Abstract
We previously reported that CagA can be translocated into B cells in Helicobacter pylori (HP) coculture media, and the translocation appears biologically significant as activation of the relevant cellular pathways was noticed. In this study, we further explore if CagA can be detected in malignant B cells of HP-positive gastric mucosa-associated lymphoid tissue (MALT) lymphoma. Expression of CagA was evaluated by immunohistochemistry. CagA expression was further confirmed by western blot analysis. The association between CagA expression in malignant B cells and tumor response to HP eradication therapy (HPE) was evaluated in 64 stage IE gastric MALT lymphoma patients. We detected CagA expression in 31 (48.4%) of 64 patients: 26 (68.4%) of the 38 HP-dependent cases and 5 (19.2%) of the 26 HP-independent cases (P<0.001). Patients with CagA expression responded to HPE quicker than those without (median time to complete remission, 3.0 vs 6.5 months, P=0.025). Our results indicated that CagA can be translocated into malignant B cells of MALT lymphoma, and the translocation is clinically and biologically significant.
Keywords: MALT lymphoma, CagA, stomach, antibiotics
Introduction
Gastric mucosa-associated lymphoid tissue (MALT) lymphoma is characterized by its close association with Helicobacter pylori infection, with H. pylori eradication therapy (HPE) curing 70% of these tumors.1, 2, 3 Previous studies have shown that MALT lymphoma cells display preserved B-cell properties and that H. pylori antigenic stimulation partially drives their growth.4, 5, 6
In early lymphomagenesis of gastric MALT lymphoma, the proliferation response is partially dependent on the stimulation of H. pylori-specific intratumoral T cells.2, 7 Growth and differentiation of MALT lymphoma cells requires CD40-mediated signaling, T helper-2-type cytokines and the costimulatory molecules CD80 (B7.1) and CD86 (B7.2).8, 9, 10 In a study of gastric MALT lymphoma by de Jong et al.,11 CD86 expression was associated significantly with H. pylori dependence, whereas the expression of CD80 and CD40 and their ligands was not. Our observations of CD86 expression in gastric diffuse large B-cell lymphoma, with histologic evidence of MALT origin, and significant association with H. pylori dependence, supported the results of de Jong et al.12 These findings suggested that the growth of malignant B-cell clones is dependent on T cells. However, a direct interaction between H. pylori with B cells has never been proposed.
In gastric MALT lymphoma, genetic aberrations such as t(11;18)(q21;q21) and t(1;14)(p22;q32), resulting in a chimeric transcript of API2-MALT1 and BCL10 nuclear translocation, respectively, are useful markers for predicting the H. pylori-independent status.13, 14, 15, 16 For patients without t(11;18)(q21;q21) or t(1;14)(p22;q32), nuclear translocation of BCL10 and nuclear factor-κB, as detected by immunohistochemical stain, is predictive of H. pylori-independent state.17
In our previous study, we reported the translocation of CagA into human B lymphocytes in cell culture models, and proposed that translocated CagA might bind to SHP-2 and regulate intracellular signaling pathways, such as the activation of extracellular signal-regulated kinase and p38 mitogen-activated protein kinase (MAPK), and the upregulation of Bcl-2 and Bcl-xL expression, leading to B-lymphoid cell proliferation.18 The CagA protein might, therefore, function as a bacterium-derived oncoprotein in gastric MALT lymphoma pathogenesis. Franceschi et al.19 identified CagA expression in the cytoplasm and nuclei of smooth muscle cells in atherosclerotic vessels, and in the nuclei of gastric mucosa cells of CagA-positive H. pylori strain-infected patients. In this study, we aimed to confirm these findings using human models. Our results indicated that CagA is expressed in 48.4% of H. pylori-positive gastric MALT lymphoma tissues, and that this expression has clinical and biological significance.
Patients and methods
Immunohistochemistry and immunohistochemical scoring
Immunohistochemical analysis of CagA (A10; sc-28368, Santa Cruz Biotechnology, Santa Cruz, CA, USA) was performed on paraffin-embedded sections of pretreatment endoscopic biopsy specimens.18, 19 Visualization was performed using an indirect immunoperoxidase method according to the manufacturer's instructions. Cell blocks of a CagA-translocated human B cell line served as a CagA-positive control (expresses CagA; Supplementary Figure 1).18
Western blot analysis confirmed the expression of CagA. Paraffin-embedded formalin-fixed tissues from patients with non-gastric MALT lymphoma were used as negative controls. Sections in which the CagA primary antibody, and the blocking of CagA immunoreactivity using CagA preadsorption, was omitted also provided negative controls. Immunoreactivity for CagA and CD20 (EP459Y; ab78237; Abcam, Cambridge, MA, USA) was analyzed simultaneously by preparing hematoxylin and eosin stains of serial pretreatment endoscopic paraffin-embedded sections from each patient. The DAKO Envision Doublestain System (Dako, Carpinteria, CA, USA) was used to perform double immunohistochemical staining for CD20 and CagA. To confirm specificity, staining was performed on paraffin-embedded sections in the absence of the first, second or both of the primary antibodies as negative controls. All sections were observed using light microscopy.
The percentages of CagA-positive cells (tumor cells with readily appreciable brown staining distinctly marking the tumor cell nucleus and/or cytoplasm) were averaged to yield an immunohistologic score ranging from 0 to 100%. The results were classified into two groups according to the intensity and extent of staining: in the CagA-negative group, either no staining was present (staining intensity score=0) or mild immunostaining or positive staining was detected in <10% of the cells (staining intensity score=1), and in the CagA-positive, moderate or strong immunostaining was present in 10–30% (staining intensity score=2) or more than 30% of the cells (staining intensity score=3).
Confocal laser scanning microscopy and immunoblotting
For double immunolabeling studies, fluorescein isothiocyanate-labeled goat anti-rabbit immunoglobulin G, or rhodamine-labeled donkey anti-mouse immunoglobulin G, was incubated as a secondary antibody for 60 min at room temperature in the dark. Slides were then rinsed two times with a phosphate-buffered solution and covered with glass for observation using confocal microscopy. Green fluorescence of fluorescein isothiocyanate—CagA (green), CD20 (red) and 4′,6-diamidino-2-phenylindole (blue)—was analyzed. Merged images were evaluated using a confocal laser scanning microscope (Zeiss 510 META Laser Scanning Confocal Imaging Systems, Zeiss, Jena, Germany) and LSM Image software (Carl Zeiss, Thornwood, NY, USA).20
Proteins were extracted from six cryostat frozen sections of tumor tissues of H. pylori-positive gastric MALT lymphoma with CagA positivity during immunohistochemical examination. Whole-cell lysates from four B-cell lymphoma cell lines, Pfeiffer (nodal DLBCL), Ramos (Epstein–Barr virus-negative Burkitt's lymphoma), Raji (Epstein–Barr virus-positive Burkitt's lymphoma) and RL (an IgM secreting low-grade lymphoma cell line; American Type Culture Collection (ATCC, Manassas, VA, USA); CRL-2261), were prepared as described previously.21, 22 Total protein concentrations were determined using a Bio-Rad determination kit (Bio-Rad Laboratories, Hercules, CA, USA) according to the manufacturer's instructions. Aliquots of tissues and cell extracts were subjected to sodium dodecylsulfate-polyacrylamide gel electrophoresis, separated using 8 and 10% running gels and transferred to polyvinylidene fluoride membranes. These membranes were blocked for 30 min using non-fat milk blocking buffer, and then incubated with the primary antibodies and horse radish peroxidase-conjugated secondary antibodies.21, 23 The horse radish peroxidase-conjugated antibodies were visualized using enhanced chemical luminescence. Primary antibody for CagA (A10; sc-28368; Santa Cruz Biotechnology; the same mouse-raised monoclonal anti-CagA antibody used for immunohistochemistry)18 was used for experiments, with β-actin serving as a loading control and normal mouse immunoglobulin G as a negative nonspecific control (Santa Cruz Biotechnology).19, 24
Patients, treatment and tumor evaluation
Sixty-four patients who had received HPE as a front-line treatment for stage IE H. pylori-positive gastric MALT lymphomas and had their gastric H. pylori infection eradicated,17, 25 as confirmed by negative results for biopsy urease test, histology and bacterial culture, were included in the analyses.26 MALT lymphoma was diagnosed according to the criteria described by Isaacson et al.3 and Chan et al.,27 and characterized by the presence of predominantly low-grade centrocyte-like cell infiltrates, the presence of lymphoepithelial lesions, the absence of confluent clusters or sheets of large cells resembling centroblasts or lymphoblasts and the absence of predominance of high-grade lymphoma cells. The histopathologic characteristics of all tumor specimens were independently reviewed by experienced hematopathologists. All patients experienced standard staging workup for gastric MALT lymphoma before antibiotic treatments, including detailed physical examination and history review, hemogram with leukocyte differential count, analysis of serum lactate dehydrogenase levels, computed tomography of the chest, abdomen and pelvis, bone marrow aspiration and biopsy, and upper gastrointestinal endoscopic examination. Of these 64 patients, 59 patients who were treated and followed up also had endoscopic ultrasonography with an Olympus transendoscopic miniature ultrasonic probe UM-2R, UM-3R or UM-DP12-25R (Olympus Optical Co Ltd, Tokyo, Japan), to evaluate the depth of tumor infiltration and the status of perigastric lymph nodes and to guide tissue sampling. Staging was based on Musshoff modification of the Ann Arbor staging system.
Initially, the HPE regimen consisted of amoxicillin 500 mg and metronidazole 250 mg four times a day, with either bismuth subcitrate 120 mg four times a day or omeprazole 20 mg twice a day, for 4 weeks. After March 1996, the regimen was changed to amoxicillin 500 mg four times a day, clarithromycin 500 mg twice a day, plus omeprazole 20 mg twice a day for 2 weeks.17, 25 Patients were scheduled to undergo their first follow-up upper gastrointestinal endoscopic examination 4–6 weeks after the completion of antimicrobial therapy. Follow-up was then repeated every 6–12 weeks until histologic evidence of remission had been identified. At each follow-up examination, 4–6 biopsy specimens were taken from the antrum and body of the stomach for the evaluation of H. pylori infection, and a minimum of six biopsy specimens were taken from each of the tumors and suspicious areas for histologic evaluation. Tumor regression posteradication therapy was histologically evaluated according to the criteria of Wotherspoon et al.28 Tumors that had resolved to Wotherspoon grade 2 or less (pathologic complete remission, pCR) after successful HPE were considered H. pylori-dependent. Tumors that displayed objective evidence of progression, which was defined as the presence of histologically proven new lesions, worsening of Wotherspoon's score from 3 or less to 4–5, or the presence of clusters or sheets of large, and transformed cells, at any time during the follow-up, or tumors that failed to show histologic regression 24 months after successful HPE, were considered H. pylori-independent.
The prospective clinical trial, the pathologic review and the genetic studies of archived tumor tissues were approved by the institutional ethics review board.
Statistical analysis
In this study, the primary end point was to evaluate the pCR rate for HPE as front-line therapy for stage IE H. pylori-positive gastric MALT lymphoma, and the secondary end point was median time to pCR after completion of HPE for H. pylori-dependent patients. χ2 Test, Fisher's exact test, Student's t-test and one-way analysis of variance were used to compare the clinical characteristics, and H. pylori dependence of the two tumor subgroups (CagA-positive vs CagA-negative; 64 cases of H. pylori-positive stage IE gastric MALT lymphoma), and also to compare the clinical characteristics between H. pylori-dependent and H. pylori-independent subgroups. Analyses were conducted using the follow-up data available on 31 December 2010. Multivariate analyses were performed using a logistic regression model for tumor response to HPE (factors with P<0.05). Time to pCR of H. pylori-dependent patients, calculated from the completion of antibiotic therapy to first evidence of pCR by Kaplan–Meier analysis. All statistical tests were two-sided, with significance defined as P<0.05.
Results
Immunohistochemical analysis of CagA protein in H. pylori-positive gastric MALT lymphoma cells
Anti-CagA antibodies are not commercially available for immunohistochemistry; therefore, the CagA antibody had not been validated previously. For its validation, we used cell blocks from a CagA-translocated human B-cell line as a positive control for CagA expression (Supplementary Figure 1). We then used immunoblotting and confocal microscopy to confirm the expression of CagA in neoplastic cells of gastric MALT lymphoma cases with CagA antigen-positivity in immunohistochemical analyses.
We observed CagA protein expression in tumor cells of the gastric mucosa, submucosa and lamina propria, as well as scattered expression in the adjacent tumor-free gastric mucosa of H. pylori-positive gastric MALT lymphoma patients (Figure 1). Immunohistochemical analyses and hematoxylin and eosin staining indicated that the majority of the CagA-positive cells were morphologically abnormal and expressed CD20. These findings were further confirmed using confocal microscopic analyses, which indicated that CagA was predominantly expressed on neoplastic cells and on B-lymphocyte cells of the CagA-translocated human B-cell line (Figure 2 and Supplementary Figure 1). Consistent with immunohistochemical findings, immunoblot analysis of tumor cells from four H. pylori-positive gastric MALT lymphoma cases with CagA expression identified one antigen with a molecular weight of approximately 120 kDa. We did not detect the CagA antigen in protein extracts from tumor cells of the H. pylori-negative case without CagA expression, or in whole-cell lysates from four B-cell lymphoma cell lines. These findings confirmed the specificity of the CagA antibody (Figure 2).
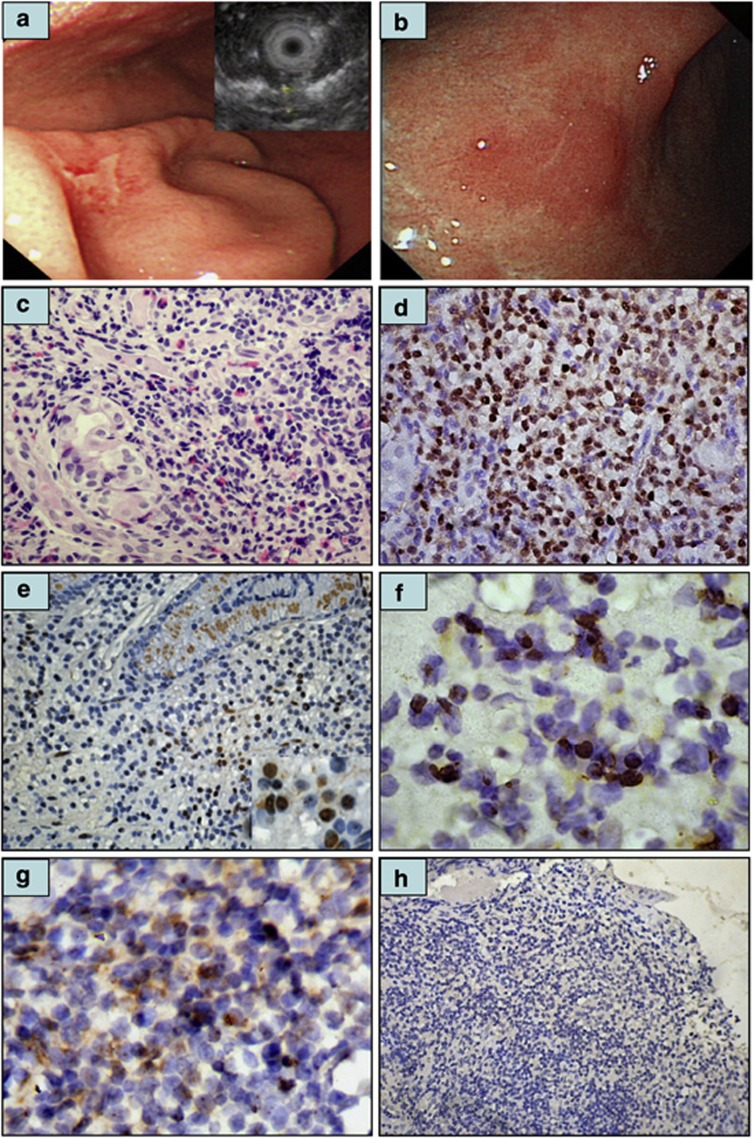
Figure 1.
Immunohistochemical analysis of CagA expression in tumor cells of gastric MALT lymphoma. (a) Endoscopy showing irregular shallow ulcers in the greater curvature of the lower body of the stomach in a 43-year-old man (right top inset: thickness of mucosa 3.6 mm in endoscopic ultrasonography). (b) One month after the completion of HPE, complete regression of all diffuse ulcerations with hyperemic and atrophic mucosa in the lower body of the stomach. (c) Histopathologic examination of the same case revealing diffuse infiltration of small lymphoid cells containing angulated nuclei and pale clear cytoplasm. Lymphoepithelial lesion is also discernible. (d) Tumor cell nuclear CagA expression in the same case (c). (e) An H. pylori-dependent case (time to pCR after HPE, 3 months) displaying nuclear CagA expression in the tumor cells of the gastric mucosa (right bottom inset, × 1000). (f) An H. pylori-dependent case (time to pCR after HPE, 11 months) displaying nuclear CagA expression in the tumor cells of gastric submucosa ( × 1000). (g) An H. pylori-dependent case displaying cytoplasmic CagA expression in the tumor cells of gastric submucosa (time to pCR after HPE, 16 months). (h) Absence of CagA expression in the tumor cells of an H. pylori-independent case.
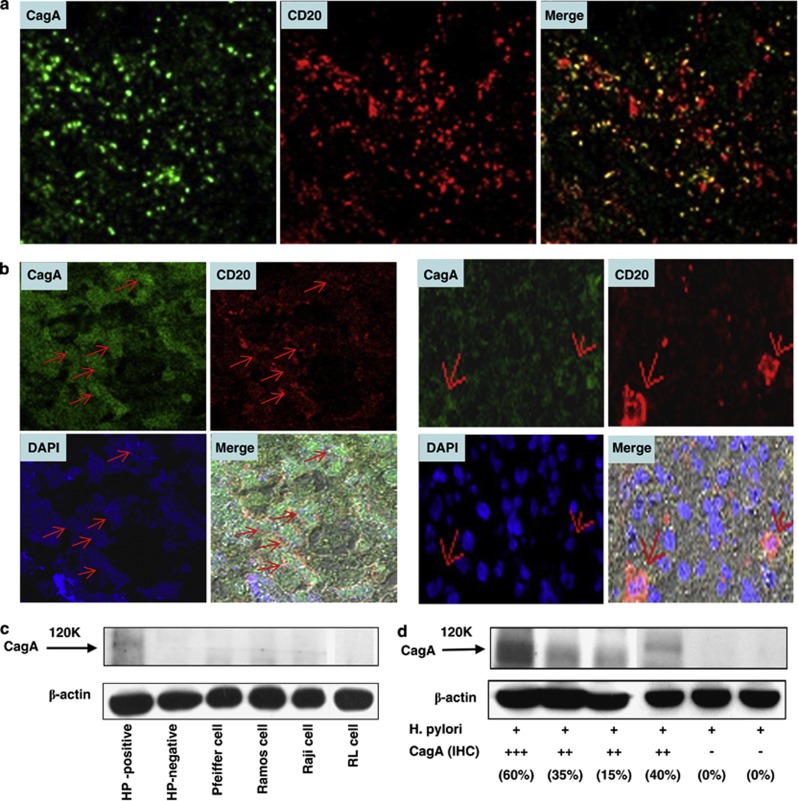
Figure 2.
Double immunolabeling and immunoblot analyses of CagA expression in tumor cells and mucosa of gastric MALT lymphoma. (a) Confocal microscopy showing that most CagA-positive cells (green fluorescence) express CD20 (red fluorescence). (b) Double immunolabeling of CagA and CD20 in two H. pylori-dependent gastric MALT lymphoma cases with immunohistochemically identified CagA expression. Left: the majority of the tumor cells (red fluorescence, CD20) contain nuclear CagA (green fluorescence). Right: CagA labeling is present in the cytoplasm, whereas CD20 labeling is exclusively cytoplasmic. The red arrow in (b) represents CagA expression, CD20 expression, nucleus (DAPI), or the merged image of CagA and CD20 expression. The black arrow in (c) and (d) represents a molecular weight of approximately 120 kDa. (c) Lane 1: Western blot analysis identified one antigen, with a molecular weight of approximately 120 kDa, from the tumor cells of a H. pylori-dependent gastric MALT lymphoma case with CagA expression. Lane 2: An H. pylori-negative gastric MALT lymphoma case without CagA expression serving as a negative control. Lane 3: Pfeiffer cell (diffuse large B-cell) serving as a negative control (no CagA expression). Lane 4: Ramos cell (Epstein–Barr virus-negative Burkitt's lymphoma) serving as a negative control (no CagA expression). Lane 5: Raji (Epstein–Barr virus-positive Burkitt's lymphoma) serving as a negative control (no CagA expression). Lane 6: RL (an IgM-secreting low-grade lymphoma cell line) serving as a negative control (no CagA expression). (d) Lanes 1–4: Western blot analysis identified one antigen, with a molecular weight of approximately 120 kDa, from the tumor cells of 4 H. pylori-dependent gastric MALT lymphoma cases with CagA expression (immunohistochemical scores ranging from 15 to 65% staining intensity, moderate ++ to strong +++). Lanes 5 and 6: Western blot analysis indicated the absence of the CagA antigen in tumor cells of two H. pylori-independent gastric MALT lymphoma cases without CagA expression (immunohistochemical score 0).
Correlation of CagA expression with clinicopathologic features and tumor response to H. pylori eradication in gastric MALT lymphoma
Between 1994 and 2008, 64 patients with stage IE H. pylori-positive gastric MALT lymphoma, who received HPE as front-line treatment and had their gastric H. pylori infection successfully eradicated, were included in the analyses. Thirty-eight (59.4%) patients had H. pylori-dependent tumors and 26 (40.6%) had H. pylori-independent tumors.
We detected CagA expression (17 cases, score 2; 14 cases, score 3) in 31 (48.4%) of 64 patients, whereas 33 patients with negative CagA expression (29 cases, score 0; 4 cases, score 1). Among patients with positive CagA expression (scores 2 and 3), 26 cases had nuclear or nuclear/cytoplasmic expression of CagA, and five cases had cytoplasmic expression of CagA. Table 1 displays the demographic characteristics of the two groups of patients (CagA-positive vs CagA-negative) and their clinicopathologic features. Age, sex, endoscopic appearance and lesion sites showed nonsignificant differences between the two groups.
Table 1. Clinicopathologic features and CagA expression in localized gastric MALT lymphoma patients who received H. pylori eradication therapy as a front-line treatment.
| Clinicopathologic characteristics |
CagA expression |
Pa | |
|---|---|---|---|
| Negative (n=33) | Positive (n=31) | ||
| Age (median, range, years) | 56 (18–75) | 58 (30–86) | 0.246b |
| Sex, male/female | 13/20 | 9/22 | 0.438c |
| Endoscopic features, n (%) | 0.247d | ||
| Gastritis-like or multiple erosion on infiltrative mucosa | 11 (33.3%) | 13 (41.9%) | |
| Ulceration or ulcerated mass | 14 (42.4%) | 15 (48.4%) | |
| Erosions on giant nodular folds | 7 (21.2%) | 2 (6.5%) | |
| Mixed | 1 (3.0%) | 1 (3.2%) | |
| Location of tumor (s), n (%) | 0.089d | ||
| Proximale | 10 (30.3%) | 8 (25.8%) | |
| Distalf | 18 (54.5%) | 22 (71.0%) | |
| ⩾2 components | 5 (15.2%) | 1 (3.2%) | |
| Depth of gastric wall involvement, n (%)g | 0.010c | ||
| Submucosa or above | 17/31 (54.8%) | 24/28 (85.7%) | |
| Muscularis propria or beyond | 14/31 (45.2%) | 4/28 (14.3%) | |
| H. pylori-dependent status | <0.001c | ||
| H. pylori-dependent | 12 (36.4%) | 26 (83.9%) | |
| H. pylori-independent | 21 (63.6%) | 5 (16.1%) | |
Abbreviations: MALT, mucosa-associated lymphoid tissue.
P: comparison of discrete variables between CagA expression-positive and CagA expression-negative.
P-values (two-sided) were calculated using the Student's t-test.
P-values (two-sided) were calculated using χ2 test or Fisher's exact test.
P-values (two-sided) were calculated using one-way analysis of variance.
Proximal: Middle body, upper body, fundus or cardia.
Distal: Antrum, angle or lower body.
Gastric wall involvement was evaluated by endoscopic ultrasonography in 59 patients.
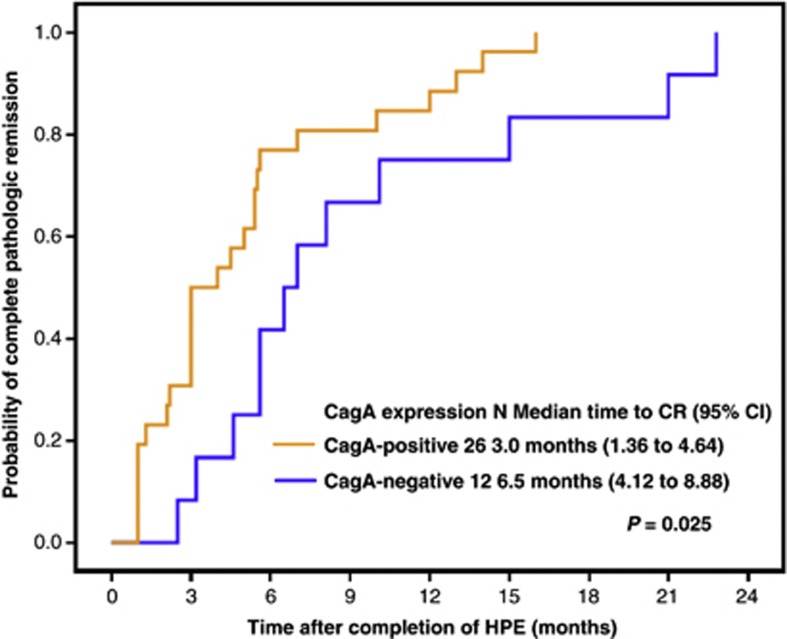
We observed CagA expression (scores of 2 and 3) in 26 (68.4%) of the 38 H. pylori-dependent cases and in 5 (19.2%) of the 26 H. pylori-independent cases (P<0.001; Table 1). Of the gastric MALT lymphoma patients with known tumor invasion depth, CagA expression was more common in tumors limited to mucosa/submucosa than in those extending into the muscularis propria or beyond (58.5% (24/41) vs 22.2% (4/18); P=0.010). We also observed that H. pylori-dependent patients expressing CagA responded to HPE more rapidly than those without CagA expression (median time to pCR after completion of HPE, 3.0 months (95% confidence interval (CI), 1.36–4.64 months) vs 6.5 months (95% CI, 4.12–8.88 months), P=0.025, log-rank test; Figure 3). Among 26 H. pylori-dependent patients with CagA expression, patients with nuclear or nuclear/cytoplasmic expression responded to HPE more rapidly than those with cytoplasmic expression (median time to pCR after completion of HPE, 3.0 months (95% CI, 2.28–3.72 months) vs 7.0 months (95% CI, 0–14.84 months), P=0.034, log-rank test). However, of 35 H. pylori-dependent patients who had had endoscopic staging, tumors limited to mucosa/submucosa did not respond to HPE quickly than those extending into the muscularis propria or beyond (median time to pCR after completion of HPE, 5.0 months (95% CI, 1.89–8.11 months) vs 4.6 months (95% CI, 4.34–4.86 months), P=0.537, log-rank test).
Figure 3.
Time to pCR of H. pylori-dependent gastric MALT lymphoma patients. Time to pCR was calculated from the completion of antibiotic treatment to first evidence of pCR using Kaplan–Meier analysis (CagA-positive group vs CagA-negative group, two-sided log-rank test; P=0.025).
In addition to CagA expression, we correlated tumor- and patient-related factors with tumor response to HPE in gastric MALT lymphoma. As shown in Table 2, endoscopic appearance (P=0.036), lesion sites (P=0.025) and endoscopic staging (P=0.034) were closely associated with H. pylori-dependent state, whereas age and sex were not associated with H. pylori-dependent state. Multivariate analysis identified the expression of CagA (P<0.001), and tumors limited to mucosa/submucosa (P=0.035) as independent predictors of H. pylori dependence for gastric MALT lymphoma. In contrast, tumors located at the distal part of the stomach (P=0.098), and gastritis-like or multiple erosion on infiltrated mucosa of endoscopic appearance (P=0.062), were marginally associated with H. pylori dependence of gastric MALT lymphoma.
Table 2. Correlation of clinicopathologic features and tumors response to H. pylori eradication therapy of gastric MALT lymphoma.
| Clinicopathologic characteristics | Tumors response to HPE | Pa | |
|---|---|---|---|
| |
HP-dependent (n=38) |
HP-independent (n=26) |
|
| Age (median, range, years) | 60 (30–86) | 58 (18–76) | 0.191b |
| Sex, male/female | 11/27 | 11/15 | 0.269c |
| Endoscopic features, n (%) | 0.036d | ||
| Gastritis-like or multiple erosion on infiltrative mucosa | 18 (47.4%) | 6 (23.1%) | |
| Ulceration or ulcerated mass | 16 (42.1%) | 13 (50.0%) | |
| Erosions on giant nodular folds | 3 (7.9%) | 6 (23.1%) | |
| Mixed | 1 (2.6%) | 1 (3.8%) | |
| Location of tumor (s), n (%) | 0.025c | ||
| Proximale or ⩾2 components | 10 (26.3%) | 14 (53.8%) | |
| Distalf | 28 (73.7%) | 12 (46.2%) | |
| Depth of gastric wall involvement, n (%)g | 0.034c | ||
| Submucosa or above | 28/35 (77.1%) | 13/24 (54.2%) | |
| Muscularis propria or beyond | 7/35 (22.9%) | 11/24 (45.8%) | |
Abbreviations: HP, H. pylori; HPE, H. pylori eradication therapy; MALT, mucosa-associated lymphoid tissue.
P: comparison of discrete variables between CagA expression-positive and CagA expression-negative.
P-values (two-sided) were calculated using the Student's t-test.
P-values (two sided) were calculated using χ2 test or Fisher's exact test.
P-values (two-sided) were calculated using one-way analysis of variance.
Proximal: Middle body, upper body, fundus or cardia.
Distal: Antrum, angle or lower body.
Gastric wall involvement was evaluated by endoscopic ultrasonography in 59 patients.
Discussion
In this study, we frequently observed CagA expression in H. pylori-dependent tumors. Patients with CagA expression in tumor cells, particularly with nuclear expression, responded to HPE more rapidly than those without CagA expression. Although we cannot exclude the possibility that a more rapid response to HPE in CagA-positive patients could be related to lesser tumor extension rather than to CagA positivity,29, 30 our findings suggested that CagA is a useful biomarker in lymphoma cells, and is associated with the direct lymphomagenic effect of H. pylori on B cells.
Epidemiologic studies have shown that the presence of CagA, a virulence factor of type I H. pylori, is associated with the formation of lymphoid follicles and lymphoma of the stomach.31, 32 When the relationship between CagA status and gastric lymphoma was investigated, CagA seropositivity was found even more common in diffuse large B-cell lymphoma than in MALT lymphoma.33, 34 However, Eck et al.35 investigated the relationship between CagA and gastric lymphoma, observing that 95.5% of H. pylori-seropositive gastric MALT lymphoma cases were positive for serum immunoglobulin G antibodies to CagA compared with 67% of H. pylori-seropositive chronic active gastritis cases (P<0.001). Recently, Sumida et al.36 also found that titers of anti-H. pylori and anti-CagA were significantly higher in H. pylori-dependent cases than in H. pylori-independent cases of t(11;18)(q21;q21)-negative gastric MALT lymphoma. These findings suggested that gastric MALT lymphoma is associated with H. pylori strains expressing the CagA protein. Recent in vitro studies have also identified that in the presence of CagA, B-cell lymphocytes evade apoptosis through the inhibition of p53 accumulation or phosphorylation of Bad at Ser-112.37, 38 In our preliminary studies, we identified the translocation of CagA into lymphoma cells, and the close association between CagA translocation and the expression of CagA signaling pathway-related proteins, such as phospho-SHP-2, phospho-extracellular signal-regulated kinase, phospho-p38 mitogen-activated protein kinase, Bcl-2 and Bcl-xL expression (data not shown).39 These findings support those of Ohnishi et al.,40 which suggested that CagA might have a role in the development of H. pylori-associated hematologic malignancies in CagA-transgenic mice through CagA-mediated tyrosine phosphorylation and subsequent deregulation of SHP-2. Qu et al.41 also described that SHP-2 tyrosine phosphatase has an important role in the development of lymphoid and hematopoietic stem/progenitor cells.
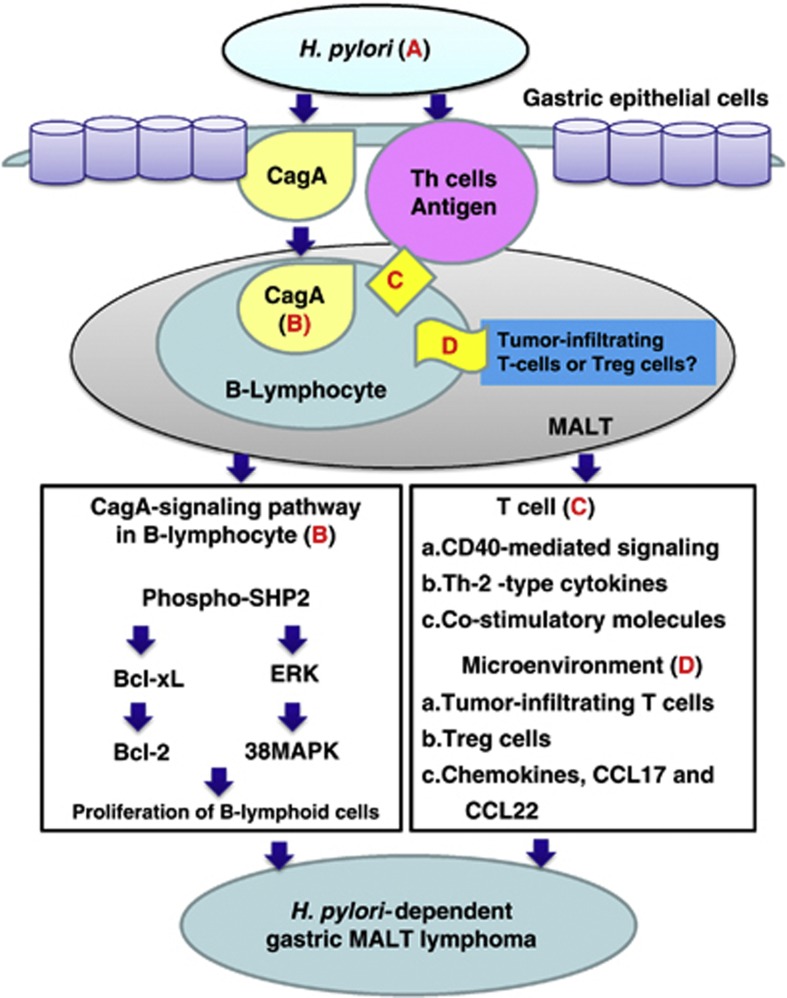
Although our study results suggest that CagA directly affects lymphomagenesis, through the regulation of CagA-related pivotal signal transduction in gastric MALT lymphoma, alternative pathways that associate H. pylori with lymphoid neoplasms cannot be overlooked. For example, antigenic stimulation of H. pylori through tonic B-cell receptor signaling, or indirect stimulation through H. pylori-stimulated T-cell mechanisms, might have equally important roles in lymphomagenesis (summarized in Figure 4).2, 5, 6, 7, 8, 9, 10, 11, 12, 42 In addition to classic T-cell mechanisms, previous studies have shown that CD4+CD25+FoxP3+ regulatory T-cell-derived signals are involved in the lymphomagenesis of MALT lymphoma.43, 44, 45
In this study, most CagA-positive gastric MALT lymphoma patients were H. pylori-dependent and 5 (16.1%) were H. pylori-independent. Of these five patients, three carried t(11;18)(p21;q21), which results in a chimeric transcript of API2-MALT1 and contributes to the H. pylori-independent growth of MALT lymphoma in tumor cells.13, 14, 15 The absence of CagA signaling molecules in the tumor cells of the two t(11;18)(p21;q21)-negative cases suggested that CagA translocation alone might not be sufficient to activate downstream signals in selected cases. However, the 12 cases who were H. pylori-dependent and CagA-negative suggested the significance of other pathways that associate H. pylori with lymphomagenesis (summarized in Figure 4).
Figure 4.
Involvement of CagA- and T-cell-derived signals in H. pylori-induced lymphomagenesis of gastric MALT lymphoma. (a) Helicobacter pylori (H. pylori) stimulates immune lymphocytes in the gastric mucosa and induces the formation of MALT. B lymphocytes can migrate to and infiltrate the site of H. pylori infection in the stomach. Therefore, H. pylori-specific CagA can be injected into lymphocytes and gastric epithelial cells. (b) The translocated CagA might co-immunoprecipitate with SHP-2 and regulate intracellular signaling pathways, such as by activating extracellular signal-regulated kinase (ERK) and p38 mitogen-activated protein kinase (MAPK) and upregulating Bcl-2 and Bcl-xL expression, leading to B-lymphoid cell proliferation. (c) H. pylori antigenic stimulation, or the triggering of tonic B-cell receptor signaling by the H. pylori antigen, partially drives MALT lymphoma progression. H. pylori can also indirectly promote MALT lymphomagenesis through T-cell stimulation (e.g., CD40-mediated signaling, T helper-2 (Th-2)-type cytokines and costimulatory molecules such as CD86). (d) Molecular crosstalk between B lymphoma cells and tumor microenvironments (tumor-infiltrating T cells, regulatory T-cell cells and chemokines) promotes the survival of B lymphoma cells. Tregs, CD4+CD25+FoxP3+ regulatory T cells.
In summary, in this study, we identified that the translocation of CagA protein into malignant B cells of MALT lymphoma, and the expression of CagA in tumor cells, is closely associated with H. pylori dependence in gastric MALT lymphoma and accompanied by the activation of molecular pathways downstream of CagA. The clinical and biological significance of the CagA oncoprotein in lymphomagenesis of gastric MALT lymphoma warrants further investigation.
Acknowledgments
This study was supported by research grants NSC96-2321-B-002-013, NSC96-2321-B-002-014, NSC96-2314-B-002-164MY3, NSC 98-2314-B-002-087-MY3, NSC 100-2321-B-002-032 and NSC 101-2314-B-002-157-MY3 from the National Science Council, Taiwan, and DOH100-TD-B-111-001 from the Department of Health, Taiwan.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Blood Cancer Journal website (http://www.nature.com/bcj)
Present in part as a poster presentation (Best Poster Award, in the category: Hematological malignancies) at the 37th Annual Meeting of the European Society for Medical Oncology Congress, Vienna, Austria, 28 September–2 October 2012.
Supplementary Material
References
- Du MQ, Isaacson PG. Gastric MALT lymphoma: from aetiology to treatment. Lancet Oncol. 2002;3:97–104. doi: 10.1016/s1470-2045(02)00651-4. [DOI] [PubMed] [Google Scholar]
- Farinha P, Gascoyne RD. Helicobacter pylori and MALT lymphoma. Gastroenterology. 2005;128:1579–1605. doi: 10.1053/j.gastro.2005.03.083. [DOI] [PubMed] [Google Scholar]
- Isaacson PG, Du MQ. Gastrointestinal lymphoma: where morphology meets molecular biology. J Pathol. 2005;205:255–274. doi: 10.1002/path.1703. [DOI] [PubMed] [Google Scholar]
- Hussell T, Isaacson PG, Crabtree JE, Spencer J. The response of cells from low-grade B cell gastric lymphomas of mucosa-associated lymphoid tissue to Helicobacter pylori. Lancet. 1993;342:571–574. doi: 10.1016/0140-6736(93)91408-e. [DOI] [PubMed] [Google Scholar]
- Hussell T, Isaacson PG, Crabtree JE, Dogan A, Spencer J. Immunoglobulin specificity of low grade B cell gastrointestinal lymphoma of mucosa associated lymphoid tissue (MALT) type. Am J Pathol. 1993;142:285–292. [PMC free article] [PubMed] [Google Scholar]
- Greiner A, Marx A, Heesemann J, Leebmann J, Schmausser B, Müller-Hermelink HK. Idiotype identity in a MALT type lymphoma and B cells in Helicobacter pylori associated chronic gastritis. Lab Invest. 1994;70:572–578. [PubMed] [Google Scholar]
- Hussell T, Isaacson PG, Crabtree JE, Spencer J. Helicobacter pylori-specific tumour-infiltrating T cells provide contact dependent help for the growth of malignant B cells in low-grade gastric lymphoma of mucosa-associated lymphoid tissue. J Pathol. 1996;178:122–127. doi: 10.1002/(SICI)1096-9896(199602)178:2<122::AID-PATH486>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Hauer AC, Finn TM, MacDonald TT, Spencer J, Isaacson PG. Analysis of TH1 and TH2 cytokine production in low-grade B-cell gastric MALT-type lymphomas stimulated in vitro with Helicobacter pylori. J Clin Pathol. 1997;50:957–959. doi: 10.1136/jcp.50.11.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiner A, Knorr C, Qin Y, Sebald W, Schimpl A, Banchereau J, et al. Low-grade B-cell lymphomas of mucosa-associated lymphoid tissue (MALT-type) require CD40-mediated signalling and Th2-type cytokines for in vitro growth and differentiation. Am J Pathol. 1997;150:1583–1593. [PMC free article] [PubMed] [Google Scholar]
- Mueller A, O'rouuke J, Chu P, Chu A, Dixon MF, Bouley DM, et al. The role of antigenic drive and tumor-infiltrating accessory cells in the pathogenesis of Helicobacter-induced mucosa-associated lymphoid tissue lymphoma. Am J Pathol. 2005;167:797–812. doi: 10.1016/S0002-9440(10)62052-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong D, Vyth-Dreese F, Dellemijn T, Verra N, Ruskoné-Fourmestraux A, Lavergne-Slove A, et al. Histological and immunological parameters to predict treatment outcome of Helicobacter pylori eradication in low-grade gastric MALT lymphoma. J Pathol. 2001;193:318–324. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH811>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Kuo SH, Chen LT, Chen CL, Doong SL, Yeh KH, Wu MS, et al. Expression of CD86 and increased infiltration of NK cells are associated with Helicobacter pylori-dependent state of early stage high-grade gastric MALT lymphoma. World J Gastroenterol. 2005;11:4357–4362. doi: 10.3748/wjg.v11.i28.4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Ye H, Ruskone-Fourmestraux A, De Jong D, Pileri S, Thiede C, et al. T(11;18) is a marker for all stage gastric MALT lymphomas that will not respond to H. pylori eradication. Gastroenterology. 2002;122:1286–1294. doi: 10.1053/gast.2002.33047. [DOI] [PubMed] [Google Scholar]
- Du MQ, Atherton JC. Molecular subtyping of gastric MALT lymphomas: implications for prognosis and management. Gut. 2006;55:886–893. doi: 10.1136/gut.2004.061663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du MQ. MALT lymphoma: many roads lead to nuclear factor-κb activation. Histopathology. 2011;58:26–38. doi: 10.1111/j.1365-2559.2010.03699.x. [DOI] [PubMed] [Google Scholar]
- Ye H, Gong L, Liu H, Ruskone-Fourmestraux A, de Jong D, Pileri S, et al. Strong BCL10 nuclear expression identifies gastric MALT lymphomas that do not respond to H. pylori eradication. Gut. 2006;55:137–138. doi: 10.1136/gut.2005.081117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh KH, Kuo SH, Chen LT, Mao TL, Doong SL, Wu MS, et al. Nuclear expression of BCL10 or nuclear factor kappa B helps predict Helicobacter pylori-independent status of low-grade gastric mucosa-associated lymphoid tissue lymphomas with or without t(11;18)(q21;q21) Blood. 2005;106:1037–1041. doi: 10.1182/blood-2005-01-0004. [DOI] [PubMed] [Google Scholar]
- Lin WC, Tsai HF, Kuo SH, Wu MS, Lin CW, Hsu PI, et al. Translocation of Helicobacter pylori CagA into Human B lymphocytes, the origin of mucosa-associated lymphoid tissue lymphoma. Cancer Res. 2010;70:5740–5748. doi: 10.1158/0008-5472.CAN-09-4690. [DOI] [PubMed] [Google Scholar]
- Franceschi F, Sepulveda AR, Gasbarrini A, Pola P, Silveri NG, Gasbarrini G, et al. Cross-reactivity of anti-CagA antibodies with vascular wall antigens: possible pathogenic link between Helicobacter pylori infection and atherosclerosis. Circulation. 2002;106:430–434. doi: 10.1161/01.cir.0000024100.90140.19. [DOI] [PubMed] [Google Scholar]
- Mishima Y, Terui Y, Takeuchi K, Matsumoto-Mishima Y, Matsusaka S, Utsubo-Kuniyoshi R, et al. The identification of irreversible rituximab-resistant lymphoma caused by CD20 gene mutations. Blood Cancer J. 2011;1:e15. doi: 10.1038/bcj.2011.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo SH, Hsu CH, Chen LT, Lu YS, Lin CH, Yeh PY, et al. Lack of compensatory pAKT activation and eIF4E phosphorylation of lymphoma cells toward mTOR inhibitor, RAD001. Eur J Cancer. 2011;47:1244–1257. doi: 10.1016/j.ejca.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Roccaro AM, Sacco A, Husu EN, Pitsillides C, Vesole S, Azab AK, et al. Dual targeting of the PI3K/Akt/mTOR pathway as an antitumor strategy in Waldenstrom macroglobulinemia. Blood. 2010;115:559–569. doi: 10.1182/blood-2009-07-235747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leleu X, Jia X, Runnels J, Ngo HT, Moreau AS, Farag M, et al. The Akt pathway regulates survival and homing in Waldenstrom macroglobulinemia. Blood. 2007;110:4417–4426. doi: 10.1182/blood-2007-05-092098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelebart P, Hegazy SA, Wang P, Bone KM, Anand M, Sharon D, et al. Aberrant expression and biological significance of Sox2, an embryonic stem cell transcriptional factor, in ALK-positive anaplastic large cell lymphoma. Blood Cancer J. 2012;2:e82. doi: 10.1038/bcj.2012.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LT, Lin JT, Tai JJ, Chen GH, Yeh HZ, Yang SS, et al. Long-term results of anti-Helicobacter pylori therapy in early-stage gastric high-grade transformed MALT lymphoma. J Natl Cancer Inst. 2005;97:1345–1353. doi: 10.1093/jnci/dji277. [DOI] [PubMed] [Google Scholar]
- Ruskoné-Fourmestraux A, Fischbach W, Aleman BM, Boot H, Du MQ, Megraud F, on behalf of the EGILS group et al. EGILS consensus report. Gastric extranodal marginal zone B-cell lymphoma of MALT. Gut. 2011;60:747–758. doi: 10.1136/gut.2010.224949. [DOI] [PubMed] [Google Scholar]
- Chan JK, Ng CS, Isaacson PG. Relationship between high-grade lymphoma and low-grade B-cell mucosa-associated lymphoid tissue lymphoma (MALToma) of the stomach. Am J Pathol. 1990;136:1153–1164. [PMC free article] [PubMed] [Google Scholar]
- Wotherspoon AC, Doglioni C, Diss TC, Pan L, Moschini A, de Boni M, et al. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet. 1993;342:575–577. doi: 10.1016/0140-6736(93)91409-f. [DOI] [PubMed] [Google Scholar]
- Morgner A, Bayerdörffer E, Neubauer A, Stolte M. Helicobacter pylori associated gastric B cell MALT lymphoma: predictive factors for regression. Gut. 2001;48:290–292. doi: 10.1136/gut.48.3.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruskoné-Fourmestraux A, Lavergne A, Aegerter PH, Megraud F, Palazzo L, de Mascarel A, et al. Predictive factors for regression of gastric MALT lymphoma after anti-Helicobacter pylori treatment. Gut. 2001;48:297–303. doi: 10.1136/gut.48.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoshal UC, Tiwari S, Dhingra S, Pandey R, Ghoshal U, Tripathi S, et al. Frequency of Helicobacter pylori and CagA antibody in patients with gastric neoplasms and controls: the Indian enigma. Dig Dis Sci. 2008;53:1215–1222. doi: 10.1007/s10620-008-0229-7. [DOI] [PubMed] [Google Scholar]
- Achyut BR, Moorchung N, Srivastava AN, Gupta NK, Mittal B. Risk of lymphoid follicle development in patients with chronic antral gastritis: role of endoscopic features, histopathological parameters, CagA status and interleukin-1 gene polymorphisms. Inflamm Res. 2008;57:51–56. doi: 10.1007/s00011-007-7033-2. [DOI] [PubMed] [Google Scholar]
- Peng H, Ranaldi R, Diss TC, Isaacson PG, Bearzi I, Pan L. High frequency of CagA+ Helicobacter pylori infection in high-grade gastric MALT B-cell lymphomas. J Pathol. 1998;185:409–412. doi: 10.1002/(SICI)1096-9896(199808)185:4<409::AID-PATH121>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Delchier JC, Lamarque D, Levy M, Tkoub EM, Copie-Bergman C, Deforges L, et al. Helicobacter pylori and gastric lymphoma: high seroprevalence of CagA in diffuse large B-cell lymphoma but not in low-grade lymphoma of mucosa-associated lymphoid tissue type. Am J Gastroenterol. 2001;96:2324–2328. doi: 10.1111/j.1572-0241.2001.04036.x. [DOI] [PubMed] [Google Scholar]
- Eck M, Schmausser B, Haas R, Greiner A, Czub S, Müller-Hermelink HK. MALT-type lymphoma of the stomach is associated with Helicobacter pylori strains expressing the CagA protein. Gastroenterology. 1997;112:1482–1486. doi: 10.1016/s0016-5085(97)70028-3. [DOI] [PubMed] [Google Scholar]
- Sumida T, Kitadai Y, Hiyama T, Shinagawa K, Tanaka M, Kodama M, et al. Antibodies to Helicobacter pylori and CagA protein are associated with the response to antibacterial therapy in patients with H. pylori-positive API2-MALT1-negative gastric MALT lymphoma. Cancer Sci. 2009;100:1075–1081. doi: 10.1111/j.1349-7006.2009.01139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umehara S, Higashi H, Ohnishi N, Asaka M, Hatakeyama M. Effects of Helicobacter pylori CagA protein on the growth and survival of B lymphocytes, the origin of MALT lymphoma. Oncogene. 2003;22:8337–8342. doi: 10.1038/sj.onc.1207028. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Wang C, Huang J, Ge Z, Dong Q, Zhong X, et al. The Helicobacter pylori virulence factor CagA promotes Erk1/2-mediated Bad phosphorylation in lymphocytes: a mechanism of CagA-inhibited lymphocyte apoptosis. Cell Microbiol. 2007;9:952–561. doi: 10.1111/j.1462-5822.2006.00843.x. [DOI] [PubMed] [Google Scholar]
- Kuo SH, Chen LT, Lin CW, Wu mS, Hsu PN, Tasi HJ, et al. Detection of CagA expression in gastric mucosa-associated lymphoid tissue lymphoma—biological significance and clinical implication. Ann Oncol. 2012;23 (Suppl 9:ix351. [Google Scholar]
- Ohnishi N, Yuasa H, Tanaka S, Sawa H, Miura M, Matsui A, et al. Transgenic expression of Helicobacter pylori CagA induces gastrointestinal and hematopoietic neoplasms in mouse. Proc Natl Acad Sci USA. 2008;105:1003–1008. doi: 10.1073/pnas.0711183105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu CK, Nguyen S, Chen J, Feng GS. Requirement of Shp-2 tyrosine pohosphatase in lymphoid and hematopoietic cell development. Blood. 2001;97:911–914. doi: 10.1182/blood.v97.4.911. [DOI] [PubMed] [Google Scholar]
- D'Elios MM, Amedei A, Manghetti M, Costa F, Baldari CT, Quazi AS, et al. Impaired T-cell regulation of B-cell growth in Helicobacter pylori-related gastric low-grade MALT lymphoma. Gastroenterology. 1999;117:1105–1112. doi: 10.1016/s0016-5085(99)70395-1. [DOI] [PubMed] [Google Scholar]
- Lundgren A, Stromberg E, Sjoling A, Lindholm C, Enarsson K, Edebo A, et al. Mucosal FOXP3-expressing CD4+CD25 high regulatory T cells in Helicobacter pylori-infected patients. Infect Immun. 2005;73:523–531. doi: 10.1128/IAI.73.1.523-531.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rad R, Brenner L, Bauer S, Schwendy S, Layland L, da Costa CP, et al. CD25+/Foxp3+ T cells regulate gastric inflammation and Helicobacter pylori colonization in vivo. Gastroenterology. 2006;131:525–537. doi: 10.1053/j.gastro.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Craig VJ, Cogliatti SB, Arnold I, Gerke C, Balandat JE, Wündisch T, et al. B-cell receptor signaling and CD40 ligand-independent T cell help cooperate in Helicobacter-induced MALT lymphomagenesis. Leukemia. 2010;24:1186–1196. doi: 10.1038/leu.2010.76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.