Abstract
Purpose
To compare the short term effects of bevacizumab and ranibizumab injections on the regression of corneal neovascularization (NV).
Methods
Sixteen eyes of 16 patients with corneal NV were randomly assigned for an injection with 2.5 mg of bevacizumab (group 1, n = 8) or 1 mg of ranibizumab (group 2, n = 8) through subconjunctival and intrastromal routes. The patients were prospectively followed-up for one month after the injections. Corneal NV areas, as shown on corneal slit-lamp photographs stored in JPEG format, were calculated using Image J software before the injection, one week after the injection, and one month after the injection. The corneal NV areas were compared before and after the injections.
Results
Seven women and nine men, with an average age of 51 years, presented with corneal NV secondary to herpetic keratitis (7 cases), graft rejection (6), chemical burn (1), pemphigoid (1), and recurrent ulcer (1). In group I, the preoperative corneal NV area (8.75 ± 4.33%) was significantly decreased to 5.62 ± 3.86% one week after the injection and to 6.35 ± 3.02% one month after the injection (p = 0.012, 0.012, respectively). The corneal NV area in group 2 also exhibited a significant change, from 7.37 ± 4.33% to 6.72 ± 4.16% one week after the injection (p = 0.012). However, no significant change was observed one month after the injection. The mean decrease in corneal NV area one month after injection in group 1 (28.4 ± 9.01%) was significantly higher than in group 2 (4.51 ± 11.64%, p = 0.001).
Conclusions
Bevacizumab injection resulted in a more effective and stable regression of corneal NV compared to the ranibizumab injection. The potency and dose of these two drugs for the regression of corneal NV require further investigation.
Keywords: Bevacizumab, Corneal neovascularization, Ranibizumab
Vascular endothelial growth factor (VEGF) plays a key role in vasculogenesis and the pathologic neovascularization (NV) associated with eye disease [1-4]. Although anti-VEGF therapy for ocular disease has been principally directed at retinal vascular conditions, it is widely accepted that anti-VEGF therapy is also effective when used to treat corneal NV, with results from animal and human studies supporting this suggestion [5-11]. Targeted corneal NV has been induced in animals by chemical burning, suturing, application of a VEGF/bFBF pellet, or limbal injury, and anti-VEGF therapy has been used to treat human eyes with corneal NV associated with chemical burns, graft-versus-host disease, herpetic keratitis, limbal damage, ocular cicatricial pemphigoid, use of penetrating keratoplasty, and Stevens-Johnson syndrome. Various reports show that the use of anti-VEGF therapy is not restricted by disease etiology.
Currently, popular anti-VEGF agents include bevacizumab (Avastin; Genentech Inc., San Francisco, CA, USA; Roche, Basel, Switzerland) and ranibizumab (Lucentis; Genentech Inc.). These materials are humanized monoclonal antibodies directed toward VEGF. No Food and Drug Administration (FDA)-approved drug is yet available for treatment of corneal NV. Most prior studies have investigated the effects of bevacizumab rather than ranibizumab. However, it is notable that ranibizumab is the only agent approved by the FDA for intraocular use even though it is costly, especially considering that anti-VEGF therapy may need to be repeated [12,13]. Therefore, comparative work using both bevacizumab and ranibizumab therapy is necessary.
In the present study, we evaluated the short term effects of bevacizumab and ranibizumab on the regression of corneal NV and compared these effects in patients with corneal NV caused by various ocular surface disorders.
Materials and Methods
Sixteen eyes of 16 patients with chronic corneal NV greater than six months in duration were enrolled in this prospective study. The eyes were randomly divided into a group of eight eyes of eight patients treated by bevacizumab injection (group 1) and eight eyes of eight patients receiving ranibizumab injection (group 2). The patients included in this study had no other ocular diseases and no history of prior treatment for corneal NV within three months prior to the study. Written informed consent was obtained from all the patients prior to study enrollment. The work was approved by the institutional review board of Inje University and was conducted in accordance with the Declaration of Helsinki.
Under topical anesthesia, subconjunctival and intrastromal injections, delivered adjacent to the areas of the corneal NV, were administered to both groups. Group 1 patients received 0.1 mL (2.5 mg equivalent to the intravitreal dose) of bevacizumab (Avastin), while 0.1 mL (1 mg being the same dose approved for intravitreal usage) of ranibizumab (Lucentis) was administered to the patients in group 2. No other medication, except for topical antibiotics, was given over the next three days.
The means and standard deviations of uncorrected visual acuity (UCVA) and best-corrected visual acuity (BCVA) in logarithm of minimum angle resolution (logMAR) were measured before and after the injection in all patients. Preoperative corneal photographs of each eye were taken at 1 : 1.8 magnification using a digital camera (×4.0 zoom; E4500, Nikon, Tokyo, Japan) under slit-lamp examination at ×10 magnification and were stored in JPEG format (4.0 megapixels/image). The corneal NV area (as a percentage of the entire corneal area) was calculated using Image J software (Wayne Rasband of the Research Services Branch, National Institute of Mental Health, Bethesda, MD, USA). For objectivity, a double-blind method using two observers was used to measure the areas and verify the interobserver agreement. One observer measured the area three times and verified the intraobserver agreement. To calculate the increase or decrease in NV area, we measured the entire cornea area from a picture taken under the same digital camera settings and slit lamp magnification. Under the condition of higher slit lamp magnification, we measured the NV area by tracing the boundaries of the vessels including all visible branches (Fig. 1). The length of corneal NV was considered to be the distance from the limbal vascular plexus to the leading point of the new vessel. The width of corneal NV was considered to be the diameter including sprouting vessels around the suture. We then calculated the ratio between the entire cornea and NV area using slit lamp magnification. We converted the area of the entire cornea and NV into pixel counts using Image J software. The menu option 'Analyze→Measure' was selected, the values were recorded, and the process was repeated on subsequent images. The 'Set Scale' function in Image J was used to convert pixel numbers to standard units [14].
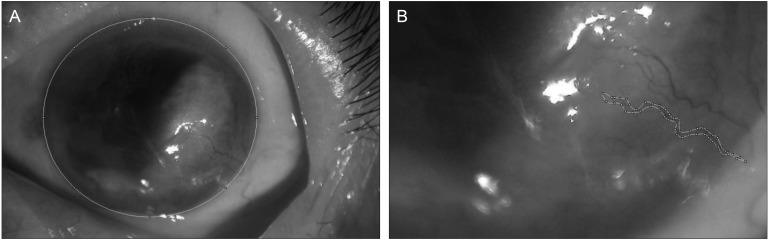
Fig. 1.
The measurement of corneal neovascularization (NV) area using the Image J program. We measured the area of the entire cornea (A) and the area of the new corneal vessels with its branches (B). The ratio of NV area was then calculated as a percentage of the entire corneal area.
The corneal NV areas one week and one month after injection were estimated using the same method and were compared to the preoperative NV areas. Each decrease in NV area from baseline was calculated as a percentage.
Routine clinical examination was performed to detect any side-effects occurring after the procedure. Statistical analysis was performed using SPSS version 19.0 for Windows (SPSS Inc., Chicago, IL, USA). Comparisons between groups 1 and 2 were achieved with the use of the Mann-Whitney U-test. The change of corneal NV area with time in each group was analyzed using the Friedman test and the Wilcoxon signed-rank test. A p-value ≤0.05 was considered statistically significant.
Results
The patients included seven women and nine men, and each group was composed of eight patients that were randomly assigned. The average patient age was 51.06 ± 15.55 years (mean ± standard deviation; range, 21 to 82 years), and there was no age difference between groups 1 and 2 (p = 0.328; Mann-Whitney U-test). Seven eyes had corneal NV secondary to herpetic keratitis, six eyes had graft rejection, and one eye each experienced chemical burn, pemphigoid, and recurrent sterile ulcers from secondary Sjogren's syndrome (Table 1). The visual acuity in logMAR was measured in both groups before and after the injections. The UCVA and BCVA were 0.79 ± 0.51, 0.64 ± 0.40 in group 1 and 0.81 ± 0.76, 0.78 ± 0.79 in group 2. There was no difference in UCVA or BCVA between groups 1 and 2 (p = 0.721, p = 0.878; Mann-Whitney U-test). The mean change of visual acuity did not show an improvement in either group after the injections (p > 0.05 in all tests; Wilcoxon signed-rank test) (Table 2).
Table 1.
Baseline characteristics of patients receiving a bevacizumab or ranibizumab injection for corneal neovascularization
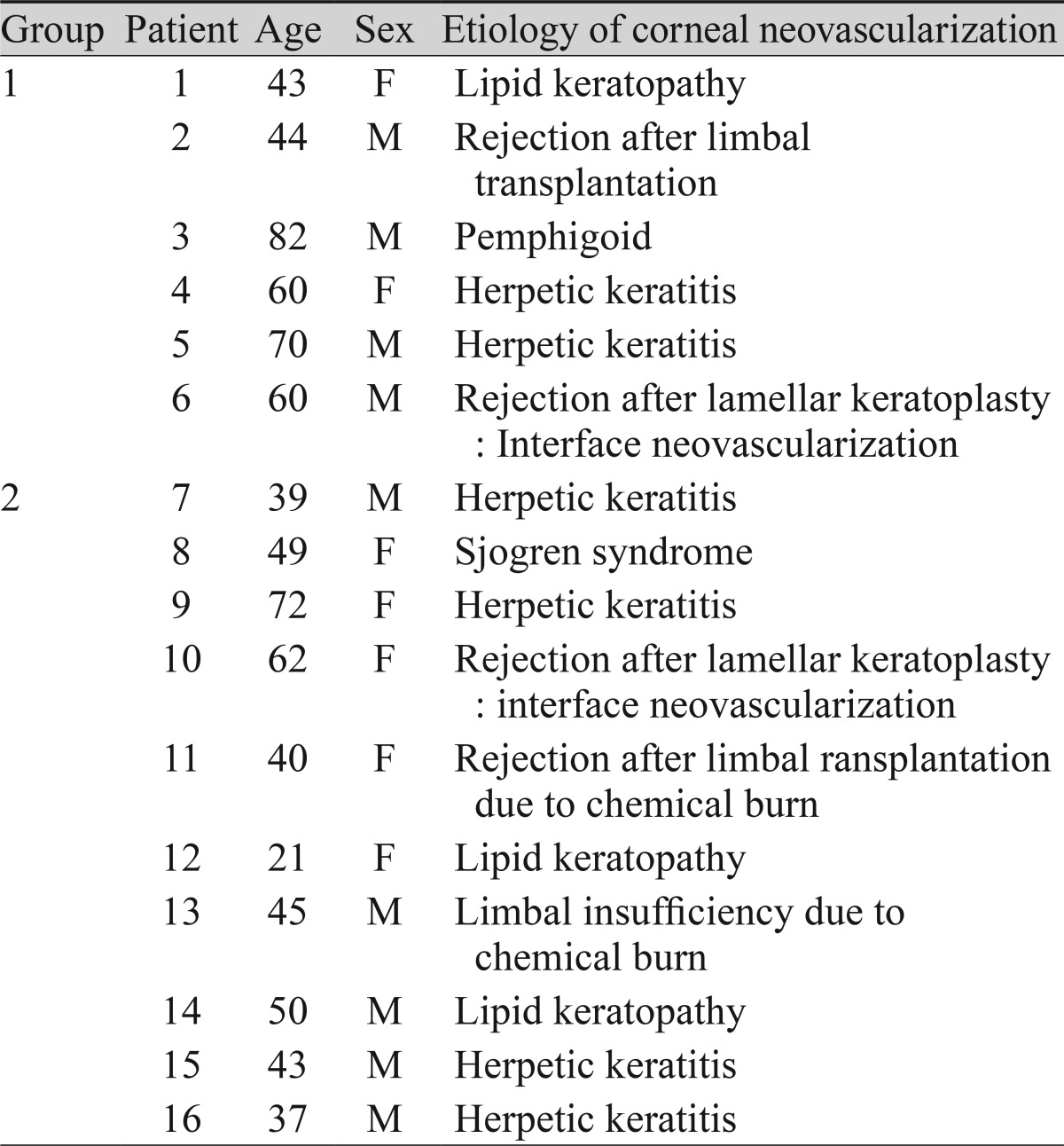
Table 2.
The visual acuity of patients receiving a bevacizumab or ranibizumab injection for corneal neovascularization
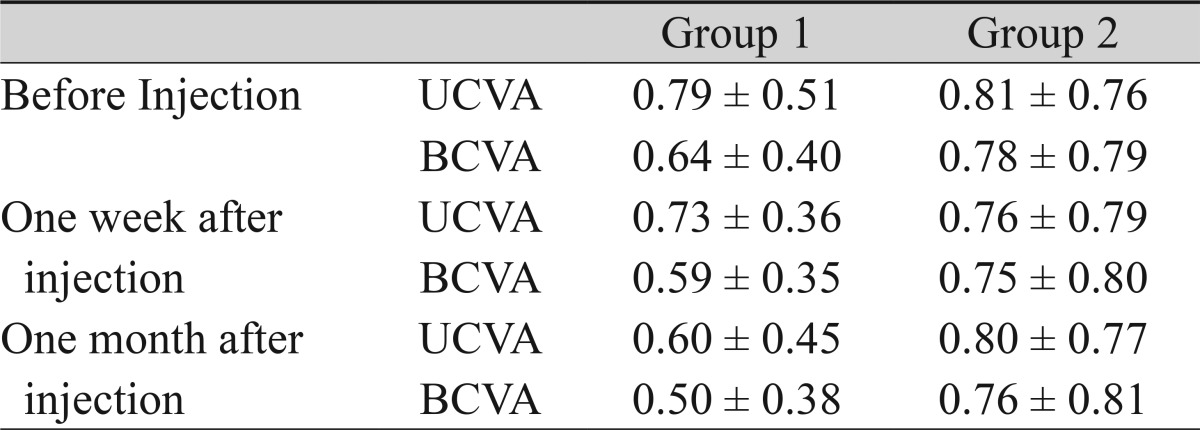
Mean ± standard deviation of visual acuity in logarithm of minimum angle resolution.
UCVA = uncorrected visual acuity; BCVA = best-corrected visual acuity.
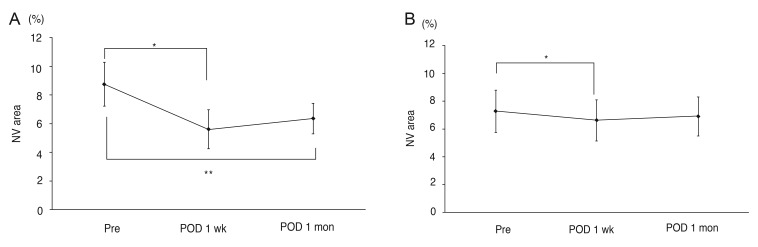
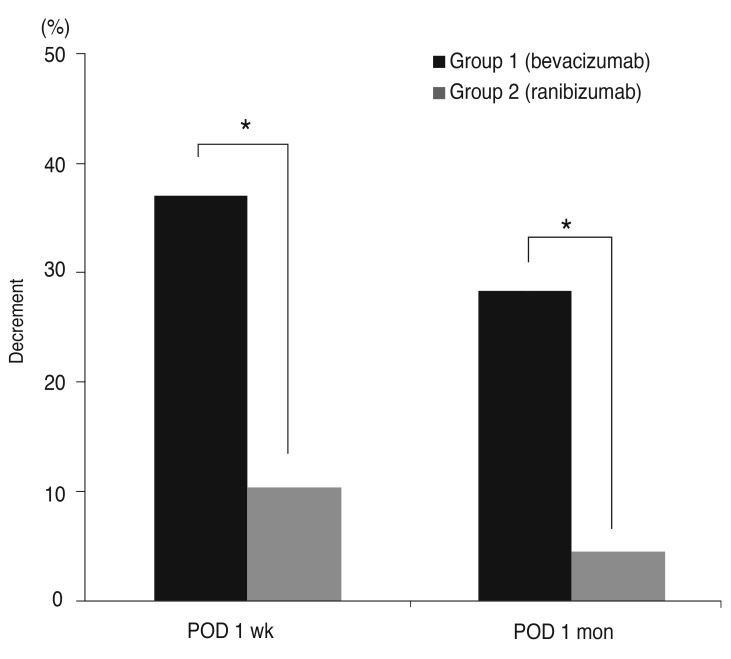
The areas of corneal NV did not differ between the two groups before the injections (p = 0.505; Mann-Whitney U-test). In both groups 1 and 2, significant differences were seen in the average areas of corneal NV with time (p = 0.002, p = 0.01, respectively; Friedman's test). However, the group responses differed in detail. In group 1, the preoperative corneal NV area (8.75 ± 4.33%, mean ± standard deviation) decreased significantly to 5.62 ± 3.86% one week after the injection and to 6.35 ± 3.02% one month after the injection (p = 0.012, p = 0.012, respectively; Wilcoxon signed-rank test) (Fig. 2A). The corneal NV area of group 2 patients also exhibited a significant change from 7.37 ± 4.33% preoperatively to 6.72 ± 4.16% one week after the injection (p = 0.012; Wilcoxon signed-rank test). However, no significant change was evident one month after the injection (7.02 ± 3.99%, p = 0.327, Wilcoxon signed-rank test) compared to the pre-injection area (Fig. 2B). The corneal NV area in group 1 decreased by 36.97 ± 27.34% (mean ± standard deviation) one week after the injection and by 28.4 ± 9.01% after one month. In group 2, decreases of 10.40 ± 4.83% and 4.51 ± 11.64% were apparent one week and one month after the injection, respectively. The average decreases were significantly greater in group 1 than in the group 2 patients at one week and one month after the injection (p = 0.003, p = 0.001, respectively; Mann-Whitney test) (Fig. 3).
Fig. 2.
Areas of corneal neovascularization (NV; mean ± standard error, % value) in group 1 patients who received bevacizumab injections (A) and group 2 patients who received ranibizumab injections (B) before and after treatment. Pre, before injection; postoperative day (POD) 1 week, one week after injection; POD 1 month, one month after injection. (A) Group 1, *p = 0.012, **p = 0.012 by the Wilcoxon signed-rank test. (B) Group 2, *p = 0.012 by the Wilcoxon signed-rank test. A p-value <0.05 was considered statistically significant.
Fig. 3.
A histogram showing a significant difference in the decrease in corneal neovascularization between group 1 (bevacizumab) and group 2 (ranibizumab) patients after treatment. postoperative day (POD) 1 week, one week after injection; POD 1 month, one month after injection. *p = 0.003 in POD 1 week, *p = 0.001 in POD 1 month; Mann-Whitney test. *A p-value <0.05 was considered statistically significant.
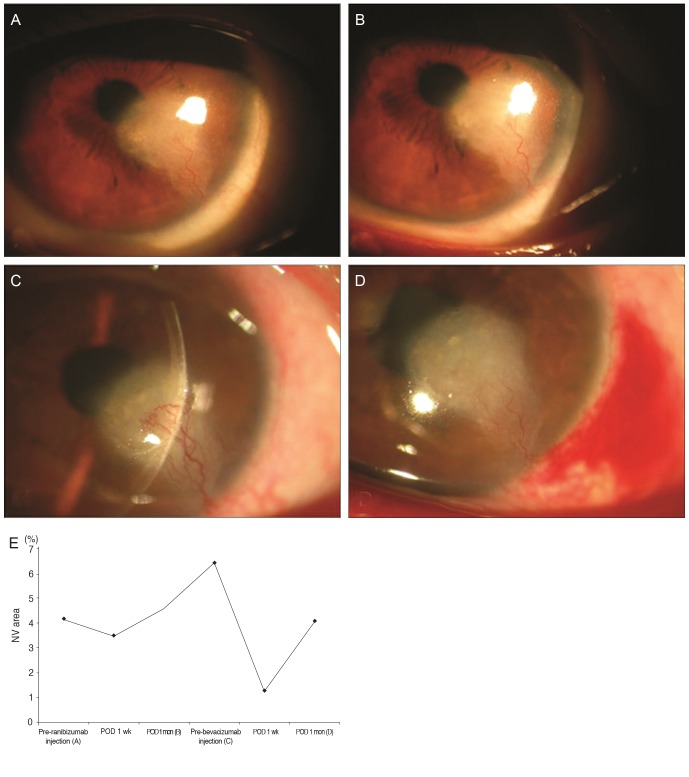
After providing informed consent, five patients in group 2 (numbers 9, 11, 12, 13, and 16) (Table 1) received bevacizumab injections two months after completion of the present study because they had experienced no notable regression of corneal NV, while one patient's condition worsened (patient 12) despite the ranibizumab injections one month after initial therapy. All the patients exhibited good responses to the bevacizumab injection. Fig. 4 shows data from patient 12 and highlights the effect of bevacizumab injection on the regression of corneal NV. Two patients with limbal insufficiency (patients 11 and 13) showed persistent epithelial defects with corneal melting after the bevacizumab injection even though they had a remarkable regression of corneal NV. No other complications were seen.
Fig. 4.
Slit-lamp photographs of the cornea of patient 12, who failed to show regression of corneal neovascularization (NV) after ranibizumab injection but demonstrated measurable regression after bevacizumab injection. The associated graph and photographs show changes in the area of corneal neovascularization (% values) after each treatment. Pre-ranibizumab injection (A): before ranibizumab injection ([A] shows the slit-lamp image obtained at that time). Postoperative day (POD) 1 week: one week after ranibizumab injection. POD 1 month (B): one month after ranibizumab injection ([B] shows the slit-lamp image obtained at that time). Pre-bevacizumab injection (C): before bevacizumab injection, thus three months after the prior ranibizumab injection ([C] shows the aggravation of corneal NV in the slit-lamp image obtained at that time). POD 1 week: one week after bevacizumab injection. POD 1 month (D): one month after bevacizumab injection ([D] shows the remarkable regression of corneal NV in the slit-lamp image obtained at that time). (E) The graph shows changes in the area of corneal neovascularization (% values) after each treatment.
Discussion
The present study showed that subconjunctival and intrastromal injections of either bevacizumab or ranibizumab were effective in achieving regression of corneal NV shortly after therapy (one week). Group 1 patients, who received bevacizumab injections, exhibited greater regression (a 36.97% decrease) one week after injection. This improvement was relatively long-lasting, up to one month, despite no repeat injections, considering the recommendation from the manufacturer that additional intraocular injections should be given at one-month intervals for the treatment of retinal disease. In contrast, the ranibizumab injections that were given to patients in group 2 were less effective and shorter acting. The decrease in the extent of corneal NV one week after the injection was significantly lower (10.40%) than in group 1 and was not maintained up to one month, at which time the extent of corneal NV did not differ from that at baseline.
Both bevacizumab and ranibizumab were constructed from full-length murine anti-VEGF IgG A.4.6.1, sharing many essential properties with anti-VEGF agents [15,16]. However, differences in clinical efficacy can arise from variations in molecular size, drug development in different cell lines, drug affinity for VEGF, and drug formulation [12,13,15]. Bevacizumab is a 149 kDa full-length IgG1 antibody composed of two 214-residue light chains and two 453-residue heavy chains. Ranibizumab is a 48 kDa Fab from the expression plasmid pY0317 [17]. Ranibizumab and bevacizumab are both able to bind to all human VEGF-A isoforms. On a molar basis, ranibizumab was determined to be five- to 20-fold more potent than full-length bevacizumab with respect to binding to VEGF-A [15,18]. Affinity-matured ranibizumab may provide better VEGF inhibition compared with bevacizumab through stronger molecular binding. On the other hand, the half-life of bevacizumab, estimated to be approximately 20 days, is longer than ranibizumab, estimated to be approximately six hours. Therefore, bevacizumab may remain in the eye for a longer period of time as a result of its larger molecular size [19].
Recent studies using porcine retina-retinal pigment epithelium (RPE)-choroid organ culture and RPE cell culture or human endothelial cell culture compared the in vitro effects of these two drugs [20,21]. It has been demonstrated that, at clinical doses, ranibizumab and bevacizumab are equally potent in neutralizing VEGF. In addition, a randomized clinical study showed no difference in the efficacy of ranibizumab versus that of bevacizumab. Therefore, the potency of both drugs was comparable in regressing choroidal neovascularization in pathologic myopia (CNVM) [22]. Since there has been no comparative study about the potency of the two drugs for the regression of corneal NV, this study used the same dose for the intravitreal injections. However, from this study, the potency of the two drugs does not seem to have the same effect in regressing corneal NV compared to CNVM.
The routes of injection may be a reason for this contrary result. Avisar et al. [23] compared the effect of bevacizumab in terms of the injection route and found that intracameral and intravitreal routes of injection were more effective than the subconjunctival route, but that the subconjunctival injection was associated with the earliest response peak. In the vitreal cavity, the vitreous body is in contact with many retinal and choroidal vessels. However, the cavity is a closed space and may trap an agent prior to its leakage out of the eye into circulation. Therefore, the need for diffusion through the ciliary body epithelium means that the region of corneal NV is not immediately accessed. The subconjunctival application of an anti-VEGF agent affords good proximity to any region of corneal NV and to a characteristic space containing abundant subconjunctival vessels, lymphatics, and a great deal of areolar tissue. As a result, clearance from the subconjunctival space may occur much faster than after injection into the intravitreal cavity. As a result, it is possible that a higher dose is required when administering through the subconjunctival space compared to an intravitreal injection. This is especially important if the drug is of low molecular weight or has a short half-life, like ranibizumab.
In addition, the shorter-acting effect of ranibizumab in the present study may be explained by the difference in half-life. The half-life of bevacizumab following intravitreal injection in an animal model is 4.32 days, while the halflife of ranibizumab is 2.88 days [24,25].
Two previous studies using ranibizumab with different results suggest that the injection dose is a main factor in achieving a good result [11,26]. Mansour [11] described one patient exhibiting a prompt regression of conjunctival microvessels in the pterygial bed after a single subconjunctival injection of ranibizumab (1 mg) and no recurrence even after 13 months. However, Mandalos et al. [26] found that subconjunctival ranibizumab at a single dose of 0.3 mg had no effect on the vascularization extent of the primary pterygium. This suggests that the use of an insufficient dose resulted in a failure of vascularization regression even though no comparative study regarding the dose-related effect of ranibizumab has been performed. Ahmed et al. [7] reported a dose-dependent effect in a study where two groups were treated with 5 or 10 mg of subconjunctival bevacizumab in rabbits. You et al. [27] compared the effects of 1.25 mg/0.05 mL, 2.5 mg/0.1 mL, and 5.0 mg/0.2 mL of bevacizumab in patients with corneal NV. The efficacy of corneal NV regression correlated with the injection dose, and there were no significant changes in the areas of the eyes injected with 1.25 mg bevacizumab. Although the suggested dose used by You et al. [27] was not always consistent with other studies, it may be sufficient to suggest that a higher injection dose of ranibizumab needs to be considered to acquire a satisfactory result [12,16,28]. In addition, the effect of the anti-VEGF agents was dependent on time of treatment after the onset of NV. Papathanassiou et al. [5] concluded that early subconjunctival bevacizumab administration (one day after injury) inhibited corneal NV more effectively in a rabbit experimental model of limbal insufficiency than when treatment was performed on day 14. Recently, Lin et al. [29] demonstrated that the earlier the time of treatment with subconjunctival bevacizumab, the better the result in rabbit eyes with limbal insufficiency. In the cited study, treatment was performed immediately (early group), at one week (mid-group), and one month after injury (late group). Early and mid-point bevacizumab injections inhibited epithelial alteration, the development of stromal opacity, and corneal NV manifestation as limbal insufficiency, while late treatment had no inhibitory effect. The cited authors suggested that vessels may mature in chronic NV, and pericytes may be recruited to the region around the area of pathologic NV [30]. Such coverage may decrease the effect of bevacizumab on the regression of newly formed immature vessels. However, although we assessed eyes that had chronic corneal NV for longer than six months in duration, we found measurable regression. Nevertheless, the mechanism suggested by the cited authors may explain why no complete resolution of corneal NV has been reported to date.
Five eyes refractory to ranibizumab therapy showed remarkable reductions in the extent of corneal NV after subsequent bevacizumab injections. The results shown in Fig. 4 indicate that the different effects are not due to the different nature of corneal NV in each case or to the unique characteristics of each patient. Therefore, in cases refractory to ranibizumab, altering the drug to bevacizumab or increasing the dose of ranibizumab is a good alternative.
From the viewpoint of safety, as seen in the present study, complications of ranibizumab injections have been rare. Galor et al. [31] found that subconjunctival injection of ranibizumab in conjunction with pterygium surgery was well tolerated. However, use of topical bevacizumab may negatively affect the epithelium and delay wound healing [32,33]. Clinically, the potential side effects of topical bevacizumab use including epitheliopathy, the development of epithelial defects, and stromal melting have been reported [9,34]. In the present study, subconjunctival bevacizumab injections resulted in the development of persistent epithelial defects with corneal melting in two patients within two weeks. The common feature of the four patients who suffered devastating side effects, in this and previous reports, was limbal deficiency induced by a chemical burn [9,34]. Therefore, the application of bevacizumab should be carefully considered, especially in patients with chemical burn-induced diffuse limbal deficiency, because such treatment may aggravate both the signs and symptoms of such deficiencies.
The limitation of this work is that only a small number of patients were enrolled. Therefore, the effect of preoccupying disease entities on the response to the different anti-VEGF drugs have yet to be verified even though five eyes in this study, which were refractory to ranibizumab but showed good response to bevacizumab, did not have common preoccupying pathology. Our results must therefore be regarded as preliminary. Additional larger studies including the evaluation of factors affecting the different responses of anti-VEGF drugs such as the preoccupying disease entity or onset of new vessels should be performed. Our comparative data indicates that subconjunctival and intrastromal bevacizumab injections result in a more effective and stable regression of corneal NV compared to ranibizumab. This implies that further research about the potency of the two drugs in regressing corneal NV is needed, and that the dosage adjustment for each drug is important in order to obtain good clinical results.
Acknowledgements
This study was supported by an Inje University research grant.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Chang JH, Gabison EE, Kato T, Azar DT. Corneal neovascularization. Curr Opin Ophthalmol. 2001;12:242–249. doi: 10.1097/00055735-200108000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Philipp W, Speicher L, Humpel C. Expression of vascular endothelial growth factor and its receptors in inflamed and vascularized human corneas. Invest Ophthalmol Vis Sci. 2000;41:2514–2522. [PubMed] [Google Scholar]
- 3.Cursiefen C, Rummelt C, Kuchle M. Immunohistochemical localization of vascular endothelial growth factor, transforming growth factor alpha, and transforming growth factor beta1 in human corneas with neovascularization. Cornea. 2000;19:526–533. doi: 10.1097/00003226-200007000-00025. [DOI] [PubMed] [Google Scholar]
- 4.Chen WL, Lin CT, Lin NT, et al. Subconjunctival injection of bevacizumab (avastin) on corneal neovascularization in different rabbit models of corneal angiogenesis. Invest Ophthalmol Vis Sci. 2009;50:1659–1665. doi: 10.1167/iovs.08-1997. [DOI] [PubMed] [Google Scholar]
- 5.Papathanassiou M, Theodossiadis PG, Liarakos VS, et al. Inhibition of corneal neovascularization by subconjunctival bevacizumab in an animal model. Am J Ophthalmol. 2008;145:424–431. doi: 10.1016/j.ajo.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Yoeruek E, Ziemssen F, Henke-Fahle S, et al. Safety, penetration and efficacy of topically applied bevacizumab: evaluation of eyedrops in corneal neovascularization after chemical burn. Acta Ophthalmol. 2008;86:322–328. doi: 10.1111/j.1600-0420.2007.01049.x. [DOI] [PubMed] [Google Scholar]
- 7.Ahmed A, Berati H, Nalan A, Aylin S. Effect of bevacizumab on corneal neovascularization in experimental rabbit model. Clin Experiment Ophthalmol. 2009;37:730–736. doi: 10.1111/j.1442-9071.2009.02112.x. [DOI] [PubMed] [Google Scholar]
- 8.Carrasco MA. Subconjunctival bevacizumab for corneal neovascularization in herpetic stromal keratitis. Cornea. 2008;27:743–745. doi: 10.1097/QAI.0b013e31815b833a. [DOI] [PubMed] [Google Scholar]
- 9.Kim SW, Ha BJ, Kim EK, et al. The effect of topical bevacizumab on corneal neovascularization. Ophthalmology. 2008;115:e33–e38. doi: 10.1016/j.ophtha.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Awadein A. Subconjunctival bevacizumab for vascularized rejected corneal grafts. J Cataract Refract Surg. 2007;33:1991–1993. doi: 10.1016/j.jcrs.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 11.Mansour AM. Treatment of inflamed pterygia or residual pterygial bed. Br J Ophthalmol. 2009;93:864–865. doi: 10.1136/bjo.2008.155291. [DOI] [PubMed] [Google Scholar]
- 12.Steinbrook R. The price of sight: ranibizumab, bevacizumab, and the treatment of macular degeneration. N Engl J Med. 2006;355:1409–1412. doi: 10.1056/NEJMp068185. [DOI] [PubMed] [Google Scholar]
- 13.Rosenfeld PJ. Intravitreal avastin: the low cost alternative to lucentis. Am J Ophthalmol. 2006;142:141–143. doi: 10.1016/j.ajo.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 14.Tang XN, Berman AE, Swanson RA, Yenari MA. Digitally quantifying cerebral hemorrhage using Photoshop and Image J. J Neurosci Methods. 2010;190:240–243. doi: 10.1016/j.jneumeth.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrara N, Damico L, Shams N, et al. Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina. 2006;26:859–870. doi: 10.1097/01.iae.0000242842.14624.e7. [DOI] [PubMed] [Google Scholar]
- 16.Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- 17.Presta LG, Chen H, O’Connor SJ, et al. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 1997;57:4593–4599. [PubMed] [Google Scholar]
- 18.Chen Y, Wiesmann C, Fuh G, et al. Selection and analysis of an optimized anti-VEGF antibody: crystal structure of an affinity-matured Fab in complex with antigen. J Mol Biol. 1999;293:865–881. doi: 10.1006/jmbi.1999.3192. [DOI] [PubMed] [Google Scholar]
- 19.Stewart MW. Aflibercept (VEGF Trap-eye): the newest anti-VEGF drug. Br J Ophthalmol. 2012;96:1157–1158. doi: 10.1136/bjophthalmol-2011-300654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klettner A, Roider J. Comparison of bevacizumab, ranibizumab, and pegaptanib in vitro: efficiency and possible additional pathways. Invest Ophthalmol Vis Sci. 2008;49:4523–4527. doi: 10.1167/iovs.08-2055. [DOI] [PubMed] [Google Scholar]
- 21.Carneiro A, Falcao M, Pirraco A, et al. Comparative effects of bevacizumab, ranibizumab and pegaptanib at intravitreal dose range on endothelial cells. Exp Eye Res. 2009;88:522–527. doi: 10.1016/j.exer.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 22.Gharbiya M, Giustolisi R, Allievi F, et al. Choroidal neovascularization in pathologic myopia: intravitreal ranibizumab versus bevacizumab: a randomized controlled trial. Am J Ophthalmol. 2010;149:458–464.e1. doi: 10.1016/j.ajo.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 23.Avisar I, Weinberger D, Kremer I. Effect of subconjunctival and intraocular bevacizumab injections on corneal neovascularization in a mouse model. Curr Eye Res. 2010;35:108–115. doi: 10.3109/02713680903429007. [DOI] [PubMed] [Google Scholar]
- 24.Mordenti J, Cuthbertson RA, Ferrara N, et al. Comparisons of the intraocular tissue distribution, pharmacokinetics, and safety of 125I-labeled full-length and Fab antibodies in rhesus monkeys following intravitreal administration. Toxicol Pathol. 1999;27:536–544. doi: 10.1177/019262339902700507. [DOI] [PubMed] [Google Scholar]
- 25.Gaudreault J, Fei D, Rusit J, et al. Preclinical pharmacokinetics of Ranibizumab (rhuFabV2) after a single intravitreal administration. Invest Ophthalmol Vis Sci. 2005;46:726–733. doi: 10.1167/iovs.04-0601. [DOI] [PubMed] [Google Scholar]
- 26.Mandalos A, Tsakpinis D, Karayannopoulou G, et al. The effect of subconjunctival ranibizumab on primary pterygium: a pilot study. Cornea. 2010;29:1373–1379. doi: 10.1097/ICO.0b013e3181d927b9. [DOI] [PubMed] [Google Scholar]
- 27.You IC, Kang IS, Lee SH, Yoon KC. Therapeutic effect of subconjunctival injection of bevacizumab in the treatment of corneal neovascularization. Acta Ophthalmol. 2009;87:653–658. doi: 10.1111/j.1755-3768.2008.01399.x. [DOI] [PubMed] [Google Scholar]
- 28.Bahar I, Kaiserman I, McAllum P, et al. Subconjunctival bevacizumab injection for corneal neovascularization in recurrent pterygium. Curr Eye Res. 2008;33:23–28. doi: 10.1080/02713680701799101. [DOI] [PubMed] [Google Scholar]
- 29.Lin CT, Hu FR, Kuo KT, et al. The different effects of early and late bevacizumab (Avastin) injection on inhibiting corneal neovascularization and conjunctivalization in rabbit limbal insufficiency. Invest Ophthalmol Vis Sci. 2010;51:6277–6285. doi: 10.1167/iovs.09-4571. [DOI] [PubMed] [Google Scholar]
- 30.Cursiefen C, Hofmann-Rummelt C, Kuchle M, Schlotzer-Schrehardt U. Pericyte recruitment in human corneal angiogenesis: an ultrastructural study with clinicopathological correlation. Br J Ophthalmol. 2003;87:101–106. doi: 10.1136/bjo.87.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galor A, Yoo SH, Piccoli FV, et al. Phase I study of subconjunctival ranibizumab in patients with primary pterygium undergoing pterygium surgery. Am J Ophthalmol. 2010;149:926–931.e2. doi: 10.1016/j.ajo.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 32.Kim TI, Chung JL, Hong JP, et al. Bevacizumab application delays epithelial healing in rabbit cornea. Invest Ophthalmol Vis Sci. 2009;50:4653–4659. doi: 10.1167/iovs.08-2805. [DOI] [PubMed] [Google Scholar]
- 33.Kim EC, Lee WS, Kim MS. The inhibitory effects of bevacizumab eye drops on NGF expression and corneal wound healing in rats. Invest Ophthalmol Vis Sci. 2010;51:4569–4573. doi: 10.1167/iovs.09-4937. [DOI] [PubMed] [Google Scholar]
- 34.Galor A, Yoo SH. Corneal melt while using topical bevacizumab eye drops. Ophthalmic Surg Lasers Imaging. 2010 Mar 09; doi: 10.3928/15428877-20100215-07. [Epub]. DOI: 10.3928/15428877-20100215-07. [DOI] [PubMed] [Google Scholar]