Abstract
Clinical genetic testing is available for mutations in BMPR2 associated with pulmonary arterial hypertension (PAH). The aim of this study is to assess attitudes of individuals affected by or at risk for PAH regarding genetic testing. Structured telephone interviews were conducted with 119 individuals affected by or at risk for PAH recruited from pulmonary hypertension clinic at Vanderbilt, Vanderbilt familial PAH registry, attendees at 2006 PHA meeting, and a local PAH support group. Sixty-four percent reported knowing little or nothing about BMPR2 testing. Predictors of greater self-assessed knowledge included having an affected family member and learning about BMPR2 testing through the internet. Most respondents reported that while they spent some time thinking about being tested for BMPR2, they had little trouble deciding. The most frequently cited reason for testing was to provide information for their children. About 20% said they had been tested, even though <5% have actually received clinical testing. Although patients with PAH and their at-risk relatives typically feel relatively uninformed about testing for mutations in BMPR2 and at times are confused about their testing status, they nonetheless report that it is easy to decide about testing.
Keywords: Genetic predisposition testing, Pulmonary hypertension, Ethics
Introduction
Mutations in BMPR2 are the most common genetic variants contributing to the development of pulmonary arterial hypertension (PAH), a rare and often fatal disease. Seventy-five percent of PAH patients with a family history of the disease (FPAH) have a mutation in BMPR2 (Austin and Loyd 2007). Although these mutations are autosomal dominant, only 20% of persons with a mutation in BMPR2 ever develop the disease. Offspring of an affected individual with a variant BMPR2 have approximately 10% risk of developing PAH.
Familial PAH tends to become symptomatic at younger ages and lead to earlier death in succeeding generations, thus demonstrating anticipation (Austin and Loyd 2007). Genetic and environmental interactions are believed to influence whether PAH will occur in a person carrying a mutation (Humbert et al. 2001; Newman et al. 2004). Genetic counseling, including the availability of clinical genetic testing, has been recommended for blood relatives of those diagnosed with PAH (McGoon et al. 2004). Given the severity of the disease, the possibility that external factors hasten onset, and the potential importance of early treatment for slowing progress of the disease, families affected by PAH and those treating the disease may want to know whether mutations in BMPR2 are present.
To date, little has been reported about how persons with PAH or at risk for developing the disease think about genetic testing for the BMPR2 mutation. Prior to the identification of the role of BMPR2 in PAH, 62 individuals diagnosed with PAH or who had a blood relative diagnosed with the disease were interviewed about their interest in being tested. Nearly two thirds of the respondents indicated that they would probably or definitely have genetic testing were it to become available (Lientz and Clayton 2000). Although clinical testing has been available for almost 3 years, no studies on utilization of genetic testing among persons with PAH or at risk for developing the disease have been reported. Differences in perceptions of testing among those with a known familial history and those with no known history of the disease have also been largely unexplored.
For this study, we interviewed individuals with PAH or at risk for developing the disease to learn what they think about genetic testing for mutations in BMPR2 and to compare differences among those with a family history of the disorder and those without. We hypothesized that individuals would differ in their knowledge and attitudes about genetic testing depending on whether they have PAH in their families. We also hypothesized that attitudes would differ between those who pursued clinical testing and those who did not, but as noted below, uptake was too low to permit meaningful comparisons. Findings presented here are informative to persons affected by the disease and those who care for them, as well as researchers and practitioners interested in understanding how patients think about testing for modestly penetrant genetic diseases, of which PAH is a prime example.
Methods
Setting and Participant Enrollment
The Institutional Review Board of Vanderbilt University approved this study, and informed consent was obtained from all participants. Respondents were recruited in one of four ways: in person at the Pulmonary Hypertension Clinic at Vanderbilt University; through written correspondence with individuals on the Vanderbilt PAH registry of patients and their family members (Austin and Loyd 2007; Loyd 2007); in person at the 2006 Pulmonary Hypertension Association conference held in Minneapolis, MN; and through written correspondence with members of one southeastern PAH support group.
Participants were screened to exclude those who had scleroderma, scleroderma-related disease, congenital heart disease, liver disease, HIV, or other diseases that could cause PAH. Participants who had previously used fenfluramine and other appetite suppressants were also excluded (Walker et al. 2006). Upon receipt of signed consent, patients were contacted via phone for interview. Interviews were close-ended and usually lasted approximately 30–45 min. During the interview respondents were asked a series of questions regarding their knowledge of PAH, perceptions of genetic testing, their systems of social support, and other demographic information. Questions about how they thought and decided about genetic testing (See Appendix) were developed from an earlier qualitative study of how at risk individuals for Huntington’s disease decide about predictive genetic testing (Cox 2003). Respondents were paid $25 in compensation for their time.
Statistical Analysis
All analyses were performed using SAS, version 9 (SAS Institute, Cary, NC, USA). The sample contains information on three groups at-risk for carrying the BMPR2 mutation, based upon whether the subject was diagnosed with PAH and/or has a family history of PAH. Table 1 cross-tabulates the cases. FPAH-diagnosed (n=22) is composed of individuals diagnosed with PAH who also have a family history of PAH. FPAH-at risk (n=31) is composed of individuals not diagnosed with PAH but who have a family history of the disease. The PAH - idiopathic (n=66) represents individuals with no family history but who are affected with the disease.
Table 1.
Distribution of Cases by Family History of Pulmonary Arterial Hypertension (PAH) and Diagnosis of PAH
| Family history | Diagnosed with PAH |
Total | |
|---|---|---|---|
| Yes | No | ||
| Yes | FPAH-diagnosed, n=22 |
FPAH-at risk, n=31 |
53 |
| No | PAH-idiopathic, n=66 |
NA | 66 |
| Total | 88 | 31 | 119 |
We estimated a logistic regression for the probability of believing that one has “a lot” or “a fair amount” of knowledge about testing for BMPR2, contrasting the FPAH-diagnosed and FPAH-at risk groups with the PAH-idiopathic group (model A). We then compared this model with one that includes additional potential predictors (model B), such as annual income (greater than $50,000=1 v. $50,000 or less=0), education (at least some college=1 vs. terminated education at or before high school graduation/GED=0), the subject’s age, and whether the subject was married (married=1 vs. single, divorced, widowed=0) or had any children.
Results
Demographics
From January 2006 through May 2007 one hundred nineteen adults diagnosed with or at risk for developing PAH were interviewed. Twenty-nine percent of our sample (n=33) were recruited in person at the Pulmonary Hypertension Clinic at Vanderbilt University; 36% (n=45) were reached through written correspondence from the Vanderbilt PAH registry of patients known to have familial PAH and their family members; 27% (n=32) were recruited in person at the 2006 Pulmonary Hypertension Association conference held in Minneapolis, MN; and 8% (n=9) were recruited through written correspondence with members of one southeastern PAH support group. (See Table 2). Stated differently, almost two-thirds (65%) were recruited through Vanderbilt (VU-group). Response rates varied across recruitment sites, including a rate of 61% of those invited to participate at the Vanderbilt Clinic; 28% from the Vanderbilt PAH registry; and 7% from the Southeastern PAH support group mailing. It was not possible to determine a rate for those recruited at the PHA meeting.
Table 2.
Descriptive Statistics
| Variable | History |
No history | Total (n=119) | P value (two-tailed) | |
|---|---|---|---|---|---|
| FPAH-diagnosed (n=22) |
FPAH-at-risk (n=31) | PAH-idiopathic (n=66) |
|||
| Female | 86% | 68% | 83% | 80% | 0.142 |
| Nonwhite | 14% | 10% | 14% | 13% | 0.850 |
| Income | 0.361 | ||||
| $35,000 or less | 30% | 28% | 31% | 30% | 0.952 |
| $35,001–$50,000 | 25% | 31% | 12% | 19% | 0.081 |
| More than $50,000 | 45% | 41% | 57% | 51% | 0.143 |
| At least some college | 68% | 39% | 79% | 66% | 0.000 |
| Insured | 95% | 94% | 98% | 97% | 0.428 |
| Religious | 91% | 81% | 92% | 89% | 0.212 |
| Married | 77% | 74% | 76% | 76% | 0.967 |
| Any children | 91% | 81% | 80% | 82% | 0.506 |
| Avg. number of children | 2 | 2.1 | 1.7 | 1.9 | 0.278 |
| Avg. age (years) | 48.7 | 53 | 48.4 | 49.7 | 0.241 |
| Number of meds | 4.23 | 0 | 4.53 | NA | 0.009 |
Table 2 presents descriptive information about subjects in each of the three groups. The sample is predominantly white, female, and insured. Mean age was fifty. At least 80% of respondents in each group had children, with about two children per family on average. Eighty-nine percent self-identified as having a religious affiliation. Groups had differing rates of college education (68% among FPAH-diagnosed, 39% among FPAH-at risk group, and 79% among PAH-idiopathic respondents).
Respondents’ knowledge of and decisions regarding genetic testing for BMPR2
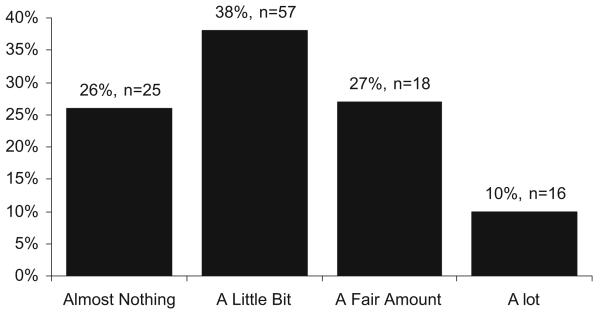
Figure 1 shows the breakdown of self-reported knowledge of testing for all respondents. Three respondents refused to answer the question and were excluded. Seventy-one percent reported knowing “almost nothing” or only “a little bit” when asked how much they knew specifically about testing for mutations in BMPR2 associated with PAH.
Fig. 1.
Knowledge About Testing for BMPR2.
Table 3 presents descriptive information of respondents’ experience with information about BMPR2. More than half (60%) of all respondents had first learned about the test 1 year or more prior to interview. FPAH-at risk respondents had known about the test for a longer period of time. PAH-idiopathic reported having the least amount of knowledge about BMPR2 testing. When asked how they had heard about the test, half (52%) of respondents reporting having learned about the test from a doctor, nurse or other health care provider. Thirty seven percent had learned about the test from a research study (including this one). Nineteen percent had gotten information about the test from the PHA website. None had obtained information about genetic testing from the media.
Table 3.
Engagement with Information About PAH and BMPR2
| Variable | History |
No history | Total (n = 119) | ||
|---|---|---|---|---|---|
| FPAH-diagnosed (n=22) |
FPAH-at-risk (n=31) |
PAH-idiopathic (n=66) |
|||
| Talked with others about PAH | 77% | 81% | 80% | 80% | 0.872 |
| Talked with others about having test for BMPR2 | 26% | 32% | 33% | 31% | 0.428 |
| Knowledge BMPR2 testa | 41% | 45% | 17% | 29% | 0.006 |
| Known about test 1+ year | 62% | 82% | 49% | 60% | 0.013 |
| Thought had BMPR2 test | 27% | 16% | 20% | 20% | 0.603 |
| Sources of knowledge about BMPR2 | |||||
| Family | 0% | 35% | 3% | 11% | 0.000 |
| Doctor | 59% | 55% | 44% | 50% | 0.372 |
| Research study | 50% | 42% | 27% | 35% | 0.103 |
| PHA Association | 9% | 13% | 18% | 15% | 0.542 |
| Web site | 14% | 13% | 21% | 18% | 0.522 |
| Media | 0% | 0% | 0% | 0% | NA |
Reported knowing “a fair amount” or “a lot” about testing for the disorder
Table 4 presents the results of logistic regressions for the predictors of self assessed knowledge of genetic testing for the BMPR2 mutation. Model A presents the base model that includes only indicators for risk-group, compared with the idiopathic group (i.e., have PAH but no family history of PAH). The model reveals a difference in the log-odds of greater self assessed knowledge of mutation testing for the BMPR2 mutation among FPAH-diagnosed and FPAH-at risk groups compared to PAH-idiopathic respondents (, p=0.005).
Table 4.
Odds-Ratios for Predictors of Self-perceived Knowledge of Genetic Testing
| Variable | Model A |
Model B |
||||||
|---|---|---|---|---|---|---|---|---|
| Odds ratio | LCI | UCI | p> | Odds ratio | LCI | UCI | p> | |
| FPAH-diagnosed | 3.46 | 1.19 | 10.08 | 0.02 | 5.96 | 1.62 | 21.94 | 0.01 |
| FPAH-at risk | 4.12 | 1.58 | 10.74 | 0.00 | 25.33 | 2.08 | 308.75 | 0.01 |
| Income >$50k | – | – | – | – | 1.28 | 0.45 | 3.63 | 0.65 |
| College attendance | – | – | – | – | 1.18 | 0.39 | 3.58 | 0.77 |
| Married | – | – | – | – | 0.54 | 0.18 | 1.66 | 0.28 |
| Any children | – | – | – | – | 3.41 | 0.72 | 16.03 | 0.12 |
| Age | – | – | – | – | 0.95 | 0.63 | 1.43 | 0.81 |
| Number of meds | – | – | – | – | 1.33 | 0.90 | 1.96 | 0.15 |
| Source of knowledge | ||||||||
| Family | – | – | – | – | 1.37 | 0.28 | 6.63 | 0.70 |
| Doctor | – | – | – | – | 0.98 | 0.36 | 2.63 | 0.96 |
| Research study | – | – | – | – | 0.80 | 0.28 | 2.35 | 0.69 |
| Association | – | – | – | – | 2.32 | 0.66 | 8.13 | 0.19 |
| Web site | – | – | – | – | 4.98 | 1.37 | 18.07 | 0.02 |
Model stats: model A, likelihood-ratio χ22=10.46 (p=0.005), pseudo-R2 =0.074; model B, likelihood-ratio χ213=25.01 (p=0.023), pseudo-R2 = 0.176
Including additional covariates likely to influence self assessed knowledge of genetic testing for BMPR2 testing (model B) improved the model fit (, p=.023) and revealed that accessing information about testing for BMPR2 on the Web increased the log odds of being highly knowledgeable about testing for BMPR2 4.98 times.
All respondents were asked a series of five questions regarding how they reached a decision about genetic testing. Most respondents reported that they had not spent a great deal of time thinking about whether or not they would have the genetic test for mutations in BMPR2 prior to interview (62%). The majority were quick to decide whether or not they wanted to have the test (71%), reported that decision making was not hard and that they had not changed their mind about whether or not they wanted to have the test once they had made up their minds (83%). Not surprisingly, more than half (59%) stated that their decision about whether or not to have the test for BMPR2 did not evolve over time. The three groups—FPAH-affected, FPAH-at risk, PAH-idiopathic—did not differ statistically in their decision making process.
Familial PAH Respondents: Positive Reasons for Testing
Respondents with a known family history of PAH (n=53) were given a list of potential reasons for testing including to learn about children’s risk, to make decisions about having more children, to be able provide children with more accurate information about their risks for developing PAH, to be reassured, or because respondent’s family or doctor thought he or she should have genetic testing. For each potential reason respondents were asked how important each factor was (very important, somewhat important or not at all important) in their consideration to have genetic testing. The most important reasons included learning more about children’s risk (84%) and ability to provide children with more information about their risk (83%). Fifteen percent volunteered contribution to research as an important reason to undergo testing when asked if there were any other potential reasons for testing. Patients who actually had testing at Vanderbilt concurred (Hannig et al. 2008).
Perceptions Regarding Uptake of Testing
Twenty percent of the respondents responded “yes” when asked if they “had the test for the exact mutation in the BMPR2 gene.” Clinicians at VUMC, however, report that they offered BMPR2 mutation analysis to at-risk individuals in 23 mutation-positive families. To date, 22 individuals from 12 families have requested genetic counseling (seven M, 15 F), and only 10/22 (four M, six F) have been tested (<5% of at-risk individuals; Hannig et al. 2008).
Among those who thought they had been tested, 75% (18/24) are confirmed to have participated in other research studies recruited either at the Vanderbilt clinic or at the 2006 Pulmonary Hypertension Association Biennial Conference. All participants recruited through the Vanderbilt clinic or registry were informed of the availability of clinical testing prior to interview, either directly through their Pulmonary Hypertension specialist at the Vanderbilt clinic or via newsletter sent out to persons on the Vanderbilt registry.
Discussion
This study involves 119 individuals at-risk for BMPR2 mutations that predispose to the development of PAH. The individuals were predominantly white, middle class, and covered by health insurance. Individuals from each of the three risk groups—people with PAH and a family history (high-risk), people with PAH but no family history (idiopathic), and people with a family history only (family-risk)—were comparable in most observable ways. The exception was in level of educational attainment with FPAH-at risk respondents being less likely to have attended college.
Before the role of BMPR2 in the pathogenesis of PAH was discovered, individuals affected by or who had relatives with PAH expressed great interest in being tested for mutations that can cause the disease (Lientz and Clayton 2000). Currently, they identify benefits for their children as the main reasons for being tested, a theme commonly found by other investigators studying uptake of other types of genetic tests (Lerman et al. 1995; Nordin et al. 2004). Respondents reported experiencing little difficulty with making a decision about testing—they typically thought only briefly, made a decision, and did not waver thereafter. Although little work has focused on how people make decisions about genetic testing, the pattern observed in our study differs rather markedly from the findings of other investigators who found that while some individuals at risk for a different disorder—Huntington’s disease (HD)—knew right away what they would do about genetic testing, others had to mull it over and evolve into a decision (Cox 2003). In addition to the possibility that FPAH simply “feels” different than HD, one possible explanation for the relative ease reported by virtually all our respondents is that the majority of them were already diagnosed with PAH so that their own future health status was already clear. Another is that most of them were not actually pursuing testing at the time of the interview.
The overwhelming majority of our respondents felt that they knew little or nothing about the test even though most had known about the test for at least a year. Individuals with a family history (i.e., the high-risk and family-risk) of PAH were more likely to report high levels of knowledge regarding genetic testing for this mutation even though patients with idiopathic PAH had higher income and more education. This suggests that economic resources and greater academic achievement are less important in influencing an individual’s knowledge about testing than specific, relevant information about the disorder from family or others. Accessing information on the internet also increased self-perceived knowledge.
As previously noted, most respondents identified desire to learn about their children’s risk and the ability to provide children with more information about their risk as strong motivations for testing. While doctrines such as informed consent presume that people want to know what is at stake before they make decisions about testing, our study suggests that willingness to be tested may, in fact, be unrelated to self-perceived or actual knowledge of genetics. This means that counselors will have to be proactive if patients are to understand the clinical implications of testing for mutations in BMPR2. Counselors may be well advised, for example, to ask patients to explain that one can have a mutation and never develop the disease. The fact that so many respondents felt uninformed about BMPR2 testing even though more than half stated that they had learned about the test from a health care professional suggests that more work needs to be done.
Despite the expressed level and certainty of interest, few of the respondents had actually obtained testing in a clinical laboratory. This finding is consistent with the commonly observed disparity between stated interest in testing and actual uptake of predisposition genetic testing (Cappelli et al. 1999; Clayton 2003), especially for mutations that are poorly penetrant (Barnoy 2007). Even so, a number of respondents reported that they had received clinical testing when in fact they had not. These responses probably reflect confusion between participation in the research trials that identified the gene and clinical testing, a distinction that both investigators and subjects find perplexing and ethically challenging (Lane and Heitman 2007). In addition, analysis of these interviews indicates that self-perceived knowledge of testing alone did not correlate with respondents’ believing they had actually had the test, suggesting that many people would be willing to proceed with testing even if they felt they were uninformed.
Respondents overall were much more likely to talk with others about the impact of dealing with PAH than they were to talk about whether or not to be tested for mutations in BMPR2. The presence or absence of a family history of PAH did not affect respondents’ willingness to talk about the challenges of PAH and its inheritance. In light of the reduced penetrance of mutations in BMPR2, it is no surprise that recent studies have revealed that many individuals with so-called idiopathic PAH also have mutations in this gene (Morisaki et al. 2004; Sztrymf et al. 2007). As a result, clinicians should counsel patients with idiopathic PAH to consider carefully the implications of genetic testing for family members and the desirability of talking with their relatives.
This study has a number of important limitations. The sample size (n=119) is relatively small, and the response rates vary substantially among the recruitment sites. Nonetheless, since we would expect that people who are interested in BMPR2 testing would be more likely to participate in this study (a number of our respondents were already highly involved in PAH support groups, educational activities, and PAH research), the prevalence of self-perceived lack of knowledge is all the more striking. Even though the majority of individuals at risk do not pursue genetic testing, more needs to be done to educate those who want more knowledge about BMPR2 testing. Genetic counseling is one strategy, and reliable information on the internet appears to be another.
Acknowledgement
This work was supported by NIH/NHLBI P01 HL 072058 “Genetic and Environmental Pathogenesis of PPH.” We would like to thank John Newman, James E. Loyd, and Lisa Wheeler for their comments and support.
Appendix: Questions About Thoughts on Testing
People make their choices about genetic testing in different ways. Some people quickly decide whether to have a genetic test. Other people may decide more slowly or remain unsure of what they want to do. After ach statement, please tell me whether you agree or disagree.
- I have changed my mind at least once about whether I want to have the genetic test for the mutation in the PPH gene.
- 1 Agree
- 0 Disagree
- I have had a hard time deciding whether I want to have the genetic test for the mutation in the PPH gene.
- 1 Agree
- 0 Disagree
- Once I knew that a genetic test for the mutation in the PPH gene was available, I made the decision about whether to have the test very quickly.
- 1 Agree
- 0 Disagree
- My thoughts about whether to have the genetic test for the mutation in the PPH gene have gradually evolved over time.
- 1 Agree
- 0 Disagree
- After I first learned that the genetic test for the mutation was available, I spent time thinking about whether I wanted to have the test
- 1 Agree
- 0 Disagree
Contributor Information
Diana L. Jones, Department of Human and Organizational Development, Vanderbilt University, Nashville, TN, USA
Joanne C. Sandberg, Department of Human Relations, Sociology, and Nonprofit Studies, High Point University, High Point, NC, USA
Mary J. Rosenthal, Center for Biomedical Ethics and Society, Vanderbilt University School of Medicine, 2525 West End Ave., Suite 400, Nashville, TN 37203-8679, USA
Robert C. Saunders, Department of Human and Organizational Development, Vanderbilt University, Nashville, TN, USA
Vickie L. Hannig, Department of Pediatrics, Vanderbilt University School of Medicine, Nashville, TN, USA
Ellen W. Clayton, Center for Biomedical Ethics and Society, Vanderbilt University School of Medicine, 2525 West End Ave., Suite 400, Nashville, TN 37203-8679, USA; Department of Pediatrics, Vanderbilt University School of Medicine, Nashville, TN, USA
References
- Austin ED, Loyd JE. Genetics and mediators in pulmonary arterial hypertension. Clinics in Chest Medicine. 2007;28(1):43–57. vii–viii. doi: 10.1016/j.ccm.2006.11.007. doi:10.1016/j.ccm.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnoy S. Genetic testing for late-onset diseases: effect of disease controllability, test predictivity, and gender on the decision to take the test. Genetic Testing. 2007;11(2):187–192. doi: 10.1089/gte.2006.0509. doi:10.1089/gte.2006.0509. [DOI] [PubMed] [Google Scholar]
- Cappelli M, Surh L, Humphreys L, Verma S, Logan D, Hunter A, et al. Psychological and social determinants of women’s decisions to undergo genetic counseling and testing for breast cancer. Clinical Genetics. 1999;55(6):419–430. doi: 10.1034/j.1399-0004.1999.550605.x. doi:10.1034/j.1399-0004.1999.550605.x. [DOI] [PubMed] [Google Scholar]
- Clayton EW. Ethical, Legal, and Social Implications of Genomic Medicine. The New England Journal of Medicine. 2003;349:562–569. doi: 10.1056/NEJMra012577. doi:10.1056/NEJMra012577. [DOI] [PubMed] [Google Scholar]
- Cox SM. Stories in decisions: how at-risk individuals decide to request predictive testing for Huntington disease. Qualitative Sociology. 2003;26(2):257–280. doi:10.1023/A:1022971113683. [Google Scholar]
- Hannig VL, Wheeler L, Phillips JAI, Vnencak-Jones CL, Newman JH, Clayton EW, et al. Interest in clinical genetic testing for familial pulmonary arterial hypertension (FPAH) American Journal of Respiratory and Critical Care Medicine. 2008;177:A921. Abstracts. [Google Scholar]
- Humbert M, Nunes H, Sitbon O, Parent F, Herve P, Simonneau G. Risk factors for pulmonary arterial hypertension. Clinics in Chest Medicine. 2001;22(3):459–475. doi: 10.1016/s0272-5231(05)70284-7. doi:10.1016/S0272-5231(05)70284-7. [DOI] [PubMed] [Google Scholar]
- Lane K, Heitman E. Role of patient-specific research data in the clinic; Paper presented at the American Society of Bioethics and Humanities Annual Meeting; Washington, DC. 2007. [Google Scholar]
- Lerman C, Seay J, Balshem A, Audrain J. Interest in genetic testing among first-degree relatives of breast cancer patients. American Journal of Medical Genetics. 1995;57(3):385–392. doi: 10.1002/ajmg.1320570304. doi:10.1002/ajmg.1320570304. [DOI] [PubMed] [Google Scholar]
- Lientz EA, Clayton EW. Psychosocial implications of primary pulmonary hypertension. American Journal of Human Genetics. 2000;59(Suppl 2):209. [Google Scholar]
- Loyd JE. National Registry for Familial Primary Pulmonary Hypertension. 2007 Retrieved November 11, 2007, from http://www.mc.vanderbilt.edu/root/vumc.php?site=vupphstudy.
- McGoon M, Gutterman D, Steen V, Barst R, McCrory DC, Fortin TA, et al. Screening, early detection, and diagnosis of pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest. 2004;126(Suppl 1):14S–34S. doi: 10.1378/chest.126.1_suppl.14S. doi:10.1378/chest.126.1_suppl.14S. [DOI] [PubMed] [Google Scholar]
- Morisaki H, Nakanishi N, Kyotani S, Takashima A, Tomoike H, Morisaki T. BMPR2 mutations found in Japanese patients with familial and sporadic primary pulmonary hypertension. Human Mutation. 2004;23(6):632. doi: 10.1002/humu.9251. doi:10.1002/humu.9251. [DOI] [PubMed] [Google Scholar]
- Newman JH, Trembath RC, Morse JA, Grunig E, Loyd JE, Adnot S, et al. Genetic basis of pulmonary arterial hypertension: current understanding and future directions. Journal of the American College of Cardiology. 2004;43(Suppl 12):33S–39S. doi: 10.1016/j.jacc.2004.02.028. doi:10.1016/j.jacc.2004.02.028. [DOI] [PubMed] [Google Scholar]
- Nordin K, Bjork J, Berglund G. Factors influencing intention to obtain a genetic test for a hereditary disease in an affected group and in the general public. Preventive Medicine. 2004;39(6):1107–1114. doi: 10.1016/j.ypmed.2004.04.021. doi:10.1016/j.ypmed.2004.04.021. [DOI] [PubMed] [Google Scholar]
- Sztrymf B, Yaici A, Girerd B, Humbert M. Genes and pulmonary arterial hypertension. Respiration. 2007;74(2):123–132. doi: 10.1159/000098818. doi:10.1159/000098818. [DOI] [PubMed] [Google Scholar]
- Walker AM, Langleben D, Korelitz JJ, Rich S, Rubin LJ, Strom BL, et al. Temporal trends and drug exposures in pulmonary hypertension: an American experience. American Heart Journal. 2006;152(3):521–526. doi: 10.1016/j.ahj.2006.02.020. doi:10.1016/j.ahj.2006.02.020. [DOI] [PubMed] [Google Scholar]