Preface
Successful cell division requires the precise and timely coordination of chromosomal, cytoskeletal and membrane trafficking events. These processes are regulated by the competing actions of protein kinases and phosphatases. Aurora B is one of the most intensively studied kinases. In conjunction with the proteins INCENP, Borealin (also known as Dasra) and Survivin, it forms the Chromosomal Passenger Complex (CPC). This complex targets to different locations at differing times during mitosis,, where it regulates key mitotic events: correction of chromosome-microtubule attachment errors, activation of the spindle assembly checkpoint, and construction and regulation of the contractile apparatus that drives cytokinesis. Our growing understanding of the CPC has seen it develop from a mere passenger riding on chromosomes to one of the main controllers of mitosis.
Introduction
The chromosomal passenger hypothesis proposed that diverse mitotic events, including chromosome segregation and cytokinesis, could be coordinated by a set of proteins that localized to chromosomes during early mitosis, before transferring to the spindle midzone during late mitosis1. Interest in these proteins took off when it was realized that INCENP (Inner Centromere Protein), the first passenger protein identified2, formed a complex with Aurora B kinase, a protein essential for accurate cell division3, 4. It is now known that the chromosomal passenger complex (CPC) is composed of four subunits: the enzymatic component Aurora B and the three regulatory and targeting components INCENP, Survivin and Borealin (also known as Dasra)5–7 (Figure 1A).
FIGURE 1. Structure and regulation of the chromosomal passenger complex (CPC).
(A, Left) Diagram of the CPC, which is formed by Aurora B, INCENP, Survivin and Borealin. The diagram shows domains and functions for each CPC component. (Right, upper): the crystal structure of full length Aurora B complexed with the INCENP C-terminus (AA790-894) (adapted with permission from ref 78). (Right, bottom): the crystal structure of the three-helix bundle of INCENP(AA1-58), Borealin (AA10-109) and full length Survivin (adapted with permission from ref 16).
(B) Phospho-regulation of the CPC showing phosphorylations (yellow spheres) that regulate CPC localisation and function throughout mitosis (pale yellow boxes). Multiple kinases (coloured spheres) phosphorylate the CPC (plain arrows) to regulate CPC function. Additionally, Aurora B activates its own kinase activity by phosphorylating its T-loop (T232) and the INCENP TSS-motif (dotted arrows). Note that some of the depicted phosphorylations are present throughout mitosis (AurB T232) while others are present at specific stages of mitosis (INC T388, T58).
Dynamic changes in CPC localisation throughout mitosis ensure the effective and spatially restricted phosphorylation of substrates involved in chromosome condensation, correction of erroneous kinetochore-microtubule attachments, activation of the spindle assembly checkpoint (SAC), and cytokinesis. When INCENP, Survivin or Borealin localisation and/or function are perturbed, the others do not localize properly, Aurora B activity is diminished and proper cell division is compromised8–12. A fifth putative passenger protein, the GEF (Guanine Exchange Factor) TD-60/Rcc213 does not stably associate with the CPC and its function in mitosis is not yet understood.
Here we discuss current knowledge concerning the structure, activation, localisation and targets of the CPC in mitosis, focusing on the regulation of the complex by other cellular activities and revealing how this regulation changes as mitosis progresses. Due to space constraints, we will not cover the functions of the CPC in meiosis. Our aim is to show how this critical signaling module is integrated structurally and mechanistically with the global cell cycle machinery, and with the intricate structures at kinetochores and the central spindle.
Composition and Structure of the CPC
Biochemical and structural studies reveal that the CPC is composed of a localisation module and a kinase module linked together by the central region of INCENP (Figure 1A)5, 14, 15. The localisation module is composed of the INCENP N-terminus, Survivin and Borealin associated with each other in a three-helix bundle15, 16. This bundle links the baculovirus IAP repeat (BIR) domain of Survivin17, 18 and the C-terminus of Borealin, both of which are required for localisation to the centromere of chromosomes16, 19–22. This module is also required for localisation of the complex to the mitotic spindle and anaphase midbody, though the mechanism is poorly understood. The kinase module is composed of Aurora B bound to the highly conserved IN-BOX at the INCENP C-terminus3.
AURORA B KINASE
Aurora B belongs to a highly conserved family of Serine-Threonine kinases first discovered in Drosophila melanogaster23. This family has three members: Aurora A, which functions at the mitotic spindle poles; Aurora B, which functions at the centromere, anaphase spindle and cell cortex; and Aurora C, which resembles Aurora B, but regulates meiosis and mitosis during early development24. Together with Cyclin-dependent kinases (Cdks) and Polo-like kinases (Plks) the Aurora kinases are master controllers that coordinate individual processes during cell division with the checkpoints that determine the overall progression of mitosis and meiosis24, 25.
Aurora B activity is tightly regulated at multiple levels, including INCENP-binding, localisation, posttranslational modification and degradation. INCENP acts analogous to a cyclin in binding and activating Aurora B. It also contributes to the localisation and spatial regulation of the kinase. Aurora B activation is discussed in detail in the following section.
INCENP
INCENP, the platform on which the CPC assembles, was discovered in a monoclonal antibody screen for novel components of the mitotic chromosome scaffold2. The INCENP N-terminus is required for CPC localisation to centromeres26. INCENP residues 1–58 form a triple-helix bundle with Borealin and Survivin that is required for localisation to the centromere, anaphase spindle midzone and telophase midbody12, 16, 26, 27. INCENP also binds heterochromatin protein 1 (HP1)26, 28, 29. This is important for CPC localisation during interphase (see below).
INCENP is regulated by Aurora B and Cdk1, the cyclin-CDK complex that controls entry and exit from mitosis (Figure 1B). In budding yeast, phosphorylation of the INCENP homolog Sli15 by CDK and the Aurora B homolog Ipl-1 prevents the CPC from associating with the spindle midzone before anaphase30, 31. Yeast CDK also phosphorylates 6 sites on Sli15 that are required to activate the SAC, a signaling cascade that monitors chromosome attachment to the mitotic spindle and delays anaphase onset in response to unattached or tensionless kinetochores thus preventing chromosome segregation until proper kinetochore-microtubule attachments are formed32. Dephosphorylation of these sites at the onset of anaphase is necessary to prevent reactivation of the SAC when sister chromatids separate at anaphase onset and their kinetochores are no longer under tension32, 33.
Phosphorylation of INCENP by CDK1 is required for PLK1 localisation to the inner centromere in mice34, though this mechanism is not conserved in D. melanogaster35. However, INCENP and Aurora B are required for the activation of PLK1 at the inner centromere via phosphorylation of a residue on the T-loop of PLK1 in both Drosophila (Thr182) and humans (Thr210)35.
Survivin
Survivin is composed of an N-terminal Zn2+-coordinated BIR domain and a C-terminal helical extension. In vertebrates, mutations within the BIR domain prevent recruitment of the CPC to the centromere but do not perturb its localisation from anaphase onward19, 36. In D. melanogaster, a different mutation within the BIR domain prevents CPC localisation to the anaphase spindle midzone without affecting its centromeric localisation37, indicating a potential contribution of the BIR domain to CPC localisation during anaphase.
Survivin was originally described as an inhibitor of apoptosis protein (IAP) that accumulated in G2 cells and was proposed to negatively regulate cell death in mitosis38. There is a vast literature on the involvement of Survivin in cell death regulation, however analysis of Survivin-null mutants in yeasts and vertebrates19, 39, 40 has failed to identify significant abnormalities in cell death responses. Survivin has a nuclear export signal41, and it has been suggested that Survivin in the cytoplasm may inhibit cell death while Survivin in the nucleus or associated with the CPC regulates mitosis42.
Purified Survivin forms a “butterfly-shape” homodimer in solution43–45. Within the CPC however, the Survivin dimerization surface is contacted by Borealin15, 16, blocking Survivin homodimer formation. A small molecule inhibitor, S12, disrupts CPC function during mitosis by binding a pocket near this dimerization interface and is being explored as an anti-cancer drug46.
Survivin is phosphorylated in vitro by Aurora B47, 48, CDK149, 50, PLK151, 52 and Casein Kinase II (CK2)53 (Figure 1B). CDK phosphorylation of Thr34 was reported to be essential to prevent spontaneous apoptosis54, 55, however DT40 cells expressing a survivin mutant in which this residue is substituted by an Alanine grow normally19. CK2 phosphorylation of Survivin appears to regulate interactions between Survivin and Borealin53, and merits further study.
Survivin ubiquitylation regulates the binding dynamics of the CPC at the centromere. Lys 63-linked ubiquitylation mediated by Ufd1 promotes the association of Survivin with centromeres, whereas de-ubiquitylation mediated by hFAM is required for its dissociation56. In addition, the budding yeast homologue of Survivin, Bir1 is regulated by SUMOylation57
BOREALIN
Borealin (also known as Dasra) was discovered in two independent studies of proteins that associate with mitotic chromosomes and chromosome scaffolds11, 58.
The N-terminus of human Borealin participates in the three-helix bundle that makes up the CPC localisation module16. The yeast homologs of Borealin (Nbl1 in budding and fission yeast) are very small compared to their vertebrate counterparts but retain the region involved in three-helix bundle formation, suggesting this is an evolutionarily conserved function of Borealin59, 60. Sequence alignments performed after identification of the yeast proteins revealed that C. elegans CSC-161 is also a Borealin homolog. Thus, the four-member CPC is widely conserved across animalia and fungi59. In addition, many species contain paralogues of CPC components which may regulate the CPC in certain developmental contexts11, 58, 62–65.
Essentially all Survivin in mitotic cells is associated with Borealin11. Borealin-Survivin forms a soluble 1:1 complex, however, in the presence of an INCENP N-terminal peptide, a 1:1:1 complex forms16, 66. The central region of Borealin (aa110–207) interacts with the ESCRT-III subunit Shrb/CHMP4C in Drosophila and humans67, 68. This conserved interaction is involved in regulating abscission, as discussed below.
Like other members of the CPC, Borealin is regulated by phosphorylation at multiple sites69–73 (Figure 1B). Phosphorylation of Borealin by Cdk1 is required for interactions with Shugoshin 1 and 2 that are important for targeting the CPC to centromeres73. The Borealin C-terminus contains a dimerization interface that has been implicated in regulating its stability72. Phosphorylation of Thr230 on this interface by Mps1 kinase modulates Borealin dimerization and also Aurora B activity66. It was suggested that this modification is required for the CPC to function efficiently in error correction and chromosome alignment70. It is important to note, however, that Mps1 has a role in those processes which is independent of regulating Aurora B activity74.
Borealin is SUMOylated in a RanBP2-dependent manner early in mitosis75, and a reconstituted RanBP2/RanGAP1*SUMO1/Ubc9 complex has E3 ligase activity on the Borealin/Survivin/INCENP complex76. At anaphase onset the SUMO isopeptidase SENP3 catalyzes the removal of SUMO2/3 from Borealin. The function of this Borealin SUMOylation is unknown.
Mechanisms of Aurora B activation
Aurora B activation is a complex, multi-step process. Aurora B initially binds the IN-BOX of INCENP, which activates low levels of kinase activity. This enables Aurora B to phosphorylate a C-terminal TSS (threonine – serine –serine) motif on INCENP10, 77 as well as Thr232 in the T-loop of its kinase domain, resulting in full activation of Aurora B (Figure 2A). Both of these phosphorylations likely occur in trans78. This explains why Aurora B activity is stimulated by increasing the local density of the CPC by adding chromatin to Xenopus laevis egg extracts79 or targeting INCENP to an ectopic locus on chromosomes in vivo80. Microtubules can also activate Aurora B79, 81–83 possibly through local enrichment of the CPC. This activation is stimulated by TD-6082. The density-dependent activation of Aurora B partially explains how kinase activation is coupled to CPC localisation at the inner-centromere and spindle midzone (Figure 2B).
FIGURE 2. Coupling of Aurora B kinase Activation to CPC formation and localisation.
(A) Activation of Aurora B (red circle) requires binding to INCENP (yellow) and phosphorylation in a feedback loop. Both of these phosphorylations are catalysed in trans.
(B) Aurora B activation is coupled to CPC localisation in vivo. The localisation module of INCENP, Survivin and Borealin targets the CPC to histones at the inner-centromere and microtubules at the spindle midzone during early and late mitosis, respectively. Enrichment of the CPC at these locations facilitates auto-phosphorylation in trans, leading to full Aurora B activation.
Other kinases also regulate Aurora B activity. Full activation of human Aurora B requires phosphorylation of Ser311 by Chk1 kinase, best known for its role in the DNA damage checkpoint84 (Figure 1B). Interestingly, Chk1 is localized to kinetochores during prometaphase85 and Ser311-phosphorylated Aurora B is only detectable adjacent to the kinetochore. The kinetochore-proximal pool of Aurora B could function in Plk1 activation35 and/or regulate microtubule binding at the kinetochore. Interactions between the CPC and Plk1 are complex, and full Aurora B activation is also promoted by Survivin phosphorylation by Plk152.
In C. elegans, Tousled-like kinase (TLK-1) is reportedly phosphorylated by Aurora B, which in turn triggers TLK-1 to further activate Aurora B kinase activity in an INCENP-dependent manner86. TLK-1 is also required for Aurora B localisation to the spindle midzone microtubules during late mitosis87.
Ubiquitylation and SUMOylation of Aurora B also modulate its localisation and activity88–90. Mono-ubiquitylation of Aurora B by Cullin 3 (Cul3) E3 ubiquitin ligases regulates its removal from chromatin and promotes relocalisation of the CPC in anaphase88. SUMOylation of Aurora B within the kinase domain is required for correct mitotic progression89, 90. Aurora B activity during mitotic exit is ultimately terminated when the kinase is degraded by the proteasome91, 92.
Regulation of Aurora B activity
Phosphorylation of Aurora B substrates must be regulated for proper cell division. This requirement is best understood during early mitosis when Aurora B phosphorylates multiple substrates at the kinetochore to destabilize and correct erroneous kinetochore-microtubule attachments. Replacing the Aurora B phosphorylation sites on one such protein with phosphomimetic residues prevents the formation of stable kinetochore-microtubule attachments93, indicating Aurora B phosphorylation must be coordinated with microtubule attachment status to balance error correction and chromosome segregation. Indeed, phosphorylation of kinetochore substrates is most prominent when kinetochores are not attached to microtubules and is reduced upon microtubule attachment94–97. How this regulation is achieved is the subject of intensive research.
A key aspect regulating this attachment-sensitive phosphorylation is recruitment of antagonistic phosphatases. During early mitosis, protein phosphatase I (PP1), the major counteracting phosphatase for Aurora B, is recruited to the outer kinetochore through Spc105/Blinkin/KNL195, 98, while its recruitment to bulk chromatin, mediated by the PP1 targeting subunit Repo-Man, is suppressed99, 100. Other proteins implicated in targeting PP1 to the kinetochore include Sds22101, Kinesin-7 (CENP-E in human)102, kinesin-8 (in fission yeast)103, and Fin1 (in budding yeast)104.
PP1 is not the only phosphatase that opposes Aurora B. The B56 subunit of PP2A, which stabilizes kinetochore-microtubule attachments by counteracting Aurora B phosphorylation, is enriched in the inner centromere in the absence of microtubule attachment, but dissociates from it upon bipolar attachment105.
It has been proposed that tension-mediated stretching of centromeric chromatin shifts substrates away from Aurora B in the inner centromere towards phosphatases in the kinetochore106–108. Indeed, substrates at the inner centromere are less readily dephosphorylated upon microtubule attachment than those at the outer kinetochore94, 109. A problem for this model, however, is that even after the establishment of bipolar attachment, the distance between the inner kinetochore and the outer kinetochore rapidly fluctuates between full extension and relaxation110.
Additional mechanisms control substrate recognition by Aurora B. During spindle assembly in X. laevis egg extract, the CPC must bind to both chromatin and microtubules83. The requirement for chromatin-binding, but not microtubule-binding, can be bypassed by artificially activating Aurora B. This indicates that CPC-microtubule binding promotes spindle assembly by a mechanism other than Aurora B activation, possibly by facilitating the recognition of critical substrates83. Substrate recognition can also be controlled by additional modifications of Aurora B substrates, including acetylation and phosphorylation of histone H382, 111 and methylation of Dam1112. Similar mechanisms may regulate phosphorylation of kinetochore substrates.
Phosphorylation of Aurora B substrates may also be regulated on a global scale during mitosis (Figure 3). Aurora B activity can be measured at a specific cellular location using Förster resonance energy transfer (FRET) sensors. A FRET sensor targeted to microtubules reveals that during anaphase, levels of Aurora B phosphorylation form a spatial gradient that is highest at the spindle midzone80, 81, 109, 113 and decreases over a micron-scale distance surrounding this location113. Augmenting this gradient perturbs the function of the CPC during anaphase, suggesting it is functionally significant113. A phosphorylation gradient is not obvious during early mitosis, however, treatment of cells with low dose Aurora B inhibitors reveals a gradient centered on chromosomes that decreases towards the spindle poles113. Similarly, a gradient along chromosomes can be seen temporarily after transient exposure to an Aurora B inhibitor 80. While the function of these gradients is still unclear, these observations suggest that phosphorylation of Aurora B substrates is regulated on multiple scales.
FIGURE 3. The CPC produces spatial gradients of Aurora B activity throughout mitosis.
(left) At metaphase, Aurora B phosphorylation (detected with a FRET sensor probe on microtubules) is high throughout the spindle and does not form a gradient. Addition of low dose Aurora B inhibitors reveals a phosphorylation gradient where activity is highest around chromatin and decreases towards the spindle poles. During anaphase, an Aurora B phosphorylation gradient is centred at the spindle midzone.
(middle) At metaphase, Aurora B activity is high along the chromosome (FRET sensor probe on chromatin). A transient pulse of high dose Aurora B inhibitors followed by washout leads to the production of a phosphorylation gradient emanating from the centromere and decreasing along the arms. This gradient rapidly disappears as Aurora B activity recovers.
(right) Unattached kinetochores exhibit a gradient of Aurora B activity emanating from centromeric chromatin towards the kinetochore (FRET sensor probes at various kinetochore locations). Microtubules (red) attach to the kinetochore, generating tension that physically stretches the kinetochore (visualized by stretching of the white kinetochore springs). This change pulls substrates at the kinetochore away from centromeric chromatin, resulting in a decrease in Aurora B activity along the kinetochore. Sensors on centromeric chromatin do not change their relative position upon microtubule binding and remain highly phosphorylated.
The CPC in interphase and early mitosis
Few studies have explored the CPC during interphase because disrupting the complex produces obvious and dramatic defects during mitosis. However, recent work suggests that transient inhibition of Aurora B in interphase causes chromosome mis-segregation in the subsequent mitosis114, suggesting that the CPC performs essential functions prior to mitotic entry.
CPC localisation during interphase
In vertebrate cultured cells, the CPC is first visualised on pericentromeric heterochromatin during late S phase2, 114–117. CPC targeting to heterochromatin involves HP1 binding to a PxVxL/I motif on INCENP26, 28, 29 (Figure 4A). An HP1-binding site mutant of INCENP does not localize to heterochromatin during interphase but causes no mitotic defects in HeLa cells29. A fraction of HP1 is closely associated with centromeres in interphase due to interactions with Mis14, a component of the Mis12 kinetochore complex involved in microtubule binding by the KMN network118. Paradoxically, although the Mis14-HP1 interaction occurs solely during interphase, it is important for centromeric enrichment of the CPC in HeLa cells during mitosis118. Thus, the Mis14-HP1 interaction may facilitate CPC recruitment to the inner centromere prior to mitosis.
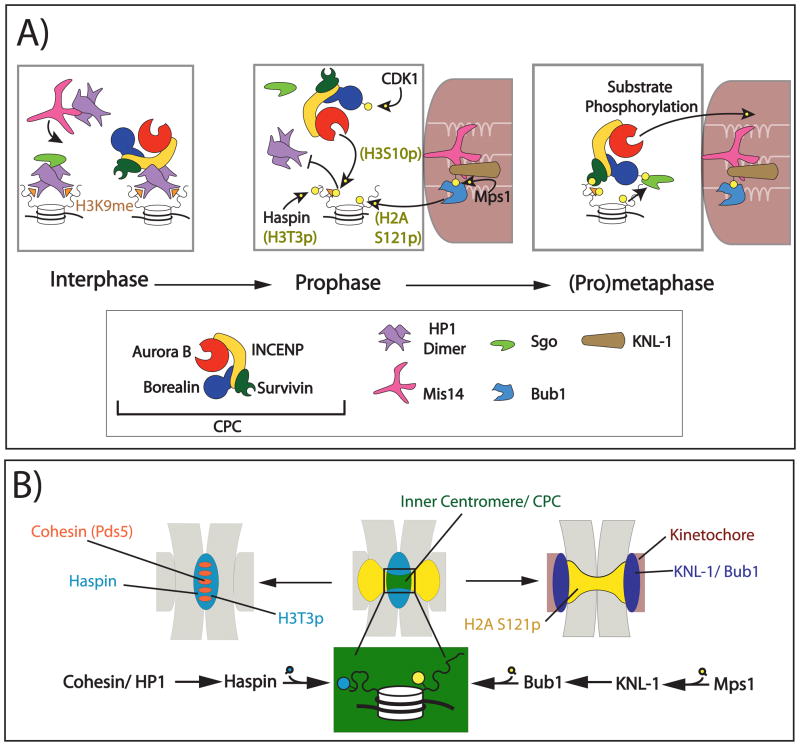
FIGURE 4. Recruitment of the CPC to centromeres during early mitosis.
(A) During interphase, the CPC is targeted to heterochromatin through the interaction of INCENP (yellow) with dimeric HP1 (purple). HP1 requires Mis14 (pink) for proper localisation. HP1 also recruits Shugoshin proteins (green) through a similar mechanism. At the beginning of prophase, active Aurora B phosphorylates histone 3 Ser10, displacing HP1 from the adjacent H3K9me mark. A series of kinases then phosphorylate the CPC and centromeric histone tails to recruit the CPC to the inner centromere by (pro)metaphase. The BIR-domain of Survivin (green) binds H3T3ph while Borealin that has been phosphorylated by CDK1 binds shugoshin proteins, which interact with H2A S121ph.
(B) Overlap between H3T3ph (blue) and H2A S121ph (yellow) defines the inner centromere (green) and recruits the CPC. H3T3ph is deposited by Haspin kinase recruited to centromeric chromatin by cohesin/HP1 (Left). H2A S121ph is deposited by Bub1 kinase, which is recruited to the kinetochore (red) by KNL-1 (purple) phosphorylated by MPS1 kinase (Right).
As cells enter mitosis, Aurora B phosphorylates histone H3 at Ser10 (H3S10ph)119, 120. This reportedly disrupts the HP1 binding to the adjacent trimethylated Lys9 (H3K9me3)121, 122 and may function as a switch to shift from HP1-mediated recruitment of the CPC used during interphase to the mitotic modes of recruitment discussed below (Figure 4A). H3 Ser10 phsphorylation requires POGZ (pogo transposable element-derived protein with zinc finger domain), which is also required to remove HP1 and the CPC from chromosome arms28, promoting their enrichment at the inner centromere.
CPC localisation in early mitosis
Centromeric enrichment of the CPC during mitosis is independent of DNA sequence123, but instead requires the mitosis-specific phosphorylation of two histone tails: histone H3 Thr3 (H3T3ph) by Haspin kinase124 and histone H2A Thr120 (H2AT120ph) by kinetochore-associated Bub1 kinase22, 125 (Figure 4). H3T3ph is concentrated along the length of chromosomes between paired sister chromatids, but is most prominent at the inner centromere22, 124, 126. Haspin activity in this region depends on the cohesin regulator Pds5 and Swi6/HP1 in fission yeast22. H2AT120ph is enriched in the kinetochore-proximal region of the centromere, as Bub1 is a kinetochore-associated protein22, 127, recruited by KNL-1. Maximal concentration of the CPC occurs at the inner centromere where these two histone modifications overlap22 (Figure 4B).
The CPC binds to H3T3ph through the BIR domain of Survivin, which directly interacts with the free N-terminus and adjacent three amino acids of the H3 tail. This interaction is structurally analogous to the recognition of the N-terminus of the pro-apoptotic factor SMAC/Diablo by the anti-apoptotic factor XIAP15, 128. However, the binding affinity of Survivin to a hydrophobic SMAC peptide is 25-fold weaker than to a H3T3ph peptide128, potentially explaining why Survivin knockouts in yeast and vertebrates lack an apoptotic phenotype19, 39, 40. The phospho-specificity of Survivin can be regulated by pH65, suggesting that Survivin-H3 interactions in vivo may be influenced by the local environment.
Phosphorylation of H2A Thr120 by Bub1 in humans (H2A Ser121 in fission yeast) recruits Shugoshin-like proteins (Sgo1 and Sgo2), which interact with either Borealin (in humans) or Survivin (in fission yeast) that has been phosphorylated by CDK173, 125. It had previously been shown that the D. melanogaster homologue of Shugoshin, MeiS332 is interdependent with the CPC for localisation to centromeres129. The structural basis for Shugoshin interactions with H2AT120ph is unknown. Interestingly, it was recently discovered that the Survivin BIR domain can bind to the N-terminus of human Sgo1 in vitro15. This suggests a crosstalk between CPC recruitment pathways, the functional significance of this interaction remains to be tested. In Drosophila, NHK-1/VRK1 was also identified as a kinase for H2A T119 (corresponding to human H2A T120)130. However NHK-1-mediated phosphorylation of H2A is suppressed during mitosis by Polo kinase in Drosophila cells131.
Aurora B kinase activity is involved in several feedback loops that facilitate the rapid and spatially restricted recruitment of the CPC to the centromere. First, Aurora B-dependent Haspin phosphorylation facilitates H3T3 phosphorylation132, thereby creating the substrate for Survivin binding. Second, the CPC contributes to centromeric recruitment of Shugoshin proteins (and also Bub1 in X. laevis)129, 133–136, which in turn are required for CPC localisation at centromeres73, 125. Third, Aurora B-dependent phosphorylation at H3S10 dissociates HP1 from H3K9me, facilitating the dissociation of the CPC from chromosome arms and its enrichment at centromeres28. Furthermore, since CPC localisation is dependent on cohesin and Pds522, 137, 138 likely through localisation of Haspin, Aurora B-mediated removal of cohesin from chromosome arms during prophase may restrict Haspin localisation and promote centromeric enrichment of the CPC.
Consistent with these observations, Aurora B inhibition can impair CPC localisation at centromeres8, 10, 28, 97, 116, 132, 139, though this phenotype is not universal89, 140, 141.
Although localisation to inner centromeres is one of the defining features of the CPC, paradoxically, lethality of chicken DT40 cells lacking the Survivin gene is rescued by a Survivin BIR mutant that is missing residues critical for binding the H3 N-terminal peptide and cannot accumulate at centromeres19. Thus, at least in DT40 cells, accumulation of the CPC at centromeres may not be essential for CPC function in mitosis.
Roles of the CPC in early mitosis
Aurora B catalyses one classic epigenetic mark of mitotic chromosomes, phosphorylation of histone H3 on serine 10 (H3S10ph)119, 120. INCENP depletion causes a substantial drop in H3S10ph levels in vitro and in vivo8, 142. The relationship between H3S10ph and mitotic chromosome compaction has been extensively explored, but while this modification may contribute to chromosome compaction during anaphase in budding yeast143 its role in higher eukaryotes remains to be established.
Mitotic Chromosome Structure
One proposed function of the CPC in mitotic chromosome compaction is regulating the binding of condensin, a multimeric protein complex that is essential for the maintenance of mitotic chromosome architecture137, 144–147. In fission yeast, Aurora B-dependent phosphorylation of the kleisin Cnd2 promotes condensin recruitment to chromosomes148, 149. Phosphorylation of the human kleisin protein CAP-H by Aurora B promotes efficient association of condensin I, but not condensin II, to mitotic chromosomes in human cells145, 146, 149. Phosphorylated kleisins can bind to the N-terminal tail of histone H2A, which may contribute to condensin recruitment148, 149. Indeed, chromosome condensation is impaired in yeast CPC mutants137, 149–151. This effect is much less pronounced in vertebrates.
Regulation of kinetochore-microtubule attachments
Accurate chromosome segregation requires kinetochores to establish correct, bioriented attachments to spindle microtubules. Classic experiments using microneedles to manipulate meiotic chromosomes in grasshopper spermatocytes first revealed that kinetochore-microtubule attachments are stabilized by tension152. The CPC, via Aurora B activity, plays a key role in regulating microtubule attachments in response to defective tension. Aurora B inhibition or Borealin depletion causes a dramatic increase in both merotelic and syntelic attachments11, 153–156. The CPC is required to destabilize and repair these erroneous attachments5, 6, 25.
The kinetochore captures dynamic microtubules through the ability of the KMN network to support load-bearing attachments to microtubule plus ends157. Aurora B regulates the stability of KMN-microtubule attachments. The unstructured, positively charged N-terminal tail of Ndc80, which interacts with the negatively charged C-terminal tails of tubulin158–162, is phosphorylated on multiple sites by Aurora B. This weakens its microtubule-binding affinity in vitro96, 158, 160, 163, 164. Phosphomimetic Ndc80 mutants fail to support stable kinetochore-microtubule attachments93, while nonphosphorylatable mutants hyperstabilise them, resulting in accumulation of syntelic and merotelic attachments in cells96, 158. Additional phosphorylation of components of the KNL-1 and Mis12 complexes, results in a synergistic decrease in microtubule binding affinity, allowing Aurora B to exquisitely control kinetochore-microtubule attachments 94, 165.
Aurora B regulates additional kinetochore proteins that cooperate with the KMN network to bind microtubules. In fungi, the ring-forming Dam1 complex forms a phospho-regulated load-bearing attachment to dynamic microtubules166. The Dam1 complex and its interactions with the Ndc80 complex are negatively regulated by Aurora B phosphorylation and constitute the major targets of the CPC for error correction in yeast167, 168. In higher eukaryotes, the Ska complex, which is proposed to be a functional analog of the Dam1 complex169, is also negatively regulated by Aurora B phosphorylation170.
Aurora B regulates the localisation and activity of the kinesin 13 MCAK, which functions as an important microtubule depolymerase. Interestingly, Aurora B phosphorylation recruits MCAK to the centromere by facilitating its interaction with centromeric Sgo2171–174 while simultaneously suppressing both the MCAK microtubule-depolymerizing activity171–175 and its accumulation at microtubule plus ends176. Why recruit MCAK to the centromere only to inhibit its activity? One possible explanation is that suppressing MCAK stabilises non-kinetochore microtubules near chromosomes to promote spindle assembly58, 79, 176, 177. An alternative hypothesis is that inner centromere Kin-I stimulator (ICIS), which can reverse Aurora B-mediated inhibition of the microtubule depolymerase Kif2a at centromeres 175, might do the same for MCAK at the centromere but not the kinetochore. This could allow MCAK to destabilize microtubules participating in merotelic attachments, while stabilizing k-MT attachments at the kinetochore. Aurora B-dependent recruitment of protein phosphatase 2A (PP2A) to Sgo2 may also facilitate dephosphorylation of MCAK and other kinetochore regulators to promote stable microtubule attachment 105, 174. In addition to its regulation of MCAK, Aurora B also regulates microtubule stability by inhibiting the microtubule-stabilizing activity of the formin mDia3 at kinetochores178.
Spindle Assembly Checkpoint Control
The SAC delays sister chromatid separation and cell cycle progression until all kinetochores attain bipolar microtubule attachments - for review, see7.
The CPC was initially implicated in the SAC in budding yeast where it was found that the Aurora B yeast homologue Ipl1 is required for the checkpoint under conditions that permit microtubule attachment but prevent tension179. It was suggested that the CPC might create unattached kinetochores that are recognized by the SAC pathway180. Similarly, inhibition of Aurora B impaired the SAC in vertebrate tissue culture cells exposed to taxol, which causes a loss of kinetochore tension19, 140, 141, 153, 181.
The CPC is required for all aspects of SAC activation and maintenance in fission yeast and in X. laevis egg extracts133, 150, 182, 183. Aurora B activity promotes kinetochore recruitment of key SAC components Mad1, Mad2, Bub1, BubR1, Mps1 and CENP-E (kinesin-7) in X. laevis and human cultured cells133, 140, 184–186. Tethering Mps1, an upstream activator of the SAC, to the kinetochore can bypass the checkpoint requirement for Aurora B in human cells, suggesting that a primary function of Aurora B for the SAC may be Mps1 recruitment185, 187.
Recent studies in human cells further established the role of Aurora B in SAC activity independently of its capacity to destabilise kinetochore-microtubule attachments186, 187. Artificial targeting of Mad1 to the kinetochore revealed that Aurora B and Mps1 contribute to SAC maintenance in a step after recruitment of Mad1 and Mad2 to the kinetochore187. Reduced requirement of Aurora B in SAC activation in response to unattached kinetochores in budding yeast and human cells may be explained by the existence of an Aurora B-independent mechanism188.
Much recent interest focuses on silencing of the SAC to allow chromosome segregation and mitotic exit, since the SAC is normally activated in every cell as it enters mitosis. Recruitment of PP1 to the kinetochore by KNL-1/Spc105/Blinkin is required for checkpoint silencing, but can be bypassed if Aurora B activity is compromised103, 189, 190. Thus, Aurora B promotes SAC activation whereas PP1 promotes SAC silencing in several ways. First, PP1 may antagonize the Mps1-dependent phosphorylation of KNL-1 by dissociating the checkpoint components Bub1 and Bub3191–193. Second, PP1 reverses the Aurora B-dependent phosphorylation of ZWINT-1 in humans and this promotes the dynein-mediated stripping of SAC components from the kinetochore194. Lastly, PP1 dephosphorylation of CENP-E also helps stabilize kinetochore microtubule attachments102.
The CPC in late mitosis
The CPC’s journey that started on the chromosomes finally comes to an end at the central spindle and the midbody, where it executes its functions in late mitosis including anaphase chromatid compaction, anaphase spindle stabilization (or destabilization in budding yeast) and cytokinesis. Removal of the CPC from chromosomes is also required to reform the nucleus and facilitates mitotic exit.
CPC relocalisation during anaphase
At the metaphase-anaphase transition a population of the CPC leaves the inner centromeres and transfers to central spindle microtubules (Figure 5A). Slightly later, the CPC also localises to the equatorial cortex, the region of the plasma membrane where the cytokinetic machinery is assembled195. Relocalisation of the CPC is coupled to cell cycle progression and is facilitated by three general events: cessation of chromosome targeting, active removal from chromosomes, and targeting to the central spindle. CPC relocalisation is mediated by a decrease in Cdk1 activity and requires both phosphatase and Aurora B kinase activity31, 141, 196.
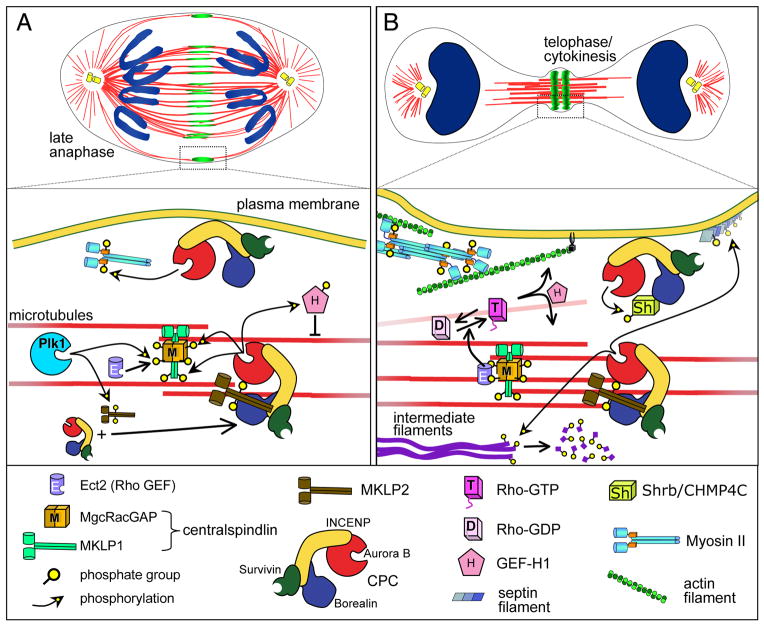
FIGURE 5.
CPC re-localisation and function in mitotic exit
(A) At anaphase onset, the CPC relocalises from chromosomes to the spindle midzone where Aurora B activity promotes centralspindlin recruitment. This stabilizes the spindle midzone and recruits factors important for late telophase/cytokinesis such as the RhoGEF Ect2 (purple). Aurora B also phosphorylates the RhoGEF H1 (pink) to prevent its targeting to microtubules prior to telophase. Additionally, a small population of the CPC accumulates at the cell cortex where cleavage furrow ingression will occur.
(B) During telophase and cytokinesis, Ect2 stimulates the conversion of inactive Rho-GDP to active Rho-GTP. Dephosphorylated Rho GEF now also activates Rho near microtubules. This promotes actin polymerization and contractile ring formation. Aurora B-mediated phosphorylation of Septin filaments (running out of the plane of the page in this diagram) may also be important in contractile ring formation. The CPC promotes disassembly of intermediate filaments (dark purple) that could otherwise obstruct cleavage furrow constriction. In the presence of lagging chromosomes, it also activates the abscission checkpoint to prevent the completion of cytokinesis. Checkpoint activation involves recruitment of the membrane fusion protein Shrb/CHMP4C (yellow square) to Borealin and inactivation of its membrane fusion activity by Aurora B-mediated phosphorylation.
Recruitment of the CPC to chromosomes is suppressed in late mitosis following the dephosphorylation of H3T3ph at anaphase onset20, 197, 198. Active removal of the CPC from chromosomes may also facilitate relocalisation to the spindle midzone. Aurora B is ubiquinated by two midzone-associated E3 ubiquitin ligase complexes, Cul3-KLHL9-KLHL1388 and Cul3-KLHL21199. Ubiquinated Aurora B is subsequently removed from chromosomes by the AAA+ ATPase Cdc48/p97 and its adaptor proteins Ufd1-Npl4200, 201. This process contributes to the level and distribution of the CPC on chromosomes prior to anaphase and facilitates chromosome decondensation and nuclear reformation at the end of mitosis200.
Transfer of the S. cerevisiae CPC to the spindle requires dephosphorylation of CDK sites on Sli15/INCENP by cdc14 phosphatase31, 32. Fission yeast cdc14/Clp1 binds Nbl1/Borealin60. Although dephosphorylation of a Cdk1 site in human INCENP is also required for translocation196, the role of human Cdc14 phosphatase family members in mitotic exit is unclear.
CPC release from chromosomes and targeting to the central spindle requires the interaction of INCENP and Aurora B with MKLP2, a kinesin-6 that binds microtubules at the central spindle196, 202–204 (Figure 5A). The CPC and MKLP2 only interact during anaphase when CDK1-mediated inhibitory phosphorylation is removed196. The CPC and MKLP2 are interdependent on each other for their localisation in most species, though not in Dictyostelium205. In budding yeast, which lacks Mklp2, Aurora B/Ipl1 is targeted to the spindle midzone at anaphase by the microtubule plus-end tracking protein Bim1 (the yeast homologue of EB1). This interaction is negatively regulated prior to anaphase by CDK phosphorylation of Aurora B206. In addition, Aurora B kinase activity141, DNA topoisomerase II207 and INCENP phosphorylation at Ser197 by an unidentified kinase208 are also required for midbody localisation of the CPC.
Formation and stabilization of the spindle midzone
The central spindle is an organized structure formed from the bundled plus-ends of antiparallel microtubules. INCENP was the first protein shown to localise specifically to the central spindle during anaphase2, and this structure is an important site of CPC action.
Central spindle formation requires the action of the microtubule bundling protein PRC1209, the kinesin KIF4210 and centralspindlin; a heterotetrameric complex formed by MKLP1 (a kinesin-6 protein) and MgcRacGAP (a Rho GAP)211–213. The CPC is required for centralspindlin localisation to the spindle midzone4 (Figure 5A). Phosphorylation of MKLP1 by Aurora B promotes centralspindlin clustering and increases its microtubule-bundling activity, thereby stabilizing the central spindle214. The CPC also binds to PRC1 and KIF4 later during cytokinesis215 though the function of these interactions is unclear.
Roles of the CPC in Cytokinesis
Cytokinesis requires the assembly and constriction of an equatorial contractile ring composed of actin, myosin and other cytoskeletal filaments. The site of contractile ring assembly and the timing of its constriction are coordinated closely with chromosome segregation to allow accurate partitioning of the genome and formation of the two daughter cells. The CPC plays an important role in coordinating and regulating these processes through its roles in central spindle formation, regulation of furrow ingression and abscission5, 7, 24 (Figure 5B).
Regulation of contractile ring formation & function
Determining the site of cleavage furrow formation is a classic problem that inspired the elegant experiments of Raymond Rappaport216. The RhoA GEF Ect2 is important for this determination, as are the central spindle and astral microtubules 217. What is less appreciated is that the CPC may have an as-yet unknown function early in contractile ring function. INCENP accumulates at the equatorial cortex in close proximity to the plasma membrane during early-mid anaphase, well before the initiation of furrowing195 (Figure 5A). It is difficult to see this cortical population of INCENP in cells that remain flat during mitosis, however, where it was analysed, INCENP was shown to precede myosin II concentration at the equatorial cortex218. While the function of INCENP at this early stage is not known, it will be interesting to see if the CPC contributes to the early assembly of the contractile ring.
The CPC contributes to contractile ring maturation and constriction through indirect regulation of RhoA, a small GTPase that promotes actin polymerization and myosin II activation (Figure 5). The CPC recruits centralspindlin to the spindle midzone which in turn promotes localisation of the RhoGEF ECT2 to microtubules219–222. Additionally, Aurora B phosphorylation of the centralspindlin component MgcRacGAP induces its RhoGAP activity223, 224. RhoA is required for the assembly of the contractile ring225, but a parallel suppression of Rac activity by MgcRacGAP is also thought to contribute226. A recent analysis in C. elegans indicates that the CPC and MgcRacGAP may function at the relatively late stage of compact contractile ring assembly by regulating actin filament assembly227.
Aurora B also participates in RhoA regulation through inhibitory phosphorylation of the microtubule-binding GEF-H1228 (Figure 5A). Phosphorylation of GEF-H1 prevents RhoA loading and activation at the equator but is reversed at the onset of cytokinesis to facilitate contractile ring formation 228. In this way, Aurora B may prevent premature assembly of the contractile ring.
In addition to its action in regulating RhoA, the CPC has a broader role in regulating cytoskeletal dynamics during cytokinesis. It has been widely assumed that interactions between myosin II and actin filaments shorten the contractile ring during constriction, driving the furrowing of the associated membrane. In Dictyostelium, the INCENP N-terminus interacts with the actin cytoskeleton205. Aurora B activity also modulates Myosin binding to the cytoskeleton215. This may be via phosphorylation of Myosin Regulatory Light Chain II229 though this was not confirmed in human cells215.
The detailed mechanism of contractile ring constriction is not known217, and other filaments may be involved. One candidate for such filaments are the septins230, GTP-binding proteins that form ordered rings in the bud-neck of S. cerevisiae231. Septins are required for cytokinesis in budding yeast232, Drosophila233 and humans234.
In budding yeast, a CPC sub-complex composed of Sli15/INCENP and Bir1p/Survivin regulates septin dynamics in anaphase and cytokinesis235, 236 (Figure 5B). The interaction between the CPC and septins is also critical for cytokinesis in C. elegans227. It will be extremely interesting to see if the CPC regulates cytokinesis through septins in other animals, where Aurora B is known to phosphorylate Septin 1 in vivo237. Interestingly, S. pombe Ark1/Aurora B functions during cytokinesis but is not essential60, 150. This may be because septin filaments are not required for cytokinesis in S. pombe238 as they are in S. cerevisiae232
A recent quantitative proteomics approach revealed that Aurora B undergoes a dramatic switch in binding partners during mitotic exit215. Aurora B activity is required for optimal interactions of a number of microtubule-associated proteins with the cytoskeleton. These include Keratin-8 and Keratin-18 and the Formin FHOD-1239, which interacts with the Rac1-GTPase and mediates actin polymerization.
The CPC contributes to furrow ingression by regulating intermediate filament (IF) assembly (Figure 5B). As is the case for the nuclear lamins240, cytoplasmic IF phosphorylation can lead to reversible filament disassembly. Mutation of an Aurora B phosphorylation site on vimentin leads to the formation of IF bridges in cytokinesis and subsequent multinucleation241. Aurora B and Rho (ROCK) kinases also phosphorylate the IF proteins GFAP (Glial Fibrillar Acidic Protein) and Desmin. Mutation of these phosphorylation sites results in defects in filament disassembly in cytokinesis, suggesting that the CPC promotes IF disassembly to facilitate constriction of the contractile ring and allow abscission to occur242.
Regulation of abscission
Abscission is the fusion of membranes that completes the separation of daughter cells during cytokinesis. Aurora B has been implicated in a checkpoint during cytokinesis that delays abscission in response to lagging chromatin in the intercellular bridge, the site of cleavage furrow ingression that connects daughter cells. Known as the abscission checkpoint (or “No-Cut” pathway in yeast where it was discovered)243, this poorly understood but seemingly conserved checkpoint may prevent chromosome breakage and protect cells from tetraploidization244.
The abscission checkpoint can also be activated by defects in nuclear pore reassembly during mitotic exit. Depletion of the nucleoporins Nup153 or Nup50 results in a delay in cytokinesis and the formation of cytoplasmic foci of active Aurora B that are not associated with the rest of the CPC subunits245. Inhibition of Aurora B permits the completion of cytokinesis, suggesting that in this checkpoint, Aurora B may act independent of the CPCto delay cytokinesis.
The mechanisms by which Aurora B regulates abscission in higher eukaryotes are beginning to emerge. During abscission in Drosophila and humans, Borealin binds to Shrb/CHMP4C (Charged multivesicular body protein)67, 68 (Figure 5B), a component of the Endosomal Sorting Complex Required for Transport III (ESCRT-III). ESCRTs are conserved complexes involved in membrane budding processes. ESCRT-III in particular mediates membrane fission at the end of cytokinesis246–248. Borealin binding may facilitate phosphorylation of Shrb/CHMP4C by Aurora B and has been proposed to inhibit its ability to participate in abscission, thereby delaying premature cytokinesis.
Perspectives.
The switching of the CPC from interactions with chromatin and the kinetochore in early mitosis to a role in regulating cytoskeletal events during mitotic exit provides strong confirmation of the original CPC hypothesis1. This complex temporally and spatially regulated signaling module can send and receive signals from both chromatin and cytoskeletal components. It can fine-tune highly local protein-protein interactions while simultaneously regulating the global effects of the SAC. Some of the many key questions that remain to be solved include the mechanistic basis for Aurora B kinase activation beyond density-dependent autophosphorylation, the control of substrate recognition, and how the spatial distribution of kinase and phosphatase activities is balanced. Beyond this, the functions of the CPC during interphase remain largely uncharted territory, though initial results are beginning to come in249. The journey of discovery is clearly far from over for the CPC.
Acknowledgments
HF is supported by a National Institutes of Health grant (R01GM075249). Work in the WCE lab is funded by The Wellcome Trust, of which WCE is a Principal Research Fellow [grant number 073915]. The Wellcome Trust Centre for Cell Biology is supported by core grant numbers 077707 and 092076.
GLOSSARY
- GEF
guanine exchange factor - enzyme that activates small GTPases by stimulating the release of GDP and allowing the formation of the active GTP-bound form
- Spindle midzone
the region of the anaphase spindle, composed of overlapping anti-parallel microtubules from opposite spindle poles, also known as the central spindle
- kinetochore
complex protein super-assembly located at centromeres that mediates microtubule attachment and regulates chromosome segregation
- BIR (Baculovirus IAP repeat) domain
a Zn2+-coordinated globular domain involved in protein–protein interactions present in all IAP proteins
- centromere
specialised chromatin at the primary constriction of mitotic chromosomes that is the site of kinetochore assembly and the focal point for sister chromatid cohesion
- midbody
dense structure derived from the remnants of the central spindle during late telophase. It is present in the intercellular bridge that connects daughter cells during cytokinesis
- CDKs
cyclin dependent kinases - family of highly conserved Serine-Threonine kinases involved in the regulation of cell cycle progression characterized by their association and regulation by cyclins
- Plks
polo-like kinases –first identified in D. melanogaster, they are involved in many aspects of cell cycle regulation including chromosome-microtubule interactions, centrosome duplication
- checkpoints
biochemical signaling networks that monitor whether key processes have taken place before allowing progression to the next cell cycle stage
- Inner centromere
the region of the centromere located between paired sister chromatids
- SUMOylation
posttranslational modification by reversible conjugation of Small Ubiquitin-like Modifier (SUMO) proteins; involved in regulation of the cell cycle, DNA repair, gene expression nuclear transport and protein stability
- E3 ligases
enzymes that promote the attachment of ubiquitin or SUMO to a protein, leading to a variety of outcomes, including changes in binding partners, sorting into different subcellular compartments or degradation
- Förster resonance energy transfer (FRET)
a method for detecting associations between proteins by measuring the transfer of energy over distances of a few nanometers between fluorescent probes attached to the proteins
- KMN network
an important microtubule-binding module of the outer kinetochore formed of the NDC80, MIS12, and KNL1 complexes
- condensins
large heteropentameric complexes essential for chromosome architecture that are composed of two structural maintenance of chromosomes (SMC) subunits and three auxiliary non-SMC subunits
- kleisin
subunit that bridges the ATPase heads of SMC proteins in SMC complexes, converting them into closed rings
- merotelic attachment
a single kinetochore attaches to microtubules from both spindle poles
- syntelic attachment
both sister kinetochores attach to microtubules from the same pole
- kinesins
superfamily of microtubule associated motor proteins whose functions include the transport of cargo along microtubules and regulation of microtubule dynamics
- formins
proteins defined by the presence of a catalytic FH2 [formin homology 2] domain that interact with actin and regulate its polymerization
- AAA+ ATPase
ATPases with associated diverse cellular activities- hexameric ATPase that couple ATP hydrolysis to translocation or remodeling of macromolecules in a wide range of cellular processes
- DNA topoisomerase II
abundant nuclear enzyme that relieves tolopogical stress in DNA by passing one duplex through another using an ATP-regulated protein gate
- GAP
GTPase Activating Protein that activates small GTPases by stimulating them to hydrolize GTP into GDP
References
- 1.Earnshaw WC, Bernat RL. Chromosomal passengers: toward an integrated view of mitosis. Chromosoma. 1991;100:139–46. doi: 10.1007/BF00337241. The proposal that proteins associated with mitotic chromosomes might integrate both chromosomal and cytoskeletal events in mitosis. [DOI] [PubMed] [Google Scholar]
- 2.Cooke CA, Heck MM, Earnshaw WC. The inner centromere protein (INCENP) antigens: movement from inner centromere to midbody during mitosis. J Cell Biol. 1987;105:2053–67. doi: 10.1083/jcb.105.5.2053. The original publication describing the identification of INCENP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adams RR, et al. INCENP binds the Aurora-related kinase AIRK2 and is required to target it to chromosomes, the central spindle and cleavage furrow. Curr Biol. 2000;10:1075–8. doi: 10.1016/s0960-9822(00)00673-4. [DOI] [PubMed] [Google Scholar]
- 4.Kaitna S, Mendoza M, Jantsch-Plunger V, Glotzer M. Incenp and an aurora-like kinase form a complex essential for chromosome segregation and efficient completion of cytokinesis. Curr Biol. 2000;10:1172–81. doi: 10.1016/s0960-9822(00)00721-1. Refs. 3 and 4 provide the first description of the biochemical complex between Aurora B and INCENP - the CPC. [DOI] [PubMed] [Google Scholar]
- 5.Ruchaud S, Carmena M, Earnshaw WC. Chromosomal passengers: conducting cell division. Nat Rev Mol Cell Biol. 2007;8:798–812. doi: 10.1038/nrm2257. [DOI] [PubMed] [Google Scholar]
- 6.Kelly AE, Funabiki H. Correcting aberrant kinetochore microtubule attachments: an Aurora B-centric view. Curr Opin Cell Biol. 2009;21:51–58. doi: 10.1016/j.ceb.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Waal MS, Hengeveld RC, van der Horst A, Lens SM. Cell division control by the Chromosomal Passenger Complex. Exp Cell Res. 2012 doi: 10.1016/j.yexcr.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 8.Adams RR, Maiato H, Earnshaw WC, Carmena M. Essential roles of Drosophila inner centromere protein (INCENP) and Aurora-B in histone H3 phosphorylation, metaphase chromosome alignment, kinetochore disjunction, and chromosome segregation. J Cell Biol. 2001;153:865–880. doi: 10.1083/jcb.153.4.865. This was the first use of RNAi to probe the function of the CPC in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carvalho A, Carmena M, Sambade C, Earnshaw WC, Wheatley SP. Survivin is required for stable checkpoint activation in taxol-treated HeLa cells. J Cell Sci. 2003;116:2987–2998. doi: 10.1242/jcs.00612. [DOI] [PubMed] [Google Scholar]
- 10.Honda R, Korner R, Nigg EA. Exploring the functional interactions between Aurora B, INCENP, and survivin in mitosis. Mol Biol Cell. 2003;14:3325–3341. doi: 10.1091/mbc.E02-11-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gassmann R, et al. Borealin: A novel chromosomal passenger required for stability of the bipolar mitotic spindle. J Cell Biol. 2004;166:179–191. doi: 10.1083/jcb.200404001. Together with Ref 58, this was the first description of Borealin/Dasra in vertebrates. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vader G, Kauw JJ, Medema RH, Lens SM. Survivin mediates targeting of the chromosomal passenger complex to the centromere and midbody. EMBO Rep. 2006;7:85–92. doi: 10.1038/sj.embor.7400562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mollinari C, et al. The mammalian passenger protein TD-60 is an RCC1 family member with an essential role in prometaphase to metaphase progression. Dev Cell. 2003;5:295–307. doi: 10.1016/s1534-5807(03)00205-3. [DOI] [PubMed] [Google Scholar]
- 14.Mackay AM, Earnshaw WC. The INCENPs: structural and functional analysis of a family of chromosome passenger proteins. Cold Spring Harb Symp Quant Biol. 1993;58:697–706. doi: 10.1101/sqb.1993.058.01.077. [DOI] [PubMed] [Google Scholar]
- 15.Jeyaprakash AA, Basquin C, Jayachandran U, Conti E. Structural Basis for the Recognition of Phosphorylated Histone H3 by the Survivin Subunit of the Chromosomal Passenger Complex. Structure. 2011;19:1625–1634. doi: 10.1016/j.str.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Jeyaprakash AA, et al. Structure of a Survivin-Borealin-INCENP core complex reveals how chromosomal passengers travel together. Cell. 2007;131:271–285. doi: 10.1016/j.cell.2007.07.045. This paper describes the crystal structure of the triple helical bundle formed by Survivin-Borealin-INCENP and explores its role in localization of the CPC. [DOI] [PubMed] [Google Scholar]
- 17.Song Z, et al. A Single Amino Acid Change (Asp 53-> Ala53) Converts Survivin from Anti-apoptotic to Pro-apoptotic. Mol Biol Cell. 2004;15:1287–1296. doi: 10.1091/mbc.E03-07-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lens SM, Vader G, Medema RH. The case for Survivin as mitotic regulator. Curr Opin Cell Biol. 2006;18:616–622. doi: 10.1016/j.ceb.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 19.Yue Z, et al. Deconstructing Survivin: comprehensive genetic analysis of Survivin function by conditional knockout in a vertebrate cell line. J Cell Biol. 2008;183:279–296. doi: 10.1083/jcb.200806118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelly AE, et al. Survivin reads phosphorylated histone H3 threonine 3 to activate the mitotic kinase Aurora B. Science. 2010;330:235–239. doi: 10.1126/science.1189505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang F, et al. Histone H3 Thr-3 phosphorylation by Haspin positions Aurora B at centromeres in mitosis. Science. 2010;330:231–235. doi: 10.1126/science.1189435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamagishi Y, Honda T, Tanno Y, Watanabe Y. Two histone marks establish the inner centromere and chromosome bi-orientation. Science. 2010;330:239–243. doi: 10.1126/science.1194498. These three papers described the postranslational modifications of histones that define the inner centromere are responsible for the targeting of the CPC to centromeres. [DOI] [PubMed] [Google Scholar]
- 23.Glover DM, Leibowitz MH, McLean DA, Parry H. Mutations in aurora prevent centrosome separation leading to the formation of monopolar spindles. Cell. 1995;81:95–105. doi: 10.1016/0092-8674(95)90374-7. This is the identification of the original Aurora kinase family member. [DOI] [PubMed] [Google Scholar]
- 24.Carmena M, Ruchaud S, Earnshaw WC. Making the Auroras glow: regulation of Aurora A and B kinase function by interacting proteins. Curr Opin Cell Biol. 2009;21:796–805. doi: 10.1016/j.ceb.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lens SM, Voest EE, Medema RH. Shared and separate functions of polo-like kinases and aurora kinases in cancer. Nat Rev Cancer. 2010;10:825–841. doi: 10.1038/nrc2964. [DOI] [PubMed] [Google Scholar]
- 26.Ainsztein AM, Kandels-Lewis SE, Mackay AM, Earnshaw WC. INCENP centromere and spindle targeting: Identification of essential conserved motifs and involvement of heterochromatin protein HP1. J Cell Biol. 1998;143:1763–1774. doi: 10.1083/jcb.143.7.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klein UR, Nigg EA, Gruneberg U. Centromere targeting of the chromosomal passenger complex requires a ternary subcomplex of Borealin, Survivin, and the N-terminal domain of INCENP. Mol Biol Cell. 2006;17:2547–2558. doi: 10.1091/mbc.E05-12-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nozawa RS, et al. Human POGZ modulates dissociation of HP1alpha from mitotic chromosome arms through Aurora B activation. Nat Cell Biol. 2010;12:719–727. doi: 10.1038/ncb2075. This study shows that the HP1α-binding protein, POGZ is required for activation of Aurora B and its dissociation from chromosomes. [DOI] [PubMed] [Google Scholar]
- 29.Kang J, et al. Mitotic centromeric targeting of HP1 and its binding to Sgo1 are dispensable for sister-chromatid cohesion in human cells. Mol Biol Cell. 2011;22:1181–1190. doi: 10.1091/mbc.E11-01-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakajima Y, et al. Ipl1/Aurora-dependent phosphorylation of Sli15/INCENP regulates CPC-spindle interaction to ensure proper microtubule dynamics. J Cell Biol. 2011;194:137–53. doi: 10.1083/jcb.201009137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pereira G, Schiebel E. Separase regulates INCENP-Aurora B anaphase spindle function through Cdc14. Science. 2003;302:2120–2124. doi: 10.1126/science.1091936. [DOI] [PubMed] [Google Scholar]
- 32.Mirchenko L, Uhlmann F. Sli15(INCENP) dephosphorylation prevents mitotic checkpoint reengagement due to loss of tension at anaphase onset. Curr Biol. 2010;20:1396–1401. doi: 10.1016/j.cub.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vazquez-Novelle MD, Petronczki M. Relocation of the chromosomal passenger complex prevents mitotic checkpoint engagement at anaphase. Curr Biol. 2010;20:1402–1407. doi: 10.1016/j.cub.2010.06.036. [DOI] [PubMed] [Google Scholar]
- 34.Goto H, et al. Complex formation of Plk1 and INCENP required for metaphase-anaphase transition. Nat Cell Biol. 2006;8:180–187. doi: 10.1038/ncb1350. [DOI] [PubMed] [Google Scholar]
- 35.Carmena M, et al. The chromosomal passenger complex activates Polo kinase at centromeres. PLoS Biol. 2012;10:e1001250. doi: 10.1371/journal.pbio.1001250. This study describes a conserved mechanism of Aurora B activation of Polo kinase at the centromeres mediated by INCENP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lens SM, et al. Uncoupling the central spindle-associated function of the chromosomal passenger complex from its role at centromeres. Mol Biol Cell. 2006;17:1897–1909. doi: 10.1091/mbc.E05-08-0727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Szafer-Glusman E, Fuller MT, Giansanti MG. Role of Survivin in cytokinesis revealed by a separation-of-function allele. Mol Biol Cell. 2011;22:3779–90. doi: 10.1091/mbc.E11-06-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3:917–921. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- 39.Uren AG, et al. Role for yeast inhibitor of apoptosis (IAP)-like proteins in cell division. Proc Natl Acad Sci U S A. 1999;96:10170–5. doi: 10.1073/pnas.96.18.10170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uren AG, et al. Survivin and the inner centromere protein INCENP show similar cell-cycle localization and gene knockout phenotype. Curr Biol. 2000;10:1319–1328. doi: 10.1016/s0960-9822(00)00769-7. [DOI] [PubMed] [Google Scholar]
- 41.Stauber RH, et al. Nucleocytoplasmic shuttling and the biological activity of mouse survivin are regulated by an active nuclear export signal. Traffic. 2006;7:1461–1472. doi: 10.1111/j.1600-0854.2006.00486.x. [DOI] [PubMed] [Google Scholar]
- 42.Connell CM, Colnaghi R, Wheatley SP. Nuclear survivin has reduced stability and is not cytoprotective. J Biol Chem. 2008;283:3289–3296. doi: 10.1074/jbc.M704461200. [DOI] [PubMed] [Google Scholar]
- 43.Verdecia MA, et al. Structure of the human anti-apoptotic protein survivin reveals a dimeric arrangement. Nat Struct Biol. 2000;7:602–608. doi: 10.1038/76838. [DOI] [PubMed] [Google Scholar]
- 44.Chantalat L, et al. Crystal structure of human survivin reveals a bow tie-shaped dimer with two unusual alpha-helical extensions. Mol Cell. 2000;6:183–189. [PubMed] [Google Scholar]
- 45.Sun C, Nettesheim D, Liu Z, Olejniczak ET. Solution structure of human survivin and its binding interface with Smac/Diablo. Biochemistry. 2005;44:11–17. doi: 10.1021/bi0485171. [DOI] [PubMed] [Google Scholar]
- 46.Berezov A, et al. Disabling the mitotic spindle and tumor growth by targeting a cavity-induced allosteric site of survivin. Oncogene. 2012;31:1938–1948. doi: 10.1038/onc.2011.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wheatley SP, Henzing AJ, Dodson H, Khaled W, Earnshaw WC. Aurora-B phosphorylation in vitro identifies a residue of survivin that is essential for its localization and binding to inner centromere protein (INCENP) in vivo. J Biol Chem. 2004;279:5655–5660. doi: 10.1074/jbc.M311299200. [DOI] [PubMed] [Google Scholar]
- 48.Wheatley SP, et al. Phosphorylation by aurora-B negatively regulates survivin function during mitosis. Cell Cycle. 2007;6:1220–1230. doi: 10.4161/cc.6.10.4179. [DOI] [PubMed] [Google Scholar]
- 49.O’Connor DS, et al. Regulation of apoptosis at cell division by p34cdc2 phosphorylation of survivin. Proc Natl Acad Sci USA. 2000;97:13103–13107. doi: 10.1073/pnas.240390697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O’Connor DS, Wall NR, Porter AC, Altieri DC. A p34(cdc2) survival checkpoint in cancer. Cancer Cell. 2002;2:43–54. doi: 10.1016/s1535-6108(02)00084-3. [DOI] [PubMed] [Google Scholar]
- 51.Colnaghi R, Wheatley SP. Liaisons between survivin and Plk1 during cell division and cell death. J Biol Chem. 2010;285:22592–604. doi: 10.1074/jbc.M109.065003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chu Y, et al. Aurora B kinase activation requires survivin priming phosphorylation by PLK1. J Mol Cell Biol. 2011;3:260–267. doi: 10.1093/jmcb/mjq037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barrett RM, Colnaghi R, Wheatley SP. Threonine 48 in the BIR domain of survivin is critical to its mitotic and anti-apoptotic activities and can be phosphorylated by CK2 in vitro. Cell Cycle. 2011;10:538–548. doi: 10.4161/cc.10.3.14758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grossman D, Kim PJ, Schechner JS, Altieri DC. Inhibition of melanoma tumor growth in vivo by survivin targeting. Proc Natl Acad Sci USA. 2001;98:635–640. doi: 10.1073/pnas.230450097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yan H, et al. Induction of melanoma cell apoptosis and inhibition of tumor growth using a cell-permeable Survivin antagonist. Oncogene. 2006;25:6968–6974. doi: 10.1038/sj.onc.1209676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vong QP, Cao K, Li HY, Iglesias PA, Zheng Y. Chromosome alignment and segregation regulated by ubiquitination of survivin. Science. 2005;310:1499–504. doi: 10.1126/science.1120160. [DOI] [PubMed] [Google Scholar]
- 57.Montpetit B, Hazbun TR, Fields S, Hieter P. Sumoylation of the budding yeast kinetochore protein Ndc10 is required for Ndc10 spindle localization and regulation of anaphase spindle elongation. J Cell Biol. 2006;174:653–663. doi: 10.1083/jcb.200605019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sampath SC, et al. The chromosomal passenger complex is required for chromatin-induced microtubule stabilization and spindle assembly. Cell. 2004;118:187–202. doi: 10.1016/j.cell.2004.06.026. Together with Ref 11, this was the first description of Dasra/Borealin in vertebrates. [DOI] [PubMed] [Google Scholar]
- 59.Nakajima Y, et al. Nbl1p: A Borealin/Dasra/CSC-1-like Protein Essential for Aurora/Ipl1 Complex Function and Integrity in Saccharomyces cerevisiae. Mol Biol Cell. 2009 doi: 10.1091/mbc.E08-10-1011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bohnert KA, Chen JS, Clifford DM, Vander Kooi CW, Gould KL. A link between aurora kinase and Clp1/Cdc14 regulation uncovered by the identification of a fission yeast borealin-like protein. Mol Biol Cell. 2009;20:3646–3659. doi: 10.1091/mbc.E09-04-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Romano A, et al. CSC-1: a subunit of the aurora b kinase complex that binds to the survivin-like protein BIR-1 and the incenp-like protein ICP-1. J Cell Biol. 2003;161:229–236. doi: 10.1083/jcb.200207117. This was the first description of a fourth member of the CPC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gao S, et al. Australin: a chromosomal passenger protein required specifically for Drosophila melanogaster male meiosis. J Cell Biol. 2008;180:521–535. doi: 10.1083/jcb.200708072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ma AC, Chung MI, Liang R, Leung AY. The role of survivin2 in primitive hematopoiesis during zebrafish development. Leukemia. 2009;23:712–720. doi: 10.1038/leu.2008.363. [DOI] [PubMed] [Google Scholar]
- 64.Fernandez-Miranda G, et al. Genetic disruption of aurora B uncovers an essential role for aurora C during early mammalian development. Development. 2011;138:2661–2672. doi: 10.1242/dev.066381. [DOI] [PubMed] [Google Scholar]
- 65.Niedzialkowska E, et al. Molecular basis for phosphospecific recognition of histone H3 tails by Survivin paralogues at inner centromeres. Mol Biol Cell. 2012;23:1457–1466. doi: 10.1091/mbc.E11-11-0904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bourhis E, Hymowitz SG, Cochran AG. The mitotic regulator Survivin binds as a monomer to its functional interactor Borealin. J Biol Chem. 2007;282:35018–35023. doi: 10.1074/jbc.M706233200. [DOI] [PubMed] [Google Scholar]
- 67.Capalbo L, et al. The Chromosomal Passenger Complex controls the function of ESCRT-III Snf7 proteins during cytokinesis. Open Biology. 2012 doi: 10.1098/rsob.120070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carlton JG, Caballe A, Agromayor M, Kloc M, Martin-Serrano J. ESCRT-III governs the Aurora B-mediated abscission checkpoint through CHMP4C. Science. 2012;336:220–225. doi: 10.1126/science.1217180. Refs 67–68 showed that the CPC is involved in the control of abscission by regulating the activity of ESCRT-III proteins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hayama S, et al. Phosphorylation and activation of cell division cycle associated 8 by aurora kinase B plays a significant role in human lung carcinogenesis. Cancer Res. 2007;67:4113–4122. doi: 10.1158/0008-5472.CAN-06-4705. [DOI] [PubMed] [Google Scholar]
- 70.Jelluma N, et al. Mps1 phosphorylates Borealin to control Aurora B activity and chromosome alignment. Cell. 2008;132:233–246. doi: 10.1016/j.cell.2007.11.046. [DOI] [PubMed] [Google Scholar]
- 71.Kaur H, Bekier ME, Taylor WR. Regulation of Borealin by phosphorylation at serine 219. J Cell Biochem. 2010;111:1291–1298. doi: 10.1002/jcb.22853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Date D, Dreier MR, Borton MT, Bekier ME, Taylor WR. Effects of phosphatase and proteasome inhibitors on Borealin phosphorylation and degradation. J Biochem. 2012;151:361–369. doi: 10.1093/jb/mvs015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tsukahara T, Tanno Y, Watanabe Y. Phosphorylation of the CPC by Cdk1 promotes chromosome bi-orientation. Nature. 2010;467:719–723. doi: 10.1038/nature09390. This MS explores the role of CDK phosphorylation of CPC components in the centromeric targeting of the complex and its role in chromosome biorentation. [DOI] [PubMed] [Google Scholar]
- 74.Maciejowski J, et al. Mps1 directs the assembly of Cdc20 inhibitory complexes during interphase and mitosis to control M phase timing and spindle checkpoint signaling. J Cell Biol. 2010;190:89–100. doi: 10.1083/jcb.201001050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Klein UR, Haindl M, Nigg EA, Muller S. RanBP2 and SENP3 function in a mitotic SUMO2/3 conjugation-deconjugation cycle on Borealin. Mol Biol Cell. 2009;20:410–418. doi: 10.1091/mbc.E08-05-0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Werner A, Flotho A, Melchior F. The RanBP2/RanGAP1 *SUMO1/Ubc9 Complex Is a Multisubunit SUMO E3 Ligase. Mol Cell. 2012 doi: 10.1016/j.molcel.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 77.Bishop JD, Schumacher JM. Phosphorylation of the carboxyl terminus of inner centromere protein (INCENP) by the Aurora B Kinase stimulates Aurora B kinase activity. J Biol Chem. 2002;277:27577–27580. doi: 10.1074/jbc.C200307200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sessa F, et al. Mechanism of Aurora B activation by INCENP and inhibition by hesperadin. Mol Cell. 2005;18:379–391. doi: 10.1016/j.molcel.2005.03.031. Structural analysis of an Aurora B-IN-BOX complex in the presence of the inhibitor Hesperadin provided support for a two-step model of Aurora B activation. [DOI] [PubMed] [Google Scholar]
- 79.Kelly AE, et al. Chromosomal enrichment and activation of the aurora B pathway are coupled to spatially regulate spindle assembly. Dev Cell. 2007;12:31–43. doi: 10.1016/j.devcel.2006.11.001. This study showed that clustering of the CPC leads to Aurora B kinase activation and contributes to spindle assembly. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang E, Ballister ER, Lampson MA. Aurora B dynamics at centromeres create a diffusion-based phosphorylation gradient. J Cell Biol. 2011;194:539–549. doi: 10.1083/jcb.201103044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fuller BG, et al. Midzone activation of aurora B in anaphase produces an intracellular phosphorylation gradient. Nature. 2008;453:1132–1136. doi: 10.1038/nature06923. Using FRET-sensors the authors described an Aurora-B phosphorylation gradient centered at the spindle midzone that is essential to provide spatial clues in late mitosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rosasco-Nitcher SE, Lan W, Khorasanizadeh S, Stukenberg PT. Centromeric Aurora-B activation requires TD-60, microtubules, and substrate priming phosphorylation. Science. 2008;319:469–472. doi: 10.1126/science.1148980. [DOI] [PubMed] [Google Scholar]
- 83.Tseng BS, Tan L, Kapoor TM, Funabiki H. Dual detection of chromosomes and microtubules by the chromosomal passenger complex drives spindle assembly. Dev Cell. 2010;18:903–912. doi: 10.1016/j.devcel.2010.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Petsalaki E, Akoumianaki T, Black EJ, Gillespie DA, Zachos G. Phosphorylation at serine 331 is required for Aurora B activation. J Cell Biol. 2011;195:449–466. doi: 10.1083/jcb.201104023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zachos G, et al. Chk1 is required for spindle checkpoint function. Dev Cell. 2007;12:247–260. doi: 10.1016/j.devcel.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Han Z, Riefler GM, Saam JR, Mango SE, Schumacher JM. The C. elegans Tousled-like kinase contributes to chromosome segregation as a substrate and regulator of the Aurora B kinase. Curr Biol. 2005;15:894–904. doi: 10.1016/j.cub.2005.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yeh CH, Yang HJ, Lee IJ, Wu YC. Caenorhabditis elegans TLK-1 controls cytokinesis by localizing AIR-2/Aurora B to midzone microtubules. Biochem Biophys Res Commun. 2010;400:187–193. doi: 10.1016/j.bbrc.2010.07.146. [DOI] [PubMed] [Google Scholar]
- 88.Sumara I, et al. A Cul3-based E3 ligase removes Aurora B from mitotic chromosomes, regulating mitotic progression and completion of cytokinesis in human cells. Dev Cell. 2007;12:887–900. doi: 10.1016/j.devcel.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 89.Fernandez-Miranda G, et al. SUMOylation modulates the function of Aurora-B kinase. J Cell Sci. 2010;123:2823–2833. doi: 10.1242/jcs.065565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ban R, Nishida T, Urano T. Mitotic kinase Aurora-B is regulated by SUMO-2/3 conjugation/deconjugation during mitosis. Genes Cells. 2011;16:652–669. doi: 10.1111/j.1365-2443.2011.01521.x. [DOI] [PubMed] [Google Scholar]
- 91.Stewart S, Fang G. Destruction box-dependent degradation of aurora B is mediated by the anaphase-promoting complex/cyclosome and Cdh1. Cancer Res. 2005;65:8730–8735. doi: 10.1158/0008-5472.CAN-05-1500. [DOI] [PubMed] [Google Scholar]
- 92.Nguyen HG, Chinnappan D, Urano T, Ravid K. Mechanism of Aurora-B degradation and its dependency on intact KEN and A-boxes: identification of an aneuploidy-promoting property. Mol Cell Biol. 2005;25:4977–4992. doi: 10.1128/MCB.25.12.4977-4992.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Guimaraes GJ, Dong Y, McEwen BF, Deluca JG. Kinetochore-microtubule attachment relies on the disordered N-terminal tail domain of Hec1. Curr Biol. 2008;18:1778–1184. doi: 10.1016/j.cub.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Welburn JP, et al. Aurora B phosphorylates spatially distinct targets to differentially regulate the kinetochore-microtubule interface. Mol Cell. 2010;38:383–392. doi: 10.1016/j.molcel.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu D, et al. Regulated targeting of protein phosphatase 1 to the outer kinetochore by KNL1 opposes Aurora B kinase. J Cell Biol. 2010;188:809–820. doi: 10.1083/jcb.201001006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.DeLuca KF, Lens SM, DeLuca JG. Temporal changes in Hec1 phosphorylation control kinetochore-microtubule attachment stability during mitosis. J Cell Sci. 2011;124:622–634. doi: 10.1242/jcs.072629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Salimian KJ, et al. Feedback control in sensing chromosome biorientation by the Aurora B kinase. Curr Biol. 2011;21:1158–1165. doi: 10.1016/j.cub.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Trinkle-Mulcahy L, et al. Time-lapse imaging reveals dynamic relocalization of PP1gamma throughout the mammalian cell cycle. Mol Biol Cell. 2003;14:107–17. doi: 10.1091/mbc.E02-07-0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Trinkle-Mulcahy L, et al. Repo-Man recruits PP1gamma to chromatin and is essential for cell viability. J Cell Biol. 2006;172:679–692. doi: 10.1083/jcb.200508154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vagnarelli P, et al. Condensin and Repo-Man-PP1 co-operate in the regulation of chromosome architecture during mitosis. Nat Cell Biol. 2006;8:1133–1142. doi: 10.1038/ncb1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Posch M, et al. Sds22 regulates aurora B activity and microtubule-kinetochore interactions at mitosis. J Cell Biol. 2010;191:61–74. doi: 10.1083/jcb.200912046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kim Y, Holland AJ, Lan W, Cleveland DW. Aurora kinases and protein phosphatase 1 mediate chromosome congression through regulation of CENP-E. Cell. 2010;142:444–455. doi: 10.1016/j.cell.2010.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Meadows JC, et al. Spindle checkpoint silencing requires association of PP1 to both Spc7 and kinesin-8 motors. Dev Cell. 2011;20:739–750. doi: 10.1016/j.devcel.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Akiyoshi B, Nelson CR, Ranish JA, Biggins S. Quantitative proteomic analysis of purified yeast kinetochores identifies a PP1 regulatory subunit. Genes Dev. 2009;23:2887–2899. doi: 10.1101/gad.1865909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Foley EA, Maldonado M, Kapoor TM. Formation of stable attachments between kinetochores and microtubules depends on the B56-PP2A phosphatase. Nat Cell Biol. 2011;13:1265–1271. doi: 10.1038/ncb2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tanaka TU, et al. Evidence that the Ipl1-Sli15 (Aurora kinase-INCENP) complex promotes chromosome bi-orientation by altering kinetochore-spindle pole connections. Cell. 2002;108:317–329. doi: 10.1016/s0092-8674(02)00633-5. This was the first suggestion that spatial proximity regulated by centromere stretch regulates the activity of Aurora B in releasing kinetochore-microtubule interactions. [DOI] [PubMed] [Google Scholar]
- 107.Maresca TJ, Salmon ED. Welcome to a new kind of tension: translating kinetochore mechanics into a wait-anaphase signal. J Cell Sci. 2010;123:825–835. doi: 10.1242/jcs.064790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lampson MA, Cheeseman IM. Sensing centromere tension: Aurora B and the regulation of kinetochore function. Trends Cell Biol. 2010;21:133–140. doi: 10.1016/j.tcb.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu D, Vader G, Vromans MJ, Lampson MA, Lens SM. Sensing chromosome bi-orientation by spatial separation of aurora B kinase from kinetochore substrates. Science. 2009;323:1350–1353. doi: 10.1126/science.1167000. Using FRET-sensors the authors demonstrated distance-dependent Aurora B phosphorylation of kinetochore substrates and proposed that this can play a role in detecting tension and regulating microtubule attachments. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Uchida KS, et al. Kinetochore stretching inactivates the spindle assembly checkpoint. J Cell Biol. 2009;184:383–390. doi: 10.1083/jcb.200811028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li Y, et al. A novel histone deacetylase pathway regulates mitosis by modulating Aurora B kinase activity. Genes Dev. 2006;20:2566–2579. doi: 10.1101/gad.1455006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang K, et al. The Set1 methyltransferase opposes Ipl1 aurora kinase functions in chromosome segregation. Cell. 2005;122:723–734. doi: 10.1016/j.cell.2005.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tan L, Kapoor TM. Examining the dynamics of chromosomal passenger complex (CPC)-dependent phosphorylation during cell division. Proc Natl Acad Sci USA. 2011;108:16675–16680. doi: 10.1073/pnas.1106748108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hayashi-Takanaka Y, Yamagata K, Nozaki N, Kimura H. Visualizing histone modifications in living cells: spatiotemporal dynamics of H3 phosphorylation during interphase. J Cell Biol. 2009;187:781–790. doi: 10.1083/jcb.200904137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zeitlin SG, Shelby RD, Sullivan KF. CENP-A is phosphorylated by Aurora B kinase and plays an unexpected role in completion of cytokinesis. J Cell Biol. 2001;155:1147–57. doi: 10.1083/jcb.200108125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Beardmore VA, Ahonen LJ, Gorbsky GJ, Kallio MJ. Survivin dynamics increases at centromeres during G2/M phase transition and is regulated by microtubule-attachment and Aurora B kinase activity. J Cell Sci. 2004;117:4033–42. doi: 10.1242/jcs.01242. [DOI] [PubMed] [Google Scholar]
- 117.Monier K, Mouradian S, Sullivan KF. DNA methylation promotes Aurora-B-driven phosphorylation of histone H3 in chromosomal subdomains. J Cell Sci. 2007;120:101–14. doi: 10.1242/jcs.03326. [DOI] [PubMed] [Google Scholar]
- 118.Kiyomitsu T, Iwasaki O, Obuse C, Yanagida M. Inner centromere formation requires hMis14, a trident kinetochore protein that specifically recruits HP1 to human chromosomes. J Cell Biol. 2010;188:791–807. doi: 10.1083/jcb.200908096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hsu JY, et al. Mitotic phosphorylation of histone H3 is governed by Ipl1/aurora kinase and Glc7/PP1 phosphatase in budding yeast and nematodes. Cell. 2000;102:279–291. doi: 10.1016/s0092-8674(00)00034-9. [DOI] [PubMed] [Google Scholar]
- 120.Murnion ME, et al. Chromatin-associated protein phosphatase 1 regulates aurora-B and histone H3 phosphorylation. J Biol Chem. 2001;276:26656–26665. doi: 10.1074/jbc.M102288200. [DOI] [PubMed] [Google Scholar]
- 121.Fischle W, et al. Regulation of HP1-chromatin binding by histone H3 methylation and phosphorylation. Nature. 2005;438:1116–22. doi: 10.1038/nature04219. [DOI] [PubMed] [Google Scholar]
- 122.Hirota T, Lipp JJ, Toh BH, Peters JM. Histone H3 serine 10 phosphorylation by Aurora B causes HP1 dissociation from heterochromatin. Nature. 2005;438:1176–80. doi: 10.1038/nature04254. [DOI] [PubMed] [Google Scholar]
- 123.Bassett EA, et al. Epigenetic centromere specification directs aurora B accumulation but is insufficient to efficiently correct mitotic errors. J Cell Biol. 2010;190:177–185. doi: 10.1083/jcb.201001035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dai J, Higgins JM. Haspin: a mitotic histone kinase required for metaphase chromosome alignment. Cell Cycle. 2005;4:665–668. doi: 10.4161/cc.4.5.1683. [DOI] [PubMed] [Google Scholar]
- 125.Kawashima SA, et al. Shugoshin enables tension-generating attachment of kinetochores by loading Aurora to centromeres. Genes Dev. 2007;21:420–435. doi: 10.1101/gad.1497307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Polioudaki H, et al. Mitotic phosphorylation of histone H3 at threonine 3. FEBS Lett. 2004;560:39–44. doi: 10.1016/S0014-5793(04)00060-2. [DOI] [PubMed] [Google Scholar]
- 127.Kawashima SA, Yamagishi Y, Honda T, Ishiguro K, Watanabe Y. Phosphorylation of H2A by Bub1 prevents chromosomal instability through localizing shugoshin. Science. 2010;327:172–177. doi: 10.1126/science.1180189. [DOI] [PubMed] [Google Scholar]
- 128.Du J, Kelly AE, Funabiki H, Patel DJ. Structural basis for recognition of H3T3ph and Smac/DIABLO N-terminal peptides by human Survivin. Structure. 2012;20:185–195. doi: 10.1016/j.str.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Resnick TD, et al. INCENP and Aurora B promote meiotic sister chromatid cohesion through localization of the Shugoshin MEI-S332 in Drosophila. Dev Cell. 2006;11:57–68. doi: 10.1016/j.devcel.2006.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Aihara H, et al. Nucleosomal histone kinase-1 phosphorylates H2A Thr 119 during mitosis in the early Drosophila embryo. Genes Dev. 2004;18:877–888. doi: 10.1101/gad.1184604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Brittle AL, Nanba Y, Ito T, Ohkura H. Concerted action of Aurora B, Polo and NHK-1 kinases in centromere-specific histone 2A phosphorylation. Exp Cell Res. 2007;313:2780–2785. doi: 10.1016/j.yexcr.2007.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang F, et al. A positive feedback loop involving Haspin and Aurora B promotes CPC accumulation at centromeres in mitosis. Curr Biol. 2011;21:1061–1069. doi: 10.1016/j.cub.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Vigneron S, et al. Kinetochore localization of spindle checkpoint proteins: who controls whom? Mol Biol Cell. 2004;15:4584–4596. doi: 10.1091/mbc.E04-01-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Huang H, et al. Tripin/hSgo2 recruits MCAK to the inner centromere to correct defective kinetochore attachments. J Cell Biol. 2007;177:413–424. doi: 10.1083/jcb.200701122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pouwels J, et al. Shugoshin 1 plays a central role in kinetochore assembly and is required for kinetochore targeting of Plk1. Cell Cycle. 2007;6:1579–1585. doi: 10.4161/cc.6.13.4442. [DOI] [PubMed] [Google Scholar]
- 136.Rivera T, et al. Xenopus Shugoshin 2 regulates the spindle assembly pathway mediated by the chromosomal passenger complex. EMBO J. 2012;31:1467–1479. doi: 10.1038/emboj.2012.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Morishita J, et al. Bir1/Cut17 moving from chromosome to spindle upon the loss of cohesion is required for condensation, spindle elongation and repair. Genes Cells. 2001;6:743–763. doi: 10.1046/j.1365-2443.2001.00459.x. [DOI] [PubMed] [Google Scholar]
- 138.Losada A, Yokochi T, Hirano T. Functional contribution of Pds5 to cohesin-mediated cohesion in human cells and Xenopus egg extracts. J Cell Sci. 2005;118:2133–2141. doi: 10.1242/jcs.02355. [DOI] [PubMed] [Google Scholar]
- 139.Emanuele MJ, et al. Aurora B kinase and protein phosphatase 1 have opposing roles in modulating kinetochore assembly. J Cell Biol. 2008;181:241–54. doi: 10.1083/jcb.200710019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ditchfield C, et al. Aurora B couples chromosome alignment with anaphase by targeting bubR1, Mad2 and CENP-E to kinetochores. J Cell Biol. 2003;161:267–280. doi: 10.1083/jcb.200208091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Xu Z, et al. INCENP-aurora B interactions modulate kinase activity and chromosome passenger complex localization. J Cell Biol. 2009;187:637–653. doi: 10.1083/jcb.200906053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chang CJ, Goulding S, Adams RR, Earnshaw WC, Carmena M. Drosophila Incenp is required for cytokinesis and asymmetric cell division during development of the nervous system. J Cell Sci. 2006;119:1144–53. doi: 10.1242/jcs.02834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Neurohr G, et al. A midzone-based ruler adjusts chromosome compaction to anaphase spindle length. Science. 2011;332:465–468. doi: 10.1126/science.1201578. [DOI] [PubMed] [Google Scholar]
- 144.Giet R, Glover DM. Drosophila aurora B kinase is required for histone H3 phosphorylation and condensin recruitment during chromosome condensation and to organize the central spindle during cytokinesis. J Cell Biol. 2001;152:669–82. doi: 10.1083/jcb.152.4.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ono T, Fang Y, Spector DL, Hirano T. Spatial and temporal regulation of Condensins I and II in mitotic chromosome assembly in human cells. Mol Biol Cell. 2004;15:3296–308. doi: 10.1091/mbc.E04-03-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lipp JJ, Hirota T, Poser I, Peters JM. Aurora B controls the association of condensin I but not condensin II with mitotic chromosomes. J Cell Sci. 2007;120:1245–1255. doi: 10.1242/jcs.03425. [DOI] [PubMed] [Google Scholar]
- 147.Collette KS, Petty EL, Golenberg N, Bembenek JN, Csankovszki G. Different roles for Aurora B in condensin targeting during mitosis and meiosis. J Cell Sci. 2011;124:3684–3694. doi: 10.1242/jcs.088336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Nakazawa N, Mehrotra R, Ebe M, Yanagida M. Condensin phosphorylated by the Aurora-B-like kinase Ark1 is continuously required until telophase in a mode distinct from Top2. J Cell Sci. 2011;124:1795–1807. doi: 10.1242/jcs.078733. [DOI] [PubMed] [Google Scholar]
- 149.Tada K, Susumu H, Sakuno T, Watanabe Y. Condensin association with histone H2A shapes mitotic chromosomes. Nature. 2011;474:477–483. doi: 10.1038/nature10179. [DOI] [PubMed] [Google Scholar]
- 150.Petersen J, Hagan IMS. pombe Aurora Kinase/Survivin Is Required for Chromosome Condensation and the Spindle Checkpoint Attachment Response. Curr Biol. 2003;13:590–597. doi: 10.1016/s0960-9822(03)00205-7. [DOI] [PubMed] [Google Scholar]
- 151.Nakazawa N, et al. Dissection of the essential steps for condensin accumulation at kinetochores and rDNAs during fission yeast mitosis. J Cell Biol. 2008;180:1115–1131. doi: 10.1083/jcb.200708170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Nicklas RB, Koch CA. Chromosome Micromanipulation III. Spindle fiber tension and the reorientation of mal-oriented chromosomes. J Cell Biol. 1969;43:40–50. doi: 10.1083/jcb.43.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hauf S, et al. The small molecule Hesperadin reveals a role for Aurora B in correcting kinetochore-microtubule attachment and in maintaining the spindle assembly checkpoint. J Cell Biol. 2003;161:281–294. doi: 10.1083/jcb.200208092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lampson MA, Renduchitala K, Khodjakov A, Kapoor TM. Correcting improper chromosome-spindle attachments during cell division. Nat Cell Biol. 2004;6:232–237. doi: 10.1038/ncb1102. High resolution microscopy revealed the role of chromosome and microtubule dynamics in the correction of spindle attachment errors by Aurora B. [DOI] [PubMed] [Google Scholar]
- 155.Cimini D, Wan X, Hirel CB, Salmon ED. Aurora kinase promotes turnover of kinetochore microtubules to reduce chromosome segregation errors. Curr Biol. 2006;16:1711–1718. doi: 10.1016/j.cub.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 156.Knowlton AL, Lan W, Stukenberg PT. Aurora B is enriched at merotelic attachment sites, where it regulates MCAK. Curr Biol. 2006;16:1705–10. doi: 10.1016/j.cub.2006.07.057. [DOI] [PubMed] [Google Scholar]
- 157.Powers AF, et al. The Ndc80 kinetochore complex forms load-bearing attachments to dynamic microtubule tips via biased diffusion. Cell. 2009;136:865–875. doi: 10.1016/j.cell.2008.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Deluca JG, et al. Kinetochore microtubule dynamics and attachment stability are regulated by hec1. Cell. 2006;127:969–982. doi: 10.1016/j.cell.2006.09.047. Demonstration that HEC1/Ndc80 phosphorylation by Aurora-B regulates kinetochore–microtubule attachment (see also Ref 163) [DOI] [PubMed] [Google Scholar]
- 159.Wei RR, Al-Bassam J, Harrison SC. The Ndc80/HEC1 complex is a contact point for kinetochore-microtubule attachment. Nat Struct Mol Biol. 2007;14:54–59. doi: 10.1038/nsmb1186. [DOI] [PubMed] [Google Scholar]
- 160.Ciferri C, et al. Implications for kinetochore-microtubule attachment from the structure of an engineered Ndc80 complex. Cell. 2008;133:427–339. doi: 10.1016/j.cell.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Miller SA, Johnson ML, Stukenberg PT. Kinetochore attachments require an interaction between unstructured tails on microtubules and Ndc80(Hec1) Curr Biol. 2008;18:1785–1791. doi: 10.1016/j.cub.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tooley JG, Miller SA, Stukenberg PT. The Ndc80 complex uses a tripartite attachment point to couple microtubule depolymerization to chromosome movement. Mol Biol Cell. 2011;22:1217–1226. doi: 10.1091/mbc.E10-07-0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cheeseman IM, Chappie JS, Wilson-Kubalek EM, Desai A. The conserved KMN network constitutes the core microtubule-binding site of the kinetochore. Cell. 2006;127:983–997. doi: 10.1016/j.cell.2006.09.039. Demonstration that HEC1/Ndc80 phosphorylation by Aurora-B regulates kinetochore–microtubule attachment (see also Ref 158) [DOI] [PubMed] [Google Scholar]
- 164.Alushin GM, et al. The Ndc80 kinetochore complex forms oligomeric arrays along microtubules. Nature. 2010;467:805–810. doi: 10.1038/nature09423. The way in which Ndc80 complexes bind to microtubules suggested a model for how Aurora B regulates kinetochore/microtubule attachments. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hua S, et al. CENP-U cooperates with Hec1 to orchestrate kinetochore-microtubule attachment. J Biol Chem. 2010;286:1627–1638. doi: 10.1074/jbc.M110.174946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gestaut DR, et al. Phosphoregulation and depolymerization-driven movement of the Dam1 complex do not require ring formation. Nat Cell Biol. 2008;10:407–414. doi: 10.1038/ncb1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Cheeseman IM, et al. Phospho-regulation of kinetochore-microtubule attachments by the Aurora kinase Ipl1p. Cell. 2002;111:163–172. doi: 10.1016/s0092-8674(02)00973-x. [DOI] [PubMed] [Google Scholar]
- 168.Tien JF, et al. Cooperation of the Dam1 and Ndc80 kinetochore complexes enhances microtubule coupling and is regulated by aurora B. J Cell Biol. 2010;189:713–723. doi: 10.1083/jcb.200910142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Welburn JP, et al. The human kinetochore Ska1 complex facilitates microtubule depolymerization-coupled motility. Dev Cell. 2009;16:374–385. doi: 10.1016/j.devcel.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chan YW, Jeyaprakash AA, Nigg EA, Santamaria A. Aurora B controls kinetochore-microtubule attachments by inhibiting Ska complex-KMN network interaction. J Cell Biol. 2012;196:563–571. doi: 10.1083/jcb.201109001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Andrews PD, et al. Aurora B regulates MCAK at the mitotic centromere. Dev Cell. 2004;6:253–268. doi: 10.1016/s1534-5807(04)00025-5. [DOI] [PubMed] [Google Scholar]
- 172.Lan W, et al. Aurora B phosphorylates centromeric MCAK and regulates its localization and microtubule depolymerization activity. Curr Biol. 2004;14:273–286. doi: 10.1016/j.cub.2004.01.055. [DOI] [PubMed] [Google Scholar]
- 173.Ohi R, Sapra T, Howard J, Mitchison TJ. Differentiation of cytoplasmic and meiotic spindle assembly MCAK functions by Aurora B-dependent phosphorylation. Mol Biol Cell. 2004;15:2895–2906. doi: 10.1091/mbc.E04-02-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Tanno Y, et al. Phosphorylation of mammalian Sgo2 by Aurora B recruits PP2A and MCAK to centromeres. Genes Dev. 2010;24:2169–2179. doi: 10.1101/gad.1945310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Knowlton AL, Vorozhko VV, Lan W, Gorbsky GJ, Stukenberg PT. ICIS and Aurora B coregulate the microtubule depolymerase Kif2a. Curr Biol. 2009;19:758–763. doi: 10.1016/j.cub.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tanenbaum ME, et al. A complex of Kif18b and MCAK promotes microtubule depolymerization and is negatively regulated by Aurora kinases. Curr Biol. 2011;21:1356–1365. doi: 10.1016/j.cub.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 177.Tanenbaum ME, Medema RH. Localized Aurora B activity spatially controls non-kinetochore microtubules during spindle assembly. Chromosoma. 2011;120:599–607. doi: 10.1007/s00412-011-0334-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Cheng L, et al. Aurora B regulates formin mDia3 in achieving metaphase chromosome alignment. Dev Cell. 2011;20:342–352. doi: 10.1016/j.devcel.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Biggins S, Murray AW. The budding yeast protein kinase Ipl1/Aurora allows the absence of tension to activate the spindle checkpoint. Genes Dev. 2001;15:3118–29. doi: 10.1101/gad.934801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Pinsky BA, Kung C, Shokat KM, Biggins S. The Ipl1-Aurora protein kinase activates the spindle checkpoint by creating unattached kinetochores. Nat Cell Biol. 2006;8:78–83. doi: 10.1038/ncb1341. This paper demonstrates that budding yeast Aurora B supports SAC activation by generating unattached kinetochores. [DOI] [PubMed] [Google Scholar]
- 181.Yang Z, Kenny AE, Brito DA, Rieder CL. Cells satisfy the mitotic checkpoint in Taxol, and do so faster in concentrations that stabilize syntelic attachments. J Cell Biol. 2009;186:675–684. doi: 10.1083/jcb.200906150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kallio MJ, McCleland ML, Stukenberg PT, Gorbsky GJ. Inhibition of aurora B kinase blocks chromosome segregation, overrides the spindle checkpoint, and perturbs microtubule dynamics in mitosis. Curr Biol. 2002;12:900–905. doi: 10.1016/s0960-9822(02)00887-4. [DOI] [PubMed] [Google Scholar]
- 183.Vanoosthuyse V, Hardwick KG. A novel protein phosphatase 1-dependent spindle checkpoint silencing mechanism. Curr Biol. 2009;19:1176–1181. doi: 10.1016/j.cub.2009.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Famulski JK, Chan GK. Aurora B kinase-dependent recruitment of hZW10 and hROD to tensionless kinetochores. Curr Biol. 2007;17:2143–2149. doi: 10.1016/j.cub.2007.11.037. [DOI] [PubMed] [Google Scholar]
- 185.Saurin AT, van der Waal MS, Medema RH, Lens SM, Kops GJ. Aurora B potentiates Mps1 activation to ensure rapid checkpoint establishment at the onset of mitosis. Nat Commun. 2011;2:316. doi: 10.1038/ncomms1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Santaguida S, Vernieri C, Villa F, Ciliberto A, Musacchio A. Evidence that Aurora B is implicated in spindle checkpoint signalling independently of error correction. EMBO J. 2011;30:1508–1519. doi: 10.1038/emboj.2011.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Maldonado M, Kapoor TM. Constitutive Mad1 targeting to kinetochores uncouples checkpoint signalling from chromosome biorientation. Nat Cell Biol. 2011;13:475–482. doi: 10.1038/ncb2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Matson DR, Demirel PB, Stukenberg PT, Burke DJ. A conserved role for COMA/CENP-H/I/N kinetochore proteins in the spindle checkpoint. Genes Dev. 2012;26:542–547. doi: 10.1101/gad.184184.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Rosenberg JS, Cross FR, Funabiki H. KNL1/Spc105 recruits PP1 to silence the spindle assembly checkpoint. Curr Biol. 2011;21:942–947. doi: 10.1016/j.cub.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Espeut J, Cheerambathur DK, Krenning L, Oegema K, Desai A. Microtubule binding by KNL-1 contributes to spindle checkpoint silencing at the kinetochore. J Cell Biol. 2012;196:469–482. doi: 10.1083/jcb.201111107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Shepperd LA, et al. Phosphodependent recruitment of Bub1 and Bub3 to Spc7/KNL1 by Mph1 kinase maintains the spindle checkpoint. Curr Biol. 2012;22:891–899. doi: 10.1016/j.cub.2012.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.London N, Ceto S, Ranish JA, Biggins S. Phosphoregulation of Spc105 by Mps1 and PP1 regulates Bub1 localization to kinetochores. Curr Biol. 2012;22:900–906. doi: 10.1016/j.cub.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Yamagishi Y, Yang CH, Tanno Y, Watanabe Y. MPS1/Mph1 phosphorylates the kinetochore protein KNL1/Spc7 to recruit SAC components. Nat Cell Biol. 2012;14:746–752. doi: 10.1038/ncb2515. [DOI] [PubMed] [Google Scholar]
- 194.Kasuboski JM, et al. Zwint-1 is a novel Aurora B substrate required for the assembly of a dynein-binding platform on kinetochores. Mol Biol Cell. 2011;22:3318–3330. doi: 10.1091/mbc.E11-03-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Earnshaw WC, Cooke CA. Analysis of the distribution of the INCENPs throughout mitosis reveals the existence of a pathway of structural changes in the chromosomes during metaphase and early events in cleavage furrow formation. J Cell Sci. 1991;98:443–461. doi: 10.1242/jcs.98.4.443. [DOI] [PubMed] [Google Scholar]
- 196.Hummer S, Mayer TU. Cdk1 negatively regulates midzone localization of the mitotic kinesin mklp2 and the chromosomal passenger complex. Curr Biol. 2009;19:607–612. doi: 10.1016/j.cub.2009.02.046. Demonstration that MKlp2 targeting of the CPC to the central spindle is reguated by CDK1 (see also ref 202) [DOI] [PubMed] [Google Scholar]
- 197.Qian J, Lesage B, Beullens M, Van Eynde A, Bollen M. PP1/Repo-Man Dephosphorylates Mitotic Histone H3 at T3 and Regulates Chromosomal Aurora B Targeting. Curr Biol. 2011;21:766–773. doi: 10.1016/j.cub.2011.03.047. [DOI] [PubMed] [Google Scholar]
- 198.Vagnarelli P, et al. Repo-Man coordinates chromosomal reorganization with nuclear envelope reassembly during mitotic exit. Dev Cell. 2011;21:328–342. doi: 10.1016/j.devcel.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Maerki S, et al. The Cul3-KLHL21 E3 ubiquitin ligase targets aurora B to midzone microtubules in anaphase and is required for cytokinesis. J Cell Biol. 2009;187:791–800. doi: 10.1083/jcb.200906117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ramadan K, et al. Cdc48/p97 promotes reformation of the nucleus by extracting the kinase Aurora B from chromatin. Nature. 2007;450:1258–62. doi: 10.1038/nature06388. [DOI] [PubMed] [Google Scholar]
- 201.Dobrynin G, et al. Cdc48/p97-Ufd1-Npl4 antagonizes Aurora B during chromosome segregation in HeLa cells. J Cell Sci. 2011;124:1571–180. doi: 10.1242/jcs.069500. [DOI] [PubMed] [Google Scholar]
- 202.Gruneberg U, Neef R, Honda R, Nigg EA, Barr FA. Relocation of Aurora B from centromeres to the central spindle at the metaphase to anaphase transition requires MKlp2. J Cell Biol. 2004;166:167–172. doi: 10.1083/jcb.200403084. Demonstration that MKlp2 targeting of the CPC to the central spindle is reguated by CDK1 (see also ref 195) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Jang JK, Rahman T, McKim KS. The kinesinlike protein Subito contributes to central spindle assembly and organization of the meiotic spindle in Drosophila oocytes. Mol Biol Cell. 2005;16:4684–4694. doi: 10.1091/mbc.E04-11-0964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Cesario JM, et al. Kinesin 6 family member Subito participates in mitotic spindle assembly and interacts with mitotic regulators. J Cell Sci. 2006;119:4770–4780. doi: 10.1242/jcs.03235. [DOI] [PubMed] [Google Scholar]
- 205.Chen Q, Lakshmikanth GS, Spudich JA, De Lozanne A. The localization of inner centromeric protein (INCENP) at the cleavage furrow is dependent on Kif12 and involves interactions of the N terminus of INCENP with the actin cytoskeleton. Mol Biol Cell. 2007;18:3366–3374. doi: 10.1091/mbc.E06-10-0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zimniak T, et al. Spatiotemporal regulation of Ipl1/Aurora activity by direct Cdk1 phosphorylation. Curr Biol. 2012;22:787–793. doi: 10.1016/j.cub.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 207.Coelho PA, et al. Dual role of topoisomerase II in centromere resolution and aurora B activity. PLoS Biol. 2008;6:e207. doi: 10.1371/journal.pbio.0060207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Yang F, et al. Identification of a novel mitotic phosphorylation motif associated with protein localization to the mitotic apparatus. J Cell Sci. 2007;120:4060–4070. doi: 10.1242/jcs.014795. [DOI] [PubMed] [Google Scholar]
- 209.Mollinari C, et al. PRC1 is a microtubule binding and bundling protein essential to maintain the mitotic spindle midzone. J Cell Biol. 2002;157:1175–1186. doi: 10.1083/jcb.200111052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kurasawa Y, Earnshaw WC, Mochizuki Y, Dohmae N, Todokoro K. Essential roles of KIF4 and its binding partner PRC1 in organized central spindle midzone formation. Embo J. 2004;23:3237–3248. doi: 10.1038/sj.emboj.7600347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Mishima M, Kaitna S, Glotzer M. Central spindle assembly and cytokinesis require a kinesin-like protein/RhoGAP complex with microtubule bundling activity. Dev Cell. 2002;2:41–54. doi: 10.1016/s1534-5807(01)00110-1. [DOI] [PubMed] [Google Scholar]
- 212.Kuriyama R, Gustus C, Terada Y, Uetake Y, Matuliene J. CHO1, a mammalian kinesin-like protein, interacts with F-actin and is involved in the terminal phase of cytokinesis. J Cell Biol. 2002;156:783–790. doi: 10.1083/jcb.200109090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Matuliene J, Kuriyama R. Kinesin-like protein CHO1 is required for the formation of midbody matrix and the completion of cytokinesis in mammalian cells. Mol Biol Cell. 2002;13:1832–1845. doi: 10.1091/mbc.01-10-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Douglas ME, Davies T, Joseph N, Mishima M. Aurora B and 14-3-3 coordinately regulate clustering of centralspindlin during cytokinesis. Curr Biol. 2010;20:927–933. doi: 10.1016/j.cub.2010.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Ozlü N, et al. Binding partner switching on microtubules and aurora-B in the mitosis to cytokinesis transition. Mol Cell Proteomics. 2010;9:336–350. doi: 10.1074/mcp.M900308-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Rappaport R. In: Cytokinesis in Animal Cells. Barlow PW, Bard JBL, Greem PB, Kirk DL, editors. Cambridge University Press; Cambridge: 1996. [Google Scholar]
- 217.Fededa JP, Gerlich DW. Molecular control of animal cell cytokinesis. Nat Cell Biol. 2012;14:440–447. doi: 10.1038/ncb2482. [DOI] [PubMed] [Google Scholar]
- 218.Eckley DM, Ainsztein AM, Mackay AM, Goldberg IG, Earnshaw WC. Chromosomal proteins and cytokinesis: patterns of cleavage furrow formation and inner centromere protein positioning in mitotic heterokaryons and mid-anaphase cells. J Cell Biol. 1997;136:1169–83. doi: 10.1083/jcb.136.6.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Yuce O, Piekny A, Glotzer M. An ECT2-centralspindlin complex regulates the localization and function of RhoA. J Cell Biol. 2005;170:571–582. doi: 10.1083/jcb.200501097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Nishimura Y, Yonemura S. Centralspindlin regulates ECT2 and RhoA accumulation at the equatorial cortex during cytokinesis. J Cell Sci. 2006;119:104–114. doi: 10.1242/jcs.02737. [DOI] [PubMed] [Google Scholar]
- 221.Petronczki M, Glotzer M, Kraut N, Peters JM. Polo-like kinase 1 triggers the initiation of cytokinesis in human cells by promoting recruitment of the RhoGEF Ect2 to the central spindle. Dev Cell. 2007;12:713–725. doi: 10.1016/j.devcel.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 222.Su KC, Takaki T, Petronczki M. Targeting of the RhoGEF Ect2 to the equatorial membrane controls cleavage furrow formation during cytokinesis. Dev Cell. 2011;21:1104–1115. doi: 10.1016/j.devcel.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 223.Minoshima Y, et al. Phosphorylation by Aurora B Converts MgcRacGAP to a RhoGAP during Cytokinesis. Dev Cell. 2003;4:549–560. doi: 10.1016/s1534-5807(03)00089-3. [DOI] [PubMed] [Google Scholar]
- 224.Touré A, et al. Phosphoregulation of MgcRacGAP in mitosis involves Aurora B and Cdk1 protein kinases and the PP2A phosphatase. FEBS Lett. 2008;582:1182–1188. doi: 10.1016/j.febslet.2007.12.036. [DOI] [PubMed] [Google Scholar]
- 225.Piekny A, Werner M, Glotzer M. Cytokinesis: welcome to the Rho zone. Trends Cell Biol. 2005;15:651–658. doi: 10.1016/j.tcb.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 226.Canman JC, et al. Inhibition of Rac by the GAP activity of centralspindlin is essential for cytokinesis. Science. 2008;322:1543–1546. doi: 10.1126/science.1163086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Lewellyn L, Carvalho A, Desai A, Maddox AS, Oegema K. The chromosomal passenger complex and centralspindlin independently contribute to contractile ring assembly. J Cell Biol. 2011;193:155–169. doi: 10.1083/jcb.201008138. Important analysis of the role of the CPC in regulation of cytokinesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Birkenfeld J, et al. GEF-H1 modulates localized RhoA activation during cytokinesis under the control of mitotic kinases. Dev Cell. 2007;12:699–712. doi: 10.1016/j.devcel.2007.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Murata-Hori M, et al. Myosin II regulatory light chain as a novel substrate for AIM-1, an aurora/Ipl1p-related kinase from rat. J Biochem (Tokyo) 2000;128:903–907. doi: 10.1093/oxfordjournals.jbchem.a022840. [DOI] [PubMed] [Google Scholar]
- 230.Sanders SL, Field CM. Cell division. Septins in common? Curr Biol. 1994;4:907–910. doi: 10.1016/s0960-9822(00)00201-3. [DOI] [PubMed] [Google Scholar]
- 231.Byers B, Goetsch L. A highly ordered ring of membrane-associated filaments in budding yeast. J of Cell Biol. 1976;69:717–721. doi: 10.1083/jcb.69.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Hartwell LH. Genetic control of the cell division cycle in yeast. IV Genes controlling bud emergence and cytokinesis. Exp Cell Res. 1971;69:265–276. doi: 10.1016/0014-4827(71)90223-0. [DOI] [PubMed] [Google Scholar]
- 233.Neufeld TP, Rubin GM. The Drosophila peanut gene is required for cytokinesis and encodes a protein similar to yeast putative bud neck filament proteins. Cell. 1994;77:371–379. doi: 10.1016/0092-8674(94)90152-x. [DOI] [PubMed] [Google Scholar]
- 234.Kinoshita M, et al. Nedd5, a mammalian septin, is a novel cytoskeletal component interacting with actin-based structures. Genes Dev. 1997;11:1535–1547. doi: 10.1101/gad.11.12.1535. [DOI] [PubMed] [Google Scholar]
- 235.Gillis AN, Thomas S, Hansen SD, Kaplan KB. A novel role for the CBF3 kinetochore-scaffold complex in regulating septin dynamics and cytokinesis. J Cell Biol. 2005;171:773–784. doi: 10.1083/jcb.200507017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Thomas S, Kaplan KB. A Bir1p Sli15p kinetochore passenger complex regulates septin organization during anaphase. Mol Biol Cell. 2007;18:3820–3834. doi: 10.1091/mbc.E07-03-0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Qi M, et al. Septin1, a new interaction partner for human serine/threonine kinase aurora-B. Biochem Biophys Res Commun. 2005;336:994–1000. doi: 10.1016/j.bbrc.2005.06.212. [DOI] [PubMed] [Google Scholar]
- 238.Wu JQ, Ye Y, Wang N, Pollard TD, Pringle JR. Cooperation Between the Septins and the Actomyosin Ring and Role of a Cell-Integrity Pathway During Cell Division in Fission Yeast. Genetics. 2010;186:897–915. doi: 10.1534/genetics.110.119842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Schonichen A, et al. Biochemical characterization of the diaphanous autoregulatory interaction in the formin homology protein FHOD1. J Biol Chem. 2006;281:5084–93. doi: 10.1074/jbc.M509226200. [DOI] [PubMed] [Google Scholar]
- 240.Heald R, McKeon F. Mutations of phosphorylation sites in lamin A that prevent nuclear lamina disassembly in mitosis. Cell. 1990;61:579–589. doi: 10.1016/0092-8674(90)90470-y. [DOI] [PubMed] [Google Scholar]
- 241.Goto H, et al. Aurora-B regulates the cleavage furrow-specific vimentin phosphorylation in the cytokinetic process. J Biol Chem. 2003;278:8526–8530. doi: 10.1074/jbc.M210892200. [DOI] [PubMed] [Google Scholar]
- 242.Kawajiri A, et al. Functional Significance of the Specific Sites Phosphorylated in Desmin at Cleavage Furrow: Aurora-B May Phosphorylate and Regulate Type III Intermediate Filaments during Cytokinesis Coordinatedly with Rho-kinase. Mol Biol Cell. 2003;14:1489–1500. doi: 10.1091/mbc.E02-09-0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Norden C, et al. The NoCut pathway links completion of cytokinesis to spindle midzone function to prevent chromosome breakage. Cell. 2006;125:85–98. doi: 10.1016/j.cell.2006.01.045. [DOI] [PubMed] [Google Scholar]
- 244.Steigemann P, et al. Aurora B-mediated abscission checkpoint protects against tetraploidization. Cell. 2009;136:473–484. doi: 10.1016/j.cell.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 245.Mackay DR, Makise M, Ullman KS. Defects in nuclear pore assembly lead to activation of an Aurora B-mediated abscission checkpoint. J Cell Biol. 2010;191:923–31. doi: 10.1083/jcb.201007124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Dukes JD, Richardson JD, Simmons R, Whitley P. A dominant-negative ESCRT-III protein perturbs cytokinesis and trafficking to lysosomes. Biochem J. 2008;411:233–239. doi: 10.1042/BJ20071296. [DOI] [PubMed] [Google Scholar]
- 247.Elia N, Sougrat R, Spurlin TA, Hurley JH, Lippincott-Schwartz J. Dynamics of endosomal sorting complex required for transport (ESCRT) machinery during cytokinesis and its role in abscission. Proc Natl Acad Sci USA. 2011;108:4846–4851. doi: 10.1073/pnas.1102714108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Guizetti J, et al. Cortical constriction during abscission involves helices of ESCRT-III-dependent filaments. Science. 2011;331:1616–1620. doi: 10.1126/science.1201847. [DOI] [PubMed] [Google Scholar]
- 249.Koch A, Krug K, Pengelley S, Macek B, Hauf S. Mitotic substrates of the kinase aurora with roles in chromatin regulation identified through quantitative phosphoproteomics of fission yeast. Sci Signal. 2011;4:rs6. doi: 10.1126/scisignal.2001588. [DOI] [PubMed] [Google Scholar]