Abstract
Lysosome-related organelles (LROs) comprise a group of cell type-specific subcellular compartments with unique composition, morphology and structure that share some features with endosomes and lysosomes and that function in varied processes such as pigmentation, hemostasis, lung plasticity and immunity. In recent years, studies of genetic diseases in which LRO functions are compromised have provided new insights into the mechanisms of LRO biogenesis and the regulated secretion of LRO contents. These insights have revealed previously unappreciated specialized endosomal sorting processes in all cell types, and are expanding our views of the plasticity of the endosomal and secretory systems in adapting to cell type-specific needs.
Introduction
The endocytic pathway fulfills many important functions in all cells, but additional adaptations of the endosomal system in specialized metazoan cell types underlie the formation of lysosome-related organelles (LROs). LROs comprise a group of functionally diverse compartments that share features with lysosomes but are distinct and harbor specific cargoes that confer their unique properties [1]. Consistent with their distinct functions, LROs vary in composition and morphology, ranging from pleiomorphic secretory granules in platelets, cytotoxic T lymphocytes (CTLs) and other hematopoietic cells to enormous fluid-filled vacuoles in the vertebrate notochord and complex subcompartmentalized structures such as pigment cell melanosomes, endothelial cell Weibel-Palade bodies, or platelet α granules. Maturing phagosomes in phagocytes such as dendritic cells, neutrophils and macrophages receive unique contents from endosomes, and thus can also be considered “inducible” LROs. LROs are diverse not only in morphology, but also in the origin of their membranes, the derivation of their contents from secretory and endosomal sources, and the complement of machineries exploited for their formation, maturation and secretion. Here we highlight recent advances in our understanding of these properties, focusing on a few areas that hold particular promise for future breakthroughs.
LRO biogenesis: Origin of LRO precursors
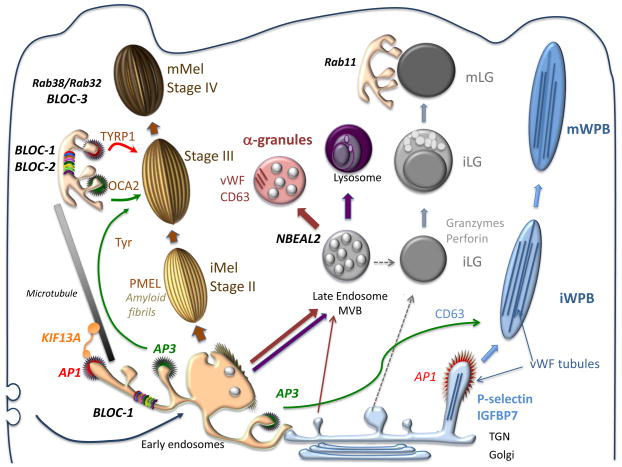
All LROs progressively mature from precursors by acquiring specialized cargoes and generating a lumenal environment conducive for their function (Figure 1). However, the origin of the precursor differs, as exemplified by four well-studied LROs – pigment cell melanosomes, endothelial cell Weibel Palade Bodies (WPBs), platelet α granules and CTL and natural killer (NK) cell lytic granules (LGs) (Figure 2). Non-pigmented melanosome precursors, or premelanosomes, develop from intermediate compartments with features of early endosomal vacuolar domains with few intraluminal vesicles (ILVs) and extended bilayered cytosolic coats [2]. The ILVs scaffold the polymerization of amyloid fibrils by the pigment cell-specific protein PMEL [3]; the fibrils assemble into sheets that distend the organelle into an ellipsoidal shape [2] and template melanin deposition [3]. Accordingly, pigment cells in Pmel−/− mice accumulate melanin in round melanosomes that lack characteristic striations, but that nevertheless remain segregated from the endolysosomal system [4]. Immature WPBs emerge from the trans Golgi Network (TGN) as von Willebrand Factor (vWF) assembles into tubules that shape the nascent compartment into a cigar-shaped organelle [5]. Platelet α granules derive from late endosomes/ multivesicular endosomes (MVEs) in megakaryocytes, and harbor both biosynthetic (e.g. vWF) and endocytic (e.g. fibrinogen) contents. Mature α granules retain CD63 on ILVs as they segregate from endosomes [6]. Whereas matured melanosomes, WPBs and α granules coexist with classical lysosomes, LGs are likely modified lysosomes with a ring of ILVs surrounding a dense core [7] that further mature upon T cell activation. The dense cores might derive like conventional secretory granules from the TGN and later fuse with MVEs to form a hybrid organelle (Figure 1).
Figure 1. Model for biogenesis and docking of four vertebrate LROs.
Shown are models for the biogenesis of immature (i) and mature (m) melanosomes (left, orange), platelet α granules (pink), lysosomes (violet), CTL LGs (gray) and WPBs (right, blue) relative to the endocytic and biosynthetic pathways (Golgi, TGN, early endosomes, late endosomes/ MVBs, and lysosomes). Key cargo molecules discussed in the text are noted in the same color as the LRO, and effectors involved in biogenetic steps are labeled in black text. Arrows indicate relevant trafficking pathways. Left, immature melanosomes (iMel) emerge from vacuolar domains of early endosomes, and mature by cargo delivery from tubulovesicular domains of early endosomes through AP-1- or AP-3-coated vesicles; recycling endosomal domains associated with KIF13A and AP-1 migrate along microtubules towards maturing melanosomes for delivery of some cargoes as indicated. BLOC-1 facilitates tubule-mediated transport; BLOC-2, BLOC-3, RAB32 and RAB38 likely function downstream. Platelet α granules derive in an NBEAL2-dependent process from late endosomes/ MVBs within megakaryocytes, and receive both biosynthetic and endocytic cargoes. MVBs in the same cells also fuse with lysosomes to deliver other cargoes. In CTLs and NK cells, immature LGs (iLGs) also derive by fusion of MVBs with dense core structures, and then fuse with recycling endosome-derived structures upon stimulation by target cells to form mature LGs (mLGs). The dense cores of iLGs contain perforin and granzymes that likely aggregate within the TGN. Likewise, vWF forms tubules in the TGN of endothelial cells, that then bud off perhaps together with cargoes such as P-selectin and IGFBP7 to form immature WPBs; other cargoes, such as CD63, are then delivered from early endosomes in an AP-3-dependent manner.
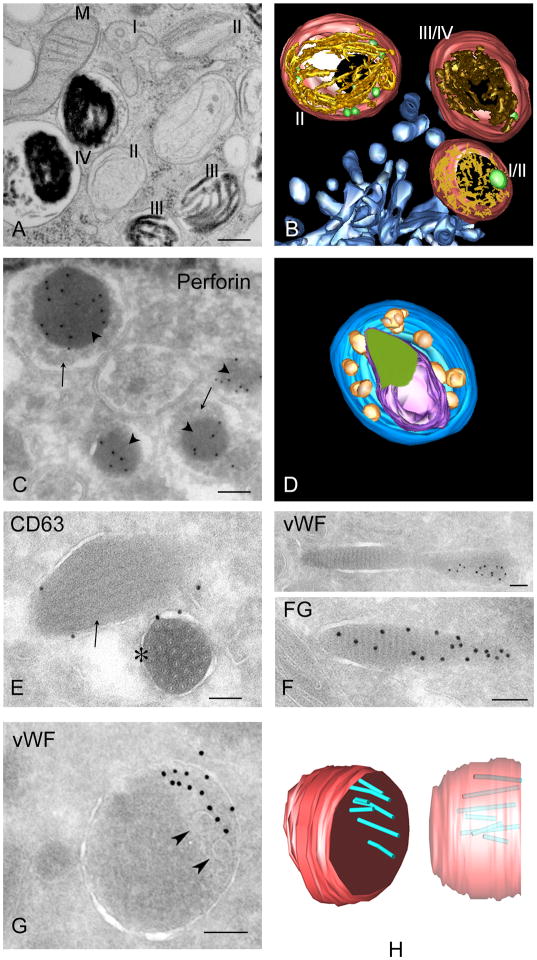
Figure 2. LRO ultrastructure.
Shown are images from electron microscopy analyses or three-dimensional (3d) reconstructions of electron tomograms for four model LROs discussed in this review. A, B, melanosomes from MNT-1 human melanoma cells fixed by high pressure freezing and embedded in plastic by freeze substitution. A, a thin section emphasizing stage I and II premelanosomes and stage III and IV mature melanosomes, as indicated. Note the striated appearance in stages II and III. M, mitochondrion. B, 3d reconstruction emphasizing fibrillar structure (yellow) emanating from intralumenal vesicles (green) in stage I/ II melanosomes. Note the accumulation of melanin (brown) on the fibrils in a stage III melanosome. Melanosome limiting membranes are indicated in red; surrounding endosomal tubules are indicated in blue. C, D, cytolytic granules from primary human CTLs. C, an ultrathin cryosection was immunogold labeled for perforin. Note labeling over dense cores (arrowheads), with surrounding ILVs (arrows). D, 3d reconstruction emphasizing dense core (green) surrounded by multilamellar membranes (violet) and ILVs (yellow). The limiting membrane is pseudocolored blue. E, WPBs within an ultrathin cryosection of a human umbilical vein endothelial cell are shown, immunogold labeled for CD63. Note the tubular vWF polymers captured in profile (arrow) or cross-section (*). F, G, H, α granules within human platelets. F, G, ultrathin cryosections were immunogold labeled for vWF or fibrinogen (FG). Note that fibrinogen is present throughout the tubular α granules but vWF is polarized to one side, adjacent to ILVs (arrows in G). H, 3d reconstruction emphasizing vWF tubules (blue) polarized to one side of a spherical α granule domain, shown in two orientations; the limiting membrane is pseudocolored red. Bars: A–D, 200 nm; E–H, 100 nm.
Melanosomes and WPBs as model LROs
LROs such as LGs and platelet dense granules lack well-organized structural scaffolds, but WPBs and melanosomes develop around rigid matrices.
PMEL is an integral membrane protein that is proteolytically processed into (i) a luminally-exposed amyloidogenic domain that generates the melanosomal fibrils, and (ii) integral membrane fragments that are eventually degraded [3]. Association of the PMEL luminal domain with ILVs requires the tetraspanin (TSP) CD63, but – unlike for most known ILV cargoes – not ubiquitylation or ESCRT function [8,9••]. CD63 is also required for the sorting and proteolytic maturation of elastase – a component of primary granules in neutrophils [10] – suggesting that TSPs on endosomes might generally function to retain proteins that are destined for LROs and prevent them from ESCRT-dependent degradation. This might explain why CD63 associates with most LROs despite associating with late endosomes/ lysosomes in cells that lack LROs [11]. In melanocytes, the integral membrane PMEL fragments are degraded in an ubiquitin- and ESCRT-dependent manner [9••], thus illustrating independent sorting mechanisms for different topological domains of the same protein.
The tubular vWF structures that assemble at the TGN during WPB biogenesis differ substantially from the PMEL amyloid fibrils. Electron tomography identified the vWF tubules as regularly-spaced helical structures [12–14]. The vWF helices likely aggregate to form a paracrystalline core in the TGN [14], causing retention and ultimate segregation into distinct membrane structures that retain cargoes such as P-selectin or insulin growth factor binding protein-7 (IGFBP7) [15]. Interestingly, vWF in platelet α granules forms shorter tubules that typically accumulate around a central core of heterogeneous, electron dense material (Figure 2), and are not responsible for the tubular shape of α granules [6].
LRO biogenesis: Precursor maturation
Whether they originate from endosomal or TGN membranes (Figure 1), LRO precursors mature by acquiring key transmembrane components – such as melanogenic enzymes in melanosomes or serotonin and calcium transporters in platelet dense granules – via membrane trafficking. Maturation also provides a means to acquire effectors that are required for LRO motility or secretion (Figure 1). LROs mature via an intimate dialogue between the immature organelle and MVEs and/or specialized domains of early sorting and recycling endosomes, ensuring that critical cargoes are diverted from classical endo-lysosomes and toward maturing LROs. Multiple non-redundant pathways generally deliver distinct transmembrane protein cargoes to the same maturing LRO, as exemplified by cargo acquisition from the TGN and early endosomes during WPB maturation, and from distinct early endosomal domains during melanosome maturation (Figure 1). The use of multiple pathways ensures that critical complex functions – such as the onset of melanin synthesis by convergence of the copper-dependent Tyrosinase enzyme with the copper transporter ATP7A [16] – are only observed in the mature organelle.
Hermansky-Pudlak syndrome and cargo delivery
Hermansky-Pudlak syndrome (HPS) and its mouse models comprise a group of genetic disorders characterized by malformation of melanosomes, platelet dense granules, and in some cases other LROs [17]. The affected genes encode subunits of five cytoplasmic multisubunit protein complexes – adaptor protein (AP)-3, vacuolar protein sorting (VPS)-C, and biogenesis of lysosome-related organelles complex (BLOC)-1, -2 and -3 [17] – that impact membrane trafficking and/or protein sorting to facilitate LRO maturation. The complexes are ubiquitously expressed, but altered expression levels, post-translational modifications, and/or unique interactions with trafficking machinery in LRO-containing cell types support their functions in critical non-redundant trafficking pathways. For these reasons, their loss of function preferentially impacts LRO maturation.
AP-3 and VPS-C
AP-3 is a heterotetrameric adaptor that engages transmembrane cargoes via cytoplasmic sorting signals in early endosomes, and packages them into clathrin-coated transport vesicles [18]. AP-3 is required for the biogenesis of eye pigment granules in Drosophila melanogaster and gut granules in Caenorhabditis elegans, and for delivery of multiple cargoes to vertebrate LROs [18] (Figure 1), including OCA2 to melanosomes [19,20], CD63 to WPBs [21], toll-like receptor 9 (TLR9) to an IRF7-signaling LRO in plasmacytoid dendritic cells [22], TLR4 to phagosomes in conventional dendritic cells [23•], and SLC35D3 likely to dense granules in platelets [24]. The heterotetrameric VPS-C core (containing VPS11, 16, 18 and 33) assembles into two larger complexes in the yeast Saccharomyces cerevisiae, HOPS and CORVET [25]. VPS-C/ HOPS in S. cerevisiae, D. melanogaster and vertebrates regulates tethering and SNARE-dependent fusion within the endosomal system [26,27], including of AP-3-coated vesicles with the lysosome-like vacuole [28]. Metazoans express distinct VPS-C complexes containing alternative VPS33 and VPS16-like isoforms. The mouse Vps33a buff mutation impacts melanosome and platelet dense granule maturation [29] and mutations in D. melanogaster VPS33A or VPS16A cause defects in eye pigment granules [30]. By contrast, mutations in VPS33B or the VPS16-like VIPAS39 underlie Arthrogryposis-Renal dysfunction-Cholestasis (ARC) syndrome [31,32] characterized by a loss of platelet α granules among other system defects [33,34].
BLOC-1
The eight subunits of BLOC-1 lack recognizable functional domains, and the molecular functions of the complex are not known. In melanocytes, BLOC-1 localizes to endosomal tubules [35] and is required to export melanosomal cargoes from vacuolar early endosomes [16,36,37] into recycling endosome-derived tubular carriers that fuse with melanosomes [38]. However, BLOC-1 unlikely functions as a sorting adaptor, as its architecture – a linear chain of eight globular domains [39•] – is unlike other adaptors, and direct interactions between BLOC-1 and cargoes have not been observed. BLOC-1 binds in vitro to endosomal Q SNAREs Syntaxin 13 and SNAP-25 [40], and might either sort them into transport carriers or regulate their interaction with a partner R-SNARE. A recent proteomics analysis identified tethering factors and two members of the peroxiredoxin family of oxidoreductases as additional binding partners [41].
AP-3 and BLOC-1 physically interact [35] and coordinately regulate cargo distribution in neurons [42,43]. However, in melanocytes BLOC-1 and AP-3 localize distinctly on early endosomes to tubular domains and buds, respectively, [35,36], and BLOC-1 functions in cargo transport to melanosomes either independently of AP-3 (as for ATP7A and the melanogenic enzyme TYRP1 [16,36]) or in conjunction with AP-3 (as for the transporter OCA2 [20]). Similarly, BLOC-1 regulates transport of cargoes that are both AP-3-dependent and independent to gut granules in C. elegans [44•]. This suggests that BLOC-1 acts in conjunction with sorting adaptors to effect cargo transport.
BLOC-2, BLOC-3, RAB32 and RAB38
The two-subunit BLOC-3 is a guanine nucleotide exchange factor (GEF) for two tissue-specific Rab GTPases, RAB38 and RAB32 [45••], that regulate cargo delivery to nascent melanosomes [46,47], platelet dense granules [48], lamellar bodies [49,50], and notochord vacuoles [51••]. How these two partially redundant small GTPases function is not clear. When bound to GTP, both interact with AP-3, the heterotetrameric BLOC-2 – which functions downstream of BLOC-1 in cargo transport [35,36,52] – and the heterotetrameric adaptor AP-1 [53]. They also both bind to VARP [54], a putative scaffold that engages and maintains the R-SNARE VAMP7/TI-VAMP in an inactive conformation [55,56••]. This suggests that RAB32 and RAB38, through multiple effectors, integrate cargo sorting into transport carriers and fusion of the carriers with target LROs. The latter would be consistent with the localization of a cohort of endogenous or overexpressed epitope tagged-RAB32 and/or -RAB38 to LROs [45••,46,48,50,51,53]. RAB32 and RAB38 activation is under complex regulation. VARP is a GEF for the early endosomal Rab GTPase, RAB21 [57], and BLOC-3 is an effector of RAB9 [58], implying that RAB32 and RAB38 participate in several Rab cascades during LRO maturation. Moreover, the existence of GEFs for RAB38 orthologues in C. elegans [59] and D. melanogaster [60] that are unrelated to BLOC-3 suggests that additional GEFs might exist in mammals, perhaps explaining why melanosome biogenesis is differentially affected by loss of BLOC-3 function in different pigment cell types [61].
Other components of the biogenesis machinery
LYST and NBEAL2
Mutations in two members of a family of large proteins with a conserved BEACH domain cause LRO biogenesis disorders. Mutations in LYST/ CHS1 underlie Chediak-Higashi syndrome, in which many LROs and conventional lysosomes are grossly enlarged, but the molecular function of this 3801-residue protein is unclear. The orthologous lvsB controls cargo transport from lysosomes to an LRO in Dictyostelium discoideum [62], perhaps by antagonizing RAB14-dependent fusion between lysosomes and LROs [63]. Alternatively, LYST might promote lysosome or LRO fission, as it does in macrophages [64]. Mutations in another BEACH protein, NBEAL2, underlie Gray Platelet Syndrome, characterized by an absence of platelet α granules [65–67•]. How NBEAL2 functions is unclear, but a role in fission of α granule contents from MVBs would parallel the proposed function of LYST in macrophages.
AP-1 and motors
A second ubiquitous heterotetrameric adaptor family member – AP-1 – plays an important but varied role in LRO generation. In melanocytes, AP-1 binds to targeting signals in several melanosome cargoes, [20,38,68,69] and is required for delivery of TYRP1 to mature melanosomes [38]. However, it is not clear whether the cargo sorting function of AP-1 is required. AP-1 on endosomal buds binds to the microtubule plus end-directed kinesin motor, KIF13A, promoting the extension of tubular Rab11-positive recycling endosomes to the cell periphery. This apposes the endosomes to maturing melanosomes, facilitating cargo delivery via the tubular transport carriers [38]. AP-1 also binds to TSG101, an ESCRT-I subunit involved in MVB formation [70] that is also required in TYRP1 delivery to melanosomes [71]. In endothelial cells, AP-1 and clathrin function as a scaffold to maintain the compacted, cigar-like structure of WPBs which is necessary for positioning vWF polymers to “unfurl” upon WPB secretion and for consequent platelet adhesion to the endothelial cell [5]. AP-1 plays a secondary role in facilitating WPB secretion through recruitment of its cofactors, amphiphilin and γ-synergin [72]. Platelet α granules are also bordered by clathrin lattices [6], but whether they contribute to α granule morphogenesis is not known.
Cytoskeletal motors have important roles in LRO motility and secretion (see below), but unconventional myosins function in LRO biogenesis likely by effecting actin rearrangements that facilitate membrane dynamics involved in budding and fusion. For example, in melanocytes, myosin VI – an actin-based motor involved in endocytic recycling – recruits actin to mature melanosomes and regulates melanosome size and melanization [73].
LRO secretion: polarization and docking
Most, if not all, LROs release their lumenal contents by secretion into the extracellular space or directly to neighbouring cells in response to signaling. This requires LRO transport to the cell periphery and stimulus-dependent fusion of the LRO and plasma membranes.
In endothelial cells, mature WPBs appear “ready” to release vWF – with the assistance of actin/ myosin II-based contractile forces – upon stimulation [74••]. Similarly, in resting platelets, fully matured α granules, dense granules and lysosomes are primed for stimulus-dependent fusion, but the kinetics of their release differ. Dense granules are likely docked at the plasma membrane and released immediately upon stimulus [75•], whereas content release from α granules is more heterogeneous and slightly delayed [75•,76]; the heterogeneity might reflect packaging into distinct α granule subsets [76,77] or spatial segregation within internally heterogeneous α granules [6]. Unlike platelet granules and WPBs, LGs in resting CTLs are functionally immature. Final maturation and fusion are triggered by target cell engagement, inducing the fusion of LGs with RAB11-containing compartments [78] (Figure 1) that deliver effectors required for positioning, fusion with the plasma membrane or both.
Genes mutated in Griscelli syndrome (GS) encode essential components of the molecular machinery that allow for LRO docking at the cell periphery. In melanocytes, RAB27A (GS1), myosin VA (GS2) and melanophilin (GS3) form a complex that tether melanosomes to cortical actin, permitting their ultimate transfer from dendritic tips to keratinocytes (reviewed in [79]). WPB and LG secretion also require RAB27A at multiple steps, but the effectors are distinct from those on melanosomes. WPB secretion requires coordination of RAB27A, RAB15, and their effector MUNC13-4 with RAB3 [80], and is regulated by a balance between RAB27A availability, Slp4-a as a stimulating effector, and MyRIP as an inhibitory effector [81•]. In CTLs, a complex of the RAB27A effector Slp3 with kinesin-1 drives LGs to the plasma membrane for ultimate secretion [82•]. RAB27A and MUNC13-4 play independent roles in stimulus-induced LG maturation [78], but a Rab27A-MUNC13-4 complex is subsequently required to tether LGs to the plasma membrane for secretion [83•].
Stimulus-dependent LG secretion in CTLs and NK cells is under tight control to ensure that lumenal cytolytic contents are directed only toward a target cell at the immunological synapse. LGs polarize toward the synapse in association with the centrosome, which is repositioned toward the plasma membrane upon target cell contact [84,85••]. Centrosome motility is tightly controlled by the strength of T cell signaling [86], at least in part via activation of the Lck tyrosine kinase [86], and requires the minus end-directed microtubule motor dynein [84,87]. Apposition of LGs to the plasma membrane further requires actin rearrangements [84,88]. A similar mode of centrosome positioning and docking targets MHC class II compartments to the immunological synapse of B lymphocytes [89].
LRO secretion: FHL and the fusion apparatus
Familial hemophagocytic lymphohistiocytosis (FHL) is a genetic disorder characterized by unchecked lymphocyte expansion and inflammation due to impaired LG function in CTLs and NK cells [90]. FHL types 3–5, which are additionally associated with bleeding diathesis, reflect impaired LG and platelet granule release. FHL4 results from gene mutations in the Qa SNARE Syntaxin 11 (STX11). FHL4 CTLs and NK cells do not undergo target cell-induced LG degranulation [91,92], and STX11-deficient platelets fail to secrete α granule and dense granule contents in response to agonists [93]. This implies that a STX11-containing tSNARE mediates a fusion step required for LRO release. FHL5 results from mutations in STXBP2 encoding the Sec1-Munc18 family member, Munc18-2/ Munc18b [94]. Munc18b binds to and stabilizes STX11 and facilitates SNARE complex formation; accordingly, FHL4 and FHL5 CTLs and platelets show similar degranulation defects [93–95••]. In platelets STX11 and Munc18b complex with the Qbc SNARE SNAP-23 on the plasma membrane and the R-SNARE VAMP8 on granule membranes to mediate fusion and granule content release [93,95••]. A similar SNARE complex likely functions during LG degranulation in CTLs and NK cells, but where the complex forms in these cells is less clear. STX11 localizes in CTLs and NK cells primarily to intracellular structures that lack RAB27A [92,96] and that might be identical to the RAB11-containing compartments that fuse with immature LGs upon CTL stimulation [78]. This fusion step in CTLs requires the RAB27A effector MUNC13-4 [78]. MUNC13-4 is a SNARE interacting protein that is mutated in FHL3, is required for degranulation of LGs [97] and platelet granules [98], and forms a complex with STX11 and Munc18b in platelets [95••]. Together, the data suggest that whereas STX11/Munc18b/MUNC13-4-dependent SNARE complex formation directly mediates degranulation in platelets, it may mediate a preparatory maturation step in CTLs and NK cells.
Perspectives
While our understanding of LRO biogenesis and secretion has deepened considerably in recent years, many questions remain and will likely be the focus of study in coming years. Firstly, how do LRO precursors such as premelanosomes and α granules segregate from the endosomal system? In both cases, both LROs and lysosomes emerge from common MVEs, and segregation appears to be independent of structural rigidity imposed by vWF or PMEL. Differential sorting of distinct cargo domains into separate ILVs might reflect a general feature of LRO precursors. Secondly, how is cargo assembly differentially regulated in distinct LRO-producing cells? For example, vWF polymerizes into elongated tubules in endothelial cell WPBs but into shorter tubules in α granules. Third, while we have amassed a reasonable “parts” list for both LRO maturation and the docking and fusion apparatus for secretion, the mechanisms by which these parts are integrated to effect their function is not yet understood. Understanding these mechanisms will require a combination of systems analyses, biochemical analyses of binding interactions among machinery components, and functional analyses of model cell types expressing targeted mutations that disrupt component interactions. Finally, comparative analyses between LRO-generating cell types will likely provide novel insights into common and distinctive features of LROs. For example, FHL is not characterized by hypopigmentation, and thus melanin transfer to keratinocytes is not likely mediated by the same fusion complex employed by platelets or CTLs to secrete their granules; indeed, a recent study suggests that melanocytes release melanosome clusters in dendritic tips by abscission of the plasma membrane in regions of adherence to keratinocytes [99]. Validation and molecular dissection of this mechanism might reveal novel ways in which LRO contents are released.
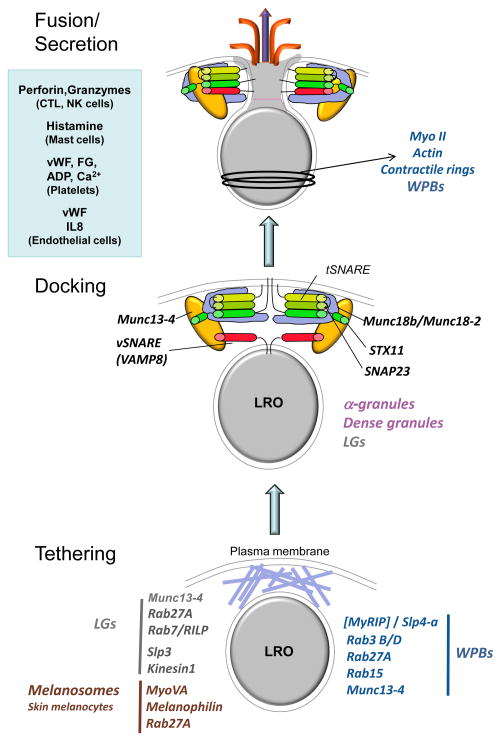
Figure 3. Model for molecular control of LRO secretion.
Shown is a schematic diagram of discrete steps in LRO secretion. Tethering (bottom) refers to the attachment of LROs to the subplasmalemmal actin cytoskeleton (royal blue network). RAB27A facilitates tethering of LGs, melanosomes and WPBs, but by distinct effectors as shown, and in several kinetically distinct stages that have not yet been detailed. In CTLs and NK cells, LGs become tethered following centriole polarisation in a process requiring RAB7, its effector RILP, and dynein (not shown). RAB27A and Munc13-4 function independently to regulate fusion of immature LGs with RAB11-positive exocytic vesicles (see Figure 1), and then together at a later step of tethering; RAB27A also functions together with a Slp3/kinesin-1 complex at a yet undefined stage. In endothelial cells, RAB27A, RAB15 and their joint effector, Munc13-4, facilitate WPB tethering; the RAB27A effectors Slp4-a and MyRIP also function in a mutually antagonistic manner at a distinct stage. In melanocytes, a complex of RAB27A, melanophilin and Myosin VA (MYOVA) recruit melanosomes from microtubules to cortical actin. Docking (middle) refers to the engagement of SNAREs on the LRO and target membranes in a pre-fusion complex. In platelets, docking is mediated by VAMP8 on α granule and dense granule membranes, a syntaxin 11 (STX11)/ SNAP-23 complex on the plasma membrane, and accessory proteins Munc13-4 and Munc18b/MUNC18-2. The same components promote docking in CTLs and NK cells for LG secretion, but it is not yet clear whether this is at the plasma membrane or at a pre-secretory step. Fusion/ secretion involves the zippering of opposing SNAREs, applying force to fuse the LRO and plasma membranes. This permits release of the lumenal LRO contents; a few examples of some LRO contents are indicated in the blue box. In endothelial cells, contractile forces from actin and myosin II are required to “squeeze” the elongated vWF tubules from fused WPBs. In skin melanocytes, it is not yet clear whether melanin is secreted like other LRO contents or whether melanin transfer is mediated by a non-secretory process.
Acknowledgments
We thank our many colleagues who have contributed to the work described herein, particularly Ilse Hurbain and Maryse Romao for contributing figures, and apologize to those whose work we failed to cite. We are grateful for funding from the National Institutes of Health (grants R01 AR048155, R01 EY015625, R21 HL096865 and R21 AI092398), Netherlands Organisation for Scientific Research (grant ALW 813.08.001), Institut Curie, CNRS, INSERM, and Fondation ARC pour la recherche sur le cancer (grant SL220100601359). The funding sources had no involvement in the preparation or submission of this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Raposo G, Marks MS, Cutler DF. Lysosome-related organelles: driving post-golgi compartments into specialisation. Curr Opin Cell Biol. 2007;19:394–401. doi: 10.1016/j.ceb.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hurbain I, Geerts WJC, Boudier T, Marco S, Verkleij A, Marks MS, Raposo G. Electron tomography of early melanosomes: implications for melanogenesis and the generation of fibrillar amyloid sheets. Proc Natl Acad Sci US A. 2008;105:19726–19731. doi: 10.1073/pnas.0803488105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watt B, van Niel G, Raposo G, Marks MS. PMEL: A pigment cell-specific model for functional amyloid formation. Pigment Cell Melanoma Res. 2013 doi: 10.1111/pcmr.12067. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hellström AR, Watt B, Fard SS, Tenza D, Mannström P, Narfström K, Ekesten B, Ito S, Wakamatsu K, Larsson J, et al. Inactivation of the Pmel gene alters melanosome shape but has only a subtle effect on visible pigmentation. PLoS Genet. 2011;7:e1002285. doi: 10.1371/journal.pgen.1002285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michaux G, Abbitt KB, Collinson LM, Haberichter SL, Norman KE, Cutler DF. The physiological function of von Willebrand’s factor depends on its tubular storage in endothelial Weibel-Palade bodies. Dev Cell. 2006;10:223–232. doi: 10.1016/j.devcel.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 6.van Nispen tot Pannerden H, de Haas F, Geerts W, Posthuma G, van Dijk S, Heijnen HF. The platelet interior revisited: electron tomography reveals tubular alpha-granule subtypes. Blood. 2010;116:1147–1156. doi: 10.1182/blood-2010-02-268680. [DOI] [PubMed] [Google Scholar]
- 7.Peters PJ, Borst J, Oorschot V, Fukuda M, Krähenbühl O, Tschopp J, Slot JW, Geuze HJ. Cytotoxic T lymphocyte granules are secretory lysosomes, containing both perforin and granzymes. J Exp Med. 1991;173:1099–1109. doi: 10.1084/jem.173.5.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Theos AC, Truschel ST, Tenza D, Hurbain I, Harper DC, Berson JF, Thomas PC, Raposo G, Marks MS. A lumenal domain-dependent pathway for sorting to intralumenal vesicles of multivesicular endosomes involved in organelle morphogenesis. Dev Cell. 2006;10:343–354. doi: 10.1016/j.devcel.2006.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9••.van Niel G, Charrin S, Simoes S, Romao M, Rochin L, Saftig P, Marks MS, Rubinstein E, Raposo G. The Tetraspanin CD63 integrates ESCRT-independent and dependent sorting at a common endosome. Dev Cell. 2011;21:708–721. doi: 10.1016/j.devcel.2011.08.019. This paper shows that distinct topological domains of the amyloid protein, PMEL, are sorted to different intralumenal vesicle populations of multivesicular endosomes by CD63- and ESCRT-dependent mechanisms, respectively, for distinct fates - melanosomes and degradation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Källquist L, Hansson M, Persson AM, Janssen H, Calafat J, Tapper H, Olsson I. The tetraspanin CD63 is involved in granule targeting of neutrophil elastase. Blood. 2008;112:3444–3454. doi: 10.1182/blood-2007-10-116285. [DOI] [PubMed] [Google Scholar]
- 11.Pols MS, Klumperman J. Trafficking and function of the tetraspanin CD63. Exp Cell Res. 2009;315:1584–1592. doi: 10.1016/j.yexcr.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 12.Zenner HL, Collinson LM, Michaux G, Cutler DF. High-pressure freezing provides insights into Weibel-Palade body biogenesis. J Cell Sci. 2007;120:2117–2125. doi: 10.1242/jcs.007781. [DOI] [PubMed] [Google Scholar]
- 13.Valentijn KM, Valentijn JA, Jansen KA, Koster AJ. A new look at Weibel-Palade body structure in endothelial cells using electron tomography. J Struct Biol. 2008;161:447–458. doi: 10.1016/j.jsb.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Berriman JA, Li S, Hewlett LJ, Wasilewski S, Kiskin FN, Carter T, Hannah MJ, Rosenthal PB. Structural organization of Weibel-Palade bodies revealed by cryo-EM of vitrified endothelial cells. Proc Natl Acad Sci US A. 2009;106:17407–17412. doi: 10.1073/pnas.0902977106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Breevoort D, van Agtmaal EL, Dragt BS, Gebbinck JK, Dienava-Verdoold I, Kragt A, Bierings R, Horrevoets AJ, Valentijn KM, Eikenboom JC, et al. Proteomic screen identifies IGFBP7 as a novel component of endothelial cell-specific Weibel-Palade bodies. J Proteome Res. 2012;11:2925–2936. doi: 10.1021/pr300010r. [DOI] [PubMed] [Google Scholar]
- 16.Setty SRG, Tenza D, Sviderskaya EV, Bennett DC, Raposo G, Marks MS. Cell-specific ATP7A transport sustains copper-dependent tyrosinase activity in melanosomes. Nature. 2008;454:1142–1146. doi: 10.1038/nature07163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei A-H, Li W. Hermansky-Pudlak syndrome: Pigmentary and non-pigmentary defects and their pathogenesis. Pigment Cell Melanoma Res. 2013;26:176–192. doi: 10.1111/pcmr.12051. [DOI] [PubMed] [Google Scholar]
- 18.Dell’Angelica EC. AP-3-dependent trafficking and disease: the first decade. Curr Opin Cell Biol. 2009;21:552–559. doi: 10.1016/j.ceb.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 19.Huizing M, Sarangarajan R, Strovel E, Zho Y, Gahl WA, Boissy RE. AP-3 mediates tyrosinase but not TRP-1 trafficking in human melanocytes. Mol Biol Cell. 2001;12:2075–2085. doi: 10.1091/mbc.12.7.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sitaram A, Dennis MK, Chaudhuri R, De Jesus-Rojas W, Tenza D, Setty SRG, Wood CS, Sviderskaya EV, Bennett DC, Raposo G, et al. Differential recognition of a dileucine-based sorting signal by AP-1 and AP-3 reveals a requirement for both BLOC-1 and AP-3 in delivery of OCA2 to melanosomes. Mol Biol Cell. 2012;23:3178–3192. doi: 10.1091/mbc.E11-06-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harrison-Lavoie KJ, Michaux G, Hewlett L, Kaur J, Hannah MJ, Lui-Roberts WW, Norman KE, Cutler DF. P-selectin and CD63 use different mechanisms for delivery to Weibel-Palade bodies. Traffic. 2006;7:647–662. doi: 10.1111/j.1600-0854.2006.00415.x. [DOI] [PubMed] [Google Scholar]
- 22.Sasai M, Linehan MM, Iwasaki A. Bifurcation of Toll-Like Receptor 9 signaling by Adaptor Protein 3. Science. 2010;329:1530–1534. doi: 10.1126/science.1187029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23•.Mantegazza AR, Guttentag SH, El-Benna J, Sasai M, Iwasaki A, Shen H, Laufer TM, Marks MS. Adaptor Protein-3 in dendritic cells facilitates phagosomal Toll-like receptor signaling and antigen presentation to CD4+ T cells. Immunity. 2012;36:782–794. doi: 10.1016/j.immuni.2012.02.018. This paper shows that AP-3 mediates sorting of Toll-like receptors to phagosomes and consequent proinflammatory signaling in conventional dendritic cells, complementing ref. 22 showing that AP-3 mediates distinctive sorting for a subset of Toll-like receptors in plasmacytoid dendritic cells to a separate LRO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meng R, Wang Y, Yao Y, Zhang Z, Harper DC, Heijnen HFG, Sitaram A, Li W, Raposo G, Weiss MJ, et al. SLC35D3 delivery from megakaryocyte early endosomes is required for platelet dense granule biogenesis and differentially defective in Hermansky-Pudlak syndrome models. Blood. 2012;120:404–414. doi: 10.1182/blood-2011-11-389551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nickerson DP, Brett CL, Merz AJ. Vps-C complexes: gatekeepers of endolysosomal traffic. Curr Opin Cell Biol. 2009;21:543–551. doi: 10.1016/j.ceb.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peplowska K, Markgraf DF, Ostrowicz CW, Bange G, Ungermann C. The CORVET tethering complex interacts with the yeast Rab5 homolog Vps21 and is involved in endo-lysosomal biogenesis. Dev Cell. 2007;12:739–750. doi: 10.1016/j.devcel.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 27.Starai VJ, Hickey CM, Wickner W. HOPS proofreads the trans-SNARE complex for yeast vacuole fusion. Mol Biol Cell. 2008;19:2500–2508. doi: 10.1091/mbc.E08-01-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Angers CG, Merz AJ. HOPS interacts with Apl5 at the vacuole membrane and is required for consumption of AP-3 transport vesicles. Mol Biol Cell. 2009;20:4563–4574. doi: 10.1091/mbc.E09-04-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suzuki T, Oiso N, Gautam R, Novak EK, Panthier JJ, Suprabha PG, Vida T, Swank RT, Spritz RA. The mouse organellar biogenesis mutant buff results from a mutation in Vps33a, a homologue of yeast vps33 and Drosophila carnation. Proc Natl Acad Sci US A. 2003;100:1146–1150. doi: 10.1073/pnas.0237292100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akbar MA, Ray S, Krämer H. The SM protein Car/Vps33A regulates SNARE-mediated trafficking to lysosomes and lysosome-related organelles. Mol Biol Cell. 2009;20:1705–1714. doi: 10.1091/mbc.E08-03-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gissen P, Johnson CA, Morgan NV, Stapelbroek JM, Forshew T, Cooper WN, McKiernan PJ, Klomp LW, Morris AA, Wraith JE, et al. Mutations in VPS33B, encoding a regulator of SNARE-dependent membrane fusion, cause arthrogryposis-renal dysfunction-cholestasis (ARC) syndrome. Nat Genet. 2004;363:400–404. doi: 10.1038/ng1325. [DOI] [PubMed] [Google Scholar]
- 32.Cullinane AR, Straatman-Iwanowska A, Zaucker A, Wakabayashi Y, Bruce CK, Luo G, Rahman F, Gürakan F, Utine E, Ozkan TB, et al. Mutations in VIPAR cause an arthrogryposis, renal dysfunction and cholestasis syndrome phenotype with defects in epithelial polarization. Nat Genet. 2010;42:303–312. doi: 10.1038/ng.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lo B, Li L, Gissen P, Christensen H, McKiernan PJ, Ye C, Abdelhaleem M, Hayes JA, Williams MD, Chitayat D, et al. Requirement of VPS33B, a member of the Sec1/Munc18 protein family, in megakaryocyte and platelet alpha-granule biogenesis. Blood. 2005;106:4159–4166. doi: 10.1182/blood-2005-04-1356. [DOI] [PubMed] [Google Scholar]
- 34.Urban D, Li L, Christensen H, Pluthero FG, Chen SZ, Puhacz M, Garg PM, Lanka KK, Cummings JJ, Kramer H, et al. The VPS33B-binding protein VPS16B is required in megakaryocyte and platelet ƒ¿-granule biogenesis. Blood. 2012;120:5032–5040. doi: 10.1182/blood-2012-05-431205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di Pietro SM, Falcon-Perez JM, Tenza D, Setty SRG, Marks MS, Raposo G, Dell’Angelica EC. BLOC-1 interacts with BLOC-2 and the AP-3 complex to facilitate protein trafficking on endosomes. Mol Biol Cell. 2006;17:4027–4038. doi: 10.1091/mbc.E06-05-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Setty SRG, Tenza D, Truschel ST, Chou E, Sviderskaya EV, Theos AC, Lamoreux ML, Di Pietro SM, Starcevic M, Bennett DC, et al. BLOC-1 is required for cargo-specific sorting from vacuolar early endosomes toward lysosome-related organelles. Mol Biol Cell. 2007;18:768–780. doi: 10.1091/mbc.E06-12-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cullinane AR, Curry JA, Carmona-Rivera C, Summers CG, Ciccone C, Cardillo ND, Dorward H, Hess RA, White JG, Adams D, et al. A BLOC-1 mutations screen reveals that PLDN is mutated in Hermansky-Pudlak syndrome type 9. Am J Human Genet. 2011;88:778–787. doi: 10.1016/j.ajhg.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Delevoye C, Hurbain I, Tenza D, Sibarita J-B, Uzan-Gafsou S, Ohno H, Geerts WJC, Verkleij AJ, Salamero J, Marks MS, et al. AP-1 and KIF13A coordinate endosomal sorting and positioning during melanosome biogenesis. J Cell Biol. 2009;187:247–264. doi: 10.1083/jcb.200907122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39•.Lee HH, Nemecek D, Schindler C, Smith WJ, Ghirlando R, Steven AC, Bonifacino JS, Hurley JH. Assembly and architecture of Biogenesis of Lysosome-related Organelles Complex-1 (BLOC-1) J Biol Chem. 2012;287:5882–5890. doi: 10.1074/jbc.M111.325746. The authors analyze the 8-subunit BLOC-1 biochemically, and show that it consists of subcomplexes that assemble into a linear array of apparently globular subunits. An implication of the structure is that BLOC-1 does not function as a conventional sorting adaptor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghiani CA, Starcevic M, Rodriguez-Fernandez IA, Nazarian R, Cheli VT, Chan LN, Malvar JS, de Vellis J, Sabatti C, Dell’angelica EC. The dysbindin-containing complex (BLOC-1) in brain: developmental regulation, interaction with SNARE proteins and role in neurite outgrowth. Mol Psychiatry. 2010;15:204–215. doi: 10.1038/mp.2009.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gokhale A, Larimore J, Werner E, So L, Moreno-De-Luca A, Lese-Martin C, Lupashin VV, Smith Y, Faundez V. Quantitative proteomic and genetic analyses of the schizophrenia susceptibility factor dysbindin identify novel roles of the biogenesis of lysosome-related organelles complex 1. J Neurosci. 2012;32:3697–3711. doi: 10.1523/JNEUROSCI.5640-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Larimore J, Tornieri K, Ryder PV, Gokhale A, Zlatic SA, Craige B, Lee JD, Talbot K, Pare J-F, Smith Y, et al. The schizophrenia susceptibility factor dysbindin and its associated complex sort cargoes from cell bodies to the synapse. Mol Biol Cell. 2011;22:4854–4867. doi: 10.1091/mbc.E11-07-0592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salazar G, Zlatic S, Craige B, Peden AA, Pohl J, Faundez V. Hermansky-pudlak syndrome protein complexes associate with phosphatidylinositol-4-kinase type II alpha in neuronal and non-neuronal cells. J Biol Chem. 2009;284:1790–1802. doi: 10.1074/jbc.M805991200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44•.Hermann GJ, Scavarda E, Weis AM, Saxton DS, Thomas LL, Salesky R, Somhegyi H, Curtin TP, Barrett A, Foster OK, et al. C. elegans BLOC-1 functions in trafficking to lysosome-related gut granules. PLoS One. 2012;7:e43043. doi: 10.1371/journal.pone.0043043. The authors identify distant homologues of BLOC-1 subunits in a screen for mutants that impact the formation of the gut granule LROs in C. elegans. They show that, like in BLOC-1-deficient vertebrate melanocytes, a subset of LRO cargoes are missorted to the plasma membrane or conventional endosomes in the BLOC-1-deficient intestine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45••.Gerondopoulos A, Langemeyer L, Liang J-R, Linford A, Barr FA. BLOC-3 mutated in Hermansky-Pudlak syndrome is a Rab32/38 guanine nucleotide exchange factor. Curr Biol. 2012;22:2135–2139. doi: 10.1016/j.cub.2012.09.020. The authors exploit distant homologies of BLOC-3 subunits with the RAB7 exchange factor, Mon1/CCz1, to show that BLOC-3 functions as a GEF for RAB32 and RAB38 and impacts cargo sorting to melanosomes in a melanoma cell line. This is the first report assigning a molecular function to one of the BLOCs that are defective in HPS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wasmeier C, Romao M, Plowright L, Bennett DC, Raposo G, Seabra MC. Rab38 an Rab32 control early post-Golgi trafficking of melanogenic enzymes. J Cell Biol. 2006;175:271–281. doi: 10.1083/jcb.200606050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lopes VS, Wasmeier C, Seabra MC, Futter CE. Melanosome maturation defect in Rab38-deficient retinal pigment epithelium results in instability of immature melanosomes during transient melanogenesis. Mol Biol Cell. 2007;18:3914–3927. doi: 10.1091/mbc.E07-03-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ambrosio AL, Boyle JA, Di Pietro SM. Mechanism of platelet dense granule biogenesis: study of cargo transport and function of Rab32 and Rab38 in a model system. Blood. 2012;120:4072–4081. doi: 10.1182/blood-2012-04-420745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Osanai K, Higuchi J, Oikawa R, Kobayashi M, Tsuchihara K, Iguchi M, Huang J, Voelker DR, Toga H. Altered lung surfactant system in a Rab38-deficient rat model of Hermansky-Pudlak syndrome. Am J Lung Cell Mol Physiol. 2010;298:L243–L251. doi: 10.1152/ajplung.00242.2009. [DOI] [PubMed] [Google Scholar]
- 50.Zhang L, Yu K, Robert KW, DeBolt KM, Hong N, Tao JQ, Fukuda M, Fisher AB, Huang S. Rab38 targets to lamellar bodies and normalizes their sizes in lung alveolar type II epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2011;301:461–477. doi: 10.1152/ajplung.00056.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51••.Ellis K, Bagwell J, Bagnat M. Notochord vacuoles are lysosome-related organelles that function in axis and spine morphogenesis. J Cell Biol. 2013;200:667–679. doi: 10.1083/jcb.201212095. The authors define a novel LRO in the inner cells of the notochord. A large vacuole with features of an LRO functions in spinal chord elongation during development, and its formation requires RAB32 and the vacuolar ATPase. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Helip-Wooley A, Westbroek W, Dorward HM, Koshoffer A, Huizing M, Boissy RE, Gahl WA. Improper trafficking of melanocyte-specific proteins in Hermansky-Pudlak syndrome type-5. J Invest Dermatol. 2007;127:1471–1478. doi: 10.1038/sj.jid.5700737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bultema JJ, Ambrosio AL, Burek CL, Di Pietro SM. BLOC-2, AP-3, and AP-1 function in concert with Rab38 and Rab32 to mediate protein trafficking to lysosome-related organelles. J Biol Chem. 2012;287:19550–19563. doi: 10.1074/jbc.M112.351908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tamura K, Ohbayashi N, Maruta Y, Kanno E, Itoh T, Fukuda M. Varp is a novel Rab32/38-binding protein that regulates Tyrp1 trafficking in melanocytes. Mol Biol Cell. 2009;20:2900–2908. doi: 10.1091/mbc.E08-12-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Burgo A, Sotirakis E, Simmler MC, Verraes A, Chamot C, Simpson JC, Lanzetti L, Proux-Gillardeaux V, Galli T. Role of Varp, a Rab21 exchange factor and TI-VAMP/VAMP7 partner, in neurite growth. EMBO Rep. 2009;10:1117–1124. doi: 10.1038/embor.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56••.Schäfer IB, Hesketh GG, Bright NA, Gray SR, Pryor PR, Evans PR, Luzio JP, Owen DJ. The binding of Varp to VAMP7 traps VAMP7 in a closed, fusogenically inactive conformation. Nat Struct Mol Biol. 2012;19:1300–1309. doi: 10.1038/nsmb.2414. In this elegant paper, the authors show that the RAB32/38 effector, VARP, binds to the R-SNARE VAMP7 and maintains it in a closed conformation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang X, He X, Fu XY, Chang Z. Varp is a Rab21 guanine nucleotide exchange factor and regulates endosome dynamics. J Cell Sci. 2006;119:1053–1062. doi: 10.1242/jcs.02810. [DOI] [PubMed] [Google Scholar]
- 58.Kloer DR, Rojas R, Ivan V, Moriyama K, van Vlijmen T, Murthy N, Ghirlando R, van der Sluijs P, Hurley JH, Bonifacino JS. Assembly of the biogenesis of lysosome-related organelles complex-3 (BLOC-3) and its interaction with Rab9. J Biol Chem. 2010;285:7794–7804. doi: 10.1074/jbc.M109.069088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hermann GJ, Schroeder LK, Hieb CA, Kershner AM, Rabbitts BM, Fonarev P, Grant BD, Priess JR. Genetic analysis of lysosomal trafficking in Caenorhabditis elegans. Mol Biol Cell. 2005;16:3273–3288. doi: 10.1091/mbc.E05-01-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ma J, Plesken H, Treisman JE, Edelman-Novemsky I, Ren M. Lightoid and Claret: a rab GTPase and its putative guanine nucleotide exchange factor in biogenesis of Drosophila eye pigment granules. Proc Natl Acad Sci US A. 2004;101:11652–11657. doi: 10.1073/pnas.0401926101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nguyen T, Wei ML. Hermansky-Pudlak HPS1/pale ear gene regulates epidermal and dermal melanocyte development. J Invest Dermatol. 2007;127:421–428. doi: 10.1038/sj.jid.5700566. [DOI] [PubMed] [Google Scholar]
- 62.Charette SJ, Cosson P. A LYST/beige homolog is involved in biogenesis of Dictyostelium secretory lysosomes. J Cell Sci. 2007;120:2338–2343. doi: 10.1242/jcs.009001. [DOI] [PubMed] [Google Scholar]
- 63.Kypri E, Falkenstein K, Lozanne AD. Antagonistic control of lysosomal fusion by Rab14 and the Lyst-related protein LvsB. Traffic. 2013 doi: 10.1111/tra.12058. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Durchfort N, Verhoef S, Vaughn MB, Shrestha R, Adam D, Kaplan J, Ward DM. The enlarged lysosomes in beigej cells result from decreased lysosome fission and not increased lysosome fusion. Traffic. 2013;13:108–119. doi: 10.1111/j.1600-0854.2011.01300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65•.Albers CA, Cvejic A, Favier R, Bouwmans EE, Alessi MC, Bertone P, Jordan G, Kettleborough RN, Kiddle G, Kostadima M, et al. Exome sequencing identifies NBEAL2 as the causative gene for gray platelet syndrome. Nat Genet. 2011;43:735–737. doi: 10.1038/ng.885. This is one of three back-to-back papers identifying the gene whose mutation underlies the majority of gray platelet syndrome patients, and suggests that the defective gene results in a failure of α granule cargoes to segregate from the classical lysosomal pathway. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66•.Gunay-Aygun M, Falik-Zaccai TC, Vilboux T, Zivony-Elboum Y, Gumruk F, Cetin M, Khayat M, Boerkoel CF, Kfir N, Huang Y, et al. NBEAL2 is mutated in gray platelet syndrome and is required for biogenesis of platelet α-granules. Nat Genet. 2011;43:732–734. doi: 10.1038/ng.883. This is one of three back-to-back papers identifying the gene whose mutation underlies the majority of gray platelet syndrome patients, and suggests that the defective gene results in a failure of α granule cargoes to segregate from the classical lysosomal pathway. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67•.Kahr WH, Hinckley J, Li L, Schwertz H, Christensen H, Rowley JW, Pluthero FG, Urban D, Fabbro S, Nixon B, et al. Mutations in NBEAL2, encoding a BEACH protein, cause gray platelet syndrome. Nat Genet. 2011;43:738–740. doi: 10.1038/ng.884. This is one of three back-to-back papers identifying the gene whose mutation underlies the majority of gray platelet syndrome patients, and suggests that the defective gene results in a failure of α granule cargoes to segregate from the classical lysosomal pathway. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sitaram A, Piccirillo R, Palmisano I, Harper DC, Dell’Angelica EC, Schiaffino MV, Marks MS. Localization to mature melanosomes by virtue of cytoplasmic dileucine motifs is required for human OCA2 function. Mol Biol Cell. 2009;20:1464–1477. doi: 10.1091/mbc.E08-07-0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Theos AC, Tenza D, Martina JA, Hurbain I, Peden AA, Sviderskaya EV, Stewart A, Robinson MS, Bennett DC, Cutler DF, et al. Functions of AP-3 and AP-1 in tyrosinase sorting from endosomes to melanosomes. Mol Biol Cell. 2005;16:5356–5372. doi: 10.1091/mbc.E05-07-0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Camus G, Segura-Morales C, Molle D, Lopez-Vergès S, Begon-Pescia C, Cazevieille C, Schu P, Bertrand E, Berlioz-Torrent C, Basyuk E. The clathrin adaptor complex AP-1 binds HIV-1 and MLV Gag and facilitates their budding. Mol Biol Cell. 2007;18:3193–3203. doi: 10.1091/mbc.E06-12-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Truschel ST, SImoes S, Setty SRG, Harper DC, Tenza D, Thomas PC, Herman KE, Sackett SD, Cowan DC, Theos AC, et al. ESCRT-1 function is required for Tyrp1 transport from early endosomes to the melanosome limiting membrane. Traffic. 2009;10:1318–1336. doi: 10.1111/j.1600-0854.2009.00955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lui-Roberts WW, Ferraro F, Nightingale TD, Cutler DF. Aftiphilin and gamma-synergin are required for secretagogue sensitivity of Weibel-Palade bodies in endothelial cells. Mol Biol Cell. 2008;19:5072–5081. doi: 10.1091/mbc.E08-03-0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Loubéry S, Delevoye C, Louvard D, Raposo G, Coudrier E. Myosin VI regulates actin dynamics and melanosome biogenesis. Traffic. 2012;13:665–680. doi: 10.1111/j.1600-0854.2012.01342.x. [DOI] [PubMed] [Google Scholar]
- 74••.Nightingale TD, White IJ, Doyle EL, Turmaine M, Harrison-Lavoie KJ, Webb KF, Cramer LP, Cutler DF. Actomyosin II contractility expels von Willebrand factor from Weibel-Palade bodies during exocytosis. J Cell Biol. 2011;194:613–629. doi: 10.1083/jcb.201011119. This paper shows that actin plays dual opposing roles in regulating WPB exocytosis. Actin functions prefusion to dock and immobilize WPBs at the plasma membrane, but following stimulus-induced fusion of the WPB and plasma membranes, actin forms a contractile ring to squeeze out the vWF contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75•.Jonnalagadda D, Izu LT, Whiteheart SW. Platelet secretion is kinetically heterogeneous in an agonist-responsive manner. Blood. 2012;120:5209–5216. doi: 10.1182/blood-2012-07-445080. This paper shows that different contents of platelet α granules are secreted with differential kinetics in response to different agonists, but do not follow “thematic” trends and appear to be released in a stochastic fashion. This opposes conclusions drawn in refs. 76 and 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Italiano JEJ, Richardson JL, Patel-Hett S, Battinelli E, Zaslavsky A, Short S, Ryeom S, Folkman J, Klement GL. Angiogenesis is regulated by a novel mechanism: pro- and antiangiogenic proteins are organized into separate platelet alpha granules and differentially released. Blood. 2008;111:1227–1233. doi: 10.1182/blood-2007-09-113837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sehgal S, Storrie B. Evidence that differential packaging of the major platelet granule proteins von Willebrand factor and fibrinogen can support their differential release. J Thrombosis Haemostasis. 2007;5:2009–2016. doi: 10.1111/j.1538-7836.2007.02698.x. [DOI] [PubMed] [Google Scholar]
- 78.Ménager MM, Ménasché G, Romao M, Knapnougel P, Ho CH, Garfa M, Raposo G, Feldmann J, Fischer A, de Saint Basile G. Secretory cytotoxic granule maturation and exocytosis require the effector protein hMunc13-4. Nat Immunol. 2007;8:257–267. doi: 10.1038/ni1431. [DOI] [PubMed] [Google Scholar]
- 79.Hume AN, Seabra MC. Melanosomes on the move: a model to understand organelle dynamics. Biochem Soc Trans. 2011;39:1191–1196. doi: 10.1042/BST0391191. [DOI] [PubMed] [Google Scholar]
- 80.Zografou S, Basagiannis D, Papafotika A, Shirakawa R, Horiuchi H, Auerbach D, Fukuda M, Christoforidis S. A complete Rab screening reveals novel insights in Weibel-Palade body exocytosis. J Cell Sci. 2012;125:4780–4790. doi: 10.1242/jcs.104174. [DOI] [PubMed] [Google Scholar]
- 81•.Bierings R, Hellen N, Kiskin N, Knipe L, Fonseca AV, Patel B, Meli A, Rose M, Hannah MJ, Carter T. The interplay between the Rab27A effectors Slp4-a and MyRIP controls hormone-evoked Weibel-Palade body exocytosis. Blood. 2012;120:2757–2767. doi: 10.1182/blood-2012-05-429936. Together with refs. 82 and 83, this paper shows that multiple RAB27A effectors function at discrete steps during the secretion of WPBs and LGs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82•.Kurowska M, Goudin N, Nehme NT, Court M, Garin J, Fischer A, de Saint Basile G, Ménasché G. Terminal transport of lytic granules to the immune synapse is mediated by the kinesin-1/Slp3/Rab27a complex. Blood. 2012;119:3879–3889. doi: 10.1182/blood-2011-09-382556. Together with refs. 81 and 83, this paper shows that multiple RAB27A effectors function at discrete steps during the secretion of WPBs and LGs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83•.Elstak ED, Neeft M, Nehme NT, Voortman J, Cheung M, Goodarzifard M, Gerritsen HC, van Bergen En Henegouwen PM, Callebaut I, de Saint Basile G, et al. The munc13-4-rab27 complex is specifically required for tethering secretory lysosomes at the plasma membrane. Blood. 2011;118:1570–1578. doi: 10.1182/blood-2011-02-339523. Together with refs. 81 and 82, this paper shows that multiple RAB27A effectors function at discrete steps during the secretion of WPBs and LGs. [DOI] [PubMed] [Google Scholar]
- 84.Stinchcombe JC, Majorovits E, Bossi G, Fuller S, Griffiths GM. Centrosome polarization delivers secretory granules to the immunological synapse. Nature. 2006;443:462–465. doi: 10.1038/nature05071. [DOI] [PubMed] [Google Scholar]
- 85••.Stinchcombe JC, Salio M, Cerundolo V, Pende D, Arico M, Griffiths GM. Centriole polarisation to the immunological synapse directs secretion from cytolytic cells of both the innate and adaptive immune systems. BMC Biol. 2011;9:45. doi: 10.1186/1741-7007-9-45. Together with Ref. 84, this paper show that the centrosome polarizes to the plasma membrane opposing the target cell in both CTLs and NK cells to facilitate positioning of LGs for secretion into the immunological synapse. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tsun A, Qureshi I, Stinchcombe JC, Jenkins MR, de la Roche M, Kleczkowska J, Zamoyska R, Griffiths GM. Centrosome docking at the immunological synapse is controlled by Lck signaling. J Cell Biol. 2011;192:663–674. doi: 10.1083/jcb.201008140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mentlik AN, Sanborn KB, Holzbaur EL, Orange JS. Rapid lytic granule convergence to the MTOC in natural killer cells is dependent on dynein but not cytolytic commitment. Mol Biol Cell. 2010;21:2241–2256. doi: 10.1091/mbc.E09-11-0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rak GD, Mace EM, Banerjee PP, Svitkina T, Orange JS. Natural killer cell lytic granule secretion occurs through a pervasive actin network at the immune synapse. PLOS Biol. 2011;9:e1001151. doi: 10.1371/journal.pbio.1001151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yuseff MI, Reversat A, Lankar D, Diaz J, Fanget I, Pierobon P, Randrian V, Larochette N, Vascotto F, Desdouets C, et al. Polarized secretion of lysosomes at the B cell synapse couples antigen extraction to processing and presentation. Immunity. 2011;35:361–374. doi: 10.1016/j.immuni.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 90.Sieni E, Cetica V, Mastrodicasa E, Pende D, Moretta L, Griffiths G, Aricò M. Familial hemophagocytic lymphohistiocytosis: a model for understanding the human machinery of cellular cytotoxicity. Cell Mol Life Sci. 2012;69:29–40. doi: 10.1007/s00018-011-0835-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bryceson YT, Rudd E, Zheng C, Edner J, Ma D, Wood SM, Bechensteen AG, Boelens JJ, Celkan T, Farah RA, et al. Defective cytotoxic lymphocyte degranulation in syntaxin-11 deficient familial hemophagocytic lymphohistiocytosis 4 (FHL4) patients. Blood. 2007;110:1906–1915. doi: 10.1182/blood-2007-02-074468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Arneson LN, Brickshawana A, Segovis CM, Schoon RA, Dick CJ, Leibson PJ. Cutting edge: syntaxin 11 regulates lymphocyte-mediated secretion and cytotoxicity. J Immunol. 2007;179:3397–3401. doi: 10.4049/jimmunol.179.6.3397. [DOI] [PubMed] [Google Scholar]
- 93.Ye S, Karim ZA, Al Hawas R, Pessin JE, Filipovich AH, Whiteheart SW. Syntaxin-11, but not syntaxin-2 or syntaxin-4, is required for platelet secretion. Blood. 2012;120:2484–2492. doi: 10.1182/blood-2012-05-430603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Côte M, Ménager MM, Burgess A, Mahlaoui N, Picard C, Schaffner C, Al-Manjomi F, Al-Harbi M, Alangari A, Le Deist F, et al. Munc18-2 deficiency causes familial hemophagocytic lymphohistiocytosis type 5 and impairs cytotoxic granule exocytosis in patient NK cells. J Clin Invest. 2009;119:3765–3773. doi: 10.1172/JCI40732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95••.Al Hawas R, Ren Q, Ye S, Karim ZA, Filipovich AH, Whiteheart SW. Munc18b/STXBP2 is required for platelet secretion. Blood. 2012;120:2493–2500. doi: 10.1182/blood-2012-05-430629. Together with ref. 93, this paper clarifies the components of the SNARE complex and its Sec1/Munc18 homologue that regulates fusion of α granules and dense granules with the plasma membrane upon stimulation of platelets. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dabrazhynetskaya A, Ma J, Guerreiro-Cacais AO, Arany Z, Rudd E, Henter JI, Karre K, Levitskaya J, Levitsky V. Syntaxin 11 marks a distinct intracellular compartment recruited to the immunological synapse of NK cells to colocalize with cytotoxic granules. J Cell Mol Med. 2012;16:129–141. doi: 10.1111/j.1582-4934.2011.01280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Feldmann J, Callebaut I, Raposo G, Certain S, Bacq D, Dumont C, Lambert N, Ouachée-Chardin M, Chedeville G, Tamary H, et al. Munc13-4 is essential for cytolytic granules fusion and is mutated in a form of familial hemophagocytic lymphohistiocytosis (FHL3) Cell. 2003;115:461–473. doi: 10.1016/s0092-8674(03)00855-9. [DOI] [PubMed] [Google Scholar]
- 98.Ren Q, Wimmer C, Chicka MC, Ye S, Ren Y, Hughson FM, Whiteheart SW. Munc13-4 is a limiting factor in the pathway required for platelet granule release and hemostasis. Blood. 2010;116:869–877. doi: 10.1182/blood-2010-02-270934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wu XS, Masedunskas A, Weigert R, Copeland NG, Jenkins NA, Hammer JA., III Melanoregulin regulates a shedding mechanism that drives melanosome transfer from melanocytes to keratinocytes. Proc Natl Acad Sci US A. 2012;109:E2101–E2109. doi: 10.1073/pnas.1209397109. [DOI] [PMC free article] [PubMed] [Google Scholar]