The Nucleoporin 98 gene (NUP98) is a promiscuous gene implicated in chromosomal aberrations in hematopoietic disorders. NUP98 encodes a 98-kDa protein of the nuclear pore complex that regulates nucleocytoplasmic transport of protein and RNA. Twenty-eight different NUP98 partner genes have been identified across various human hematological malignancies,1, 2 many of which encode for homeodomain (HD) transcription factors and chromatin-modifying factors. We report here two new oncogenic fusions for NUP98, involving the homeobox gene genetic screened homeobox 2 (GSX2, formerly Gsh2) and the putative zinc-finger transcription factor gene ALL-1 fused gene from chromosome 10 (AF10)/MLLT10.
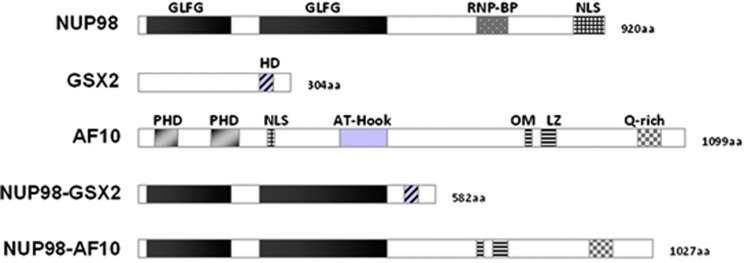
Patient 1 is a 57-year-old woman with a post-myeloproliferative neoplasm M4 acute myeloid leukemia (AML) associated with a t(4;11)(q12;p15) translocation and trisomy 8. Patient 2 is an 83-year-old man who developed an atypical myelodysplastic syndrome resembling chronic myelomonocytic leukemia associated with a t(10;11)(p12;p15) translocation. Molécular cytogenetic techniques demonstrated the NUP98-GSX2 and NUP98-AF10 fusions for the t(4;11) and the t(10;11) respectively (not shown). Reverse transcriptase (RT)-PCR using primers located within NUP98, GSX2 and AF10 exons validated the presence of the NUP98–GSX2 and NUP98–AF10 fusion transcripts in patient samples (Supplementary Figure 1). Nucleotide sequencing showed an in-frame fusion of the NUP98 exon 12 with GSX2 exon 2, predicting a putative chimeric protein of 59 kDa that joins the Gly-Leu-Phe-Gly (GLFG) repeats of the amino-terminal part of NUP98 at the carboxy (C)-terminal part of GSX2 containing a HD (Figure 1). Sequence analysis of the NUP98–AF10 fusion transcript showed an in-frame fusion of NUP98 exon 11 with AF10 exon 15, predicting a 1027-amino-acid protein with the NUP98 GLFG repeats fused to the C-terminal part of the AF10 protein including the octapeptide motif-leucine-zipper (OM-LZ) domain and the glutamine-rich (Q-rich) sequence of AF10, but without the nuclear localization signal (NLS) region.
Figure 1.
Schematic representation of the native or chimeric AF10 and GSX2 and NUP98 proteins. RNP-BD, ribonucleoparticle binding domain; PHD, plant homeodomain; Q-rich, glutamine-rich region.
Both AF10 and GSX2 genes have been involved in human hematological malignancies. GSX2 is a brain-specific class II homeobox gene of the Antennapedia family that regulates the development of mouse embryonic telencephalon.3 It has been associated with acute leukemia through its involvement in the recurrent t(4;12)(q11q12;p13) translocation.4 AF10 belongs to a family of proteins that includes AF17 and BR140 characterized by the presence of a C-terminal OM-LZ domain. AF10 is considered a putative transcription factor, binding DNA through an AT hook motif and interacting with the SWI/SNF chromatin remodeling complex.5 Through its OM-LZ domain, AF10 interacts with the histone methyltransferase hDOT1L that methylates the lysine 79 residues of histone H3 (H3K79),6, 7 a mark associated with an open-chromatin configuration. In hematological malignancies, AF10 is fused to the mixed lineage leukemia (MLL) gene by the t(10;11)(p12;q23) translocation8 and to the clathrin assembly lymphoid myeloid leukemia (CALM) gene by the t(10;11)(p12;q14) translocation.9
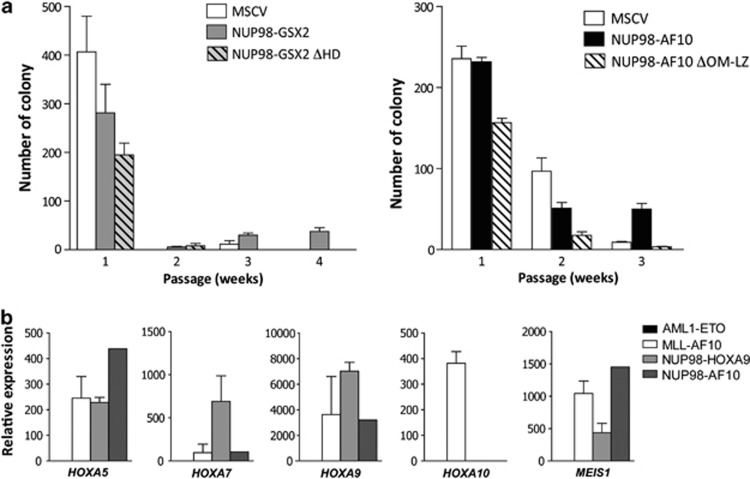
The transformation potential of the two NUP98 fusions was investigated by transducing murine primary bone marrow (BM) hematopoietic progenitors, defined as lineage-negative cells, by murine stem cell virus (MSCV) retroviral vectors containing different versions of Flag-tagged NUP98–GSX2 and NUP98–AF10 sequences as reported.10 Cells were transduced with MSCV expressing the native NUP98–GSX2 and NUP98–AF10 fusions, fusions deleted for the conserved functional domains (NUP98–GSX2-⊗HD and NUP98–AF10-ΔOM-LZ) or with the empty MSCV. Transduced cells were seeded in methylcellulose medium for serial plating assays. We observed that NUP98–GSX2-transduced progenitors formed numerous colonies after the fourth round of replating, whereas control cells were not replated after the third round (Figure 2a). Cytological analysis of the colonies showed that cells expressing NUP98–GSX2 exhibit a blast morphology, whereas empty MSCV-transduced progenitors formed monocytic and mast cell colonies (not shown). In contrast, NUP98–GSX2-⊗HD-transduced progenitors showed no proliferative advantage and were not able to form colonies after the second replating, thus illustrating that the transforming effect of the NUP98–GSX2 fusion requires the GSX2 HD. Immunofluorescence analysis showed a marked nuclear presence for the NUP98–GSX2 protein, whereas NUP98–GSX2-⊗HD was located both in the nucleus and in the cytosol with a diffuse staining pattern (Supplementary Figure 2). These data demonstrated that the NUP98–GSX2 fusion encodes a nuclear protein with a GSX2 HD-dependent oncogenic capacity. Progenitors transduced with NUP98–AF10 were able to produce a significantly increased number of colonies up to the third round of plating, contrary to the NUP98–AF10-ΔOM-LZ-transduced cells, which did not generate a significant number after the second round of plating (Figure 2a). Thus, expression of NUP98–AF10 is sufficient to induce a proliferative advantage in progenitor BM cells and this proliferative effect depends on the integrity of the OM-LZ region. CALM–AF10 (as well as some MLL fusions) participates in gene deregulation by virtue of hDOT1L recruitment at target gene loci, in particular for certain HOXA genes. As its transforming potential is linked to the integrity of the OM-LZ domain, we hypothesized that the oncogenic capacity of NUP98–AF10 is linked to HOXA gene deregulation by a process similar to that involved in MLL–AF10 and CALM–AF10 fusions. We performed quantitative RT-PCR to measure the levels of the HOXA5, HOXA7, HOXA9, HOXA10 transcripts and that of the HOX cofactor MEIS1 transcript in patient 2's (NUP98–AF10 fusion) BM cells (Figure 2b) (no material was available for patient 1). Expression analyses were performed in parallel in samples from patients with AML1-ETO-positive leukemia (n=2) as a negative control for HOXA expression, and in samples from patients with MLL–AF10 (n=1)- and NUP98–HOXA9 (n=2)-positive leukemia as positive controls. In patient 2's BM cells, we observed an elevated expression of HOXA5, HOXA7, HOXA9 and MEIS1 as in leukemic cells with MLL–AF10 and NUP98–HOXA9 fusions. HOXA10 was specifically overexpressed in the MLL–AF10 sample. No HOXA cluster gene expression was observed in AML1-ETO BM cells. In murine models, NUP98 fusions are often associated with upregulation of HOXA cluster and MEIS1 genes in blast cells.11, 12, 13 Thus, NUP98–AF10-transduced BM cells were engrafted into sublethally irradiated mice. Mice transplanted with cells transduced with empty (n=10) or NUP98–AF10 MSCV vectors (n=5) remained free of hematological disease up to 12 months after transplantation (not shown), contrary to those receiving cells transduced with the NUP98–HMGB3 fusion.10 Altogether, our results indicate that contrary to the MLL–AF10 and CALM–AF10 fusions,14, 15 NUP98–AF10 has only weak oncogenic power. This may be explained by the lack of an exclusively nuclear localization for NUP98–AF10 due to the absence of the NLS of AF10 in the chimeric protein.
Figure 2.
(a) Serial colony-plating assay of bone marrow progenitors transduced by wild-type, NUP98, NUP98–GSX2, NUP98–AF10 or empty vector. The average colony numbers±s.d. values are shown for three independent experiments. (b) Quantitative RT-PCR analyses of the HOXA5, HOXA7, HOXA9, HOXA10 and MEIS1 gene expression from RNA of various human AML samples carrying the following fusion genes: AML1-ETO (n=2), MLL–AF10 (n=1), NUP98–HOXA9 (n=2) and NUP98–AF10 (n=1). Expression was normalized with respect to ABL expression.
In conclusion, the identification of AF10 and GSX2 as new NUP98 partner genes in hematological malignancies strengthens the predominance of homeobox and chromatin-modifier genes as NUP98 partners and the deregulation of HOXA cluster genes as an oncogenic mechanism in several NUP98-associated leukemia.
Acknowledgments
This works was supported by grants from The Ligue Nationale Contre le Cancer (LNCC). GS was a recipient from the Fondation pour la Recherche Médicale (FRM).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Blood Cancer Journal website (http://www.nature.com/bcj)
Supplementary Material
References
- Romana SP, Radford-Weiss I, Ben Abdelali R, Schluth C, Petit A, Dastugue N, et al. NUP98 rearrangements in hematopoietic malignancies: a study of the Groupe Francophone de Cytogenetique Hematologique. Leukemia. 2006;20:696–706. doi: 10.1038/sj.leu.2404130. [DOI] [PubMed] [Google Scholar]
- Gough SM, Slape CI, Aplan PD. NUP98 gene fusions and hematopoietic malignancies: common themes and new biologic insights. Blood. 2011;118:6247–6257. doi: 10.1182/blood-2011-07-328880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Z, Wang B, Chen G, Nagao M, Nakafuku M, Campbell K. Homeobox genes Gsx1 and Gsx2 differentially regulate telencephalic progenitor maturation. Proc Natl Acad Sci USA. 2011;108:1675–1680. doi: 10.1073/pnas.1008824108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools J, Mentens N, Odero MD, Peeters P, Wlodarska I, Delforge M, et al. Evidence for position effects as a variant ETV6-mediated leukemogenic mechanism in myeloid leukemias with a t(4;12)(q11-q12;p13) or t(5;12)(q31;p13) Blood. 2002;99:1776–1784. doi: 10.1182/blood.v99.5.1776. [DOI] [PubMed] [Google Scholar]
- Debernardi S, Bassini A, Jones LK, Chaplin T, Linder B, de Bruijn DR, et al. The MLL fusion partner AF10 binds GAS41, a protein that interacts with the human SWI/SNF complex. Blood. 2002;99:275–281. doi: 10.1182/blood.v99.1.275. [DOI] [PubMed] [Google Scholar]
- Mohan M, Herz HM, Takahashi YH, Lin C, Lai KC, Zhang Y, et al. Linking H3K79 trimethylation to Wnt signaling through a novel Dot1-containing complex (DotCom) Genes Dev. 2010;24:574–589. doi: 10.1101/gad.1898410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y, Feng Q, Lin Y, Jiang Q, Li Y, Coffield VM, et al. hDOT1L links histone methylation to leukemogenesis. Cell. 2005;121:167–178. doi: 10.1016/j.cell.2005.02.020. [DOI] [PubMed] [Google Scholar]
- Chaplin T, Ayton P, Bernard OA, Saha V, Della Valle V, Hillion J, et al. A novel class of zinc finger/leucine zipper genes identified from the molecular cloning of the t(10;11) translocation in acute leukemia. Blood. 1995;85:1435–1441. [PubMed] [Google Scholar]
- Dreyling MH, Martinez-Climent JA, Zheng M, Mao J, Rowley JD, Bohlander SK. The t(10;11)(p13;q14) in the U937 cell line results in the fusion of the AF10 gene and CALM, encoding a new member of the AP-3 clathrin assembly protein family. Proc Natl Acad Sci USA. 1996;93:4804–4809. doi: 10.1073/pnas.93.10.4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit A, Ragu C, Della-Valle V, Mozziconacci MJ, Lafage-Pochitaloff M, Soler G, et al. NUP98-HMGB3: a novel oncogenic fusion. Leukemia. 2010;24:654–658. doi: 10.1038/leu.2009.241. [DOI] [PubMed] [Google Scholar]
- Wang GG, Cai L, Pasillas MP, Kamps MP. NUP98-NSD1 links H3K36 methylation to Hox-A gene activation and leukaemogenesis. Nat Cell Biol. 2007;9:804–812. doi: 10.1038/ncb1608. [DOI] [PubMed] [Google Scholar]
- Palmqvist L, Pineault N, Wasslavik C, Humphries RK. Candidate genes for expansion and transformation of hematopoietic stem cells by NUP98-HOX fusion genes. PLoS One. 2007;2:e768. doi: 10.1371/journal.pone.0000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose K, Abramovich C, Argiropoulos B, Humphries RK. Leukemogenic properties of NUP98-PMX1 are linked to NUP98 and homeodomain sequence functions but not to binding properties of PMX1 to serum response factor. Oncogene. 2008;27:6056–6067. doi: 10.1038/onc.2008.210. [DOI] [PubMed] [Google Scholar]
- DiMartino JF, Ayton PM, Chen EH, Naftzger CC, Young BD, Cleary ML. The AF10 leucine zipper is required for leukemic transformation of myeloid progenitors by MLL-AF10. Blood. 2002;99:3780–3785. doi: 10.1182/blood.v99.10.3780. [DOI] [PubMed] [Google Scholar]
- Okada Y, Jiang Q, Lemieux M, Jeannotte L, Su L, Zhang Y. Leukaemic transformation by CALM-AF10 involves upregulation of Hoxa5 by hDOT1L. Nat Cell Biol. 2006;8:1017–1024. doi: 10.1038/ncb1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.