Hypofrontality revisited: a high resolution single photon emission computed tomography study in schizophrenia. Journal of Neurology, Neurosurgery, and Psychiatry. 1995;58:452–456. doi: 10.1136/jnnp.58.4.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K. Disconnection and cognitive dysmetria in schizophrenia. The American journal of psychiatry. 2005;162:429–432. doi: 10.1176/appi.ajp.162.3.429. [DOI] [PubMed] [Google Scholar]
- Friston K, Büchel C. Functional connectivity: Eigenimages and multivariate analyses. In: Frackowiak R, Friston K, Frith C, Dolan R, Price C, Zeki S, et al., editors. Human Brain Function. 2. Salt Lake City: Academic Press; 2004. [Google Scholar]
- Friston KJ. The disconnection hypothesis. Schizophr Res. 1998;30:115–125. doi: 10.1016/s0920-9964(97)00140-0. [DOI] [PubMed] [Google Scholar]
- Friston KJ. Dysfunctional connectivity in schizophrenia. World Psychiatry. 2002;1:66–71. [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD. Schizophrenia: a disconnection syndrome? Clin Neurosci. 1995;3:89–97. [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Fletcher P, Liddle PF, Frackowiak RS. Functional topography: multidimensional scaling and functional connectivity in the brain. Cerebral cortex (New York, NY. 1996;6:156–164. doi: 10.1093/cercor/6.2.156. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Liddle PF, Frackowiak RS. Functional connectivity: the principal-component analysis of large (PET) data sets. J Cereb Blood Flow Metab. 1993;13:5–14. doi: 10.1038/jcbfm.1993.4. [DOI] [PubMed] [Google Scholar]
- Frith CD, Friston KJ, Herold S, Silbersweig D, Fletcher P, Cahill C, et al. Regional brain activity in chronic schizophrenic patients during the performance of a verbal fluency task. The British journal of psychiatry. 1995;167:343–349. doi: 10.1192/bjp.167.3.343. [DOI] [PubMed] [Google Scholar]
- Garey LJ, Ong WY, Patel TS, Kanani M, Davis A, Mortimer AM, et al. Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia. Journal of Neurology, Neurosurgery, and Psychiatry. 1998;65:446–453. doi: 10.1136/jnnp.65.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- Golanov EV, Yamamoto S, Reis DJ. Spontaneous waves of cerebral blood flow associated with a pattern of electrocortical activity. Am J Physiol. 1994;266:R204–214. doi: 10.1152/ajpregu.1994.266.1.R204. [DOI] [PubMed] [Google Scholar]
- Gold JM, DRW . Frontal lobe structure, function and connectivity in schizophrenia. In: Kerwin R, Dawbarn D, McCulloch J, CT, editors. Cambridge Medical Reviews: Neurobiology and psychiatry. Cambridge: Cambridge University Press; 1991. [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Molecular psychiatry. 2005;10:40–68. doi: 10.1038/sj.mp.4001558. image 45. [DOI] [PubMed] [Google Scholar]
- Hazlett EA, Buchsbaum MS, Zhang J, Newmark RE, Glanton CF, Zelmanova Y, et al. Frontal-striatal-thalamic mediodorsal nucleus dysfunction in schizophrenia-spectrum patients during sensorimotor gating. Neuroimage. 2008;42:1164–1177. doi: 10.1016/j.neuroimage.2008.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman RE, McGlashan TH. Neural network models of schizophrenia. Neuroscientist. 2001;7:441–454. doi: 10.1177/107385840100700513. [DOI] [PubMed] [Google Scholar]
- Hua JY, Smith SJ. Neural activity and the dynamics of central nervous system development. Nature Neuroscience. 2004;7:327–332. doi: 10.1038/nn1218. [DOI] [PubMed] [Google Scholar]
- Hulshoff Pol HE, Schnack HG, Mandl RC, Cahn W, Collins DL, Evans AC, et al. Focal white matter density changes in schizophrenia: reduced inter-hemispheric connectivity. Neuroimage. 2004;21:27–35. doi: 10.1016/j.neuroimage.2003.09.026. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jones E. Cortical development and thalamic pathology in schizophrenia. Schizophr Bull. 1997;23:483–501. doi: 10.1093/schbul/23.3.483. [DOI] [PubMed] [Google Scholar]
- Jones SR, Fernyhough C. A new look at the neural diathesis--stress model of schizophrenia: the primacy of social-evaluative and uncontrollable situations. Schizophr Bull. 2007;33:1171–1177. doi: 10.1093/schbul/sbl058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain. 2004;127:1811–1821. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. The American journal of psychiatry. 2003;160:13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Kwon JS, Park HJ, Youn T, Kang do H, Kim MS, et al. Functional disconnection between the prefrontal and parietal cortices during working memory processing in schizophrenia: a[15(O)]H2O PET study. Am J Psychiatry. 2003;160:919–923. doi: 10.1176/appi.ajp.160.5.919. [DOI] [PubMed] [Google Scholar]
- Kreitschmann-Andermahr I, Rosburg T, Demme U, Gaser E, Nowak H, Sauer H. Effect of ketamine on the neuromagnetic mismatch field in healthy humans. Brain Research. 2001;12:109–116. doi: 10.1016/s0926-6410(01)00043-x. [DOI] [PubMed] [Google Scholar]
- Kumari V, Antonova E, Geyer MA, Ffytche D, Williams SC, Sharma T. A fMRI investigation of startle gating deficits in schizophrenia patients treated with typical or atypical antipsychotics. The international journal of neuropsychopharmacology (CINP) 2007;10:463–477. doi: 10.1017/S1461145706007139. [DOI] [PubMed] [Google Scholar]
- Kumari V, Gray JA, Geyer MA, ffytche D, Soni W, Mitterschiffthaler MT, et al. Neural correlates of tactile prepulse inhibition: a functional MRI study in normal and schizophrenic subjects. Psychiatry Res. 2003;122:99–113. doi: 10.1016/s0925-4927(02)00123-3. [DOI] [PubMed] [Google Scholar]
- Kumra S, Ashtari M, Cervellione KL, Henderson I, Kester H, Roofeh D, et al. White matter abnormalities in early-onset schizophrenia: a voxel-based diffusion tensor imaging study. J Am Acad Child Adolesc Psychiatry. 2005;44:934–941. doi: 10.1097/01.chi.0000170553.15798.94. [DOI] [PubMed] [Google Scholar]
- Kunimatsu N, Aoki S, Kunimatsu A, Yoshida M, Abe O, Yamada H, et al. Tract-specific analysis of the superior occipitofrontal fasciculus in schizophrenia. Psychiatry Res. 2008;164:198–205. doi: 10.1016/j.pscychresns.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Lambe EK, Aghajanian GK. Hypocretin (orexin) induces calcium transients in single spines postsynaptic to identified thalamocortical boutons in prefrontal slice. Neuron. 2003;40:139–150. doi: 10.1016/s0896-6273(03)00598-1. [DOI] [PubMed] [Google Scholar]
- Lambe EK, Liu RJ, Aghajanian GK. Schizophrenia, hypocretin (orexin), and the thalamocortical activating system. Schizophr Bull. 2007;33:1284–1290. doi: 10.1093/schbul/sbm088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambe EK, Olausson P, Horst NK, Taylor JR, Aghajanian GK. Hypocretin and nicotine excite the same thalamocortical synapses in prefrontal cortex: correlation with improved attention in rat. The Journal of Neuroscience. 2005;25:5225–5229. doi: 10.1523/JNEUROSCI.0719-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang DJ, Khorram B, Goghari VM, Kopala LC, Vandorpe RA, Rui Q, et al. Reduced anterior internal capsule and thalamic volumes in first-episode psychosis. Schizophr Res. 2006 doi: 10.1016/j.schres.2006.05.002. (in press) [DOI] [PubMed] [Google Scholar]
- Lawrie SM, Buechel C, Whalley HC, Frith CD, Friston KJ, Johnstone EC. Reduced frontotemporal functional connectivity in schizophrenia associated with auditory hallucinations. Biol Psychiatry. 2002;51:1008–1011. doi: 10.1016/s0006-3223(02)01316-1. [DOI] [PubMed] [Google Scholar]
- Lee KH, Williams LM, Breakspear M, Gordon E. Synchronous gamma activity: a review and contribution to an integrative neuroscience model of schizophrenia. Brain Research. 2003;41:57–78. doi: 10.1016/s0165-0173(02)00220-5. [DOI] [PubMed] [Google Scholar]
- Lowe MJ, Mock BJ, Sorenson JA. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. Neuroimage. 1998;7:119–132. doi: 10.1006/nimg.1997.0315. [DOI] [PubMed] [Google Scholar]
- Magnotta VA, Adix ML, Caprahan A, Lim K, Gollub R, Andreasen NC. Investigating connectivity between the cerebellum and thalamus in schizophrenia using diffusion tensor tractography: a pilot study. Psychiatry Res. 2008;163:193–200. doi: 10.1016/j.pscychresns.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlashan TH, Hoffman RE. Schizophrenia as a disorder of developmentally reduced synaptic connectivity. Arch Gen Psychiatry. 2000;57:637–648. doi: 10.1001/archpsyc.57.7.637. [DOI] [PubMed] [Google Scholar]
- McGuire PK, Frith CD. Disordered functional connectivity in schizophrenia. Psychol Med. 1996;26:663–667. doi: 10.1017/s0033291700037673. [DOI] [PubMed] [Google Scholar]
- McKeown MJ, Makeig S, Brown GG, Jung TP, Kindermann SS, Bell AJ, et al. Analysis of fMRI data by blind separation into independent spatial components. Human Brain Mapping. 1998;6:160–188. doi: 10.1002/(SICI)1097-0193(1998)6:3<160::AID-HBM5>3.0.CO;2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg AS, Olsen RK, Kohn PD, Brown T, Egan MF, Weinberger DR, et al. Regionally specific disturbance of dorsolateral prefrontal-hippocampal functional connectivity in schizophrenia. Arch Gen Psychiatry. 2005;62:379–386. doi: 10.1001/archpsyc.62.4.379. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Byne W, Kemether EM, Hazlett EA, Buchsbaum MS. Metabolic Disconnection Between the Mediodorsal Nucleus of the Thalamus and Cortical Brodmann’s Areas of the Left Hemisphere in Schizophrenia. Am J Psychiatry. 2005;162:1733–1735. doi: 10.1176/appi.ajp.162.9.1733. [DOI] [PubMed] [Google Scholar]
- Monfils MH, Teskey GC. Induction of long-term depression is associated with decreased dendritic length and spine density in layers III and V of sensorimotor neocortex. Synapse. 2004;53:114–121. doi: 10.1002/syn.20039. [DOI] [PubMed] [Google Scholar]
- Monfils MH, VandenBerg PM, Kleim JA, Teskey GC. Long-term potentiation induces expanded movement representations and dendritic hypertrophy in layer V of rat sensorimotor neocortex. Cerebral cortex. 2004;14:586–593. doi: 10.1093/cercor/bhh020. [DOI] [PubMed] [Google Scholar]
- Olney JW, Farber NB. Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry. 1995;52:998–1007. doi: 10.1001/archpsyc.1995.03950240016004. [DOI] [PubMed] [Google Scholar]
- Powell TP, Cowan WM. The interpretation of the degenerative changes in the intralaminar nuclei of the thalamus. Journal of Neurology, Neurosurgery, and Psychiatry. 1967;30:140–153. doi: 10.1136/jnnp.30.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner B. Fundamentals of Biostatistics. 6. Florence: Cengage Learning; 2006. [Google Scholar]
- Schirmer TN, Dorflinger JM, Marlow-O’Connor M, Pendergrass JC, Hartzell A, All SD, et al. FMRI indices of auditory attention in schizophrenia. Progress in neuro-psychopharmacology & biological psychiatry. 2009;33:25–32. doi: 10.1016/j.pnpbp.2008.09.028. [DOI] [PubMed] [Google Scholar]
- Schlosser R, Gesierich T, Kaufmann B, Vucurevic G, Stoeter P. Altered effective connectivity in drug free schizophrenic patients. NeuroReport. 2003;14:2233–2237. doi: 10.1097/00001756-200312020-00020. [DOI] [PubMed] [Google Scholar]
- Shipp S. The functional logic of cortico-pulvinar connections. Philos Trans R Soc Lond B Biol Sci. 2003;358:1605–1624. doi: 10.1098/rstb.2002.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Stein T, Moritz C, Quigley M, Cordes D, Haughton V, Meyerand E. Functional connectivity in the thalamus and hippocampus studied with functional MR imaging. Ajnr. 2000;21:1397–1401. [PMC free article] [PubMed] [Google Scholar]
- Stephan KE, Baldeweg T, Friston KJ. Synaptic plasticity and dysconnection in schizophrenia. Biol Psychiatry. 2006;59:929–939. doi: 10.1016/j.biopsych.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Stephan KE, Magnotta VA, White T, Arndt S, Flaum M, O’Leary DS, et al. Effects of olanzapine on cerebellar functional connectivity in schizophrenia measured by fMRI during a simple motor task. Psychological Medicine. 2001;31:1065–1078. doi: 10.1017/s0033291701004330. [DOI] [PubMed] [Google Scholar]
- Stone JM, Morrison PD, Pilowsky LS. Glutamate and dopamine dysregulation in schizophrenia--a synthesis and selective review. Journal of psychopharmacology (Oxford, England) 2007;21:440–452. doi: 10.1177/0269881106073126. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Kendler KS, Neale MC. Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Arch Gen Psychiatry. 2003;60:1187–1192. doi: 10.1001/archpsyc.60.12.1187. [DOI] [PubMed] [Google Scholar]
- Swerdlow N, Koob G. Dopamine, schizophrenia, mania, and depression: Towards a unified hypothesis of cortico-striato-palido-thalamic function. Behavioral and Brain Sciences. 1987;10:197–245. [Google Scholar]
- Szeszko PR, Ardekani BA, Ashtari M, Kumra S, Robinson DG, Sevy S, et al. White matter abnormalities in first-episode schizophrenia or schizoaffective disorder: a diffusion tensor imaging study. Am J Psychiatry. 2005;162:602–605. doi: 10.1176/appi.ajp.162.3.602. [DOI] [PubMed] [Google Scholar]
- Walter H, Wunderlich AP, Blankenhorn M, Schafer S, Tomczak R, Spitzer M, et al. No hypofrontality, but absence of prefrontal lateralization comparing verbal and spatial working memory in schizophrenia. Schizophr Res. 2003;61:175–184. doi: 10.1016/s0920-9964(02)00225-6. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Egan MF, Bertolino A, Callicott JH, Mattay VS, Lipska BK, et al. Prefrontal neurons and the genetics of schizophrenia. Biol Psychiatry. 2001;50:825–844. doi: 10.1016/s0006-3223(01)01252-5. [DOI] [PubMed] [Google Scholar]
- Welsh RC, Chen AC, Taylor SF. Low-Frequency BOLD Fluctuations Demonstrate Altered Thalamocortical Connectivity in Schizophrenia. Schizophr Bull. 2008 doi: 10.1093/schbul/sbn145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalley HC, Simonotto E, Marshall I, Owens DG, Goddard NH, Johnstone EC, et al. Functional disconnectivity in subjects at high genetic risk of schizophrenia. Brain. 2005;128:2097–2108. doi: 10.1093/brain/awh556. [DOI] [PubMed] [Google Scholar]
- Xiong J, Parsons LM, Gao JH, Fox PT. Interregional connectivity to primary motor cortex revealed using MRI resting state images. Human Brain Mapping. 1999;8:151–156. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<151::AID-HBM13>3.0.CO;2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LI, Poo MM. Electrical activity and development of neural circuits. nature neuroscience. 2001;4(Suppl):1207–1214. doi: 10.1038/nn753. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Liang M, Jiang T, Tian L, Liu Y, Liu Z, et al. Functional dysconnectivity of the dorsolateral prefrontal cortex in first-episode schizophrenia using resting-state fMRI. Neuroscience Letters. 2007;417:297–302. doi: 10.1016/j.neulet.2007.02.081. [DOI] [PubMed] [Google Scholar]