Full text
PDF
















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T., ROSENTHAL S. M. Presence of polyamines in certain bacterial viruses. Science. 1958 Apr 11;127(3302):814–815. doi: 10.1126/science.127.3302.814-a. [DOI] [PubMed] [Google Scholar]
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- ANDERTON J. I., LOCKE D. J. Extraction of pigment from cooked cured-meat products. Nature. 1955 May 7;175(4462):818–819. doi: 10.1038/175818b0. [DOI] [PubMed] [Google Scholar]
- Algranati I. D., Echandi G., Goldemberg S. H., Cunningham-Rundles S., Maas W. K. Ribosomal distribution in a polyamine auxotroph of Escherichia coli. J Bacteriol. 1975 Dec;124(3):1122–1127. doi: 10.1128/jb.124.3.1122-1127.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Algranati I. D., Goldemberg S. H. Initiation, elongation and termination of polypeptide synthesis in cell-free systems from polyamine-deficient bacteria. Biochem Biophys Res Commun. 1981 Nov 16;103(1):8–15. doi: 10.1016/0006-291x(81)91653-3. [DOI] [PubMed] [Google Scholar]
- Allen R. R., Klinman J. P. Stereochemistry and kinetic isotope effects in the decarboxylation of S-adenosylmethionine catalyzed by the pyruvyl enzyme, S-adenosylmethionine decarboxylase. J Biol Chem. 1981 Apr 10;256(7):3233–3239. [PubMed] [Google Scholar]
- Applebaum D. M., Dunlap J. C., Morris D. R. Comparison of the biosynthetic and biodegradative ornithine decarboxylases of Escherichia coli. Biochemistry. 1977 Apr 19;16(8):1580–1584. doi: 10.1021/bi00627a008. [DOI] [PubMed] [Google Scholar]
- Applebaum D., Sabo D. L., Fischer E. H., Morris D. R. Biodegradative ornithine decarboxylase of Escherichia coli. Purification, properties, and pyridoxal 5'-phosphate binding site. Biochemistry. 1975 Aug 12;14(16):3675–3681. doi: 10.1021/bi00687a025. [DOI] [PubMed] [Google Scholar]
- Arst H. N., Jr Some genetical aspects of ornithine metabolism in Aspergillus nidulans. Mol Gen Genet. 1977 Feb 28;151(1):105–110. doi: 10.1007/BF00446919. [DOI] [PubMed] [Google Scholar]
- Atmar V. J., Kuehn G. D. Phosphorylation of ornithine decarboxylase by a polyamine-dependent protein kinase. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5518–5522. doi: 10.1073/pnas.78.9.5518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BACHRACH U. Spermidine oxidase from Serratia marcescens. J Biol Chem. 1962 Nov;237:3443–3448. [PubMed] [Google Scholar]
- Barnett G. R., Kazarinoff M. N. Purification and properties of ornithine decarboxylase from Physarum polycephalum. J Biol Chem. 1984 Jan 10;259(1):179–183. [PubMed] [Google Scholar]
- Bitonti A. J., McCann P. P., Sjoerdsma A. Restriction of bacterial growth by inhibition of polyamine biosynthesis by using monofluoromethylornithine, difluoromethylarginine and dicyclohexylammonium sulphate. Biochem J. 1982 Nov 15;208(2):435–441. doi: 10.1042/bj2080435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blethen S. L., Boeker E. A., Snell E. E. Argenine decarboxylase from Escherichia coli. I. Purification and specificity for substrates and coenzyme. J Biol Chem. 1968 Apr 25;243(8):1671–1677. [PubMed] [Google Scholar]
- Bode V. C., Harrison D. P. Distinct effects of diamines, polyamines, and magnesium ions on the stability of lambda phage heads. Biochemistry. 1973 Aug 14;12(17):3193–3196. doi: 10.1021/bi00741a008. [DOI] [PubMed] [Google Scholar]
- Boeker E. A., Fischer E. H. Lysine decarboxylase (Escherichia coli B). Methods Enzymol. 1983;94:180–184. doi: 10.1016/s0076-6879(83)94030-2. [DOI] [PubMed] [Google Scholar]
- Boeker E. A., Fischer E. H., Snell E. E. Arginine decarboxylase from Escherichia coli. 3. Subunit structure. J Biol Chem. 1969 Oct 10;244(19):5239–5245. [PubMed] [Google Scholar]
- Boeker E. A., Fischer E. H., Snell E. E. Arginine decarboxylase from Escherichia coli. IV. Structure of the pyridoxal phosphate binding site. J Biol Chem. 1971 Nov 25;246(22):6776–6781. [PubMed] [Google Scholar]
- Boeker E. A., Snell E. E. Arginine decarboxylase from Escherichia coli. II. Dissociation and reassociation of subunits. J Biol Chem. 1968 Apr 25;243(8):1678–1684. [PubMed] [Google Scholar]
- Bowman B. J., Davis R. H. Cellular distribution of ornithine in Neurospora: anabolic and catabolic steady states. J Bacteriol. 1977 Apr;130(1):274–284. doi: 10.1128/jb.130.1.274-284.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
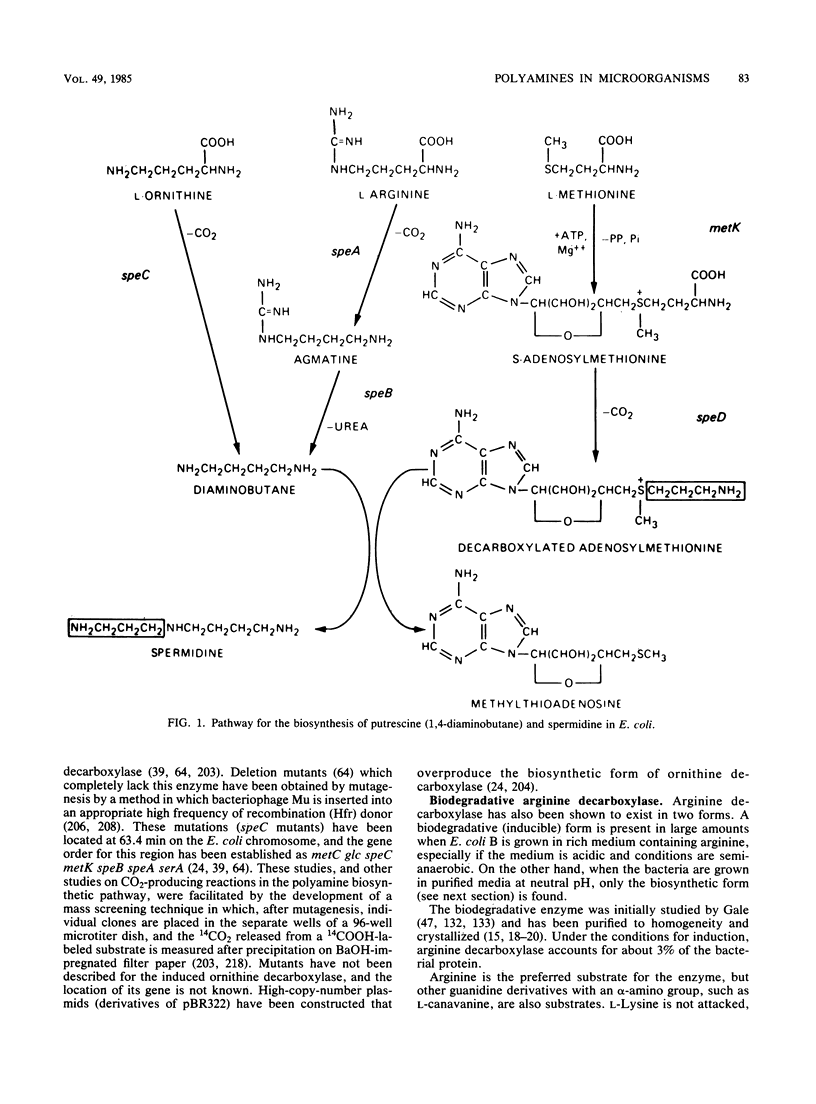
- Bowman W. H., Tabor C. W., Tabor H. Spermidine biosynthesis. Purification and properties of propylamine transferase from Escherichia coli. J Biol Chem. 1973 Apr 10;248(7):2480–2486. [PubMed] [Google Scholar]
- Boyle S. M., Adachi K. Biosynthetic ornithine and arginine decarboxylases: correlation of rates of synthesis with activities in Escherichia coli during exponential growth and following nutritional shift-up. Can J Microbiol. 1982 Aug;28(8):945–950. doi: 10.1139/m82-142. [DOI] [PubMed] [Google Scholar]
- Boyle S. M., Markham G. D., Hafner E. W., Wright J. M., Tabor H., Tabor C. W. Expression of the cloned genes encoding the putrescine biosynthetic enzymes and methionine adenosyltransferase of Escherichia coli (speA, speB, speC and metK). Gene. 1984 Oct;30(1-3):129–136. doi: 10.1016/0378-1119(84)90113-6. [DOI] [PubMed] [Google Scholar]
- Canellakis E. S., Viceps-Madore D., Kyriakidis D. A., Heller J. S. The regulation and function of ornithine decarboxylase and of the polyamines. Curr Top Cell Regul. 1979;15:155–202. [PubMed] [Google Scholar]
- Cherest H., Surdin-Kerjan Y. S-adenosyl methionine requiring mutants in Saccharomyces cerevisiae: evidences for the existence of two methionine adenosyl transferases. Mol Gen Genet. 1978 Jul 11;163(2):153–167. doi: 10.1007/BF00267406. [DOI] [PubMed] [Google Scholar]
- Chiang P. K., Cantoni G. L. Activation of methionine for transmethylation. Purification of the S-adenosylmethionine synthetase of bakers' yeast and its separation into two forms. J Biol Chem. 1977 Jul 10;252(13):4506–4513. [PubMed] [Google Scholar]
- Cohen S. S., McCormick F. P. Polyamines and virus multiplication. Adv Virus Res. 1979;24:331–387. doi: 10.1016/s0065-3527(08)60397-8. [DOI] [PubMed] [Google Scholar]
- Cohn M. S., Tabor C. W., Tabor H. Identification of a pyruvoyl residue in S-adenosylmethionine decarboxylase from Saccharomyces cerevisiae. J Biol Chem. 1977 Nov 25;252(22):8212–8216. [PubMed] [Google Scholar]
- Cohn M. S., Tabor C. W., Tabor H. Isolation and characterization of Saccharomyces cerevisiae mutants deficient in S-adenosylmethionine decarboxylase, spermidine, and spermine. J Bacteriol. 1978 Apr;134(1):208–213. doi: 10.1128/jb.134.1.208-213.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn M. S., Tabor C. W., Tabor H. Regulatory mutations affecting ornithine decarboxylase activity in Saccharomyces cerevisiae. J Bacteriol. 1980 Jun;142(3):791–799. doi: 10.1128/jb.142.3.791-799.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn M. S., Tabor C. W., Tabor H. S-adenosylmethionine decarboxylase (Saccharomyces cerevisiae). Methods Enzymol. 1983;94:231–234. doi: 10.1016/s0076-6879(83)94040-5. [DOI] [PubMed] [Google Scholar]
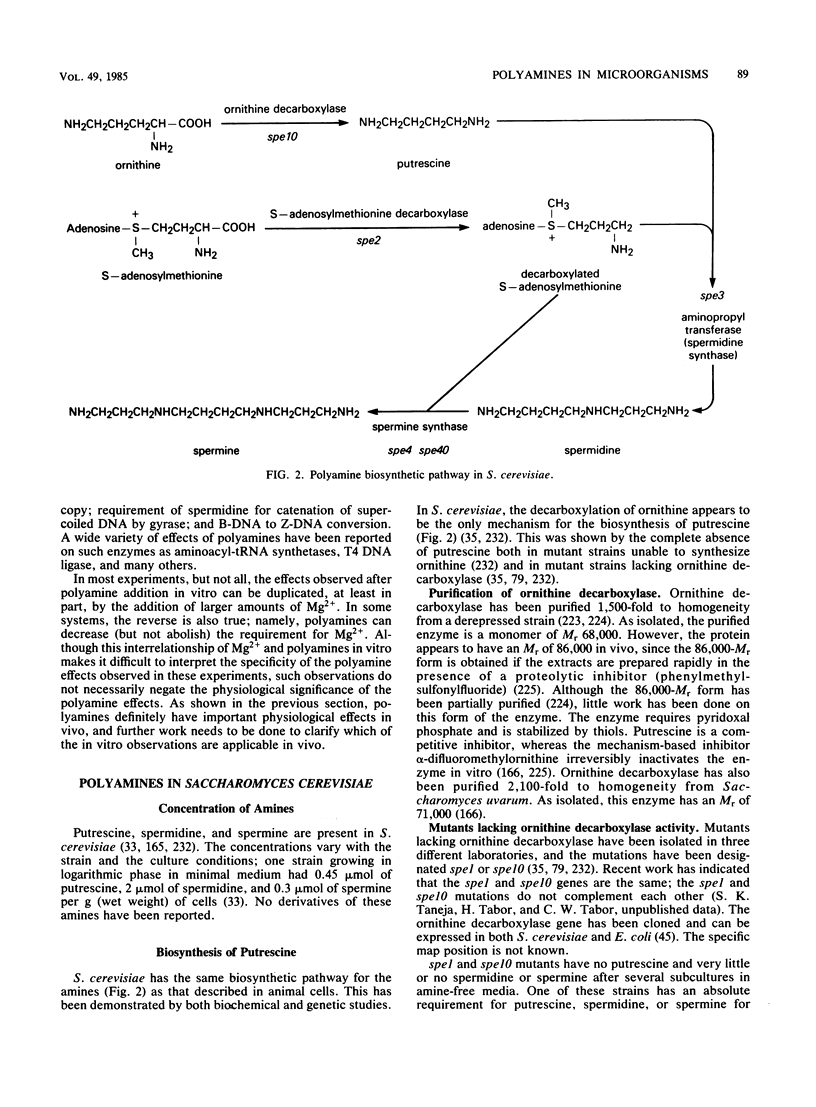
- Cohn M. S., Tabor C. W., Tabor H., Wickner R. B. Spermidine or spermine requirement for killer double-stranded RNA plasmid replication in yeast. J Biol Chem. 1978 Aug 10;253(15):5225–5227. [PubMed] [Google Scholar]
- Cramer C. L., Davis R. H. Polyphosphate-cation interaction in the amino acid-containing vacuole of Neurospora crassa. J Biol Chem. 1984 Apr 25;259(8):5152–5157. [PubMed] [Google Scholar]
- Cunningham-Rundles S., Maas W. K. Isolation, characterization, and mapping of Escherichia coli mutants blocked in the synthesis of ornithine decarboxylase. J Bacteriol. 1975 Nov;124(2):791–799. doi: 10.1128/jb.124.2.791-799.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels G. R., Atmar V. J., Kuehn G. D. Polyamine-activated protein kinase reaction from nuclei and nucleoli of Physarum polycephalum which phosphorylates a unique Mr 70 000 nonhistone protein. Biochemistry. 1981 Apr 28;20(9):2525–2532. doi: 10.1021/bi00512a025. [DOI] [PubMed] [Google Scholar]
- Davis R. H., Lawless M. B., Port L. A. Arginaseless Neurospora: genetics, physiology, and polyamine synthesis. J Bacteriol. 1970 May;102(2):299–305. doi: 10.1128/jb.102.2.299-305.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. H., Paulus T. J. Uses of arginaseless cells in the study of polyamine metabolism (Neurospora crassa). Methods Enzymol. 1983;94:112–117. doi: 10.1016/s0076-6879(83)94018-1. [DOI] [PubMed] [Google Scholar]
- Dion A. S., Cohen S. S. Polyamines in the synthesis of bacteriophage deoxyribonucleic acid. II. Requirement for polyamines in T4 infection of a polyamine auxotroph. J Virol. 1972 Mar;9(3):423–430. doi: 10.1128/jvi.9.3.423-430.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairlamb A. H., Cerami A. Identification of a novel, thiol-containing co-factor essential for glutathione reductase enzyme activity in trypanosomatids. Mol Biochem Parasitol. 1985 Feb;14(2):187–198. doi: 10.1016/0166-6851(85)90037-4. [DOI] [PubMed] [Google Scholar]
- Fonzi W. A., Sypherd P. S. Expression of the gene for ornithine decarboxylase of Saccharomyces cerevisiae in Escherichia coli. Mol Cell Biol. 1985 Jan;5(1):161–166. doi: 10.1128/mcb.5.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSSOWICZ N., ARIEL M. Mechanism of protection of cells by spermine against lysozyme-induced lysis. J Bacteriol. 1963 Feb;85:293–300. doi: 10.1128/jb.85.2.293-300.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger L. E., Morris D. R. Stimulation of deoxyribonucleic acid replication fork movement by spermidine analogs in polyamine-deficient Escherichia coli. J Bacteriol. 1980 Mar;141(3):1192–1198. doi: 10.1128/jb.141.3.1192-1198.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldemberg S. H., Algranati I. D. Polyamine requirement for streptomycin action on protein synthesis in bacteria. Eur J Biochem. 1981 Jul;117(2):251–255. doi: 10.1111/j.1432-1033.1981.tb06330.x. [DOI] [PubMed] [Google Scholar]
- Goldemberg S. H., Algranati I. D. Polyamines and protein synthesis: studies in various polyamine-requiring mutants of Escherichia coli. Mol Cell Biochem. 1977 Jul 5;16(2):71–77. doi: 10.1007/BF01732046. [DOI] [PubMed] [Google Scholar]
- Goldemberg S. H., Fernandez-Velasco J. G., Algranati I. D. Differential binding of streptomycin to ribosomes of polyamine-deficient bacteria grown in the absence and presence of putrescine. FEBS Lett. 1982 Jun 7;142(2):275–279. doi: 10.1016/0014-5793(82)80151-8. [DOI] [PubMed] [Google Scholar]
- Goldemberg S. H. Lysine decarboxylase mutants of Escherichia coli: evidence for two enzyme forms. J Bacteriol. 1980 Mar;141(3):1428–1431. doi: 10.1128/jb.141.3.1428-1431.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldemberg S. H. Polyamine regulation of stringent control in a polyamine-auxotrophic strain of Escherichia coli. Biochem J. 1984 Apr 1;219(1):205–210. doi: 10.1042/bj2190205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorini L. The contrasting role of strA and ram gene products in ribosomal functioning. Cold Spring Harb Symp Quant Biol. 1969;34:101–109. doi: 10.1101/sqb.1969.034.01.016. [DOI] [PubMed] [Google Scholar]
- Gottesman S., Gottesman M. Excision of prophage lambda in a cell-free system. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2188–2192. doi: 10.1073/pnas.72.6.2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene R. C., Hunter J. S., Coch E. H. Properties of metK mutants of Escherichia coli K-12. J Bacteriol. 1973 Jul;115(1):57–67. doi: 10.1128/jb.115.1.57-67.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene R. C., Su C. H., Holloway C. T. S-Adenosylmethionine synthetase deficient mutants of Escherichia coli K-12 with impaired control of methionine biosynthesis. Biochem Biophys Res Commun. 1970 Mar 27;38(6):1120–1126. doi: 10.1016/0006-291x(70)90355-4. [DOI] [PubMed] [Google Scholar]
- Greenstein M., Speth J. L., Maiese W. M. Mechanism of action of cinodine, a glycocinnamoylspermidine antibiotic. Antimicrob Agents Chemother. 1981 Oct;20(4):425–432. doi: 10.1128/aac.20.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERBST E. J., WEAVER R. H., KEISTER D. L. The gram reaction and cell composition: diamines and polyamines. Arch Biochem Biophys. 1958 May;75(1):171–177. doi: 10.1016/0003-9861(58)90407-7. [DOI] [PubMed] [Google Scholar]
- Hafner E. W., Tabor C. W., Tabor H. Isolation of a metK mutant with a temperature-sensitive S-adenosylmethionine synthetase. J Bacteriol. 1977 Dec;132(3):832–840. doi: 10.1128/jb.132.3.832-840.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner E. W., Tabor C. W., Tabor H. Mutants of Escherichia coli that do not contain 1,4-diaminobutane (putrescine) or spermidine. J Biol Chem. 1979 Dec 25;254(24):12419–12426. [PubMed] [Google Scholar]
- Harrison D. P., Bode V. C. Putrescine and certain polyamines can inhibit DNA injection from bacteriophage lambda. J Mol Biol. 1975 Aug 15;96(3):461–470. doi: 10.1016/0022-2836(75)90173-4. [DOI] [PubMed] [Google Scholar]
- Hayashi S., Fujita K. Antizyme and antizyme inhibitor of ornithine decarboxylase (rat liver). Methods Enzymol. 1983;94:185–193. doi: 10.1016/s0076-6879(83)94031-4. [DOI] [PubMed] [Google Scholar]
- Heby O. Role of polyamines in the control of cell proliferation and differentiation. Differentiation. 1981;19(1):1–20. doi: 10.1111/j.1432-0436.1981.tb01123.x. [DOI] [PubMed] [Google Scholar]
- Heller J. S., Kyriakidis D. A., Canellakis E. S. Purification and properties of the antizymes of Escherichia coli to ornithine decarboxylase. Biochim Biophys Acta. 1983 Oct 4;760(1):154–162. doi: 10.1016/0304-4165(83)90137-x. [DOI] [PubMed] [Google Scholar]
- Heller J. S., Rostomily R., Kyriakidis D. A., Canellakis E. S. Regulation of polyamine biosynthesis in Escherichia coli by basic proteins. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5181–5184. doi: 10.1073/pnas.80.17.5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilger F., Mortimer R. K. Genetic mapping of arg1 and arg8 in Saccharomyces cerevisiae by trisomic analysis combined with interallelic complementation. J Bacteriol. 1980 Jan;141(1):270–274. doi: 10.1128/jb.141.1.270-274.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshfield I. N., Rosenfeld H. J., Leifer Z., Maas W. K. Isolation and characterization of a mutant of Escherichia coli blocked in the synthesis of putrescine. J Bacteriol. 1970 Mar;101(3):725–730. doi: 10.1128/jb.101.3.725-730.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope J. A., Stevens L. The effects of putrescine, spermidine, and spermine on the growth of a polyamine-requiring mutant of Aspergillus nidulans. Biochem Soc Trans. 1976;4(6):1128–1130. doi: 10.1042/bst0041128. [DOI] [PubMed] [Google Scholar]
- Hosaka K., Yamashita S. Induction of choline transport and its role in the stimulation of the incorporation of choline into phosphatidylcholine by polyamines in a polyamine auxotroph of Saccharomyces cerevisiae. Eur J Biochem. 1981 May;116(1):1–6. doi: 10.1111/j.1432-1033.1981.tb05292.x. [DOI] [PubMed] [Google Scholar]
- Huang S. C., Kyriakidis D. A., Rinehart C. A., Jr, Canellakis S. Reversal of the antizyme inhibition of ornithine decarboxylase by nucleic acids. Biochem Pharmacol. 1984 Apr 15;33(8):1383–1386. doi: 10.1016/0006-2952(84)90200-4. [DOI] [PubMed] [Google Scholar]
- Hunter J. S., Greene R. C., Su C. H. Genetic characterization of the metK locus in Escherichia coli K-12. J Bacteriol. 1975 Jun;122(3):1144–1152. doi: 10.1128/jb.122.3.1144-1152.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höltje J. V. Streptomycin uptake via an inducible polyamine transport system in Escherichia coli. Eur J Biochem. 1978 May 16;86(2):345–351. doi: 10.1111/j.1432-1033.1978.tb12316.x. [DOI] [PubMed] [Google Scholar]
- Hölttä E., Jänne J., Pispa J. Ornithine decarboxylase from Escherichia coli: stimulation of the enzyme activity by nucleotides. Biochem Biophys Res Commun. 1972 Jun 9;47(5):1165–1171. doi: 10.1016/0006-291x(72)90957-6. [DOI] [PubMed] [Google Scholar]
- Hölttä E., Jänne J., Pispa J. The regulation of polyamine synthesis during the stringent control in Escherichia coli. Biochem Biophys Res Commun. 1974 Aug 5;59(3):1104–1111. doi: 10.1016/s0006-291x(74)80092-6. [DOI] [PubMed] [Google Scholar]
- Igarashi K., Kashiwagi K., Kishida K., Kakegawa T., Hirose S. Decrease in the S1 protein of 30-S ribosomal subunits in polyamine-requiring mutants of Escherichia coli grown in the absence of polyamines. Eur J Biochem. 1981;114(1):127–131. doi: 10.1111/j.1432-1033.1981.tb06182.x. [DOI] [PubMed] [Google Scholar]
- Igarashi K., Mitsui K., Kubota M., Shirakuma M., Ohnishi R., Hirose S. Effect of polyamines on synthesis and degradation of guanosine 5'-diphosphate 3'-diphosphate. Biochim Biophys Acta. 1983 Feb 22;755(3):326–331. doi: 10.1016/0304-4165(83)90234-9. [DOI] [PubMed] [Google Scholar]
- Janne J., Williams-Ashman H. G., Schenone A. Spermidine synthesizing enzymes in baker's yeast. Biochem Biophys Res Commun. 1971 Jun 18;43(6):1362–1368. doi: 10.1016/s0006-291x(71)80024-4. [DOI] [PubMed] [Google Scholar]
- Jorstad C. M., Harada J. J., Morris D. R. Structural specificity of the spermidine requirement of an Escherichia coli auxotroph. J Bacteriol. 1980 Feb;141(2):456–463. doi: 10.1128/jb.141.2.456-463.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jänne J., Pösö H., Raina A. Polyamines in rapid growth and cancer. Biochim Biophys Acta. 1978 Apr 6;473(3-4):241–293. doi: 10.1016/0304-419x(78)90015-x. [DOI] [PubMed] [Google Scholar]
- Kaiser D., Syvanen M., Masuda T. DNA packaging steps in bacteriophage lambda head assembly. J Mol Biol. 1975 Jan 15;91(2):175–186. doi: 10.1016/0022-2836(75)90158-8. [DOI] [PubMed] [Google Scholar]
- Kallio A., McCann P. P., Bey P. DL-a-Monofluoromethylputrescine is a potent irreversible inhibitor of Escherichia coli ornithine decarboxylase. Biochem J. 1982 Jun 15;204(3):771–775. doi: 10.1042/bj2040771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallio A., McCann P. P., Bey P. DL-alpha-(Difluoromethyl)arginine: a potent enzyme-activated irreversible inhibitor of bacterial decarboxylases. Biochemistry. 1981 May 26;20(11):3163–3168. doi: 10.1021/bi00514a027. [DOI] [PubMed] [Google Scholar]
- Kallio A., McCann P. P. Difluoromethylornithine irreversibly inactivates ornithine decarboxylase of Pseudomonas aeruginosa, but does not inhibit the enzymes of Escherichia coli. Biochem J. 1981 Oct 15;200(1):69–75. doi: 10.1042/bj2000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamio Y., Terawaki Y., Izaki K. Biosynthesis of cadaverine-containing peptidoglycan in Selenomonas ruminantium. J Biol Chem. 1982 Mar 25;257(6):3326–3333. [PubMed] [Google Scholar]
- Karlin J. N., Bowman B. J., Davis R. H. Compartmental behavior of ornithine in Neurospora crassa. J Biol Chem. 1976 Jul 10;251(13):3948–3955. [PubMed] [Google Scholar]
- Karrer E., Bose R. J., Warren R. A. Polyamines of Pseudomonas acidovorans. J Bacteriol. 1973 Jun;114(3):1365–1366. doi: 10.1128/jb.114.3.1365-1366.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay D. G., Singer R. A., Johnston G. C. Ornithine decarboxylase activity and cell cycle regulation in Saccharomyces cerevisiae. J Bacteriol. 1980 Mar;141(3):1041–1046. doi: 10.1128/jb.141.3.1041-1046.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitani T., Fujisawa H. Purification and some properties of a protein inhibitor (antizyme) of ornithine decarboxylase from rat liver. J Biol Chem. 1984 Aug 25;259(16):10036–10040. [PubMed] [Google Scholar]
- Kropinski A. M., Bose R. J., Warren R. A. 5-(4-Aminobutylaminomethyl)uracil, an unusual pyrimidine from the deoxyribonucleic acid of bacteriophage phiW-14. Biochemistry. 1973 Jan 2;12(1):151–157. doi: 10.1021/bi00725a025. [DOI] [PubMed] [Google Scholar]
- Kuhn A. H., Jütte H., Kellenberger E. Involvement of the bacterial groM gene product in bacteriophage T7 reproduction. II. A reduced level of ion concentrations causes the blockage of T7 maturation in K-12-M cells. J Virol. 1983 Sep;47(3):540–552. doi: 10.1128/jvi.47.3.540-552.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullnig R., Rosano C. L., Hurwitz C. Identification of 2-hydroxyputrescine in a pseudomonad lacking spermidine. Biochem Biophys Res Commun. 1970;39(6):1145–1148. doi: 10.1016/0006-291x(70)90679-0. [DOI] [PubMed] [Google Scholar]
- Kurylo-Borowska Z., Heaney-Kieras J. Edeine A, edeine B, and guanidospermidine. Methods Enzymol. 1983;94:441–451. doi: 10.1016/s0076-6879(83)94080-6. [DOI] [PubMed] [Google Scholar]
- Kyriakidis D. A., Flamigni F., Pawlak J. W., Canellakis E. S. Mode of interaction of ornithine decarboxylase with antizyme and alpha-difluoromethylornithine. Biochem Pharmacol. 1984 May 1;33(9):1575–1578. doi: 10.1016/0006-2952(84)90435-0. [DOI] [PubMed] [Google Scholar]
- Kyriakidis D. A., Heller J. S., Canellakis E. S. Modulation of ornithine decarboxylase activity in Escherichia coli by positive and negative effectors. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4699–4703. doi: 10.1073/pnas.75.10.4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakidis D. A., Heller J. S., Canellakis E. S. Purification of ornithine decarboxylase antizymes (Escherichia coli). Methods Enzymol. 1983;94:193–199. doi: 10.1016/s0076-6879(83)94032-6. [DOI] [PubMed] [Google Scholar]
- Linderoth N., Morris D. R. Structural specificity of the triamines sym-homospermidine and aminopropylcadaverine in stimulating growth of spermidine auxotrophs of Escherichia coli. Biochem Biophys Res Commun. 1983 Dec 16;117(2):616–622. doi: 10.1016/0006-291x(83)91245-7. [DOI] [PubMed] [Google Scholar]
- Lusk J. E., Williams R. J., Kennedy E. P. Magnesium and the growth of Escherichia coli. J Biol Chem. 1968 May 25;243(10):2618–2624. [PubMed] [Google Scholar]
- MAGER J. The stabilizing effect of spermine and related polyamines and bacterial protoplasts. Biochim Biophys Acta. 1959 Dec;36:529–531. doi: 10.1016/0006-3002(59)90195-7. [DOI] [PubMed] [Google Scholar]
- MUDD S. H., CANTONI G. L. Activation of methionine for transmethylation. III. The methionine-activating enzyme of Bakers' yeast. J Biol Chem. 1958 Mar;231(1):481–492. [PubMed] [Google Scholar]
- Maas W. K. Mapping of genes involved in the synthesis of spermidine in Escherichia coli. Mol Gen Genet. 1972;119(1):1–9. doi: 10.1007/BF00270439. [DOI] [PubMed] [Google Scholar]
- Maltman K. L., Neuhard J., Lewis H. A., Warren R. A. Synthesis of thymine and alpha-putrescinylthymine in bacteriophage phi W-14-infected Pseudomonas acidovorans. J Virol. 1980 May;34(2):354–359. doi: 10.1128/jvi.34.2.354-359.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltman K. L., Neuhard J., Warren R. A. 5-[(Hydroxymethyl)-O-pyrophosphoryl]uracil, an intermediate in the biosynthesis of alpha-putrescinylthymine in deoxyribonucleic acid of bacteriophage phi W-14. Biochemistry. 1981 Jun 9;20(12):3586–3591. doi: 10.1021/bi00515a043. [DOI] [PubMed] [Google Scholar]
- Markham G. D., DeParasis J., Gatmaitan J. The sequence of metK, the structural gene for S-adenosylmethionine synthetase in Escherichia coli. J Biol Chem. 1984 Dec 10;259(23):14505–14507. [PubMed] [Google Scholar]
- Markham G. D., Hafner E. W., Tabor C. W., Tabor H. S-Adenosylmethionine synthetase from Escherichia coli. J Biol Chem. 1980 Oct 10;255(19):9082–9092. [PubMed] [Google Scholar]
- Markham G. D., Hafner E. W., Tabor C. W., Tabor H. S-adenosylmethionine synthetase (methionine adenosyltransferase) (Escherichia coli). Methods Enzymol. 1983;94:219–222. doi: 10.1016/s0076-6879(83)94037-5. [DOI] [PubMed] [Google Scholar]
- Markham G. D. Spatial proximity of two divalent metal ions at the active site of S-adenosylmethionine synthetase. J Biol Chem. 1981 Feb 25;256(4):1903–1909. [PubMed] [Google Scholar]
- Markham G. D. Structure of the divalent metal ion activator binding site of S-adenosylmethionine synthetase studied by vanadyl(IV) electron paramagnetic resonance. Biochemistry. 1984 Jan 31;23(3):470–478. doi: 10.1021/bi00298a011. [DOI] [PubMed] [Google Scholar]
- Markham G. D., Tabor C. W., Tabor H. S-adenosylmethionine decarboxylase (Escherichia coli). Methods Enzymol. 1983;94:228–230. doi: 10.1016/s0076-6879(83)94039-9. [DOI] [PubMed] [Google Scholar]
- Markham G. D., Tabor C. W., Tabor H. S-adenosylmethionine decarboxylase of Escherichia coli. Studies on the covalently linked pyruvate required for activity. J Biol Chem. 1982 Oct 25;257(20):12063–12068. [PubMed] [Google Scholar]
- Matsui I., Kamei M., Otani S., Morisawa S., Pegg A. E. Occurrence and induction of spermidine-N1-acetyltransferase in Escherichia coli. Biochem Biophys Res Commun. 1982 Jun 30;106(4):1155–1160. doi: 10.1016/0006-291x(82)91233-5. [DOI] [PubMed] [Google Scholar]
- McDougall K. J., Deters J., Miskimen J. Isolation of putrescine-requiring mutants of Neurospora crassa. Antonie Van Leeuwenhoek. 1977;43(2):143–151. doi: 10.1007/BF00395669. [DOI] [PubMed] [Google Scholar]
- Michaels R., Tchen T. T. Polyamine content of nucleated and enucleated Escherichia coli cells. J Bacteriol. 1968 May;95(5):1966–1967. doi: 10.1128/jb.95.5.1966-1967.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P. B., Scraba D. G., Leyritz-Wills M., Maltman K. L., Warren R. A. Formation and possible functions of alpha-putrescinylthymine in bacteriophage phi W-14 DNA: analysis of bacteriophage mutants with decreased levels of alpha-putrescinylthymine in their DNAs. J Virol. 1983 Sep;47(3):399–405. doi: 10.1128/jvi.47.3.399-405.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills J., Dubin D. T. Some effects of spermine on Escherichia coli. Mol Pharmacol. 1966 Jul;2(4):311–318. [PubMed] [Google Scholar]
- Mitchell J. L., Augustine T. A., Wilson J. M. Protein factor which induces conversion between Physarum ornithine decarboxylase forms in vitro. Biochim Biophys Acta. 1981 Jan 15;657(1):257–267. doi: 10.1016/0005-2744(81)90149-2. [DOI] [PubMed] [Google Scholar]
- Mitchell J. L., Carter D. D., Rybski J. A. Control of ornithine decarboxylase activity in Physarum by polyamines. Eur J Biochem. 1978 Dec;92(2):325–331. doi: 10.1111/j.1432-1033.1978.tb12751.x. [DOI] [PubMed] [Google Scholar]
- Mitchell J. L., Kottas G. E. Osmotically-induced modification of ornithine decarboxylase in Physarum. FEBS Lett. 1979 Jun 15;102(2):265–268. doi: 10.1016/0014-5793(79)80015-0. [DOI] [PubMed] [Google Scholar]
- Mitchell J. L., Mitchell G. K., Carter D. D. Amine-specificity of the inactivating ornithine decarboxylase modification in Physarum polycephalum. Biochem J. 1982 Sep 1;205(3):551–557. doi: 10.1042/bj2050551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J. L. Ornithine decarboxylase and the ornithine decarboxylase-modifying protein of Physarum polycephalum. Methods Enzymol. 1983;94:140–146. doi: 10.1016/s0076-6879(83)94022-3. [DOI] [PubMed] [Google Scholar]
- Mitchell J. L., Rusch H. P. Regulation of polyamine synthesis in Physarum polyciphalum during growth and differentiation. Biochim Biophys Acta. 1973 Feb 28;297(2):503–516. doi: 10.1016/0304-4165(73)90098-6. [DOI] [PubMed] [Google Scholar]
- Mitchell J. L., Wilson J. M. Polyamine-stimulated alteration of the ornithine decarboxylase molecule in Physarum polycephalum. Biochem J. 1983 Aug 15;214(2):345–351. doi: 10.1042/bj2140345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsui K., Igarashi K., Kakegawa T., Hirose S. Preferential stimulation of the in vivo synthesis of a protein by polyamines in Escherichia coli: purification and properties of the specific protein. Biochemistry. 1984 Jun 5;23(12):2679–2683. doi: 10.1021/bi00307a022. [DOI] [PubMed] [Google Scholar]
- Mitsui K., Ohnishi R., Hirose S., Igarashi K. Necessity of polyamines for maximum in vivo synthesis of beta beta' subunits of RNA polymerase. Biochem Biophys Res Commun. 1984 Sep 17;123(2):528–534. doi: 10.1016/0006-291x(84)90261-4. [DOI] [PubMed] [Google Scholar]
- Morris D. R., Boeker E. A. Biosynthetic and biodegradative ornithine and arginine decarboxylases from Escherichia coli. Methods Enzymol. 1983;94:125–134. doi: 10.1016/s0076-6879(83)94020-x. [DOI] [PubMed] [Google Scholar]
- Morris D. R., Fillingame R. H. Regulation of amino acid decarboxylation. Annu Rev Biochem. 1974;43(0):303–325. doi: 10.1146/annurev.bi.43.070174.001511. [DOI] [PubMed] [Google Scholar]
- Morris D. R., Hansen M. T. Influence of polyamine limitation on the chain growth rates of beta-galactosidase and of its messenger ribonucleic acid. J Bacteriol. 1973 Nov;116(2):588–592. doi: 10.1128/jb.116.2.588-592.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris D. R., Jorstad C. M. Growth and macromolecular composition of a mutant of Escherichia coli during polyamine limitation. J Bacteriol. 1973 Jan;113(1):271–277. doi: 10.1128/jb.113.1.271-277.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris D. R., Jorstad C. M. Isolation of conditionally putrescine-deficient mutants of Escherichia coli. J Bacteriol. 1970 Mar;101(3):731–737. doi: 10.1128/jb.101.3.731-737.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris D. R., Koffron K. L. Putrescine biosynthesis in Escherichia coli. Regulation through pathway selection. J Biol Chem. 1969 Nov 25;244(22):6094–6099. [PubMed] [Google Scholar]
- Morris D. R., Koffron K. L. Urea production and putrescine biosynthesis by Escherichia coli. J Bacteriol. 1967 Nov;94(5):1516–1519. doi: 10.1128/jb.94.5.1516-1519.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris D. R., Pardee A. B. A biosynthetic ornithine decarboxylase in Escherichia coli. Biochem Biophys Res Commun. 1965 Sep 22;20(6):697–702. doi: 10.1016/0006-291x(65)90072-0. [DOI] [PubMed] [Google Scholar]
- Morris D. R., Pardee A. B. Multiple pathways of putrescine biosynthesis in Escherichia coli. J Biol Chem. 1966 Jul 10;241(13):3129–3135. [PubMed] [Google Scholar]
- Munro G. F., Hercules K., Morgan J., Sauerbier W. Dependence of the putrescine content of Escherichia coli on the osmotic strength of the medium. J Biol Chem. 1972 Feb 25;247(4):1272–1280. [PubMed] [Google Scholar]
- Munro G. F., Sauerbier W. Osmotically induced excretion of putrescine by mutants of Escherichia coli defective in potassium transport. J Bacteriol. 1973 Oct;116(1):488–490. doi: 10.1128/jb.116.1.488-490.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MØLLER V. Simplified tests for some amino acid decarboxylases and for the arginine dihydrolase system. Acta Pathol Microbiol Scand. 1955;36(2):158–172. doi: 10.1111/j.1699-0463.1955.tb04583.x. [DOI] [PubMed] [Google Scholar]
- Nash H. A. Integrative recombination of bacteriophage lambda DNA in vitro. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1072–1076. doi: 10.1073/pnas.72.3.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak S., Boeker E. A. The inducible arginine decarboxylase of Escherichia coli B: activity of the dimer and the decamer. Arch Biochem Biophys. 1981 Mar;207(1):110–116. doi: 10.1016/0003-9861(81)90015-1. [DOI] [PubMed] [Google Scholar]
- Panagiotidis C. A., Canellakis E. S. Comparison of the basic Escherichia coli antizyme 1 and antizyme 2 with the ribosomal proteins S20/L26 and L34. J Biol Chem. 1984 Dec 25;259(24):15025–15027. [PubMed] [Google Scholar]
- Paulin L., Pösö H. Ornithine decarboxylase activity from an extremely thermophilic bacterium, Clostridium thermohydrosulfuricum. Effect of GTP analogues on enzyme activity. Biochim Biophys Acta. 1983 Jan 12;742(1):197–205. doi: 10.1016/0167-4838(83)90377-1. [DOI] [PubMed] [Google Scholar]
- Paulus T. J., Cramer C. L., Davis R. H. Compartmentation of spermidine in Neurospora crassa. J Biol Chem. 1983 Jul 25;258(14):8608–8612. [PubMed] [Google Scholar]
- Paulus T. J., Davis R. H. Metabolic sequestration of putrescine in Neurospora crassa. Biochem Biophys Res Commun. 1982 Jan 15;104(1):228–233. doi: 10.1016/0006-291x(82)91963-5. [DOI] [PubMed] [Google Scholar]
- Paulus T. J., Davis R. H. Regulation of polyamine synthesis in relation to putrescine and spermidine pools in Neurospora crassa. J Bacteriol. 1981 Jan;145(1):14–20. doi: 10.1128/jb.145.1.14-20.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus T. J., Kiyono P., Davis R. H. Polyamine-deficient Neurospora crassa mutants and synthesis of cadaverine. J Bacteriol. 1982 Oct;152(1):291–297. doi: 10.1128/jb.152.1.291-297.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peebles C. L., Gegenheimer P., Abelson J. Precise excision of intervening sequences from precursor tRNAs by a membrane-associated yeast endonuclease. Cell. 1983 Feb;32(2):525–536. doi: 10.1016/0092-8674(83)90472-5. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., Bitonti A. J., McCann P. P., Coward J. K. Inhibition of bacterial aminopropyltransferases by S-adenosyl-1,8-diamino-3-thiooctane and by dicyclohexylamine. FEBS Lett. 1983 May 8;155(2):192–196. doi: 10.1016/0014-5793(82)80600-5. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., Jacobs G. Comparison of inhibitors of S-adenosylmethionine decarboxylase from different species. Biochem J. 1983 Aug 1;213(2):495–502. doi: 10.1042/bj2130495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E. S-adenosylmethionine decarboxylase: a brief review. Cell Biochem Funct. 1984 Jan;2(1):11–15. doi: 10.1002/cbf.290020105. [DOI] [PubMed] [Google Scholar]
- Popkin P. S., Maas W. K. Escherichia coli regulatory mutation affecting lysine transport and lysine decarboxylase. J Bacteriol. 1980 Feb;141(2):485–492. doi: 10.1128/jb.141.2.485-492.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pösö H., Hannonen P., Himberg J. J., Jänne J. Adenosylmethionine decarboxylase from various organisms: relation of the putrescine activation of the enzyme to the ability of the organism to synthesize spermine. Biochem Biophys Res Commun. 1976 Jan 12;68(1):227–234. doi: 10.1016/0006-291x(76)90033-4. [DOI] [PubMed] [Google Scholar]
- Pösö H., Pegg A. E. Measurement of the amount of ornithine decarboxylase in Saccharomyces cerevisiae and Saccharomyces uvarum by using alpha-[5-14C]difluoromethylornithine. Biochim Biophys Acta. 1983 Sep 28;747(3):209–214. doi: 10.1016/0167-4838(83)90099-7. [DOI] [PubMed] [Google Scholar]
- Pösö H., Sinervirta R., Jänne J. S-adenosylmethionine decarboxylase from baker's yeast. Biochem J. 1975 Oct;151(1):67–73. doi: 10.1042/bj1510067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROZANSKY R., BACHRACH U., GROSSOWICZ N. Studies on the antibacterial action of spermine. J Gen Microbiol. 1954 Feb;10(1):11–16. doi: 10.1099/00221287-10-1-11. [DOI] [PubMed] [Google Scholar]
- Raina A., Jansen M., Cohen S. S. Polyamines and the accumulation of ribonucleic acid in some polyauxotrophic strains of Escherichia coli. J Bacteriol. 1967 Nov;94(5):1684–1696. doi: 10.1128/jb.94.5.1684-1696.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recsei P. A., Snell E. E. Pyruvoyl enzymes. Annu Rev Biochem. 1984;53:357–387. doi: 10.1146/annurev.bi.53.070184.002041. [DOI] [PubMed] [Google Scholar]
- Riley W. D., Snell E. E. Histidine decarboxylase of Lactobacillus 30a. IV. The presence of covalently bound pyruvate as the prosthetic group. Biochemistry. 1968 Oct;7(10):3520–3528. doi: 10.1021/bi00850a029. [DOI] [PubMed] [Google Scholar]
- Rosano C. L., Bunce S. C., Hurwitz C. Localization of polyamine enhancement of protein synthesis to subcellular components of Escherichia coli and Pseudomonas sp. strain Kim. J Bacteriol. 1983 Jan;153(1):326–334. doi: 10.1128/jb.153.1.326-334.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein K. E., Streibel E., Massey S., Lapi L., Cohen S. S. Polyamine metabolism in potassium-deficient bacteria. J Bacteriol. 1972 Dec;112(3):1213–1221. doi: 10.1128/jb.112.3.1213-1221.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D. H. Clinical relevance of polyamines. Crit Rev Clin Lab Sci. 1983;18(3):261–311. doi: 10.3109/10408368209085073. [DOI] [PubMed] [Google Scholar]
- Russell D. H. Posttranslational modification of ornithine decarboxylase by its product putrescine. Biochem Biophys Res Commun. 1981 Apr 30;99(4):1167–1172. doi: 10.1016/0006-291x(81)90741-5. [DOI] [PubMed] [Google Scholar]
- Sabo D. L., Boeker E. A., Byers B., Waron H., Fischer E. H. Purification and physical properties of inducible Escherichia coli lysine decarboxylase. Biochemistry. 1974 Feb 12;13(4):662–670. doi: 10.1021/bi00701a005. [DOI] [PubMed] [Google Scholar]
- Sabo D. L., Fischer E. H. Chemical properties of Escherichia coli lysine decarboxylase including a segment of its pyridoxal 5'-phosphate binding site. Biochemistry. 1974 Feb 12;13(4):670–676. doi: 10.1021/bi00701a006. [DOI] [PubMed] [Google Scholar]
- Sakai T. T., Cohen S. S. Effects of polyamines on the structure and reactivity of tRNA. Prog Nucleic Acid Res Mol Biol. 1976;17:15–42. doi: 10.1016/s0079-6603(08)60064-1. [DOI] [PubMed] [Google Scholar]
- Satishchandran C., Boyle S. M. Antagonistic transcriptional regulation of the putrescine biosynthetic enzyme agmatine ureohydrolase by cyclic AMP and agmatine in Escherichia coli. J Bacteriol. 1984 Feb;157(2):552–559. doi: 10.1128/jb.157.2.552-559.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scraba D. G., Bradley R. D., Leyritz-Wills M., Warren R. A. Bacteriophage phi W-14: the contribution of covalently bound putrescine to DNA packing in the phage head. Virology. 1983 Jan 15;124(1):152–160. doi: 10.1016/0042-6822(83)90298-2. [DOI] [PubMed] [Google Scholar]
- Sjoerdsma A., Schechter P. J. Chemotherapeutic implications of polyamine biosynthesis inhibition. Clin Pharmacol Ther. 1984 Mar;35(3):287–300. doi: 10.1038/clpt.1984.33. [DOI] [PubMed] [Google Scholar]
- Spathas D. H., Clutterbuck A. J., Pateman J. A. A polyamine-sensitive mutant of Aspergillus nidulans. J Gen Microbiol. 1983 Jun;129(6):1865–1871. doi: 10.1099/00221287-129-6-1865. [DOI] [PubMed] [Google Scholar]
- Spathas D. H., Pateman J. A., Clutterbuck A. J. Polyamine transport in Aspergillus nidulans. J Gen Microbiol. 1982 Mar;128(3):557–563. doi: 10.1099/00221287-128-3-557. [DOI] [PubMed] [Google Scholar]
- Stevens L., McKinnon I. M. The effect of 1,4-diaminobutanone on the stability of ornithine decarboxylase from Aspergillus nidulans. Biochem J. 1977 Sep 15;166(3):635–637. doi: 10.1042/bj1660635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens L. Ornithine decarboxylase activity in germinating conidia of Aspergillus nidulans. FEBS Lett. 1975 Nov 1;59(1):80–82. doi: 10.1016/0014-5793(75)80345-0. [DOI] [PubMed] [Google Scholar]
- Stevens L. Studies on the interaction of homologues of spermine with deoxyribonucleic acid and with bacterial protoplasts. Biochem J. 1967 Jun;103(3):811–815. doi: 10.1042/bj1030811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens L., Winther M. D. Spermine, spermidine and putrescine in fungal development. Adv Microb Physiol. 1979;19:63–148. doi: 10.1016/s0065-2911(08)60198-8. [DOI] [PubMed] [Google Scholar]
- TABOR C. W., ROSENTHAL S. M. Pharmacology of spermine and spermidine; some effects on animals and bacteria. J Pharmacol Exp Ther. 1956 Feb;116(2):139–155. [PubMed] [Google Scholar]
- TABOR C. W., TABOR H., BACHRACH U. IDENTIFICATION OF THE AMINOALDEHYDES PRODUCED BY THE OXIDATION OF SPERMINE AND SPERMIDINE WITH PURIFIED PLASMA AMINE OXIDASE. J Biol Chem. 1964 Jul;239:2194–2203. [PubMed] [Google Scholar]
- TABOR H., ROSENTHAL S. M., TABOR C. W. The biosynthesis of spermidine and spermine from putrescine and methionine. J Biol Chem. 1958 Oct;233(4):907–914. [PubMed] [Google Scholar]
- TABOR H. Stabilization of bacteriophage T5 by spermine and related polyamines. Biochem Biophys Res Commun. 1960 Oct;3:382–385. doi: 10.1016/0006-291x(60)90049-8. [DOI] [PubMed] [Google Scholar]
- TABOR H., TABOR C. W. SPERMIDINE, SPERMINE, AND RELATED AMINES. Pharmacol Rev. 1964 Sep;16:245–300. [PubMed] [Google Scholar]
- Tabor C. W., Dobbs L. G. Metabolism of 1,4-diaminobutane and spermidine in Escherichia coli: the effects of low temperature during storage and harvesting of cultures. J Biol Chem. 1970 Apr 25;245(8):2086–2091. [PubMed] [Google Scholar]
- Tabor C. W., Kellogg P. D. Identification of flavin adenine dinucleotide and heme in a homogeneous spermidine dehydrogenase from Serratia marcescens. J Biol Chem. 1970 Oct 25;245(20):5424–5433. [PubMed] [Google Scholar]
- Tabor C. W., Kellogg P. D. The effect of isolation conditions on the polyamine content of Escherichia coli ribosomes. J Biol Chem. 1967 Mar 10;242(5):1044–1052. [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. 1,4-Diaminobutane (putrescine), spermidine, and spermine. Annu Rev Biochem. 1976;45:285–306. doi: 10.1146/annurev.bi.45.070176.001441. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H., Hafner E. H. Mass screening for mutants in the biosynthetic pathway for polyamines in Escherichia coli: a general method for mutants in enzymatic reactions producing CO2. Methods Enzymol. 1983;94:83–91. doi: 10.1016/s0076-6879(83)94014-4. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H., Hafner E. W. Escherichia coli mutants completely deficient in adenosylmethionine decarboxylase and in spermidine biosynthesis. J Biol Chem. 1978 May 25;253(10):3671–3676. [PubMed] [Google Scholar]
- Tabor C. W., Tabor H., Hafner E. W., Markham G. D., Boyle S. M. Cloning of the Escherichia coli genes for the biosynthetic enzymes for polyamines. Methods Enzymol. 1983;94:117–121. doi: 10.1016/s0076-6879(83)94019-3. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Methionine adenosyltransferase (S-adenosylmethionine synthetase) and S-adenosylmethionine decarboxylase. Adv Enzymol Relat Areas Mol Biol. 1984;56:251–282. doi: 10.1002/9780470123027.ch4. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Polyamines. Annu Rev Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Transport systems for 1,4-diaminobutane, spermidine, and spermine in Escherichia coli. J Biol Chem. 1966 Aug 25;241(16):3714–3723. [PubMed] [Google Scholar]
- Tabor H., Hafner E. W., Tabor C. W. Construction of an Escherichia coli strain unable to synthesize putrescine, spermidine, or cadaverine: characterization of two genes controlling lysine decarboxylase. J Bacteriol. 1980 Dec;144(3):952–956. doi: 10.1128/jb.144.3.952-956.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor H., Hafner E. W., Tabor C. W. Localized mutagenesis of any specific region of the Escherichia coli chromosome with bacteriophage Mu. Methods Enzymol. 1983;94:91–104. doi: 10.1016/s0076-6879(83)94015-6. [DOI] [PubMed] [Google Scholar]
- Tabor H., Hafner E. W., Tabor C. W. Localized mutagenesis with bacteriophage Mu: method for increasing the frequency of specific bacterial mutants. J Bacteriol. 1977 Oct;132(1):359–361. doi: 10.1128/jb.132.1.359-361.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor H., Tabor C. W. Biosynthesis and metabolism of 1,4-diaminobutane, spermidine, spermine, and related amines. Adv Enzymol Relat Areas Mol Biol. 1972;36:203–268. doi: 10.1002/9780470122815.ch7. [DOI] [PubMed] [Google Scholar]
- Tabor H., Tabor C. W., Cohn M. S., Hafner E. W. Streptomycin resistance (rpsL) produces an absolute requirement for polyamines for growth of an Escherichia coli strain unable to synthesize putrescine and spermidine [delta(speA-speB) delta specC]. J Bacteriol. 1981 Aug;147(2):702–704. doi: 10.1128/jb.147.2.702-704.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor H., Tabor C. W. Formation of 1,4-diaminobutane and of spermidine by an ornithine auxotroph of Escherichia coli grown on limiting ornithine or arginine. J Biol Chem. 1969 May 10;244(9):2286–2292. [PubMed] [Google Scholar]
- Tabor H., Tabor C. W., Hafner E. W. Convenient method for detecting 14CO2 in multiple samples: application to rapid screening for mutants. J Bacteriol. 1976 Oct;128(1):485–486. doi: 10.1128/jb.128.1.485-486.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor H., Tabor C. W., Irreverre F. Quantitative determination of aliphatic diamines and polyamines by an automated liquid chromatography procedure. Anal Biochem. 1973 Oct;55(2):457–467. doi: 10.1016/0003-2697(73)90136-x. [DOI] [PubMed] [Google Scholar]
- Tabor H., Tabor C. W. Isolation, characterization, and turnover of glutathionylspermidine from Escherichia coli. J Biol Chem. 1975 Apr 10;250(7):2648–2654. [PubMed] [Google Scholar]
- Tabor H., Tabor C. W. Partial separation of two pools of arginine in Escherichia coli; preferential use of exogenous rather than endogenous arginine for the biosynthesis of 1,4-diaminobutane. J Biol Chem. 1969 Dec 10;244(23):6383–6387. [PubMed] [Google Scholar]
- Tabor H., Tabor C. W. Polyamine requirement for efficient translation of amber codons in vivo. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7087–7091. doi: 10.1073/pnas.79.23.7087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobari J., Tchen T. T. Identification of (+)-hydroxyputrescine (1,4-diaminobutan-2-ol) from a Pseudomonas species. J Biol Chem. 1971 Mar 10;246(5):1262–1265. [PubMed] [Google Scholar]
- Tyagi A. K., Tabor C. W., Tabor H. Ornithine decarboxylase (Saccharomyces cerevisiae). Methods Enzymol. 1983;94:135–139. doi: 10.1016/s0076-6879(83)94021-1. [DOI] [PubMed] [Google Scholar]
- Tyagi A. K., Tabor C. W., Tabor H. Ornithine decarboxylase from Saccharomyces cerevisiae. Purification, properties, and regulation of activity. J Biol Chem. 1981 Dec 10;256(23):12156–12163. [PubMed] [Google Scholar]
- Tyagi A. K., Tabor H., Tabor C. W. Inactivation of yeast ornithine decarboxylase by polyamines in vivo does not result from the incorporation of polyamines into enzyme protein. Biochem Biophys Res Commun. 1982 Nov 30;109(2):533–540. doi: 10.1016/0006-291x(82)91754-5. [DOI] [PubMed] [Google Scholar]
- Tyagi A. K., Wickner R. B., Tabor C. W., Tabor H. Specificity of polyamine requirements for the replication and maintenance of different double-stranded RNA plasmids in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1149–1153. doi: 10.1073/pnas.81.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezawa H., Kondo S., Iinuma H., Kunimoto S., Ikeda Y., Iwasawa H., Ikeda D., Takeuchi T. Structure of an antitumor antibiotic, spergualin. J Antibiot (Tokyo) 1981 Dec;34(12):1622–1624. doi: 10.7164/antibiotics.34.1622. [DOI] [PubMed] [Google Scholar]
- Viotti A., Bagni N., Sturani E., Alberghina F. A. Magnesium and polyamine levels in Neurospora crassa mycelia. Biochim Biophys Acta. 1971 Aug 19;244(2):329–337. doi: 10.1016/0304-4165(71)90234-0. [DOI] [PubMed] [Google Scholar]
- Weiss R. L., Davis R. H. Intracellular localization of enzymes of arginine metabolism in Neurospora. J Biol Chem. 1973 Aug 10;248(15):5403–5408. [PubMed] [Google Scholar]
- Wertheimer S. J., Leifer Z. Putrescine and spermidine sensitivity of lysine decarboxylase in Escherichia coli: evidence for a constitutive enzyme and its mode of regulation. Biochem Biophys Res Commun. 1983 Jul 29;114(2):882–888. doi: 10.1016/0006-291x(83)90863-x. [DOI] [PubMed] [Google Scholar]
- Whitney P. A., Morris D. R. Polyamine auxotrophs of Saccharomyces cerevisiae. J Bacteriol. 1978 Apr;134(1):214–220. doi: 10.1128/jb.134.1.214-220.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B., Tabor C. W., Tabor H. Purification of adenosylmethionine decarboxylase from Escherichia coli W: evidence for covalently bound pyruvate. J Biol Chem. 1970 Apr 25;245(8):2132–2139. [PubMed] [Google Scholar]
- Williams-Ashman H. G., Canellakis Z. N. Polyamines in mammalian biology and medicine. Perspect Biol Med. 1979 Spring;22(3):421–453. doi: 10.1353/pbm.1979.0013. [DOI] [PubMed] [Google Scholar]
- Williams-Ashman H. G., Schenone A. Methyl glyoxal bis(guanylhydrazone) as a potent inhibitor of mammalian and yeast S-adenosylmethionine decarboxylases. Biochem Biophys Res Commun. 1972 Jan 14;46(1):288–295. doi: 10.1016/0006-291x(72)90661-4. [DOI] [PubMed] [Google Scholar]
- Wright J. M., Boyle S. M. Negative control of ornithine decarboxylase and arginine decarboxylase by adenosine-3':5'-cyclic monophosphate in Escherichia coli. Mol Gen Genet. 1982;186(4):482–487. doi: 10.1007/BF00337952. [DOI] [PubMed] [Google Scholar]
- Wu W. H., Morris D. R. Biosynthetic arginine decarboxylase from Escherichia coli. Purification and properties. J Biol Chem. 1973 Mar 10;248(5):1687–1695. [PubMed] [Google Scholar]
- Wu W. H., Morris D. R. Biosynthetic arginine decarboxylase from Escherichia coli. Subunit interactions and the role of magnesium ion. J Biol Chem. 1973 Mar 10;248(5):1696–1699. [PubMed] [Google Scholar]
- Young D. V., Srinivasan P. R. Regulation of macromolecular synthesis by putrescine in a conditional Escherichia coli putrescine auxotroph. J Bacteriol. 1972 Oct;112(1):30–39. doi: 10.1128/jb.112.1.30-39.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zappia V., Cacciapuoti G., Pontoni G., Oliva A. Mechanism of propylamine-transfer reactions. Kinetic and inhibition studies on spermidine synthase from Escherichia coli. J Biol Chem. 1980 Aug 10;255(15):7276–7280. [PubMed] [Google Scholar]


