Abstract
Background
High titer donor-specific antibodies (DSA) and positive crossmatch in cardiac transplant recipients is associated with increased mortality from antibody-mediated rejection (AMR). Although treatment to reduce antihuman leukocyte antigen antibodies using plasmapheresis, intravenous immunoglobulin, and rituximab has been reported to be beneficial, in practice these are often ineffective. Moreover, these interventions do not affect the mature antibody producing plasma cell. Bortezomib, a proteasome inhibitor active against plasma cells, has been shown to reduce DSA in renal transplant patients with AMR. We report here the first use of bortezomib for cardiac transplant recipients in four pediatric heart recipients with biopsy-proven AMR, hemodynamic compromise, positive crossmatch, and high titer class I DSA.
Methods
Patients received four intravenous dose of bortezomib (1.3 mg/m2) over 2 weeks with plasmapheresis and rituximab. DSA specificity and strength (mean fluorescence intensity) was determined with Luminex. All had received previous treatment with plasmapheresis, intravenous immunoglobulin, and rituximab that was ineffective.
Results
AMR resolved in all patients treated with bortezomib with improvement in systolic function, conversion of biopsy to C4d negative in three patients and IgG negative in one patient, and a prompt, precipitous reduction in DSAs. In three patients who received plasmapheresis before bortezomib, plasmapheresis failed to reduce DSA. In one case, DSA increased after bortezomib but decreased after retreatment.
Conclusions
Bortezomib reduces DSA and may be an important adjunct to treatment of AMR in cardiac transplant recipients. Bortezomib may also be useful in desensitization protocols and in prevention of AMR in sensitized patients with positive crossmatch and elevated DSA.
Keywords: Antibody-mediated rejection, Pediatric heart transplant, Donor-specific antibodies
Anti-human leukocyte antigen (HLA) sensitization of potential heart transplant recipients is encountered frequently due to previous cardiac surgery or mechanically assisted device placement, and presence of anti-HLA antibodies is associated with decreased survival after transplantation (1–6). Obtaining prospective crossmatches for sensitized patients is typically unsuccessful, so there is increased mortality of highly sensitized patients on the waiting list (7–9). High levels of anti-HLA antibodies at the time of transplantation, specifically donor-specific antibodies (DSA), is associated with positive donor-recipient crossmatch, conferring a high risk of acute antibody-mediated rejection (AMR), chronic rejection, and death (1, 3–6, 10–13). The de novo development of alloantibody after transplantation is also associated with severe rejection and death (14, 15).
A number of studies have reported beneficial effects of a variety of interventions used to treat AMR or reduce total anti-HLA antibody load expressed as percent panel reactive antibody (PRA). Reversal of AMR and reduction in antibody load has been described with plasmapheresis (16–20), intravenous immunoglobulin (IVIg) (19), cyclophosphamide (6, 18, 20), polyclonal antilymphocyte antibodies (6, 20), and monoclonal antibodies to B lymphocytes (rituximab) (21–23). However, none of these consistently reduce PRA and are at best variably effective in reversing AMR. There are few data on their effectiveness in reducing DSA. Because of the general ineffectiveness of conventional AMR treatment, irreversible cardiac injury often occurs. Even with “successful treatment” recurrence is common after cessation of treatment with any or all of these modalities.
The elimination of DSA is the logical goal in prevention or treatment of AMR but plasmapheresis, rituximab, IVIg, or polyclonal antilymphocyte antibodies directly affect the mature plasma cells that produce alloantibodies. Bortezomib, a proteasome inhibitor used primarily for treatment of multiple myeloma is active against normal alloantibody producing plasma cells (24, 25). Bortezomib also reduces DSA with resolution of AMR in renal transplant patients (26, 27). We report, for the first time, the use of bortezomib, in conjunction with plasmapheresis and rituximab, in pediatric heart transplant recipients with AMR, significant DSA levels, and positive retrospective T- and B-cell crossmatches. This retrospective review was conducted with institutional review board approval.
RESULTS
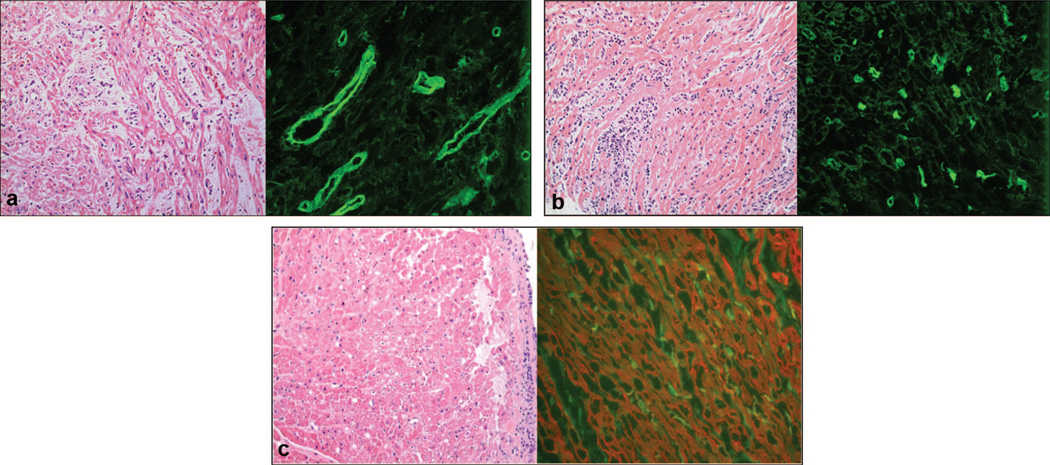
Demographic and clinical data are summarized in Table 1. Three patients had undergone cardiac surgery and two had mechanical support before transplantation. AMR developed between 7 days and 35 months after transplantation. Despite conventional treatment of AMR with multiple rounds of plasmapheresis, IVIg and rituximab (135 mg/m2) DSA remained elevated with clinical, echocardiographic, and invasive hemodynamic evidence of reduced graft function. Biopsy before bortezomib was 0R (no lymphocytic infiltrate) in all with C4d positive in three (Fig. 1) and immunoglobulin positive in one. Three of four cases, all except case 1, received IVIg and plasmapheresis in the days immediately before receiving bortezomib.
TABLE 1.
Case details
| Case 1 | Case 2 | Case 3 | Case 4 | |
|---|---|---|---|---|
| Age at bortezomib treatment | 14 mo | 21 mo | 13 mo | 5 yr |
| Age at transplantation (mo) | 10 | 19 | 13 | 26 |
| Interval to AMR | 4 mo | 2 mo | 7 d | 35 mo |
| Pretransplant DX | Myocarditis | HLHS | HLHS | HLHS |
| Previous surgery | No | Stage I and II Norwood | Stage I and II Norwood | Stage I and II Norwood |
| Previous mechanical support | ECMO, Berlin Heart | ECMO | No | No |
| cPRA before bortezomib (%) | 98 | 27 | 91 | 53 |
| Previous treatment | IVIg, PP, rituximab | PP | PP, IVIg | PP, IVIg×8 |
| Class I MFI before bortezomib, total (iDSA) | 5414 (2970) | 15,249 (11,249) | 25,200 (19,200) | 2524 (1959)a |
| Class I MFI after bortezomib, total (iDSA) | 359 (347)a | 6801 (4990) | 805 (600) | 152 (84) |
| Class I percent change MFI, total (iDSA) | 93 (88) | 55 (56) | 97 (97) | 81 (90) |
| Class II MFI pre, total (iDSA) | 25,653 (11,300) | 11,672 (8,9420) | Not performed | 5396 (4726) |
| Class II MRI post, total (iDSA) | 4246 (3306) | 1980 (1873) | Not performed | 274 (117) |
| Class II MFI percent change, total (iDSA) | 83 (71) | 83 (79) | Not performed | 95 (85) |
Initial decline after institution of ECMO and plasmapheresis from 2524 (1959) to 805 (805).
AMR, antibody mediated rejection; HLHS, hypoplastic left heart syndrome; ECMO, extracorporeal membrane oxygenator; IVIg, intravenous immunoglobulin; PP, plasmapheresis; cPRA, calculated panel reactive antibody; iDSA, immunodominant donor specific antibody; MFI, mean fluorescent intensity.
FIGURE 1.
(a) Case 1: interstitial edema (hematoxylin-eosin [H&E]) (left) and positive C4d staining (right); magnification,×400. (b) Case 2: H&E histology shows diffuse interstitial inflammation (left) and positive C4d staining (right); magnification, ×400. (c) Case 4: H&E from biopsy before bortezomib treatment showing minimal interstitial inflammation and myocytolysis (left) and positive C4d staining (right). Because the initial C4d stain showed diffuse staining of myocytes and the capillaries could not be recognized, the stain was repeated using a double stain with C4d (green, fluorescein isothiocyanate, capillaries) and actinin (red, Cy3, myocytes); magnification, ×400.
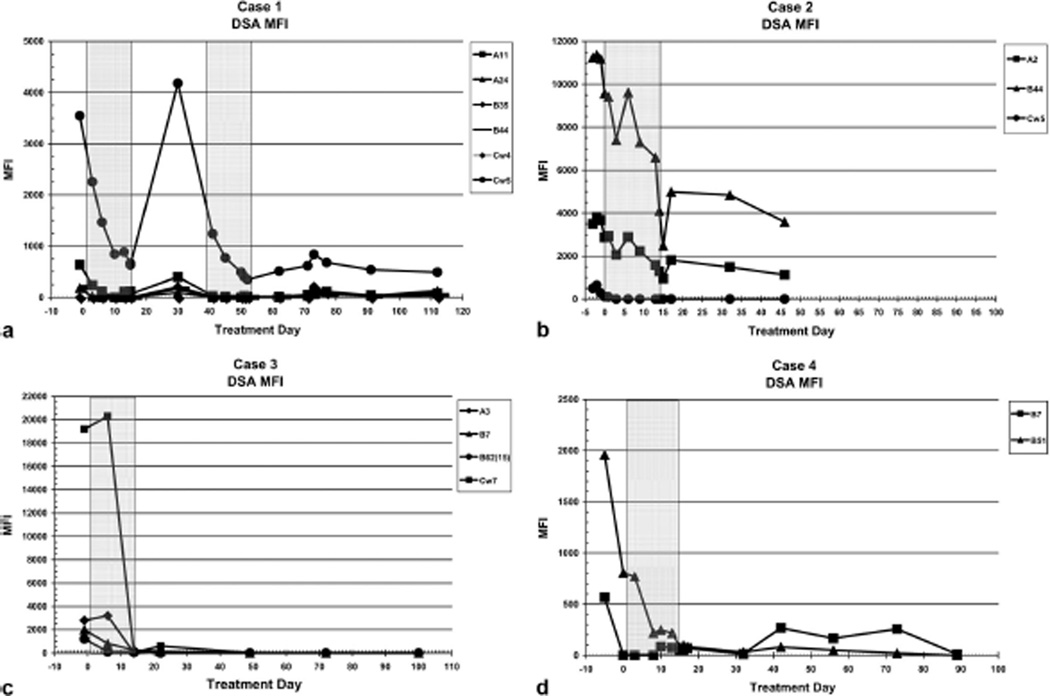
After treatment with bortezomib, there was dramatic reduction DSA with marked improvement in cardiac function in all. There was a rapid and precipitous reduction in iDSA of 56% to 97% after treatment with bortezomib (Fig. 2). Biopsy after bortezomib was C4d negative and immunoglobulin negative in all cases. Case 1 had recurrent elevation of DSA and received another course of bortezomib resulting in further reduction of DSA (Fig. 2a). Three of four patients recovered, and the nonsurvivor had renal and pulmonary complications with normal cardiac function and no evidence of AMR. One late death occurred of unknown causes with no significant DSA or clinical evidence of AMR.
FIGURE 2.
Graphic depiction of change in class I donor-specific antibodies (DSA) mean fluorescence intensity (MFI) before and after treatment with bortezomib. (Shaded areas) Treatment with bortezomib. iDSA was the DSA with highest MFI. There was a 56%to97%reduction in class I iDSA MFI after treatment with bortezomib. (a) After treatment with plasmapheresis, IVIg and rituximab 2 ½ months earlier iDSA Cw5 remained elevated but showed an acute precipitous reduction with bortezomib treatment followed by an increase at 2 weeks posttreatment. Retreatment resulted in an acute reduction in iDSA Cw5. (b) Plasmapheresis and IVIg given less than 1 week before had no effect on iDSA B44 MFI, which decreased over a 20-day period and then subsequently declines. This is accompanied by a reduction in DSA A2 MFI. (c) After an initial increase in iDSA despite plasmapheresis on five of the preceeding 7 days, there is an acute and sustained reduction in iDSA Cw7 after treatment with bortezomib. (d) After an initial reduction in iDSA with institution of ECMO and plasmapheresis on the 5 days before treatment, there was a further sustained reduction of iDSA B51 with bortezomib treatment.
DISCUSSION
Class I HLA antigens are extensively expressed on the vascular surface of blood vessel endothelium. Donor-specific anti-class I HLA antibodies adversely affect organ transplantation through capillary endothelial damage (28, 29). Acute endothelialitis due to DSA can disrupt allograft vascular supply resulting in acute graft dysfunction. Chronic AMR can result in long-term endothelial damage with more gradual vascular disruption but ultimately graft failure. In cardiac as opposed to other solid organ transplantation, there is a greater extent of vascular endothelium, and therefore available class I HLA antigen targets present. Further, the lack of HLA-matching and greater propensity to develop class I anti-HLA antibodies due to pretransplant blood product exposure in consequence of surgeries, including cardiac bypass, extra-corporeal membrane oxygenator (ECMO) and implantable ventricular assist devices, results in a greater chance of DSA being present and predilection to develop detrimental levels of DSA after transplantation.
Various therapies have been directed at pretransplant reduction of antibodies (desensitization) and reduction or blocking of antibodies after transplantation when positive retrospective crossmatch or AMR. The primary therapies used are plasmapheresis, IVIg, and rituximab (17–24). In most reports, these methods produce varying degrees of PRA reduction and clinical improvement. However, AMR reversal is often incomplete or gradual, and DSA persistence is common. Few studies report DSA specificity and strength (mean fluorescence intensity [MFI]) or effect of therapies on DSA. Plasmapheresis removes only a fraction of anti-HLA antibodies from circulating plasma with each cycle, and reequilibration with extravascular antibodies occurs over 24 to 72 hr (30). Rituximab depletes B cells expressing CD20, but antiallotypic antibodies are produced by mature plasma cells no longer expressing CD20. IVIg is believed to treat elevated HLA antibody levels through a variety of mechanisms, but may not be capable of an overall reduction in DSA. Although these interventions have been successful in some instances, there is a generally recognized lack of short- and long-term benefit for most patients.
Bortezomib, a proteasome inhibitor initially developed for treatment of multiple myeloma has been shown to lead to apoptosis and interference with antibody production by normal human plasma cells (25–27). Woodle and coworkers (25, 27, 31, 32) demonstrated that reduction of elevated DSA using bortezomib could provide benefit for organ transplant recipients at risk for, or undergoing AMR. Their group reported six adult kidney transplant patients treated with bortezomib demonstrating prompt reversal of rejection and sustained reductions in DSA level with associated improved renal function and suppression of recurrent rejection.
Our reported experience (5) and that of others in pediatric heart transplant recipients (6, 20) has shown a significantly increased risk of AMR and death associated with a positive retrospective crossmatch and elevated anti-HLA antibodies. Given the limited effectiveness of conventional strategies to reduce DSA, we attempted the use of bortezomib in four critically ill pediatric heart transplant patients with AMR. All had previous conventional therapy. In these patients, the use of bortezomib resulted in marked reductions in class I DSA with clinical and histologic resolution of AMR. Total donor-specific allo-antibody load decreased from a mean of 7900 to 1700 MFI decreasing 55% to 98% using the bortezomib protocol. Reduction in class I iDSA paralleled the reduction in total class I DSA MFI. A corresponding reduction in class II DSA was seen in the three patients in whom class II antibodies were determined. This reduction in DSA was transient in one case, requiring retreatment. However, the three remaining cases showed sustained reduction of class I DSA, suggesting elimination of DSA producing clones of plasma cells and lack of replenishment with donor-specific B lymphocytes and that a single bortezomib cycle was effective in reducing DSA is of interest, as several cycles are usually required to achieve remission in multiple myeloma patients.
Plasmapheresis, IVIg, and rituximab before bortezomib failed to produce reductions in DSA in three patients. In one (case 4), there was a significant reduction in DSA with institution of ECMO and plasmapheresis with a subsequent reduction after treatment with bortezomib. In this case, the initial DSA MFI was relatively low in comparison with cases 1,2, and 3. Initial reduction in DSA could have been related to hemodilution by ECMO circuit priming blood or improved efficiency of plasmapheresis on ECMO. Pretreatment measures did not significantly lower DSA in the remaining patients, but may have created the milieu for successful treatment. The extent and rapidity of reduction of DSA with bortezomib was surprising, particularly as DSA level had proven refractory to multiple IVIg doses, plasmapheresis, and rituximab.
The normal half-life of immunoglobulin in the blood is 21 to 28 days. Yet, we obtained large reductions in DSA within a few days of therapy. This reduction was much greater than that which could be achieved by plasmapheresis alone. Merely stopping production of IgG through plasma cell pro-teasome inhibition should result in DSA diminution over weeks to months, according to the expected half-life of IgG. Therefore, we suspect reduction in DSA by other mechanism affected by bortezomib. This effect has not been seen with other conventional therapies and may be additive or additional. Further studies are needed to delineate the mechanism of this rapid reduction in DSA.
All four patients received conventional treatment before bortezomib. Unfortunately, two developed significant morbidity with multiorgan damage and dysfunction before bortezomib. Despite reversal of AMR and normalization of ventricular function, the prebortezomib to kidneys, lungs, and central nervous system resulted in the ultimate demise of two patients. We speculate that earlier institution of bortezomib, before developing severe cardiac dysfunction would have prevented development of AMR and collateral organ damage. Indeed, Woodle and coworkers (27) have shown that first-line therapy with bortezomib is an effective therapy for AMR in kidney transplant recipients. Moreover, as bortezomib is effective in lowering HLA antibody levels in sensitized kidney transplant recipients (32), it could also be used for desensitization in sensitized patients awaiting cardiac transplantation.
Despite the potential for increased immunosuppression with bortezomib, opportunistic infections attributable to bortezomib did not occur in any of our patients. One patient had transient thrombocytopenia, none developed anemia, neurologic changes, or detectable gastrointestinal symptoms. Larger numbers of patients over a longer time frame are needed to verify the safety of bortezomib in pediatric patients. However, rescue therapy to reverse AMR may be preferred to less effective approaches that carry the risks inherent to multiple immunosuppressive agents combined at higher doses including opportunistic infections and lymphoproliferative disease.
This approach using plasmapheresis, rituximab, and bortezomib resulted in rapid and significant reduction in DSA, while reversing AMR. However, the necessity for plasmapheresis or rituximab is unclear. Certainly, elimination of progenitor B cells with rituximab with bortezomib directed against plasma cells seems a rational starting point. Removal of naive B cells might prevent the reconstitution of plasma cells producing allotypic antibody. However, bortezomib is also active against B cells, particularly proliferating B cells. Therefore, the absolute need for rituximab remains an open question. Plasmapheresis may increase the efficacy of bortezomib against plasma cells by lowering serum IgG, stimulating increased antibody production and metabolic activity (31). Bortezomib is also active against T cells and therefore other potential mechanisms may be responsible in whole or in part for reversal of clinical and pathologic AMR.
A number of potential strategies to reduce DSA could be used in future investigations of bortezomib in the cardiac transplant population. Anti-HLA antibodies present before transplantation may be amenable to reduction through a bortezomib-based desensitization protocol, and thus allowing for negative crossmatch transplantation and reduced risk of AMR. Elevated DSA detected at or after transplantation, but before development of AMR, could be reduced by bortezomib, allowing for maintenance of the cardiac allograft free from risk of graft damage or loss due to AMR.
CONCLUSIONS
AMR by donor-specific anti-HLA antibodies is a serious and increasingly frequent problem in cardiac transplant patients, resulting in increased morbidity and significant risk for mortality. Conventional approaches to DSA reduction or blockade, including plasmapheresis, IVIg, rituximab, antilymphocyte immunoglobulins, and increases in routine immunosuppressive therapies are often ineffective in eliminating AMR. We have demonstrated, for the first time in heart transplant recipients, the addition of bortezomib rapidly and significantly reduces DSA, effectively halting AMR. The acute side effect profile in pediatric patients seems to be minimal. As our report involves a small number of patients, further studies are warranted to determine the safety and efficacy of bortezomib for reduction of DSA and treatment of AMR in pediatric and adult heart transplant recipients.
MATERIALS AND METHODS
Patients and donors were HLA typed according to standard procedures in an American Society of Histocompatibility and Immunogenetics (ASHI)-certified laboratory (ASHI-01-5-AR-01-1; CLIA 04D0466863; UNOS 040016). HLA typing was performed by serologic analyses (One Lambda tissue typing trays and reagents) and molecular DNA technique using PCR and SSO probe detection via Luminex technology (One Lambda). Anti-HLA antibodies were detected using Luminex fluorescence cytometry with LabScreen single-antigen beads (One Lambda, Inc.). Intensity of detected anti-HLA antibody is reported as MFI as determined by Luminex and accompanying software, according to the manufacturer’s guidelines. Comparison of patient and donor HLA types and detected anti-HLA antibodies identified DSA. Based on control studies, anti-HLA antibodies with MFI values more than or equal to 1000 would be expected to result in a positive fluorescence cytometric crossmatch, and are reported as positive. Calculated PRA (cPRA) was determined using the web-based UNOS cPRA calculator (http://www.unos.org/resources/frm_cPRA_Calculator.asp?index=78). DSA-cPRA was also calculated by inputting only DSA anti-HLA antibody specificities. Class I antibodies were determined in all, class II in 3 of 4. Because of the recognized role of class I anti-HLA antibodies in endothelial damage and AMR and uncertain role of class II antibodies, we present class I alloantibody data. The dominant class I, single DSA with highest MFI (iDSA), is reported (26, 27). When more than one class I DSA was present, the total MFI was used given adverse anti-HLA antibody potential (31, 33).
Retrospective crossmatches were performed on an FC-500 Fluorescence Cytometer (Beckman-Coulter), using ASHI guidelines. Based on control studies, a positive T-cell crossmatch was more than 60 channel number shift, and positive B-cell crossmatch more than 100 channel number shift. DSA against class I HLA antigens are expected to result in positive T- and B-cell crossmatches with DSA to class II HLA antigens positive B-cell crossmatches only. All patients in this study had more than 60 channel number shift in T-cell crossmatches and more than 100 channel number shift in B-cell crossmatches.
Diagnosis of AMR
Graft function was assessed by examination, echocardiography, and cardiac catheterization. Cardiac catheterization was performed at the time of initial biopsy to confirm the clinical diagnosis of AMR and on completion of treatment with bortezomib. The diagnosis of AMR was made in the presence of typical histologic changes including evidence of endothelialitis and positive C4d staining (cases 1, 2, and 4) or immunoglobulin deposition (case 3) (28, 34).
Treatment Protocol
The treatment protocol (Table 2) was based on the report of Everly and coworkers (27) describing bortezomib use in adult renal transplant patients, from the START Collaborative (35), and also from existing protocols for children with solid tumors or refractory leukemia (36, 37). This protocol was followed in all patients with only minor variation in timing of plasmapheresis and administration of bortezomib. Class I PRA and DSA were determined after such treatment, before bortezomib. A single dose of intravenous rituximab (375 mg/m2) was given to deplete CD20 positive B cells before treatment with bortezomib, if not previously given. Four intravenous doses of bortezomib (1.3 mg/m2) were administered before each dose, plasmapheresis was performed (1.5–2 plasma volumes), and methylprednisolone (1.5 mg/kg) administered as premedication. Beginning 72 hr after the fourth dose, three daily cycles of plasmapheresis were performed to remove remaining circulating alloantibody. Anti-HLA antibodies were measured before and after treatment with bortezomib, after the three cycles of plasmapheresis, and 3 to 4 weeks after completion of protocol.
TABLE 2.
Medication protocol
| Pretreatment | Rituximab | 375 mg/m2 IV |
| Day1, 4, 7, 11 | Plasmapheresis | |
| Methylprednisolone | 5 mg/kg (max 100 mg) | |
| Bortezomib | 1.3 mg/m2 IV | |
| Day14–16 | Plasmapheresis | |
| Day 18 | PRA, T-/B-cell subsets | |
| Follow-up after 30 Days | PRA, T-/B-cell subsets |
PRA, panel reactive antibody.
ACKNOWLEDGMENTS
The authors acknowledge the support of the Arkansas Children’s Hospital apheresis nursing staff (Helyn Jones, Zelenda Owens, and Sandra Smith); Valeria Smith, B.S., C.H.S., in the UAMS HLA Laboratory; and Denise Graves and Angie McCuin, without whom this report would not have been possible.
Footnotes
The authors declare no conflicts of interest.
W.R.M. contributed to design, data analysis, and writing of the manuscript; E.A.F. contributed to the writing of the manuscript; W.T.M. participated in the performance of the research; T.O.H. contributed analytic tools and to the writing of the manuscript; S.E.P. participated in performance of the research; K.R.K. contributed to the writing of the manuscripts; E.L.H. contributed reagents and tools for analysis; N.S. participated in the performance of the research; R.L.S. participated in the design and performance of the research; X.G. participated in the performance of the research; R.D.B.J. participated in the performance of the research; and E.S.W. contributed to the design of the study.
REFERENCES
- 1.Velez M, Johnson MR. Management of allosensitized cardiac transplant candidates. Transplant Rev. 2009;23:235. doi: 10.1016/j.trre.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rose EA, Smith CR, Petrossian GA, et al. Humoral immune responses after cardiac transplantation: Correlation with fatal rejection and graft atherosclerosis. Surgery. 1989;106:203. [PubMed] [Google Scholar]
- 3.Lavee J, Kormos RL, Duquesnoy RJ, et al. Influence of panel-reactive antibody and lymphocytoxic crossmatch on survival after heart transplantation. J Heart Lung Transplant. 1991;10:921. [PubMed] [Google Scholar]
- 4.Smith JD, Danskine AJ, Laylor RM, et al. The effect of panel reactive antibodies and the donor specific crossmatch on graft survival after heart and heart-lung transplantation. Transplant Immunol. 1993;1:60. doi: 10.1016/0966-3274(93)90060-l. [DOI] [PubMed] [Google Scholar]
- 5.Wright EJ, Fiser WP, Edens E, et al. Cardiac transplant outcomes in pediatric patients with pre-formed anti-human leukocyte antigen antibodies and/or positive retrospective crossmatch. J Heart Lung Transplant. 2007;26:1163. doi: 10.1016/j.healun.2007.07.042. [DOI] [PubMed] [Google Scholar]
- 6.Holt DB, Lublin DM, Phelan DL, et al. Mortality and morbidity in pre-sensitized pediatric heart transplant recipients with a positive donor crossmatch utilizing per-operative plasmapheresis and cytolytic therapy. J Heart Lung Transplant. 2007;26:876. doi: 10.1016/j.healun.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 7.Goffinet J, St Pierre Schneider B. Preformed anti-human leukocyte antigen antibodies jeopardize cardiac transplantation in patients with a left ventricular assist device. Heart Lung. 2002;31:122. doi: 10.1067/mhl.2002.122893. [DOI] [PubMed] [Google Scholar]
- 8.McKenna DH, Eastlund T, Segall M, et al. HLA alloimmunization in patients requiring ventricular assist device support. J Heart Lung Transplant. 2002;21:1218. doi: 10.1016/s1053-2498(02)00448-5. [DOI] [PubMed] [Google Scholar]
- 9.Kfoury AG, Hammond ME, Snow GL, et al. Cardiovascular mortality among heart transplant recipients with asymptomatic antibody-mediated or stable mixed cellular and antibody-mediated rejection. J Heart Lung Transplant. 2009;28:781. doi: 10.1016/j.healun.2009.04.035. [DOI] [PubMed] [Google Scholar]
- 10.Feingold B, Bowman P, Zeevi A, et al. Survival in allosensitized children after listing for cardiac transplantation. J Heart Lung Transplant. 2007;26:565. doi: 10.1016/j.healun.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 11.Michaels PJ, Espejo ML, Kobashigawa J, et al. Humoral rejection in cardiac transplantation: Risk factors, hemodynamic consequences and relationship to transplant coronary artery disease. J Heart Lung Transplant. 2003;22:58. doi: 10.1016/s1053-2498(02)00472-2. [DOI] [PubMed] [Google Scholar]
- 12.Taylor DO, Yowell RL, Kfoury AG, et al. Allograft coronary artery disease: Clinical correlations with circulating anti-HLA antibodies and the immunohistopathologic pattern of vascular rejection. J Heart Lung Transplant. 2000;19:518. doi: 10.1016/s1053-2498(00)00095-4. [DOI] [PubMed] [Google Scholar]
- 13.Soleimani B, Lechler RI, Hornick PI, et al. Role of alloantibodies in the pathogenesis of graft arteriosclerosis in cardiac transplantation. Am J Transplant. 2006;6:1781. doi: 10.1111/j.1600-6143.2006.01401.x. [DOI] [PubMed] [Google Scholar]
- 14.Rose ML. De novo production of antibodies after heart or lung transplantation should be regarded as an early warning system. J Heart Lung Transplant. 2004;23:385. doi: 10.1016/j.healun.2003.08.028. [DOI] [PubMed] [Google Scholar]
- 15.Xydas S, Yang JK, Burke EM, et al. Utility of post-transplant anti-HLA antibody measurements in pediatric cardiac transplant recipients. J Heart Lung Transplant. 2005;24:1289. doi: 10.1016/j.healun.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Ratkovec RM, Hammond EH, O’Connell JB, et al. Outcome of cardiac transplant recipients with a positive donor-specific crossmatch— Preliminary results with plasmapheresis. Transplantation. 1992;54:651. doi: 10.1097/00007890-199210000-00017. [DOI] [PubMed] [Google Scholar]
- 17.Wang SS, Chou NK, Ko WJ, et al. Effect of plasmapheresis for acute humoral rejection after heart transplantation. Transplant Proc. 2006;38:3692. doi: 10.1016/j.transproceed.2006.10.060. [DOI] [PubMed] [Google Scholar]
- 18.Grauhan O, Knosalla C, Ewert R, et al. Plasmapheresis and cyclophos-phamide in the treatment of humoral rejection after heart transplantation. J Heart Lung Transplant. 2001;20:316. doi: 10.1016/s1053-2498(00)00211-4. [DOI] [PubMed] [Google Scholar]
- 19.Pisani BA, Mullen GM, Malinowska K, et al. Plasmapheresis with intravenous immunoglobulin G is effective in patients with elevated panel reactive antibody prior to cardiac transplantation. J Heart Lung Transplant. 1999;18:701. doi: 10.1016/s1053-2498(99)00022-4. [DOI] [PubMed] [Google Scholar]
- 20.Jacobs JP, Quintessenza JA, Boucek RJ, et al. Pediatric cardiac transplantation in children with high panel reactive antibody. Ann Thorac Surg. 2004;78:1703. doi: 10.1016/j.athoracsur.2004.03.031. [DOI] [PubMed] [Google Scholar]
- 21.Baran DA, Lubitz S, Alvi S, et al. Refractory humoral cardiac allograft rejection successfully treated with a single dose of rituximab. Transplant Proc. 2004;36:3164. doi: 10.1016/j.transproceed.2004.10.087. [DOI] [PubMed] [Google Scholar]
- 22.Garrett HE, Duvall-Seaman D, Helsley B, et al. Treatment of vascular rejection with rituximab in cardiac transplantation. J Heart Lung Transplant. 2005;24:337. doi: 10.1016/j.healun.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 23.Balfour IC, Fiore A, Graff RJ, et al. Use of rituximab to decrease panel-reactive antibodies. J Heart Lung Transplant. 2005;24:628. doi: 10.1016/j.healun.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 24.Everly J, Walsh RC, Alloway RR, et al. Proteasome inhibition for antibody-mediated rejection. Curr Opin Organ Transplant. 2009;14:662. doi: 10.1097/MOT.0b013e328330f304. [DOI] [PubMed] [Google Scholar]
- 25.Perry DJ, Burns JM, Pollinger HS, et al. Proteasome inhibition causes apoptosis of normal human plasma cells preventing alloantibody production. Am J Transplant. 2009;9:201. doi: 10.1111/j.1600-6143.2008.02461.x. [DOI] [PubMed] [Google Scholar]
- 26.Everly MJ, Everly JJ, Susskind B, et al. Bortezomib provides effective therapy for antibody and cell mediated acute rejection. Transplantation. 2008;86:1754. doi: 10.1097/TP.0b013e318190af83. [DOI] [PubMed] [Google Scholar]
- 27.Walsh RC, Everly JJ, Brailey P, et al. Proteasome inhibitor-based primary therapy for antibody mediated renal allograft rejection. Transplantation. 2010;89:277. doi: 10.1097/TP.0b013e3181c6ff8d. [DOI] [PubMed] [Google Scholar]
- 28.Smith RN, Brousaides N, Grazette L, et al. C4d deposition in cardiac allografts correlates with alloantibody. J Heart Lung Transplant. 2005;24:1202. doi: 10.1016/j.healun.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 29.Lones MA, Czer LS, Trento A, et al. Clinical-pathologic features of humoral rejection in cardiac allografts: A study in 81 consecutive patients. J Heart Lung Transplant. 1995;14:151. [PubMed] [Google Scholar]
- 30.Harville TO. Use of immunoglobulins in rheumatologic disorders and autoimmunopathies. In: Wahn V, Orange J, editors. Clinical use of immunoglobulins. Bremen, London, Boston: UNI-MED Verlag AG; 2008. p. 127. [Google Scholar]
- 31.Harville TO. HLA typing for cellular product characterization and identity testing. In: Areman EM, Loper K, editors. Cellular therapy: Principles, methods, and regulations. Bethesda, MD: AABB; 2009. p. 627. [Google Scholar]
- 32.Walsh RC, Shields AR, Brailey P, et al. Prospective safety and efficacy analysis of bortezomib-based therapy for desensitization of sensitized kidney transplant candidates. Am J Transplant. 2010;10:44. [Google Scholar]
- 33.Taylor C. Back to the future: Applications of contemporary technology to long standing questions about the clinical relevance of human leukocyte antigen specific allo-antibodies in renal transplantation. Human Immunol. 2009;70:563. doi: 10.1016/j.humimm.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 34.Reed EF, Demetris AJ, Hammond E, et al. Acute antibody-mediated rejection of cardiac transplants. J Heart Lung Transplant. 2006;25:153. doi: 10.1016/j.healun.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 35.Woodle ES, Light J, Rubin M, et al. Proteasome inhibitor therapy for antibody mediated rejection: Initial report from a multicenter collaborative. Am J Transplant. 2010;10(suppl 4):83. [Google Scholar]
- 36.Horton TM, Pati D, Plon SE, et al. A phase I study of the proteasome inhibitor bortezomib in pediatric patients with refractory leukemia: A children’s oncology group study. Clin Cancer Res. 2007;13:1516. doi: 10.1158/1078-0432.CCR-06-2173. [DOI] [PubMed] [Google Scholar]
- 37.Blaney SM, Bernstein M, Neville K, et al. Phase I study of the proteasome inhibitor bortezomib in pediatric patients with refractory solid tumors: A children’s oncology group study (ADVL0015) J Clin Oncol. 2004;22:4804. doi: 10.1200/JCO.2004.12.185. [DOI] [PubMed] [Google Scholar]