Abstract
By crosslinking B7-DC on dendritic cells (DC) the human IgM antibody (B7-DC XAb) shifts polarized immune responses from T-helper 2 (Th2) to T-helper 1 (Th1) in an antigen specific manner. The molecular determinants governing the ability of DC to reprogram the polarity of T cell recall responses is not yet known. In addition to the expected role of T-bet expressed by T cells in regulating Th1 responses, we find using in vitro assays and an established in vivo model of allergic airway inflammation that T-bet expression by DC is also required for the polarity shift promoted by B7-DC XAb. T-bet expression by both T-cells and DC is critically important for B7-DC XAb-induced downregulation of IL4, upregulation of interferon-γ and suppression of allergic airway inflammation. Moreover, retroviral reconstitution of T-bet expression in T-bet KO DC rescued their ability to modulate both naive and memory T cell responses from Th2 to Th1. Our observations further our understanding of the critical mediators controlling the ability of DC to modify the responses of previously activated T cells and reveal the interesting use of the same transcription factor to regulate the inductive phenotype of DC and the inducible phenotype of T cells.
Keywords: dendritic cells, T-bet, T-Box Domain Proteins, transcription factors, immune regulation
INTRODUCTION
The phenotype of the CD4+ T lymphocytes defines the polarity of the immune response. There are four known types of Th responses: Th1, Th2, Th17 and the regulatory T cell response (Treg) [1]. Th differentiation is regulated by specific transcription factors expressed by T cells and is driven by the local cytokine milieu. Th1 responses are typically triggered by infections with intracellular pathogens and utilize the T-box family transcription factor T-bet and the cytokine IL-12 to generate IFN-γ secreting CD4+ Th cells [1, 2]. In contrast, Th2 polarized CD4+ T cells usually develop in response to pathogens such as parasites and helminthes. Th2 responses are driven by the transcription factor GATA3 and characterized by T cells producing the cytokines IL-4, IL-5 and IL-13 [1, 2]. The Th17 phenotype is regulated by the transcription factor RORγt, while TGF-β and IL-6 support the differentiation of this Th polarized phenotype [1, 3, 4]. It is characterized by the production of IL-17 and associated with responses to extracellular bacteria [3]. In contrast to the other Th responses, Tregs are anti-inflammatory, producing the cytokines IL-10 and TGF-β under the regulation of the transcription factor FOXP-3 [1].
Despite being first characterized as the initiator of Th1 responses in CD4+ T cells, T-bet is also expressed in many other immune cells. T-bet promotes the cytolytic activity and IFN-γ production in CD8+ effector cells and is also important for CD8+ memory T cells [5-7]. It is involved in B cell class switching as well as the development of NKT cells [8]. In DC, T-bet has been shown to regulate the expression of IFN-γ and TNF-α [10, 11]. These observations demonstrate that T-bet is not only important in T cells, but also contributes to a wide variety of immune processes.
While polarized immunity represents physiological responses required for the effective clearance of microbial pathogens, Th polarity has also been associated with various disease processes. Many autoimmune diseases have been characterized by Th1 polarity, whereas Th2 cells have been linked to allergic inflammation [2]. Recent evidence from murine models of multiple sclerosis and arthritis indicate the involvement of Th17 polarized cells in these autoimmune diseases [3]. Consequently, there has been increasing interest in therapeutic strategies modulating the polarity of these pathogenic immune responses [12-16].
We recently discovered a human IgM antibody (B7-DC XAb) that profoundly alters immune responses by cross-linking B7-DC costimulatory molecules on DC [17, 18]. B7-DC XAb treated DC acquire a unique maturation state characterized by a marked increase in antigen presentation in the absence of an upregulation of co-stimulatory molecules [17]. Moreover, B7-DC XAb is capable of redirecting established Th2 cells towards a Th1 cell phenotype [19]. These and other unique effects of B7-DC XAb treatment modulate immune responses associated with cancer and allergic asthma [18-23].
Ovalbumin (OVA) induced airway inflammation is a widely used murine model to study a Th2 polarized immune response [24]. Using this model we previously demonstrated that the adoptive transfer of B7-DC XAb treated DC reprograms established Th2 polarized cells and induces a fundamental shift in T cell polarity towards Th1 [21]. This shift in the immune response protects the animals from allergic airway inflammation and airway hypersensitivity [21]. This protective effect is associated with the induction of IFN-γ and down-regulation of IL-4 [21]. Therefore, we hypothesized that this polarity shift from Th2 to Th1 requires the expression of the Th1 associated transcription factor T-bet. Here we report that T-bet expression is indeed essential, not only in T cells, but also in DC for the repolarization of Th2 responses by B7-DC XAb.
RESULTS
T-bet expression by T cells and DC is required for reprogramming Th2 polarized splenocytes
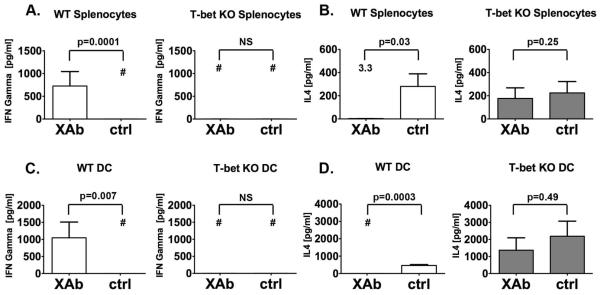
Th2 splenocytes were generated in wild-type (WT) and T-bet deficient (T-bet KO) mice by intraperitoneal injections of OVA adsorbed to alum. Co-culture of WT Th2 splenocytes and WT DC, pretreated with OVA and B7-DC XAb, resulted in the upregulation of IFN-γ and down-regulation of IL-4 (Fig. 1A and B). Consistent with the known role of T-bet in regulating Th1 polarization [1, 2], none of these changes were observed when Th2 polarized T-bet KO splenocytes were substituted for the WT splenocytes (Fig. 1A and B). However, we also observed that T-bet KO DC treated with B7-DC XAb failed to induce the production of IFN-γ and down-regulation of IL-4 by WT Th2 polarized splenocytes (Fig. 1C and D). This key observation suggests that T-bet functions as an important regulator of Th polarity in both the stimulatory DC as well as the responding T cells.
Figure 1. T-bet deficient DC fail to reprogram Th2 polarized splenocytes.
(A, B) T-bet deficient (T-bet KO) or wild type (WT), Th2 polarized and ovalbumin (OVA) sensitized splenocytes were co-cultured with WT bone-marrow derived DC that had been pre-incubated with OVA and either B7-DC XAb or control IgM. (A) IFN- γ and (B) IL-4 levels in the culture supernatants were analyzed by ELISA on day 3. Although B7-DC XAb induced IFN-γ and inhibited IL4 production in WT splenocytes, no such effect was seen in T-bet KO splenocytes. (C, D) Th2 polarized and OVA sensitized WT splenocytes were co-cultured with either WT or T-bet KO syngeneic bone-marrow derived DC pretreated as in (A, B). (C) IFN- γ and (D) IL-4 levels in the culture supernatants were analyzed by ELISA on day 3. T-bet KO DC failed to induce IFN-γ and downregulate IL-4 in WT splenocytes. Data displayed are pooled from three independent experiments and error bars show mean ± SEM. B7-DC XAb = XAb, Control IgM = ctrl, difference is not significant = NS. For small data points the mean is displayed as number on the graph. The # symbol indicates that the value was below the detection limit of the assay.
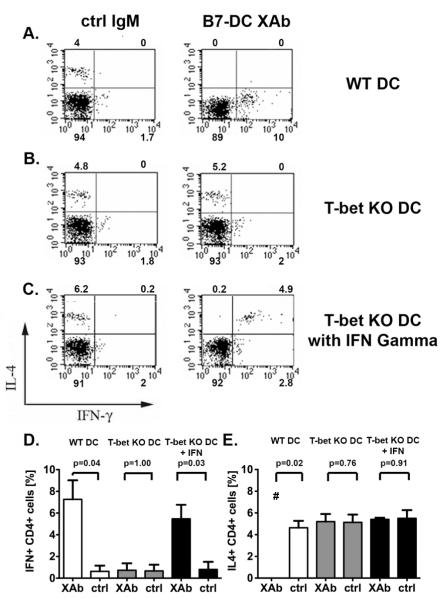
To test whether the failure of T-bet KO DC to repolarize Th2 splenocytes is due to insufficient IFN-γ production by T-bet KO DC, we added recombinant IFN-γ to T-bet KO DC and WT Th2 splenocyte co-cultures and examined the cytokine production profile of CD4+ cells by intracellular cytokine staining (Fig. 2). In the absence of exogenous IFN-γ, Th2 polarized splenocytes co-cultured with T-bet KO DC treated with B7-DC XAb maintained their Th2 cytokine profile (presence of IL-4 and absence of IFN-γ and presence of IL-4, respectively) (Fig. 2B, D and E). The supplementation of recombinant IFN-γ restored the ability of T-bet KO DC to induce the production of IFN-γ by CD4+ T cells (Fig. 2C and DE). However, the IL-4 expression by these T cells was not affected (Fig. 2C and ED).
Figure 2. Exogenous supplementation of IFN-γ only partially compensates for the absence of T-bet during CD4+ cell repolarization.
Th2 polarized and ovalbumin sensitized wild type (WT) splenocytes were co-cultured with OVA-pulsed, antibody treated (A) WT or (B, C) T-bet KO DC in the absence (A, B) and presence (C) of recombinant murine IFN-γ. IFN- γ and IL-4 expression was evaluated by flow cytometry. Supplementation with recombinant murine IFN-γ resulted in the induction of IFN-γ but failed to down-regulate IL4 expression in splenocytes co-cultured with T-bet KO DC (C). All scatter plots are gated for live CD4+ cells; the numbers on the plots represent the percentage of all gated cells localizing to the quadrant (representative experiment). (D, E) Combined data of three independent experiments. The graphs display the mean percentages ± SEM of CD4 + cells staining for (D) IFN γ and (E) IL4. Control IgM = ctrl. The # symbol indicates that the value was below the detection limit of the assay.
Retroviral reconstitution restores T-bet KO DC function
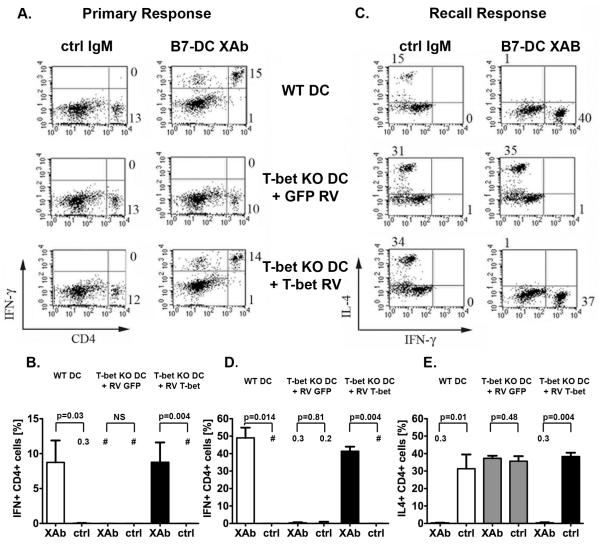
To verify whether our observations were indeed due to the inability of T-bet KO DC to express T-bet and not the result of a more generalized defect in these DC, we reconstituted T-bet expression in genetically deficient DC using a retroviral system. Two days prior to treatment with B7-DC XAb or control IgM and OVA, bone marrow derived T-bet KO DC were subjected to retroviral transduction. In order to obtain an antigen specific, naive cell population free of Tregs and highly activated CD4+ CD25+ cells, we isolated CD4+ CD25- cells from DO11.10 mice. OVA-pulsed, antibody treated DC were subsequently incubated with these purified OVA-specific transgenic DO11.10 T cells for 48 hours. Whereas T-bet KO DC transduced with GFP (control retrovirus) and treated with B7-DC XAb were unable to stimulate IFN-γ production in these cells (Fig. 3A and B), T-bet KO DC transduced with the T-bet retrovirus and treated with B7-DC XAb induced IFN-γ production similar to B7-DC XAb treated WT DC (Fig. 3A and B). Co-culturing splenocytes from Th2 polarized DO11.10 mice with T-bet transduced, B7-DC XAb treated T-bet KO DC also induced the production of IFN-γ and decreased the production of IL-4, compared to GFP transduced similarly treated KO DC (Fig. 3 C-E). Therefore, reconstitution of T-bet restores the ability of B7-DC XAb treated T-bet KO DC to redirect both primary and established Th2 responses, indicating that the induction of a Th1 polarized response by B7-DC XAb depends on the expression of T-bet by the activating DC.
Figure 3. Retrovirus induced T-bet expression reconstitutes the ability of T-bet deficient DC to mediate a Th polarity shift.
(A) Naïve transgenic DO11, CD4+,CD25- T cells were co-cultured with OVA pulsed, antibody treated WT or T-bet KO DC transduced with retrovirus expressing either the control, GFP, or T-bet. IFN-γ production was assessed by intracellular staining and flow cytometry. Retroviral transduction of T-bet in T-bet KO DC restores their ability to induce IFN-γ expression in naïve ovalbumin (OVA) specific CD4+CD25- T lymphocytes. The numbers on the plots represent the percentage of all live cells localizing to the quadrant. (B) Combined data from three independent experiments presenting the mean percentage ± SEM of CD4 + cells staining for IFN γ. (C) Th2 polarized and OVA sensitized transgenic DO11 splenocytes were co-cultured with antibody treated, OVA pulsed WT or T-bet KO DC transduced with either GFP or T-bet. Retroviral transduction of T-bet in T-bet KO DC restores their ability to induce IFN-γ expression and down-regulate IL4 production in Th2 polarized and OVA sensitized transgenic DO11.10. The numbers on the plots represent the percentage of all gated live, blasting CD4+ lymphocytes localizing to the quadrant. (C) Displays a representative experiment. (D, E) Combined data from three independent experiments displayed as the mean percentage ± SEM of CD4 + cells staining for (D) IFN γ and (E) IL4. Retrovirus = RV, Control IgM = ctrl, difference is not significant = NS. For small data points the mean is displayed as a number on the graph. The # symbol indicates that the value was below the detection limit of the assay.
B7-DC XAb treated T-bet KO DC do not protect mice against allergic airway inflammation
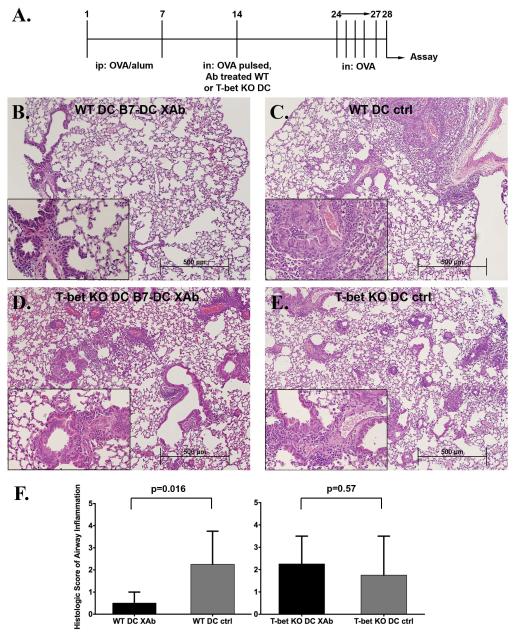
We evaluated the physiological relevance of our finding by examining the ability of B7-DC XAb treated T-bet deficient DC to protect mice in a model of allergic airway inflammation. As illustrated in Fig. 4A, wild-type mice were injected with two doses of OVA/alum to polarize immunity towards Th2 and sensitize the animals to OVA. The animals then received OVA pulsed, antibody treated WT or T-bet KO DC intranasally. A separate experiment demonstrated that DC transferred in this manner home to the thoracic lymph nodes of recipient mice (supplemental Fig. 1, online supplement). Ten days after DC transfer, the mice were challenged with OVA on four consecutive days. Histopathology of lung tissue from mice that received antigen-pulsed, B7-DC XAb treated WT DC revealed only very little evidence of inflammation (Fig. 4B). In contrast, marked perivascular and peribronchial eosinophilic inflammation was observed in the lungs of mice that had received antigen-pulsed WT DC treated with control IgM antibody or T-bet KO DC treated with B7-DC XAb or the control IgM antibody (Fig. 4C, D and E). The severity of pulmonary inflammation is summarized in Figure 4F. Trichrome and PAS stains revealed decreased collagen deposition in the mice that received WT DC treated with B7-DC XAb as compared to the other experimental groups, but did not show a difference regarding mucous hyperplasia. (data not shown). Thus, T-bet expression by DC is critical for the protective response mediated by B7-DC XAb in vivo.
Figure 4. Adoptive transfer of B7-DC XAb treated T-bet deficient DC does not protect mice from allergic airway inflammation.
(A) Scheme of the protocol followed. Th2 polarized, ovalbumin (OVA) sensitized BALB/c mice received 0.5 ×10 6 OVA pulsed, antibody treated WT or T-bet KO intranasally on day 14. (B-E) Representative H&E sections of three independent experiments for each experimental group: (B) WT-B7-DC XAb treated DC (n=8), (C) WT control IgM treated DC (n=8), (D) T-bet KO B7-DC XAb treated DC (n=10) and (E) T-bet KO control IgM treated DC (n=10). Main images are shown at 100× magnification and inserts are 400× magnification. (F) The panels summarize scoring of the severity of histologic inflammation (0 = none to 5 = worst airway inflammation) performed in a blind fashion. The data are displayed as median ± interquartile range. Control IgM = ctrl
To confirm these histopathologic findings, differential cell counts and cytokine levels were assessed in the bronchoalveolar lavage fluid (BALF) and homogenized lung tissue. Mice that received wild-type B7-DC XAb treated DC had fewer eosinophils in the BALF than mice that received similarly treated T-bet KO DC. These animals had eosinophil cell counts comparable to those of mice that had received control IgM treated T-bet KO DC (Fig. 5A). IL-5 was lower in the BALF of recipients of B7-DC XAb treated WT DC, and higher in mice that received B7-DC XAb treated T-bet KO DC or control IgM treated WT or T-bet KO DC (Fig. 5B). The Th1 cytokine IFN-γ was highly expressed in the lungs of mice that received B7-DC XAb treated WT DC but almost absent in all other groups (Fig. 5C). In contrast, the expression of the Th2 cytokine IL-4 was very low in mice treated with B7-DC XAb stimulated WT DC and much higher in the lungs of mice of all other groups (Fig. 5D). In conclusion, T-bet deficient DC treated with B7-DC XAb failed to redirect the established Th2 response and did not protect presensitized animals from allergic pulmonary inflammation.
Figure 5. B7-DC XAb treated T-bet KO DC do not prevent airway eosinophilia and fail to mediate the reprogramming of the immune response.
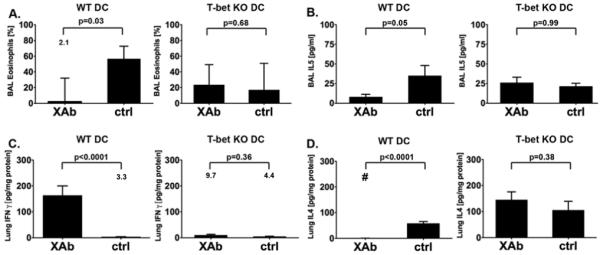
The protocol described in Fig. 4A was followed and (A) the percentage of broncho-alveolar lavage (BAL) eosinophils was determined (mean ± SEM, three experiments, n=36). (B) IL-5 levels were quantified in the BAL fluid by ELISA (mean ± SEM, three experiments, n=36) IFN-γ (C) and IL-4 (D) were measured in lung homogenates by ELISA (mean ± SEM, three experiments, n=23). Measurements were adjusted for total protein concentration. Control IgM = ctrl, difference is not significant = NS. For small data points, the mean is displayed as number on the graph. The # symbol indicates that the value was below the detection limit of the assay.
DISCUSSION
The transcription factor T-bet is best characterized in CD4+ T cells where it regulates the polarization of activated T cells towards Th1. T-bet has also been shown to repress Th2 and Th17 immunity [5, 25]. Th2 repression is due to T-bet’s interference with GATA-3 [26]. Therefore it is not surprising that T-bet KO mice develop either spontaneous asthma (C57BL/6J) or enhanced allergic airway inflammation in asthma models (BALB/c), depending upon their background strain [25, 27, 28]. Accordingly, our findings that T-bet deficient, Th2 polarized splenocytes cannot be reprogrammed to produce Th1 cytokines is consistent with these previous observations. However, our studies provide the first direct evidence for the importance of T-bet expression by DC for the repolarization of an established immune response from Th2 to Th1. T-bet deficient DC treated with B7-DC XAb were unable to modulate the polarity of Th2 polarized WT splenocytes, indicating that reprogramming of immunity does not only require T-bet expression in T cells but also in DC. This was confirmed by retroviral restoration of T-bet expression in T-bet KO DC, which rescued the ability of these DC to stimulate IFN-γ and suppress IL-4 production by Th2 cells. The finding that the same transcription factor functions as a master regulator of an immune response profile in both the inductive DC and the induced T cell in this regulatory network raises interesting questions about the molecular mechanisms that have led to this alignment. In this regard, we have shown previously that production of IFN- γ by DC is required for the induction of IFN- γ by T cells. As T-bet expression is required for IFN- γ production by both cell types, dependency on the transcription factor for both induction of Th1 polarity by DC and a Th1 polarized response by T cells provides a framework for understanding the role of this factor in the cellular network.
The mechanism by which T-bet expression in DC contributes to the polarity shift mediated by B7-DC XAb, however, is not completely understood. We previously observed that the B7-DC XAb mediated restoration of antigen uptake in CpG matured DC is independent of T-bet expression (unpublished observation) [20]. Moreover, despite the absence of T-bet, DC mature properly and are able to upregulate appropriate costimulatory molecules [9]. We have previously demonstrated that B7-DC XAb treatment of WT DC induces the expression of various cytokines by these cells, including IFN-γ, IL-12, IL-6 and TNF-α [23]. In the absence of T-bet the production of several pro-inflammatory cytokines such as IFN-γ and IL-1α and the chemokine MIP-1α is impaired, whereas the expression of many other cytokines and chemokines including IL-12p40, IL-12p70, IL-6, IL-1β, and RANTES appears to be unaffected [9, 29]. Additionally T-bet has been shown to suppress the Th2 polarizing transcription factor GATA3, TNF-α and the thymus- and activating chemokine (TARC) [10, 26, 29].
In a prior publication we demonstrated that the B7-DC XAb mediated polarity shift requires the expression of IL-12, IFN-γ, the presence of the cell surface adhesion molecule ICAM-1 as well as adequate levels of the signaling lymphocytic activation adhesion molecule (SLAM) by DC [19]. In addition, the reprogramming of the immune response appears to be antigen specific and depends on the appropriate interaction between the DC’s antigen loaded MHC and the matching T cell receptors [19]. Interestingly, B7-DC XAb treated IFN-γ KO DC are able to downregulate IL4 by Th2 polarized wild type T cells, but fail to induce IFN-γ [19]. This is consistent with our current observation that the inability of B7-DC XAb treated T-bet KO DC to induce IFN-γ production by naïve antigen specific T cells can be overcome by supplementation of IFN-γ. Despite the addition of IFN-γ, these cells continue to produce IL-4. One possibility is that the continued expression of IL-4 may be due to the absence of the repressor effect of T-bet, such as has been described previously on GATA-3 [26]. Alternatively, in the absence of T-bet, insufficient quantities of IL-12, ICAM-1, SLAM or a lack of other unknown mediators in DC may prevent the downregulation of IL-4. We previously observed a similar phenotype, the simultaneous expression of IL-4 and IFN-γ, following the treatment of Th2 polarized splenocytes with TLR agonists such as CpG-ODN, pI:C, LPS and Gardiquomod as well as anti-CD40 antibodies [30]. This suggests that the effect of these mediators may be due to the induction of IFN-γ in DC, partially recapitulating the effects of B7-DC XAb on the cytokine production of DC. Clearly, more information is needed to further characterize the molecular mechanisms by which T-bet contributes to polarity shifts of the immune response and therefore we continue to dissect these complex relationships.
The requirement for T-bet expression by DC in order to modulate T cell polarity provides a key mechanistic explanation for the prophylactic and therapeutic protection against airway inflammation afforded to mice by administration of B7-DC XAb. In an established mouse model of airway inflammation, the intravenous administration of B7-DC XAb prohibits the development of allergic airway inflammation in pre-sensitized animals [31]. Similar effects are also seen when antigen loaded, B7-DC XAb treated DC are treated in vitro prior to their intranasal transfer into pre-sensitized mice, indicating that the effect of this immune modulating antibody is mediated by DC [21]. Utilizing this adoptive transfer model, we investigated the ability of B7-DC XAb treated DC to reprogram the allergic memory response towards Th1 and provide protection against airway inflammation in the absence and presence of T-bet expression. We demonstrate that the Th1 polarizing and Th2 repressing effects of T-bet in DC are essential for the protection from allergic airway inflammation conferred by B7-DC XAb.
Studies in other disease models also suggest the importance of T-bet expression by DC [29, 32]. In the Th1 polarized disease model of collagen induced arthritis, double knockout mice RAG2 KO/T-bet KO display few spontaneous symptoms and minimal pathology[29]. Observations that the adoptive transfer of WT DC, but not T-bet KO DC, accelerates disease progression suggests that DC lacking T-bet expression protect against this Th1 polarized disorder [29]. In contrast, RAG2 KO/T-bet KO mice developed increased symptoms in a Th2 polarized model of scleroderma, indicating that T-bet expression in innate cells is important for the regulation of Th2 polarized responses [32]. Consequently, based on these studies and our observations it becomes clear that aside from expression in T cells, expression of T-bet in DC is also essential for the induction of Th1 and repression of Th2 immunity. In our case, not only is the development of Th2 immunity repressed, but also, established Th2 immunity is reprogrammed to an alternative phenotype.
It has been proposed that the phenotype of DC determines the polarity of the immune response. Therefore DC supporting the Th1 inflammatory phenotype have been labeled as DC1 whereas DC which promote the characteristic Th2 cytokine milieu are categorized as DC2 [33]. While these terms are applied somewhat loosely, our findings support the concept that the phenotype of the activating DC determines the polarity of the resulting adaptive immune response. Specifically, our observation that T-bet is essential for the ability of a dendritic cell to induce a Th1 response suggests that T-bet might be a key mediator of the so-called DC1 phenotype.
MATERIALS AND METHODS
Mice and primary cell preparation
T-bet deficient mice (C.129S6-Tbx21tm1Glm/J), C57BL/6J and C57BL/6J, GFP mice were obtained from The Jackson Laboratory (Bar Harbor, ME) and bred within our animal colony. BALB/c (BALB/cByJ) mice and DO11 mice (C.Cg-Tg(Do11.10)10Dlo/J) expressing a transgenic TcR that recognizes an ovalbumin peptide presented by MHC class II were also bred in our colony; some BALB/c mice were also purchased directly from The Jackson Laboratory. All mice were housed and used in experimental protocols according to the Mayo Clinic Institutional Animal Care and Use Committee standards.
Murine DC were generated by harvesting bone marrow, eliminating red blood cells (lysis in a hypotonic solution), and culturing remaining cells at 106 per milliliter in media (RPMI 1640 Gibco Invitrogen, Carlsbad, CA) plus 10% Cosmic calf serum (HyClone, Logan, UT) supplemented with 10 ng/mL murine GM-CSF (PeproTech Inc., Rocky Hill, NJ) and 1 ng/mL murine IL-4 (PeproTech Inc.). Two days later, media was gently removed from the adherent cells and replaced with freshly supplemented media. For co-culture experiments presented in Fig. 1 and 2, DC were incubated with antigen and treated with antibody on day 6 before being used for culture on day 7.
Reagents
The human B7-DC cross-linking IgM B7-DC XAb (also referred to as sHIgM12 in other publications) was isolated from the serum of a patient with Waldenstrom’s macroglobulinemia and characterized in our laboratory; both serum derived and recombinant B7-DC XAb were utilized [17, 18, 34]. Another isolated human IgM (also called sHIgM39) was used as a control antibody for these experiments [22]. Both B7-DC XAb and the control IgM are routinely used at a concentration of 10 μg/mL. Murine recombinant interferon-γ (PeproTech Inc.) was utilized at 2000 units/mL for indicated DC/polarized splenocyte co-cultures.
Intracellular staining and flow cytometry
Intracellular staining was performed using the Cytofix/Cytoperm Plus (with GolgiStop) Kit from BD Pharmingen (San Jose, CA). Briefly, cells were stained first with antibodies that detect cell surface molecules for 15 minutes on ice. Following washes with regular flow cytometry buffer (0.5% BSA and 0.1% sodium azide in PBS), cells were incubated with Cytofix/Cytoperm Plus solution for 20 minutes on ice. Next, the cells were washed several times with the Perm/Wash buffer followed by staining (20 minutes on ice) with appropriate antibodies for intracellular cytokines diluted in Perm/Wash buffer. Cells were then washed two or three more times with the Perm/Wash buffer before being resuspended in the regular buffer for flow cytometry analysis. The following anti-mouse antibodies were used for flow cytometry: anti-IL-4 PE (11B11) (eBioscience, San Diego, CA), anti-CD4 APC (RM4-5) (BD Pharmingen), anti-CD4 PerCp (RM4-5) (BD Pharmingen), anti-IFN-γ FITC (XMG1.2) (BD Pharmingen), anti-IFN-γ PE (XMG1.2) (BD Pharmingen), and anti-IFN-γ APC (XMG1.2) (BD Pharmingen).
In vitro co-cultures
Th2 polarized splenocytes were generated from BALB/c, T-bet deficient, or DO11 mice by two 100 μL intraperitoneal injections of chicken ovalbumin (OVA) (Sigma-Aldrich, St. Louis, MO) absorbed to alum (Pierce, Rockford, IL) on days 1 and 7. (Equal volumes OVA in PBS (2mg/mL) and alum were combined for a final OVA concentration of 1 mg/mL, and the mixture was rotated/rocked for at least 30 minutes at room temperature or up to overnight at 4°C.) Spleens were harvested on day 14 (unless otherwise indicated) and homogenized prior to red blood cell lysis with a hypotonic solution. Splenocytes were co-cultured at 37°C with washed DC (BALB/c or T-bet KO) in a 3:1 splenocyte:DC ratio; DC had been incubated with OVA (1 mg/mL) and treated with either B7-DC XAb or control IgM (10 μg/mL) overnight. Culture supernatants were harvested after 3 days and assayed immediately or stored at - 80°C until analysis by ELISA for murine IL-4 and IFN-γ per manufacturer’s protocol (eBioscience).
Mouse airway inflammation
Airway inflammation was induced in BALB/c mice as outlined in Fig. 4A. Briefly, BALB/c mice were sensitized to OVA by two intraperitoneal injections of 100 μg OVA adsorbed to alum as described above. On day 14, mice were anesthetized with tribromoethanol (Mayo Pharmacy, Rochester, MN) prior to intranasal adoptive transfer of 5 × 105 wild-type or T-bet deficient DC that had been incubated with OVA and treated with B7-DC XAb or control IgM overnight. Cells were washed two or three times prior to transfer. In a separate experiment 5 × 105 GFP-DC were intranasally transferred into Th2 polarized, ovalbumin sensitized syngeneic recipients. Forty-eight hours later the mediastinal lymph nodes were harvested and analyzed by fluorescent microscopy for the presence of GFP positive DC. To elicit airway inflammation, anesthetized mice and challenged intranasally with 100 μg OVA in PBS daily from days 24-27. On day 28, mice were sacrificed by pentobarbital injection (Abbott Laboratories, Abbott Park, IL) and BALF was collected from the lungs by flushing with a total of 1 mL HBSS. Cells pelleted from this lavage fluid were assessed by Wright Giemsa staining to obtain a cell differential, while the supernatant was frozen and later assessed for IL-5 content by ELISA (R&D Systems, Minneapolis, MN) per manufacturer’s protocol. After lavage, one lung per mouse was fixed, sectioned, and stained by H&E for histology analysis. H&E slides were randomly graded on a five point scale by one of the investigators (KI) who was unaware of the treatment group assignment of the animal. The other lung was homogenized in 0.5 mL cold PBS; supernatant was analyzed for IL-4 and IFN-γ content by ELISA (eBioscience).
Retrovirus preparation and DC reconstitution experiments
T-bet cDNA was generated from RNA isolated from BALB/c DC and cloned into the pBabe retroviral vector kindly supplied by Dr. Richard Vile (Mayo Clinic) [35]. GFP was also cloned into this vector for use in control transductions. The T-bet/pBabe and GFP/pBabe constructs were transfected into Plat-E ecotropic packaging cells (a gift from Dr. Kay Medina, Mayo Clinic) using the FuGene 6 Transfection Reagent (Roche, Indianapolis, IN) per manufacturer’s protocol [36]. Plat E cells were maintained in DMEM media (Gibco Invitrogen) with 10% Cosmic calf serum plus 1 μg/mL puromycin (Sigma Aldrich). Ten to twelve million Plat E cells were plated the day prior to transfection and were 50-80% confluent at the time of transfection. A 6:1 volume to weight ratio of FuGene to DNA was used with 12 μg of construct DNA being introduced per transfection; fresh media without puromycin was placed on the Plat E cells just prior to transfection. Culture supernatants were collected after 72 hours and stored at 4°C. Cells were immediately covered with fresh media without puromycin and incubated for another 24 hours, when culture supernatant was harvested again and stored at 4°C. Culture supernatants were concentrated approximately 10 fold using 100 kDa filter tubes (Millipore, Billerica, MA) and centrifugation at 3,000 rpm. Concentrated virus was then filtered through a 0.2 micron syringe filter (NALGENE Labware, Rochester, NY), aliquoted, and stored at -80°C.
Each preparation of GFP virus was tested by the transduction of 3T3 cells (kindly provided by Dr. Cynthia Wetmore, Mayo Clinic) simultaneously treated with hexadimethrine bromide (32 μg/mL Sigma Aldrich) to enhance transduction efficiency. After 3 days, flow cytometry was used to compare transduction efficiency by varying amounts of the concentrated GFP virus. Based on the GFP transduction efficiency of 3T3 cells in parallel cultures, DC were transduced on day 3 with the virus containing either the T-bet or GFP construct along with the addition of freshly supplemented (IL-4 and GM-CSF) media and hexadimethrine bromide (32 μg/mL).
On day 6 of DC culture, naïve CD4+,CD25- cells were isolated from the spleens of DO11 mice by magnetic bead sorting (murine anti-CD25) per manufacturer’s protocol (Miltenyi Biotec, Auburn, CA). The CD4+,CD25- cells were added at a 3:1 ratio (CD4+,CD25- cells:DC) to wild-type or retrovirally transduced T-bet KO DCs that had been incubated with OVA (final concentration: 1 mg/mL) and treated with B7-DC XAb or control IgM (10 μg/mL) since day 5. On day 8, cells were analyzed by intracellular staining and flow cytometry as previously described.
For the in vitro polarity shift experiments, T-bet KO DC were also transduced as described and, along with WT DC, were incubated with OVA and treated with B7-DC XAb or control IgM on day 5. Cells were washed two or three times on day 6 and co-cultured with splenocytes harvested from Th2 polarized DO11 transgenic mice (day 14 relative to intraperitoneal OVA/alum injections days 1 and 7) at a 3:1 splenocyte to DC ratio. After 3 days of co-culture, CD4+ cells were assessed for IFN-γ and IL-4 production by intracellular staining and flow cytometry.
Statistical Analysis
GraphPad Prism 4.0 for Macintosh (GraphPad Software, San Diego, CA) was used for statistical analysis. Differences in BALF cell counts and flow cytometry data are displayed as mean ± standard error of the mean (SEM) and compared using the paired T-test. Cytokine analysis data obtained by ELISA are also presented as mean ± SEM. In order to account for clonal expansion as the main driving force of cytokine production we logarithmically transformed all ELISA data prior to statistical analysis by the paired T-test. Histological scores were summarized as median ± interquartile range and analyzed using the Mann-Whitney U test. A p-value of < 0.05 was considered statistically significant.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Rosalyn Cabrera for assembling the GFP-pBabe retrovirus construct. This work was supported by NIH grant R01 HL077296-3 (L.R.P.).
REFERENCES
- 1.Romagnani S. Regulation of the T cell response. Clinical & Experimental Allergy. 2006;36:1357–1366. doi: 10.1111/j.1365-2222.2006.02606.x. [DOI] [PubMed] [Google Scholar]
- 2.Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nature Reviews. Immunology. 2002;2:933–944. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- 3.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Ivanov, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 5.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 6.Sullivan BM, Juedes A, Szabo SJ, von Herrath M, Glimcher LH. Antigen-driven effector CD8 T cell function regulated by T-bet. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:15818–15823. doi: 10.1073/pnas.2636938100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, Mullen AC, Gasink CR, Kaech SM, Miller JD, Gapin L, Ryan K, Russ AP, Lindsten T, Orange JS, Goldrath AW, Ahmed R, Reiner SL. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nature Immunology. 2005;6:1236–1244. doi: 10.1038/ni1268. [erratum appears in Nat Immunol. 2006 Jan;7(1):113] [DOI] [PubMed] [Google Scholar]
- 8.Townsend MJ, Weinmann AS, Matsuda JL, Salomon R, Farnham PJ, Biron CA, Gapin L, Glimcher LH. T-bet regulates the terminal maturation and homeostasis of NK and Valpha14i NKT cells. Immunity. 2004;20:477–494. doi: 10.1016/s1074-7613(04)00076-7. [see comment] [DOI] [PubMed] [Google Scholar]
- 9.Lugo-Villarino G, Maldonado-Lopez R, Possemato R, Penaranda C, Glimcher LH. T-bet is required for optimal production of IFN-gamma and antigen-specific T cell activation by dendritic cells. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:7749–7754. doi: 10.1073/pnas.1332767100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garrett WS, Lord GM, Punit S, Lugo-Villarino G, Mazmanian SK, Ito S, Glickman JN, Glimcher LH. Communicable ulcerative colitis induced by T-bet deficiency in the innate immune system. Cell. 2007;131:33–45. doi: 10.1016/j.cell.2007.08.017. [see comment] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lugo-Villarino G, Ito S, Klinman DM, Glimcher LH. The adjuvant activity of CpG DNA requires T-bet expression in dendritic cells. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:13248–13253. doi: 10.1073/pnas.0506638102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manetti R, Gerosa F, Giudizi MG, Biagiotti R, Parronchi P, Piccinni MP, Sampognaro S, Maggi E, Romagnani S, Trinchieri G. Interleukin 12 induces stable priming for interferon gamma (IFN-gamma) production during differentiation of human T helper (Th) cells and transient IFN-gamma production in established Th2 cell clones. Journal of Experimental Medicine. 1994;179:1273–1283. doi: 10.1084/jem.179.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Annunziato F, Cosmi L, Manetti R, Brugnolo F, Parronchi P, Maggi E, Nagata K, Romagnani S. Reversal of human allergen-specific CRTH2+ T(H)2 cells by IL-12 or the PS-DSP30 oligodeoxynucleotide. Journal of Allergy & Clinical Immunology. 2001;108:815–821. doi: 10.1067/mai.2001.119156. [DOI] [PubMed] [Google Scholar]
- 14.Parronchi P, Brugnolo F, Annunziato F, Manuelli C, Sampognaro S, Mavilia C, Romagnani S, Maggi E. Phosphorothioate oligodeoxynucleotides promote the in vitro development of human allergen-specific CD4+ T cells into Th1 effectors. Journal of Immunology. 1999;163:5946–5953. [PubMed] [Google Scholar]
- 15.Tighe H, Takabayashi K, Schwartz D, Van Nest G, Tuck S, Eiden JJ, Kagey-Sobotka A, Creticos PS, Lichtenstein LM, Spiegelberg HL, Raz E. Conjugation of immunostimulatory DNA to the short ragweed allergen amb a 1 enhances its immunogenicity and reduces its allergenicity. Journal of Allergy & Clinical Immunology. 2000;106:124–134. doi: 10.1067/mai.2000.107927. [see comment] [DOI] [PubMed] [Google Scholar]
- 16.Brugnolo F, Sampognaro S, Liotta F, Cosmi L, Annunziato F, Manuelli C, Campi P, Maggi E, Romagnani S, Parronchi P. The novel synthetic immune response modifier R-848 (Resiquimod) shifts human allergen-specific CD4+ TH2 lymphocytes into IFN-gamma-producing cells. Journal of Allergy & Clinical Immunology. 2003;111:380–388. doi: 10.1067/mai.2003.102. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen LT, Radhakrishnan S, Ciric B, Tamada K, Shin T, Pardoll DM, Chen L, Rodriguez M, Pease LR. Cross-linking the B7 family molecule B7-DC directly activates immune functions of dendritic cells. Journal of Experimental Medicine. 2002;196:1393–1398. doi: 10.1084/jem.20021466. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Radhakrishnan S, Nguyen LT, Ciric B, Ure DR, Zhou B, Tamada K, Dong H, Tseng SY, Shin T, Pardoll DM, Chen L, Kyle RA, Rodriguez M, Pease LR. Naturally occurring human IgM antibody that binds B7-DC and potentiates T cell stimulation by dendritic cells. Journal of Immunology. 2003;170:1830–1838. doi: 10.4049/jimmunol.170.4.1830. [DOI] [PubMed] [Google Scholar]
- 19.Radhakrishnan S, Wiehagen KR, Pulko V, Van Keulen V, Faubion WA, Knutson KL, Pease LR. Induction of a Th1 response from Th2-polarized T cells by activated dendritic cells: dependence on TCR:peptide-MHC interaction, ICAM-1, IL-12, and IFN-gamma. Journal of Immunology. 2007;178:3583–3592. doi: 10.4049/jimmunol.178.6.3583. [DOI] [PubMed] [Google Scholar]
- 20.Radhakrishnan S, Celis E, Pease LR. B7-DC cross-linking restores antigen uptake and augments antigen-presenting cell function by matured dendritic cells. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:11438–11443. doi: 10.1073/pnas.0501420102. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Radhakrishnan S, Iijima K, Kobayashi T, Kita H, Pease LR. Dendritic cells activated by cross-linking B7-DC (PD-L2) block inflammatory airway disease. Journal of Allergy & Clinical Immunology. 2005;116:668–674. doi: 10.1016/j.jaci.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 22.Radhakrishnan S, Nguyen LT, Ciric B, Flies D, Van Keulen VP, Tamada K, Chen L, Rodriguez M, Pease LR. Immunotherapeutic potential of B7-DC (PD-L2) cross-linking antibody in conferring antitumor immunity. Cancer Research. 2004;64:4965–4972. doi: 10.1158/0008-5472.CAN-03-3025. [DOI] [PubMed] [Google Scholar]
- 23.Blocki FA, Radhakrishnan S, Van Keulen VP, Heckman KL, Ciric B, Howe CL, Rodriguez M, et al. Induction of a gene expression program in dendritic cells with a cross-linking IgM antibody to the co-stimulatory molecule B7-DC. FASEB J. 2006;20:2408–2410. doi: 10.1096/fj.06-6171fje. [DOI] [PubMed] [Google Scholar]
- 24.Herz U, Renz H, Wiedermann U. Animal models of type I allergy using recombinant allergens. Methods (Duluth) 2004;32:271–280. doi: 10.1016/j.ymeth.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 25.Fujiwara M, Hirose K, Kagami S, Takatori H, Wakashin H, Tamachi T, Watanabe N, Saito Y, Iwamoto I, Nakajima H. T-bet inhibits both TH2 cell-mediated eosinophil recruitment and TH17 cell-mediated neutrophil recruitment into the airways. Journal of Allergy & Clinical Immunology. 2007;119:662–670. doi: 10.1016/j.jaci.2006.12.643. [DOI] [PubMed] [Google Scholar]
- 26.Hwang ES, Szabo SJ, Schwartzberg PL, Glimcher LH. T helper cell fate specified by kinase-mediated interaction of T-bet with GATA-3. Science. 2005;307:430–433. doi: 10.1126/science.1103336. [DOI] [PubMed] [Google Scholar]
- 27.Finotto S, Neurath MF, Glickman JN, Qin S, Lehr HA, Green FH, Ackerman K, Haley K, Galle PR, Szabo SJ, Drazen JM, De Sanctis GT, Glimcher LH. Development of spontaneous airway changes consistent with human asthma in mice lacking T-bet. Science. 2002;295:336–338. doi: 10.1126/science.1065544. [see comment] [DOI] [PubMed] [Google Scholar]
- 28.Finotto S, Hausding M, Doganci A, Maxeiner JH, Lehr HA, Luft C, Galle PR, Glimcher LH. Asthmatic changes in mice lacking T-bet are mediated by IL-13. International Immunology. 2005;17:993–1007. doi: 10.1093/intimm/dxh281. [DOI] [PubMed] [Google Scholar]
- 29.Wang J, Fathman JW, Lugo-Villarino G, Scimone L, von Andrian U, Dorfman DM, Glimcher LH. Transcription factor T-bet regulates inflammatory arthritis through its function in dendritic cells. Journal of Clinical Investigation. 2006;116:414–421. doi: 10.1172/JCI26631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Radhakrishnan S, Nguyen LT, Ciric B, Van Keulen VP, Pease LR. B7-DC/PD-L2 cross-linking induces NF-kappaB-dependent protection of dendritic cells from cell death. Journal of Immunology. 2007;178:1426–1432. doi: 10.4049/jimmunol.178.3.1426. [DOI] [PubMed] [Google Scholar]
- 31.Radhakrishnan S, Iijima K, Kobayashi T, Rodriguez M, Kita H, Pease LR. Blockade of allergic airway inflammation following systemic treatment with a B7-dendritic cell (PD-L2) cross-linking human antibody. Journal of Immunology. 2004;173:1360–1365. doi: 10.4049/jimmunol.173.2.1360. [DOI] [PubMed] [Google Scholar]
- 32.Aliprantis AO, Wang J, Fathman JW, Lemaire R, Dorfman DM, Lafyatis R, Glimcher LH. Transcription factor T-bet regulates skin sclerosis through its function in innate immunity and via IL-13. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:2827–2830. doi: 10.1073/pnas.0700021104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pulendran B, Palucka K, Banchereau J. Sensing pathogens and tuning immune responses. Science. 2001;293:253–256. doi: 10.1126/science.1062060. [DOI] [PubMed] [Google Scholar]
- 34.Van Keulen VP, Ciric B, Radhakrishnan S, Heckman KL, Mitsunaga Y, Iijima K, Kita H, Rodriguez M, Pease LR. Immunomodulation using the recombinant monoclonal human B7-DC cross-linking antibody rHIgM12. Clinical & Experimental Immunology. 2006;143:314–321. doi: 10.1111/j.1365-2249.2005.02992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morgenstern JP, Land H. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Research. 1990;18:3587–3596. doi: 10.1093/nar/18.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morita S, Kojima T, Kitamura T. Plat-E: an efficient and stable system for transient packaging of retroviruses. Gene Therapy. 2000;7:1063–1066. doi: 10.1038/sj.gt.3301206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.