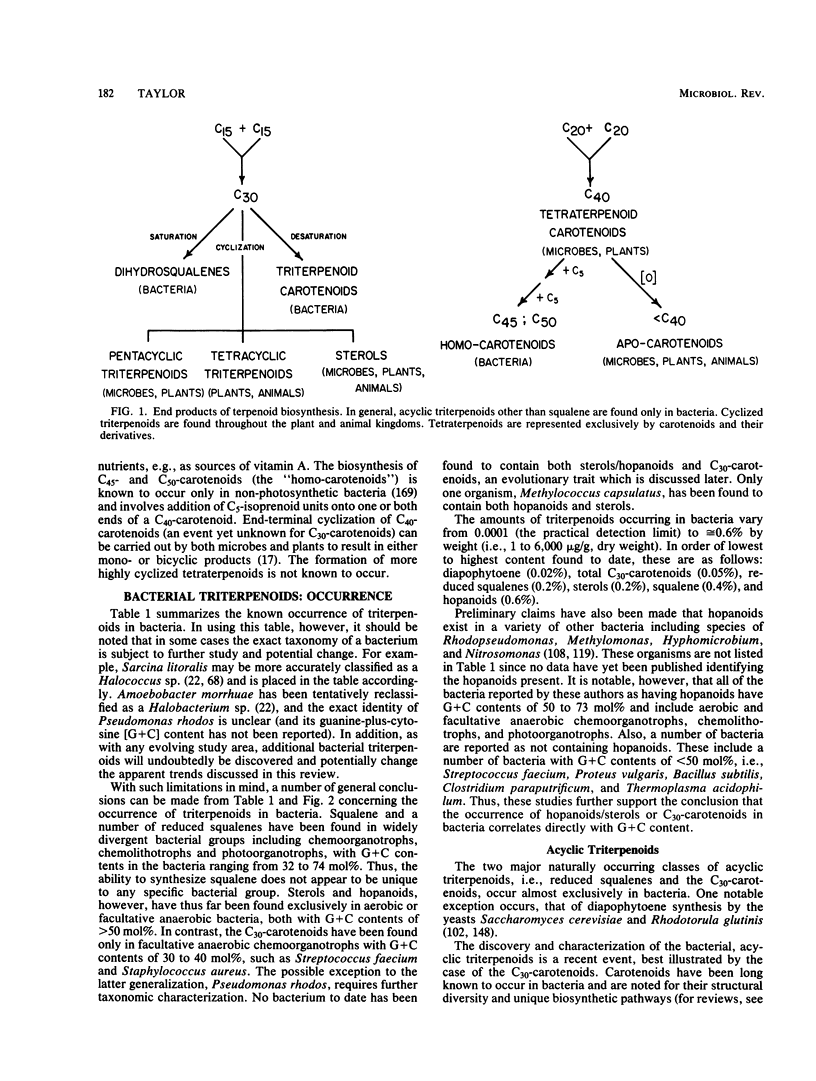
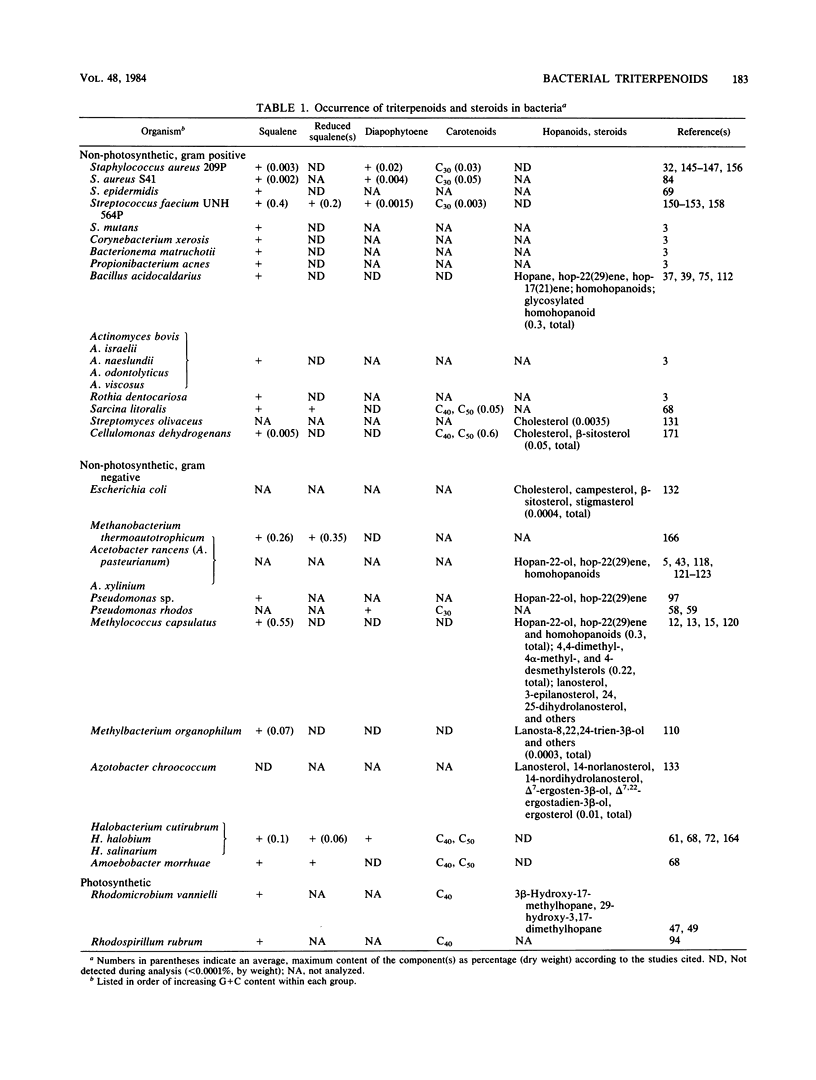
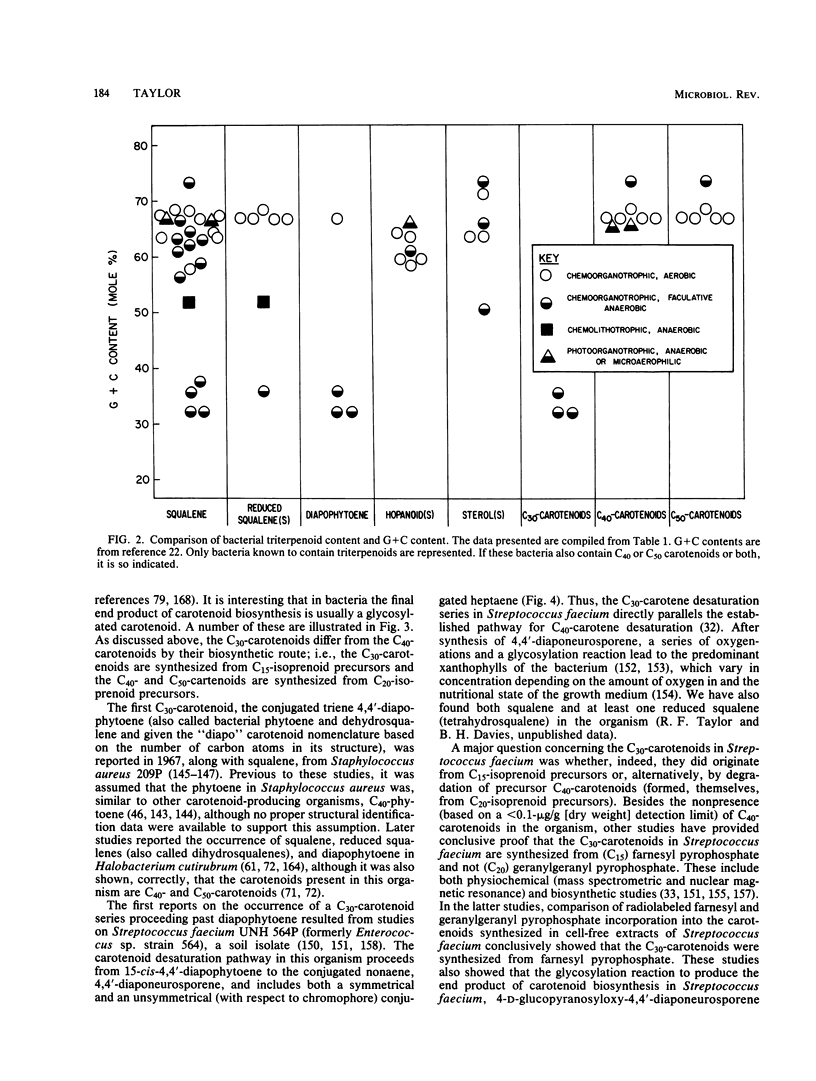
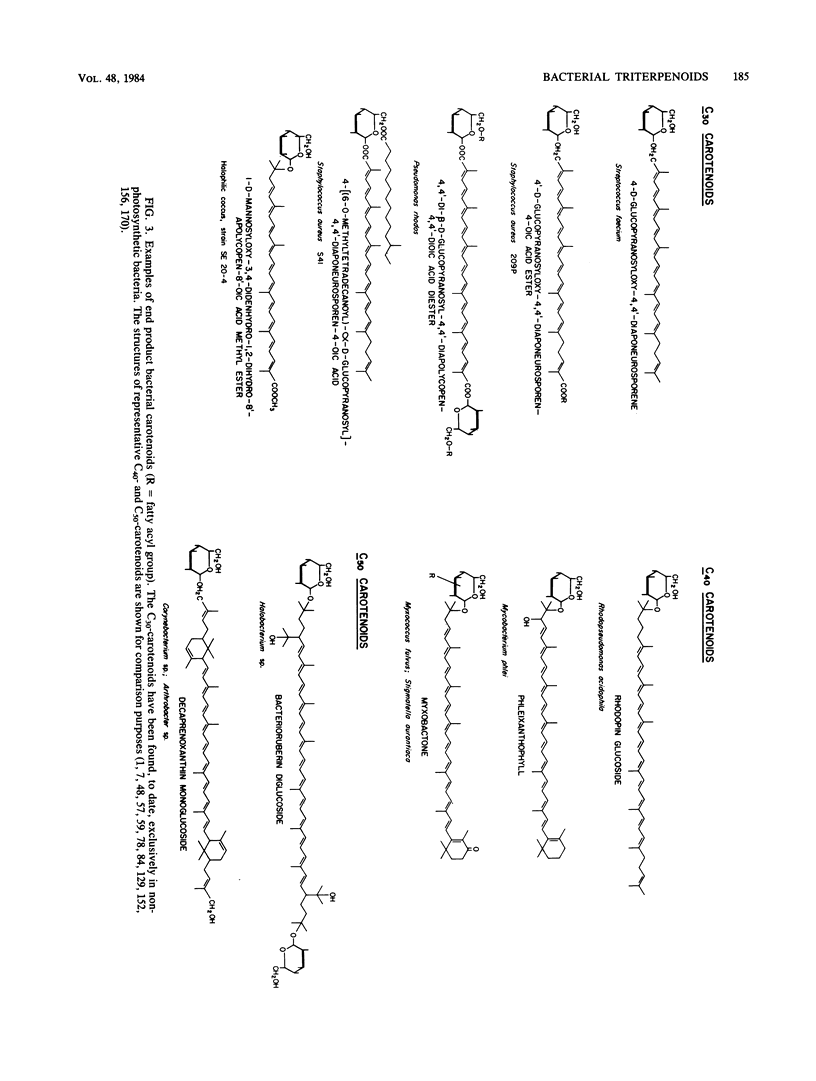
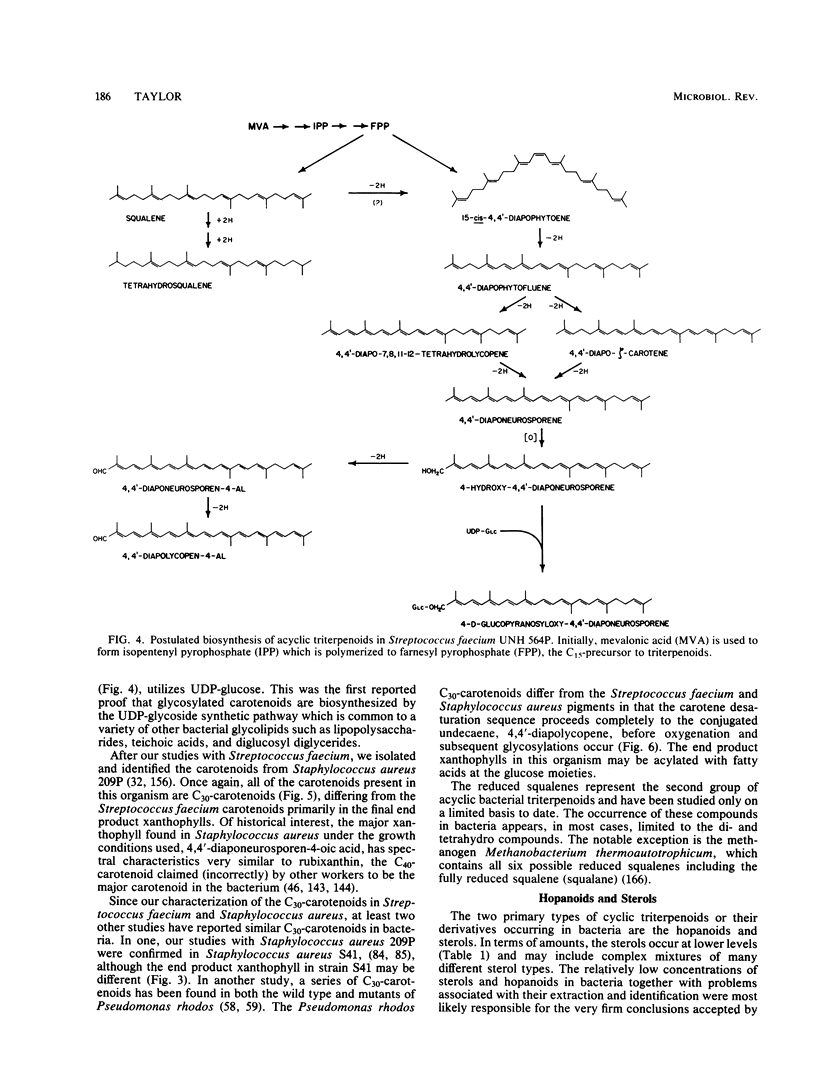
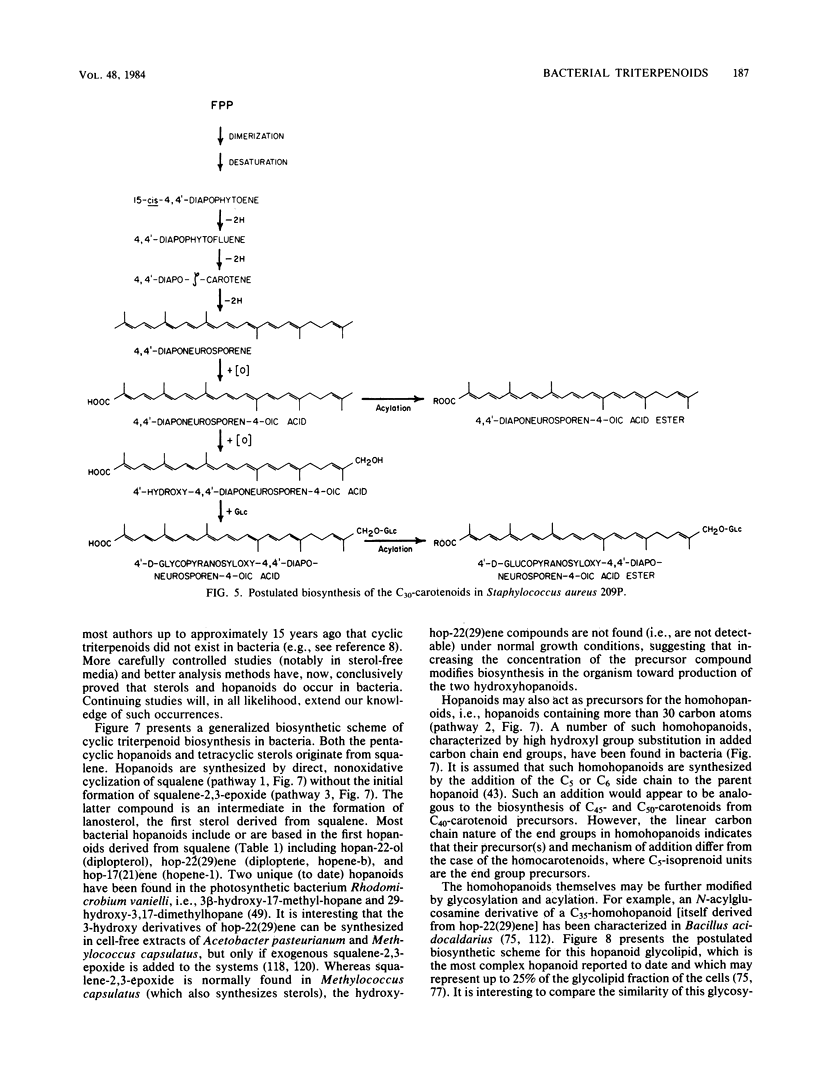
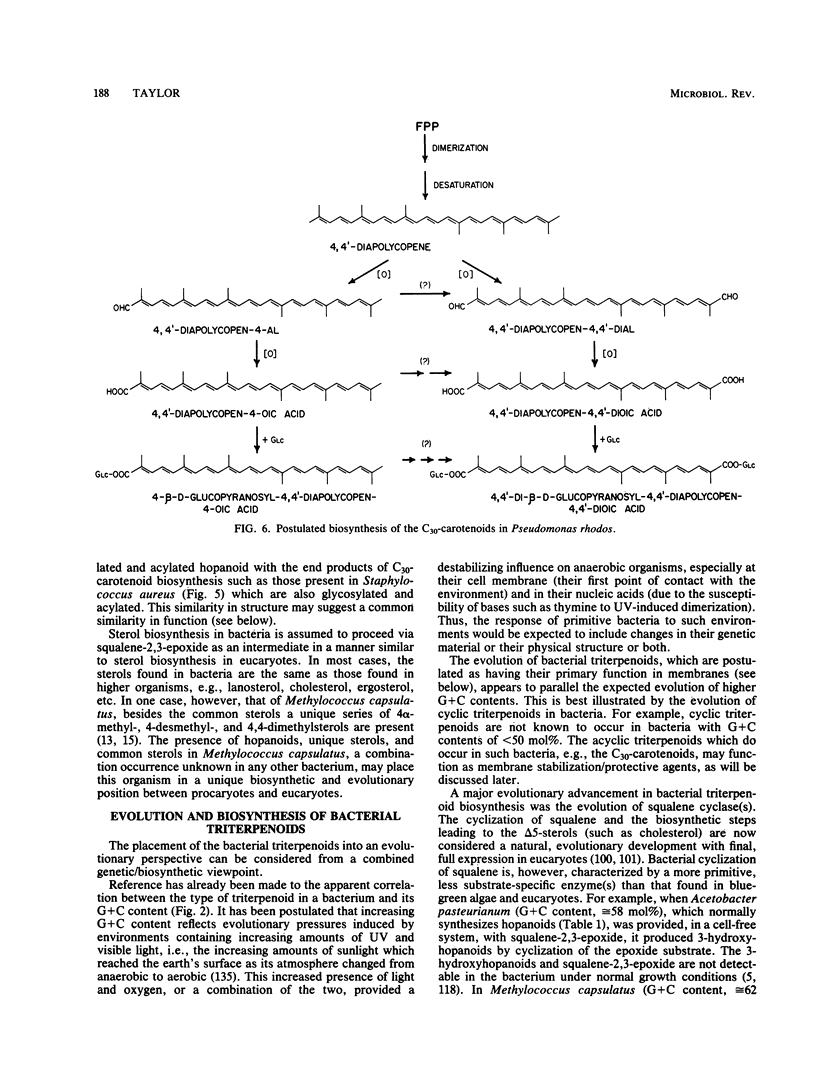
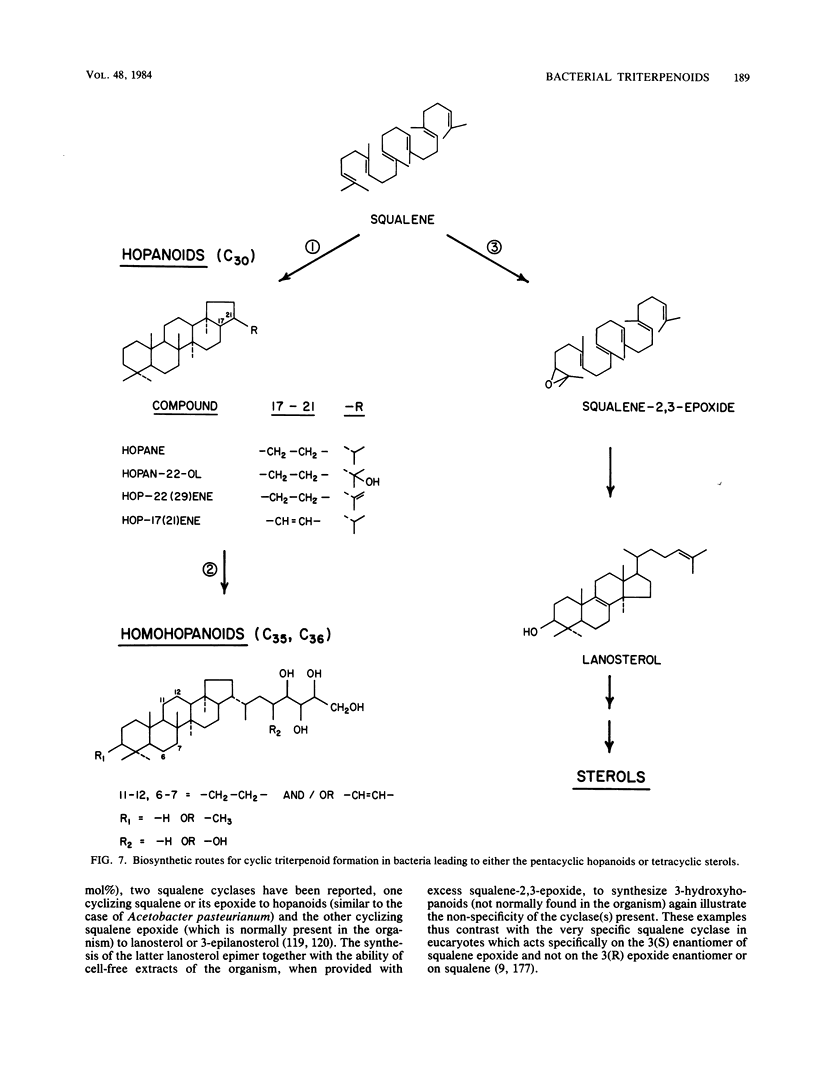
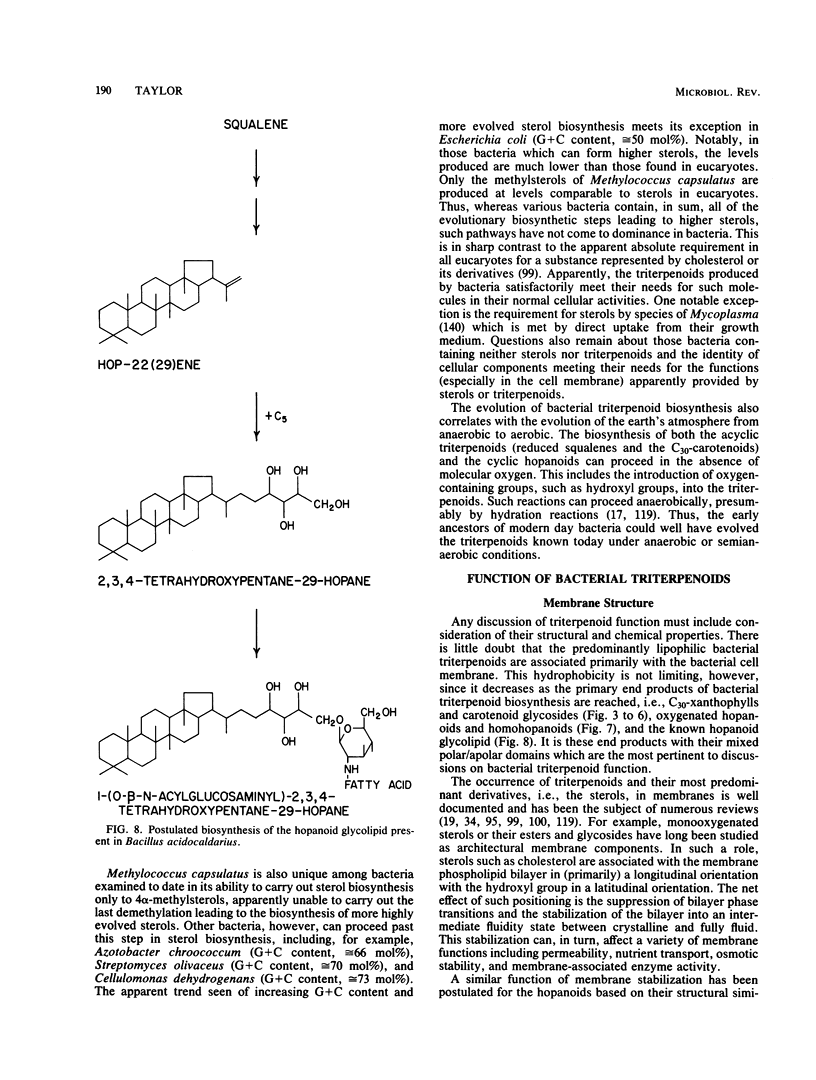
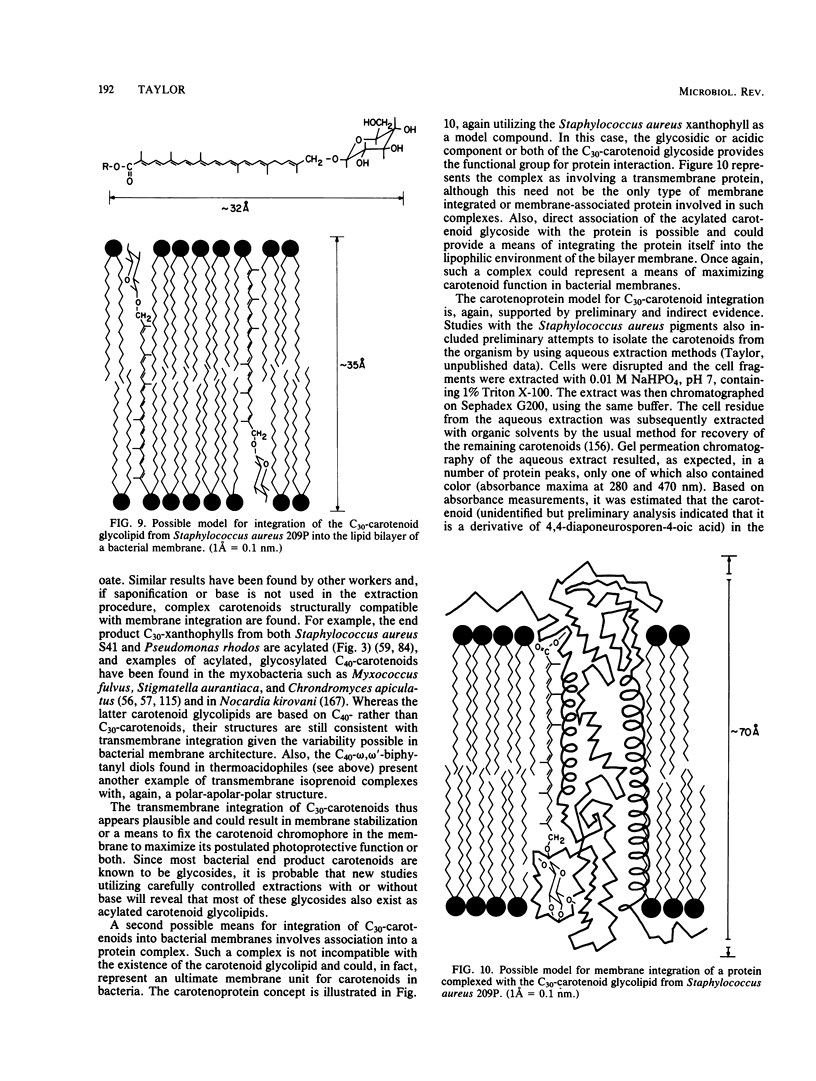
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amdur B. H., Szabo E. I., Socransky S. S. Presence of squalene in gram-positive bacteria. J Bacteriol. 1978 Jul;135(1):161–163. doi: 10.1128/jb.135.1.161-163.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S. M., Krinsky N. I., Stone M. J., Clagett D. C. Effect of singlet oxygen quenchers on oxidative damage to liposomes initiated by photosensitization or by radiofrequency discharge. Photochem Photobiol. 1974 Jul;20(1):65–69. doi: 10.1111/j.1751-1097.1974.tb06549.x. [DOI] [PubMed] [Google Scholar]
- Anding C., Rohmer M., Ourisson G. Letter: Nonspecific biosynthesis of hopane triterpenes in a cell-free system from Acetobacter rancens. J Am Chem Soc. 1976 Mar 3;98(5):1274–1275. doi: 10.1021/ja00421a045. [DOI] [PubMed] [Google Scholar]
- Anwar M., Khan T. H., Prebble J., Zagalsky P. F. Membrane-bound carotenoid in Micrococcus luteus protects naphthoquinone from photodynamic action. Nature. 1977 Dec 8;270(5637):538–540. doi: 10.1038/270538a0. [DOI] [PubMed] [Google Scholar]
- Arpin N., Fiasson J. L., Liaaen-Jensen A. Bacterial carotenoids. XXXIX. C 50 -carotenoids. 10. Bacterioruberin mono- and diglucoside. Acta Chem Scand. 1972;26(6):2526–2528. doi: 10.3891/acta.chem.scand.26-2526. [DOI] [PubMed] [Google Scholar]
- BRODIE A. F., BALLANTINE J. Oxidative phosphorylation in fractionated bacterial systems. III. Specificity of vitamin K reactivation. J Biol Chem. 1960 Jan;235:232–237. [PubMed] [Google Scholar]
- Barton D. H., Jarman T. R., Watson K. C., Widdowson D. A., Boar R. B., Damps K. Investigations on the biosynthesis of steroids and terpenoids. Part XII. Biosynthesis of 3beta-hydroxy-triterpenoids and -steroids from (3S)-2,3-epoxy-2,3-dihydrosqualene. J Chem Soc Perkin 1. 1975;(12):1134–1138. doi: 10.1039/p19750001134. [DOI] [PubMed] [Google Scholar]
- Bensasson R., Land E. J., Maudinas B. Triplet states of carotenoids from photosynthetic bacteria studied by nanosecond ultraviolet and electron pulse irradiation. Photochem Photobiol. 1976 Mar;23(3):189–193. doi: 10.1111/j.1751-1097.1976.tb07240.x. [DOI] [PubMed] [Google Scholar]
- Berns D. S. Photosensitive bilayer membranes as model systems for photobiological processes. Photochem Photobiol. 1976 Aug;24(2):117–139. doi: 10.1111/j.1751-1097.1976.tb06807.x. [DOI] [PubMed] [Google Scholar]
- Bird C. W., Lynch J. M., Pirt F. J., Reid W. W. Steroids and squalene in Methylococcus capsulatus grown on methane. Nature. 1971 Apr 16;230(5294):473–474. doi: 10.1038/230473a0. [DOI] [PubMed] [Google Scholar]
- Bisseret P., Wolff G., Albrecht A. M., Tanaka T., Nakatani Y., Ourisson G. A direct study of the cohesion of lecithin bilayers: the effect of hopanoids and alpha, omega-dihydroxycarotenoids. Biochem Biophys Res Commun. 1983 Jan 14;110(1):320–324. doi: 10.1016/0006-291x(83)91298-6. [DOI] [PubMed] [Google Scholar]
- Bouvier P., Rohmer M., Benveniste P., Ourisson G. Delta8(14)-steroids in the bacterium Methylococcus capsulatus. Biochem J. 1976 Nov;159(2):267–271. doi: 10.1042/bj1590267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher M. S. Membrane structure: some general principles. Science. 1973 Aug 17;181(4100):622–629. doi: 10.1126/science.181.4100.622. [DOI] [PubMed] [Google Scholar]
- Brockerhoff H. Model of interaction of polar lipids, cholesterol, and proteins in biological membranes. Lipids. 1974 Sep;9(9):645–650. doi: 10.1007/BF02532169. [DOI] [PubMed] [Google Scholar]
- Broglie R. M., Hunter C. N., Delepelaire P., Niederman R. A., Chua N. H., Clayton R. K. Isolation and characterization of the pigment-protein complexes of Rhodopseudomonas sphaeroides by lithium dodecyl sulfate/polyacrylamide gel electrophoresis. Proc Natl Acad Sci U S A. 1980 Jan;77(1):87–91. doi: 10.1073/pnas.77.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchard R. P., Dworkin M. Light-induced lysis and carotenogenesis in Myxococcus xanthus. J Bacteriol. 1966 Feb;91(2):535–545. doi: 10.1128/jb.91.2.535-545.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLAES H. Interaction between chlorophyll and carotenes with different chromophoric groups. Biochem Biophys Res Commun. 1960 Dec;3:585–590. doi: 10.1016/0006-291x(60)90066-8. [DOI] [PubMed] [Google Scholar]
- CLAES H., NAKAYAMA T. O. [The photoxidative fading of chlorophyll in vitro in the presence of carotenes with various chromophoric groups]. Z Naturforsch B. 1959 Nov;14B:746–747. [PubMed] [Google Scholar]
- COHEN-BAZIRE G., STANIER R. Y. Specific inhibition of carotenoid synthesis in a photosynthetic bacterium and its physiological consequences. Nature. 1958 Jan 24;181(4604):250–252. doi: 10.1038/181250a0. [DOI] [PubMed] [Google Scholar]
- Caughey W. S., Smythe G. A., O'Keeffe D. H., Maskasky J. E., Smith M. I. Heme A of cytochrome c oxicase. Structure and properties: comparisons with hemes B, C, and S and derivatives. J Biol Chem. 1975 Oct 10;250(19):7602–7622. [PubMed] [Google Scholar]
- Comita P. B., Gagosian R. B. Membrane lipid from deep-sea hydrothermal vent methanogen: a new macrocyclic glycerol diether. Science. 1983 Dec 23;222(4630):1329–1331. doi: 10.1126/science.222.4630.1329. [DOI] [PubMed] [Google Scholar]
- DWORKIN M. Endogenous photosensitization in a carotenoidless mutant of Rhodopseudomonas speroides. J Gen Physiol. 1958 Jul 20;41(6):1099–1112. doi: 10.1085/jgp.41.6.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danon A., Stoeckenius W. Photophosphorylation in Halobacterium halobium. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1234–1238. doi: 10.1073/pnas.71.4.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies B. H., Taylor R. F. The biosynthesis of triterpenoid carotenoids in Streptococcus faecium UNH 564P. Can J Biochem. 1982 Jun;60(6):684–692. doi: 10.1139/o82-084. [DOI] [PubMed] [Google Scholar]
- De Rosa M., Gambacorta A., Minale L., Bu'lock J. D. The formation of -cyclohexyl-fatty acids from shikimate in an acidophilic thermophilic bacillus. A new biosynthetic pathway. Biochem J. 1972 Jul;128(4):751–754. doi: 10.1042/bj1280751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demel R. A., De Kruyff B. The function of sterols in membranes. Biochim Biophys Acta. 1976 Oct 26;457(2):109–132. doi: 10.1016/0304-4157(76)90008-3. [DOI] [PubMed] [Google Scholar]
- FULLER R. C., ANDERSON I. C. Suppression of carotenoid synthesis and its effect on the activity of photosynthetic bacterial chromatophores. Nature. 1958 Jan 24;181(4604):252–254. doi: 10.1038/181252a0. [DOI] [PubMed] [Google Scholar]
- Foote C. S., Chang Y. C., Denny R. W. Chemistry of singlet oxygen. X. Carotenoid quenching parallels biological protection. J Am Chem Soc. 1970 Aug 26;92(17):5216–5218. doi: 10.1021/ja00720a036. [DOI] [PubMed] [Google Scholar]
- Förster H. J., Biemann K., Haigh W. G., Tattrie N. H., Colvin J. R. The structure of novel C35 pentacyclic terpenes from Acetobacter xylinum. Biochem J. 1973 Sep;135(1):133–143. doi: 10.1042/bj1350133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond R. K., White D. C. Carotenoid formation by Staphylococcus aureus. J Bacteriol. 1970 Jul;103(1):191–198. doi: 10.1128/jb.103.1.191-198.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J., Calvin M. Hydrocarbon distribution of algae and bacteria, and microbiological activity in sediments. Proc Natl Acad Sci U S A. 1969 Oct;64(2):436–443. doi: 10.1073/pnas.64.2.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzberg S., Liaaen Jensen S. Bacterial carotenoids XX. The carotenoids of Mycobacterium phlei strain Vera. 2. The structures of the phlei-xanthophylls--two novel tertiary glucosides. Acta Chem Scand. 1967;21(1):15–41. [PubMed] [Google Scholar]
- Huang L., Haug A. Regulation of membrane lipid fluidity in Acholeplasma laidlawii: effect of carotenoid pigment content. Biochim Biophys Acta. 1974 Jun 29;352(3):361–370. doi: 10.1016/0005-2736(74)90228-4. [DOI] [PubMed] [Google Scholar]
- Hurst J. K. Evaluation of the role of polyisoprenyl functional groups in biological electron transfer. Transition metal models. Biochemistry. 1979 Apr 17;18(8):1504–1510. doi: 10.1021/bi00575a018. [DOI] [PubMed] [Google Scholar]
- KILBURN R. E., BELLAMY W. D., TERNI S. A. Studies on a radiation-resistant pigmented Sarcina sp. Radiat Res. 1958 Aug;9(2):207–215. [PubMed] [Google Scholar]
- Kleinig H., Reichenbach H., Achenbach H. Carotenoid pigments of Stigmatella aurantiaca (Myxobacterales). II. Acylated carotenoid glucosides. Arch Mikrobiol. 1970;74(3):223–234. doi: 10.1007/BF00408883. [DOI] [PubMed] [Google Scholar]
- Koltun W. L. Precision space-filling atomic models. Biopolymers. 1965 Dec;3(6):665–679. doi: 10.1002/bip.360030606. [DOI] [PubMed] [Google Scholar]
- Kramer J. K., Kushwaha S. C., Kates M. Structure ditermination of the squalene, dihydrosqualene and tetrahydrosqualene in Halobacterium cutirubrum. Biochim Biophys Acta. 1972 May 23;270(1):103–110. doi: 10.1016/0005-2760(72)90183-x. [DOI] [PubMed] [Google Scholar]
- Krinsky N. I., Deneke S. M. Interaction of oxygen and oxy-radicals with carotenoids. J Natl Cancer Inst. 1982 Jul;69(1):205–210. [PubMed] [Google Scholar]
- Kuhn H. Electron tunneling effects in monolayer assemblies. Chem Phys Lipids. 1972 May;8(4):401–404. doi: 10.1016/0009-3084(72)90071-0. [DOI] [PubMed] [Google Scholar]
- Kushwaha S. C., Gochnauer M. B., Kushner D. J., Kates M. Pigments and isoprenoid compounds in extremely and moderately halophilic bacteria. Can J Microbiol. 1974 Feb;20(2):241–245. doi: 10.1139/m74-038. [DOI] [PubMed] [Google Scholar]
- Kushwaha S. C., Kates M., Martin W. G. Characterization and composition of the purple and red membrane from Halobacterium cutirubrum;. Can J Biochem. 1975 Mar;53(3):284–292. doi: 10.1139/o75-040. [DOI] [PubMed] [Google Scholar]
- Kushwaha S. C., Kates M. Nonpolar lipids of a halotolerant species of Staphylococcus epidermidis. Can J Biochem. 1976 Jan;54(1):79–85. doi: 10.1139/o76-013. [DOI] [PubMed] [Google Scholar]
- Kushwaha S. C., Kramer J. K., Kates M. Isolation and characterization of C50-carotenoid pigments and other polar isoprenoids from Halobacterium cutirubrum. Biochim Biophys Acta. 1975 Aug 25;398(2):303–314. doi: 10.1016/0005-2760(75)90146-0. [DOI] [PubMed] [Google Scholar]
- Kushwaha S. C., Pugh E. L., Kramer J. K., Kates M. Isolation and identification of dehydrosqualene and C 40 -carotenoid pigments in Halobacterium cutirubrum. Biochim Biophys Acta. 1972 Mar 23;260(3):492–506. doi: 10.1016/0005-2760(72)90064-1. [DOI] [PubMed] [Google Scholar]
- Langworthy T. A., Mayberry W. R., Smith P. F. A sulfonolipid and novel glucosamidyl glycolipids from the extreme thermoacidophile Bacillus acidocaldarius. Biochim Biophys Acta. 1976 Jun 22;431(3):550–569. doi: 10.1016/0005-2760(76)90220-4. [DOI] [PubMed] [Google Scholar]
- Langworthy T. A., Mayberry W. R., Smith P. F. Long-chain glycerol diether and polyol dialkyl glycerol triether lipids of Sulfolobus acidocaldarius. J Bacteriol. 1974 Jul;119(1):106–116. doi: 10.1128/jb.119.1.106-116.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liaaen-Jensen S., Andrewes A. G. Microbial carotenoids. Annu Rev Microbiol. 1972;26:225–248. doi: 10.1146/annurev.mi.26.100172.001301. [DOI] [PubMed] [Google Scholar]
- MATHEWS M. M., SISTROM W. R. Intracellular location of carotenoid pigments and some respiratory enzymes in Sarcina lutea. J Bacteriol. 1959 Dec;78:778–787. doi: 10.1128/jb.78.6.778-787.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATHEWS M. M., SISTROM W. R. The function of the carotenoid pigments of Sarcina lutea. Arch Mikrobiol. 1960;35:139–146. doi: 10.1007/BF00425002. [DOI] [PubMed] [Google Scholar]
- MacDonald R. E., Lanyi L. K. Light-induced leucine transport in Halobacterium halobium envelope vesicles: a chemiosmotic system. Biochemistry. 1975 Jul;14(13):2882–2889. doi: 10.1021/bi00684a014. [DOI] [PubMed] [Google Scholar]
- Mackenzie A. S., Brassell S. C., Eglinton G., Maxwell J. R. Chemical fossils: the geological fate of steroids. Science. 1982 Aug 6;217(4559):491–504. doi: 10.1126/science.217.4559.491. [DOI] [PubMed] [Google Scholar]
- Mangel M., Berns D. S., Ilani A. Dependence of photosensitivity of bileaflet lipid membranes upon the chlorophyll and carotenoid content. J Membr Biol. 1975;20(1-2):171–180. doi: 10.1007/BF01870634. [DOI] [PubMed] [Google Scholar]
- Marshall J. H., Wilmoth G. J. Pigments of Staphylococcus aureus, a series of triterpenoid carotenoids. J Bacteriol. 1981 Sep;147(3):900–913. doi: 10.1128/jb.147.3.900-913.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J. H., Wilmoth G. J. Proposed pathway of triterpenoid carotenoid biosynthesis in Staphylococcus aureus: evidence from a study of mutants. J Bacteriol. 1981 Sep;147(3):914–919. doi: 10.1128/jb.147.3.914-919.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews-Roth M. M., Krinsky N. I. Carotenoid pigments and the stability of the cell membrane of Sarcina lutea. Biochim Biophys Acta. 1970 Apr 21;203(2):357–359. doi: 10.1016/0005-2736(70)90155-0. [DOI] [PubMed] [Google Scholar]
- Mathews-Roth M. M., Krinsky N. I. Failure of conjugated octaene carotenoids to protect a mutant of Sarcina lutea against lethal photosensitization. Photochem Photobiol. 1970 Jun;11(6):555–557. doi: 10.1111/j.1751-1097.1970.tb06027.x. [DOI] [PubMed] [Google Scholar]
- Mathews-Roth M. M., Krinsky N. I. Studies on the protective function of the carotenoid pigments of Sarcina lutea. Photochem Photobiol. 1970 Jun;11(6):419–428. doi: 10.1111/j.1751-1097.1970.tb06014.x. [DOI] [PubMed] [Google Scholar]
- Mathews-Roth M. M., Wilson T., Fujimori E., Krinsky N. I. Carotenoid chromophore length and protection against photosensitization. Photochem Photobiol. 1974 Mar;19(3):217–222. doi: 10.1111/j.1751-1097.1974.tb06501.x. [DOI] [PubMed] [Google Scholar]
- Mathews M. M., Krinsky N. I. The relationship between carotenoid pigments and resistance to radiation in non-photosynthetic bacteria. Photochem Photobiol. 1965 Sep;4(4):813–817. doi: 10.1111/j.1751-1097.1965.tb07923.x. [DOI] [PubMed] [Google Scholar]
- Nes W. R. Role of sterols in membranes. Lipids. 1974 Aug;9(8):596–612. doi: 10.1007/BF02532509. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D. Bacteriorhodopsin as an example of a light-driven proton pump. Angew Chem Int Ed Engl. 1976 Jan;15(1):17–24. doi: 10.1002/anie.197600171. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Rhodopsin-like protein from the purple membrane of Halobacterium halobium. Nat New Biol. 1971 Sep 29;233(39):149–152. doi: 10.1038/newbio233149a0. [DOI] [PubMed] [Google Scholar]
- Oró J., Nooner D. W., Zlatkis A., Wikström S. A., Barghoorn E. S. Hydrocarbons of Biological Origin in Sediments about Two Billion Years Old. Science. 1965 Apr 2;148(3666):77–79. doi: 10.1126/science.148.3666.77. [DOI] [PubMed] [Google Scholar]
- Oshima M., Ariga T. Omega-cyclohexyl fatty acids in acidophilic thermophilic bacteria. Studies on their presence, structure, and biosynthesis using precursors labeled with stable isotopes and radioisotopes. J Biol Chem. 1975 Sep 10;250(17):6963–6968. [PubMed] [Google Scholar]
- Ostroy S. E. Rhodopsin and the visual process. Biochim Biophys Acta. 1977 Jun 21;463(1):91–125. doi: 10.1016/0304-4173(77)90004-0. [DOI] [PubMed] [Google Scholar]
- Patt T. E., Hanson R. S. Intracytoplasmic membrane, phospholipid, and sterol content of Methylobacterium organophilum cells grown under different conditions. J Bacteriol. 1978 May;134(2):636–644. doi: 10.1128/jb.134.2.636-644.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt J. R. Carotene-Donor-Acceptor Complexes in Photosynthesis: The predicted lowering of the excited states of carotenoids may offer a new photosynthetic pathway. Science. 1959 Feb 13;129(3346):372–374. doi: 10.1126/science.129.3346.372. [DOI] [PubMed] [Google Scholar]
- Poralla K., Kannenberg E., Blume A. A glycolipid containing hopane isolated from the acidophilic, thermophilic Bacillus acidocaldarius, has a cholesterol-like function in membranes. FEBS Lett. 1980 Apr 21;113(1):107–110. doi: 10.1016/0014-5793(80)80506-0. [DOI] [PubMed] [Google Scholar]
- Prebble J., Huda A. S. Sensitivity of the electron transport chain of pigmented and non-pigmented Sarcina membranes to photodynamic action. Photochem Photobiol. 1973 Apr;17(4):255–264. doi: 10.1111/j.1751-1097.1973.tb06354.x. [DOI] [PubMed] [Google Scholar]
- Razin S., Cleverdon R. C. Carotenoids and cholesterol in membranes of Mycoplasma laidlawii. J Gen Microbiol. 1965 Dec;41(3):409–415. doi: 10.1099/00221287-41-3-409. [DOI] [PubMed] [Google Scholar]
- Renthal R., Lanyi J. K. Light-induced membrane potential and pH gradient in Halobacterium halobium envelope vesicles. Biochemistry. 1976 May 18;15(10):2136–2143. doi: 10.1021/bi00655a017. [DOI] [PubMed] [Google Scholar]
- Rohmer M., Anding C., Ourisson G. Non-specific biosynthesis of hopane triterpenes by a cell-free system from Acetobacter pasteurianum. Eur J Biochem. 1980 Dec;112(3):541–547. doi: 10.1111/j.1432-1033.1980.tb06117.x. [DOI] [PubMed] [Google Scholar]
- Rohmer M., Bouvier P., Ourisson G. Molecular evolution of biomembranes: structural equivalents and phylogenetic precursors of sterols. Proc Natl Acad Sci U S A. 1979 Feb;76(2):847–851. doi: 10.1073/pnas.76.2.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohmer M., Bouvier P., Ourisson G. Non-specific lanosterol and hopanoid biosynthesis be a cell-free system from the bacterium Methylococcus capsulatus. Eur J Biochem. 1980 Dec;112(3):557–560. doi: 10.1111/j.1432-1033.1980.tb06121.x. [DOI] [PubMed] [Google Scholar]
- Rottem S., Gottfried L., Razin S. Carotenoids as protectors against photodynamic inactivation of the adenosine triphosphatase of Mycoplasma laidlawii membranes. Biochem J. 1968 Oct;109(4):707–708. doi: 10.1042/bj1090707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottem S., Markowitz O. Carotenoids acts as reinforcers of the Acholeplasma laidlawii lipid bilayer. J Bacteriol. 1979 Dec;140(3):944–948. doi: 10.1128/jb.140.3.944-948.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAPERSTEIN S., STARR M. P. Association of carotenoid pigments with protein components in non-photosynthetic bacteria. Biochim Biophys Acta. 1955 Apr;16(4):482–488. doi: 10.1016/0006-3002(55)90267-5. [DOI] [PubMed] [Google Scholar]
- SISTROM W. R., GRIFFITHS M., STANIER R. Y. The biology of photosynthetic bacterium which lacks colored carotenoids. J Cell Physiol. 1956 Dec;48(3):473–515. doi: 10.1002/jcp.1030480309. [DOI] [PubMed] [Google Scholar]
- SMITH P. F. RELATION OF STEROL STRUCTURE TO UTILIZATION IN PLEUROPNEUMONIA-LIKE ORGANISMS. J Lipid Res. 1964 Jan;5:121–125. [PubMed] [Google Scholar]
- SMITH P. F. THE CAROTENOID PIGMENTS OF MYCOPLASMA. J Gen Microbiol. 1963 Sep;32:307–319. doi: 10.1099/00221287-32-3-307. [DOI] [PubMed] [Google Scholar]
- Salton M. R., Freer J. H. Composition of the membranes isolated from several Gram-positive bacteria. Biochim Biophys Acta. 1965 Oct 18;107(3):531–538. doi: 10.1016/0304-4165(65)90197-2. [DOI] [PubMed] [Google Scholar]
- Schadt M. Photoresponse of bimolecular lipid membranes pigmented with retinal and vitamin A acid. Biochim Biophys Acta. 1973 Oct 25;323(3):351–366. doi: 10.1016/0005-2736(73)90181-8. [DOI] [PubMed] [Google Scholar]
- Schmidt K., Francis G. W., Liaaen-Jensen S. Bacterial carotenoids. XXXVI. Remarkable C 43 -carotenoid artefacts of cross-conjugated carotenals and new carotenoid glucosides from Athiorhodaceae spp. Acta Chem Scand. 1971;25(7):2476–2486. doi: 10.3891/acta.chem.scand.25-2476. [DOI] [PubMed] [Google Scholar]
- Schreckenbach T., Oesterhelt D. Photochemical and chemical studies on the chromophore of bacteriorhodopsin. Fed Proc. 1977 May;36(6):1810–1814. [PubMed] [Google Scholar]
- Schubert K., Rose G., Hörhold C. Cholesterin in Streptomyces olivaceus. Biochim Biophys Acta. 1967 Feb 14;137(1):168–171. [PubMed] [Google Scholar]
- Schubert K., Rose G., Tümmler R., Ikekawa N. Sterine in Escherichia coli. Hoppe Seylers Z Physiol Chem. 1964;339(1):293–296. [PubMed] [Google Scholar]
- Schubert K., Rose G., Wachtel H., Hörhold C., Ikekawa N. Zum Vorkommen von Sterinen in Bakterien. Eur J Biochem. 1968 Jul;5(2):246–251. doi: 10.1111/j.1432-1033.1968.tb00364.x. [DOI] [PubMed] [Google Scholar]
- Schwenker U., ST-ONGE M., Ginras G. Chemical and physical properties of a carotenoprotein from Rhodospirillum rubrum. Biochim Biophys Acta. 1974 Jun 7;351(2):246–260. doi: 10.1016/0005-2795(74)90187-1. [DOI] [PubMed] [Google Scholar]
- Singer C. E., Ames B. N. Sunlight ultraviolet and bacterial DNA base ratios. Science. 1970 Nov 20;170(3960):822–825. doi: 10.1126/science.170.3960.822. [DOI] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Smith P. F., Langworthy T. A. Existence of carotenoids in Acholeplasma axanthum. J Bacteriol. 1979 Jan;137(1):185–188. doi: 10.1128/jb.137.1.185-188.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J. L., Stoeckenius W. Light-regulated retinal-dependent reversible phosphorylation of Halobacterium proteins. J Biol Chem. 1980 Jun 25;255(12):5501–5503. [PubMed] [Google Scholar]
- Suzue G., Tsukada K., Nakai C., Tanaka S. Presence of squalene in Staphylococcus. Arch Biochem Biophys. 1968 Mar 11;123(3):644–644. doi: 10.1016/0003-9861(68)90187-2. [DOI] [PubMed] [Google Scholar]
- Suzue G., Tsukada K., Tanaka S. A new triterpenoid from a mutant of Staphylococcus aureus. Biochim Biophys Acta. 1967 Aug 8;144(1):186–188. [PubMed] [Google Scholar]
- Suzue G., Tsukada K., Tanaka S. Occurrence of dehydrosqualene (C30 phytoene) in Staphylococcus aureus. Biochim Biophys Acta. 1968 Sep 2;164(1):88–93. doi: 10.1016/0005-2760(68)90074-x. [DOI] [PubMed] [Google Scholar]
- Takatsuji H., Nishino T., Izui K., Katsuki H. Formation of dehydrosqualene catalyzed by squalene synthetase in Saccharomyces cerevisiae. J Biochem. 1982 Mar;91(3):911–921. doi: 10.1093/oxfordjournals.jbchem.a133780. [DOI] [PubMed] [Google Scholar]
- Taylor R. F., Davies B. H. A cell-free system for Streptococcus faecium for studies on the biosynthesis of triterpenoid carotenoids. Can J Biochem. 1982 Jun;60(6):675–683. doi: 10.1139/o82-083. [DOI] [PubMed] [Google Scholar]
- Taylor R. F., Davies B. H. The influence of culture conditions on carotenogenesis in Streptococcus faecium UNH564P. J Gen Microbiol. 1976 Feb;92(2):325–334. doi: 10.1099/00221287-92-2-325. [DOI] [PubMed] [Google Scholar]
- Taylor R. F., Davies B. H. The triterpenoid carotenoids and related terpenoids in Staphylococcus aureus 209P. Can J Biochem Cell Biol. 1983 Aug;61(8):892–905. doi: 10.1139/o83-114. [DOI] [PubMed] [Google Scholar]
- Taylor R. F., Davies B. H. Triterpenoid carotenoids and related lipids. The triterpenoid carotenes of Streptococcus faecium UNH 564P. Biochem J. 1974 Jun;139(3):751–760. doi: 10.1042/bj1390751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. F., Davies B. H. Triterpenoid carotenoids and related lipids. Triterpenoid carotenoid aldehydes from Streptococcus faecium UNH 564P. Biochem J. 1976 Feb 1;153(2):233–239. doi: 10.1042/bj1530233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. F., Davies B. H. Triterpenoid carotenoids and related lipids. Triterpenoid monohydroxy- and monoglucosyloxy-carotenoids from Streptococcus faecium UNH 564P. Biochem J. 1974 Jun;139(3):761–769. doi: 10.1042/bj1390761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. F., Ikawa M., Chesbro W. Carotenoids in yellow-pigmented enterococci. J Bacteriol. 1971 Feb;105(2):676–678. doi: 10.1128/jb.105.2.676-678.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. F., Ikawa M. Gas chromatography, gas chromatography--mass spectrometry, and high-pressure liquid chromatography of carotenoids and retinoids. Methods Enzymol. 1980;67:233–261. doi: 10.1016/s0076-6879(80)67031-1. [DOI] [PubMed] [Google Scholar]
- Thirkell D., Hunter M. I. Carotenoid-glycoprotein of sarcina flava membrane. J Gen Microbiol. 1969 Nov;58(3):289–292. doi: 10.1099/00221287-58-3-289. [DOI] [PubMed] [Google Scholar]
- Thornber J. P. Photochemical reactions of purple bacteria as revealed by studies of three spectrally different carotenobacteriochlorophyll-protein complexes isolated from Chromatium, strain D. Biochemistry. 1970 Jun 23;9(13):2688–2698. doi: 10.1021/bi00815a017. [DOI] [PubMed] [Google Scholar]
- Tien H. T. Electronic processes and photoelectric aspects of bilayer lipid membranes. Photochem Photobiol. 1976 Aug;24(2):97–116. doi: 10.1111/j.1751-1097.1976.tb06806.x. [DOI] [PubMed] [Google Scholar]
- Tornabene T. G., Kates M., Gelpi E., Oro J. Occurrence of squalene, di- and tetrahydrosqualenes, and vitamin MK8 in an extremely halophilic bacterium, Halobacterium cutirubrun. J Lipid Res. 1969 May;10(3):294–303. [PubMed] [Google Scholar]
- Tornabene T. G., Langworthy T. A. Diphytanyl and dibiphytanyl glycerol ether lipids of methanogenic archaebacteria. Science. 1979 Jan 5;203(4375):51–53. doi: 10.1126/science.758677. [DOI] [PubMed] [Google Scholar]
- Tornabene T. G. Non-aerated cultivation of Halobacterium cutirubrum and its effect on cellular squalenes. J Mol Evol. 1978 Aug 2;11(3):253–257. doi: 10.1007/BF01734486. [DOI] [PubMed] [Google Scholar]
- Tornabene T. G., Wolfe R. S., Balch W. E., Holzer G., Fox G. E., Oro J. Phytanyl-glycerol ethers and squalenes in the archaebacterium Methanobacterium thermoautotrophicum. J Mol Evol. 1978 Aug 2;11(3):259–266. doi: 10.1007/BF01734487. [DOI] [PubMed] [Google Scholar]
- Weeks O. B., Andrewes A. G. Structure of the glycosidic carotenoid corynexanthin. Arch Biochem Biophys. 1970 Mar;137(1):284–286. doi: 10.1016/0003-9861(70)90435-2. [DOI] [PubMed] [Google Scholar]
- White D. C., Frerman F. E. Fatty acid composition of the complex lipids of Staphylococcus aureus during the formation of the membrane-bound electron transport system. J Bacteriol. 1968 Jun;95(6):2198–2209. doi: 10.1128/jb.95.6.2198-2209.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S., Bloch K. Enzymatic studies on the oxidative cyclizations of squalene. Biochem Soc Symp. 1970;29:35–43. [PubMed] [Google Scholar]


