Abstract
Purpose
To identify risk factors associated with incomplete neurological recovery in pediatric patients with infratentorial ependymoma treated with postoperative conformal radiation therapy (CRT).
Methods
The study included 68 patients (median age ± standard deviation of 2.6 ± 3.8 years) who were followed for 5 years after receiving CRT (54–59.4 Gy) and were assessed for function of cranial nerves V to VII and IX to XII, motor weakness, and dysmetria. The mean (± standard deviation) brainstem dose was 5,487 (±464) cGy. Patients were divided into four groups representing those with normal baseline and follow-up, those with abnormal baseline and full recovery, those with abnormal baseline and partial or no recovery, and those with progressive deficits at 12 (n = 62 patients), 24 (n = 57 patients), and 60 (n = 50 patients) months. Grouping was correlated with clinical and treatment factors.
Results
Risk factors (overall risk [OR], p value) associated with incomplete recovery included gender (male vs. female, OR = 3.97, p = 0.036) and gross tumor volume (GTV) (OR/ml = 1.23, p = 0.005) at 12 months, the number of resections (>1 vs. 1; OR = 23.7, p = 0.003) and patient age (OR/year = 0.77, p = 0.029) at 24 months, and cerebrospinal fluid (CSF) shunting (Yes vs. No; OR = 21.9, p = 0.001) and GTV volume (OR/ml = 1.18, p = 0.008) at 60 months. An increase in GTV correlated with an increase in the number of resections (p = 0.001) and CSF shunting (p = 0.035); the number of resections correlated with CSF shunting (p < 0.0001), and male patients were more likely to undergo multiple tumor resections (p = 0.003). Age correlated with brainstem volume (p < 0.0001). There were no differences in outcome based on the absolute or relative volume of the brainstem that received more than 54 Gy.
Conclusions
Incomplete recovery of brainstem function after CRT for infratentorial ependymoma is related to surgical morbidity and the volume and the extent of tumor.
Keywords: Ependymoma, Brainstem, Radiotherapy, Pediatrics, Tolerance
Introduction
Ependymoma accounts for 8 to 10% of all childhood central nervous system (CNS) tumors, with fewer than 300 new cases diagnosed annually in the United States in children and young adults younger than 19 years of age (1). Survival statistics for ependymoma have been disappointing, with 5-year overall survival and progression-free survival estimates of 50 to 60% and 23 to 45%, respectively (2, 3).
The standard treatment for children with ependymoma is surgical resection, compatible with an acceptable neurological outcome, followed by postoperative irradiation directed at the primary site. The extent of resection appears to be the most important prognostic factor. Patients who undergo gross total resection and receive radiation therapy have 5-year progression-free survival estimates of 51 to 75%, which compares favorably to estimates of 0 to 26% for those who receive radiation therapy after a resection that is incomplete (4, 5).
Even though complete resection has been clearly documented as a treatment critical to long-term survival, it is achieved for only 42 to 62% of patients and less often for patients with infratentorial tumors. To improve the outcome for patients treated at our institution, we have systematically performed a second surgery prior to radiation therapy for patients with ependymoma. In our previous report (5) that included 88 patients with ependymoma, 74 (84%) patients underwent gross total resection prior to radiation therapy, and a total of 32 (36%) patients required more than one surgery. For the entire group, the 3-year progression-free survival rate ± standard deviation (SD) was 74% ± 5%, with a clear advantage for those treated with gross-total resection (5).
Understanding the morbidity associated with aggressive surgery and postoperative irradiation is important because of the emphasis placed on the extent of resection to improve disease control. The purpose of the present study was to identify risk factors associated with incomplete neurological recovery in pediatric patients with infratentorial ependymoma, who received postoperative conformal radiation therapy (CRT) (54–59.4 Gy). Because brainstem tolerance to high-dose radiation therapy is also an important concern, the purpose of the study was extended to determine the effect of the radiation dose on brainstem function.
Methods and Materials
Between July 1997 and January 2003, 68 patients with infratentorial ependymoma were treated with a prospective institutional protocol and serially followed to study the side effects of postoperative CRT. Among the 68 patients, there were 37 male and 31 female patients, with the median age ± SD of 2.6 ±3.8 years. There were 42 patients under the age of 3 years at the time of irradiation. Hydrocephalus at diagnosis was present in 46 patients, a ventriculo-peritoneal shunt was placed in 25 patients, and 26 patients underwent more than one surgery for tumor resection; the extent of resection was gross-total resection (GTR) in 54 patients, near-total resection (NTR) in 6 patients, and sub-total resection (STR) in 8 patients. When residual tumor was identified in patients during the workup for radiation therapy, patients were evaluated by the neurosurgical team for further resection. All patients underwent surgical resection of the primary tumor, as well as management of hydrocephalus. After definitive surgery, all patients underwent repeated magnetic resonance imaging (MRI) of the brain and spine and cerebrospinal fluid (CSF) cytology and proceeded with CRT using methods previously described (5). The brainstem was contoured to calculate dose per absolute or relative volume.
Clinical evaluation
The clinical portions of the evaluation before and after CRT included a history and physical examination with a neurological examination. After completing radiation therapy, patients were followed at regular intervals (every 3 months for the first 2 years and every 6 months for an additional 3 years). All patients underwent a detailed neurologic examination to identify deficits in cranial nerve function, sensory-motor systems, and axial cerebellar function (dysmetria). We studied only these neurologic parameters because they can be consistently evaluated in all age groups; many of our study subjects were under 3 years of age at the time of radiation therapy. Brainstem functionality was assessed by examination of three neurological functions, as follows: (1) cranial nerves V to VII and IX to XII, (2) motor weakness, and (3) dysmetria. For each of the three functions, a score of 1 was normal, and greater than 1 was abnormal; the higher the score, the worse that particular brainstem function. Patients were categorized according to their brainstem function scores at baseline (start of RT) and at 12 and 24 months. Neurological deficits were graded 1 to 4, with 1 being normal, 2 indicating minor deficits with no significant functional consequence, 3 indicating major deficits with significant functional consequence or >50% loss of function, and 4 indicating a complete loss of function (Table 1). A need for gastric tube feeding or a tracheostomy was scored as a neurological deficit of 4. For each category at interval examinations, an average score for left and right sides was obtained. At the last follow-up examination, an average score of 1 for individual function was categorized as normal, while a score greater than 1 indicated incomplete recovery.
Table 1. Neurological scoring system.
| Grade | ||||
|---|---|---|---|---|
|
|
||||
| Function | 1 | 2 | 3 | 4 |
| Brainstem motor | Normal | Minor loss of strength | Moderate loss with impaired activity | Complete paralysis |
| Dysmetria | Minimal | Moderate | Moderate | Severe |
| Cranial nerve | Normal | Minor deficits | Moderate deficits | Complete paralysis |
Clinical and treatment variables
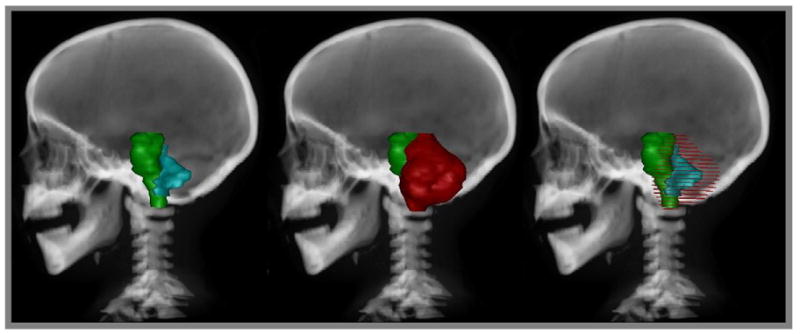
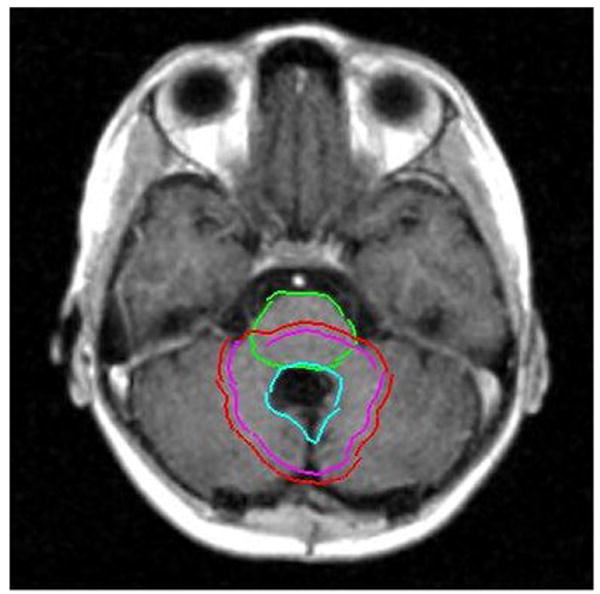
A prospective analysis of morbidity in relation to the clinical variables that might affect the recovery of the brainstem function/outcome was performed. The variables included gender, age at the time of irradiation, the extent and number of surgical procedures, hydrocephalus, CSF shunting and number of shunt revisions, gross tumor volume (GTV) and planning target volume (PTV), brainstem volume, mean brainstem dose, and absolute and relative volumes of the brainstem and subvolumes of the brainstem (medulla, pons, midbrain) that received a dose of >54 Gy. Figures 1 and 2 show contours and volumes in relation to the brainstem for a typical patient with IVth-ventricle ependymoma.
Fig. 1.
Axial T1-weighted MR images demonstrating contours of the brainstem and contours of the GTV (left), the clinical target volume (center), and the PTV (right).
Fig. 2.
Digitally reconstructed sagittal radiograph demonstrating (left) brainstem and GTV, (middle) brainstem and planning target volume, and (right) brainstem with GTV (solid) and PTV (wire overlay).
Statistical analysis
In order to identify risk factors that affected the recovery of the three brainstem functions, the scores were evaluated at fixed time points of 12, 24, and 60 months. Sixty-two of the patients had a 12-month evaluation, 57 patients were evaluated at 24 months, and 50 patients were evaluated at 60 months. In the univariate analysis, a chi-square test or Fisher's exact test was used for categorical covariates, and logistic regression analysis was used for continuous covariates. In the multivariate analysis, we used logistic regression. The effect of factors of interest on the brainstem function recovery was analyzed by using logistic regression (6). Univariate analysis was performed first, and the factors with a significance of ≤0.10 were then included in the multiple regression analysis; a stepwise selection method was used, and the factors remained in the model if there was a significance level of ≤0.05. Because PTV is highly correlated with GTV (Pearson correlation coefficient [r] = 0.86), only GTV was included in the multiple regression. The combined number of surgeries and shunting was highly correlated with the number of shunts and number of surgeries (r, ≥0.92; r, ≥0.82, respectively); therefore, only the number of shunts and/or number of surgeries was included in the multiple regression. The probability of not obtaining full recovery or being healthy (normal) was modeled. The definitions of responses are given below. The cumulative incidence function for necrosis was estimated based on the methods of Kalbfleisch and Prentice (7). The length of time at risk for necrosis was computed as the interval from the initiation of CRT to the date of necrosis, to the identification of recurrence by MRI, to the incidence of death without necrosis or recurrence, or to the last MRI follow-up, whichever came first. Recurrences and deaths from nonrecurrent cause were considered competing events. Patients who survived without necrosis or recurrence were censored at the last MRI follow-up time. We categorized recovery in five levels (progressive deficit [PD], partial recovery [PR], full recovery [FR], and healthy [normal, NL]), according to the following definitions. If for any of the three functions, a patient had a score of greater than 1 at baseline and a score that was greater at follow-up, the patient was put in the PD category; for patients not in the PD category, if, for any of the three functions, a patient had a score of greater than 1 at baseline and at follow-up the score was no larger than the baseline score but still greater than 1, then they were put into the PR category; for patients not in the PD or the PR category, if, for any of the three functions, the patient had a score greater than 1 and at follow-up the score became 1, then the patient was put into the FR category. If, for all three functions, a patient had a normal score of 1 both at the baseline and at the follow-up examinations, then the patient was put in the NL category. The number of patients in some categories was small and required that the four categorical levels be reduced to two categorical levels. The analysis focused on modeling the probability of full recovery or normal function at follow-up. All analyses were performed using SAS software (8).
Results
A total of 66 patients with a median age ± SD of 2.6 ± 3.8 years were followed for more than 6 months after they received radiation therapy and were assessed for brainstem function by evaluation of cranial nerves V to VII and IX to XII, motor weakness, and dysmetria. Two of the original patients treated during this time period were not included in the analysis. One was lost to follow-up immediately after radiation therapy, and one died immediately after radiation therapy, during a seizure episode. Forty one of the 66 patients (41/66) were under the age of 3 years at the time of irradiation. Patients were divided into four groups representing those with normal baseline and subsequent follow-up at 12, 24, and 60 months (NL category, 17, 17, and 17 patients, respectively), abnormal baseline and full recovery (FR category, 6, 7, and 10 patients, respectively), abnormal baseline and partial or no recovery (PR category, 35, 29, and 22 patients, respectively), and progressive deficits (PD category, 4, 4, and 1 patient, respectively).
Risk factors associated with recovery of brainstem function at 12 months after radiation therapy
By using univariate analysis, we found that the significant factors for incomplete recovery were gender (male vs. female; OR = 3.00, p = 0.042), CSF shunting (Yes vs. No; OR = 3.67, p = 0.042), number of tumor resections (>1 vs. 1; OR = 8.11, p = 0.010), number of interventions including tumor resection and shunt procedure (>1 vs. 1; OR = 3.67, p = 0.024), and GTV volume (OR/ml = 1.19, p = 0.004). Hydrocephalus, number of shunting procedures, extent of resection, age at the time of irradiation, and brainstem volume were not significant factors. By using multivariate analysis, we found that the significant factors for incomplete recovery were gender (male vs. female; OR = 3.97, p = 0.036) and GTV (OR/ml = 1.23, p = 0.005). GTV and PTV values were interchangeable (Pearson correlation coefficient = 0.86, p < 0.0001). A larger GTV value was associated with CSF shunting (p = 0.035) and a greater number of surgeries (p = 0.001). Female patients had fewer surgeries (p = 0.004).
Risk factors associated with recovery of brainstem function at 24 months after radiation therapy
Using univariate analysis, we found that the significant factors for incomplete recovery were CSF shunting (Yes vs. No; OR = 4.71, p = 0.017), number of tumor resections (>1 vs. 1; OR = 11.7, p = 0.003), number of interventions including tumor resection and shunt procedure (>1 vs. 1; OR = 5.25, p = 0.005), and GTV (OR/ml = 1.15, p = 0.006). Gender, hydrocephalus, number of shunting procedures, extent of resection, age at the time of irradiation (p = 0.088), and brainstem volume were not significant factors. Using multivariate analysis, we found that the significant factors for incomplete recovery were number of tumor resections (>1 vs. 1; OR = 23.7, p = 0.003) and age at the time of irradiation (OR/year = 0.77, p = 0.029). The number of tumor resections were associated with male gender (p = 0.003), hydrocephalus (p = 0.010), presence of a CSF shunt (p = 0.0002), and number of shunt procedures (p = 0.035) and GTV (p = 0.002). Age correlated with brainstem volume.
Risk factors associated with recovery of brainstem function at 60 months after radiation therapy
By using univariate analysis, we found that the significant factors for incomplete recovery were CSF shunting (Yes vs. No; OR = 19.4, p = 0.0005), number of tumor resections (>1 vs. 1; OR = 10.4, p = 0.002), number of interventions including tumor resection and shunt procedure (>1 vs. 1; OR = 12.5, p = 0.0002), extent of the resection (NTR/STR vs. GTR; OR = 9.17, p = 0.049) and GTV (OR/ml = 1.17, p = 0.003). Gender, hydrocephalus, number of shunting procedures, age at the time of irradiation, and brainstem volume were not significant factors. By using multivariate analysis, we found that the significant factors for incomplete recovery were CSF shunting (Yes vs. No; OR = 21.9, p = 0.001) and GTV (OR/ml = 1.18, p = 0.008). CSF shunting was associated with hydrocephalus (p = 0.0003), more tumor resections (p = 0.0001), and incomplete extent of tumor resection. An increase in the number of tumor resections was associated with larger GTVs (p = 0.004).
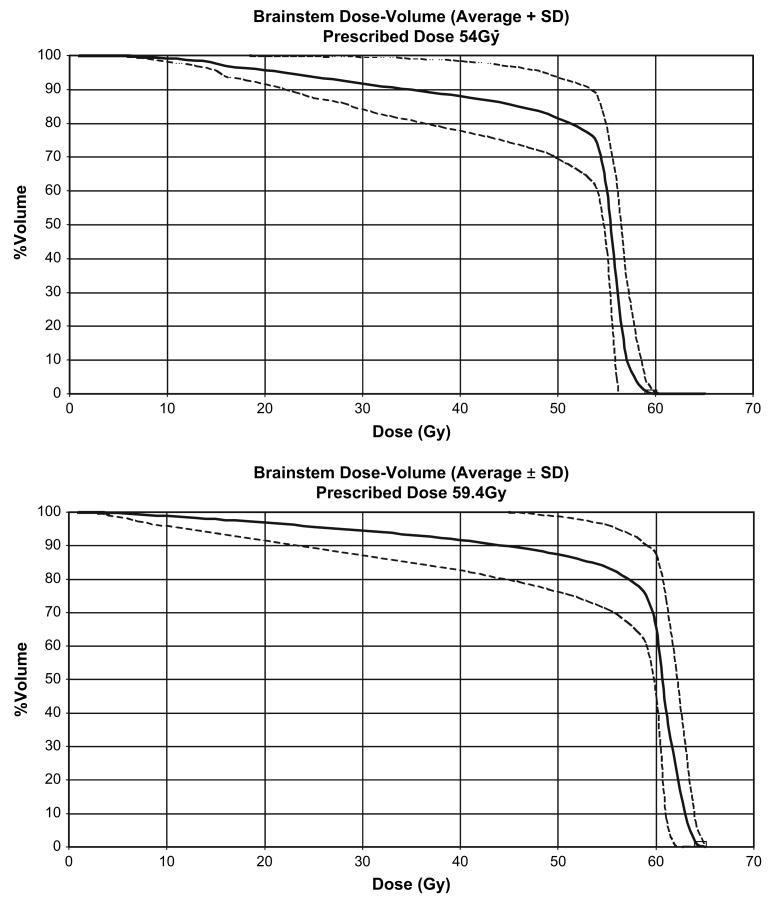
Dose–volume profiles and brainstem volume by age
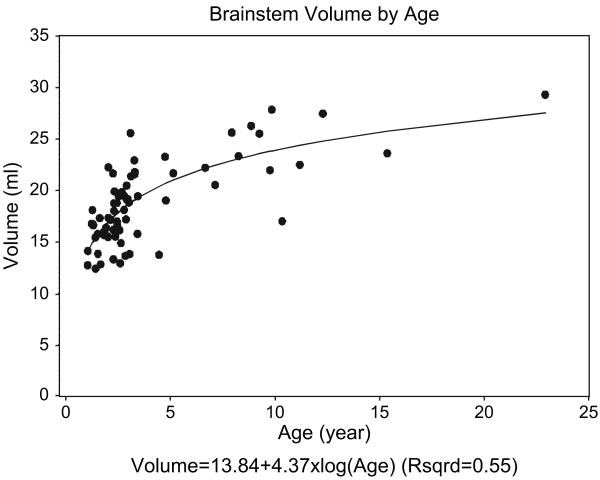
Mean dose–volume curves ± SDs were computed from the patients that contributed to the analysis (Fig. 3). The curves are presented according to the total prescribed doses, 54 Gy and 59.4 Gy. The effect of absolute and relative (percent volume) irradiated brainstem volumes on recovery was determined and is presented in Table 3. No effect was identified for this patient cohort, using the entire volume of the brainstem or subvolumes including the medulla, pons, and midbrain. The relationship between brainstem volume and age was assessed, given the young age at which patients were irradiated and the range of patient ages included in this research. The relationship between brainstem volume and age was exponential (Fig. 4). This relationship was modeled by taking the log of the patient age and performing linear regression. The following equation was used to calculate brainstem volume (ml) = 13.84 + 4.37 × log (age), where the standard errors of the intercept, 13.84, and the slope, 4.37, were ±0.64 and ±0.48, respectively. The slope was statistically significant compared to zero, p < 0.0001, and the coefficient of determination, R2, was 0.55. There was no correlation between brainstem volume and response at 12, 24, and 60 months.
Fig. 3.
Brainstem mean dose–volume curves (± SDs) for patients with infratentorial ependymoma treated with a prescribed total dose of 54 Gy (upper panel, n = 10 patients) or 59.4 Gy (lower panel, n = 56 patients).
Table 3. Impact of brainstem dose–volume on functional recovery for patients with infratentorial ependymoma treated with postoperative irradiation.
| Dose factor | Mean ± SD volume (ml) | Odds ratio (95% CI) of response at 12 months (n = 62 patients) | p value | Odds ratio (95% CI) of response at 24 months (n = 56 patients) | p value |
|---|---|---|---|---|---|
| Volume (ml) >54Gy | 15.06 + 4.15 | 1.052 (0.928 to 1.192) | 0.43 | 0.993 (0.877 to 1.125) | 0.92 |
| Volume (ml) >56Gy | 13.34 + 6.09 | 1.045 (0.953 to 1.146) | 0.35 | 0.993 (0.912 to 1.082) | 0.88 |
| Volume (ml) >58Gy | 12.24 + 6.28 | 1.014 (0.932 to 1.104) | 0.75 | 0.974 (0.897 to 1.058) | 0.54 |
| Volume (ml) >60Gy | 7.64 + 6.53 | 1.055 (0.972 to 1.145) | 0.2 | 1.031 (0.949 to 1.120) | 0.47 |
| % volume >54Gy | 79.95 + 15.66 | 0.986 (0.954 to 1.020) | 0.42 | 0.964 (0.927 to 1.002) | 0.06 |
| % volume >56Gy | 69.89 + 28.99 | 1.002 (0.983 to 1.020) | 0.87 | 0.989 (0.971 to 1.008) | 0.25 |
| % volume >58Gy | 64.03 + 30.21 | 0.996 (0.979 to 1.013) | 0.64 | 0.986 (0.969 to 1.004) | 0.12 |
| % volume >60Gy | 39.60 + 32.47 | 1.006 (0.989 to 1.022) | 0.49 | 1.001 (0.985 to 1.018) | 0.88 |
Abbreviation: CI = confidence interval.
Fig. 4.
The relationship between brainstem volume and age is exponential for patients with infratentorial ependymoma. The scatter plot includes 68 patients, with measurements taken prior to irradiation.
Unexpected death
One male patient died during a seizure episode at 3 months after the initiation of CRT. Autopsy showed residual tumor and focal areas of necrosis within the brainstem. Necrosis of normal tissue resulting from the acute effects of irradiation and the effects of the brainstem stroke suffered 6 months earlier could not be excluded. This patient had significant perioperative morbidity after undergoing two surgical procedures that resulted in subtotal resection. He had evidence of brainstem ischemia, based on postoperative T2-weighted MRI. His preirradiation history included tracheostomy, gastrostomy, CNS-mediated hypertension requiring medication, and postoperative seizure. He ranked fifteenth in mean dose (59.13 Gy), first in percent volume exceeding 54, 56 and 58 Gy, and twenty-fourth in percent volume exceeding 60 Gy.
Discussion
Brainstem recovery after surgery for infratentorial ependymoma is time dependent and mediated by a number of factors including the number of tumor resections, age at time of irradiation, gender, CSF shunting, and tumor volume. Age and brainstem volume were highly correlated, and gender, tumor volume, and CSF shunting were correlated with the number of tumor resections. The odds of incomplete recovery of brainstem function at 1 year were nearly four times greater for male patients and increased by 19% for each milliliter increase in the GTV. At 2 years, those patients who had undergone more than one tumor resection were 24 times more likely to have incomplete recovery than those who had undergone only one tumor resection, and the risk of incomplete recovery decreased by 23% for each year of increase in age. At 5 years, those who required CSF shunting had a 22 times greater risk of incomplete recovery, and the risk of incomplete recovery increased by 18% for every milliliter increase in gross tumor volume. Among the patients evaluated for this study, there was no relationship between dose, volume, and recovery of brainstem function. The mean dose exceeded 54 Gy for most patients (group mean = 54.87 ± 4.64 Gy). The FR or NL group was characterized by a mean brainstem volume of 9.4 ± 1.4 ml (percent volume = 45.4 ± 6.3), and the incomplete recovery group was characterized by a mean volume of 7.0 ± 1.0 ml (percent volume = 37.8 ± 5.4 ml) irradiated with at least 60 Gy.
Surgery has the greatest impact on disease control and neurological outcomes for patients with infratentorial ependymoma. The improved outcomes achieved by increasing the rate of gross-total resection must be balanced against long-term neurological effects. The next step is to determine the impact of these neurological deficits on parameters measuring functional outcomes and quality of life and then to find new ways to reduce surgical morbidity. Surgical morbidity rates in the range of 20 to 36% (9, 10) have previously been reported, and with technological improvements, morbidity rates in modern surgical practice have fallen. For tumors involving the posterior fossa, morbidity has been attributed to radical resection of IVth-ventricle tumors attached to the brainstem (11). The number of surgical procedures has been previously identified as a factor: the incidence of brainstem morbidity in patients undergoing skull base surgery for chordoma increased from 6% to 27% when measured at 10 years, in a comparison of patients who were treated with one surgery with those who were treated with two or more surgeries.
CSF shunting was a significant factor leading to partial brainstem recovery. Hydrocephalus was observed in 46/66 patients, and 25 patients required CSF shunting. A second surgery increases the risk for symptomatic hydrocephalus and CSF shunting, indicating that CSF shunting is not independent of the number of tumor resections (12). The complications of CSF diversion procedures are well documented, especially in patients who experience shunt failure (13–15). Indeed, the only patient to experience progressive neurologic deficits at 60 months in this series was a patient who experienced a catastrophic shunt failure that resulted in a prolonged anoxic event. Although a long-term survivor, this patient was permanently disabled and will require mechanical ventilation support for the remainder of his life.
There are limited data concerning brainstem tolerance to fractionated photon radiation therapy in adult and pediatric patients. Most clinical protocols for children with CNS tumors recommend target volume doses where the risk of a radiation-related side effect should be low. Normal tissue constraints targeting the brainstem are absent from current CNS tumor protocols because of limited information and largely because, for many of the commonly curable tumors, the cumulative total dose does not exceed 54 Gy. For children with ependymoma, tolerance levels were not included in a recent study that included a prescribed PTV dose of 59.4 Gy.
In previous reports, brainstem tolerance to radiation therapy was mediated by a number of factors including tumor location that would increase the dose to the brainstem, tumor size, maximum dose, the absolute volume receiving the highest doses, the number of surgeries, and the preexisting factors of diabetes and hypertension. Limiting the dose to the brainstem to less than 60 Gy or, more specifically, allowing no more than 53 Gy to the center of the brainstem or 64 Gy to the surface has resulted in a low risk of complications for patients with base-of-skull, intracranial, and head-and-neck tumors treated with combined modality therapy that including photon irradiation and proton beam therapy (16). Among reports of series using fractionated photon irradiation, the report by Jian et al (17) provides some assurance of safety for dose–volume data within a range similar to that of patients treated in our series. Those authors reported limited toxicity to 48 patients with nasopharyngeal cancer treated with hyperfractionated irradiation (1.2 Gy/day) to dose levels of 74.4 Gy, provided that brainstem dose constraints were V65 of <3 ml and V60 of <5 ml.
The present data support the tolerance of the entire brainstem to photon doses in the range of 54 Gy and the treatment of portions of the brainstem to higher doses without impeding neurological recovery from tumor and surgically acquired deficits. Special attention should be paid to patients with large tumors, very young patients, and those patients who require multiple tumor resections.
Table 2. Impact of brainstem dose–volume on functional recovery of patients with infratentorial ependymoma treated with postoperative irradiation.
| 12 month follow-up | 24 month follow-up | 60 month follow-up | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||||
| Dose factor | Group response | n patients | Mean irradiated brainstem volume ± SE (ml) | % volume of irradiated brainstem ± SE% | p value | n patients | Mean irradiated brainstem volume ± SE (ml) | % volume of irradiated brainstem ± SE% | p value | n patients | Mean irradiated brainstem volume ± SE (ml) | % volume of irradiated brainstem ± SE% | p value |
| Volume | FR or NL (+) | 23 | 15.7 ± 0.8 | 0.42 | 24 | 15.3 ± 1.0 | 0.97 | 27 | 15.4 ± 0.9 | 0.78 | |||
| >54 Gy | FR or NL (-) | 39 | 14.8 ± 0.7 | 33 | 15.4 ± 0.7 | 23 | 15.1 ± 0.8 | ||||||
| Volume | FR or NL (+) | 23 | 14.6 ± 1.1 | 0.32 | 24 | 13.6 ± 1.4 | 0.96 | 27 | 13.4 ± 1.3 | 0.99 | |||
| >56 Gy | FR or NL (-) | 39 | 13.0 ± 1.0 | 33 | 13.7 ± 1.1 | 23 | 13.4 ± 1.3 | ||||||
| Volume | FR or NL (+) | 23 | 12.9 ± 1.3 | 0.66 | 24 | 12 ± 1.5 | 0.60 | 27 | 11.8 ± 1.4 | 0.64 | |||
| >58 Gy | FR or NL (-) | 39 | 12.2 ± 1.0 | 33 | 13 ± 1.0 | 23 | 12.8 ± 1.3 | ||||||
| Volume | FR or NL (+) | 23 | 9.4 ±1.4 | 0.17 | 24 | 8.7 ± 1.5 | 0.54 | 27 | 7.8 ± 1.4 | 0.88 | |||
| >60 Gy | FR or NL (-) | 39 | 7.0 ± 1.0 | 33 | 7.6 ± 1.1 | 23 | 7.5 ± 1.3 | ||||||
| % volume | FR or NL (+) | 23 | 78.5 ± 2.4 | 0.63 | 24 | 75.7 ± 3.3 | 0.10 | 27 | 76.4 ± 3.0 | 0.19 | |||
| >54 Gy | FR or NL (-) | 39 | 80.5 ± 2.8 | 33 | 82.6 ± 2.6 | 23 | 82.5 ± 3.3 | ||||||
| % volume | FR or NL (+) | 23 | 72.3 ± 4.2 | 0.68 | 24 | 65.8 ± 5.8 | 0.38 | 27 | 64.8 ± 5.6 | 0.39 | |||
| >56 Gy | FR or NL (-) | 39 | 69.2 ± 5.2 | 33 | 72.8 ± 5.2 | 23 | 72.3 ± 6.6 | ||||||
| % volume | FR or NL (+) | 23 | 63.8 ± 5.6 | 0.86 | 24 | 57.9 ± 6.5 | 0.19 | 27 | 56.7 ± 6.2 | 0.18 | |||
| >58Gy | FR or NL (-) | 39 | 65.2 ± 5.1 | 33 | 68.9 ± 5.2 | 23 | 69 ± 6.5 | ||||||
| % volume | FR or NL (+) | 23 | 45.4 ± 6.3 | 0.37 | 24 | 42.2 ± 6.6 | 0.87 | 27 | 37.1 ± 6.3 | 0.70 | |||
| >60Gy | FR or NL (-) | 39 | 37.8 ± 5.4 | 33 | 40.7 ± 5.8 | 23 | 40.8 ±7.1 | ||||||
Acknowledgments
This work was supported in part by the American Lebanese Syrian Associated Charities (ALSAC) and the American Cancer Society.
Footnotes
Conflict of interest: none.
References
- 1.Ries LAG, Smith MA, Gurney JG, et al., editors. SEER Program. Bethesda, MD: National Cancer Institute; 1999. Cancer incidence and survival among children and adolescents: United States SEER program 1975-1995. NIH pub; no. p. 99-4649. [Google Scholar]
- 2.Merchant TE, Haida T, Wang MH, Finlay JL, Leibel SA. Anaplastic ependymoma: Treatment of pediatric patients with or without craniospinal radiation therapy. J Neurosurg. 1997;86:943–949. doi: 10.3171/jns.1997.86.6.0943. [DOI] [PubMed] [Google Scholar]
- 3.Pollack IF, Gerszten PC, Martinez AJ, et al. Intracranial ependymomas of childhood: Long-term outcome and prognostic factors. Neurosurgery. 1995;37(4):655–666. doi: 10.1227/00006123-199510000-00008. [DOI] [PubMed] [Google Scholar]
- 4.van Veelen-Vincent ML, Pierre-Kahn A, Kalifa C, et al. Ependymoma in childhood: Prognostic factors, extent of surgery, and adjuvant therapy. J Neurosurg. 2002;97:827–835. doi: 10.3171/jns.2002.97.4.0827. [DOI] [PubMed] [Google Scholar]
- 5.Merchant TE, Mulhern RK, Krasin MJ, et al. Preliminary results from a phase II trial of conformal radiation therapy and evaluation of radiation-related CNS effects for pediatric patients with localized ependymoma. J Clin Oncol. 2004;22(15):3156–3162. doi: 10.1200/JCO.2004.11.142. [DOI] [PubMed] [Google Scholar]
- 6.Agresti A. Categorical data analysis. 2nd. New York: John Wiley & Sons; 2002. [Google Scholar]
- 7.Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. New York: John Wiley; 1980. pp. 163–188. [Google Scholar]
- 8.SAS Institute Inc: SAS/STAT user's guide, version 9.1. Cary, NC: SAS Institute Inc; 2004. [Google Scholar]
- 9.Cohen ME, Duffner PK. Brain tumors in children: Principles of diagnosis and treatment. In: Cohen ME, Duffner PK, editors. Ependymomas. New York: Raven Press; 1994. pp. 219–239. [Google Scholar]
- 10.Undjian S, Marinov M. Intracranial ependymomas in children. Childs Nerv Syst. 1990;6:131–134. doi: 10.1007/BF00308488. [DOI] [PubMed] [Google Scholar]
- 11.Cochrane DD, Gustavsson B, Poskitt KP, Steinbok P, Kestle JR. The surgical and natural morbidity of aggressive resection for posterior fossa tumors in childhood. Pediatr Neurosurg. 1994;20:19–29. doi: 10.1159/000120761. [DOI] [PubMed] [Google Scholar]
- 12.Merchant TE, Lee H, Zhu J, et al. The effects of hydrocephalus on intelligence quotient in children with localized infratentorial ependymoma before and after focal radiation therapy. J Neurosurg. 2004;101(Suppl 2):159–168. doi: 10.3171/ped.2004.101.2.0159. [DOI] [PubMed] [Google Scholar]
- 13.Casey AT, Kimmings EJ, Kleinlugtebeld AD, et al. The long-term outlook for hydrocephalus in childhood.A ten-year cohort study of 155 patients. Pediatr Neurosurg. 1997;27(2):63–70. doi: 10.1159/000121229. [DOI] [PubMed] [Google Scholar]
- 14.Wu Y, Green NL, Wrensch MR, Zhao S, Gupta N. Ventriculoperitoneal shunt complications in California: 1990 to 2000. Neurosurgery. 2007;61(3):557–562. doi: 10.1227/01.NEU.0000290903.07943.AF. [DOI] [PubMed] [Google Scholar]
- 15.Vinchon M, Dhellemmes P. Cerebrospinal fluid shunt infection: risk factors and long-term follow-up. Childs Nerv Syst. 2006;22(7):692–697. doi: 10.1007/s00381-005-0037-8. [DOI] [PubMed] [Google Scholar]
- 16.Debus J, Hug EB, Liebsch NJ, et al. Brainstem tolerance to conformal radiotherapy of skull base tumors. Int J Radiat Oncol Biol Phys. 1997;39(5):967–975. doi: 10.1016/s0360-3016(97)00364-7. [DOI] [PubMed] [Google Scholar]
- 17.Jian JJ, Cheng SH, Tsai SY, et al. Improvement of local control of T3 and T4 nasopharyngeal carcinoma by hyperfractionated radiotherapy and concomitant chemotherapy. Int J Radiat Oncol Biol Phys. 2002;53(2):344–352. doi: 10.1016/s0360-3016(02)02709-8. [DOI] [PubMed] [Google Scholar]