Abstract
The mariner transposon vector pYV07 was tested for use in the mutagenesis of Bacteroides fragilis 638R. The transposon vector efficiently generated mutants in B. fragilis 638R. The transposon disrupted genes were scattered throughout the genome of B. fragilis 638R. This method serves as a powerful tool to study B. fragilis.
Keywords: Bacteroides fragilis, transposon mutagenesis, mutant library, mariner transposon, semi random PCR, rescue cloning
Bacteroides fragilis is an obligatory anaerobic bacterium that usually exists as a commensal in the gut of humans and animals. Stool culture studies performed in the 1970’s suggested that the species B. fragilis comprises a small proportion of the gut Bacteroides and that has been the classical assumption for decades [1–3]. Recent metagenomic studies generally support that assumption [4] although the speciation, data collection and analyses have obviously become much more complex and the genomic methods used to determine abundance of species are completely different than those used by the earlier studies. B. fragilis is still considered the primary cause of infections involving Bacteroides spp [5–7] although, again, definitive results may be influenced by recent changes in technique and speciation of isolates [7]. It is clear, however, that when B fragilis moves out of its niche in the gut, it becomes an opportunistic pathogen and can cause serious infections. It is the anaerobe most frequently isolated from patients with intra-abdominal sepsis, necrotizing skin, perforated and gangrenous appendicitis and soft tissue infections. Enterotoxigenic strains of B. fragilis are associated with human diarrheal disease including traveler’s diarrhea [8–9].
In many cases, treatment of B. fragilis infection is problematic due to its high level of resistance to multiple classes of antibiotics. Many B. fragilis clinical isolates are resistant to aminoglycosides, β-lactams, macrolide, and metronidazole [10]. Recent studies have demonstrated an increase in B. fragilis isolates that harbor multiple conjugation elements that may confer resistance to multiple antibiotics [11]. These strains further increase the risk of transfer of multiple drug resistance to bacteria in the neighboring environment. Development of genetic tools to probe the gene functions in B. fragilis is necessary to better understand both the development of antimicrobial resistance and pathogenic potential of B. fragilis.
Recently we described a transposon mutagenesis method for B. fragilis using the EZ-Tn5 transposome [12]. The method involves transposome construction using transposon DNA and the commercially available EZ::Tn5 transposase. Although this transposon mutagenesis method is simple and efficient, it is not cost-effective. Therefore, we sought to develop a simple, efficient and cost effective transposon vector for transposon mutagenesis of B. fragilis. Mariner-family transposable elements are active in a wide variety of organisms and generate stable random insertions in the recipient genome and are considered a promising mutagenic tool, especially for studying a species which lacks sophisticated genetic tools [13]. Therefore, we reasoned that a mariner transposon mutagenesis would be an effective method to construct a random mutant library in B. fragilis. Goodman et al. [14] developed the mariner transposon vector pSAM-Bt which has a hyperactive mariner transposase (Himar1C9) [15–16] that inserts the transposon DNA (i.e., the region within the inverted repeat) into the genome of recipient strain at a “TA” site. This transposon vector has been successfully exploited for transposon mutagenesis for B. thetaiotaomicron and Porphyromonas gingivalis [14]. In the present study, we examined the efficacy of a modified pSAM-Bt in the construction of a transposon mutant library of B. fragilis 638R.
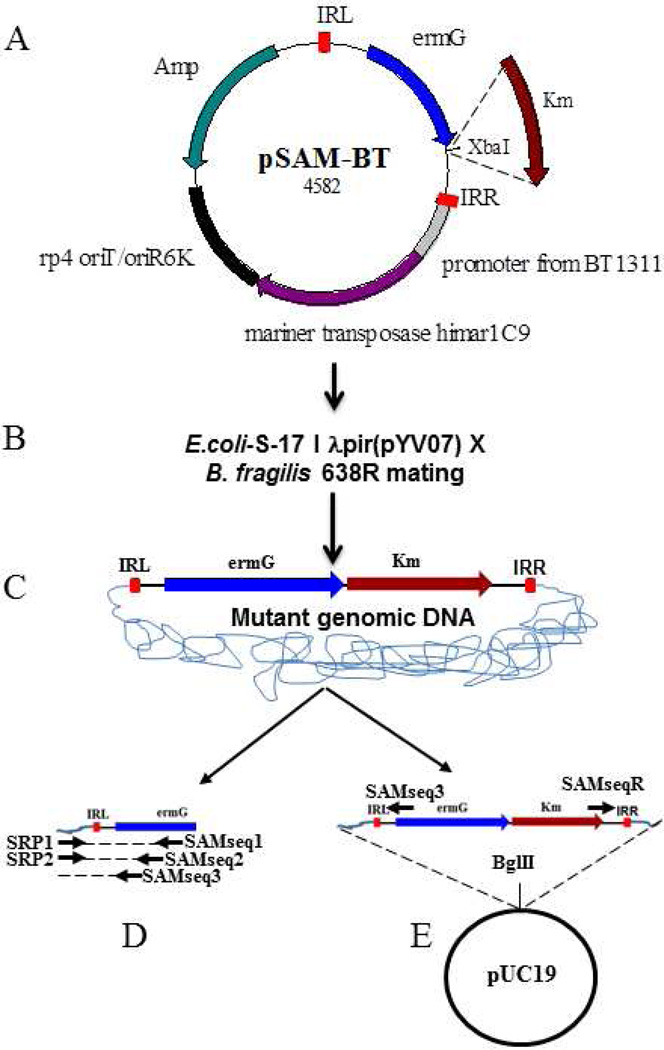
The vector pSAM-Bt was a kind gift from Andrew L. Goodman [14]. With this vector, transposon disrupted gene can be easily identified by semi random priming (SRP) PCR. SRP-PCR usually gives short (~50–200 base pairs) sequence which is sufficient to identify a mutated gene if the genome sequence is known. However, if the genome sequence is not known, as in the case of a clinical isolate, rescue cloning of the interrupted gene will yield a much longer sequence for mutated gene identification. Therefore, we added a kanamycin cassette to facilitate the easy retrieval of the mutated gene from both ends of the transposon. The Km (kanamycin) cassette was cloned downstream of the ermG (erythromycin) cassette in pSAM-Bt. The Km cassette along with its promoter was PCR amplified from a pET-27b+ template using KmFXbaI (CTGCAGTCTAGAGTGGAACGAAAACTCACGTTAAGG) and KmRXbaI (CTGCAGTCTAGACATTCAAATATGTATCCGCTC) as primers. The amplified PCR product was digested with XbaI and ligated to XbaI-digested pSAM-Bt. The resulting ligation mix was used to transform E. coli S-17 λ pir [17] competent cells and transformants were selected on a Luria Bertani (LB)-Km (40 µg/ml) agar plate. The transformants were isolated and the resultant plasmid is named pYV07 (Fig. 1).
Fig. 1.
Transposon mutagenesis of B. fragilis 638R. A) The mariner transposon vector pSAM-Bt. IRL, Inverted repeat left; ermG-provides erythromycin resistance in B. fragilis; rp4 oriT/oriR6K-conditional origin of replication facilitates plasmid replication only in E. coli; Amp-ampicillin resistance gene for E. coli. Plasmid pYV07 was created by cloning Km-kanamycin resistance gene at the XbaI site of pSAM-Bt. B) Transposon mutants are generated by mating E. coli S-17 λ pir-pYV07 with B. fragilis 638R. C) When pYV07 enters B. fragilis 638R, the mariner transposase, whose expression is driven by B. thetaiotaomicron promoter (BT1331), inserts the transposon DNA (ie. IRL, ermG, Km and IRR) into the genome. The transposon mutants become resistant to erythromycin. D) Transposon disrupted gene identification by SRP-PCR and E) Transposon disrupted gene identification by rescue cloning. Mutant genomic DNA can be cut with BglII and cloned into BglII-digested pUC19. Sequencing with SAMseq3 and SAMseqR primer yields transposon junction DNA.
The vector pYV07 was used for mutagenesis of B. fragilis 638R as follows. E. coli S-17 λ pir-pYV07 and B. fragilis 638R were grown overnight in LB/Km and brain heart infusion (BHI) broths, respectively. Overnight cultures were subcultured in antibiotic-free medium and grown to an OD600 of 0.15. One ml of E. coli S-17 λ pir -pYV07 was then mixed with 10 ml of B. fragilis 638R. The mixed cultures were collected by centrifugation, resuspended in 100 µl BHI broth and plated on a single BHI plate. The resulting plates were incubated aerobically for 3h and then anaerobically for overnight at 37°C and the cells harvested by scraping them off the plate. They were then suspended in 1ml BHI broth, centrifuged and then resuspended in 1 ml of BHI broth containing 10% glycerol. The resulting mutant library can be used directly or stored at −80°C for future use. The transposon mutants were isolated by plating the mutant library (20 µl) on BHI/Gentamycin (25 µg/ml)/erythromycin (10 µg/ml)/ plate. The early log phase cells of B. fragilis (OD600 0.06 to 0.15) yields a higher number of transposon mutants (208±15 mutants/20 µl of mating mix) than a mid-log phase (0.3 to 0.4 OD600) (65±16 mutants/20 µl of mating mix).
The presence of the ermG cassette in 50 randomly selected mutants was confirmed by PCR amplification. All of the mutants yielded a 750 bp PCR product indicating that ermG ( (present in the transposon) was carried by all mutants. It has been previously reported that transposon vectors can also integrate into the genome of the recipient strain by illegitimate recombination. Therefore, we looked for the potential vector backbone integration in fifty transposon mutants by performing PCR to detect the presence of the ampicillin resistant (Amp) gene which resides on the plasmid backbone. None of the 50 mutants yielded a PCR product, indicating that mutants are due to transposon insertion and not due to vector integration.
The quality of random insertion is one of key aspects of a transposon vector. To determine whether pYV07 inserts randomly and not into genetic “hot spots”, we identified the transposon insertion positions in 100 mutants by SRP-PCR [12]. We used this method to retrieve the sequence of the mutated gene next to the IRL (inverted repeat left) but the same method can be used to retrieve the sequence of the gene next to the IRR using the appropriate transposon specific primers. The first round of PCR was performed using OneTaqTM Hot Start 2X master mix (New England Biolabs MA USA) with the SRP1 (GGCCACGCGTCGACTAGTACNNNNNNNNNNGATAT) and the transposon-specific primer SAMseq1 (ACGTACTCATGGTTCATCCCGATA) and mutant colony as template DNA. The first round PCR conditions were 10 min at 95°C; 6 cycles of 30s at 95°C, 30s at 30°C, and 1.5 min at 68°C with 5s increments per cycle; 30 cycles of 30s at 95°C, 30s at 45°C, and 2 min at 68°C with 5s increments per cycle; and 5 min at 68°C. One µl of the first round PCR product was used as the template in the second round PCR with SRP2 (GGCCACGCGTCGACTAGTAC) and the transposon-specific primer SAMseq2 (GCGTATCGGTCTGTATATCAGCAA). The second round PCR conditions were 10 min at 95°C; 35 cycles of 45 s at 95°C, 45 s at 55°C, and 1.5 min at 68°C with 5 s increments per cycle; and 10 min at 68°C. The second round PCR product was column purified and sequenced with the transposon specific primer, SAMseq3 (TCTATTCTCATCTTTCTGAGTCCAC). The mutant DNA sequences which contained the IRL (ACAGGTTGGATGATAAGTCCCCGGTCTT) were considered bona fide transposon-disrupted genes. The transposon-disrupted gene was identified by comparing the DNA sequence next to the IRL to the genome of B. fragilis 638R.
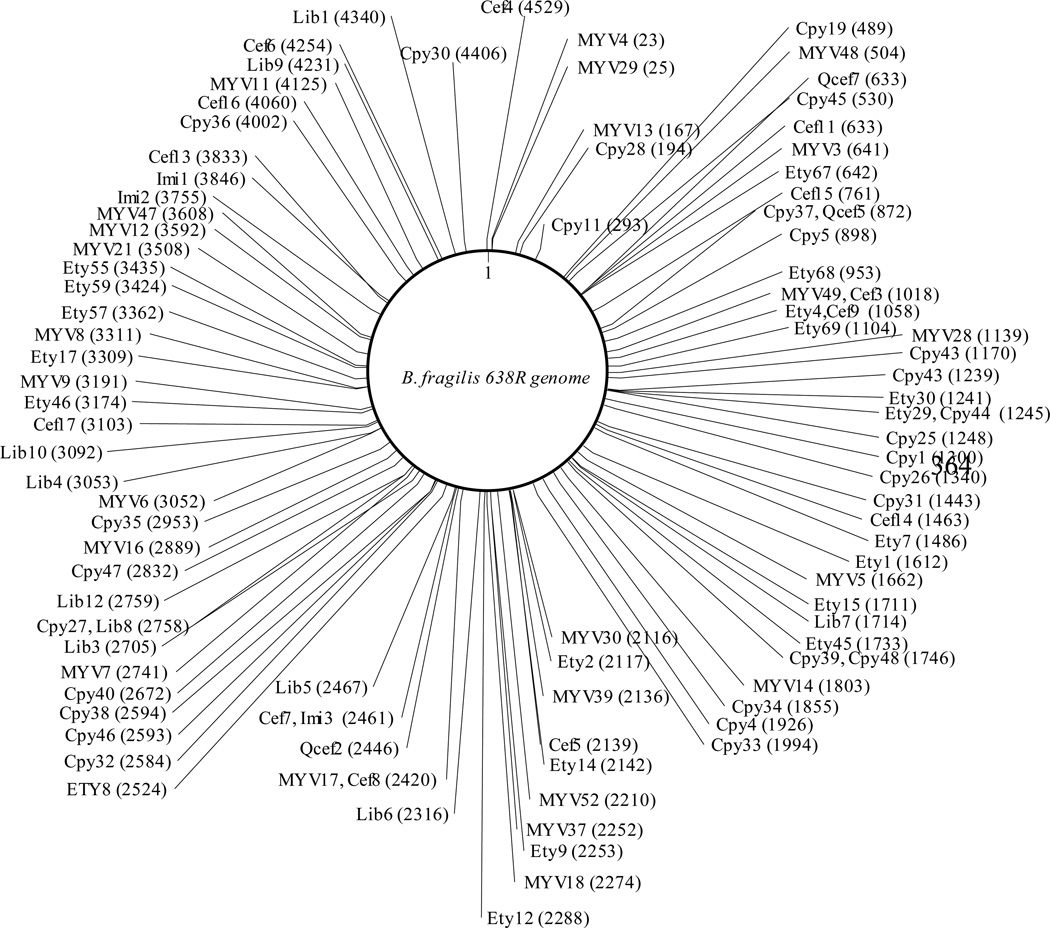
As expected, the mariner transposase inserted the transposon into the genome at a “TA” site in all the 100 mutants tested. This insertion preference does not materially affect the quality of the randomness of the mutant library; B. fragilis has 347,859 “TA” sites on the positive strand of the genome which theoretically corresponds to one TA site/~ 15 bases (B. fragilis has 5,373,121 nucleotides in its genome). As shown in Fig. 2, the transposon insertion sites are evenly distributed across the genome of B. fragilis 638R. The selected transposon mutants showed disruptions in the genes involved in polysaccharide (PS) gene clusters (PSA (Cpy31), PSD (Imi2), PSE (Cpy46) and PSJ (Ety15)), RND efflux pumps (Bme8B (MYV52) and Bme1B (Cpy35)), transcriptional regulators (Bme5R (Cpy36)), sigma factors (sigma54 modulation protein (Cef16), ECF-type sigma factor (Cpy4)), transporters (ABC transporter (MYV30)), and RecA (Cpy25). These results indicated that pYV07 is an effective and efficient tool for B. fragilis random mutant library generation.
Fig. 2.
Schematic representation of B. fragilis 638R transposon mutants. B. fragilis 638R has 4,416 genes in its genome. B. fragilis transposon mutants are generated using pYV07 and identified by SRP-PCR. The mutant name and the transposon insertion position (locus tag number) are indicated in brackets.
The transposon vector described in this study also facilitates the identification of the transposon-mutated gene by rescue cloning. In this case, the genomic DNA of the transposon mutants would be digested with BglII (or any enzyme which does not cut the transposon DNA), cloned into BglII-digested pUC19 and transformed into E. coli. Transformants can be selected on a kanamycin LB/agar plate. Plasmids from kanamycin-resistant transformants contain the kanamycin cassette and flanking genomic sequence. Sequencing of the rescued plasmid with the outwardly directed SAMSeq3 and SAMseqR (GCCAGGCATCAAATTAAGCAG) primers will yield the transposon junction sequence. Compared to SRP-PCR which usually yields small PCR products, rescue cloning will yield a longer stretch of junction DNA. This feature of the transposon is particularly useful to retrieve the mutated gene sequence if the genome sequence of organism of interest is not available.
Random transposon mutagenesis is a powerful tool for understanding specific gene function in bacteria. Improvement in next generation sequencing (NGS) technology has facilitated the en masse identification of a large number of transposon mutants [18]. With the help of NGS technology, transposon vectors have been successfully exploited for identification of essential/fitness genes in many pathogenic bacteria such as Mycobacterium tuberculosis [19], Pseudomonas aeruginosa [20], B. thetaiotaomicron [14] and P. gingivalis [16]. These high throughput identification methods are helpful for large scale linking of genotype to phenotypes, identification of gene function and unraveling complex pathways in bacteria. The transposon vector described in the present study is a powerful tool for the characterization of B. fragilis genes. We are currently determining the essential genes in B. fragilis 638R and our preliminary results indicate that transposon insertions are not detected in likely essential genes. With the help of this transposon vector and NGS technology, we are currently investigating the genes required for B. fragilis fitness under a variety of stress conditions.
Acknowledgment
We would like to thank Dr. Andrew Goodman (Yale University School of Medicine) for providing the pSAM-Bt transposon vector. We would also like to thank Dr. Elizabeth Tenorio (Tufts Medical Center, Boston) for advice on the technical aspects of semi-random PCR. This research is based upon work supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development and in part by the NIAID (NIH) Grant Number 1R56AI083649-01A2.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Moore W, Holdeman L. Human fecal flora: the normal flora of 20 Japanese-Hawaiians. Appl. Microbiol. 1974;75:961–979. doi: 10.1128/am.27.5.961-979.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polk BF, Kasper DL. Bacteroides fragilis subspecies in clinical isolates. Ann. Intern. Med. 1977;86:569–571. doi: 10.7326/0003-4819-86-5-569. [DOI] [PubMed] [Google Scholar]
- 3.Salyers AA. Bacteroides of the human lower intestinal tract. Annu. Rev. Microbiol. 1984;38:293–313. doi: 10.1146/annurev.mi.38.100184.001453. [DOI] [PubMed] [Google Scholar]
- 4.Qin J, Li R, Raes J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brook I. The role of anaerobic bacteria in bacteremia. Anaerobe. 2010;16:183–189. doi: 10.1016/j.anaerobe.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Wexler HM. Bacteroides--The Good, the Bad, and the Nitty-Gritty. Clin. Microbiol. Rev. 2007;20:593–621. doi: 10.1128/CMR.00008-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tong J, Liu C, Summanen P, et al. Application of quantitative real-time PCR for rapid identification of Bacteroides fragilis group and related organisms in human wound samples. Anaerobe. 2011;17:64–68. doi: 10.1016/j.anaerobe.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Wick EC, Sears CL. Bacteroides spp. and diarrhea. Curr. Opin. Infect. Dis. 2010;23:470–474. doi: 10.1097/QCO.0b013e32833da1eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang ZD, DuPont HL, Brown EL, et al. Microbial etiology of travelers' diarrhea in Mexico, Guatemala, and India: importance of enterotoxigenic Bacteroides fragilis and Arcobacter species. J. Clin. Microbiol. 2010;48:1417–1419. doi: 10.1128/JCM.01709-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vedantam G. Antimicrobial resistance in Bacteroides spp.: occurrence and dissemination. Future. Microbiol. 2009;4:413–423. doi: 10.2217/fmb.09.12. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen M, Vedantam G. Mobile genetic elements in the genus Bacteroides, and their mechanism(s) of dissemination. Mob. Genet. Elements. 2011;1:187–196. doi: 10.4161/mge.1.3.18448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Veeranagouda Y, Husain F, Wexler HM. Transposon mutagenesis of the anaerobic commensal, Bacteroides fragilis, using the EZ::TN5 transposome. FEMS Microbiol. Lett. 2012;333:94–100. doi: 10.1111/j.1574-6968.2012.02602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayes F. Transposon-based strategies for microbial functional genomics and proteomics. Annu. Rev. Genet. 2003;37:3–29. doi: 10.1146/annurev.genet.37.110801.142807. [DOI] [PubMed] [Google Scholar]
- 14.Goodman A, McNulty N, Zhao Y, et al. Identifying genetic determinants needed to establish a human gut symbiont in its habitat. Cell host. & microbe. 2009;6:279–289. doi: 10.1016/j.chom.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lampe DJ, Akerley BJ, Rubin EJ, et al. Hyperactive transposase mutants of the Himar1 mariner transposon. Proc. Natl. Acad. Sci. U.S.A. 1999;96:11428–11433. doi: 10.1073/pnas.96.20.11428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klein BA, Tenorio EL, Lazinski DW, et al. Identification of essential genes of the periodontal pathogen Porphyromonas gingivalis. BMC Genomics. 2012;13:578-. doi: 10.1186/1471-2164-13-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simon R, Priefer U, Pühler A. A broad host range mobilization system for In Vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Nature Biotechnology. 1983;1:784–791. [Google Scholar]
- 18.van OT, Bodi KL, Camilli A. Tn-seq: high-throughput parallel sequencing for fitness and genetic interaction studies in microorganisms. Nat. Methods. 2009;6:767–772. doi: 10.1038/nmeth.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griffin JE, Gawronski JD, Dejesus MA, et al. High-resolution phenotypic profiling defines genes essential for mycobacterial growth and cholesterol catabolism. PLoS. Pathog. 2011;7:e1002251. doi: 10.1371/journal.ppat.1002251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gallagher LA, Shendure J, Manoil C. Genome-scale identification of resistance functions in Pseudomonas aeruginosa using Tn-seq. M Bio. 2011;2:e00315-10. doi: 10.1128/mBio.00315-10. [DOI] [PMC free article] [PubMed] [Google Scholar]