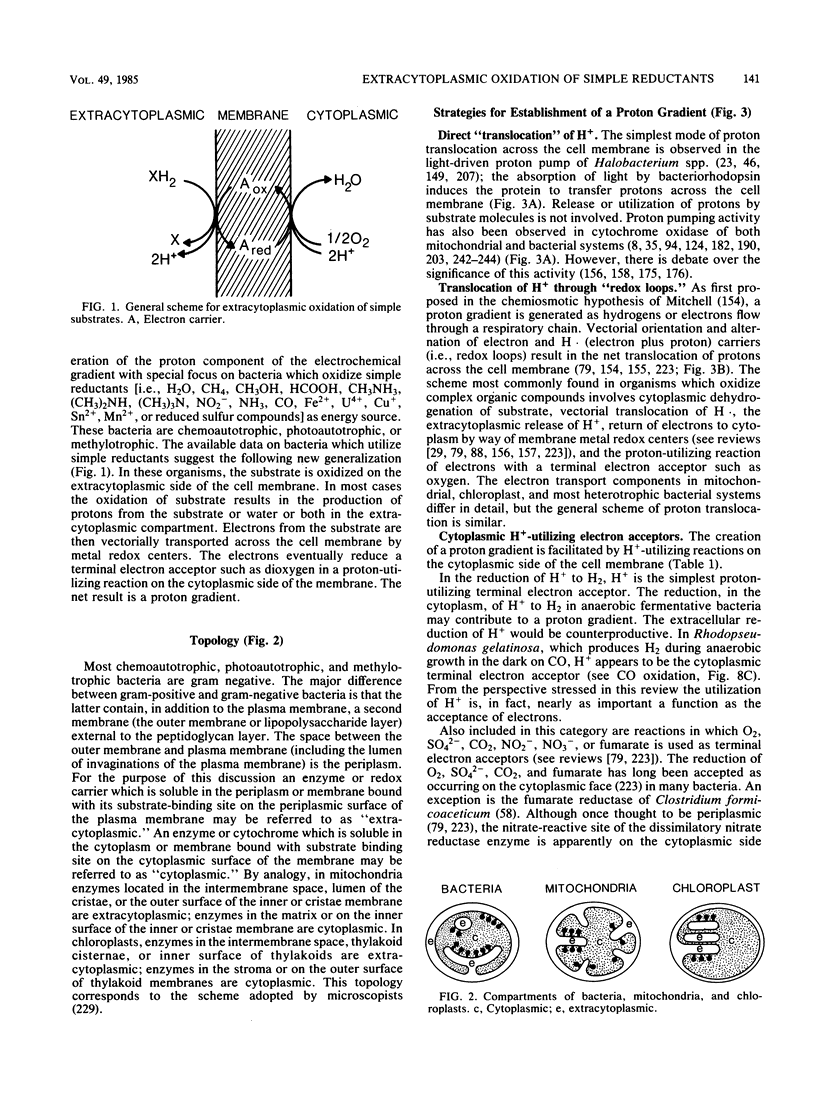
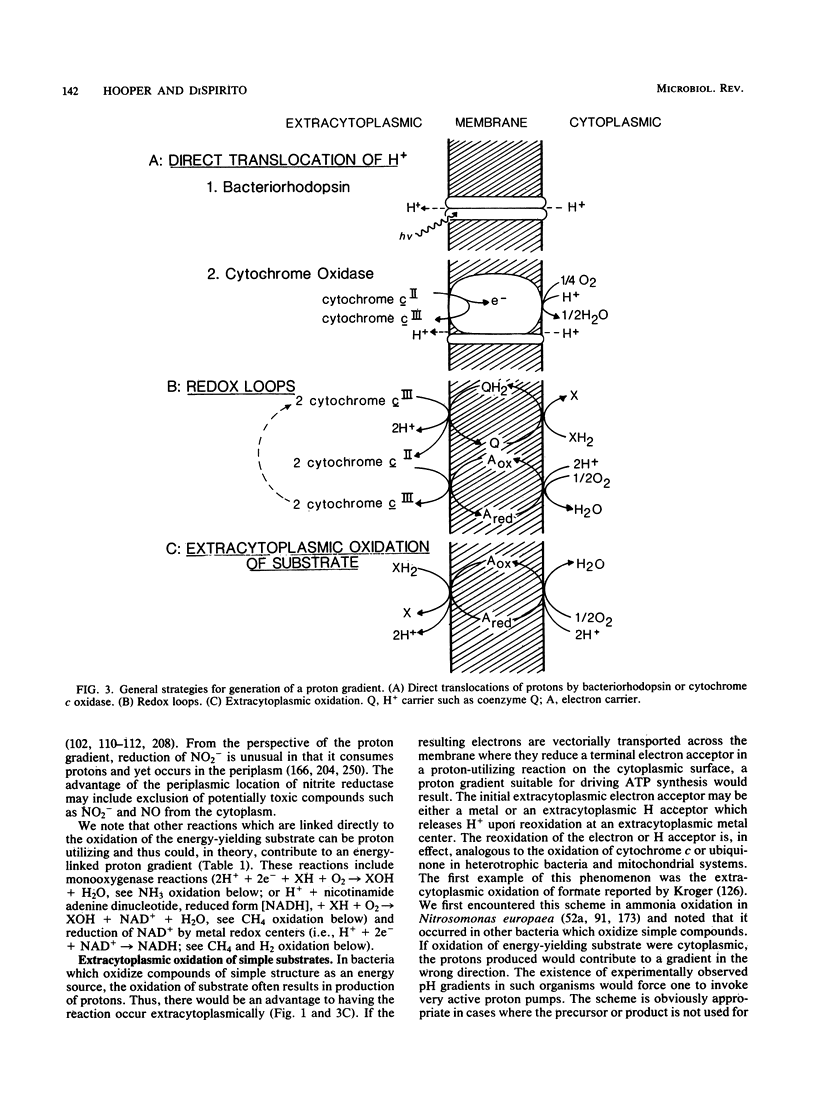
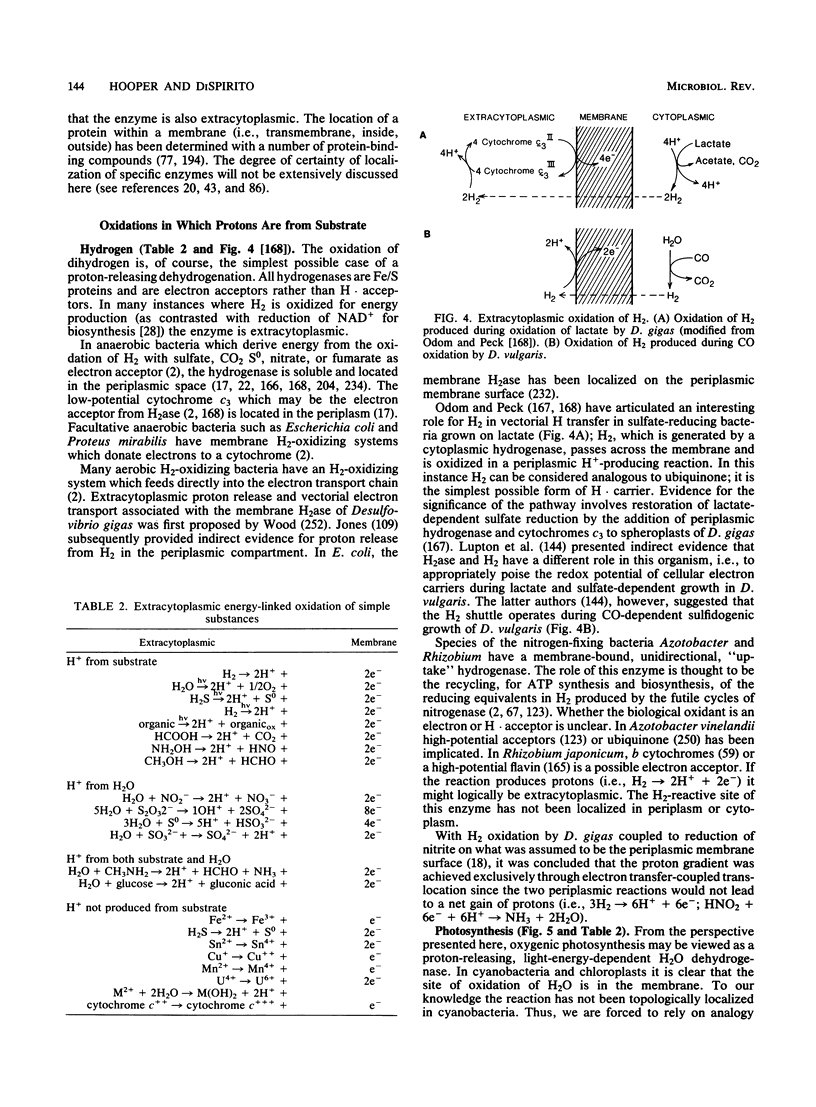
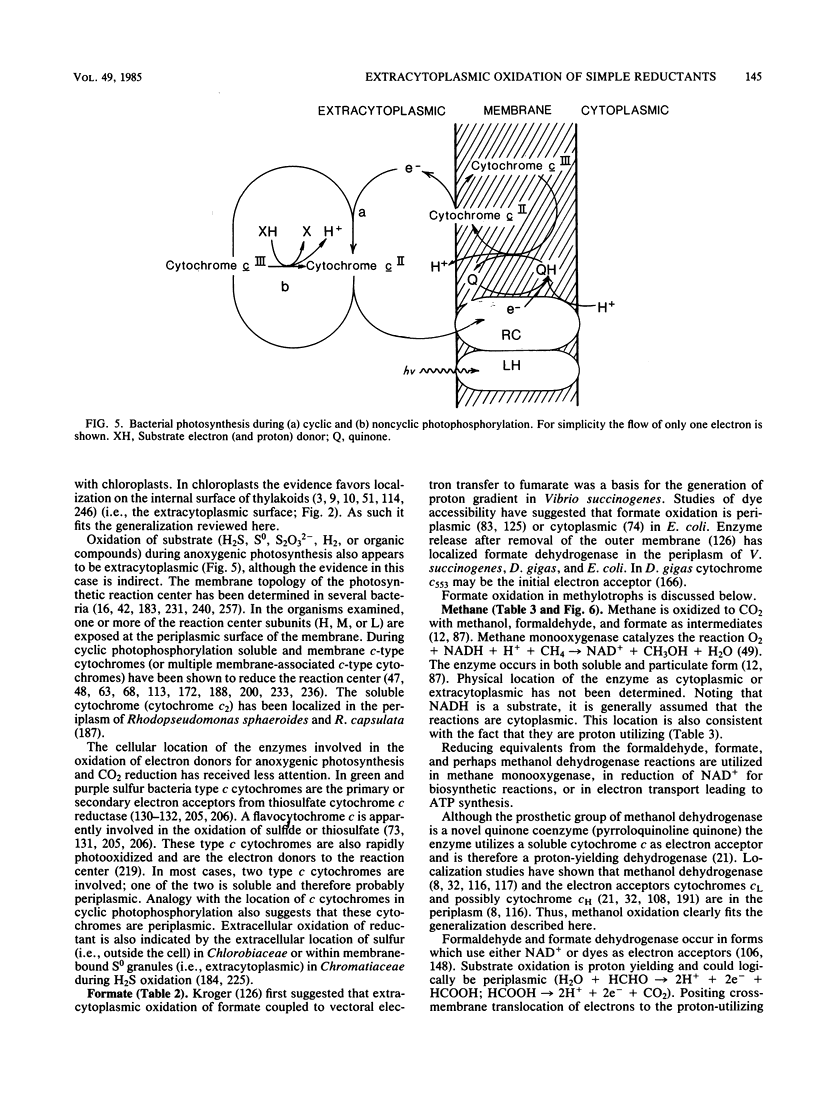
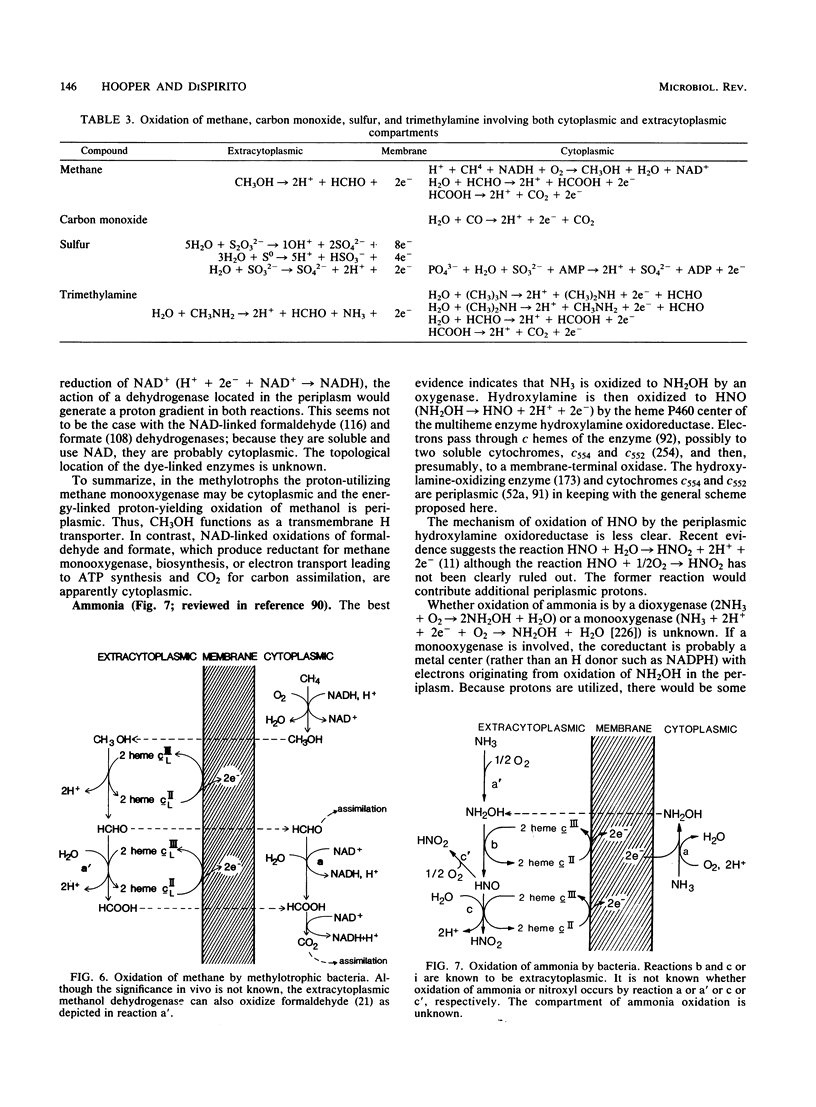
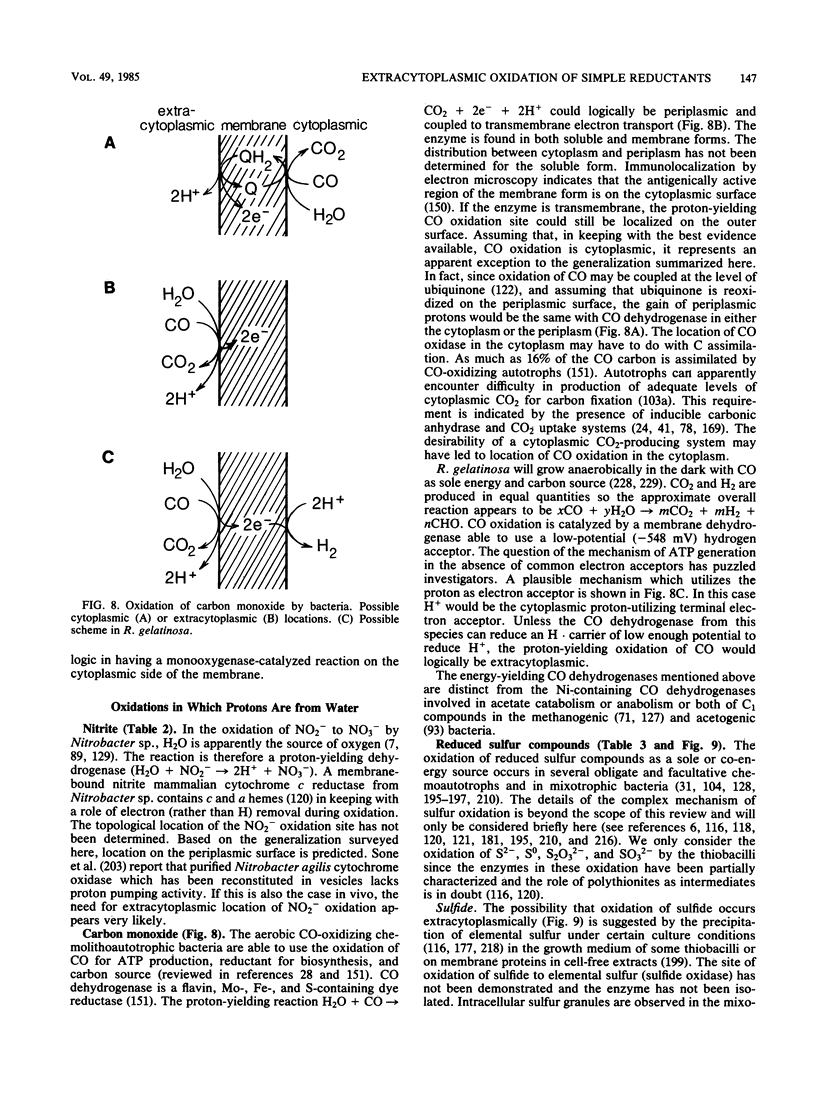
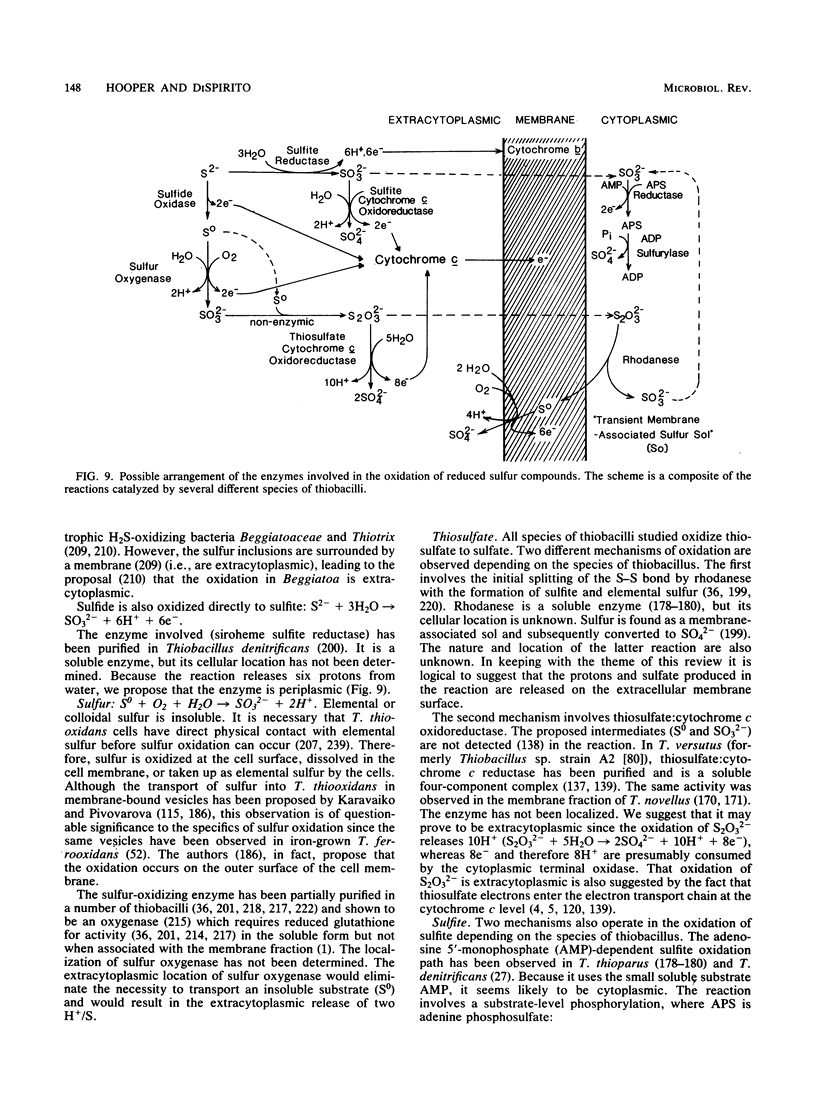
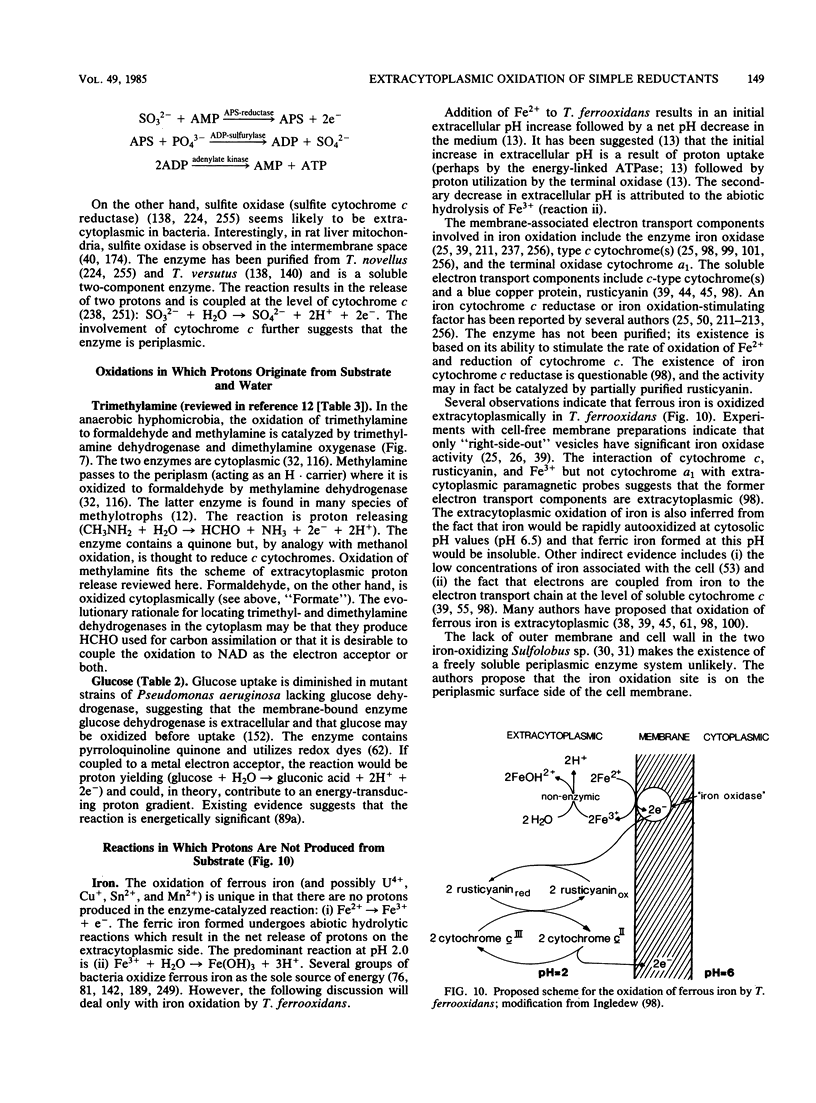
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adair F. W. Membrane-associated sulfur oxidation by the autotroph Thiobacillus thiooxidans. J Bacteriol. 1966 Oct;92(4):899–904. doi: 10.1128/jb.92.4.899-904.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams M. W., Mortenson L. E., Chen J. S. Hydrogenase. Biochim Biophys Acta. 1980 Dec;594(2-3):105–176. doi: 10.1016/0304-4173(80)90007-5. [DOI] [PubMed] [Google Scholar]
- Aleem M. I. Generation of reducing power in chemosynthesis. II. Energy-linked reduction of pyridine nucleotides in the chemoautotroph, Nitrosomonas europaea. Biochim Biophys Acta. 1966 Feb 14;113(2):216–224. doi: 10.1016/s0926-6593(66)80062-0. [DOI] [PubMed] [Google Scholar]
- Aleem M. I., Hoch G. E., Varner J. E. Water as the source of oxidant and reductant in bacterial chemosynthesis. Proc Natl Acad Sci U S A. 1965 Sep;54(3):869–873. doi: 10.1073/pnas.54.3.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleem M. I. Thiosulfate Oxidation and Electron Transport in Thiobacillus novellus. J Bacteriol. 1965 Jul;90(1):95–101. doi: 10.1128/jb.90.1.95-101.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alefounder P. R., Ferguson S. J. A periplasmic location for methanol dehydrogenase from Paracoccus denitrificans: implications for proton pumping by cytochrome aa3. Biochem Biophys Res Commun. 1981 Feb 12;98(3):778–784. doi: 10.1016/0006-291x(81)91179-7. [DOI] [PubMed] [Google Scholar]
- Anderson J. M. The molecular organization of chloroplast thylakoids. Biochim Biophys Acta. 1975 Aug 15;416(2):191–235. doi: 10.1016/0304-4173(75)90007-5. [DOI] [PubMed] [Google Scholar]
- Arcuri E. J., Ehrlich H. L. Cytochrome Involvement in Mn(II) Oxidation by Two Marine Bacteria. Appl Environ Microbiol. 1979 May;37(5):916–923. doi: 10.1128/aem.37.5.916-923.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLAYLOCK B. A., NASON A. ELECTRON TRANSPORT SYSTEMS OF THE CHEMOAUTOTROPH FERROBACILLUS FERROOXIDANS. I. CYTOCHROME C-CONTAINING IRON OXIDASE. J Biol Chem. 1963 Oct;238:3453–3462. [PubMed] [Google Scholar]
- Bachmann R. C., Gillies K., Takemoto J. Y. Membrane topography of the photosynthetic reaction center polypeptides of Rhodopseudomonas sphaeroides. Biochemistry. 1981 Aug 4;20(16):4590–4596. doi: 10.1021/bi00519a012. [DOI] [PubMed] [Google Scholar]
- Barton L. L., LeGall J., Odom J. M., Peck H. D., Jr Energy coupling to nitrite respiration in the sulfate-reducing bacterium Desulfovibrio gigas. J Bacteriol. 1983 Feb;153(2):867–871. doi: 10.1128/jb.153.2.867-871.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer M. E. Response of Cell Walls of Escherichia coli to a Sudden Reduction of the Environmental Osmotic Pressure. J Bacteriol. 1967 Mar;93(3):1104–1112. doi: 10.1128/jb.93.3.1104-1112.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beacham I. R. Periplasmic enzymes in gram-negative bacteria. Int J Biochem. 1979;10(11):877–883. doi: 10.1016/0020-711x(79)90117-4. [DOI] [PubMed] [Google Scholar]
- Bell G. R., LeGall L., Peck H. D. Evidence for the periplasmic location of hydrogenase in Desulfovibrio gigas. J Bacteriol. 1974 Nov;120(2):994–997. doi: 10.1128/jb.120.2.994-997.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birge R. R., Cooper T. M. Energy storage in the primary step of the photocycle of bacteriorhodopsin. Biophys J. 1983 Apr;42(1):61–69. doi: 10.1016/S0006-3495(83)84369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodo C., Lundgren D. G. Iron oxidation by cell envelopes of Thiobacillus ferrooxidans. Can J Microbiol. 1974 Dec;20(12):1647–1652. doi: 10.1139/m74-256. [DOI] [PubMed] [Google Scholar]
- Bowen T. J., Happold F. C., Taylor B. F. Studies on adenosine-5'-phosphosulphate reductase from Thiobacillus denitrificans. Biochim Biophys Acta. 1966 Jun 15;118(3):566–576. doi: 10.1016/s0926-6593(66)80098-x. [DOI] [PubMed] [Google Scholar]
- Bowien B., Schlegel H. G. Physiology and biochemistry of aerobic hydrogen-oxidizing bacteria. Annu Rev Microbiol. 1981;35:405–452. doi: 10.1146/annurev.mi.35.100181.002201. [DOI] [PubMed] [Google Scholar]
- Brierley C. L., Brierley J. A. A chemoautotrophic and thermophilic microorganism isolated from an acid hot spring. Can J Microbiol. 1973 Feb;19(2):183–188. doi: 10.1139/m73-028. [DOI] [PubMed] [Google Scholar]
- Brock T. D., Brock K. M., Belly R. T., Weiss R. L. Sulfolobus: a new genus of sulfur-oxidizing bacteria living at low pH and high temperature. Arch Mikrobiol. 1972;84(1):54–68. doi: 10.1007/BF00408082. [DOI] [PubMed] [Google Scholar]
- Case G. D., Leigh J. S., Jr Intramitochondrial positions of cytochrome haem groups determined by dipolar interactions with paramagnetic cations. Biochem J. 1976 Dec 15;160(3):769–783. doi: 10.1042/bj1600769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case G. D., Ohnishi T., Leigh J. S., Jr Intramitochondrial positions of ubiquinone and iron-sulphur centres determined by dipolar interactions with paramagnetic ions. Biochem J. 1976 Dec 15;160(3):785–795. doi: 10.1042/bj1600785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey R. P., Broger C., Thelen M., Azzi A. Studies on the molecular basis of H+ translocation by cytochrome c oxidase. J Bioenerg Biomembr. 1981 Dec;13(5-6):219–228. doi: 10.1007/BF00743201. [DOI] [PubMed] [Google Scholar]
- Cheng K. J., Ingram J. M., Costerton J. W. Alkaline phosphatase localization and spheroplast formation of Pseudomonas aeruginosa. Can J Microbiol. 1970 Dec;16(12):1319–1324. doi: 10.1139/m70-218. [DOI] [PubMed] [Google Scholar]
- Cobley J. G., Cox J. C. Energy conservation in acidophilic bacteria. Microbiol Rev. 1983 Dec;47(4):579–595. doi: 10.1128/mr.47.4.579-595.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobley J. G., Haddock B. A. The respiratory chain of Thiobacillus ferrooxidans: the reduction of cytochromes by Fe2+ and the preliminary characterization of rusticyanin a novel "blue" copper protein. FEBS Lett. 1975 Dec 1;60(1):29–33. doi: 10.1016/0014-5793(75)80411-x. [DOI] [PubMed] [Google Scholar]
- Cohen H. J., Betcher-Lange S., Kessler D. L., Rajagopalan K. V. Hepatic sulfite oxidase. Congruency in mitochondria of prosthetic groups and activity. J Biol Chem. 1972 Dec 10;247(23):7759–7766. [PubMed] [Google Scholar]
- Collins M. L., Mallon D. E., Niederman R. A. Assessment of Rhodopseudomonas sphaeroides chromatophore membrane asymmetry through bilateral antiserum adsorption studies. J Bacteriol. 1980 Jul;143(1):221–230. doi: 10.1128/jb.143.1.221-230.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton J. W., Ingram J. M., Cheng K. J. Structure and function of the cell envelope of gram-negative bacteria. Bacteriol Rev. 1974 Mar;38(1):87–110. doi: 10.1128/br.38.1.87-110.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J. C., Boxer D. H. The purification and some properties of rusticyanin, a blue copper protein involved in iron(II) oxidation from Thiobacillus ferro-oxidans. Biochem J. 1978 Aug 15;174(2):497–502. doi: 10.1042/bj1740497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespi H. L., Ferraro J. R. Active site structure of bacteriorhodopsin and mechanism of action. Biochem Biophys Res Commun. 1979 Nov 28;91(2):575–582. doi: 10.1016/0006-291x(79)91561-4. [DOI] [PubMed] [Google Scholar]
- DUGAN P. R., LUNDGREN D. G. ENERGY SUPPLY FOR THE CHEMOAUTOTROPH FERROBACILLUS FERROOXIDANS. J Bacteriol. 1965 Mar;89:825–834. doi: 10.1128/jb.89.3.825-834.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Din G. A., Suzuki I., Lees H. Ferrous iron oxidation by Ferrobacillus ferrooxidans. Purification and properties of Fe++-cytochrome c reductase. Can J Biochem. 1967 Oct;45(10):1523–1546. doi: 10.1139/o67-183. [DOI] [PubMed] [Google Scholar]
- Dispirito A. A., Silver M., Voss L., Tuovinen O. H. Flagella and pili of iron-oxidizing thiobacilli isolated from a uranium mine in northern ontario, Canada. Appl Environ Microbiol. 1982 May;43(5):1196–1200. doi: 10.1128/aem.43.5.1196-1200.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn M., Andreesen J. R., Gottschalk G. Fumarate reductase of Clostridium formicoaceticum. A peripheral membrane protein. Arch Microbiol. 1978 Oct 4;119(1):7–11. doi: 10.1007/BF00407920. [DOI] [PubMed] [Google Scholar]
- Dubinina G. A. Mekhanizm okisleniia dvukhvalentnogo zheleza i margantsa zhelezobakteriiami, razvivaiushchimisia pri neitral'noi kislotnosti sredy. Mikrobiologiia. 1978 Jul-Aug;47(4):591–599. [PubMed] [Google Scholar]
- Duine J. A., Frank J., van Zeeland J. K. Glucose dehydrogenase from Acinetobacter calcoaceticus: a 'quinoprotein'. FEBS Lett. 1979 Dec 15;108(2):443–446. doi: 10.1016/0014-5793(79)80584-0. [DOI] [PubMed] [Google Scholar]
- Dutton P. L., Jackson J. B. Thermodynamic and kinetic characterization of electron transfer components in situ in Rhodopseudomonas spheroides and Rhodospirillum rubrum. Eur J Biochem. 1972 Nov 7;30(3):495–510. doi: 10.1111/j.1432-1033.1972.tb02121.x. [DOI] [PubMed] [Google Scholar]
- Eady R. R., Jarman T. R., Large P. J. Microbial oxidation of amines. Partial purification of a mixed-function secondary-amine oxidase system from Pseudomonas aminovorans that contains an enzymically active cytochrome-P-420-type haemoprotein. Biochem J. 1971 Nov;125(2):449–459. doi: 10.1042/bj1250449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. H., Crofts A. R. In situ characterisation of photosynthetic electron transport in Rhodopseudomonas capsulata. Biochim Biophys Acta. 1974 Jul 25;357(1):89–102. doi: 10.1016/0005-2728(74)90115-7. [DOI] [PubMed] [Google Scholar]
- Forsberg C. W., Costerton J. W., Macleod R. A. Quantitation, chemical characteristics, and ultrastructure of the three outer cell wall layers of a gram-negative bacterium. J Bacteriol. 1970 Dec;104(3):1354–1368. doi: 10.1128/jb.104.3.1354-1368.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg C. W., Costerton J. W., Macleod R. A. Separation and localization of cell wall layers of a gram-negative bacterium. J Bacteriol. 1970 Dec;104(3):1338–1353. doi: 10.1128/jb.104.3.1338-1353.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumori Y., Yamanaka T. Flavocytochrome c of Chromatium vinosum. Some enzymatic properties and subunit structure. J Biochem. 1979 Jun;85(6):1405–1414. doi: 10.1093/oxfordjournals.jbchem.a132467. [DOI] [PubMed] [Google Scholar]
- Garland P. B., Downie J. A., Haddock B. A. Proton translocation and the respiratory nitrate reductase of Escherichia coli. Biochem J. 1975 Dec;152(3):547–559. doi: 10.1042/bj1520547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrard W. T. Selective release of proteins from Spirillum itersonii by tris (hydroxymethyl) aminomethane and ethylenediaminetetraacetate. J Bacteriol. 1971 Jan;105(1):93–100. doi: 10.1128/jb.105.1.93-100.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiorse W. C. Biology of iron- and manganese-depositing bacteria. Annu Rev Microbiol. 1984;38:515–550. doi: 10.1146/annurev.mi.38.100184.002503. [DOI] [PubMed] [Google Scholar]
- Gitler C., Bercovici T. Use of lipophilic photoactivatable reagents to identify the lipid-embedded domains of membrane proteins. Ann N Y Acad Sci. 1980;346:199–211. doi: 10.1111/j.1749-6632.1980.tb22100.x. [DOI] [PubMed] [Google Scholar]
- Graham D., Reed M. L. Carbonic anhydrase and the regulation of photosynthesis. Nat New Biol. 1971 May 19;231(20):81–83. doi: 10.1038/newbio231081a0. [DOI] [PubMed] [Google Scholar]
- Haddock B. A., Jones C. W. Bacterial respiration. Bacteriol Rev. 1977 Mar;41(1):47–99. doi: 10.1128/br.41.1.47-99.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison A. P., Jr The acidophilic thiobacilli and other acidophilic bacteria that share their habitat. Annu Rev Microbiol. 1984;38:265–292. doi: 10.1146/annurev.mi.38.100184.001405. [DOI] [PubMed] [Google Scholar]
- Hauska G., Trebst A., Draber W. Lipophilicity and catalysis of photophosphorylation. II. Quinoid compounds as artificial carriers in cyclic photophosphorylation and photoreductions by photosystem I. Biochim Biophys Acta. 1973 Jun 28;305(3):632–641. doi: 10.1016/0005-2728(73)90082-0. [DOI] [PubMed] [Google Scholar]
- Heckels J. E. The surface of Neisseria gonorrhoeae: isolation of the major components of the outer membrane. J Gen Microbiol. 1977 Apr;99(2):333–341. doi: 10.1099/00221287-99-2-333. [DOI] [PubMed] [Google Scholar]
- Heppel L. A. Selective release of enzymes from bacteria. Science. 1967 Jun 16;156(3781):1451–1455. doi: 10.1126/science.156.3781.1451. [DOI] [PubMed] [Google Scholar]
- Higgins I. J., Best D. J., Hammond R. C., Scott D. Methane-oxidizing microorganisms. Microbiol Rev. 1981 Dec;45(4):556–590. doi: 10.1128/mr.45.4.556-590.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollocher T. C. Source of the oxygen atoms of nitrate in the oxidation of nitrite by Nitrobacter agilis and evidence against a P-O-N anhydride mechanism in oxidative phosphorylation. Arch Biochem Biophys. 1984 Sep;233(2):721–727. doi: 10.1016/0003-9861(84)90499-5. [DOI] [PubMed] [Google Scholar]
- Hooper A. B., Tran V. M., Balny C. Kinetics of reduction by substrate or dithionite and heme-heme electron transfer in the multiheme hydroxylamine oxidoreductase. Eur J Biochem. 1984 Jun 15;141(3):565–571. doi: 10.1111/j.1432-1033.1984.tb08230.x. [DOI] [PubMed] [Google Scholar]
- Hu S. I., Drake H. L., Wood H. G. Synthesis of acetyl coenzyme A from carbon monoxide, methyltetrahydrofolate, and coenzyme A by enzymes from Clostridium thermoaceticum. J Bacteriol. 1982 Feb;149(2):440–448. doi: 10.1128/jb.149.2.440-448.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingledew W. J., Cobley J. G. A potentiometric and kinetic study on the respiratory chain of ferrous-iron-grown Thiobacillus ferrooxidans. Biochim Biophys Acta. 1980 Apr 2;590(2):141–158. doi: 10.1016/0005-2728(80)90020-1. [DOI] [PubMed] [Google Scholar]
- Ingledew W. J., Poole R. K. The respiratory chains of Escherichia coli. Microbiol Rev. 1984 Sep;48(3):222–271. doi: 10.1128/mr.48.3.222-271.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingledew W. J. Thiobacillus ferrooxidans. The bioenergetics of an acidophilic chemolithotroph. Biochim Biophys Acta. 1982 Nov 30;683(2):89–117. doi: 10.1016/0304-4173(82)90007-6. [DOI] [PubMed] [Google Scholar]
- Jacobson G. R., Takacs B. J., Rosenbusch J. P. Properties of a major protein released from Escherichia coli by osmotic shock. Biochemistry. 1976 Jun 1;15(11):2297–2303. doi: 10.1021/bi00656a008. [DOI] [PubMed] [Google Scholar]
- Jannasch H. W., Taylor C. D. Deep-sea microbiology. Annu Rev Microbiol. 1984;38:487–514. doi: 10.1146/annurev.mi.38.100184.002415. [DOI] [PubMed] [Google Scholar]
- Johnson A. H., Stokes J. L. Managanese oxidation by Sphaerotilus discophorus. J Bacteriol. 1966 Apr;91(4):1543–1547. doi: 10.1128/jb.91.4.1543-1547.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. A., Quayle J. R. Microbial growth on C-1 compounds. 6. Oxidation of methanol, formaldehyde and formate by methanol-grown Pseudomonas AM-1. Biochem J. 1964 Nov;93(2):281–290. doi: 10.1042/bj0930281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston K. H., Holmes K. K., Gotschlich E. C. The serological classification of Neisseria gonorrhoeae. I. Isolation of the outer membrane complex responsible for serotypic specificity. J Exp Med. 1976 Apr 1;143(4):741–758. doi: 10.1084/jem.143.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. W., Garland P. B. Sites and specificity of the reaction of bipyridylium compounds with anaerobic respiratory enzymes of Escherichia coli. Effects of permeability barriers imposed by the cytoplasmic membrane. Biochem J. 1977 Apr 15;164(1):199–211. doi: 10.1042/bj1640199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. W., Garland P. B. The proton-consuming site of the respiratory nitrate reductase of Escherichia coli is on the cytoplasmic aspect of the cytoplasmic membrane [proceedings]. Biochem Soc Trans. 1978;6(2):416–418. doi: 10.1042/bst0060416. [DOI] [PubMed] [Google Scholar]
- Jones R. W., Lamont A., Garland P. B. The mechanism of proton translocation driven by the respiratory nitrate reductase complex of Escherichia coli. Biochem J. 1980 Jul 15;190(1):79–94. doi: 10.1042/bj1900079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. W. The role of the membrane-bound hydrogenase in the energy-conserving oxidation of molecular hydrogen by Escherichia coli. Biochem J. 1980 May 15;188(2):345–350. doi: 10.1042/bj1880345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jortner J. Dynamics of electron transfer in bacterial photosynthesis. Biochim Biophys Acta. 1980 Dec;594(4):193–230. doi: 10.1016/0304-4173(80)90001-4. [DOI] [PubMed] [Google Scholar]
- Karavaiko G. I., Pivovarova T. A. Okislenie élementarnoi sery Thiobacillus thiooxidans. Mikrobiologiia. 1973 May-Jun;42(2):389–395. [PubMed] [Google Scholar]
- Kasprzak A. A., Steenkamp D. J. Localization of the major dehydrogenases in two methylotrophs by radiochemical labeling. J Bacteriol. 1983 Oct;156(1):348–353. doi: 10.1128/jb.156.1.348-353.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly D. P. Biochemistry of the chemolithotrophic oxidation of inorganic sulphur. Philos Trans R Soc Lond B Biol Sci. 1982 Sep 13;298(1093):499–528. doi: 10.1098/rstb.1982.0094. [DOI] [PubMed] [Google Scholar]
- Kim Y. M., Hegeman G. D. Electron transport system of an aerobic carbon monoxide-oxidizing bacterium. J Bacteriol. 1981 Dec;148(3):991–994. doi: 10.1128/jb.148.3.991-994.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kow Y. W., Burris R. H. Purification and properties of membrane-bound hydrogenase from Azotobacter vinelandii. J Bacteriol. 1984 Aug;159(2):564–569. doi: 10.1128/jb.159.2.564-569.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krab K., Wikström M. Proton-translocating cytochrome c oxidase in artificial phospholipid vesicles. Biochim Biophys Acta. 1978 Oct 11;504(1):200–214. doi: 10.1016/0005-2728(78)90018-x. [DOI] [PubMed] [Google Scholar]
- Krzycki J. A., Zeikus J. G. Characterization and purification of carbon monoxide dehydrogenase from Methanosarcina barkeri. J Bacteriol. 1984 Apr;158(1):231–237. doi: 10.1128/jb.158.1.231-237.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kröger A. Fumarate as terminal acceptor of phosphorylative electron transport. Biochim Biophys Acta. 1978 Oct 23;505(2):129–145. doi: 10.1016/0304-4173(78)90010-1. [DOI] [PubMed] [Google Scholar]
- Kröger A. The interaction of the radicals of ubiquinone in mitochondrial electron transport. FEBS Lett. 1976 Jun 15;65(3):278–280. doi: 10.1016/0014-5793(76)80128-7. [DOI] [PubMed] [Google Scholar]
- Kumar S., Nicholas D. J., Williams E. H. Definitive 15N NMR evidence that water serves as a source of 'O' during nitrite oxidation by Nitrobacter agilis. FEBS Lett. 1983 Feb 7;152(1):71–74. doi: 10.1016/0014-5793(83)80484-0. [DOI] [PubMed] [Google Scholar]
- Kusai A., Yamanaka T. A Novel function of cytochrome C (555, Chlorobium thiosulfatophilum) in oxidation of thiosulfate. Biochem Biophys Res Commun. 1973 Mar 5;51(1):107–112. doi: 10.1016/0006-291x(73)90514-7. [DOI] [PubMed] [Google Scholar]
- Kusai A., Yamanaka T. Cytochrome c (553, Chlorobium thiosulfatophilum) is a sulphide-cytochrome c reductase. FEBS Lett. 1973 Aug 15;34(2):235–237. doi: 10.1016/0014-5793(73)80801-4. [DOI] [PubMed] [Google Scholar]
- Kusai K., Yamanaka T. The oxidation mechanisms of thiosulphate and sulphide in Chlorobium thiosulphatophilum: roles of cytochrome c-551 and cytochrome c-553. Biochim Biophys Acta. 1973 Nov 22;325(2):304–314. doi: 10.1016/0005-2728(73)90106-0. [DOI] [PubMed] [Google Scholar]
- Lewis A. J., Miller J. D. Stannous and cuprous ion oxidation by Thiobacillus ferrooxidans. Can J Microbiol. 1977 Mar;23(3):319–324. doi: 10.1139/m77-047. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Van Alphen L. Molecular architecture and functioning of the outer membrane of Escherichia coli and other gram-negative bacteria. Biochim Biophys Acta. 1983 Mar 21;737(1):51–115. doi: 10.1016/0304-4157(83)90014-x. [DOI] [PubMed] [Google Scholar]
- Lunn C. A., Pigiet V. P. Localization of thioredoxin from Escherichia coli in an osmotically sensitive compartment. J Biol Chem. 1982 Oct 10;257(19):11424–11430. [PubMed] [Google Scholar]
- Lupton F. S., Conrad R., Zeikus J. G. Physiological function of hydrogen metabolism during growth of sulfidogenic bacteria on organic substrates. J Bacteriol. 1984 Sep;159(3):843–849. doi: 10.1128/jb.159.3.843-849.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALAMY M., HORECKER B. L. The localization of alkaline phosphatase in E. coli K12. Biochem Biophys Res Commun. 1961 Jun 2;5:104–108. doi: 10.1016/0006-291x(61)90020-1. [DOI] [PubMed] [Google Scholar]
- MITCHELL P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961 Jul 8;191:144–148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- MacAlister T. J., Costerton J. W., Thompson L., Thompson J., Ingram J. M. Distribution of alkaline phosphatase within the periplasmic space of gram-negative bacteria. J Bacteriol. 1972 Sep;111(3):827–832. doi: 10.1128/jb.111.3.827-832.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz H., Zundel G. Proton conduction in bacteriorhodopsin via a hydrogen-bonded chain with large proton polarizability. Biochem Biophys Res Commun. 1981 Jul 30;101(2):540–546. doi: 10.1016/0006-291x(81)91293-6. [DOI] [PubMed] [Google Scholar]
- Meyer O., Schlegel H. G. Biology of aerobic carbon monoxide-oxidizing bacteria. Annu Rev Microbiol. 1983;37:277–310. doi: 10.1146/annurev.mi.37.100183.001425. [DOI] [PubMed] [Google Scholar]
- Midgley M., Dawes E. A. The regulation of transport of glucose and methyl alpha-glucoside in Pseudomonas aeruginosa. Biochem J. 1973 Feb;132(2):141–154. doi: 10.1042/bj1320141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Keilin's respiratory chain concept and its chemiosmotic consequences. Science. 1979 Dec 7;206(4423):1148–1159. doi: 10.1126/science.388618. [DOI] [PubMed] [Google Scholar]
- Mitchell P. The Ninth Sir Hans Krebs Lecture. Compartmentation and communication in living systems. Ligand conduction: a general catalytic principle in chemical, osmotic and chemiosmotic reaction systems. Eur J Biochem. 1979 Mar 15;95(1):1–20. doi: 10.1111/j.1432-1033.1979.tb12934.x. [DOI] [PubMed] [Google Scholar]
- Moyle J., Mitchell P. Cytochrome c oxidase is not a proton pump. FEBS Lett. 1978 Apr 15;88(2):268–272. doi: 10.1016/0014-5793(78)80190-2. [DOI] [PubMed] [Google Scholar]
- Munkres M., Wachtel A. Histochemical localization of phosphatases in Mycoplasma gallisepticum. J Bacteriol. 1967 Mar;93(3):1096–1103. doi: 10.1128/jb.93.3.1096-1103.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgier M., Pelissier C., Lazdunski A. Aminopeptidase N from Escherichia coli. Unusual interactions with the cell surface. Eur J Biochem. 1977 Apr 15;74(3):425–433. doi: 10.1111/j.1432-1033.1977.tb11408.x. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. On the surface localization of enzymes in E. coli. Biochem Biophys Res Commun. 1964 Oct 14;17(3):215–219. doi: 10.1016/0006-291x(64)90386-9. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Nielsen A. M., Beck J. V. Chalcocite Oxidation and Coupled Carbon Dioxide Fixation by Thiobacillus ferrooxidans. Science. 1972 Mar 10;175(4026):1124–1126. doi: 10.1126/science.175.4026.1124. [DOI] [PubMed] [Google Scholar]
- O'Brian M. R., Maier R. J. Involvement of cytochromes and a flavoprotein in hydrogen oxidation in Rhizobium japonicum bacteroids. J Bacteriol. 1983 Aug;155(2):481–487. doi: 10.1128/jb.155.2.481-487.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe D. P., Dutton P. L. Cytochrome b oxidation and reduction reactions in the ubiquinone-cytochrome b/c2 oxidoreductase from Rhodopseudomonas sphaeroides. Biochim Biophys Acta. 1981 Mar 12;635(1):149–166. doi: 10.1016/0005-2728(81)90015-3. [DOI] [PubMed] [Google Scholar]
- Odom J. M., Peck H. D., Jr Hydrogenase, electron-transfer proteins, and energy coupling in the sulfate-reducing bacteria Desulfovibrio. Annu Rev Microbiol. 1984;38:551–592. doi: 10.1146/annurev.mi.38.100184.003003. [DOI] [PubMed] [Google Scholar]
- Odom J. M., Peck H. D., Jr Localization of dehydrogenases, reductases, and electron transfer components in the sulfate-reducing bacterium Desulfovibrio gigas. J Bacteriol. 1981 Jul;147(1):161–169. doi: 10.1128/jb.147.1.161-169.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshino N., Chance B. The properties of sulfite oxidation in perfused rat liver; interaction of sulfite oxidase with the mitochondrial respiratory chain. Arch Biochem Biophys. 1975 Oct;170(2):514–528. doi: 10.1016/0003-9861(75)90147-2. [DOI] [PubMed] [Google Scholar]
- PARKER C. D., PRISK J. The oxidation of inorganic compounds of sulphur by various sulphur bacteria. J Gen Microbiol. 1953 Jun;8(3):344–364. doi: 10.1099/00221287-8-3-344. [DOI] [PubMed] [Google Scholar]
- PECK H. D., Jr, DEACON T. E., DAVIDSON J. T. STUDIES ON ADENOSINE 5'-PHOSPHOSULFATE REDUCTASE FROM DESULFOVIBRIO DESULFURICANS AND THIOBACILLUS THIOPARUS. I. THE ASSAY AND PURIFICATION. Biochim Biophys Acta. 1965 Mar 22;96:429–446. doi: 10.1016/0005-2787(65)90561-7. [DOI] [PubMed] [Google Scholar]
- PECK H. D., Jr, FISHER E., Jr The oxidation of thiosulfate and phosphorylation in extracts of Thiobacillus thioparus. J Biol Chem. 1962 Jan;237:190–197. [PubMed] [Google Scholar]
- Papa S., Guerrieri F., Izzo G., Boffoli D. Mechanism of proton translocation associated to oxidation of N,N,N',N'-tetramethyl-p-phenylenediamine in rat liver mitochondria. FEBS Lett. 1983 Jun 27;157(1):15–20. doi: 10.1016/0014-5793(83)81107-7. [DOI] [PubMed] [Google Scholar]
- Papa S., Lorusso M., Capitanio N., De Nitto E. Characteristics of redox-linked proton ejection in cytochrome c oxidase reconstituted in phospholipid vesicles. New observations support mechanisms different from proton pumping. FEBS Lett. 1983 Jun 27;157(1):7–14. doi: 10.1016/0014-5793(83)81106-5. [DOI] [PubMed] [Google Scholar]
- Peck H. D. ADENOSINE 5'-PHOSPHOSULFATE AS AN INTERMEDIATE IN THE OXIDATION OF THIOSULFATE BY THIOBACILLUS THIOPARUS. Proc Natl Acad Sci U S A. 1960 Aug;46(8):1053–1057. doi: 10.1073/pnas.46.8.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck H. D., Jr Energy-coupling mechanisms in chemolithotrophic bacteria. Annu Rev Microbiol. 1968;22:489–518. doi: 10.1146/annurev.mi.22.100168.002421. [DOI] [PubMed] [Google Scholar]
- Peschek G. A. Proton pump coupled to cytochrome c oxidase in the cyanobacterium Anacystis nidulans. J Bacteriol. 1983 Jan;153(1):539–542. doi: 10.1128/jb.153.1.539-542.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J., Drews G. Transverse topography of the photochemical reaction center polypeptides in the Rhodopseudomonas capsulata membrane. J Bacteriol. 1984 Jun;158(3):983–989. doi: 10.1128/jb.158.3.983-989.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfennig N. Phototrophic green and purple bacteria: a comparative, systematic survey. Annu Rev Microbiol. 1977;31:275–290. doi: 10.1146/annurev.mi.31.100177.001423. [DOI] [PubMed] [Google Scholar]
- Piperno J. R., Oxender D. L. Amino-acid-binding protein released from Escherichia coli by osmotic shock. J Biol Chem. 1966 Dec 10;241(23):5732–5734. [PubMed] [Google Scholar]
- Pivovarova T. A., Karavaiko G. I. O funktsii poverkhnostnykh membrannykh struktur Thiobacillus thiooxidans. Mikrobiologiia. 1975 Mar-Apr;44(2):269–271. [PubMed] [Google Scholar]
- Prince R. C., Baccarini-Melandri A., Hauska G. A., Melandri B. A., Crofts A. R. Asymmetry of an energy transducing membrane the location of cytochrome c2 in Rhodopseudomonas spheroides and Rhodopseudomonas capsulata. Biochim Biophys Acta. 1975 May 15;387(2):212–227. doi: 10.1016/0005-2728(75)90104-8. [DOI] [PubMed] [Google Scholar]
- Prince R. C., Dutton P. L. A kinetic completion of the cyclic photosynthetic electron pathway of Rhodopseudomonas sphaeroides: cytochrome b-cytochrome c2 oxidation-reduction. Biochim Biophys Acta. 1975 Jun 17;387(3):609–613. doi: 10.1016/0005-2728(75)90101-2. [DOI] [PubMed] [Google Scholar]
- Püttner I., Solioz M., Carafoli E., Ludwig B. Dicyclohexylcarbodiimide does not inhibit proton pumping by cytochrome c oxidase of Paracoccus denitrificans. Eur J Biochem. 1983 Jul 15;134(1):33–37. doi: 10.1111/j.1432-1033.1983.tb07527.x. [DOI] [PubMed] [Google Scholar]
- REPASKE R. Lysis of gram-negative bacteria by lysozyme. Biochim Biophys Acta. 1956 Oct;22(1):189–191. doi: 10.1016/0006-3002(56)90240-2. [DOI] [PubMed] [Google Scholar]
- REPASKE R. Lysis of gram-negative organisms and the role of versene. Biochim Biophys Acta. 1958 Nov;30(2):225–232. doi: 10.1016/0006-3002(58)90044-1. [DOI] [PubMed] [Google Scholar]
- Ruby E. G., Jannasch H. W. Physiological characteristics of Thiomicrospira sp. Strain L-12 isolated from deep-sea hydrothermal vents. J Bacteriol. 1982 Jan;149(1):161–165. doi: 10.1128/jb.149.1.161-165.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby E. G., Wirsen C. O., Jannasch H. W. Chemolithotrophic sulfur-oxidizing bacteria from the galapagos rift hydrothermal vents. Appl Environ Microbiol. 1981 Aug;42(2):317–324. doi: 10.1128/aem.42.2.317-324.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAEFFER W. I., HOLBERT P. E., UMBREIT W. W. Attachment of Thiobacillus thiooxidans to sulfur crystals. J Bacteriol. 1963 Jan;85:137–140. doi: 10.1128/jb.85.1.137-140.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schedel M., Trüper H. G. Purification of Thiobacillus denitrificans siroheme sulfite reductase and investigation of some molecular and catalytic properties. Biochim Biophys Acta. 1979 Jun 6;568(2):454–466. doi: 10.1016/0005-2744(79)90314-0. [DOI] [PubMed] [Google Scholar]
- Silver M., Lundgren D. G. Sulfur-oxidizing enzyme of Ferrobacillus ferrooxidans (Thiobacillus ferrooxidans). Can J Biochem. 1968 May;46(5):457–461. doi: 10.1139/o68-069. [DOI] [PubMed] [Google Scholar]
- Slater E. C. Mechanism of oxidative phosphorylation. Annu Rev Biochem. 1977;46:1015–1026. doi: 10.1146/annurev.bi.46.070177.005055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenkamp D. J., Peck H. D., Jr Proton translocation associated with nitrite respiration in Desulfovibrio desulfuricans. J Biol Chem. 1981 Jun 10;256(11):5450–5458. [PubMed] [Google Scholar]
- Stoeckenius W., Lozier R. H., Bogomolni R. A. Bacteriorhodopsin and the purple membrane of halobacteria. Biochim Biophys Acta. 1979 Mar 14;505(3-4):215–278. doi: 10.1016/0304-4173(79)90006-5. [DOI] [PubMed] [Google Scholar]
- Suzuki I. Incorporation of atmospheric oxygen-18 into thiosulfate by the sulfur-oxidizing enzyme of Thiobacillus thiooxidans. Biochim Biophys Acta. 1965 Oct 25;110(1):97–101. doi: 10.1016/s0926-6593(65)80098-4. [DOI] [PubMed] [Google Scholar]
- Suzuki I. Mechanisms of inorganic oxidation and energy coupling. Annu Rev Microbiol. 1974;28(0):85–101. doi: 10.1146/annurev.mi.28.100174.000505. [DOI] [PubMed] [Google Scholar]
- Suzuki I. Oxidation of elemental sulfur by an enzyme system of Thiobacillus thiooxidans. Biochim Biophys Acta. 1965 Jul 8;104(2):359–371. doi: 10.1016/0304-4165(65)90341-7. [DOI] [PubMed] [Google Scholar]
- Suzuki I., Silver M. The initial product and properties of the sulfur-oxidizing enzyme of thiobacilli. Biochim Biophys Acta. 1966 Jul 6;122(1):22–33. doi: 10.1016/0926-6593(66)90088-9. [DOI] [PubMed] [Google Scholar]
- Suzuki I., Werkman C. H. GLUTATHIONE AND SULFUR OXIDATION BY THIOBACILLUS THIOOXIDANS. Proc Natl Acad Sci U S A. 1959 Feb;45(2):239–244. doi: 10.1073/pnas.45.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sybesma C., Beugeling T. Light-induced absorbance changes in the green photosynthetic bacterium Chloropseudomonas ethylicum. Biochim Biophys Acta. 1967 Mar 8;131(2):357–361. doi: 10.1016/0005-2728(67)90149-1. [DOI] [PubMed] [Google Scholar]
- Tabita R., Silver M., Lundgren D. G. The rhodanese enzyme of Ferrobacillus ferrooxidans (Thiobacillus ferrooxidans). Can J Biochem. 1969 Dec;47(12):1141–1145. doi: 10.1139/o69-184. [DOI] [PubMed] [Google Scholar]
- Taylor B. F. Oxidation of elemental sulfur by an enzyme system from Thiobacillus neapolitanus. Biochim Biophys Acta. 1968 Nov 12;170(1):112–122. doi: 10.1016/0304-4165(68)90165-7. [DOI] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toghrol F., Southerland W. M. Purification of Thiobacillus novellus sulfite oxidase. Evidence for the presence of heme and molybdenum. J Biol Chem. 1983 Jun 10;258(11):6762–6766. [PubMed] [Google Scholar]
- Tsang D. C., Suzuki I. Cytochrome c554 as a possible electron donor in the hydroxylation of ammonia and carbon monoxide in Nitrosomonas europaea. Can J Biochem. 1982 Nov;60(11):1018–1024. doi: 10.1139/o82-131. [DOI] [PubMed] [Google Scholar]
- Uffen R. L. Anaerobic growth of a Rhodopseudomonas species in the dark with carbon monoxide as sole carbon and energy substrate. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3298–3302. doi: 10.1073/pnas.73.9.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uffen R. L. Metabolism of carbon monoxide by Rhodopseudomonas gelatinosa: cell growth and properties of the oxidation system. J Bacteriol. 1983 Sep;155(3):956–965. doi: 10.1128/jb.155.3.956-965.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban P. F., Klingenberg M. On the redox potentials of ubiquinone and cytochrome b in the respiratory chain. Eur J Biochem. 1969 Jul;9(4):519–525. doi: 10.1111/j.1432-1033.1969.tb00640.x. [DOI] [PubMed] [Google Scholar]
- VERNON L. P., MANGUM J. H., BECK J. V., SHAFIA F. M. Studies on a ferrous-ion-oxidizing bacterium. II. Cytochrome composition. Arch Biochem Biophys. 1960 Jun;88:227–231. doi: 10.1016/0003-9861(60)90227-7. [DOI] [PubMed] [Google Scholar]
- Valkirs G. E., Feher G. Topography of reaction center subunits in the membrane of the photosynthetic bacterium, rhodopseudomonas sphaeroides. J Cell Biol. 1982 Oct;95(1):179–188. doi: 10.1083/jcb.95.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga A. R., Staehelin L. A. Spatial differentiation in photosynthetic and non-photosynthetic membranes of Rhodopseudomonas palustris. J Bacteriol. 1983 Jun;154(3):1414–1430. doi: 10.1128/jb.154.3.1414-1430.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestal J. R., Lundgren D. G. The sulfite oxidase of Thiobacillus ferrooxidans (Ferrobacillus ferrooxidans). Can J Biochem. 1971 Oct;49(10):1125–1130. doi: 10.1139/o71-162. [DOI] [PubMed] [Google Scholar]
- Webster G. D., Cogdell R. J., Lindsay J. G. Localization of the reaction-centre subunits in the intracytoplasmic membranes of Rhodopseudomonas sphaeroides and Rhodopseudomonas capsulata [proceedings]. Biochem Soc Trans. 1980 Apr;8(2):184–185. doi: 10.1042/bst0080184. [DOI] [PubMed] [Google Scholar]
- Whiteley N. M., Berg H. C. Amidination of the outer and inner surfaces of the human erythrocyte membrane. J Mol Biol. 1974 Aug 15;87(3):541–561. doi: 10.1016/0022-2836(74)90103-x. [DOI] [PubMed] [Google Scholar]
- Wikström M., Krab K. Proton-pumping cytochrome c oxidase. Biochim Biophys Acta. 1979 Aug 17;549(2):177–122. doi: 10.1016/0304-4173(79)90014-4. [DOI] [PubMed] [Google Scholar]
- Wikström M., Krab K., Saraste M. Proton-translocating cytochrome complexes. Annu Rev Biochem. 1981;50:623–655. doi: 10.1146/annurev.bi.50.070181.003203. [DOI] [PubMed] [Google Scholar]
- Wikström M. Two protons are pumped from the mitochondrial matrix per electron transferred between NADH and ubiquinone. FEBS Lett. 1984 Apr 24;169(2):300–304. doi: 10.1016/0014-5793(84)80338-5. [DOI] [PubMed] [Google Scholar]
- Witholt B., Boekhout M., Brock M., Kingma J., Heerikhuizen H. V., Leij L. D. An efficient and reproducible procedure for the formation of spheroplasts from variously grown Escherichia coli. Anal Biochem. 1976 Jul;74(1):160–170. doi: 10.1016/0003-2697(76)90320-1. [DOI] [PubMed] [Google Scholar]
- Wong T. Y., Maier R. J. Hydrogen-oxidizing electron transport components in nitrogen-fixing Azotobacter vinelandii. J Bacteriol. 1984 Jul;159(1):348–352. doi: 10.1128/jb.159.1.348-352.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood P. M. A chemiosmotic model for sulphate respiration. FEBS Lett. 1978 Nov 1;95(1):12–18. doi: 10.1016/0014-5793(78)80042-8. [DOI] [PubMed] [Google Scholar]
- Wood P. M. Periplasmic location of the terminal reductase in nitrite respiration. FEBS Lett. 1978 Aug 15;92(2):214–218. doi: 10.1016/0014-5793(78)80757-1. [DOI] [PubMed] [Google Scholar]
- Wood P. M. Why do c-type cytochromes exist? FEBS Lett. 1983 Dec 12;164(2):223–226. doi: 10.1016/0014-5793(83)80289-0. [DOI] [PubMed] [Google Scholar]
- Yamanaka T., Shinra M. Cytochrome c-552 and cytochrome c-554 derived from Nitrosomonas europaea. Purification, properties, and their function in hydroxylamine oxidation. J Biochem. 1974 Jun;75(6):1265–1273. doi: 10.1093/oxfordjournals.jbchem.a130510. [DOI] [PubMed] [Google Scholar]
- Yates M. G., Nason A. Electron transport systems of the chemoautotroph Ferrobacillus ferrooxidans. II. Purification and properties of a heat-labile iron-cytochrome c reductase. J Biol Chem. 1966 Nov 10;241(21):4872–4880. [PubMed] [Google Scholar]
- Zürrer H., Snozzi M., Hanselmann K., Bachofen R. Localisation of the subunits of the photosynthetic reaction centers in the chromatophore membrane of Rhodospirillum rubrum. Biochim Biophys Acta. 1977 May 11;460(2):273–279. doi: 10.1016/0005-2728(77)90213-4. [DOI] [PubMed] [Google Scholar]
- van der Plas J., Hellingwerf K. J., Seijen H. G., Guest J. R., Weiner J. H., Konings W. N. Identification and localization of enzymes of the fumarate reductase and nitrate respiration systems of escherichia coli by crossed immunoelectrophoresis. J Bacteriol. 1983 Feb;153(2):1027–1037. doi: 10.1128/jb.153.2.1027-1037.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Westen H. M., Mayhew S. G., Veeger C. Separation of hydrogenase from intact cells of Desulfovibrio vulgaris. Purification and properties. FEBS Lett. 1978 Feb 1;86(1):122–126. doi: 10.1016/0014-5793(78)80112-4. [DOI] [PubMed] [Google Scholar]


