Abstract
OBJECTIVES: The full potential of stereotactic body radiation therapy (SBRT), in the treatment of unresectable intrahepatic malignancies, has yet to be realized as our experience is still limited. Thus, we evaluated SBRT outcomes for primary and metastatic liver tumors, with the goal of identifying factors that may aid in optimization of therapy. METHODS: From 2005 to 2010, 62 patients with 106 primary and metastatic liver tumors were treated with SBRT to a median biologic effective dose (BED) of 100 Gy (42.6–180). The majority of patients received either three (47%) or five fractions (48%). Median gross tumor volume (GTV) was 8.8 cm3 (0.2–222.4). RESULTS: With a median follow-up of 18 months (0.46–46.8), freedom from local progression (FFLP) was observed in 97 of 106 treated tumors, with 1- and 2-year FFLP rates of 93% and 82%. Median overall survival (OS) for all patients was 25.2 months, with 1- and 2-year OS of 81% and 52%. Neither BED nor GTV significantly predicted for FFLP. Local failure was associated with a higher risk of death [hazard ratio (HR) = 5.1, P = .0007]. One Child-Pugh Class B patient developed radiation-induced liver disease. There were no other significant toxicities. CONCLUSIONS: SBRT provides excellent local control for both primary and metastatic liver lesions with minimal toxicity. Future studies should focus on appropriate selection of patients and on careful assessment of liver function to maximize both the safety and efficacy of treatment.
Introduction
The majority of patients with intrahepatic cancer are ineligible for curative resection because of impaired liver function; multiple, large, or centrally located lesions; or medical comorbidities. Alternative treatment methods have emerged, among which radiofrequency ablation (RFA) is the most widely used and offers a high rate of local control. However, large liver lesions and lesions located near major blood vessels, the main biliary tract, or at the dome are not amenable to this treatment. In addition, RFA is an invasive procedure requiring anesthesia that has been associated with significant complications such as pneumothorax and bleeding [1]. Thus, patients with unresectable disease that cannot be addressed by local therapies such as RFA may benefit from non-invasive treatments involving radiation therapy.
Although treating liver tumors with external beam radiation has historically been limited by toxicity [2], we have previously demonstrated long-term local control and possibly prolonged overall survival (OS) with carefully planned fractionated radiotherapy with low risk of complications [3]. Stereotactic body radiation therapy (SBRT) now can be used to precisely target small liver tumors: improved immobilization, localization, and compensation for respiratory movement allow for the delivery of large, focused fractions of radiation, while minimizing dose to the remaining liver. Several small studies have demonstrated that SBRT is an effective and safe means of local treatment [4–9]. However, experience with SBRT for liver tumors is still limited. In this study, which is the largest to date, we evaluated the effects of biologic effective dose (BED), tumor size, and histology on local control and survival after SBRT in patients with primary and metastatic liver tumors. In particular, we aimed to determine if there is a subset of patients who may benefit from further treatment intensification.
Materials and Methods
Eligibility
As part of an Institutional Review Board-approved retrospective review, we searched the University of Michigan Radiation Oncology database to identify patients who received SBRT for primary or metastatic liver tumors between 2005 and 2010. Information was available for 62 patients with a total of 106 treated liver tumors. In general, patients had liver lesions that were unresectable, not amenable to RFA, or had progressed after RFA. In one case, the patient refused surgical resection.
Radiation Treatment
All patients underwent computed tomography (CT) simulation and were immobilized in the supine position with a customized vacuum body mold. In general, an active breathing control (ABC) device was used to control breathing-related tumor motion. For patients unable to tolerate ABC, 4DCT or a set of maximal inhalation and exhalation breath hold CTs was used to generate an internal target volume. When the target lesion was not readily apparent on the contrast-enhanced planning CT images, the planning data set was registered to a pretreatment diagnostic magnetic resonance imaging study, using a mutual information algorithm in our in-house treatment planning system, UMPLAN, to facilitate target delineation [10]. The gross tumor volume (GTV) for ABC cases and the internal target volume for free-breathing cases were expanded by a 5-mm radial and 8-mm craniocaudal margin for the planning target volume (PTV) [11]. SBRT was planned and delivered using three-dimensional conformal techniques with multiple (typically ≥8), non-opposed, non-coplanar static 6 or 16 MV beams. Radiation dose was prescribed to the isodose surface covering 99.5% of the PTV, typically 75% to 85% of the maximum PTV dose. The majority of patients received either three or five fractions (47% and 48%, respectively), with 20 Gy x 3 (31%) and 10 Gy x 5 (39%) comprising the most common dose fractionation schemes. Generally, patients received five fractions due to the proximity of organs at risk. Dose limits to the duodenum, stomach, and heart were 24, 22.5, and 30 Gy, respectively, while <30 cm3 of the chest wall was permitted to receive ≥30 Gy for patients treated with three-fraction SBRT. These limits were 30, 27.5, 52.5, and 35 Gy for patients treated with five fractions. Three early patients received a single fraction of 24 Gy (n = 2) or 26 Gy (n = 1).
Daily image guidance and positioning was performed with either orthogonal X-rays or cone beam CT imaging. X-ray localization involved alignment of one to three percutaneously placed intrahepatic fiducial markers in the vicinity of the lesion for 38 patients (52 lesions), whereas cone beam CT alignment included markers or landmarks such as large liver vessels and surface features of the liver for the remaining patients.
Follow-up
Patients were evaluated at 1 and 3 months after completion of SBRT and then every 3 to 6 months thereafter. Follow-up visits typically consisted of a history and physical examination, tumor marker assessment, serum liver enzymes, and imaging with CT, magnetic resonance imaging, or positron emission tomography (PET). Follow-up imaging was reviewed by a radiologist and a radiation oncologist. Treatment response was assessed for each treated lesion and scored using the Response Evaluation Criteria in Solid Tumors. Local recurrence was scored as in-field or marginal failure [12]. New lesions were also documented.
Evaluation of Response and Statistical Analysis
The primary end point was freedom from local progression (FFLP), defined as the absence of new or progressive lesions within or at the margin of the PTV. Secondary end points were freedom from any progression, OS, and toxicity. Freedom from any progression was defined as freedom from any local, distant intrahepatic, or distant extrahepatic progression. FFLP, freedom from any progression, and OS were estimated using the Kaplan-Meier method. Most analyses were conducted at the lesion level, with each lesion contributing a unique observation for analysis. Dose analyses were performed using BED, a standard method of accounting for differences in dose per fraction and total dose when comparing treatments. Survival end points were calculated as time from the start of SBRT. The robust sandwich variance estimate was used to account for correlation between multiple lesions within a subject [13]. A modified score test using this variance estimate was used to test for significance of predictors in Cox regression models. To assess whether local failure was predictive of OS, it was included as a time-dependent covariate in a Cox regression model for OS. This analysis was performed at the patient level so OS time was measured as the time from the start of radiation therapy for the first treated lesion until death or loss to follow-up. The time-dependent covariate was an indicator variable (yes or no) for failure of any treated lesion. The software package SAS (V9.2, Cary, NC) was used for analysis.
Results
Patient and Treatment Characteristics
Patient characteristics are presented in Table 1. In total, there were 62 patients with 106 unique lesions treated with SBRT and assessable for outcome analysis. Median follow-up for all lesions was 18 months (range, 0.46–46.8) and for alive patients 12.4 months (range, 1–47). Metastatic tumors comprised the majority of lesions (65%), with colorectal (CRC), breast, and esophageal primaries accounting for 23%, 8%, and 8% of all lesions, respectively. Hepatocellular carcinoma (HCC) represented the most common lesion overall, constituting 29% of all lesions.
Table 1.
Patient and Tumor Characteristics.
| No. | % | |
| Total No. of patients | 62 | |
| Total No. of tumors evaluated | 106 | |
| Age, years | ||
| Median | 60 | |
| Range | 23–82 | |
| Primary liver tumors | 37 | 35 |
| Hepatocellular carcinoma | 31 | 29 |
| Intrahepatic cholangiocarcinoma | 6 | 6 |
| Metastatic liver tumors | 69 | 65 |
| Colorectal primary | 24 | 23 |
| Breast primary | 9 | 8 |
| Esophageal primary | 8 | 8 |
| Other primary | 28 | 26 |
| Prior liver-directed (local) therapy | ||
| Yes | 54 | 51 |
| No | 52 | 49 |
| Prior systemic therapy | ||
| Yes | 57 | 54 |
| No | 49 | 46 |
| No. of active liver tumors | ||
| 1 | 52 | 49 |
| 2 | 24 | 23 |
| 3 | 23 | 22 |
| ≥4 | 7 | 7 |
| Child-Pugh classification, HCC tumors | ||
| Class A (5–6) | 22 | 71 |
| Class B (7–9) | 7 | 23 |
| Class C (10–15) | 2 | 6 |
| Active extrahepatic disease, metastatic tumors | ||
| Yes | 25 | 36 |
| No | 44 | 64 |
| Lesion volume (cm3) | ||
| Median | 8.8 | |
| Range | 0.2–222.4 | |
| Median biologic equivalent dose (BED10) | ||
| Median | 100 | |
| Range | 42.6–180 |
Patients were heavily pretreated: 51% of cases received one or more prior form of liver-directed therapy, including transarterial chemoembolization, RFA, fractionated radiotherapy, intra-arterial chemotherapy, and surgical resection, and 54% received one or more prior systemic therapy regimen.
Clinical Outcomes
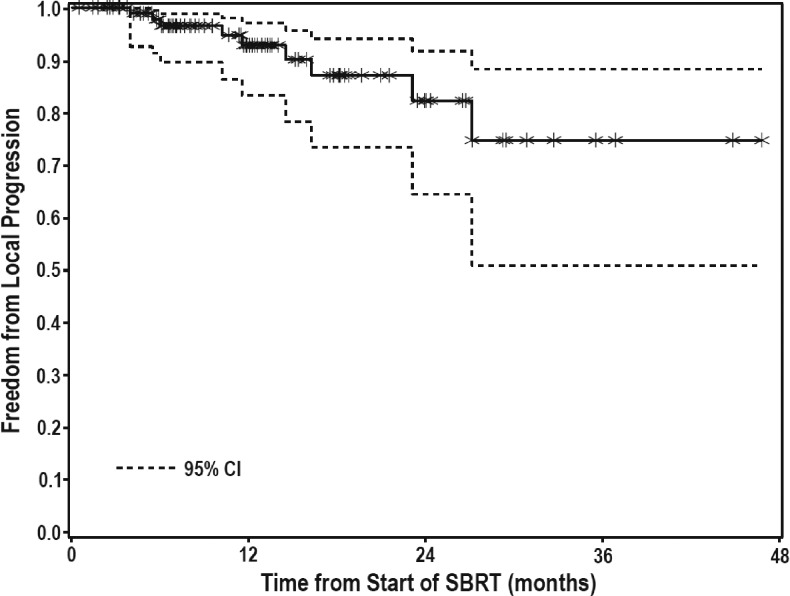
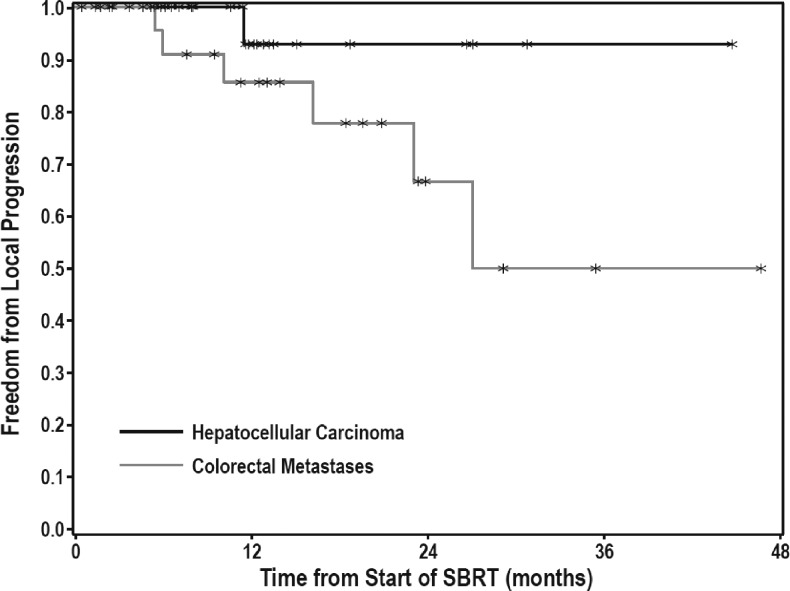
FFLP was observed in 97 of 106 treated lesions with 1- and 2-year FFLP rates of 93% and 82%, respectively (Figure 1). On multivariate analysis, no correlation was found between FFLP and lesion size, BED, prior systemic or liver-directed therapy, number of active liver lesions, or whether the lesion was metastatic or primary. There was a trend toward better local control for patients with HCC compared with colorectal metastases (Figure 2), although this difference was not significant. For patients with HCC, 1- and 2-year FFLP rates were 93% and 93%, respectively, while for patients with colorectal metastases, 1- and 2-year FFLP rates were 86% and 67% (HR = 0.203, P = .0827). Of the 31 HCC lesions, one failed locally, 19 failed elsewhere in the liver, and 5 had distant progression. Of the 24 CRC lesions, 6 failed locally, 14 failed elsewhere in the liver, and 17 had distant progression. Of all local failures, two were marginal and seven were in-field (Table 2). One marginal recurrence was in a patient with metastatic breast cancer, who developed four new liver metastases 8 months after SBRT to 36 Gy in three fractions. Her marginal recurrence occurred 14 months after SBRT, in the setting of continued liver progression. She also had received multiple prior systemic therapies and had active extrahepatic disease at the start of treatment. The other marginal recurrence was in an HCC patient who had been treated with two surgical resections, as well as five transarterial chemoembolization (TACE) procedures before SBRT to 50 Gy in five fractions. Subsequent to SBRT, he underwent two additional TACE procedures for new out-of-field tumors. His marginal recurrence, 2 years after SBRT, occurred in the setting of a massive, diffuse recurrence throughout the liver, bones, spleen, and peritoneum. Both patients were treated with ABC, with standard PTV margins, as described above. Image-guided radiotherapy (IGRT) consisted of alignment of fiducial markers with cone beam computed tomography (CBCT).
Figure 1.
FFLP for treated tumors.
Figure 2.
FFLP for HCC and colorectal metastases.
Table 2.
Tumor and Treatment Characteristics of Local Failures.
| Primary Site | BED* (Gy) | GTV† (cm3) | Location of Failures | |
| 1 | Colon | 100 | 3 | In-field |
| 2 | Colon | 180 | 10 | In-field |
| 3 | Colon | 125 | 4 | In-field |
| 4 | Colon | 180 | 13 | In-field |
| 5 | Colon | 101 | 3 | In-field |
| 6 | Colon | 100 | 19 | In-field |
| 7 | Pancreas | 100 | 118 | In-field |
| 8 | Breast | 79 | 47 | Marginal |
| 9 | Liver (HCC) | 100 | 57 | Marginal |
Median BED = 100 Gy.
Median GTV = 13 cm3.
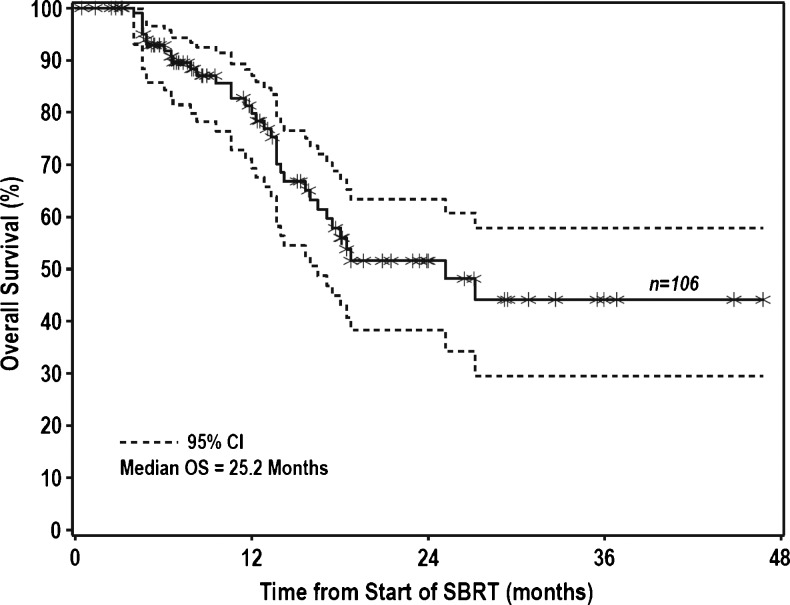
Because the local failure rate for patients with CRC was higher than for other groups of patients, we also reviewed these failures in detail. The six CRC failures occurred in four patients, all of whom had received prior systemic therapy and three of whom had active extrahepatic disease at the start of radiation treatment. All had distant progression before local recurrence after SBRT. One patient failed in one of three treated lesions and developed new intrahepatic lesions. Two patients each progressed in two lesions, one with concurrent new lesions and the other with a massive, diffuse liver recurrence. One of the four CRC patients had only one active liver lesion at the time of treatment, but this patient also had a massive, diffuse liver recurrence and lung metastases. Colorectal metastases actually received a significantly higher BED (mean of 133 vs 109, P = .03) than HCC tumors, despite lower FFLP, and no recurrences were marginal. Median OS for all patients was 25.2 months, with 1- and 2-year OS of 81% and 52%, respectively (Figure 3). Survival was greater among patients with metastatic compared to primary lesions (2-year OS of 63% vs 29%, HR = 0.46, P = .02). For patients with metastatic liver lesions, the presence of active extrahepatic disease yielded significantly worse survival (2-year OS of 37% vs 83%, HR = 3.8, P = .005). Lesion size, BED, prior systemic or liver-directed therapy, and number of active liver lesions did not influence survival. Four of the seven patients who experienced local progression died within 5 weeks of local progression. In a Cox model for OS with presence of local progression as a time-dependent covariate, the presence of a local failure resulted in a significantly increased risk of death (HR = 5.1, P = .0007).
Figure 3.
OS for treated tumors.
In 104 assessable lesions at last follow-up (1 patient was lost to follow-up and 1 patient had a liver transplant shortly after treatment), 40 lesions had a sustained objective tumor response according to Response Evaluation Criteria in Solid Tumors (8 had complete response; 32 had partial response) and 55 lesions had stable disease. Nine lesions progressed, in seven patients. One of these lesions was retreated and progressed later. Freedom from any progression for all patients was 28% and 15% at 1 and 2 years, respectively.
Assessment for Dose-Response
BED was calculated using the linear quadratic formula with α/β ratio of 10. For all lesions, the median BED was 100 Gy (range, 42.6–180), with 76% of lesions receiving ≥100 BED. Dose was not a significant predictor of FFLP in a univariate model. In fact, eight of nine failures in our series received ≥100 BED, whereas 24 of 25 patients who received <100 BED were controlled locally.
As expected, there was moderate negative correlation between GTV and BED (Spearman's rho = -0.45). To account for possible confounding with size, BED and GTV were included together in a multivariate model. In this model, neither GTV (median, 8.8 cm3; range, 0.2–222.4) nor BED was found to significantly predict FFLP. Among the patients with larger tumors (>20 cm3, the upper quartile), those who received >100 BED tended to have a higher FFLP than those who received = 100 Gy, although this trend was not statistically significant.
Toxicity
Overall, patients tolerated treatment well, and all patients completed therapy without treatment breaks. Only one patient experienced a serious toxicity, which was the development of radiation-induced liver disease (RILD), who recovered. This patient had hepatitis C cirrhosis, Child-Pugh Class B liver disease, and had received multiple prior liver-directed therapies, including two RFAs and SBRT. Her 2.87-cm3 tumor received 45 Gy in five fractions. No gastrointestinal bleeding, rib fracture, or pneumonitis was observed.
Discussion
In this series, we demonstrate that SBRT provides excellent local control with minimal toxicity for both primary and metastatic liver tumors, with 1- and 2-year FFLP rates of 93% and 82%, respectively, and a median OS of 25.2 months. Neither tumor size nor BED was found to significantly predict FFLP. Our local control rates are consistent with prior SBRT as well as RFA series of primary and metastatic liver tumors [1,4–9]. The majority of patients in our study had prior liver-directed and systemic therapies. Despite the inclusion of patients refractory to multiple lines of treatment, there were only nine cases (8%) of local progression.
While our HCC lesions had excellent local control, with 1- and 2-year FFLP rates of 93% and 93%, our CRC control rate (1- and 2-year FFLP rates of 86% and 67%) was substantially lower, consistent with other reports [5,9]. The series on SBRT for primary and metastatic liver lesions of Wulf et al. found 1- and 2-year local control rates at approximately 90% and 55%, respectively. In fact, all nine of their local failures occurred in metastatic liver lesions, with six from CRC. They suggested that dose escalation results in better local control, as 6 of the 12 CRC patients in their series who received 3 x 10 Gy had local failure, while none of the 11 CRC patients treated with higher doses (3x 12–12.5 or 1 x 26 Gy) did. The pooled analysis of Chang et al. also demonstrated a 55% 2-year local control rate [7], with total dose, dose per fraction, and BED correlated with local control in multivariate analysis. A dose effect was not demonstrated in our series, despite using a variety of dose fractionation schedules since the start of the SBRT program in 2005. This may have been due to the relatively high overall doses, with 76% lesions receiving ≥100 BED. However, among patients with the largest tumors (>20 cm3), those who received higher dose (>100 Gy) tended to have better FFLP, although not statistically significant, hinting at a possible correlation limited by a small sample size and relatively small tumors in our series (median of 9 cm3, compared with 30 cm3 in the pooled analysis of Chang et al.). Treatment intensification may be necessary for larger tumors, whereas smaller tumors may be controlled by any large, ablative dose of SBRT. Furthermore, we can rule out a difference in planning, image guidance, or treatment delivery as the cause of our CRC failures, because all of our CRC failures were in-field rather than marginal misses. Although we prioritized dose limits of normal tissues over target coverage, no recurrences were observed in regions of rapid dose falloff near normal tissues.
The most likely cause for the relatively higher number of local failures in CRC patients is that our cohort had biologically aggressive disease, evidenced by distant failures predating local failures in all cases, which highlights the need for better patient selection, to identify patients most likely to benefit from this treatment. Despite high control rates, survival in this study was lower than with hepatic resection [14], likely since almost all of these patients were medically inoperable, with lower than expected OS than those who would have qualified for surgery, and the more rigorous selection of patients for surgery. The presence of active extrahepatic disease was found to be a significant predictor for OS, confirming the importance of proper patient selection and the need for improved systemic therapy.
Limitations of this study include the retrospective nature, small patient numbers compared with other disease sites, and variety of pre-SBRT and post-SBRT systemic and liver-directed therapies. Still, this is the largest combined series of primary and metastatic liver tumors.
SBRT in our series was well tolerated, with only one case of RILD, which resolved, suggesting that SBRT may have a more favorable toxicity profile than RFA, which can result in anesthetic and post-procedural complications, in addition to bile duct and vessel injuries, gastrointestinal (GI) perforation, and pneumothorax [1]. Further study suggests that SBRT may be an alternative to RFA, and this should be investigated in a prospective clinical trial [15].
On the basis of our case of RILD and the experience of others [9], patients who are Child-Pugh Class B or who have had previous liver-directed therapies should be treated with caution. To address these patients, we are now conducting a phase II study of a new approach to SBRT for patients at increased risk of toxicity with the aim of maximizing both the safety and efficacy of treatment for each individual patient [16]. Using clearance rates of indocyanine green, a dye that is taken up from the plasma almost exclusively by hepatic parenchymal cells [17], liver function is tested before treatment, as well as following a partial course of therapy, to assess the liver's tolerance of radiotherapy. The remainder of treatment is customized for each patient based on the change in function. By individualizing dose this way, we can potentially simultaneously maximize both the safety and efficacy of treatment.
Footnotes
Presented in part at the Gastrointestinal Cancer Symposium, 20–22 January 2011, San Francisco, CA. Funded by NIH P01-CA-59827. No conflicts of interest.
References
- 1.Wong SL, Mangu PB, Choti MA, Crocenzi TS, Dodd GD, III, Dorfman GS, Eng C, Fong Y, Giusti AF, Lu D, et al. American Society Of Clinical Oncology 2009 clinical evidence review on radiofrequency ablation of hepatic metastases from colorectal cancer. J Clin Oncol. 2010;28:493–508. doi: 10.1200/JCO.2009.23.4450. [DOI] [PubMed] [Google Scholar]
- 2.Lawrence TS, Robertson JM, Anscher MS, Jirtle RL, Ensminger WD, Fajardo LF. Hepatic toxicity resulting from cancer treatment. Int J Radiat Oncol Biol Phys. 1995;31:1237–1248. doi: 10.1016/0360-3016(94)00418-K. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Josef E, Normolle D, Ensminger WD, Walker S, Tatro D, Ten Haken RK, Knol J, Dawson LA, Pan C, Lawrence TS. Phase II trial of high-dose conformal radiation therapy with concurrent hepatic artery floxuridine for unresectable intrahepatic malignancies. J Clin Oncol. 2005;23:8739–8747. doi: 10.1200/JCO.2005.01.5354. [DOI] [PubMed] [Google Scholar]
- 4.Herfarth KK, Debus J, Lohr F, Bahner ML, Rhein B, Fritz P, Hoss A, Schlegel W, Wannenmacher MF. Stereotactic single-dose radiation therapy of liver tumors: results of a phase I/II trial. J Clin Oncol. 2001;19:164–170. doi: 10.1200/JCO.2001.19.1.164. [DOI] [PubMed] [Google Scholar]
- 5.Rusthoven KE, Kavanagh BD, Burri SH, Chen C, Cardenes H, Chidel MA, Pugh TJ, Kane M, Gaspar LE, Schefter TE. Multi-institutional phase I/II trial of stereotactic body radiation therapy for lung metastases. J Clin Oncol. 2009;27:1579–1584. doi: 10.1200/JCO.2008.19.6386. [DOI] [PubMed] [Google Scholar]
- 6.Schefter TE, Kavanagh BD, Timmerman RD, Cardenes HR, Baron A, Gaspar LE. A phase I trial of stereotactic body radiation therapy (SBRT) for liver metastases. Int J Radiat Oncol Biol Phys. 2005;62:1371–1378. doi: 10.1016/j.ijrobp.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Chang DT, Swaminath A, Kozak M, Weintraub J, Koong AC, Kim J, Dinniwell R, Brierley J, Kavanagh BD, Dawson LA, et al. Stereotactic body radiotherapy for colorectal liver metastases: a pooled analysis. Cancer. 2011;117:4060–4069. doi: 10.1002/cncr.25997. [DOI] [PubMed] [Google Scholar]
- 8.Goodman KA, Wiegner EA, Maturen KE, Zhang Z, Mo Q, Yang G, Gibbs IC, Fisher GA, Koong AC. Dose-escalation study of single-fraction stereo-tactic body radiotherapy for liver malignancies. Int J Radiat Oncol Biol Phys. 2010;78:486–493. doi: 10.1016/j.ijrobp.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 9.Wulf J, Guckenberger M, Haedinger U, Oppitz U, Mueller G, Baier K, Flentje M. Stereotactic radiotherapy of primary liver cancer and hepatic metastases. Acta Oncol. 2006;45:838–847. doi: 10.1080/02841860600904821. [DOI] [PubMed] [Google Scholar]
- 10.Roberson PL, McLaughlin PW, Narayana V, Troyer S, Hixson GV, Kessler ML. Use and uncertainties of mutual information for computed tomography/magnetic resonance (CT/MR) registration post permanent implant of the prostate. Med Phys. 2005;32:473–482. doi: 10.1118/1.1851920. [DOI] [PubMed] [Google Scholar]
- 11.Dawson LA, Brock KK, Kazanjian S, Fitch D, McGinn CJ, Lawrence TS, Ten Haken RK, Balter J. The reproducibility of organ position using active breathing control (ABC) during liver radiotherapy. Int J Radiat Oncol Biol Phys. 2001;51:1410–1421. doi: 10.1016/s0360-3016(01)02653-0. [DOI] [PubMed] [Google Scholar]
- 12.Chan JL, Lee SW, Fraass BA, Normolle DP, Greenberg HS, Junck LR, Gebarski SS, Sandler HM. Survival and failure patterns of high-grade gliomas after three-dimensional conformal radiotherapy. J Clin Oncol. 2002;20:1635–1642. doi: 10.1200/JCO.2002.20.6.1635. [DOI] [PubMed] [Google Scholar]
- 13.Lin DY, Wei LJ. The robust inference for the Cox proportional hazards model. J Am Stat Assoc. 1989;84:1074–1078. [Google Scholar]
- 14.Agrawal S, Belghiti J. Oncologic resection for malignant tumors of the liver. Ann Surg. 2011;253:656–665. doi: 10.1097/SLA.0b013e3181fc08ca. [DOI] [PubMed] [Google Scholar]
- 15.Liu E, Stenmark MH, Schipper MJ, Caoili EM, Ben-Josef E, Lawrence TS, Feng M. SBRT as an alternative to RFA for the treatment of primary and metastatic liver tumors. J Clin Oncol. 2012;30:158. [Google Scholar]
- 16.Normolle D, Pan C, Ben-Josef E, Lawrence T. Adaptive trial of personalized radiotherapy for intrahepatic cancer. Per Med. 2010;7:197–204. doi: 10.2217/pme.10.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imamura H, Sano K, Sugawara Y, Kokudo N, Makuuchi M. Assessment of hepatic reserve for indication of hepatic resection: decision tree incorporating indocyanine green test. J Hepatobiliary Pancreat Surg. 2005;12:16–22. doi: 10.1007/s00534-004-0965-9. [DOI] [PubMed] [Google Scholar]