Abstract
OBJECTIVES
Withdrawal time has been proposed as a quality indicator for colonoscopy based on evidence that it is directly related to the rate of adenoma detection. Our objective was to test the hypothesis that baseline withdrawal time is inversely associated with the risk of finding neoplasia at interval colonoscopy.
METHODS
3121 subjects, age 50 to 75 years, had screening colonoscopy between 1994–1997 at 13 Veteran Affairs Medical Centers. 1193 subjects returned by protocol for surveillance within 5.5 years. In the 304 patients without polyps at baseline, we evaluated the contribution of baseline withdrawal time to their risk of interval neoplasia using bivariate and logistic regression analysis. We also examined the correlation between mean withdrawal time, baseline adenoma detection rate, and interval neoplasia rate at the medical-center level.
RESULTS
The average withdrawal time at the baseline exam in subjects with neoplasia on follow-up was 15.3 minutes as compared to 13.2 minutes in subjects without neoplasia (p=0.18). In a logistic regression model, withdrawal time was not associated with the risk of interval neoplasia (p=0.07). At the medical-center level, mean withdrawal time was not correlated with the probability of finding interval neoplasia (p=0.61) but was positively correlated with adenoma detection rate at baseline (p=0.03).
CONCLUSIONS
In this study with a mean baseline withdrawal time greater than 12 minutes, there was no detectable association between withdrawal time and risk of future neoplasia. The medical-center level withdrawal time was positively correlated with adenoma detection. Therefore, above a certain threshold, withdrawal time may no longer be an adequate quality measure for screening colonoscopy.
II. Introduction
Colonoscopy has become an integral modality for colorectal cancer screening and is increasing in frequency.1, 2 Early detection and removal of adenomatous polyps by colonoscopy decreases the risk of progression to cancer.3–5 However, rates of adenoma detection can vary among individual endoscopists.6, 7 The effect of this variation is amplified when one considers that greater than 14 million colonoscopies are performed yearly in the United States.8
Factors leading to variation in polyp detection are only beginning to be understood. A Canadian study found that risk factors for interval colorectal cancer within 3 years of a colonoscopy included performance of the colonoscopy in an office setting or performance by an internist or family physician.9 Secondly, colonoscopy withdrawal time has been shown to be related to adenoma detection rate in several studies6, 7, and the U.S. Multi-Society Task Force recommended in 2002 that optimal withdrawal time for normal colonoscopies should average 6–10 minutes.10
It follows, then, that if a longer withdrawal time is associated with an increased rate of adenoma detection then it should also be associated with a decreased risk of future advanced adenomas and cancer. However, this relationship has not yet been evaluated. Our hypothesis is that, after adjusting for other factors that may influence the development of neoplasia, withdrawal time at baseline colonoscopy will be inversely associated with the risk of developing advanced neoplasia at five-years.
III. Methods
Setting and Study Participants
As part of VA Cooperative Study 380, participants were enrolled in 13 Veterans Affairs Medical Centers geographically distributed across the United States between February 1994 and January 1997 as previously described.11 Veterans age 50 to 75 years old were invited to participate. Exclusion criteria included symptoms of disease of the lower gastrointestinal tract, prior disease of the colon, structural examination of the colon within the previous ten years or any contraindication to colonoscopy. At enrollment, interviews were conducted to measure potential risk factors for colon neoplasia, including family history of colon cancer in a first degree relative, past and current tobacco and alcohol use, physical activity index,12 body mass index, presence of diabetes and use of nonsteroidal anti-inflammatory drugs.
A total of 17,732 patients were screened for enrollment; 2346 patients declined to complete the survey. Among the patients who met eligibility criteria, 1463 (31.4%) declined to participate, 3196 eligible patients were enrolled, and 3121 had complete colonoscopy examination to the cecum. If patients had repeat examinations within 6 months of the baseline examination, the additional findings were included as part of the baseline findings; this additional examination occurred in 6.0% of patients.
The study protocol was approved by a central Human Rights Committee, and by Institutional Review Boards at each participating center. This paper describes additional analysis of the study cohort. No new data collection was undertaken.
Study Protocol
Eligible subjects, after providing written informed consent, received a polyethylene glycol-based electrolyte solution for bowel preparation and colonoscopy was performed as described previously.11 The center principal investigator or faculty designate was personally involved in the colonoscopy procedure. The study protocol did not allow trainees to perform these exams without direct supervision. Bowel preparation was assessed as good (mucosa well seen throughout), fair (liquid contents; exam adequate) or poor (solid contents; exam compromised). Total procedure time and insertion time were recorded prospectively by study nurses. Insertion time was defined as the time from scope insertion to the time the cecum was reached. Cecal intubation was confirmed in all cases by photographing cecal landmarks. Withdrawal time was calculated by subtracting insertion time from total procedure time. If polyps were identified, they were photographed with an open biopsy forceps touching the polyp to confirm size. All polyps were removed when possible with the exception of multiple small rectal polyps in which up to six biopsies were taken to sample for adenomatous tissue. Pathology was interpreted by the local pathologist and sent for blinded central pathology review. When there was disagreement, a third blinded pathologist also reviewed the tissue.
In addition to polypectomies, select patients also underwent 4 to 8 random rectal biopsies to sample normal appearing mucosa for an ancillary study. In the group of patients without polyps at baseline, only the 300 patients who returned for follow up underwent biopsies. These biopsies were generally completed in about one minute at the conclusion of the colonoscopy. The exclusion of these biopsies from total procedure time was not explicitly prescribed in the study protocol. However, at the site of the CSP 380 principal investigator (D.L.), biopsies were not included as part of the procedure time.
The surveillance plan has been previously described.13 Patients with neoplasia at baseline were assigned to follow up depending on the baseline pathology. Patients with cancer or adenomas with high-grade dysplasia were followed based on physician decisions. Patients with villous adenomas or adenomas ≥10mm were assigned to have repeat colonoscopies at 2 and 5 years after the baseline exam. Patients with small tubular adenomas were randomly assigned to follow up at 2 and 5 years or 5 years only for surveillance. A portion of patients with no polyps at baseline were matched by age to patients with adenomas ≥10mm and assigned to surveillance at 5 years. There was a six-month scheduling window for return procedures provided for in study protocol.
Outcomes and measurements
Advanced colonic neoplasia was defined as an adenoma of 10mm of more, a villous or tubulovillous adenoma (at least 25% villous), adenoma with high-grade dysplasia or intramucosal carcinoma, or invasive cancer. Invasive cancer was defined as invasion of malignant cells beyond the muscularis mucosa.
“Baseline colonoscopy” was defined as colonoscopy performed at study entry or within 6 months of study entry if a repeat examination was required to clear the colon. “Interval colonoscopy” refers to the follow-up colonoscopy performed within 5.5 years. Neoplastic lesions detected during this colonoscopy were defined as “interval neoplasia.” Neoplasia included small adenomas and advanced colonic neoplasia.
Data Analysis
The relationship between withdrawal time (WT) and risk of neoplasia at follow-up was examined in two ways. First, a patient-level analysis was undertaken examining the risk of interval neoplasia in patients without polyps at baseline. The baseline colonoscopy WT was compared across patients with and without neoplasia at interval colonoscopy using t-tests for continuous measures. We then performed bivariate comparisons of WT and risk of interval adenoma across a number of purported risk factors for interval colon neoplasia, including age, gender, race (White compared to Non-White), prep quality at baseline, body mass index (BMI) (divided into quintiles), physical activity index 14[categorized as 24–28; 29–36; and 37+], family history of colorectal cancer in first-degree relatives, tobacco or alcohol use at baseline, diabetes and daily non-steroidal anti-inflammatory use (NSAID) at baseline. These were factors that were also hypothesized to affect WT and hence potentially confound the relationship between WT and interval neoplasia detection. T-tests and ANOVA were used for continuous measures and chi-square for categorical comparisons. Bonferonni adjustment was performed to account for multiple comparisons. Finally, we evaluated the various risk factors for neoplasia at 5.5 years employing a stepwise logistic regression modeling approach. The model included WT and age with the remaining factors determined by the selection procedure. With WT and age in the model, the only significant risk entered by the selection process was the physical activity index.
The second part of the analysis was performed at the center-level. We calculated the mean WT for each center based on all 1441 subjects without polyps at baseline colonoscopy. We then examined the correlation (Spearman Correlation) between mean WT by center and baseline adenoma detection rate in all patients from each center. We also examined the correlation between mean WT by center and interval neoplasia detection rate in the cohort of patients from each center who returned for surveillance within 5.5 years. The interval neoplasia rates would thus include those patients with no polyps at baseline and those who had their polyps removed during baseline colonoscopy.
All data were analyzed at the Veterans Affairs Cooperative Study coordinating center in Perry Point, MD. Management of the study data base and all statistical analyses were performed with SAS software (SAS Institute, Cary, N.C.). All p-values are two-sided.
IV. Results
One thousand one hundred ninety-three subjects returned by protocol for colonoscopic surveillance within 5.5 years (mean = 3.4 years, SD = 1.5 years). During surveillance, bowel preparation was described as good in 81.8% of patients, fair in 12.7% and poor in 5.5%. Of 1441 that were without polyps at baseline, 304 subjects returned for follow-up colonoscopy (mean = 4.8 years). Of the 304 subjects without polyps at baseline who were followed up, 49 subjects (16.2%) had interval neoplasia including 7 with advanced adenomas and one with invasive cancer. Two of the 304 follow-up patients did not have baseline withdrawal time recorded and were excluded from the analysis.
The characteristics of the study cohort are listed in Table 1. There were no statistically significant differences in proposed risk factors for neoplasia in the group with interval neoplasia as compared to those without neoplasia. There was a higher proportion of patients with family history of colon cancer that returned for surveillance. The prep quality at baseline was good in over 80% of patients.
Table 1.
Cohort Characteristics of patients without polyps at baseline
| Baseline Characteristic | All Patients N=1441 | Interval Neoplasia N=49 | No Interval Neoplasia N=255 | p value (neoplasia v. no neoplasia) |
|---|---|---|---|---|
|
| ||||
| Mean follow-up, yr (sd) | n/a | 4.8 (0.6) | 4.8 (0.7) | 0.67 |
|
| ||||
| Mean Age, yr (sd) | 62.7 (7.3) | 63.8 (6.8) | 62.4 (7.1) | 0.21 |
|
| ||||
| Race, N (%) | ||||
| White | 1202 (83.4) | 42 (85.7) | 211 (82.8) | |
| Other | 239 (16.6) | 7 (14.3) | 44 (17.7) | 0.61 |
|
| ||||
| Gender, N (%) | ||||
| Male | 1377 (95.6) | 48 (98.0) | 234 (91.8) | |
| Female | 64 (4.4) | 1 (2.0) | 21 (8.2) | 0.22 |
|
| ||||
| Family History, N (%) | 172 (11.9) | 12 (24.5) | 50 (19.6) | 0.44 |
|
| ||||
| Current Smoker, N (%) | 262 (18.2) | 8 (16.3) | 30 (11.8) | 0.38 |
|
| ||||
| Current Alcohol, N (%) | 587 (40.8) | 21 (42.9) | 106 (41.6) | 0.87 |
|
| ||||
| Mean Body Mass Index (sd) | 29.3 (5.2) | 30.9 (6.1) | 29.6 (5.5) | 0.14 |
|
| ||||
| Mean Physical Activity Index (sd) | 36.2 (8.7) | 38.4 (9.9) | 35.9 (8.2) | 0.10 |
|
| ||||
| Diabetes, N (%) | 289 (20.1) | 13 (26.5) | 41 (16.1) | 0.10 |
|
| ||||
| Daily NSAID Use, N (%) | 798 (55.4) | 25 (51.0) | 142 (55.7) | 0.55 |
|
| ||||
| Good Prep Quality at Baseline, N (%) | 1201 (83.4) | 41 (83.7) | 215 (84.3) | 0.88 |
The unadjusted results of the patient-level analysis are detailed in table 2. The average WT at baseline colonoscopy in subjects with neoplasia on follow-up was 15.3 minutes (SD 10.2) as compared to 13.2 minutes (SD 8.0) in subjects without neoplasia (p=0.18). Baseline WT ranged from 1–57 minutes and 1–55 minutes in patients with and without neoplasia on follow up. Seventy-five percent of subjects that returned for surveillance had baseline WT greater than eight minutes.
Table 2.
Withdrawal time (WT) and Interval Neoplasia: Baseline WT in all patients without polyps at study entry and in those without polyps who returned for follow up
| Patients without polyps at baseline | Interval Neoplasia | No Interval Neoplasia | |
|---|---|---|---|
|
| |||
| # Cases* | 1429 | 49 | 253 |
|
| |||
| Mean WT (sd), min | 12.0 (8.2) | 15.3 (10.2) | 13.2 (8.0) |
| p=0.18 ** | |||
|
| |||
| Percentiles, min | |||
| 10 | 4 | 6 | 6 |
| 25 | 7 | 8 | 8 |
| 50 | 10 | 14 | 12 |
| 75 | 15 | 18 | 16 |
| 95 | 27 | 31 | 27 |
Two subjects did not have withdrawal time recorded and have thus been excluded from the analysis.
p value refers to the comparison of withdrawal times in the two interval neoplasia groups
Table 3 describes the baseline colonoscopy WT and rates of interval neoplasia in relation to the potential confounding variables. After Bonferroni adjustment, none of the variables we examined were significantly associated with average WT or an increased risk of interval neoplasia. In our logistic regression model, baseline colonoscopy WT was not independently associated with the risk of interval neoplasia (OR 1.03, p=0.07) after adjusting for age, prep quality, BMI and physical activity index. Only increased physical activity index was an independent predictor of risk of interval neoplasia in patients with no polyps at baseline (OR 1.04, 95% CI 1.01–1.08).
Table 3.
Bivariate comparisons with withdrawal time and percent interval neoplasia
| Withdrawal Time | Interval Neoplasia | ||||
|---|---|---|---|---|---|
|
| |||||
| Baseline Characteristic | N | Mean (sd), min | p value | N (%) | p value |
| Age (mean, sd) | |||||
| 50–59 | 109 | 13.6 (8.8) | 14 (12.8) | ||
| 60–69 | 140 | 13.8 (8.8) | 26 (18.6) | ||
| 70–75 | 55 | 12.9 (6.5) | 0.80 | 9 (16.4) | 0.47 |
|
| |||||
| Prep Quality | |||||
| Good | 256 | 13.3 (8.4) | 41 (16.0) | ||
| Fair/Poor | 48 | 14.8 (8.4) | 0.26 | 8 (16.7) | 0.91 |
|
| |||||
| Body Mass Index | |||||
| QUINTILE 1 | 59 | 13.3 (7.1) | 8 (13.6) | ||
| 2 | 56 | 14.9 (8.7) | 7 (12.5) | ||
| 3 | 64 | 14.1 (10.8) | 9 (14.1) | ||
| 4 | 63 | 12.5 (7.3) | 8 (12.7) | ||
| 5 | 58 | 12.8 (7.6) | 0.55 | 16(27.6) | 0.12 |
|
| |||||
| Physical Activity Index | |||||
| 24–28 | 70 | 13.3 (9.0) | 7 (10.0) | ||
| 29–36 | 114 | 15.0 (8.0) | 19 ( 16.7) | ||
| 37++ | 118 | 12.3 (8.3) | 0.05 | 23 (19.2) | 0.25 |
|
| |||||
| Family History | |||||
| Yes | 62 | 13.2 (6.8) | 12 (19.4) | ||
| No | 242 | 13.6 (8.8) | 0.73 | 37 (15.3) | 0.44 |
|
| |||||
| Gender | |||||
| Male | 282 | 13.5 (8.4) | 48 (17.0) | ||
| Female | 22 | 14.6 (8.6) | 0.55 | 1 ( 4.6) | 0.22 |
|
| |||||
| Race | |||||
| White | 253 | 13.0 (8.2) | 42 (16.6) | ||
| Other | 51 | 16.4 (8.9) | 0.01 | 7 ( 13.7) | 0.61 |
|
| |||||
| Smoking History | |||||
| Current | 38 | 12.5 (6.1) | 8 (21.1) | ||
| Other | 266 | 13.7 ( 8.7) | 0.40 | 41 (15.4) | 0.35 |
|
| |||||
| Current Alcohol | |||||
| Yes | 127 | 12.2 (6.6) | 21 (16.5) | ||
| No | 177 | 14.5 (9.4) | 0.02 | 28 (15.8) | 0.87 |
|
| |||||
| Diabetes | |||||
| Yes | 54 | 13.8 (8.9) | 13 (24.1) | ||
| No | 250 | 13.5 (8.3) | 0.82 | 36 (14.4) | 0.10 |
|
| |||||
| Daily NSAID Use | |||||
| Yes | 167 | 13.4 (8.6) | 25 (15.0) | ||
| No | 137 | 13.8 (8.3) | 0.66 | 24 (17.5) | 0.55 |
The results of the center level analysis are summarized in table 4 and figure 1. Average WT ranged from 5.2–25.6 minutes and was correlated with the detection rate for adenomas (Spearman correlation coefficient [r]=0.61, p=0.03) but not advanced neoplasia (r=0.26, p=0.40) at the baseline colonoscopy. However, if the center with the fastest average WT (5.2 min) was eliminated, the correlation with adenoma detection was no longer seen. There was no correlation of WT with average polyp size (r=−0.42, p=0.15) though the center with the fastest WT did have a larger average polyp size when compared to the other centers. WT at baseline was not associated with finding interval neoplasia (r=0.16, p=0.61) nor interval advanced neoplasia (r=0.21, p=0.49).
Table 4.
Center Level Analysis: Association of baseline withdrawal time with adenoma detection rates at baseline, average polyp size at baseline, and risk of neoplasia at 5 years in those that returned for follow up.
| Center | Baseline Colonoscopy | Interval Colonoscopy | ||||||
|---|---|---|---|---|---|---|---|---|
| Number of cases | WT (min) in patients without polyps | Adenomas (%) | Advanced Neoplasia (%) | Mean Polyp Size (mm) | Number of cases | Interval Neoplasia (%) | Interval Advanced Neoplasia (%) | |
| 1 | 413 | 5.2 | 17.7 | 8.0 | 7.0 | 112 | 41.1 | 13.4 |
| 2 | 274 | 8.6 | 39.1 | 7.3 | 4.0 | 108 | 31.5 | 1.9 |
| 3 | 129 | 8.7 | 34.1 | 8.5 | 5.2 | 57 | 33.3 | 5.3 |
| 4 | 163 | 10.2 | 39.3 | 16.6 | 5.9 | 66 | 47.0 | 4.6 |
| 5 | 288 | 11.6 | 39.9 | 14.9 | 6.5 | 86 | 27.9 | 4.7 |
| 6 | 305 | 11.6 | 42.0 | 10.5 | 4.6 | 141 | 41.1 | 8.5 |
| 7 | 252 | 12.6 | 36.9 | 7.9 | 4.3 | 88 | 33.0 | 4.6 |
| 8 | 273 | 14.4 | 43.2 | 6.6 | 5.2 | 111 | 55.9 | 15.3 |
| 9 | 295 | 14.7 | 35.9 | 12.5 | 5.9 | 96 | 40.6 | 9.4 |
| 10 | 150 | 16.8 | 48.7 | 14.0 | 4.9 | 53 | 32.1 | 9.4 |
| 11 | 189 | 18.5 | 38.1 | 11.6 | 4.3 | 79 | 26.6 | 3.8 |
| 12 | 141 | 24.5 | 46.8 | 9.2 | 4.0 | 85 | 64.7 | 5.9 |
| 13 | 237 | 25.6 | 43.5 | 11.4 | 4.5 | 111 | 45.1 | 9.9 |
| Correlation Coefficient of baseline WT with : | 0.608 (p=0.03) | 0.256 (p=0.40) | −0.420 (p=0.15) | n/a | 0.156 (p=0.61) | 0.212 (p=0.48) | ||
Figure 1.
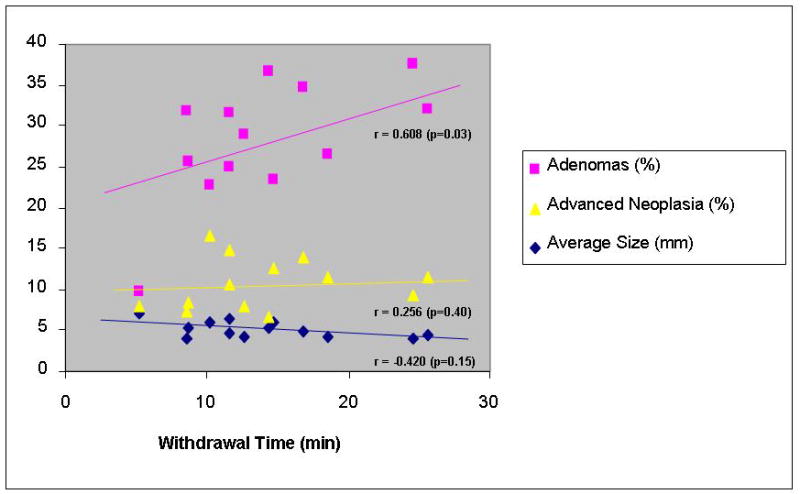
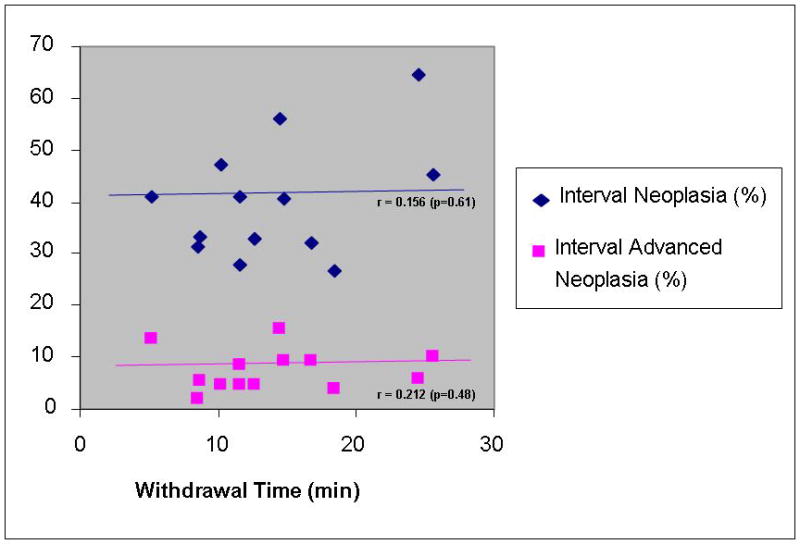
Figure 1a. Center Level Analysis: Association of withdrawal time with average polyp size and adenoma detection rates at baseline
Figure 1b. Center Level Analysis; Association of withdrawal time at baseline with risk of neoplasia at 5 years.
V. Discussion
This study is the first to examine the impact of withdrawal time at baseline with metachronous or missed adenomas. Contrary to our hypothesis, our analysis did not identify an association between withdrawal time at baseline colonoscopy and risk of neoplasia on follow up colonoscopy within 5.5 years. The medical center-level analysis did detect a positive correlation between withdrawal time and adenoma detection rate, corroborating the results of previously published studies.6, 7 However, our data suggests that a threshold may exist between 5.2 and 8.6 minutes beyond which withdrawal time may no longer be correlated with adenoma detection.
Our results could be explained by the concept that longer withdrawal time leads to the increased removal of clinically insignificant polyps. In fact, prior studies have suggested that the perceived association between withdrawal time and polyp detection is primarily driven by the detection of polyps <5mm.15 Furthermore, in the study by Barclay and colleagues, mean polyp size was negatively correlated with average withdrawal time in procedures in which no polyps were removed (spearman rank-correlation coefficient − 0.57, p=0.05).6 The challenge in translating these results into practice rests in the fact that the preventive benefit of colonoscopy is based on studies in which all detected polyps were removed, regardless of size.11 It is unclear if the same protective effect would be demonstrated with screening strategies in which diminutive polyps are left intact.
Another potential explanation for our findings is the possibility that a longer withdrawal time at baseline is a marker for a more difficult exam rather than a more careful exam. While our analysis was not designed to identify determinants of withdrawal time, we did find that patients receiving narcotics in addition to midazolam had a longer average withdrawal time that those receiving midazolam alone (data not shown). If patients with a longer withdrawal time had a more difficult exam, then it may be possible that more lesions were missed in procedures with longer withdrawal times. Thus the potential protective effect of a longer withdrawal time (i.e. careful examination) might be diluted by the increased likelihood of missed lesions.
Our findings must be interpreted in the context of the study limitations. Our power to detect meaningful differences in interval neoplasia risk at the patient level is limited by the small number of cases of interval neoplasia in our patient-level cohort. Indeed, our observed association between baseline withdrawal time and interval neoplasia, albeit marginal, bordered on statistical significance. This limitation was partly due to the original study design, in which only a small percentage of patients without polyps at baseline were invited to return for surveillance. We undertook the center-level analysis, in part, to overcome the sample size limitation and minimize the risk of making a type 2 statistical error. Additionally, the five year follow up interval may have been too short to see a large effect on risk of advanced neoplasia.
Another limitation of our study is the possibility of residual confounding related to imperfect measures of colonoscopy quality. The protective effect of a colonoscopy is dependent on the identification and removal of pre-cancerous polyps at baseline and again at the interval exam. We identified withdrawal time as a proxy for quality at baseline, but similar measures of quality were not available to adjust the analysis of interval neoplasia. In fact, in our center-level analysis, there is a significant variability in the detection rates for interval adenomas and advanced adenomas. This variability may relate to the quality of the baseline exam; however, it may also reflect the quality of the surveillance exam.
As with other studies conducted in the VA, our study cohort was largely limited to men, so these results may not be generalized to colonoscopies in women. Secondly, the average withdrawal times in our study are considerably longer than those previously published in non-VA settings. In fact, in our study, less than ten percent of subjects had withdrawal times <6 minutes. As a result, our findings may not be generalizeable to settings with a shorter withdrawal time, especially if the relationship between these two variables is not linear. Some of discrepancy may be explained by the rectal biopsies taken as part of study protocol, though these biopsies were generally completed in about one minute at the conclusion of the colonoscopy and were not uniformly included in procedure time. Nevertheless, recent studies performed within the veterans health care system have reported average withdrawal times comparable to our results, 22, 23 so there may be important unrecognized systematic differences between VA and non-VA health care systems that makes quality comparisons challenging. These differences may relate to patient populations, healthcare resource limitations or systematic differences in the performance of colonoscopy, and they deserve further study prior to the application of existing quality measures to VA populations.
While withdrawal time in our study and that reported outside the VA may be different, the rates of interval neoplasia observed in our study mirror those reported in other patient populations. For example, the proportion of patients in our study with negative colonoscopies at baseline who developed cancer within 5 years (0.33%) is low, consistent with findings reported by Singh et al (0.38%) in Canada.24 Moreover, the proportion of interval advanced adenomas in our study (2.3%) was actually higher than that recently published by Imperiale et al (1.1%).25 Similarly, the rate of interval advanced neoplasia was also higher (2.6%) than a recently published Chinese screening cohort (1.4%).26 These differences may reflect differences in the patient population or variation in the quality of the baseline colonoscopy.
In conclusion, in our study with a mean baseline withdrawal time of greater than 12 minutes, we identified a modest positive correlation between withdrawal time and adenoma detection rate at the medical-center level, consistent with prior studies. However, baseline withdrawal time was not correlated with the detection of advanced adenomas at baseline or any interval neoplasia at follow up colonoscopy within 5.5 years. The interpretation of these findings is limited by our lack of shorter withdrawal times as reported in other studies and small sample size. However, our results suggest that there is a limited range in which withdrawal time is a useful quality indicator for colonoscopy; this range may exist because of the increased removal of clinically insignificant polyps with increasing withdrawal times above a certain threshold. Developing a better understanding of the significance of diminutive colorectal polyps will be integral to the refinement of existing quality measures including withdrawal time.
VII. Study Highlights.
-
WHAT IS CURRENT KNOWLEDGE
Colonoscopy withdrawal time is positively correlated with adenoma detection.
The impact of increasing withdrawal time on interval neoplasia risk is unknown.
-
WHAT IS NEW HERE
Above a certain threshold, withdrawal time may no longer be an adequate quality measure for screening colonoscopy
VIII. References
- 1.Harewood GC, Lieberman D. Colonoscopy practice patterns since introduction of medicare coverage for average-risk screening. Clin Gastroenterol Hepatol. 2004;2:72–77. doi: 10.1016/s1542-3565(03)00294-5. [DOI] [PubMed] [Google Scholar]
- 2.Lieberman DA, Holub J, Eisen G, et al. Utilization of colonoscopy in the United States: results from a national consortium. Gastrointest Endosc. 2005;62:875–883. doi: 10.1016/j.gie.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 3.Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. N Engl J Med. 1993;329:1977–1981. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 4.Citarda F, Tomaselli G, Capocaccia R, et al. Efficacy in standard clinical practice of colonoscopic polypectomy in reducing colorectal cancer incidence. Gut. 2001;48:812–815. doi: 10.1136/gut.48.6.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muller AD, Sonnenberg A. Prevention of colorectal cancer by flexible endoscopy and polypectomy; A case-control study of 32,702 veterans. Ann Intern Med. 1995;123:904–910. doi: 10.7326/0003-4819-123-12-199512150-00002. [DOI] [PubMed] [Google Scholar]
- 6.Barclay RL, Vicari JJ, Doughty AS, et al. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N Engl J Med. 2006;355:2533–2541. doi: 10.1056/NEJMoa055498. [DOI] [PubMed] [Google Scholar]
- 7.Rex DK. Colonoscopic withdrawal technique is associated with adenoma miss rates. Gastrointest Endosc. 2000;51:33–36. doi: 10.1016/s0016-5107(00)70383-x. [DOI] [PubMed] [Google Scholar]
- 8.Seeff LC, Richards TB, Shapiro JA, et al. How many endoscopies are performed for colorectal cancer screening? Results from CDC’s survey of endoscopic capacity. Gastroenterology. 2004;127:1670–1677. doi: 10.1053/j.gastro.2004.09.051. [DOI] [PubMed] [Google Scholar]
- 9.Bressler B, Paszat LF, Chen Z, et al. Rates of new or missed colorectal cancers after colonoscopy and their risk factors: a population-based analysis. Gastroenterology. 2007;132:96–102. doi: 10.1053/j.gastro.2006.10.027. [DOI] [PubMed] [Google Scholar]
- 10.Rex DK, Bond JH, Winawer S, et al. Quality in the technical performance of colonoscopy and the continuous quality improvement process for colonoscopy: recommendations of the U.S. Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol. 2002;97:1296–1308. doi: 10.1111/j.1572-0241.2002.05812.x. [DOI] [PubMed] [Google Scholar]
- 11.Lieberman D, Weiss DG, Bond JH, et al. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. N Engl J Med. 2000;343:162–168. doi: 10.1056/NEJM200007203430301. [DOI] [PubMed] [Google Scholar]
- 12.Lieberman D, Prindiville S, Weiss DG, et al. Risk factors for advanced colonic neoplasia and hyperplastic polyps in asymptomatic individuals. JAMA. 2003;290:2959–2967. doi: 10.1001/jama.290.22.2959. [DOI] [PubMed] [Google Scholar]
- 13.Lieberman D, Weiss DG, Harford WV, et al. Five-year colon surveillance after screening colonoscopy. Gastroenterology. 2007;133:1077–1085. doi: 10.1053/j.gastro.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 14.Kannel WB, Sorlie P. Some health benefits of physical activity. Arch Int Med. 1979;139:857–861. [PubMed] [Google Scholar]
- 15.Simmons DT, Harewood GC, Baron TH, et al. Impact of endoscopist withdrawal speed on polyp yield: implications for optimal colonoscopy withdrawal time. Aliment Pharmacol Ther. 2006;24:965–971. doi: 10.1111/j.1365-2036.2006.03080.x. [DOI] [PubMed] [Google Scholar]
- 16.Pickhardt PJ, Choi JR, Hwang I, et al. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. N Engl J Med. 2003;349:2191–2200. doi: 10.1056/NEJMoa031618. [DOI] [PubMed] [Google Scholar]
- 17.Johnson CD, Chen M-HY, Toledano A, et al. Accuracy of CT Colonography for detection of large adenomas and cancers. N Engl J Med. 2008;359:1207–17. doi: 10.1056/NEJMoa0800996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gottlieb LS, Winawer S, Sternberg SS. National Polyp Study (NPS): the diminutive colonic polyp (abstract) Gastrointest Endosc. 1984;28:143. [Google Scholar]
- 19.Butterly LF, Chase MP, Pohl H, et al. Prevalence of clinically important histology in small adenomas. Clin Gastroenterol Hepatol. 2006;4:343–348. doi: 10.1016/j.cgh.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 20.Pickhardt PJ, Hassan C, Laghi A, et al. Cost-effectiveness of colorectal cancer screening with computed tomography colonography: The impact of not reporting diminutive lesions. Cancer. 2007;109:2213–21. doi: 10.1002/cncr.22668. [DOI] [PubMed] [Google Scholar]
- 21.Eddy DM. Screening for colorectal cancer. Ann Intern Med. 1990;113:373–384. doi: 10.7326/0003-4819-113-5-373. [DOI] [PubMed] [Google Scholar]
- 22.Shah DK, Gerkin R, Leung FW, et al. Colonoscopy withdrawal time - impact of polypectomy and trainee involvement (abstract) Gastrointestinal Endoscopy. 67(5):AB292–AB292. [Google Scholar]
- 23.Chan MY, Cohen H, Spiegel BMR. Fewer polyps detected by colonoscopy as the day progresses at a Veteran’s Administration teaching hospital. Clin Gastroenterol Hepatol. doi: 10.1016/j.cgh.2009.07.013. (in press) [DOI] [PubMed] [Google Scholar]
- 24.Singh H, Turner D, Xue L, Targownik LE, Bernstein CN. Risk of developing colorectal cancer following a negative colonoscopy examination: evidence for a 10-year interval between colonoscopies. JAMA. 2006;295:2366–2373. doi: 10.1001/jama.295.20.2366. [DOI] [PubMed] [Google Scholar]
- 25.Imperiale TF, Glowinski EA, Lin-Cooper C, Larkin GN, Rogge JD, Ransohoff DF. Five-year risk of colorectal neoplasia after negative screening colonoscopy. N Engl J Med. 2008;359:1218–24. doi: 10.1056/NEJMoa0803597. [DOI] [PubMed] [Google Scholar]
- 26.Leung WK, Lau JY, Suen BY, Wong GL, Chow DK, Lai LH, To KF, Yim CK, Lee ES, Tsoi KK, Ng SS, Sung JJ. Repeat-screening colonoscopy 5 years after normal baseline-screening colonoscopy in average-risk Chinese: a prospective study. Am J Gastroenterol. 2009;104:2028–34. doi: 10.1038/ajg.2009.202. [DOI] [PubMed] [Google Scholar]


