Abstract
Rationale
Effect of cannabinoid CB1 receptor deletion on cocaine’s actions is controversial. This is partly based on findings in CB1-receptor-knockout (CB1−/−) mice with CD1 genetic background.
Objectives
In the present study, we used CB1−/− mice with a C57BL/6J genetic background to further investigate the role of CB1 receptors in cocaine’s action.
Materials and methods
Locomotor activity was assessed using AccuScan locomotor chambers. Brain extracellular dopamine (DA) levels were measured by in vivo microdialysis and by fast-scan cyclic voltammetry in the nucleus accumbens (NAc).
Results
CB1−/− mice displayed a significant reduction in basal levels of locomotion and extracellular DA, as well as in cocaine-enhanced locomotion and extracellular DA, as compared to their wild-type (CB1+/+) littermates. The reduction in basal and cocaine-enhanced DA appears to be related to a reduction in basal DA release, not to an increase in DA clearance, as indicated by fast-scan cyclic voltammetry in brain slices. Pharmacological blockade of CB1 receptors by SR141716 inhibited locomotion and NAc DA release in CB1+/+ mice.
Conclusions
The present findings suggest an important role for CB1 receptors in mediating cocaine’s behavioral and neurochemical effects.
Keywords: Cannabinoid, CB1 receptor, Cocaine, Dopamine, Locomotion, Nucleus accumbens
Cannabinoid CB1 receptors have become important targets in preclinical development of anti-addiction medications because their pharmacological blockade or genetic deletion significantly attenuates the effects of marijuana, opiates, nicotine, and alcohol (Maldonado et al. 2006; Fattore et al. 2007; Parolaro and Rubino 2008). In contrast, the possible involvement of CB1 receptors in cocaine’s actions is controversial. Early studies showed that blockade of CB1 receptors by SR141716A (rimonabant) had no effect on intravenous cocaine self-administration (Fattore et al. 1999; De Vries et al. 2001; Filip et al. 2006), cocaine-induced conditioned place preference (CPP; Chaperon et al. 1998), cocaine’s discriminative stimulus effects (Filip et al. 2006), or cocaine-induced behavioral sensitization (Lesscher et al. 2005). In addition, neither SR141716A nor AM251 (another CB1 receptor antagonist) affected cocaine-enhanced DA accumulation in the nucleus accumbens (NAc) (Caillé and Parsons 2006; Xi et al. 2006).
In contrast to these negative findings, other studies demonstrated that AM251 and SR141716A (to a lesser extent) significantly inhibit cocaine self-administration under progressive-ratio (PR) reinforcement conditions (Soria et al. 2005; Xi et al. 2008), cocaine-enhanced electrical brain-stimulation reward (Xi et al. 2008), cocaine-induced behavioral sensitization (Filip et al. 2006), and cocaine- or cocaine-associated cue-induced reinstatement of drug-seeking behavior (De Vries et al. 2001; Filip et al. 2006; Xi et al. 2006). In addition, SR141716A also significantly inhibits cocaine-, nicotine-, or alcohol-induced phasic DA release in the NAc (Cheer et al. 2007). These recent findings suggest an important role for CB1 receptors in cocaine’s actions in animal models of drug addiction (Xi et al. 2008). The reason for the discrepancy remains unclear but could be related to a recent finding that SR141716A is not as highly potent and highly selective a CB1 receptor antagonist in vivo as previously thought (Xi et al. 2008).
In addition to the use of CB1 receptor antagonists, CB1−/− mice have been used to study the role of CB1 receptors in drug reward and addiction (Ledent et al. 1999; Zimmer et al. 1999). There are presently three lines of CB1−/− mice, with two different genetic backgrounds: CD1 (Ledent et al., 1999) and C57BL/6J (Zimmer et al. 1999). Previous studies have shown that CB1−/− mice with CD1 genetic background display similar responses to cocaine as their CB1+/+ littermates in cocaine self-administration (Cossu et al. 2001), cocaine-induced CPP and behavioral sensitization (Martin et al. 2000), and in cocaine-enhanced NAc DA (Soria et al. 2005), suggesting a limited role of CB1 receptors in cocaine’s actions.
However, this view has been challenged by other studies demonstrating that CD1 CB1−/− mice display significant reductions in breakpoint levels for cocaine self-administration under PR reinforcement (Soria et al. 2005), in cocaine- or amphetamine-induced hyperlocomotion and locomotor sensitization (Corbillé et al. 2007; Thiemann et al. 2008), and in cocaine-induced changes in cortical dendritic arborization (Ballesteros-Yáñez et al. 2007), when compared to CB1+/+ mice. This is further supported by a recent finding that cocaine-induced CPP is abolished in CB1−/− mice with C57BL/6J background, when cocaine doses were increased to 10 mg/kg (Miller et al. 2008). These data suggest an important role for CB1 receptors in cocaine reward and addiction-related behaviors. The reasons for this discrepancy remain unclear. It could be related to differences in cocaine doses, mouse genetic backgrounds, experimental approaches, data analyses, or experimental conditions.
Strikingly, CB1−/− mice with CD1 versus C57BL/6J backgrounds have been shown to exhibit different alterations in spontaneous locomotion, pain sensitivity, and several other behavioral responses to Δ9-tetrahydrocannabinol (Δ9-THC) (see Table 1). This suggests that mouse genetic backgrounds could be an important factor contributing to the discrepant findings described above. Since the majority of previous studies used CD1 CB1−/− mice, in the present study, we used C57BL/6J CB1−/− mice to further evaluate the role of CB1 receptors in cocaine’s action. Given the critical role of NAc DA in cocaine’s action (Wise 2005), we first observed basal and cocaine-augmented locomotion and NAc DA in both CB1+/+ and CB1−/− mice and then studied underlying mechanisms by observing basal DA release and clearance in CB1+/+ and CB1−/− mice with fast-scan cyclic voltammetry. Finally, we observed whether pharmacological blockade of CB1 receptors produced similar alterations in locomotion and NAc DA as genetic deletion in C57BL/ 6J mice.
Table 1.
Comparison of behavioral and striatal DA responses to cocaine, Δ9-THC, or noxious stimuli between CB1−/− mice with CD1 versus C57BL/6J genetic background
| Measurement | CB1−/− mice (C57BL/6J) | CB1−/− mice (CD1) |
|---|---|---|
| Basal locomotor activity | ↓ (Zimmer et al. 1999; present study) | ↑ (Ledent et al. 1999) |
| Cocaine-induced hyperactivity | ↓ (present study) | Unchanged (Martin et al. 2000); ↓ (Corbillé et al. 2007) |
| Behavioral sensitization to cocaine or amphetamine |
Unchanged (Martin et al. 2000); ↓ (Corbillé et al. 2007; Thiemann et al. 2008) | |
| Basal levels of extracellular DA in striatum | ↓ (present study) | Unchanged (Soria et al. 2005) |
| Cocaine-enhanced DA in striatum | ↓ (present study) | Unchanged (Soria et al. 2005) |
| Cocaine self-administration | Unchanged (FR; Cossu et al. 2001); ↓ Acquisition (FR; Soria et al. 2005); ↓ (PR; Soria et al. 2005) |
|
| Cocaine-induced CPP | ↓ (Miller et al. 2008) | Unchanged (Martin et al. 2000) |
| Pain sensitivity—hotplate test | ↓ (Zimmer et al. 1999) | Unchanged (Ledent et al. 1999) |
| Pain sensitivity—tail flick test | Unchanged (Zimmer et al. 1999) | Unchanged (Ledent et al. 1999) |
| Pain sensitivity—chemical irritation | ↓ (Zimmer et al. 1999) | Unchanged (Ledent et al. 1999) |
| THC-induced analgesia (hotplate test) | ↓ (Zimmer et al. 1999) | ↓ (Ledent et al. 1999) |
| THC-induced hypothermia | ↓ (Zimmer et al. 1999) | ↓ (Ledent et al. 1999) |
| THC-induced hypomotility | ↓ (Zimmer et al. 1999) | ↓ (Ledent et al. 1999) |
| THC-induced catalepsy | ↓ (Zimmer et al. 1999) | ↓ (Ledent et al. 1999) |
FR fixed ratio, PR progressive ratio, CPP conditioned place preference, THC Δ9 -tetrahydrocannabinol
Materials and methods
Animals
Male wild type (CB1+/+) and CB1 receptor knockout (CB1−/−) mice with C57BL/6J genetic background bred within the National Institute on Drug Abuse Intramural Research Program were descendants of three CB1+/− breeding pairs, generously donated by Dr. Andreas Zimmer of the National Institute of Mental Health (Bethesda, MD, USA). Genotyping was performed by Charles River Laboratories. All animals used in the present experiments were matched for age (8–14 weeks) and weight (25–35 g). They were housed individually in a climate-controlled animal colony room on a reversed light–dark cycle (lights on at 7:00 PM, lights off at 7:00 am) with free access to food and water. The animals were maintained in a facility fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. All experimental procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals of the US National Academy of Sciences and were approved by the Animal Care and Use Committee of the National Institute on Drug Abuse of the US National Institutes of Health.
Locomotor activity
Before drug administration, each animal was placed in locomotor detection chambers (Accuscan Instruments, Inc., Columbus, OH, USA) for 3 days (4 h/day) for environmental habituation. On each testing day, mice were placed in locomotor detection monitors for 1 h of habituation), and then each mouse was removed and given saline, cocaine (10 mg/kg, i.p.), or one dose (3, 10 mg/kg i.p.) of SR141716 (free base of SR141716A). Immediately after the drug injection, animals were placed back into the locomotor monitors. Data were collected in 10-min intervals using VersaMax version 3.0 software (Accuscan Instruments, Inc., Columbus, OH, USA). Total distance was used to evaluate the effects of different treatments on locomotor behavior.
In vivo microdialysis
Surgery
All mice were surgically implanted with intracranial guide cannulae (MAB 4.15.IC, SciPro Inc., Sanborn, NY, USA) into the NAc. The surgery was performed under anesthesia with a ketamine hydrochloride (80 mg/ml) and xylazine hydrochloride (12 mg/ml) mixture (0.08 ml/10 g body weight, i.p.), using standard aseptic surgical and stereotaxic technique. The stereotaxic coordinates for the NAc were AP +1.4 mm, ML ±1.5 mm, DV −3.8 mm with an angle of 8° from vertical (Paxinos and Watson 1998). The guide cannulae were fixed to the skull with dental acrylic.
General procedure
After 7 days of recovery from surgery, a probe (MAB 4.15.2.PES, SciPro Inc., Sanborn NY, USA) was inserted into the NAc at least 12 h before the experiments to minimize damage-induced DA release. On the day of the experiment, dialysis buffer (5 mM glucose, 2.5 mM KCl, 140 mM NaCl, 1.4 mM CaCl2, 1.2 mM MgCl2, 0.15% phosphate-buffered saline, pH 7.4) was perfused through the probe (2.0 µl/min) via syringe pump (Bioanalytical Systems, West Lafayette, IN, USA) beginning at least 2 h prior to sample collection. Dialysis samples were collected every 20 min into 10 µl 0.5 M perchloric acid to prevent DA degradation. After 1 h of baseline collection, animals received either vehicle, cocaine (10 mg/kg, i.p.), or SR141716 (3, 10 mg/kg, i.p.). After collection, samples were frozen at −80°C until analyzed.
Quantification of DA
DA was measured in the dialysate with the ESA electrochemical detection system. The DA mobile phase contained 4.76 mM citric acid, 150 mM Na2HPO4, 3 mM sodium dodecyl sulfate, 50 mM EDTA, 10% methanol, and 15% acetylnitrile, pH 5.6. DA was separated using an MD-150×3.2-mm reversed-phase column from ESA Inc. (Chelmsford, MA, USA) and oxidized/ reduced using a Coulochem III detector from ESA Inc. Three electrodes were used: a preinjection port guard cell (+0.25 V) to oxidize the mobile phase, a reduction analytical electrode (E1, −0.1 V), and an oxidation analytical electrode (E2, 0.2 V). The area under curve (AUC) of the DA peak was measured using an “EZChrom Elite” ESA chromatography data system. DA values were quantified with an external standard curve (1–1,000 fM).
Brain slice preparation
Mice were killed by cervical dislocation and rapidly decapitated. The brains were removed and placed in chilled (4°C) and aerated (95% O2/5% CO2) artificial cerebrospinal fluid (aCSF), comprised of (mM): NaCl (126); KCl (3.0); MgCl2 (1.5); CaCl2 (2.4); NaH2PO4 (1.2); glucose (11.0); NaHCO3 (25). Transverse brain slices containing the cerebral cortex and the striatum were cut (300 µm) in the chilled aCSF using a vibrating tissue slicer (Leica VT1000S). The brain slices were then transferred to a holding chamber containing aCSF, saturated with 95% O2/ 5% CO2, at 32°C. Prior to initiating recordings, a single hemi-slice (i.e., one hemisphere) was submerged in a low-volume (170 µl) recording chamber and continuously perfused with warm (30–32°C) aCSF at 2 ml/min. The aCSF was warmed using an in-line solution heater (TC-324B, Warner Instruments, Hamden, CT, USA).
Fast-scan cyclic voltammetry
Carbon-fiber electrode construction
Fast-scan cyclic voltammetry was performed using carbon-fiber electrodes as described previously (Cahill et al. 1996; Heien et al. 2004). Carbon fibers (7 µm diameter; Goodfellow Corp., Devon, PA, USA) were aspirated into glass capillary tubes, which were then pulled using a multistage patch pipette program on a Sutter P-97 electrode puller. The carbon fibers were trimmed to allow ~20–50 µm to protrude from the glass capillary and then sealed in the glass by passing the assembly over a flame. Pipettes containing the carbon fiber were filled with a solution of 4 M potassium acetate and 150 mM KCl and attached to the headstage of a patch-clamp amplifier (Heka EVA-8). Voltammetric scans, stimulus waveform generation and timing, and data collection were performed using A/D boards (PCI 6052E and PCI-6711E, National Instruments, Austin, TX, USA) and custom LabView-based software (TarHeel CV, courtesy of Drs. Joseph Cheer and Michael Heien, University of North Carolina). All electrodes were tested for stable background currents and responsiveness in a laboratory beaker containing a DA (5 µM) solution prior to each experiment.
DA measurement in striatal slices
Carbon-fiber tips were positioned between the separated tips of a bipolar stimulating electrode (FHC Inc., Bowdoin, ME, USA) using a stereo microscope. The carbon fibers were inserted ~75–100 µm into the NAc slice, as previously described (Jones et al. 1995). In initial experiments, we observed the most consistent and stable signals in the NAc core, just medial and dorsal to the anterior commissure. Therefore, all recordings were obtained from this locus. Voltammetric scans from −0.4 to 1.0 V and back were performed at 400 V/s (7-ms scan duration) at a frequency of 10 Hz. A 5-s measurement (50 scans) of background current was taken prior to electrical stimulation of the brain slice and subtracted from the voltammetric scan obtained at the signal peak during the electrical stimulation. The remaining currents following background subtraction were then used to generate a voltammogram (plotting current versus voltage) for each signal. All signals matched the expected oxidation and reduction signals of DA (Jones et al. 1995; Cahill et al. 1996). Electrical stimulation consisted of a single 1-ms biphasic pulse that was offset from the voltammetric scans. Electrical stimulation current intensity was varied from 0.1 to 1 mA in order to construct input– output curves for each placement in the striatal slice. Time constants (τ) for the decay phase of each DA signal were obtained by fitting a single exponential function (y = A−t/τ) using a least squares minimization algorithm (WCP, Dr. John Dempster, Strathclyde Institute for Biomedical Sciences, Glasgow, UK, http://spider.science.strath.ac.uk). Initial comparisons of the sum of squares (F test) between single and double exponential functions (Prism v. 5.0) confirmed that the decay phase was best fit by a single exponential in all cases. It has been previously demonstrated that the first-order rate constant (k or 1/τ) obtained using this approach provides an index of the efficiency (Vmax/Km) of DA clearance mediated via the DA transporter, at low DA concentrations (Sabeti et al. 2002).
Drugs
Cocaine HCl (Sigma-Aldrich., Saint Louis, MO, USA) was dissolved in physiological saline. SR141716 (free base form) [N-piperidinyl-5-(4-chlorophenyl)−1-(2,4-dichlorophenyl)−4-methylpyrazole−3-carboxamide] was obtained from Research Triangle Institute (Research Triangle Park, NC, USA). SR141716 was dissolved in 0.5% Tween−80 (Sigma-RBI, St. Louis, MO, USA).
Histology
Following completion of in vivo microdialysis, mice were decapitated and brains were removed and placed in 10% formalin for 1 week. The tissue was blocked around the NAc and coronal sections (100 µm thick) made by vibratome. The sections were stained with cresyl violet. Only those animals that had been implanted in the desired NAc locus were used in the study.
Data analyses
All data are presented as means (±SEM). One-way (genotype), two-way (genotype × time, or genotype × stimulus intensity for input–output relationships in slice studies), or three-way (genotype × treatment × time) analysis of covariance (ANCOVA) were used to analyze the data (more details described in “Results” below). Preplanned individual group comparisons were carried out using the Bonferroni statistical procedure.
Results
Basal locomotion and cocaine-induced hyperlocomotion is attenuated in CB1−/− mice
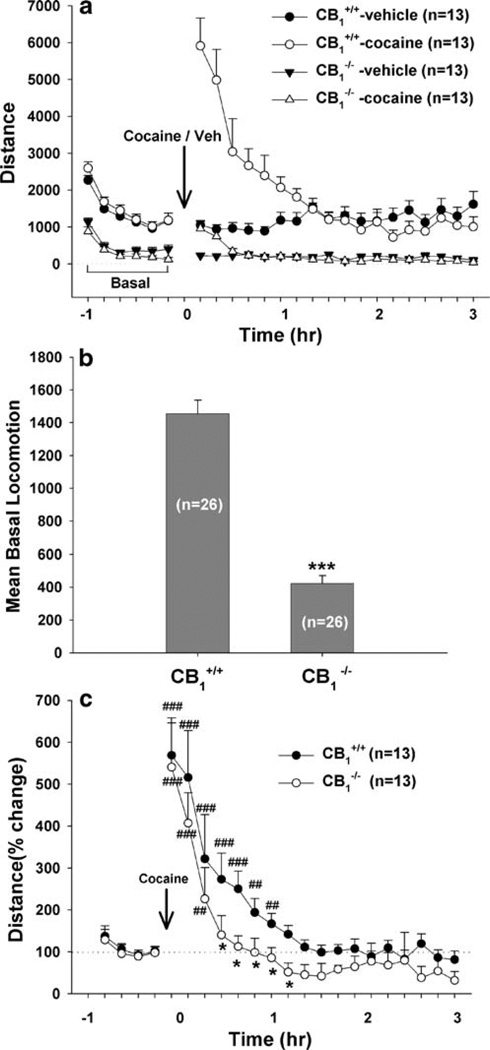
Figure 1 shows the effect of cocaine (10 mg/kg, i.p.) or vehicle on locomotion in CB1+/+ and CB1−/− mice, indicating a significant attenuation of basal and cocaine-augmented locomotion in CB1−/− mice. Three-way ANCOVA with repeated measures over time and baseline values as covariates for the data shown in Fig. 1a revealed a statistically significant genotype (CB1+/+ vs. CB1−/−) main effect (F1, 48=114.55, p<0.001), treatment (cocaine vs. vehicle) main effect (F1, 48=5.01, p<0.001), and time main effect (F23, 1,104=18.44, p<0.001). Figure 1b shows mean basal locomotion in CB1+/+ and CB1−/− mice, indicating a significant attenuation (~70%) of locomotion in CB1−/− mice (F1, 50=119.98, p<0.001), as compared to CB1+/+ mice. Figure 1c illustrates percent change from baseline locomotion after cocaine administration, indicating that the duration of cocaine-induced hyperlocomotion was significantly shorter in CB1−/− mice than in CB1+/+ mice (F1, 24=4.92, p<0.05, two-way analysis of variance (ANOVA)).
Fig. 1.
Basal and cocaine-enhanced locomotor activity in CB1+/+ and CB1−/− mice. a shows basal locomotion data (distance) and cocaine-or saline-induced locomotion in CB1+/+ and CB1−/− mice. b shows mean basal locomotion in CB1+/+ and CB1−/− mice, indicating a significant reduction in locomotion in CB1−/− mice relative to their wild-type CB1+/+ littermates. c shows percent change from baseline in cocaine-induced locomotion, indicating that the duration of cocaine-enhanced locomotion was shorter in CB1−/− mice than in CB1+/+ mice. *p<0.05, **p<0.01, ***p<0.001, compared to CB1+/+ mice. ##p<0.01, ###p<0.001, compared to baseline
Basal and cocaine-enhanced extracellular NAc DA is attenuated in CB1−/− mice
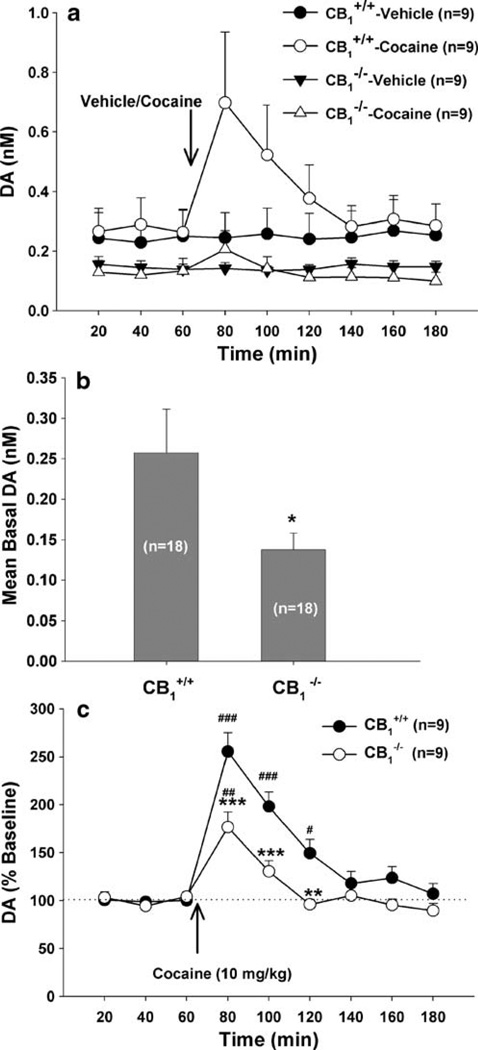
Figure 2 shows the effect of cocaine or vehicle on extracellular NAc DA in CB1+/+ and CB1−/− mice. Three-way ANCOVA with repeated measures over time and baseline values as covariates for the data shown in Fig. 2a revealed a statistically significant genotype (CB1+/+ vs CB1−/−) main effect (F1, 32=6.87, p<0.001), treatment (cocaine vs. vehicle) main effect (F1, 32=3.55, p<0.05), and time main effect (F8, 256=7.9, p<0.001). Further data analyses revealed a significant reduction in either basal levels of extracellular DA (Fig. 2b: F1, 34=4.51, p<0.05, one-way ANOVA) or cocaine-enhanced NAc DA (Fig. 2c: F1,16=11.79, p<0.01, two-way ANOVA) in CB1−/− mice, as compared to CB1+/+ mice.
Fig. 2.
Basal and cocaine-enhanced NAc DA in CB1+/+ and CB1−/− mice. a shows basal and cocaine- or saline-induced changes in NAc DA in CB1+/+ and CB1−/− mice. b shows mean basal extracellular DA, indicating a significant attenuation of basal DA in CB1−/− mice relative to wild-type CB1+/+ littermates. c shows percent change in DA from baseline, indicating a significant attenuation of cocaine-enhanced DA in CB1−/− mice relative to CB1+/+ mice. *p<0.05, **p< 0.01, ***p<0.001, compared to CB1+/+ mice. #p<0.05, ##p<0.01, ###p<0.001, compared to baseline
Electrically evoked NAc DA release is attenuated in CB1−/− mice
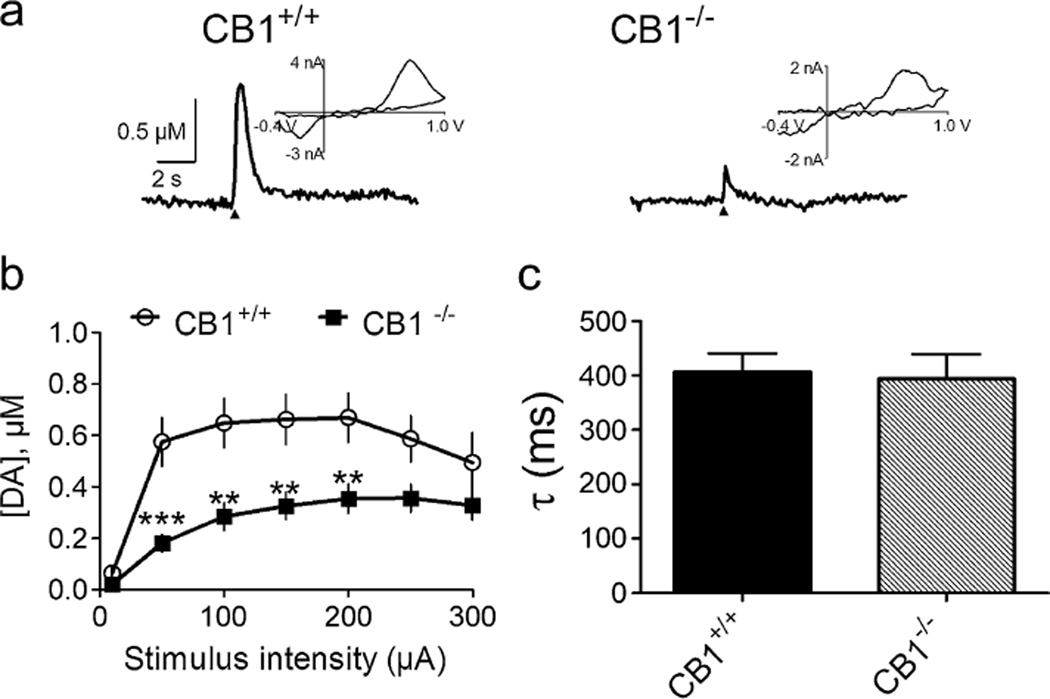
Figure 3 shows DA release in NAc brain slices from CB1−/− and CB1+/+ littermate mice. Figure 3a shows representative voltammetric signals, illustrating that DA release elicited by single-pulse electrical stimulation was significantly lower in a CB1−/− mouse than in a CB1+/+ mouse. Figure 3b shows mean DA release elicited by single-pulse electrical stimuli across a range of stimulus intensities, demonstrating a significant reduction in evoked DA release in CB1−/− mice compared to CB1+/+ mice (F1, 330=47.70, p<0.001, repeated-measures ANOVA). Figure 3c shows mean decay time constants (τ) of the DA signal in both CB1+/+ and CB1−/− mice (t=0.2399, p=NS), indicating no difference in DA uptake between the two groups of mice.
Fig. 3.
NAc DA release in CB1−/− mice. a shows representative voltammetric signals recorded in brain slices from a CB1+/+ mouse and a CB1−/− mouse. A single-pulse stimulus (1 ms, 100 µA; arrowhead) was used to elicit NAc DA release. Inset of each signal shows the background-subtracted voltammogram obtained at the peak of the recorded current. b shows the input–output relationship of DA release elicited by single-pulse stimulation in the NAc, indicating attenuated DA release across a range of stimulus intensities in CB1−/− mice (n=28 slices from eight subjects) relative to CB1+/+ mice (n=23 slices from six subjects). c shows the mean decay time constants (tau, τ) of the DA signals, indicating that DA clearance (or uptake) was not different between CB1+/+ (n=18 slices from six subjects) and CB1−/− mice (n=15 slices from four subjects). **p<0.01, ***p<0.001, compared to CB1+/+ mice, repeated-measures ANOVA
SR141716 inhibits locomotion in CB1+/+ mice
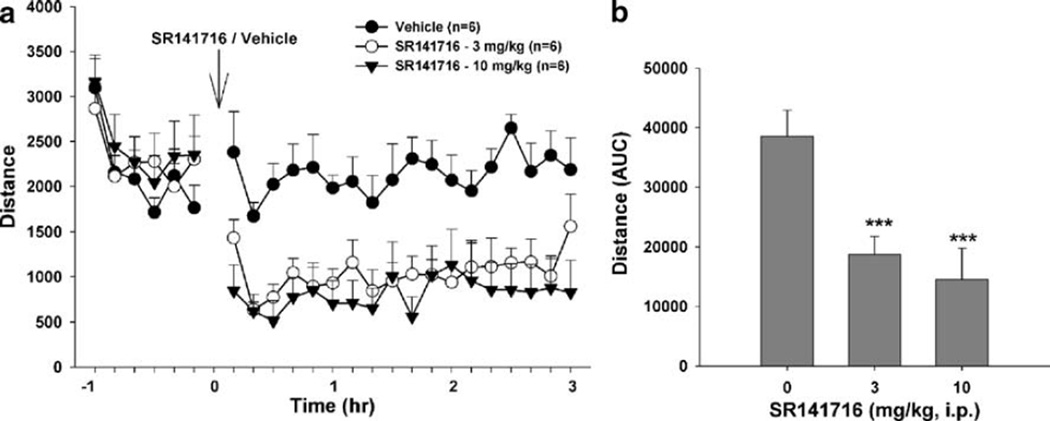
Figure 4 shows that the CB1 receptor antagonist SR141716 (3 or 10 mg/kg, i.p.) significantly inhibited locomotor activity in CB1+/+ mice. Two-way ANOVA with repeated measures over time and subjects for the data shown in Fig. 4a revealed a significant treatment main effect (F2, 10= 10.46, p<0.01), time main effect (F23, 115=27.55, p< 0.001), and treatment × time interaction (F46, 230=7.62, p<0.001). Individual group comparisons indicated a significant reduction in locomotion after 3 mg/kg (t= 3.41, p<0.05) or 10 mg/kg (t=4.35, p<0.01) SR141716 administration, when compared to the vehicle treatment group. Similarly, one-way ANOVA for the AUC data shown in Fig. 4b also revealed a significant SR141716 treatment main effect (F2, 10=26.33, p<0.001). Individual group comparisons revealed a significant reduction in locomotion after 3 mg/kg (t=5.39, p<0.001) or 10 mg/kg (t=6.90, p<0.001) SR141716 administration.
Fig. 4.
Effect of SR141716 on locomotor activity in CB1+/+ mice. a shows locomotion after SR141716 (3, 10 mg/kg, i.p.) or vehicle. b shows overall mean locomotion (AUC data) after SR141716 or vehicle administration. ***p<0.001, compared to vehicle group
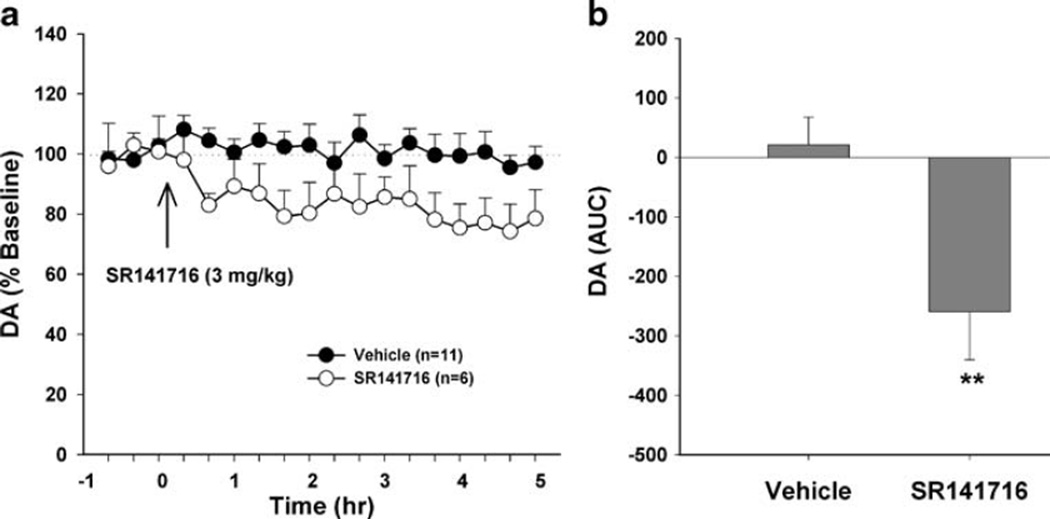
SR141716 inhibits NAc DA release in CB1+/+ mice
Systemic administration of SR141716 (3 mg/kg, i.p.) significantly lowered extracellular NAc DA levels in CB1+/+ mice, as measured by in vivo microdialysis (Fig. 5). Two-way ANOVA with repeated measures over time for the data shown in Fig. 5a revealed a statistically significant treatment main effect (F1, 15=12.10, p<0.01) and time main effect (F17, 255=4.43, p<0.05). One-way ANOVA for the AUC data shown in Fig. 5b revealed a statistically significant reduction (F1, 15=12.19, p<0.01) in extracellular DA after SR141716 administration.
Fig. 5.
Effect of SR141716 on extracellular NAc DA levels in CB1+/+ mice. a shows extracellular DA after SR141716 (3 mg/kg, i.p.) or vehicle. b shows overall mean NAc DA (AUC data) after SR141716 or vehicle administration. **p<0.01, compared to vehicle group
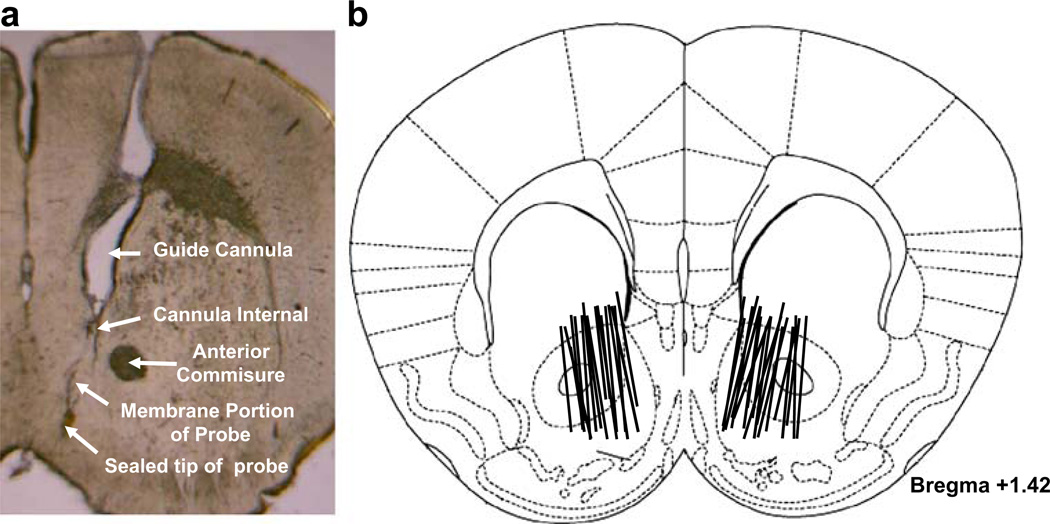
Histology
Figure 6 shows microdialysis probe locations in the mouse ventral striatum. Figure 6a shows a representative probe track. The active semi-permeable probe membranes of the microdialysis probes were located within the NAc core and shell (Fig. 6b). There were no differences in placement of microdialysis probes between CB1+/+ and CB1−/− mice.
Fig. 6.
Microdialyses probe locations in the NAc of mice. a Shows a representative probe track. Lines in b indicate the placement of probes in both the core and the shell of the NAc
Discussion
The present study demonstrates that CB1−/− mice bred on a C57BL/6J genetic background display a significant reduction in both basal levels of and cocaine-enhanced locomotion and in extracellular NAc DA, compared to their CB1+/+ littermates. In vitro fast-scan cyclic voltammetry demonstrated that the reduced extracellular DA is likely due to a reduction in basal DA release rather than an increase in DA clearance. These observations in CB1−/− mice are supported by the finding that pharmacological blockade of CB1 receptors by SR141716 also inhibited locomotion and NAc DA release in CB1+/+ mice. Together, these data suggest an important role for CB1 receptors in maintaining locomotor activity and DA tone, under both basal and cocaine-stimulated conditions.
Endocannabinoid modulation of locomotion
Locomotion has been widely used to study neural mechanisms underlying addictive drug action (Cornish and Kalivas 2001). The high density of CB1 receptors in the basal ganglia and cerebellum suggests involvement of CB1 receptors in locomotion (Mailleux and Vanderhaeghen 1992; Tsou et al. 1998). However, the reported effects of cannabinoid agonists on locomotor activity are varied. Previous studies have reported increases (Sañudo-Peña et al. 2000), decreases (Ferrari et al. 1999; Shi et al. 2005; Wu and French 2000), and biphasic dose-dependent effects of cannabinoid agonists on locomotion (Sulcova et al. 1998; Abel 1970), which appears to be largely dose-dependent (Muschamp and Siviy 2002; Järbe et al. 2006; Pandolfo et al. 2007). In the present study, CB1−/− mice displayed decreased basal locomotor activity compared with their wild-type CB1+/+ littermates. This is consistent with a previous report on CB1−/− mice with C57BL/6J background (Zimmer et al. 1999), but contrasts with findings in CB1−/− mice bred on a CD1 background in which increased basal locomotion was observed (Ledent et al. 1999; Table 1). Consistent with our finding in CB1−/− mice, the CB1 receptor antagonist SR141716 inhibited spontaneous locomotion in C57BL/6J (present study) and other mouse strains (Compton et al. 1996), as well as in rats (Järbe et al. 2002, 2006; Gardner and Mallet 2006). These data suggest that activation of CB1 receptors under physiological conditions tends to activate locomotion and may therefore contribute to the maintenance of normal locomotor activity in wild-type mice or rats. Given that Δ9-THC and other cannabinoid agonists such as WIN55,212-2, CPP55940, and HU-210 are non-selective cannabinoid receptor agonists (Pertwee 2005), we speculate that locomotion reduction produced by these compounds could be mediated by activation of other non-CB1 receptors.
Endocannabinoid involvement in cocaine-induced hyperlocomotion
In addition, endocannabinoids may also be involved in cocaine-induced hyperlocomotion. In the present study, we found that CB1−/− mice displayed a significantly reduced locomotor response to acute cocaine as compared to their CB1+/+ littermates. This finding is in contrast to a previous report by Martin et al. (2000) but is consistent with a recent report by Corbillé et al. (2007). In addition, CB1−/− mice have been shown to display impaired locomotor sensitization to cocaine or d-amphetamine (Corbillé et al. 2007; Thiemann et al. 2008). Further, the novel CB1 receptor antagonist AM251 blocks cocaine or d-amphetamine-induced locomotor sensitization in CB1+/+ mice (Corbillé et al. 2007). These findings suggest that cannabinoid mechanisms acting via CB1 receptors play an important role in mediating psychostimulant-induced hyperlocomotion. We note, however, that SR141716A has been reported to have no effect on acute cocaine-induced locomotor stimulation (Przegaliński et al. 2005; Lesscher et al. 2005) but attenuates chronic cocaine-induced motoric sensitization in rats (Filip et al. 2006; Gerdeman et al. 2008). In addition, the cannabinoid agonists HU210 and WIN55,212-2 have been reported to significantly attenuate cocaine-induced hyperlocomotion in rats (Ferrari et al. 1999; Przegaliński et al. 2005). The reasons for such discrepant findings are unclear. Many factors—including species differences (rats vs. mice), different neural mechanisms underlying acute cocaine-induced hyperlocomotion versus chronic cocaine-induced locomotor sensitization (Cornish and Kalivas 2001), relatively poor potency and/or receptor selectivity of SR141716A in vivo (Xi et al. 2008), and non-selective CB1/CB2 receptor binding properties of the cannabinoid agonists tested (Pertwee 2005)—may all contribute to the above-noted discrepant findings.
Endocannabinoid modulation of DA release
It is well documented that increases in extracellular DA levels in both the dorsal and ventral striatum are related to the rewarding and psychomotor-stimulating effects of cocaine and other psychostimulants (Wise 2005). This suggests that altered striatal DA responsivity may alter locomotion in CB1−/− mice. To test this hypothesis, in vivo microdialysis was used to measure NAc DA levels in CB1+/+ and CB1−/− mice. We found that CB1−/− mice displayed significant reductions (~50%) in basal levels of extracellular NAc DA. This is consistent with a previous report (Soria et al. 2005) demonstrating a ~35% reduction in basal extracellular DA in the dorsal striatum of CB1−/− mice (on a CD1 background) as compared to CB1+/+ mice. To ascertain whether the reduction in DA levels reflected a decrease in release or a change in transporter-mediated DA clearance, we performed voltammetric recordings in brain slices from CB1+/+ and CB1−/− mice. Consistent with the microdialysis data, we observed reduced peak DA amplitudes elicited by single-pulse electrical stimulation in CB1−/− mice, without change in decay time constants of the observed signals. Thus, we hypothesize that the reduced baseline extracellular DA levels in CB1−/− mice are caused by reduced DA release rather than by a change in DA uptake. Overall, these findings are consistent with previous reports that Δ9-THC or other cannabinoid agonists produce an increase in either striatal DA release or ventral tegmental area (VTA) DA neuron activity (Chen et al. 1990; French 1997; Tanda et al. 1997; Melis et al. 2000), an effect that is blocked by SR141716A (French 1997; Tanda et al. 1997). These data suggest that activation of CB1 receptors increases, whereas inactivation decreases, basal DA release in the dorsal and/or ventral striatum. This conclusion is further supported by our finding that pharmacologic blockade of CB1 receptors by SR141716 significantly lowered extracellular NAc DA levels in CB1+/+ mice. This finding is in contrast to previous findings in rats that SR141716A or AM251 failed to lower extracellular NAc DA (Caillé and Parsons 2006; Xi et al. 2006), perhaps suggesting a difference between rats and mice in cannabinoid tone on DA release.
The mechanisms by which cannabinoids modulate DA release remain unclear. Anatomical evidence indicates that midbrain DA neurons have very low CB1 receptor levels (Mailleux and Vanderhaeghen 1992; Tsou et al. 1998; Julian et al. 2003), with much higher levels observed on axon terminals impinging upon these cells (Mátyás et al. 2008). This suggests that cannabinoids may not exert direct actions on midbrain DA neurons (Maldonado et al. 2006 for review). Instead, the high densities of CB1 receptors expressed on both glutamatergic and GABAergic neurons in the VTA and NAc (Szabo et al. 2002; Julian et al. 2003; Riegel and Lupica 2004; Köfalvi et al. 2005; Mátyás et al. 2006; Corbillé et al. 2007) appear indicative of indirect cannabinoid modulation of DA release. Cannabinoid activation of VTA CB1 receptors appears to produce inhibition of GABA release (Szabo et al. 2002; Riegel and Lupica 2004). This may result in increased VTA DA neuronal activity or striatal DA release by disinhibition. In addition, increased VTA DA neuronal activity may augment endocannabinoid release resulting in inhibition of both GABAergic and glutamatergic inputs to VTA DA neurons (Lupica et al. 2004; Lupica and Riegel 2005; Maldonado et al. 2006). The final net effect on VTA DA neuron activity would depend on the functional balance between these opposite actions (Riegel and Lupica 2004). Finally, increased NAc DA may also stimulate endocannabinoid release from postsynaptic medium-spiny GABAergic neurons (Giuffrida et al. 1999; Patel et al. 2003; Centonze et al. 2004), which may then activate presynaptic CB1 receptors, producing an inhibition of glutamate release from corticostriatal glutamatergic terminals (Robbe et al. 2001, 2002). This decreased glutamate would lower the excitability of medium-spiny GABAergic neurons, thereby decreasing inhibitory GABAergic input from NAc to VTA DA neurons (Szabo et al. 2002). Accordingly, deletion of CB1 receptors might well decrease basal NAc DA release, as observed in the present study.
Endocannabinoid involvement in cocaine-enhanced DA release
We also found in the present study that the striatal DA response to acute cocaine (10 mg/kg i.p.) was significantly reduced in CB1−/− mice compared to CB1+/+ mice. This is consistent with a recent finding that SR141716A significantly inhibits cocaine-, nicotine-, or alcohol-induced phasic DA release in rats as assessed by fast-scan cyclic voltammetry (Cheer et al. 2007) but contrasts with the finding that cocaine produces a similar increase in extracellular NAc DA in both CB1+/+ and CB1−/− mice with CD1 genetic background (Soria et al. 2005), and with reports that CB1 receptor antagonists fail to alter cocaine-induced increases in extracellular NAc DA in rats (Caillé and Parsons 2006; Xi et al. 2006). Many factors could account for such discrepancies, including species differences (rats vs. mice), genetic backgrounds (C57BL/6J vs. CD1), experimental methods (voltammetry vs. microdialysis), or placement of microdialysis probes (dorsal vs. ventral striatum). Whatever the reasons for the discrepancies in the literature, the present findings suggest an important role for CB1 receptors in cocaine-enhanced NAc DA. Given that cocaine-enhanced DA levels result from blockade of DA re-uptake (Wise 2005), reduced basal DA release in CB1−/− mice may underlie the observed reduction in cocaine’s actions on DA release and locomotion observed in CB1−/− mice in the present study. Finally, the present data speak to the importance of background genetic strain in the functional consequences of gene deletion.
In conclusion, the present study demonstrates that CB1−/− mice on a C57BL/6J background (Zimmer et al. 1999) display a significant reduction both in basal DA release and locomotor activity, as well as in cocaine-induced increases in locomotion and extracellular NAc DA. These findings, combined with pharmacological findings, support an important role for endocannabinoid-CB1 receptor substrates in cocaine addiction.
Acknowledgement
This research was supported by the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health.
Contributor Information
Xia Li, Neuropsychopharmacology Section, Chemical Biology Research Branch, National Institute on Drug Abuse, Baltimore, MD 21224, USA.
Alexander F. Hoffman, Neurophysiology Section, Cellular Neurobiology Research Branch, Intramural Research Program, National Institute on Drug Abuse, Baltimore MD 21224 USA
Xiao-Qing Peng, Neuropsychopharmacology Section, Chemical Biology Research Branch, National Institute on Drug Abuse, Baltimore, MD 21224, USA.
Carl R. Lupica, Neurophysiology Section, Cellular Neurobiology Research Branch, Intramural Research Program, National Institute on Drug Abuse, Baltimore MD 21224 USA
Eliot L. Gardner, Neuropsychopharmacology Section, Chemical Biology Research Branch, National Institute on Drug Abuse, Baltimore, MD 21224, USA
Zheng-Xiong Xi, Email: zxi@intra.nida.nih.gov, Neuropsychopharmacology Section, Chemical Biology Research Branch, National Institute on Drug Abuse, Baltimore, MD 21224, USA.
References
- Abel EL. Effects of the marihuana-homologue, parahexyl, on open field behaviour in the rat. J Pharm Pharmacol. 1970;22:785. doi: 10.1111/j.2042-7158.1970.tb08432.x. [DOI] [PubMed] [Google Scholar]
- Ballesteros-Yáñez I, Valverde O, Ledent C, Maldonado R, DeFelipe J. Chronic cocaine treatment alters dendritic arborization in the adult motor cortex through a CB1 cannabinoid receptor-dependent mechanism. Neuroscience. 2007;146:1536–1545. doi: 10.1016/j.neuroscience.2007.03.017. [DOI] [PubMed] [Google Scholar]
- Cahill PS, Walker QD, Finnegan JM, Mickelson GE, Travis ER, Wightman RM. Microelectrodes for the measurement of catecholamines in biological systems. Anal Chem. 1996;68:3180–3186. doi: 10.1021/ac960347d. [DOI] [PubMed] [Google Scholar]
- Caillé S, Parsons LH. Cannabinoid modulation of opiate reinforcement through the ventral striatopallidal pathway. Neuro-psychopharmacology. 2006;31:804–813. doi: 10.1038/sj.npp.1300848. [DOI] [PubMed] [Google Scholar]
- Centonze D, Battista N, Rossi S, Mercuri NB, Finazzi-Agrò A, Bernardi G, Calabresi P, Maccarrone M. A critical interaction between dopamine D2 receptors and endocannabinoids mediates the effects of cocaine on striatal GABAergic transmission. Neuropsychopharmacology. 2004;29:1488–1497. doi: 10.1038/sj.npp.1300458. [DOI] [PubMed] [Google Scholar]
- Chaperon F, Soubrié P, Puech AJ, Thiébot MH. Involvement of central cannabinoid (CB1) receptors in the establishment of place conditioning in rats. Psychopharmacology. 1998;135:324–332. doi: 10.1007/s002130050518. [DOI] [PubMed] [Google Scholar]
- Cheer JF, Wassum KM, Sombers LA, Heien MLAV, Ariansen JL, Aragona BJ, Phillips PEM, Wightman RM. Phasic dopamine release evoked by abused substances requires cannabinoid receptor activation. J Neurosci. 2007;27:791–795. doi: 10.1523/JNEUROSCI.4152-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Paredes W, Li J, Smith D, Lowinson J, Gardner EL. Δ9-tetrahydrocannabinol produces naloxone-blockable enhancement of presynaptic basal dopamine efflux in nucleus accumbens of conscious, freely-moving rats as measured by intracerebral microdialysis. Psychopharmacology. 1990;102:156–162. doi: 10.1007/BF02245916. [DOI] [PubMed] [Google Scholar]
- Compton DR, Aceto MD, Lowe J, Martin BR. In vivo characterization of a specific cannabinoid receptor antagonist (SR141716A): inhibition of delta Δ9 -tetrahydrocannabinol-induced responses and apparent agonist activity. J Pharmacol Exp Ther. 1996;277:586–594. [PubMed] [Google Scholar]
- Corbillé AG, Valjent E, Marsicano G, Ledent C, Lutz B, Hervé D, Girault JA. Role of cannabinoid type 1 receptors in locomotor activity and striatal signaling in response to psychostimulants. J Neurosci. 2007;27:6937–6947. doi: 10.1523/JNEUROSCI.3936-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish JL, Kalivas PW. Cocaine sensitization and craving: differing roles for dopamine and glutamate in the nucleus accumbens. J Addict Dis. 2001;20:43–54. doi: 10.1300/J069v20n03_05. [DOI] [PubMed] [Google Scholar]
- Cossu G, Ledent C, Fattore L, Imperato A, Böhme GA, Parmentier M, Fratta W. Cannabinoid CB1 receptor knockout mice fail to self-administer morphine but not other drugs of abuse. Behav Brain Res. 2001;118:61–65. doi: 10.1016/s0166-4328(00)00311-9. [DOI] [PubMed] [Google Scholar]
- De Vries TJ, Shaham Y, Homberg JR, Crombag H, Schuurman K, Dieben J, Vanderschuren LJMJ, Schoffelmeer ANM. A cannabinoid mechanism in relapse to cocaine seeking. Nat Med. 2001;7:1151–1154. doi: 10.1038/nm1001-1151. [DOI] [PubMed] [Google Scholar]
- Fattore L, Martellotta MC, Cossu G, Mascia MS, Fratta W. CB1 cannabinoid receptor agonist WIN 55,212-2 decreases intravenous cocaine self-administration in rats. Behav Brain Res. 1999;104:141–146. doi: 10.1016/s0166-4328(99)00059-5. [DOI] [PubMed] [Google Scholar]
- Fattore L, Fadda P, Fratta W. Endocannabinoid regulation of relapse mechanisms. Pharmacol Res. 2007;56:418–427. doi: 10.1016/j.phrs.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Ferrari F, Ottani A, Giuliani D. Influence of the cannabinoid agonist HU 210 on cocaine- and CQP 201–403-induced behavioural effects in rat. Life Sci. 1999;65:823–831. doi: 10.1016/s0024-3205(99)00309-4. [DOI] [PubMed] [Google Scholar]
- Filip M, Goxda A, Zaniewska M, McCreary AC, Nowak E, Kolasiewicz W, Przegalihski E. Involvement of cannabinoid CB1 receptors in drug addiction: effects of rimonabant on behavioral responses induced by cocaine. Pharmacol Rep. 2006;58:806–819. [PubMed] [Google Scholar]
- French ED. Δ9 -Tetrahydrocannabinol excites rat VTA dopamine neurons through activation of cannabinoid CB1 but not opioid receptors. Neurosci Lett. 1997;226:159–162. doi: 10.1016/s0304-3940(97)00278-4. [DOI] [PubMed] [Google Scholar]
- Gardner A, Mallet PE. Suppression of feeding, drinking, and locomotion by a putative cannabinoid receptor ‘silent antagonist’. Eur J Pharmacol. 2006;530:103–106. doi: 10.1016/j.ejphar.2005.11.032. [DOI] [PubMed] [Google Scholar]
- Gerdeman GL, Schechter JB, French ED. Context-specific reversal of cocaine sensitization by the CB(1) cannabinoid receptor antagonist rimonabant. Neuropsychopharmacology. 2008;33:2747–2759. doi: 10.1038/sj.npp.1301648. [DOI] [PubMed] [Google Scholar]
- Giuffrida A, Parsons LH, Kerr TM, Rodríguez de Fonseca F, Navarro M, Piomelli D. Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nat Neurosci. 1999;2:358–363. doi: 10.1038/7268. [DOI] [PubMed] [Google Scholar]
- Heien ML, Johnson MA, Wightman RM. Resolving neurotransmitters detected by fast-scan cyclic voltammetry. Anal Chem. 2004;76:5697–5704. doi: 10.1021/ac0491509. [DOI] [PubMed] [Google Scholar]
- Järbe TUC, Andrzejewski ME, DiPatrizio NV. Interactions between the CB1 receptor agonist Δ9-THC and the CB1 receptor antagonist SR-141716 in rats: open-field revisited. Pharmacol Biochem Behav. 2002;73:911–919. doi: 10.1016/s0091-3057(02)00938-3. [DOI] [PubMed] [Google Scholar]
- Järbe TUC, Ross T, DiPatrizio NV, Pandarinathan L, Makriyannis A. Effects of the CB1R agonist WIN-55,212-2 and the CB1R antagonists SR-141716 and AM-1387: open-field examination in rats. Pharmacol Biochem Behav. 2006;85:243–252. doi: 10.1016/j.pbb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Jones SR, Garris PA, Wightman RM. Different effects of cocaine and nomifensine on dopamine uptake in the caudate-putamen and nucleus accumbens. J Pharmacol Exp Ther. 1995;274:396–403. [PubMed] [Google Scholar]
- Julian MD, Martin AB, Cuellar B, Rodríguez de Fonseca F, Navarro M, Moratalla R, Garcia-Segura LM. Neuroanatomical relationship between type 1 cannabinoid receptors and dopaminergic systems in the rat basal ganglia. Neuroscience. 2003;119:309–318. doi: 10.1016/s0306-4522(03)00070-8. [DOI] [PubMed] [Google Scholar]
- Köfalvi A, Rodrigues RJ, Ledent C, Mackie K, Vizi ES, Cunha RA, Sperlágh B. Involvement of cannabinoid receptors in the regulation of neurotransmitter release in the rodent striatum: a combined immunochemical and pharmacological analysis. J Neurosci. 2005;25:2874–2884. doi: 10.1523/JNEUROSCI.4232-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledent C, Valverde O, Cossu G, Petitet F, Aubert JF, Beslot F, Böhme GA, Imperato A, Pedrazzini T, Roques BP, Vassart G, Fratta W, Parmentier M. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science. 1999;283:401–404. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- Lesscher HMB, Hoogveld E, Burbach JPH, van Ree JM, Gerrits MAFM. Endogenous cannabinoids are not involved in cocaine reinforcement and development of cocaine-induced behavioural sensitization. Eur Neuropsychopharmacol. 2005;15:31–37. doi: 10.1016/j.euroneuro.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Lupica CR, Riegel AC. Endocannabinoid release from midbrain dopamine neurons: a potential substrate for cannabinoid receptor antagonist treatment of addiction. Neuropharmacology. 2005;48:1105–1116. doi: 10.1016/j.neuropharm.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Lupica CR, Riegel AC, Hoffman AF. Marijuana and cannabinoid regulation of brain reward circuits. Br J Pharmacol. 2004;143:227–234. doi: 10.1038/sj.bjp.0705931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailleux P, Vanderhaeghen JJ. Distribution of neuronal cannabinoid receptor in the adult rat brain: a comparative receptor binding radioautography and in situ hybridization histochemistry. Neuroscience. 1992;48:655–668. doi: 10.1016/0306-4522(92)90409-u. [DOI] [PubMed] [Google Scholar]
- Maldonado R, Valverde O, Berrendero F. Involvement of the endocannabinoid system in drug addiction. Trends Neurosci. 2006;29:225–232. doi: 10.1016/j.tins.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Martin M, Ledent C, Parmentier M, Maldonado R, Valverde O. Cocaine, but not morphine, induces conditioned place preference and sensitization to locomotor responses in CB1 knockout mice. Eur J Neurosci. 2000;12:4038–4046. doi: 10.1046/j.1460-9568.2000.00287.x. [DOI] [PubMed] [Google Scholar]
- Mátyás F, Yanovsky Y, Mackie K, Kelsch W, Misgeld U, Freund TF. Subcellular localization of type 1 cannabinoid receptors in the rat basal ganglia. Neuroscience. 2006;137:337–361. doi: 10.1016/j.neuroscience.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Mátyás F, Urbán GM, Watanabe M, Mackie K, Zimmer A, Freund TF, Katona I. Identification of the sites of 2-arachidonoylglycerol synthesis and action imply retrograde endocannabinoid signaling at both GABAergic and glutamatergic synapses in the ventral tegmental area. Neuropharmacology. 2008;54:95–107. doi: 10.1016/j.neuropharm.2007.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis M, Gessa GL, Diana M. Different mechanisms for dopaminergic excitation induced by opiates and cannabinoids in the rat midbrain. Prog Neuropsychopharmacol Biol Psychiatry. 2000;24:993–1006. doi: 10.1016/s0278-5846(00)00119-6. [DOI] [PubMed] [Google Scholar]
- Miller LL, Ward SJ, Dykstra LA. Chronic unpredictable stress enhances cocaine-conditioned place preference in type 1 cannabinoid receptor knockout mice. Behav Pharmacol. 2008;19:575–581. doi: 10.1097/FBP.0b013e32830ded11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschamp JW, Siviy SM. Behavioral sensitization to amphetamine follows chronic administration of the CB1 agonist WIN 55,212-2 in Lewis rats. Pharmacol Biochem Behave. 2002;73:835–842. doi: 10.1016/s0091-3057(02)00910-3. [DOI] [PubMed] [Google Scholar]
- Pandolfo P, Pamplona FA, Prediger RD, Takahashi RN. Increased sensitivity of adolescent spontaneously hypertensive rats, an animal model of attention deficit hyperactivity disorder, to the locomotor stimulation induced by the cannabinoid receptor agonist WIN 55,212-2. Eur J Pharmacol. 2007;563:141–148. doi: 10.1016/j.ejphar.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Parolaro D, Rubino T. The role of the endogenous cannabinoid system in drug addiction. Drug News Perspect. 2008;21:149–157. [PubMed] [Google Scholar]
- Patel S, Rademacher DJ, Hillard CJ. Differential regulation of the endocannabinoids anandamide and 2-arachidonylglycerol within the limbic forebrain by dopamine receptor activity. J Pharmacol Exp Ther. 2003;306:880–888. doi: 10.1124/jpet.103.054270. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th edn. San Diego: Academic; 1998. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Pharmacological actions of cannabinoids. Handb Exp Pharmacol. 2005;168:1–51. doi: 10.1007/3-540-26573-2_1. [DOI] [PubMed] [Google Scholar]
- Przegaliński E, Göthert M, Frankowska M, Filip M. WIN 55,212-2-induced reduction of cocaine hyperlocomotion: possible inhibition of 5-HT3 receptor function. Eur J Pharmacol. 2005;517:68–73. doi: 10.1016/j.ejphar.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Riegel AC, Lupica CR. Independent presynaptic and postsynaptic mechanisms regulate endocannabinoid signaling at multiple synapses in the ventral tegmental area. J Neurosci. 2004;24:11070–11078. doi: 10.1523/JNEUROSCI.3695-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbe D, Alonso G, Duchamp F, Bockaert J, Manzoni OJ. Localization and mechanisms of action of cannabinoid receptors at the glutamatergic synapses of the mouse nucleus accumbens. J Neurosci. 2001;21:109–116. doi: 10.1523/JNEUROSCI.21-01-00109.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbe D, Kopf M, Remaury A, Bockaert J, Manzoni OJ. Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proc Natl Acad Sci USA. 2002;99:8384–8388. doi: 10.1073/pnas.122149199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabeti J, Adams CE, Burmeister J, Gerhardt GA, Zahniser NR. Kinetic analysis of striatal clearance of exogenous dopamine recorded by chronoamperometry in freely-moving rats. J Neurosci Methods. 2002;121:41–52. doi: 10.1016/s0165-0270(02)00229-7. [DOI] [PubMed] [Google Scholar]
- Sañudo-Peña MC, Romero J, Seale GE, Fernandez-Ruiz JJ, Walker JM. Activational role of cannabinoids on movement. Eur J Pharmacol. 2000;391:269–274. doi: 10.1016/s0014-2999(00)00044-3. [DOI] [PubMed] [Google Scholar]
- Shi LH, Luo F, Woodward DJ, Chang JY. Dose and behavioral context dependent inhibition of movement and basal ganglia neural activity by Δ−9-tetrahydrocannabinol during spontaneous and treadmill locomotion tasks in rats. Synapse. 2005;55:1–16. doi: 10.1002/syn.20088. [DOI] [PubMed] [Google Scholar]
- Soria G, Mendizábal V, Touriño C, Robledo P, Ledent C, Parmentier M, Maldonado R, Valverde O. Lack of CB1 cannabinoid receptor impairs cocaine self-administration. Neuropsychopharmacology. 2005;30:1670–1680. doi: 10.1038/sj.npp.1300707. [DOI] [PubMed] [Google Scholar]
- Sulcova E, Mechoulam R, Fride E. Biphasic effects of anandamide. Pharmacol Biochem Behav. 1998;59:374–352. doi: 10.1016/s0091-3057(97)00422-x. [DOI] [PubMed] [Google Scholar]
- Szabo B, Siemes S, Wallmichrath I. Inhibition of GABAergic neurotransmission in the ventral tegmental area by cannabinoids. Eur J Neurosci. 2002;15:2057–2061. doi: 10.1046/j.1460-9568.2002.02041.x. [DOI] [PubMed] [Google Scholar]
- Tanda G, Pontieri FE, Di Chiara G. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common µ1 opioid receptor mechanism. Science. 1997;276:2048–2050. doi: 10.1126/science.276.5321.2048. [DOI] [PubMed] [Google Scholar]
- Thiemann G, van der Stelt M, Petrosino S, Molleman A, Di Marzo V, Hasenöhrl RU. The role of the CB1 cannabinoid receptor and its endogenous ligands, anandamide and 2-arachidonoylglycerol, in amphetamine-induced behavioural sensitization. Behav Brain Res. 2008;187:289–296. doi: 10.1016/j.bbr.2007.09.022. [DOI] [PubMed] [Google Scholar]
- Tsou K, Brown S, Sañudo-Peña MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- Wise RA. Forebrain substrates of reward and motivation. J Comp Neurol. 2005;493:115–121. doi: 10.1002/cne.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, French ED. Effects of chronic Δ9-tetrahydrocannabinol on rat midbrain dopamine neurons: an electrophysiological assessment. Neuropharmacology. 2000;39:391–398. doi: 10.1016/s0028-3908(99)00140-9. [DOI] [PubMed] [Google Scholar]
- Xi Z-X, Gilbert JG, Peng X-Q, Pak AC, Li X, Gardner EL. Cannabinoid CB1 receptor antagonist AM251 inhibits cocaine-primed relapse in rats: role of glutamate in the nucleus accumbens. J Neurosci. 2006;26:8531–8536. doi: 10.1523/JNEUROSCI.0726-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Z-X, Spiller K, Pak AC, Gilbert J, Dillon C, Li X, Peng X-Q, Gardner EL. Cannabinoid CB1 receptor antagonists attenuate cocaine’s rewarding effects: experiments with self-administration and brain-stimulation reward in rats. Neuropsychopharmacology. 2008;33:1735–1745. doi: 10.1038/sj.npp.1301552. [DOI] [PubMed] [Google Scholar]
- Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, Bonner TI. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad USA. 1999;96:5780–5785. doi: 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]