Abstract
Our previous studies have shown that the selective dopamine D3 receptor antagonists SB-277011A or NGB 2904 significantly attenuate cocaine self-administration under a progressive-ratio reinforcement schedule and cocaine-, methamphetamine- or nicotine-enhanced brain stimulation reward. However, the poor bioavailability of SB-277011A has limited its potential use in humans. In the present study, we investigated the effects of the novel D3 receptor antagonist PG01037 on methamphetamine self-administration, methamphetamine-associated cue-induced reinstatement of drug seeking and methamphetamine-enhanced brain stimulation reward. Rats were allowed to intravenously self-administer methamphetamine under fixed-ratio 2 and progressive-ratio reinforcement conditions, and then the effects of PG01037 on methamphetamine self-administration and cue-induced reinstatement were assessed. Additional groups of rats were trained for intracranial electrical brain stimulation reward and the effects of PG01037 and methamphetamine on brain stimulation reward were assessed. Acute intraperitoneal administration of PG01037 (3, 10, 30 mg/kg) failed to alter methamphetamine or sucrose self-administration under fixed-ratio 2 reinforcement, but significantly lowered the break-point levels for methamphetamine or sucrose self-administration under progressive-ratio reinforcement. In addition, PG01037 significantly inhibited methamphetamine-associated cue-triggered reinstatement of drug-seeking behavior and methamphetamine-enhanced brain stimulation reward. These data suggest that the novel D3 antagonist PG01037 significantly attenuates the rewarding effects as assessed by progressive-ratio self-administration and brain stimulation reward, and inhibits methamphetamine-associated cue-induced reinstatement of drug-seeking behavior These findings support the potential use of PG01037 or other selective D3 antagonists in the treatment of methamphetamine addiction.
Keywords: brain reward, brain-stimulation reward, cue-induced reinstatement, D3 receptor, methamphetamine, PG01037, self-administration
Introduction
Methamphetamine is a highly potent, profoundly addictive psychostimulant drug with long-term debilitating effects. Patients with methamphetamine as their primary drug of choice are now the predominant population in public-funded treatment systems in most states (Rose and Grant, 2008). Methamphetamine use continues to evolve as part of a growing epidemic. Internationally, it is estimated that more than 40 million people abuse methamphetamine (versus 15 million abusers of cocaine and less than 10 million abusers of opiates) (Meredith et al., 2005). Despite the utility of behavioral treatment programs for the treatment of methamphetamine addiction, many patients repeatedly relapse (Rose and Grant, 2008). Currently, there is no pharmacotherapy to treat methamphetamine addiction.
Methamphetamine stimulates brain reward through its actions at both the dopamine (DA) and the vesicular mono-amine transporters, resulting in excessive extracellular DA in the nucleus accumbens (Hyman et al., 2006; Kalivas, 2007). Overstimulation of mesolimbic DA receptors results in psychomotor stimulation and pleasurable effects that lead to chronic use and may ultimately result in methamphetamine addiction (Kalivas and Volkow, 2005; Pierce and Kumaresan, 2006; Wise, 2005). Medication development for drug addiction treatment has long focused on the mesolimbic dopaminergic system. Recently, the dopamine D3 receptor subtype (Sokoloff et al., 1990) has received considerable recognition for its purported role in mediating abuse-related properties of addictive drugs (for review see Heidbreder et al., 2005; Le Foll et al., 2005; Newman et al., 2005; Xi and Gardner, 2007). D3 receptors are expressed primarily in the mesolimbic dopamine system (Gurevich and Joyce, 1999; Levant, 1998; Sokoloff et al., 1992); the greatest densities of D3 mRNA are found in the nucleus accumbens shell, islands of Calleja, and olfactory tubercle; brain structures deemed critical in drug-taking behavior (Diaz et al., 2002; Sokoloff et al., 2001, 2006; Stanwood et al., 2000). In addition, growing evidence demonstrates that pharmacological blockade of DA D3 receptors inhibits the actions of various drugs of abuse in animal models of drug addiction (for a more comprehensive review see Heidbreder et al. (2005) and Le Foll et al. (2005)).
Recent studies have shown that highly selective D3 receptor antagonists such as SB-277011A and NGB 2904 block both the acquisition and expression of cocaine and heroin-induced conditioned place preference (Ashby et al., 2003; Vorel et al., 2002), inhibit alcohol intake and reinstatement in ethanol-preferring rats (Heidbreder et al., 2007; Thanos et al., 2005), attenuate cocaine self-administration under progressive ratio reinforcement (Gilbert et al., 2005; Xi et al., 2004, 2005, 2006), inhibit drug-, cue-, and stress- induced reinstatement to drug seeking (Di Ciano and Everitt, 2003; Gilbert et al., 2005; Vorel et al., 2002; Xi et al., 2004), and inhibit nicotine-, cocaine- and methamphetamine-enhanced brain-stimulation reward (BSR) in rats (Pak et al., 2006; Spiller et al., 2008; Vorel et al., 2002). Although the efficacy of D3 receptor antagonists in attenuating the addictive potential of cocaine may be well established, their efficacy against methamphetamine, a more potent and arguably the most problematic illicit drug to date, has been neglected. Furthermore, the number of satisfactorily selective D3 antagonists available for preclinical testing in addiction-related animal models has, to date, been extremely limited. The present study, for the first time, characterizes the effectiveness of a novel D3 receptor antagonist, PG01037, in several preclinical animal models related to methamphetamine reward and addiction.
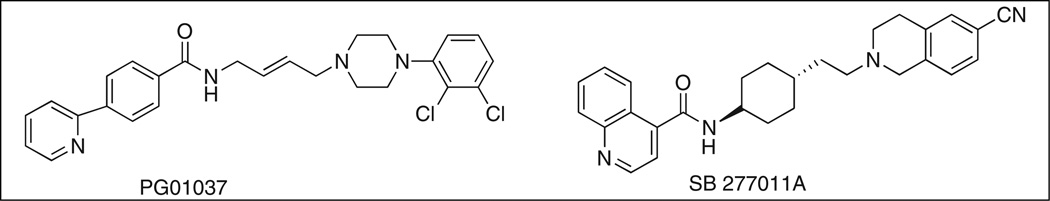
SB-277011A (Figure 1) is the most preclinically characterized DA D3 receptor antagonist to date. SB-277011A is a highly selective, highly potent DA D3 antagonist with 120-and 80-fold selectivity for D3 over D2 receptors in human and rat, respectively (Reavill et al., 2000). However, clinical development of SB-277011A has been halted by GlaxoSmithKline Pharmaceuticals, due to poor bioavailability (~2%) and a very short half-life (<20 min) in primates (Austin et al., 2001; Remington and Kapur, 2001). Nevertheless, SB-277011A remains an important research tool to probe the role of D3 receptors in animal models of addiction.
Figure 1.
Chemical structures of D3 receptor antagonists SB-277011A and PG01037.
PG01037 (Figure 1) is a novel D3 receptor antagonist (Grundt et al., 2005, 2007). In vitro binding studies reveal that PG01037 has high affinity for the D3 receptor (Ki = 0.7±0.1 nM) and 133-fold selectivity over D2 receptors in HEK 293 cells transfected with human D3 or D2 receptors, respectively (Grundt et al., 2005), and it preferentially binds to D3 receptors over many other central nervous system receptors (Grundt et al., 2007). Pharmacological magnetic resonance imaging studies have shown that PG01037 readily enters the brain and is localized in D3 receptor-rich brain regions such as the nucleus accumbens and islands of Calleja, without significant localization in the caudate putamen, a D2 receptor-rich brain region (Grundt et al., 2007). PG01037 has a similar regional activation pattern to SB-277011A (Schwartz et al., 2004), and has been shown by in vitro (Grundt et al., 2005, 2007), and in vivo (Collins et al., 2005, 2007) studies to function as a selective D3 antagonist. Based upon the result of mitogenic assays, Kumar et al. (2009) characterized PG01037 as an antagonist at D3 receptors (Ki = 0.7 nM ± 0.1) and a weak partial agonist at D2 receptors (Ki = 93.3 ± 11.9 nM). In addition, PG01037 binds with >65-fold selectivity at D3 receptors compared with 5-HT1A receptors.
In the present study, we observed the effects of PG01037 on intravenous (i.v.) methamphetamine self-administration, cue-induced reinstatement to methamphetamine-seeking behavior, and methamphetamine-enhanced BSR. Additionally, we examined the effects of PG01037 on non-drug reward using a sucrose self-administration paradigm.
Materials and methods
Subjects
For all experiments, male Long-Evans rats (Charles River Laboratories, Raleigh, NC, USA), experimentally naive and initially weighing 250–300 g, were used. Rats were housed individually in a climate-controlled animal colony room on a reversed light–dark cycle (lights on at 19:00, lights off at 07:00) with ad libitum access to food and water. Animals were maintained in a facility fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, and all experimental procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (National Academy of Sciences, 1996) and were approved by the Animal Care and Use Committee of the National Institute on Drug Abuse of the United States National Institute of Health.
Drugs and chemicals
Methamphetamine (Sigma-Aldrich Corporation, St. Louis, MO, USA) was dissolved in sterile physiological saline. PG01037 ((E)-N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)but-2-enyl)-4-(pyridin-2-yl)benzamide was synthesized in the Medicinal Chemistry Section, Intramural Research Program, National Institute on Drug Abuse (Baltimore, MD, USA) using procedures previously reported (Grundt et al., 2005) and was dissolved in 0.5% Tween-80 (Sigma-RBI, St. Louis, MO, USA).
Experimental set 1: methamphetamine self-administration
Surgery
All animals were prepared for experimentation by surgical catheterization of the right external jugular vein under sodium pentobarbital anesthesia (65 mg/kg, i.p.) using standard aseptic surgical techniques. The right jugular vein was exposed by blunt dissection and the catheter inserted into the vein and sutured into place. The catheter was passed sub-cutaneously to the skull top and made to exit into a connector (a modified 22-gauge cannula; Plastics One, Roanoke, VA, USA) that was mounted to the skull with jewelers screws and cranioplastic acrylic. The venous catheters were constructed of microrenathane (Braintree Scientific Inc., Braintree, MA, USA). To prevent clogging, catheters were flushed daily with a gentamicin–heparin–saline solution (30 IU/ml heparin; ICN Biochemicals, Cleveland, OH, USA).
Apparatus
All experiments were conducted in standard Med Associates (Georgia, VT, USA) operant response test chambers (32 cm × 25 cm × 33 cm). Each test chamber had one active and one inactive lever, located 6.5 cm above the floor. Depression of the active lever resulted in activation of the infusion pump; depression of the inactive lever was recorded but had no consequence.
General Procedure
Following recovery from surgery (5–7 days), animals were given the opportunity to self-administer i.v. methamphetamine in daily 3-hour sessions. Prior to initiation of the session, the catheter for each rat was connected to a microprocessor-controlled syringe pump by polyethylene tubing, which was fed through a liquid swivel that allowed freedom of movement. Training sessions began with the insertion of the retractable operant lever into the chamber and illumination of a 15-Watt house-light that remained on for the duration of the session. Each depression of the active lever produced delivery of i.v. methamphetamine (0.05 mg/kg/infusion) in a volume of 0.08 ml delivered over 4.6 s. A white cue light located above the lever was illuminated, and a cue tone was emitted for the duration of the infusion. A fixed-ratio 1 schedule of reinforcement was used for 3–5 days to facilitate acquisition of self-administration behavior. After this initial training, animals were switched to fixed-ratio 2 (FR2) reinforcement, such that two lever presses resulted in one i.v. infusion of methamphetamine. The dose of methamphetamine was chosen based on pilot data indicating that rats trained with 0.05 mg/kg/infusion display rapid and reliable acquisition of self-administration (Higley et al., 2007). To avoid overdose each animal was limited to a maximum of 50 methamphetamine infusions per 3-hour session.
Effect of PG01037 on FR2 methamphetamine self-administration
Rats (N = 12) continued self-administration for i.v. methamphetamine under FR2 reinforcement until the following criteria for stable responding were met: (1) less than 10% variability in the mean inter-response interval; (2) less than 10% variability in number of infusions; and (3) less than 10% variability in number of active lever presses for a minimum of three consecutive days. After stable rates of responding were established (7–10 days), each subject randomly received one of four treatment doses of PG01037 (0, 3, 10, 30 mg/kg, intraperitoneal (i.p.)) 30 min prior to the 3-hour test session; all other conditions were the same. Prior to testing the next dose of PG01037, all animals underwent a 5- to 7-day period of FR2 methamphetamine self-administration until a stable baseline was re-established. This schedule was maintained until each animal received each treatment dose. The order of treatment was counterbalanced according to a Latin square design.
Effect of PG01037 on progressive ratio breakpoint for methamphetamine self-administration
Self-administration training was identical to that outlined above. Following stable FR2 responding for methamphetamine, all subjects (N = 48) were switched to a progressive-ratio (PR) schedule of reinforcement. The PR reinforcement model imposes incrementally increasing work demand upon the animal to receive a single reinforcer. In this experiment, the number of lever presses required to receive a single infusion of methamphetamine was increased according to the following series: 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145, 178, 219, 268, 328, 402, 492, and 603. The break-point is defined as the final completed lever presses for drug infusion within a 1-hour period when no infusion was earned. It is considered an index of the strength of the reinforcer. Animals continued with daily PR self-administration sessions until they achieved stability and day-to-day variability in break-point ratios were within 1–2 ratio increments for three consecutive days. Once stable, animals were randomly divided into one of four groups (n = 12 each) based on their subsequent treatment dose of PG01037 (0, 3, 10, or 30 mg/kg). On the test day, PG01037 was administered 30 min prior to initiation of PR self-administration and break-point values were calculated.
PG01037 self-administration in rats formerly self-administering methamphetamine
The initial self-administration training was identical to that outlined above for FR2 methamphetamine. Following stable levels of responding for i.v. methamphetamine, animals (N = 27) were divided into three subgroups (n = 9 each) to assess the ability of PG01037 to support self-administration. Methamphetamine was replaced by one of the following: PG01037 (0.06 mg/kg/infusion, n = 9); physiological saline (0.08 ml/infusion; n = 9); or continued methamphetamine (0.05 mg/kg/infusion; n = 9). Each group received their respective replacement treatment for five consecutive, daily, 3-hour self-administration sessions. After completion of the replacement tests, all groups were retested for methamphetamine self-administration.
Sucrose self-administration
The procedures for oral sucrose self-administration (N=12) were identical to the procedures for methamphetamine self-administration except for the following: 1) no surgery was performed on the animals in the sucrose experiment; 2) active lever presses led to delivery of 0.1 ml of 5% sucrose solution into a liquid food tray located on the operant chamber wall.
Experimental set 2: reinstatement to methamphetamine seeking behavior
Surgery, apparatus, and general procedure
This experimental set required the establishment of stable FR2 methamphetamine self-administration before behavioral extinction and reinstatement testing began. The surgery, apparatus, and general procedure to establish stable FR2 methamphetamine self-administration were identical to experimental set 1 as outlined above.
Extinction
After stable responding for i.v. methamphetamine was established (approximately 14 days), rats underwent two weeks of daily extinction trials, during which methamphetamine was replaced by saline, and the methamphetamine-associated cue light and tone were turned off. Thus, active lever presses led only to a saline infusion. Daily 3-hour extinction sessions continued for each animal until the animal made less than 10 lever presses per 3-hour session for at least three consecutive days. After meeting this extinction criterion, animals were divided into four groups of 8–10 rats each for reinstatement testing.
Reinstatement
On the reinstatement test day, rats received one of four treatment doses of PG01037 (0, 3, 10, or 30 mg/kg, i.p.) 30min prior to the initiation of the 3-hour reinstatement test. The cue-induced reinstatement test was initiated by two non-contingent presentations of the methamphetamine-associated cue light and tone. Subsequent lever presses then led to response contingent deliveries of the same conditioned light-tone cues. The cue light was illuminated and the cue sound (tone, ~20 dB above background) were turned on for 4.6 s. Response on the active lever never resulted in methamphetamine infusion.
Experimental set 3: brain-stimulation reward
Surgery
Under the same anesthesia as used in experimental set 1, nine rats were positioned in a stereotaxic frame and a unilateral monopolar stainless-steel stimulating electrode (Plastics One, Roanoke, VA, USA) was inserted into the lateral hypothalamus using standard aseptic surgical and stereotaxic techniques. The target implant stereotaxic coordinates were, from bregma, AP + 2.5 mm, ML + 1.7 mm, and DV −8.4 mm, (Paxinos and Watson, 1998). The top of the electrode and the electrode connector (to which the wires from the brain stimulator were connected via a quick-connect electrical mini-plug) were cemented to the skull with acrylic resin cement. A wire leading from the electrode was wrapped around a skull screw to serve as a current return.
Apparatus
All training and testing occurred in standard operant chambers (Med Associates, Inc., Georgia, VT, USA), each enclosed in ventilated, sound-attenuating cabinets.
General procedure
The general procedures for electrical BSR were the same as we reported previously (Pak et al., 2006; Spiller et al., 2008; Vorel et al., 2002; Xi et al., 2006). Briefly, after seven days of recovery from surgery, rats were allowed to self-train (autoshape) to lever-press for rewarding BSR. Each lever press resulted in a 500-ms train of 0.1-ms rectangular cathodal pulses through the electrode in the rat’s lateral hypothalamus, followed by a 500-ms ‘timeout’ in which further lever presses had no consequence. Initial stimulation parameters were 72 Hz and 200 µA. If the animal did not learn to lever-press under these parameters the stimulation intensity was increased by 50 µA daily until the animal learned to lever press (45–60 responses/30 s) or a maximum stimulation of 800 µA was reached. Animals that did not lever-press at 800 µA or in which the stimulation produced unwanted effects (e.g. head or body movements, spinning, or vocalization) were removed from the experiment.
Rate-frequency BSR procedure
Once lever-pressing for BSR was established, animals were presented with a series of 16 different pulse frequencies ranging from 141–25 Hz in descending order. At each pulse frequency, animals lever-pressed for two 30-s time periods (‘bins’), after which the pulse frequency was decreased by 0.05 log units. Following each 30-s bin the lever retracted for 5 s. The response rate (i.e. mean lever responses during two 30-s bins) was then calculated for each frequency. To minimize the effect of day-to-day variability on response rate, each animal participated in three daily sessions. The first session served as a warm-up session in which no data were collected. Data obtained from the second and third session were designated as baseline session data and test session data, respectively.
The BSR threshold (θ0) was defined as the minimum frequency at which the animal responded for rewarding stimulation. The Ymax was defined as the maximal rate of response (number of lever presses for rewarding brain stimulation per unit of time). The BSR threshold and Ymax were mathematically derived for each baseline run and each drug run by analyzing each rate-frequency BSR function generated by a given animal over a given descending series of pulse frequencies using ‘best-fit’ mathematical algorithms. Specifically, each rate-frequency BSR function was mathematically fitted, by iterative computer programs derived from the Gauss-Newton algorithm for non-linear regression, to three different sigmoid curve-fitting mathematical growth models that appear to accurately fit rate-frequency brain-stimulation reward functions (Coulombe and Miliaressis, 1987) the Gompertz model (Y′ = ae−e(b–cX)), the logistic model (Y′ = a/[1 + e(b–cX)]), and the Weibull function (Y′ = a[1 − e−(bx)c ]); where Y′ is the rate of response (number of lever presses for rewarding brain stimulation per unit of time), X is the pulse frequency, and a, b, and c are parameters approximated from each empirical rate-frequency data curve (a representing the asymptotic response rate value, b relating to the intercept of the rate-frequency curve with the y-axis, and c representing the rate at which Y increments). From each curve-fitting model, a solution for θ0 and a solution for Ymax were obtained. Thus, for each rate-frequency BSR function generated by a given animal over a given descending series of pulse frequencies, three solutions for θ0 and three solutions for Ymax were obtained. The three solutions for θ0 were averaged, to produce a mean θ0 for each rate-frequency BSR function generated by a given animal over a given descending series of pulse frequencies. Similarly, the three solutions for Ymax were averaged, to produce a mean Ymax for each rate-frequency BSR function generated by a given animal over a given descending series of pulse frequencies. The mean θ0 values and mean Ymax values were expressed as means ± SEM. Data analyses were performed on percent changes from baseline levels.
Testing the effects of PG01037 on BSR and methamphetamine-enhanced BSR
After stable baseline BSR threshold values (θ0) and Ymax values were achieved (<10% variability over five consecutive days), the effects of PG01037 and/or methamphetamine on BSR were assessed. On each BSR test day, rats (N = 9) randomly received one of three possible doses of PG01037 (3, 10, 30 mg/kg, i.p.) or vehicle 30 min prior to systemic administration of methamphetamine (0.2 mg/kg) or vehicle. Following each test session, animals received an additional 5–7 days of BSR restabilization until a new BSR baseline θ0 and Ymax values were established. The order of testing for various doses of PG01037 was counterbalanced according to a Latin square design. The effect of PG01037 on methamphetamine-enhanced BSR was evaluated by comparing methamphetamine-induced alterations in θ0 in the presence or absence of each dose of PG01037 pretreatment. The order of testing for various doses of PG01037 was counterbalanced according to a Latin square design.
Data analyses
All data are presented as means (±S.E.M.). One-way analysis of variance (ANOVA) was used to analyze the effect of PG01037 on self-administration behavior, cue-induced reinstatement and methamphetamine-enhanced BSR. Two-way ANOVA with repeated measures over PG01037 dose was used to analyze the effects of PG01037 and methamphetamine on BSR. Individual group comparisons were carried out using pre-planned Bonferroni t-tests. The minimal acceptable statistical significance was set at a probability level of p < 0.05 for all tests.
Results
PG01037 inhibits methamphetamine self-administration under PR, but not FR2, reinforcement
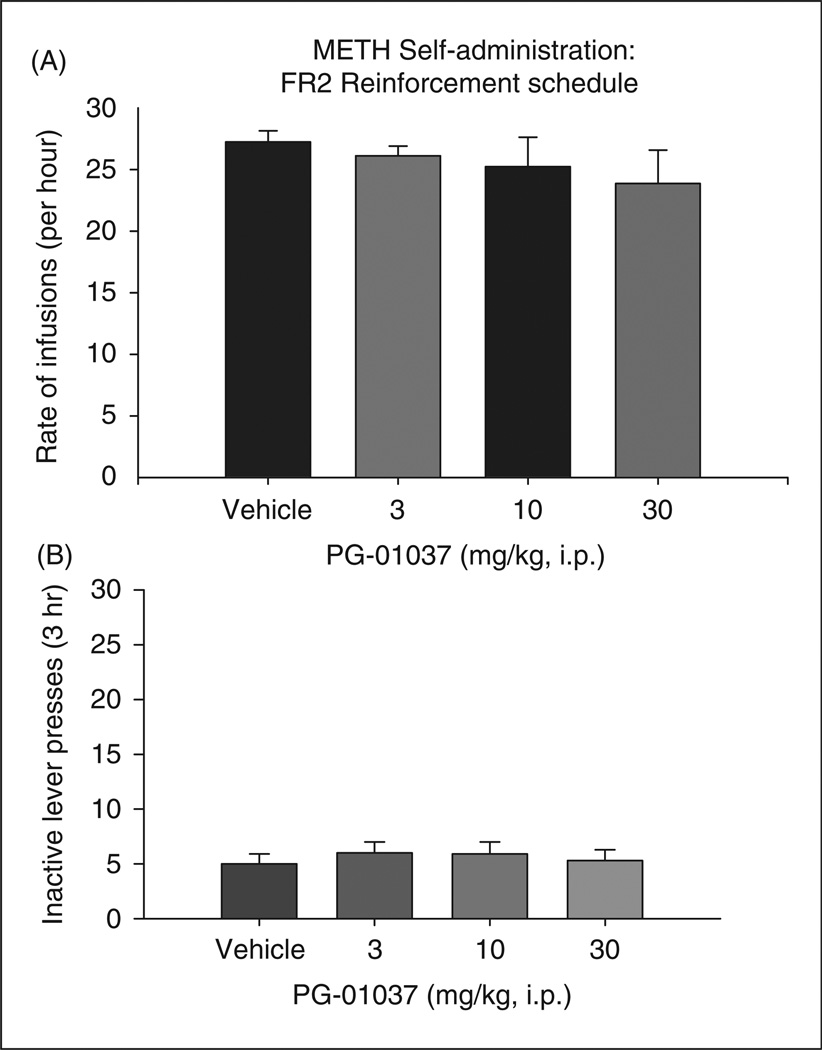
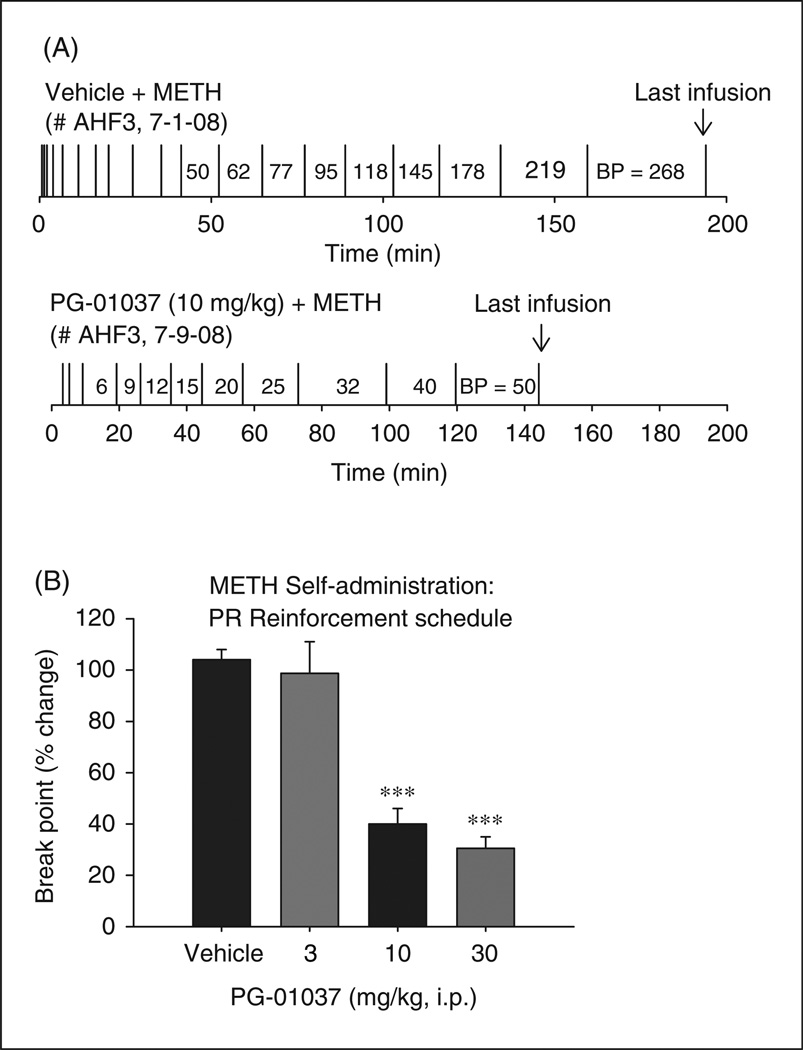
Figure 2 shows that systemic administration of PG01037 (3, 10, or 30 mg/kg i.p., 30 min prior to self-administration sessions) failed to alter i.v. methamphetamine self-administration. A one-way ANOVA for repeated measures over the PG01037 dose range revealed no statistically significant effect of PG01037 on methamphetamine self-administration under FR2 reinforcement conditions (F3,39 = 0.56; p = NS). Figure 3A shows a representative response record for methamphetamine self-administration under PR reinforcement conditions following pretreatment with vehicle or 10 mg/kg PG01037. Each vertical line represents an i.v. methamphetamine infusion; the numbers between the vertical lines indicate the work demand (i.e. number of lever pressing) to receive the following methamphetamine infusion. Figure 3A (upper trace) illustrates a PR break-point value of 268 after the vehicle pretreatment. Pretreatment with 10 mg/kg PG01037, however, resulted in a drastically lower break-point value of 50 (Figure 3A lower trace). Figure 3B illustrates that averaged PR break-point levels for methamphetamine self-administration after the different doses of PG01037 pretreatment. A one-way ANOVA revealed a statistically significant PG01037 treatment main effect (F3,36 = 26.56, p < 0.001). Individual group comparisons revealed a significant reduction in PR break-point levels after 10 mg/kg PG01037 (t = 7.14, p < 0.001, n = 12) or 30 mg/kg PG01037 (t = 7.31, p < 0.001, n = 12) but not after 3 mg/kg PG01037, when compared with the vehicle treatment group.
Figure 2.
Effects of PG01037 (3–30 mg/kg, i.p.) on methamphetamine self-administration under fixed-ratio 2 (FR2) reinforcement schedules in rats. (A) There was no significant effect of PG01037 on the rate of infusion for methamphetamine self-administration. (B) There was no effect of PG0137 on inactive lever presses.
Figure 3.
Effects of PG01037 (3–30 mg/kg, i.p.) on methamphetamine self-administration under a progressive ratio (PR) schedule of reinforcement. Panel A shows representative individual responding curve for methamphetamine (0.05 mg/kg/infusion) after vehicle (upper trace) or 10 mg/kg PG01037 (lower trace) pretreatment. Panel B shows a significant reduction in PR breakpoint following pretreatment with PG01037. Data are presented as percent change from baseline. ***p < 0.001, when compared with the vehicle treatment group.
PG01037 inhibits oral sucrose self-administration under PR, but not FR2, reinforcement
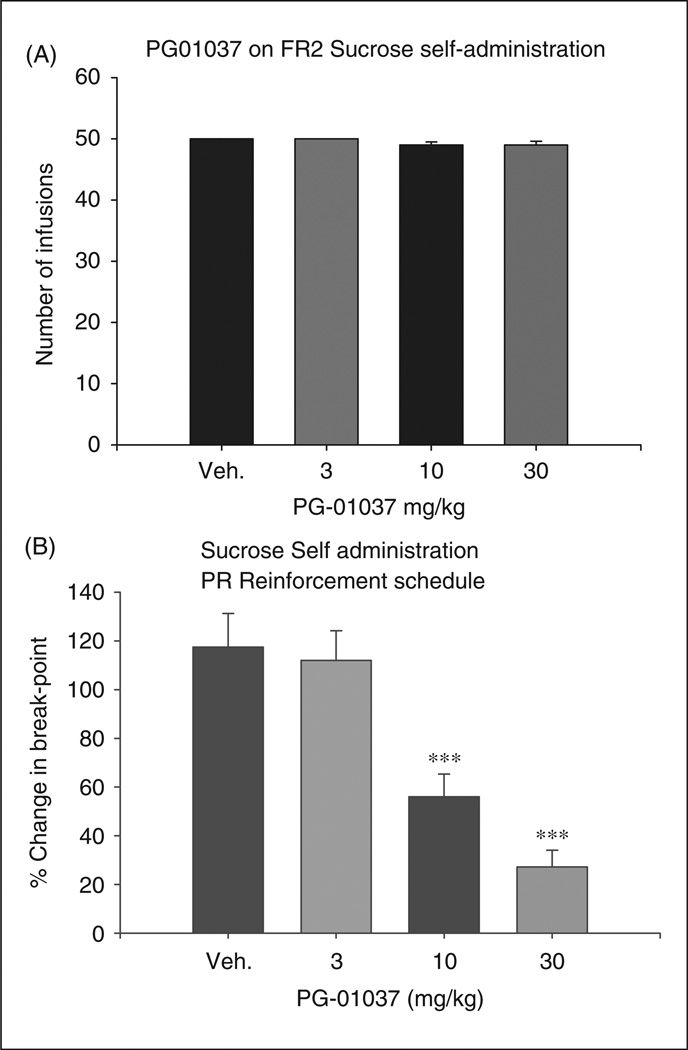
Figure 4A shows that the same doses of PG01037 pretreatment also failed to alter oral sucrose self-administration under a FR2 reinforcement schedule (F3,36 = 0.39, p = NS). Figure 4B shows that pretreatment with PG01037 produced a significant reduction in PR break-point levels for oral sucrose self-administration under a PR schedule of reinforcement (F3,24 = 17.54, p < 0.001). Individual group comparisons indicated a statistically significant reduction in break-point levels for sucrose self-administration after 10 mg/kg PG01037 (t = 3.88, p < 0.01) or 30 mg/kg PG01037 (t = 6.14, p < 0.001), but not after 3 mg/kg PG01037 (t = 0.36, p = NS), when compared with the vehicle group.
Figure 4.
Effect of PG01037 (3–30 mg/kg, i.p.) on oral sucrose self-administration. Panel A shows no significant effect of PG01037 on sucrose self-administration under an FR2 schedule of reinforcement. Panel B shows a significant and dose dependent reduction of PR break-points levels for oral sucrose following pretreatment with PG01037. Data are presented as percent change from baseline. ***p < 0.001, when compared with the vehicle treatment group.
PG01037 inhibits methamphetamine cue-induced reinstatement of drug seeking
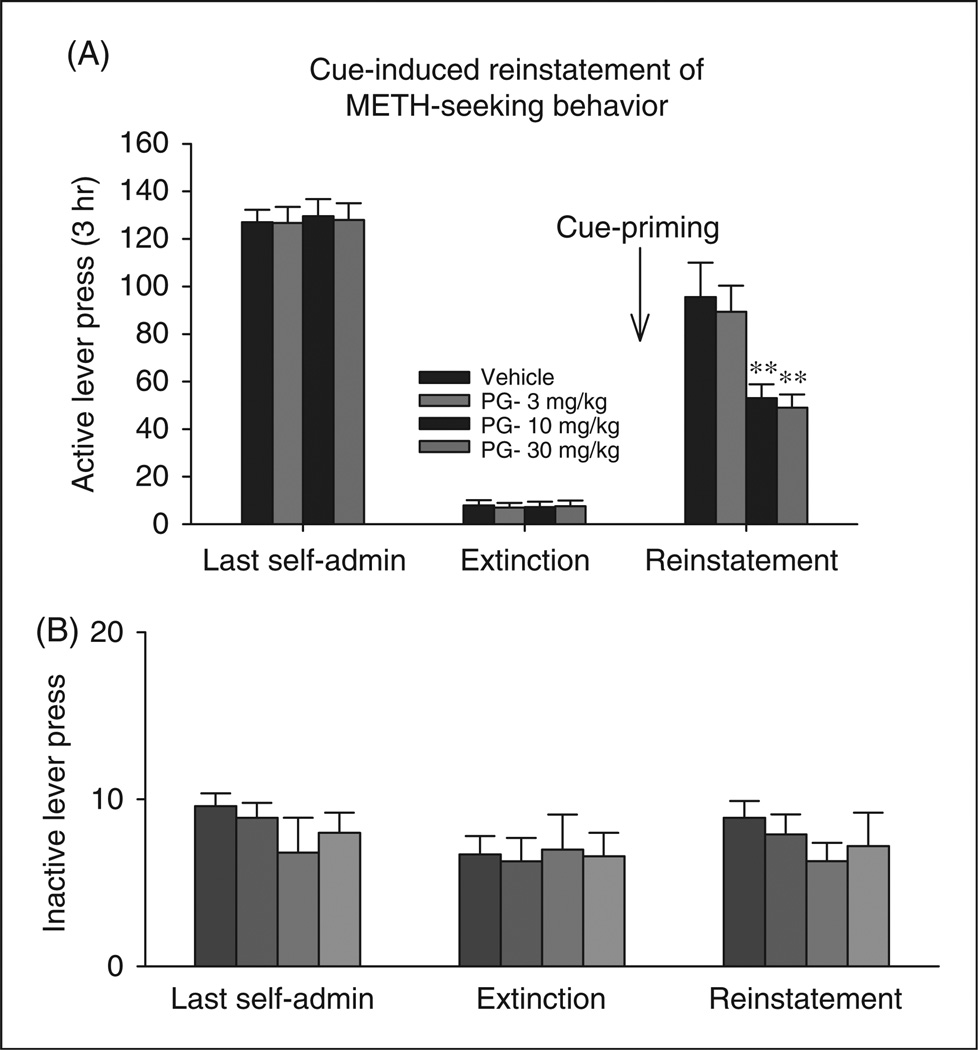
Figure 5 shows that non-contingent presentations of methamphetamine-associated cues resulted in a profound increase in methamphetamine-seeking behaviors, an effect that was dose-dependently inhibited by PG01037 pretreatment. One-way ANOVA with repeated measures over PG01037 doses resulted in a statistically significant treatment main effect (F3,36 = 39.24, p < 0.001). Individual group comparisons revealed a statistically significant reduction in cue-induced reinstatement after 10 mg/kg PG01037 (t = 8.51, p < 0.001, n = 12) or 30 mg/kg PG01037 (t = 8.89, p < 0.001, n = 12), when compared with the vehicle treatment group.
Figure 5.
Effects of PG01037 (3–30 mg/kg, i.p.) on reinstatement of cue-induced drug-seeking behavior triggered by presentation of methamphetamine-associated cues. Panel A shows a statistically significant reduction in cue-induced methamphetamine seeking after 10 and 30 mg/kg PG01037, but not 3 mg/kg PG01037 when compared with vehicle treatment group **p<0.01. In contrast, Panel B shows that PG01037 had no significant effect on inactive lever presses in the last self-administration sessions, last extinction sessions, or during the cue-induced reinstatement test.
PG01037 attenuates methamphetamine-enhanced BSR
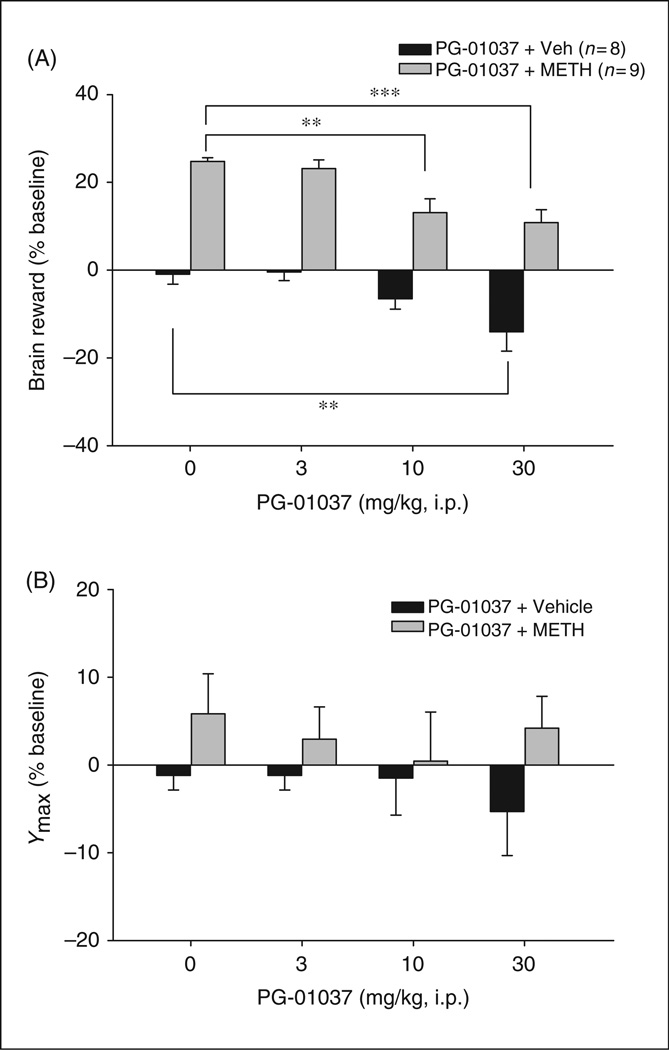
Figure 6 shows that systemic administration of methamphetamine (0.2 mg/kg) produced a significant enhancement of BSR. Figure 6A illustrates the averaged effects of PG01037 and methamphetamine on BSR as assessed by stimulation threshold. Two-way ANOVA with repeated measures over PG01037 dose revealed a significant treatment (PG01037 vs. methamphetamine) main effect (F1,15 = 83.41, p <0.001), PG01037 dose main effect (F3,45 = 17.45, p < 0.001). Individual group comparisons revealed a statistically significant reduction in methamphetamine-enhanced BSR after 10 mg/kg PG01037 (t = 4.71, p < 0.001, n = 9) or 30 mg/kg PG01037 (t = 5.61, p < 0.001, n = 9), but not after 3 mg/kg PG01037 (t = 0.66, p = NS), when compared with the vehicle treatment group. In addition, individual group comparisons revealed a significant reduction in BSR after 30 mg/kg (but not 3, 10 mg/kg) PG01037 administration (t = 3.73, p < 0.05). Figure 6B illustrates that the effects of PG01037 and methamphetamine on Ymax levels. Two-way ANOVA with repeated measures over PG01037 dose revealed neither significant treatment (PG01037 vs. methamphetamine) main effect (F115 = 2.02, p = NS) nor significant PG01037 dose main effect (F3,45 = 0.30, p = NS).
Figure 6.
Effect of PG01037 (0–30 mg/kg, i.p.) and methamphetamine on brain-stimulation reward (BSR). Panel A shows that PG01037 (10 or 30 mg/kg, but not 3 mg/kg), significantly attenuated methamphetamine-enhanced BSR, as assessed by BSR threshold (θ0). PG01037 alone, at 30 mg/kg, but not 3 or 10 mg/kg, also produced a significant inhibition of BSR. Panel B shows that neither PG01037 nor methamphetamine altered Ymax levels at any doses tested. **p < 0.01, ***p < 0.001, compared with the vehicle treatment group.
Discussion
The present study demonstrated, for the first time, that systemic administration of the selective DA D3 receptor antagonist PG01037 significantly attenuated the behavioral effects of methamphetamine in several preclinical models relevant to methamphetamine addiction, including PR methamphetamine or sucrose self-administration, cue-induced reinstatement to methamphetamine seeking behavior and electrical BSR. PG01037 replacement for methamphetamine failed to maintain self-administration in methamphetamine self-administration rats, suggesting lower addictive potential by itself. In contrast, PG01037, at the highest dose tested (30 mg/kg), produced an inhibitory effect on brain reward by itself. Taken together, these data support the utility of the D3 receptor antagonist PG01037 as a treatment modality for methamphetamine addiction, and also implicates D3 receptors in the mechanisms underlying addiction relevant behaviors.
Intravenous drug self-administration is the most commonly used animal model to evaluate a drug’s rewarding effects (Gardner, 2000). Both the FR and PR self-administration paradigms are commonly uses in rodents, in contrast to more common use of second-order of reinforcement in non-human primates. Under low FR reinforcement, the addictive drug is readily available to animals under low work demand. In the present study, we found that PG01037 had no effect on methamphetamine self-administration under FR2 reinforcement. However, under a PR schedule of reinforcement, PG01037 dose-dependently decreased break-point levels for methamphetamine self-administration. This finding indicates decreased motivation to work for the next infusion, ultimately resulting in less total drug consumption. The present data are congruent with previous experiments which have found that highly selective D3 antagonists inhibit cocaine, alcohol, and nicotine self-administration under PR or high FR conditions, but not under low FR conditions (Di Ciano et al., 2003; Gal and Gyertyán, 2003; Gilbert et al., 2005; Pilla et al., 1999; Vorel et al., 2002; Xi et al., 2005, 2006).
There are several possible explanations for PG01037’s inhibition of methamphetamine self-administration under PR but not low FR reinforcement conditions. First, FR2 reinforcement demands less work to obtain much higher cumulative methamphetamine. In the present study, the total methamphetamine intake during FR2 methamphetamine self-administration averaged 2.5 mg methamphetamine, considerably higher than the average total intake of 0.90 mg methamphetamine during PR self-administration. The stronger rewarding effects produced by the higher cumulative dose of methamphetamine may counteract antagonism by PG01037. Another consideration is the relative insensitivity of low FR reinforcement schedules to changes in reward efficacy. Many have argued that FR reinforcement schedules simply evaluate the fact of reinforcement (i.e. that something is or is not reinforcing) rather than the degree of reinforcing efficacy (Arnold and Roberts, 1997; Gardner, 2000; Roberts et al., 1989; Wise and Gardner, 2004). In contrast, the PR paradigm is highly sensitive to a given drug’s reinforcing efficacy (Arnold and Roberts, 1997; French et al., 1995; Roberts et al., 1989; Stafford et al., 1998). Additionally, the PR paradigm quantifiably assesses incentive motivation to self-administer drugs (Richardson and Roberts 1996; Rowlett, 2000; Stafford et al., 1998). Thus, the present finding that PG01037 significantly inhibits methamphetamine self-administration under PR reinforcement conditions suggests that PG01037 attenuates methamphetamine’s reinforcing efficacy of methamphetamine and its motivational salience.
Importantly, in the self-administration paradigm PG01037 reduced the PR breakpoint for both sucrose and methamphetamine, suggesting that PG01037 may act on general reward mechanisms rather than having specificity for drugs of abuse. It is important to note that, regardless of reinforcer, PG01037’s effects were specific to the reinforcing efficacy (PR) rather than the acute reinforcing properties of the stimulus (FR2). Further studies investigating other natural rewards (i.e. food-taking or sexual behaviors) are required to elucidate these points.
Craving-driven relapse to illicit drug use is a core feature of drug addiction (Mendelson and Mello, 1996; O’Brien, 1997). In humans, relapse to drug use can be triggered by adventitious drug administration, by exposure to environmental stimuli previously associated with drug use, or by exposure to various stressors (Jaffe et al., 1989; O’Brien et al., 1992; Sinha, 2001). Relapse to drug use is similarly modeled in the laboratory using the reinstatement paradigm (Shaham et al., 1994; Shalev et al., 2000; 2003; Stewart, 2000). In this model, extinguished drug-seeking behavior is re-evoked by re-exposing the animal to an addictive drug, drug-associated environmental cues, or stress (Di Ciano and Everitt, 2003; Shalev et al., 2002; Stewart, 2000). The reinstatement paradigm is commonly used to study neurobiological mechanisms underlying drug relapse (Bossert et al., 2005). The present study demonstrated that presenting cues previously associated with methamphetamine reliably and rapidly reinstated methamphetamine-seeking behavior in rats with extinguished methamphetamine-seeking behavior. PG01037 (10 or 30 mg/kg, i.p.) significantly attenuated cue-triggered reinstatement to methamphetamine-seeking behavior. The preferential responding on the active lever is important, as it indicates drug seeking. Thus, PG01037’s action appears specific to cue-induced reinstatement rather than nonspecific behavioral disruption. These findings suggest that PG01037 may have potential in attenuating drug craving and relapse in humans, consistent with previous findings with D3-selective or D2-preferring receptor antagonists in experimental animals (Cervo et al., 2003; Khroyan et al., 2000; Self et al., 1996).
The BSR paradigm is considered a reliable and sensitive animal model for assessing the reward-relevant properties of addictive drugs (Kornetsky, 2004; O’Brien and Gardner, 2005; Stein and Ray, 1960; Wise, 1996a). BSR involves the same reward circuits as, and summates with, the rewarding effects of addictive drugs (Bauco and Wise, 1997). The present data demonstrate that the D3 receptor antagonist PG01037 (10 and 30 mg/kg) produced a significant inhibition of methamphetamine-enhanced BSR, again suggesting possible clinical utility for D3 receptor antagonists as anti-addiction pharmacotherapeutic agents.
However, PG01037, at the highest dose tested (30 mg/kg), produced a significant increase in BSR threshold, an effect not observed with the D3 antagonists SB277011A or NGB 2904 (Pak et al., 2006; Spiller et al., 2008). PG01037 demonstrates higher binding affinity at D2 receptors than SB277011A, as measured by in vitro binding assays in cell culture (Newman et al., 2005). Notably, the inhibition of BSR observed with the high dose of PG01037 is similar to that observed with the mixed D3/D2 antagonist S33138 (Peng et al., 2009), and the partial D3 receptor agonist BP-897 that has similar D2 receptor affinity as PG01037 (Newman et al., 2005), and both of which produce dose-dependent inhibition of BSR (Kita et al., 1999; Ranaldi and Beninger, 1994; Spiller et al., 2008). The precise mechanisms underlying this BSR inhibition are unclear. It could be related to its binding to other unknown functional proteins in the brain. PG01037’s observed effects are unlikely to have been the result of impaired motor function, as PG01037 – at the doses tested – failed to alter spontaneous locomotion and had no effect on the inactive lever in any of the operant behaviors assessed (data not shown).
In summary, we demonstrate in the present study that although acute administration of the D3 antagonist PG01037 failed to alter methamphetamine self-administration under FR2 reinforcement, it significantly lowered the break-point for methamphetamine and sucrose self-administration under PR reinforcement. In addition, PG01037 significantly attenuated methamphetamine-enhanced BSR. PG01037 also significantly inhibited cue-triggered reinstatement of extinguished methamphetamine-seeking behavior. The intermodal consistency of the present findings strengthen the conclusion that D3 receptor blockade attenuates methamphetamine’s rewarding effects and supports the further exploration and potential development of selective D3 antagonists as medications to treat psychostimulant addiction.
Acknowledgement
This research was supported by the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health.
References
- Arnold JM, Roberts DC. A critique of fixed and progressive ratio schedules used to examine the neural substrates of drug reinforcement. Pharmacol Biochem Behav. 1997;57:441–457. doi: 10.1016/s0091-3057(96)00445-5. [DOI] [PubMed] [Google Scholar]
- Ashby CR, Paul M, Gardner EL, Heidbreder CA, Hagan JJ. Acute administration of the selective D3 receptors antagonist SB-277011-A blocks the acquisition and expression of the conditioned place preference response to heroin in male rats. Synapse. 2003;48:154–156. doi: 10.1002/syn.10188. [DOI] [PubMed] [Google Scholar]
- Austin NE, Baldwin SJ, Cutler L, et al. Pharmacokinetics of the novel, high-affinity and selective dopamine D3 receptor antagonist SB-277011A in rat, dog and monkey: in vitro/in vivo correlation and the role of aldehyde oxidase. Xenobiotica. 2001;6:677–686. doi: 10.1080/00498250110056531. [DOI] [PubMed] [Google Scholar]
- Bauco P, Wise RA. Synergistic effects of cocaine with lateral hypothalamic brain stimulation reward: lack of tolerance or sensitization. J Pharmacol Exp Ther. 1997;283:1160–1167. [PubMed] [Google Scholar]
- Bossert JM, Ghitza UE, Lu L, Epstein DH, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: an update and clinical implications. Eur J Pharmacol. 2005;526:36–50. doi: 10.1016/j.ejphar.2005.09.030. [DOI] [PubMed] [Google Scholar]
- Cervo L, Carnovali F, Stark JA, Mennini T. Cocaine-seeking behavior in response to drug-associated stimuli in rats: Involvement of D3 and D2 dopamine receptors. Neuropsychopharmacology. 2003;28:1150–1159. doi: 10.1038/sj.npp.1300169. [DOI] [PubMed] [Google Scholar]
- Collins GT, Newman AH, Grundt P, et al. Yawning and hypothermia in rats: effects of dopamine D3 and D2 agonists and antagonists. Psychopharmacology(Berl) 2007;193:159–170. doi: 10.1007/s00213-007-0766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Witkin JM, Newman AH, et al. Dopamine agonist-induced yawning in rats: a dopamine D3 receptor-mediated behavior. J Pharmacol Exp Ther. 2005;314:310–319. doi: 10.1124/jpet.105.085472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulombe D, Miliaressis E. Fitting intracranial self-stimulation data with growth models. Behav Neurosci. 1987;101:209–214. doi: 10.1037//0735-7044.101.2.209. [DOI] [PubMed] [Google Scholar]
- Diaz J, Pilon C, Le Foll B, Gros C, Triller A, Schwartz JC, Sokoloff P. Dopamine D3 receptors expressed by all mesencephalic dopamine neurons. Journal of Neuroscience. 2002;20:8677–8684. doi: 10.1523/JNEUROSCI.20-23-08677.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Differential control over drug-seeking behavior by drug-associated conditioned reinforcers and discriminative stimuli predictive of drug availability. Behav Neurosci. 2003;117:952–960. doi: 10.1037/0735-7044.117.5.952. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Underwood RJ, Hagan JJ, Everitt BJ. Attenuation of cue-controlled cocaine-seeking by a selective D3 dopamine receptor antagonist SB-277011-A. Neuropsychopharmacology. 2003;28:329–338. doi: 10.1038/sj.npp.1300148. [DOI] [PubMed] [Google Scholar]
- French ED, Lopez M, Peper S, Kamenka JM, Roberts DC. A comparison of the reinforcing efficacy of PCP, the PCP derivatives TCP and BTCP, and cocaine using a progressive ratio schedule in the rat. Behav Pharmacol. 1995;6:223–228. [PubMed] [Google Scholar]
- Gal K, Gyertyán I. Dopamine D3 receptor ligands show place conditioning effect but do not influence cocaine-induced place preference. Neuroreport. 2003;14:93–98. doi: 10.1097/00001756-200301200-00018. [DOI] [PubMed] [Google Scholar]
- Gardner EL. What we have learned about addiction from animal models of drug self administration. Am J Addict. 2000;9:285–313. doi: 10.1080/105504900750047355. [DOI] [PubMed] [Google Scholar]
- Gilbert JG, Newman AH, Gardner EL, et al. Acute administration of SB-277011A, NGB 2904, or BP 897 inhibits cocaine cue-induced reinstatement of drug-seeking behavior in rats: role of dopamine D3 receptors. Synapse. 2005;57:17–28. doi: 10.1002/syn.20152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich EV, Joyce AN. Distribution of dopamine D3 receptor expressing neurons in the human forebrain: Comparison with D2 receptor expressing neurons. Neuropsychopharmacology. 1999;20:60–80. doi: 10.1016/S0893-133X(98)00066-9. [DOI] [PubMed] [Google Scholar]
- Grundt P, Carlson EE, Cao J, et al. Novel heterocyclic trans olefin analogues of N-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]-butyl}arylcarboxamides as selective probes with high affinity for the dopamine D3 receptor. J Med Chem. 2005;48:839–848. doi: 10.1021/jm049465g. [DOI] [PubMed] [Google Scholar]
- Grundt P, Prevatt KM, Cao J, et al. Heterocyclic analogues of N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)arylcarboxamides with functionalized linking chains as novel dopamine D3 receptor ligands: Potential substance abuse therapeutic agents. J Med Chem. 2007;50:4135–4146. doi: 10.1021/jm0704200. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Gardner EL, Xi ZX, et al. The role of central dopamine D3 receptors in drug addiction: a review of pharmacological evidence. Brain Res Brain Res Rev. 2005;49:77–105. doi: 10.1016/j.brainresrev.2004.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder CA, Andreoli M, Marcon C, Hutcheson DM, Gardner EL, Ashby CR., Jr Evidence for the role of dopamine D3 receptors in oral operant alcohol self-administration and reinstatement of alcohol-seeking behavior in mice. Addict Biol. 2007;12:35–50. doi: 10.1111/j.1369-1600.2007.00051.x. [DOI] [PubMed] [Google Scholar]
- Higley AH, Dillon C, Xi ZX, Gardner EL. Abstracts for the 37th Annual Meeting for Society of Neuroscience. San Diego, CA: Nov, 2007. The dopamine D3 receptor antagonist SB-277011A inhibits progressive-ratio methamphetamine self-administration and methamphetamine-induced reinstatement of drug-seeking behavior in rats; pp. 3–7. [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Jaffe JH, Cascell NG, Kumor KM, Sherer MA. Cocaine-induced cocaine craving. Psychopharmacology. 1989;110:76–84. doi: 10.1007/BF00443414. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. Neurobiology of cocaine addiction: implications for new pharmacotherapy. Am J Addict. 2007;16:71–78. doi: 10.1080/10550490601184142. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatr. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Khroyan TV, Barrett-Larimore RL, Rowlett JK, Spealman D. Dopamine D1- and D2-like receptor mechanisms in relapse to cocaine-seeking behavior: effects of selective antagonists and agonists. J Pharmacol Exp Ther. 2000;294:680–687. [PubMed] [Google Scholar]
- Kita K, Shiratani T, Takenouchi K, Fukuzako H, Takigawa M. Effects of D1 and D2 dopamine receptor antagonists on cocaine-induced self-stimulation and locomotor activity in rats. Eur Neuropsychopharmacol. 1999;9:1–7. doi: 10.1016/s0924-977x(97)00098-9. [DOI] [PubMed] [Google Scholar]
- Kornetsky C. Brain-stimulation reward, morphine-induced oral stereotypy, and sensitization: implications for abuse. Neurosci Biobehav Rev. 2004;27:777–786. doi: 10.1016/j.neubiorev.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Kumar R, Riddle L, Griffin SA, Grundt P, Newman AH, Luedtke RR. Evaluation of the D3 dopamine receptor selective antagonist PG01037 on L-dopa-dependent abnormal involuntary movements in rats. Neuropharmacology. 2009;56:944–955. doi: 10.1016/j.neuropharm.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Sokoloff P, Stark H, Goldberg SR. Dopamine D3 receptor ligands block nicotine-induced conditioned place preferences through a mechanism that does not involve discriminative-stimulus or antidepressant-like effects. Neuropsychopharmacology. 2005;30:720–730. doi: 10.1038/sj.npp.1300622. [DOI] [PubMed] [Google Scholar]
- Levant B. Differential distribution of D3 dopamine receptors in the brains of several mammalian species. Brain Res. 1998;2:269–274. doi: 10.1016/s0006-8993(98)00529-0. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Mello NK. Management of cocaine abuse and dependence. N Eng J Med. 1996;334:965–972. doi: 10.1056/NEJM199604113341507. [DOI] [PubMed] [Google Scholar]
- Meredith CW, Jaffe C, Ang-Lee K, Saxon AJ. Implications of chronic methamphetamine use: a literature review. Harv Rev Psychiatry. 2005;13:141–154. doi: 10.1080/10673220591003605. [DOI] [PubMed] [Google Scholar]
- National Academy of Sciences (National Research Council, Commission on Life Sciences, Institute of Laboratory Animal Resources) Guide for the Care and Use o Laboratory Animals. Washington, DC: National Academy Press; 1996. [Google Scholar]
- Newman AH, Grundt P, Nader MA. Dopamine D3 receptor partial agonists and antagonists as potential drug abuse therapeutic agents. J Med Chem. 2005;48:3663–3679. doi: 10.1021/jm040190e. [DOI] [PubMed] [Google Scholar]
- O’Brien CP. A range of research-based pharmacotherapies for addiction. Science. 1997;278:66–70. doi: 10.1126/science.278.5335.66. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, McLellan AT, Ehrman R. Classical conditioning in drug-dependent humans. Ann N Y Acad Sci. 1992;654:400–415. doi: 10.1111/j.1749-6632.1992.tb25984.x. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Gardner EL. Critical assessment of how to study addiction and its treatment: human and non-human animal models. Pharmacol Ther. 2005;108:18–58. doi: 10.1016/j.pharmthera.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Pak AC, Ashby CR, Jr, Heidbreder CA, et al. The selective dopamine D3 receptor antagonist SB-277011A reduces nicotine-enhanced brain reward and nicotine-paired environmental cue functions. Int J Neuropsychopharmacol. 2006;9:585–602. doi: 10.1017/S1461145706006560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1998. [Google Scholar]
- Peng XQ, Ashby CR, Jr, Spiller K, Li X, Li J, Thomasson N, Millan MJ, Mocaër E, Muńoz C, Gardner EL, Xi ZX. The preferential dopamine D3 antagonist S33138 inhibits cocaine reward and cocaine triggered relapse to drug- seeking behavior in rats. Neuropharmacology. 2009;56:752–760. doi: 10.1016/j.neuropharm.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Kumaresan V. The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neurosci Biobehav Rev. 2006;30:15–38. doi: 10.1016/j.neubiorev.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Pilla M, Perachon S, Sautel F, et al. Selective inhibition of cocaine-seeking behaviour by a partial dopamine D3 receptor agonist. Nature. 1999;400:371–375. doi: 10.1038/22560. [DOI] [PubMed] [Google Scholar]
- Ranaldi R, Beninger RJ. The effects of systemic and intracerebral injections of D1 and D2 agonists on brain stimulation reward. Brain Res. 1994;651:283–292. doi: 10.1016/0006-8993(94)90708-0. [DOI] [PubMed] [Google Scholar]
- Reavill C, Taylor SG, Wood MD, et al. Pharmacological actions of a novel, high-affinity, and selective human dopamine D3 receptor antagonist, SB-277011-A. J Pharmacol Exp Ther. 2000;294:1154–1165. [PubMed] [Google Scholar]
- Remington G, Kapur S. SB-277011 GlaxoSmithKline. Curr Opin Investig Drugs. 2001;2:946–949. [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Loh EA, Vickers G. Self-administration of cocaine on a progressive ratio schedule in rats: dose–response relationship and effect of haloperidol pretreatment. Psychopharmacology. 1989;97:535–538. doi: 10.1007/BF00439560. [DOI] [PubMed] [Google Scholar]
- Rose ME, Grant CE. Pharmacotherapy for methamphetamine dependence: A review of the pathophysiology of methamphetamine addiction and the theoretical basis and efficacy of pharmacotherapeutic interventions. Ann Clin Psychiatry. 2008;20:145–155. doi: 10.1080/10401230802177656. [DOI] [PubMed] [Google Scholar]
- Rowlett JK. A labor-supply analysis of cocaine self-administration under progressive ratio schedules: antecedents, methodologies, and perspectives. Psychopharmacology. 2000;153:1–16. doi: 10.1007/s002130000610. [DOI] [PubMed] [Google Scholar]
- Schwartz A, Gozzi A, Reese T, et al. Selective dopamine D3-receptor antagonist SB-277011-A potentiates phMRI response to acute amphetamine challenge in the rat brain. Synapse. 2004;54:1–10. doi: 10.1002/syn.20055. [DOI] [PubMed] [Google Scholar]
- Self DW, Barnhart WJ, Lehman DA, Nestler EJ. Opposite modulation of cocaine-seeking behavior by D1- and D2-like dopamine receptor agonists. Science. 1996;271:1586–1589. doi: 10.1126/science.271.5255.1586. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Rodaros D, Stewart J. Reinstatement of heroin-reinforced behavior following long-term extinction: implications for the treatment of relapse to drug taking. Behav Pharmacol. 1994;5:360–364. doi: 10.1097/00008877-199406000-00015. [DOI] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- Shalev U, Highfield D, Yap J, Shaham Y. Stress and relapse to drug seeking in rats: studies on the generality of the effect. Psychopharmacology (Berl) 2000;150:337–346. doi: 10.1007/s002130000441. [DOI] [PubMed] [Google Scholar]
- Shalev U, Marinelli M, Baumann MH, Piazza PV, Shaham Y. The role of corticosterone in food deprivation-induced reinstatement of cocaine seeking in the rat. Psychopharmacology (Berl) 2003;168:170–176. doi: 10.1007/s00213-002-1200-5. [DOI] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology. 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Spiller K, Xi ZX, Peng XQ, Newman AH, Ashby CR, Heidbreder C, Gardner EL. The putative dopamine D3 receptor antagonists SB-277011A, NGB 2904, S33,138 or BP 897 inhibit methamphetamine-enhanced brain stimulation reward in rats. Psychopharmacology. 2008;196:533–542. doi: 10.1007/s00213-007-0986-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff P, Diaz J, Le Foll B, et al. The dopamine D3 receptor: A therapeutic target for the treatment of neuropsychiatric disorders. CNS Neurol Disord Drug Targets. 2006;5:25–43. doi: 10.2174/187152706784111551. [DOI] [PubMed] [Google Scholar]
- Sokoloff P, Giros B, Martres MP, Bouthenet ML, Schwartz JC. Molecular cloning and characterization of novel dopamine receptor (D3) as a target for neuroleptics. Nature. 1990;347:146–151. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- Sokoloff P, Le Foll B, Perachon S, Bordet R, Ridray S, Schwartz JC. The dopamine D3 receptor and drug addiction. Neurotox Res. 2001;3:433–441. doi: 10.1007/BF03033202. [DOI] [PubMed] [Google Scholar]
- Sokoloff P, Martres MP, Giros B, Bouthenet ML, Schwartz JC. The third dopamine receptor (D3) as a novel target for antipsychotics. Biochem Pharmacol. 1992;43:659–666. doi: 10.1016/0006-2952(92)90227-a. [DOI] [PubMed] [Google Scholar]
- Stafford D, LeSage MG, Glowa JR. Progressive-ratio schedules of drug delivery in the analysis of drug self-administration: a review. Psychopharmacology. 1998;139:169–184. doi: 10.1007/s002130050702. [DOI] [PubMed] [Google Scholar]
- Stanwood GD, Artymyshyn RP, Kung MP, Kung HF, Lucki I, McGonigle P. Quantitative autoradiographic mapping of rat brain dopamine D3 binding with [(125)I]7-OH-PIPAT: evidence for the presence of D3 receptors on dopaminergic and non-dopaminergic cell bodies and terminals. J Pharmacol Exp Ther. 2000;295:1223–1231. [PubMed] [Google Scholar]
- Stein L, Ray OS. Brain stimulation reward ‘thresholds’ self-determined in rat. Psychopharmacologia. 1960;1:251–256. doi: 10.1007/BF00402746. [DOI] [PubMed] [Google Scholar]
- Stewart J. Pathways to relapse: the neurobiology of drug- and stress-induced relapse to drug-taking. J Psychiat Neurosci. 2000;25:125–136. [PMC free article] [PubMed] [Google Scholar]
- Thanos PK, Katana JM, Ashby CR, et al. The selective dopamine D3 antagonist SB-277011A attenuates ethanol consumption in ethanol preferring (P) and non-preferring (NP) rats. Pharmacol Biochem Behav. 2005;81:190–197. doi: 10.1016/j.pbb.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Vorel SR, Ashby CR, Jr, Paul M, et al. Dopamine D3 receptor antagonism inhibits cocaine-seeking and cocaine-enhanced brain reward in rats. J Neurosci. 2002;22:9595–9603. doi: 10.1523/JNEUROSCI.22-21-09595.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Addictive drugs and brain stimulation reward. Ann Rev Neurosci. 1996a;19:319–340. doi: 10.1146/annurev.ne.19.030196.001535. [DOI] [PubMed] [Google Scholar]
- Wise RA. Forebrain substrates of reward and motivation. J Comp Neurol. 2005;493:115–121. doi: 10.1002/cne.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Gardner EL. Animal models of addiction. In: Charney DS, Nestler EJ, editors. Neurobiology of Mental Illness. 2nd edn. London: Oxford University Press; 2004. pp. 683–697. [Google Scholar]
- Xi ZX, Gardner EL. Pharmacological actions of NGB-2904, a selective dopamine D3 receptor antagonist, in animal models of drug addiction. CNS Drug Rev. 2007;13:1–21. doi: 10.1111/j.1527-3458.2007.00013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Gilbert JG, Campos AC, et al. Blockade of mesolimbic dopamine D3 receptors inhibits stress-induced reinstatement of cocaine-seeking in rats. Psychopharmacology. 2004;176:57–65. doi: 10.1007/s00213-004-1858-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Gilbert JG, Pak AC, Ashby CR, Heidbreder CA, Gardner EL. Selective dopamine D3 receptor antagonism by SB-277011A attenuates cocaine reinforcement as assessed by progressive ratio and variable cost variable payoff fixed ratio cocaine self administration in rats. Eur J Neurosci. 2005;21:3427–3438. doi: 10.1111/j.1460-9568.2005.04159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Newman AH, Gilbert JG, et al. The novel dopamine D3 receptor antagonist NGB 2904 inhibits cocaine’s rewarding effects and cocaine-induced reinstatement of drug-seeking behavior in rats. Neuropsychopharmacology. 2006;31:1393–1405. doi: 10.1038/sj.npp.1300912. [DOI] [PubMed] [Google Scholar]