Abstract
Background and Methods
Low frequency oscillations (LFO) of cerebral vessels are believed to reflect cerebral autoregulation. We investigated day-to-day and hemispheric variations in 0·1 Hz LFO with near infrared spectroscopy (NIRS) and transcranial Doppler (TCD) to determine phase shift and gain of oxygenated haemoglobin (oxy-Hb) and the velocity of the middle cerebral artery (Vmca) to the arterial blood pressure (ABP). The direct left–right phase shifts of oxyHb and Vmca were also assessed. We examined 44 healthy volunteers by simultaneous recordings of ABP, oxyHb and Vmca during spontaneous and paced breathing at 6 breaths per minute on two separate days.
Results
The variation between hemispheres had a prediction interval (PI) of ±39° for ABP–oxyHb phase shift and ±69% for gain. ABP–Vmca showed ±57° PI phase shift and ±158% PI for gain. The variation from day to day showed ±61° PI for ABP–oxyHb phase shift and ±297% PI for gain. ABP–Vmca showed ±45° PI phase shift and ±166% PI for gain. We found a linear relation between phase shift of oxyHb and Vmca at paced breathing (P = 0·0005), but not at rest (P = 0·235).
Conclusion
Our results show that LFO phase shift ABP–oxyHb may be used as a robust measurement of differences in autoregulation between hemispheres and over time. In addition, we found a strong relation between oxyHb and Vmca during paced breathing. Gain showed too large variation for clinical use, as the SD was up to 100-fold of mean values.
Keywords: Cerebral autoregulation, low frequency oscillations, near infrared spectroscopy, transcranial Doppler
Introduction
Near infrared spectroscopy (NIRS) is a noninvasive optical method to investigate regional changes in oxygenated (oxyHb) and deoxygenated haemoglobin (deoxyHb) in the outermost layers of the cerebral cortex [1]. Among the advantages of NIRS are the high temporal resolution and the lack of discomfort during recording, which ensure high compliance for both patients and healthy volunteers. Few previous studies have used NIRS to investigate spontaneous 0·1 Hz low frequency oscillations (LFO) of cortical cerebral vessels [2–4], whereas most studies used transcranial Doppler (TCD) to examine LFO of middle cerebral artery velocity (Vmca) [5–10]. These studies analysed LFO and examined the time relation (phase shift) and the amplitude ratio (gain) between corresponding oscillations in arterial blood pressure (ABP) and cerebral vessels. The phase shift at 0·1 Hz is suggested to reflect the cerebral blood flow autoregulation [9] and is known to be altered in vascular disorders such as ischaemic stroke [11–13] and carotid artery disease [6,14]. We aimed to describe the inter-hemisphere and day-to-day variability of 0·1 Hz LFO phase shift and gain on oxyHb and Vmca measurements to the ABP in healthy volunteers. In addition, we examined the linear relation between oxyHb and Vmca as well as the possible variation caused by age and gender. We also investigated the direct phase shift and gain variation between left–right hemispheres for oxyHb and Vmca values.
Materials and methods
Subjects
We recruited 44 healthy volunteers with no history of cardio-vascular or neurological disease. Subjects using daily medication of any kind were excluded, except oral contraceptives, and two subjects using a cholesterol-lowering drug (simvastatine). None of the subjects had a history of alcohol or substance abuse, and none of the subjects had a body mass index >30. We recruited five men and five women in three age groups of 27–34, 37–44 and 47–54 years and a fourth group of six men and eight women aged >57 years (range, 57–69; mean, 63 years). Four subjects were left-handed (9%).
The Biomedical Research Ethics committee in the Capital Region of Denmark approved the study (H-B-2008-039). All subjects gave informed consent to participate in the study, which was undertaken in accordance with the Helsinki Declaration of 1964, as revised in Edinburgh in 2000.
Near infrared spectroscopy
Near infrared spectroscopy measurements were performed using continuous wave NIRS (NIRS2; TechEn Inc, Milford, MA, USA). The NIRS optodes were placed bilateral on the forehead with one light source at two wavelengths (690 and 830 nm) and two detectors on each side, avoiding the midline sinus. The distance between source and detectors was 3 cm, with the detectors lateral to the source. Thus, the detectors measured the frontal cortex region in the territory supplied by the middle cerebral artery (MCA). NIRS recordings were acquired at a sampling rate of 200 Hz.
Transcranial Doppler
The Vmca was recorded on both sides using a TCD monitor (Multidop-X4, DWL). The ultrasound probes were fixed using a head band. Identification of the middle cerebral artery was performed as previously described [15]. All recordings were performed by the same trained physician (DP).
Arterial blood pressure
Continuous noninvasive ABP recording was achieved via a finger plethysmograph (CNAP500) using the subjects’ left hand, positioned at heart level. End-tidal CO2 partial pressure (PetCO2) was measured continuously using an open mask that caused no respiratory resistance (ProPac Encore). Data from TCD and finger plethysmograph were recorded synchronously with the NIRS data for subsequent offline analysis.
Procedures
Each subject was examined on two different days within 1 week (range, 1–7 days; mean, 3·0 days), and both examinations were performed at the same time of the day. Before the experiments, each subject underwent a general physical and neurological examination. Coffee, tea or tobacco was not allowed 8 h before the investigations. All experiments were performed with the subjects placed in a supine position in a silent room with a constant temperature and the light dimmed. After 15 min of rest in the supine position, data acquisition was started with a 10-min trial of spontaneous breathing. This was followed by a 5-min trial with paced breathing at a rate of six respiration cycles per minute (0·1 Hz).
Data analysis
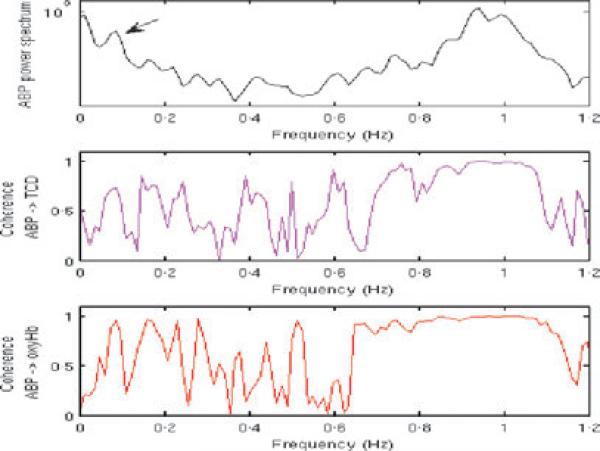
All signals were analysed in successive 50% overlapping time segments of 100 s. The NIRS light intensity at 690 and 830 nm were first converted to time series of variations in oxyHb concentrations using the modified Beer–Lambert law. On each time segment, we then computed the power spectra of all signals (ABP, oxyHb, Vmca), the coherence spectra for ABP–oxyHb and ABP–Vmca and the complex transfer function for ABP–oxyHb and ABP–Vmca, using Matlab (The MathWorks, Inc., Natick, MA, USA) functions pwelch, mscohere and tfestimate, respectively. All three functions are based on the Welch's averaged periodogram method, and we used 50% overlap 50-s Hamming windowing of the data in each segment. For two signals x and y, the Matlab function tfestimate computes the complex transfer function TFx-y as the quotient of the cross-power spectral density of the two signals Pxy and the power spectral density of the input signal Pxx. The magnitude-squared coherence Cxy between the signal x and y is obtained with mscohere as Cxy = (abs(Pxy))2/(Pxx . Pyy). The phase shift and gain between ABP oscillations and oxyHb and Vmca were obtained as the phase and absolute values of the complex transfer function, respectively. The LFO frequency was selected as the frequency with maximal ABP oscillation power in the 0·09–0·11 Hz range (Fig. 1). In this manner, for each run, we obtained 5 (5-min run) or 11 (10-min run) overlapping segments each characterized by a coherence value, a phase shift, and a gain for ABP–oxyHb and ABP–Vmca. We selected the segments for which the coherence was above an arbitrary threshold of 0·7 and averaged the gain (linear average) and the phase shift (circular average) over these selected segments. If the total number of segments with a coherence above 0·7 was smaller than 3 for a specific run, we discarded that run. Each NIRS channel was investigated independently and averaged subsequently.
Figure 1.
Arterial blood pressure (ABP) power spectrum and coherence spectra for ABP–Vmca and ABP–oxyHb.
Statistics
Data are presented as mean values with standard deviation (SD), confidence interval (CI) or prediction interval (PI) which is ± 2 SD.
The primary end-points were as follows:
ABP–oxyHb and ABP–Vmca phase shift and gain during spontaneous breathing between left and right hemisphere as well as between day 1 and day 2.
The phase shift relation between ABP–oxyHb and ABP–Vmca during spontaneous and paced breathing.
Secondary end-points were as follows:
The direct left–right phase shift and gain for both oxyHb and Vmca values
The ABP–oxyHb and ABP–Vmca phase shift and gain between hemispheres and days using paced breathing.
Differences in gender and age groups for oxyHb and Vmca values.
For calculation of PI for ABP–oxyHb and ABP–Vmca and the relation between ABP–oxyHb and ABP–Vmca, we used a mixed model, which takes into account the correlations between sides and days of each subject. PI was chosen to establish a normal range that may be used in future studies examining cerebrovascular diseases.
Gain was measured in arbitrary unit (a.u.). Owing to nonnormal distribution of gain data and increasing variability with increasing level of gain for both ABP–oxyHb and ABP–Vmca, we applied a logarithmic transformation to the gain data. As a subtraction of logarithmic values results in a ratio after back-transformation, all gain data are presented in per cent for each recording, except mean values which are presented as a geometric mean that can be interpreted much like a median. All analyses were performed with SAS 9.2 (SAS Institute Inc., Cary, NC, USA). Five per cent (P = 0·05) was chosen as the level of significance.
Results
The final data analysis comprised 44 healthy subjects. In seven subjects, the Vmca recordings on one or both sides were missing because of an insufficient TCD signal. The recordings at rest were missing in three subjects on 1 day because of technical problems. The percentage of recordings excluded because of low coherence in three or more windows were phase shift rest oxyHb 24% and Vmca 10%; phase shift 6 bpm oxyHb 16% and Vmca 15%; gain rest oxyHb 24% and Vmca 17%; gain 6 bpm oxyHb 17% and Vmca 15%. DeoxyHb showed very low coherence and was not analysed further. Mean arterial blood pressure (MAP) in the different age groups were 27–34 years: 81 mmHg, 37–44 years: 82 mmHg, 47–54 years: 90 mmHg and >57 years: 91 mmHg. There were no significant differences between groups (P = 0.072).
Inter-subject and group variation
At rest, Vmca oscillations were 57° (PI – 137; 24), 1·6 s, ahead of ABP. The oxyHb oscillations were 15° (PI – 45; 75), 0·4 s, after ABP, thus 72° and 2 s after Vmca (Table 1). The direct relation for oxyHb left was 4° (PI – 29; 21), 0·1 s, after right, and for Vmca left was 5° (PI – 55; 65), 0·1 s, ahead of right (Table 2).
Table 1.
Mean, standard deviation (SD) and prediction interval (PI) for phase shifts and gain at rest and paced breathing at 6 bpm
| Mean (SD) | |||||
|---|---|---|---|---|---|
| Phase Rest | Left day1 | Right day1 | Left day2 | Right day2 | PI |
| OxyHb | 13 (25) | 15 (30) | 11 (32) | 17 (27) | –45; 75 |
| Vmca | –58 (26) | –53 (45) | –64 (22) | –63 (27) | –137; 24 |
| Gain Rest | Mean (SD %) | ||||
| OxyHb | 0·280 (190) | 0·296 (160) | 0·231 (197) | 0·246 (165) | 0·027; 2·146 |
| Vmca | 1·492 (53) | 1·369 (133) | 1·431 (81) | 1·465 (73) | 0·309; 5·859 |
| Phase 6 bpm | Mean (SD) | ||||
| OxyHb | 9 (29) | 2 (40) | 9 (35) | 10 (26) | –60; 74 |
| Vmca | –58 (35) | –56 (51) | –54 (31) | –53 (29) | –147; 38 |
| Gain 6 bpm | Mean (SD %) | ||||
| OxyHb | 0·308(217) | 0·337 (183) | 0·291 (155) | 0·300 (143) | 0·039; 2·208 |
| Vmca | 0·919 (90) | 0·933 (123) | 1·211 (96) | 1·149 (81) | 0·207; 4·278 |
Table 2.
Mean, standard deviation (SD), confidence interval (CI) and prediction interval (PI) for day 1 and the difference between days for the direct left–right phase shift and gain
| Day 1 Rest | 6 bpm | |||
|---|---|---|---|---|
| Phase | Mean (SD) | PI | Mean (SD) | PI |
| OxyHb | –4 (13) | –29; 21 | –1 (27) | –56; 53 |
| Vmca | 5 (30) | –55; 65 | 4 (21) | –38; 46 |
| Gain | Mean (SD %) | PI | Mean (SD %) | PI |
| OxyHb | 0·927 (26) | 0·584; 1·472 | 1·071 (54) | 0·449; 2·553 |
| Vmca | 0·828 (90) | 0·230; 2·984 | 0·890 (71) | 0·304; 2·601 |
| Day to day Rest | 6 bpm | |||||
|---|---|---|---|---|---|---|
| Phase | Mean | CI | PI | Mean | CI | PI |
| OxyHb | –1 | –7; 4 | –33; 30 | –7 | –16; 3 | –63; 50 |
| Vmca | 3 | –5; 10 | –43; 48 | 2 | –1; 5 | –14; 17 |
| Gain | % | % | % | % | % | % |
| OxyHb | –2 | –11; 8 | –45; 75 | 9 | –5; 24 | –51; 142 |
| Vmca | 11 | –8; 33 | –62; 220 | 7 | –6; 21 | –45; 109 |
At rest, gain was 0·242 a.u. (PI 0·003–2·146) for ABP–oxyHb and 1·346 a.u. (PI 0·309–5·859) for ABP–Vmca. Gain was 0·950 a.u. (PI 0·544–1·356) for oxyHb left–right and 0·947 a.u. (PI 0·090–1·804) for Vmca left–right. PetCO2 did not change (32·6–32·2 mmHg, P = 0·197) at rest, whereas during paced breathing PetCO2 decreased (33·6–31·8 mmHg, P < 0·001).We found no significant differences between age groups and gender in any variables (Table 3).
Table 3.
P-values for differences in gender and age groups for phase shift and gain
| Phase shift | Gain | |||
|---|---|---|---|---|
| Gender | Age | Gender | Age | |
| OxyHb rest | 0·871 | 0·067 | 0·891 | 0·079 |
| Vmca rest | 0·307 | 0·062 | 0·871 | 0·100 |
| OxyHb 6 bpm | 0·222 | 0·293 | 0·242 | 0·073 |
| Vmca 6 bpm | 0·255 | 0·278 | 0·907 | 0·486 |
Side-to-side variation
There were no significant differences between hemispheres in phase shift and gain for ABP–oxyHb or ABP–Vmca. The mean differences in phase shift between the two hemispheres at rest ranged from 3 to 6° (PI 39) for ABP–oxyHb and from 0 to 2° (PI 57) for ABP–Vmca. Differences in gain at rest were 6% (PI 69%) in ABP–oxyHb and ranged from 2% to 8% (PI 158%) for ABP–Vmca (Table 4).
Table 4.
Mean difference, confidence interval (CI) and prediction interval (PI) at rest
| Side to side Day 1 | Day 2 | ||||
|---|---|---|---|---|---|
| Phase | Mean | CI | Mean | CI | PI (±) |
| OxyHb | 3 | –2; 8 | 6 | –3; 14 | 39 |
| Vmca | –2 | –5; 2 | 0 | –12; 11 | 57 |
| Gain | % | % | % | % | % |
| OxyHb | –6 | –14; 3 | –6 | –16; 5 | 69 |
| Vmca | –8 | –21; 6 | 2 | –16; 24 | 158 |
| Day to day Left | Right | ||||
|---|---|---|---|---|---|
| Phase | Mean | CI | Mean | CI | PI (±) |
| OxyHb | –5 | –17; 6 | 2 | –8; 12 | 61 |
| Vmca | –10 | –17; –2 | –2 | –9; 6 | 45 |
| Gain | % | % | % | % | % |
| OxyHb | 2 | –22; 35 | 11 | –14; 43 | 297 |
| Vmca | –8 | –21; 6 | 4 | –17; 29 | 166 |
Day-to-day variation
We found no significant difference between day 1 and day 2 in phase shift and gain for ABP–oxyHb and ABP–Vmca. The mean differences in phase shifts from day to day at rest ranged from 2 to 5° (PI 61) for ABP–oxyHb and from 2 to 10° (PI 45) for ABP–Vmca (Table 4). Differences in gain at rest ranged from 2% to 11% (PI 297%) in ABP–oxyHb and from 4% to 8% (PI 166%) for ABP–Vmca. For the direct left–right phase shift and gain, we found no significant differences for either oxyHb or Vmca (Table 2).
Relation between ABP–oxyHb and ABP–Vmca
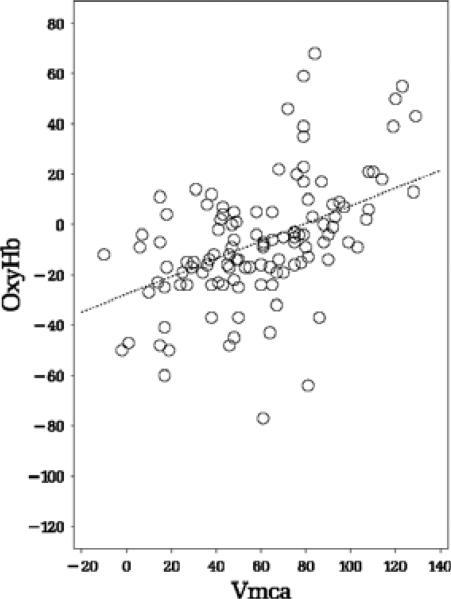
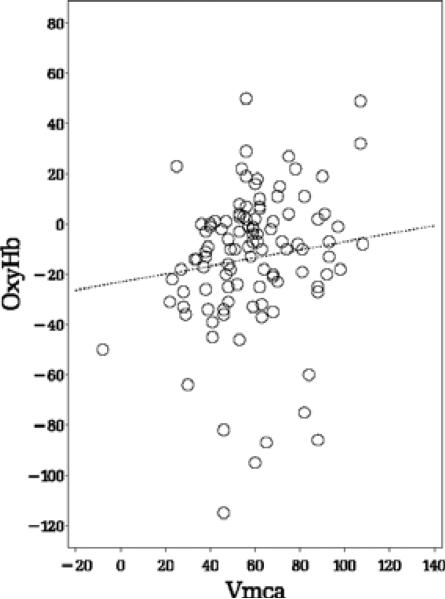
We investigated the linear relation between ABP–oxyHb and ABP–Vmca and found a positive significant linear relation during paced breathing for both phase shift (P = 0·0005, slope = 0·35, Fig. 2) and gain (P = 0·0025, slope = 0·36). We found no linear relation at rest for phase shift (P = 0·235), see Fig. 3, and gain (P = 0·131).
Figure 2.
The relation of Arterial blood pressure (ABP)–oxyHb and ABP–Vmca phase shift at paced breathing at 6 bpm.
P = 0·0005, slope = 0·35.
Figure 3.
The relation of Arterial blood pressure (ABP)–oxyHb and ABP–Vmca phase shift at rest. P = 0·235.
Paced breathing
For phase shift at 6 bpm, Vmca was ahead of ABP with 54° (PI – 147; 38), 1·5 s, and oxyHb was 7° (PI – 60; 74), 0·2 s after ABP, thus a difference of 60°, 1·7 s. Gain during 6 bpm was 0·294 a.u. (PI 0·039–2·208) for ABP–oxyHb and 0·942 a.u. (PI 0·207–4·278) for ABP–Vmca (Table 5).
Table 5.
Mean difference, confidence interval (CI) and prediction interval (PI) at 6 bpm
| Side to side Day 1 | Day 2 | ||||
|---|---|---|---|---|---|
| Phase | Mean | CI | Mean | CI | PI (±) |
| OxyHb | –9 | –24; 6 | –3 | –10; 5 | 70 |
| Vmca | 0 | –4; 4 | 2 | –1; 5 | 19 |
| Gain | % | % | % | % | % |
| OxyHb | –7 | –21; 10 | –1 | –11; 10 | 120 |
| Vmca | –6 | –15; 5 | 1 | –11; 14 | 83 |
| Day to day Left | Right | ||||
|---|---|---|---|---|---|
| Phase | Mean | CI | Mean | CI | PI (±) |
| OxyHb | 2 | –6; 11 | 5 | –13; 22 | 82 |
| Vmca | 1 | –10; 12 | 7 | –1; 14 | 57 |
| Gain | % | % | % | % | % |
| OxyHb | –3 | –28; 31 | 16 | –9; 49 | 388 |
| Vmca | –15 | –32; 7 | –5 | –24; 19 | 235 |
Discussion
In the present study, we have showed the variation of LFO phase shift and gain for ABP–oxyHb and ABP–Vmca between hemispheres and days. In addition, we found a positive linear relation for phase shift and gain between oxyHb and ABP–Vmca during paced breathing, but not at rest. Gain showed too large variation for clinical use, as the SD was up to 100-fold of the mean values.
Obrig et al. [2] reported an ABP–oxyHb phase shift of zero in healthy volunteers (n = 3), and Payne et al. [16] using a larger source–detector separation of 5 cm and subjects in a sitting position, reported 7° ± 4° (n = 9), which is in agreement with the phase shift value of 15 found in the present study (n = 44). Whether the differences in examination protocol of the two studies have any influence on the results remain unknown. In the present study, the Vmca phase shift value at rest was 57°, which is similar to previous studies [6,9,16].
The variation between hemispheres is of great interest as cerebrovascular diseases, such as stroke and carotid artery disease, often present with unilateral changes. Previous TCD studies have shown that there is a difference in ABP–Vmca phase shift and gain between the affected and the contralateral side in minor stroke [5,11] and decreasing phase shift over time on the contralateral side after major stroke. Reinhard et al. [11] reported a phase shift difference of ABP–Vmca between hemispheres in healthy controls to be ± 1·5° (SD 7·7), which is in agreement with the present study, even though we found a higher variance (PI 57). Both studies used identical TCD set-up, except that Reinhard et al. [17] investigated the subjects with the upper body slightly elevated, which could cause changes in sympathetic tone affecting the LFO. For gain data, Reinhard et al. [11] reported the difference between hemispheres to be approximately ± 40 (SD 140), but we presented gain data in arbitrary units, and therefore, the results between the two studies cannot be directly compared. Furthermore, we found that variability increased as gain increased, which made it necessary to log-transform the gain data. This suggests that LFO data should be carefully examined before postprocessing, as transformation of data might become necessary.
Few TCD studies have investigated variation over time in different groups of patients to detect changes in the disease [5,7,18,19]. While a day-to-day variation of 26% on absolute Vmca values previously has been reported [20], we found no NIRS or TCD studies on LFO variation from day to day in healthy subjects at rest. Reproducibility between days of Vmca were examined by Reinhard et al. [7] in patients with unilateral carotid artery stenosis, which for both phase shift and gain showed significant correlation between days. However, one would expect that both gain and phase shift values would show significant day to day correlations. Thus, when reporting reproducibility, we believe that it is more useful to report the exact day-to-day variation than the correlation. Although we did not statistically compare inter-hemispheric and day-to-day variations between ABP–oxyHb and ABP–Vmca, the variations appear to be of the same magnitude, which suggests that NIRS recordings could also be used as a robust tool in assessing cerebrovascular autoregulation of cortical vessels between hemispheres and over time.
We investigated the phase shift relation between spontaneous oscillations of ABP–oxyHb and ABP–Vmca at rest and found no linear relation. The clear lack of linear relation might be due to local autoregulatory mechanisms, which could be an indicator of intact autoregulation in healthy volunteers. A previous study has reported a significant positive correlation between ABP–oxyHb and ABP–Vmca LFO at paced breathing [3]. This is in agreement with the present study showing a significant positive linear relation only at paced breathing with a slope of 0·35.
Payne et al. [16] examined the effects of arterial blood gas levels on phase shift and found that the phase shift is strongly affected by the level of CO2. Fluctuations in CO2 also drive changes in the phase shift during paced breathing [21] and this explains the strong linear relationship found here between ABP–oxyHb and ABP–Vmca during paced breathing. The slope of the linear relation is <1 as the oxyHb and Vmca phase shift have different sensitivities to CO2 [21]. Furthermore, Peng et al. [22] showed that, at rest, the power contained in the CO2 trace at 0·1 Hz is negligible, which explains why we did not find a linear relation between ABP–oxyHb and ABP–Vmca at rest.
Paced breathing induces oscillations in the vascular system by changes in the intrathoracic pressure, and paced breathing at 6 bpm is used to obtain strong regular oscillations for ABP, NIRS variables and Vmca [2,3]. Our phase shift values for ABP–oxyHb and ABP–Vmca using paced breathing at 6 bpm correspond with previous findings of ABP–oxyHb: +20° [3] and for ABP–Vmca: –65° [14]. The phase shift of ABP–Vmca between hemispheres during paced breathing were previously examined in healthy controls in a carotid artery stenosis study [14] and showed a difference of 5·45° (SD 5·71), which is of the same magnitude as our results. Reinhard et al. [7] also investigated differences between spontaneous and paced breathing. The authors concluded that paced breathing was more reproducible, as paced breathing phase shift in Vmca showed a higher correlation between days than spontaneous breathing, which is in contrast to our findings.
Breathing amplitudes and phase probably vary across study days and subjects, which could explain the high variation for paced breathing. The CO2 drive controlling the phase shift during paced breathing should be compensated for [22]. Furthermore, we found a significant decrease in PetCO2 during paced breathing despite attempting to keep the PetCO2 stable. Several healthy volunteers were unable to perform paced breathing for 5 min, which would likely be an even larger problem in patients with cerebrovascular or other diseases.
A previous study investigating age and LFOs in oxyHb showed that LFO amplitude declined with age [23], whereas another group investigating both oxyHb and Vmca found no differences for phase shift related to age [24]. Thomsen et al. [20] reported that Vmca values were higher in women compared to men, and velocity was shown to decrease with age. Based on these finding, it could be suspected that there might be difference in oscillations between both gender and across age groups. However, we found no statistical differences between male and female volunteers or between the four age groups. As our healthy subjects did not undergo further imaging evaluation, we cannot rule out the existence of underlying subclinical cerebrovascular disease, which could have affected our findings. It has been suggested that the age changes may be related to a decline in spontaneous activity in microvascular smooth muscle cells and stiffening of the vessels [23]. However, we did not examine the amplitude directly, but used gain, which is the amplitude ratio between oscillations in oxyHb and Vmca and corresponding oscillations in ABP. ABP oscillations could be affected by similar changes and therefore could leave the gain unaffected with no change with increasing age.
In conclusion, the present study has established the variation between hemispheres and over time for ABP–oxyHb and ABP–Vmca LFO phase shift, and the results indicate that phase shift for ABP–oxyHb in LFOs, as well as ABP–Vmca, can be used as a robust measurement of autoregulation status in patients with localized unilateral cerebrovascular disease. Thus, using bilateral NIRS and TCD technique may be a noninvasive tool to assess which vascular territories are affected by cerebrovascular disease and whether there might be a change over time in cerebral autoregulation. We also found a linear relation between LFO ABP–oxyHb and ABP-Vmca during paced breathings, but not at rest. NIRS and TCD measure different vascular compartments and therefore may provide complimentary information of cerebral autoregulation.
Acknowledgements
This study was supported by the Lundbeck Foundation via the Lundbeck Foundation Center for Neurovascular Signaling (LUCENS).
Footnotes
Author Contributions
Dorte Phillip contributed to design of the study, acquisition of data, analysis of data and writing the draft of the paper. Henrik Schytz contributed to design of the study, analysis/interpretation of data and writing the paper. Juliette Selb contributed to analysis and interpretation of data and writing the paper. Stephen Payne contributed to interpretation of data and writing the paper. Helle K Iversen contributed to design of the study, interpretation of data and writing the paper. Lene T Skovgaard contributed to statistical analysis of data and writing the statistics. David A Boas contributed to analysis and interpretation of data and writing the paper. Messoud Ashina, the study promoter, contributed to design of the study, interpretation of data and writing the paper.
Conflict of interest
There was no conflict of interest in relation to this study
References
- 1.Boas DA, Dale AM, Franceschini MA. Diffuse optical imaging of brain activation: approaches to optimizing image sensitivity, resolution, and accuracy. Neuroimage. 2004;23(Suppl 1):S275–88. doi: 10.1016/j.neuroimage.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 2.Obrig H, Neufang M, Wenzel R, Kohl M, Steinbrink J, Einhaupl K, et al. Spontaneous low frequency oscillations of cerebral hemodynamics and metabolism in human adults. Neuroimage. 2000;12:623–39. doi: 10.1006/nimg.2000.0657. [DOI] [PubMed] [Google Scholar]
- 3.Reinhard M, Wehrle-Wieland E, Grabiak D, Roth M, Guschlbauer B, Timmer J, et al. Oscillatory cerebral hemodynamics–the macro- vs. microvascular level. J Neurol Sci. 2006;250:103–9. doi: 10.1016/j.jns.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 4.Muehlschlegel S, Selb J, Patel M, Diamond SG, Franceschini MA, Sorensen AG, et al. Feasibility of NIRS in the neurointensive care unit: a pilot study in stroke using physiological oscillations. Neurocrit Care. 2009;11:288–95. doi: 10.1007/s12028-009-9254-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reinhard M, Wihler C, Roth M, Harloff A, Niesen WD, Timmer J, et al. Cerebral autoregulation dynamics in acute ischemic stroke after rtPA thrombolysis. Cerebrovasc Dis. 2008;26:147–55. doi: 10.1159/000139662. [DOI] [PubMed] [Google Scholar]
- 6.Haubrich C, Klemm A, Diehl RR, Moller-Hartmann W, Klotzsch C. M-wave analysis and passive tilt in patients with different degrees of carotid artery disease. Acta Neurol Scand. 2004;109:210–6. doi: 10.1034/j.1600-0404.2003.00210.x. [DOI] [PubMed] [Google Scholar]
- 7.Reinhard M, Muller T, Guschlbauer B, Timmer J, Hetzel A. Transfer function analysis for clinical evaluation of dynamic cerebral autoregulation–a comparison between spontaneous and respiratory-induced oscillations. Physiol Meas. 2003;24:27–43. doi: 10.1088/0967-3334/24/1/303. [DOI] [PubMed] [Google Scholar]
- 8.Claassen JA, Diaz-Arrastia R, Martin-Cook K, Levine BD, Zhang R. Altered cerebral hemodynamics in early Alzheimer disease: a pilot study using transcranial Doppler. J Alzheimers Dis. 2009;17:621–9. doi: 10.3233/JAD-2009-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diehl RR, Linden D, Lucke D, Berlit P. Spontaneous blood pressure oscillations and cerebral autoregulation. Clin Auton Res. 1998;8:7–12. doi: 10.1007/BF02267598. [DOI] [PubMed] [Google Scholar]
- 10.Kuo TB, Chern CM, Yang CC, Hsu HY, Wong WJ, Sheng WY, et al. Mechanisms underlying phase lag between systemic arterial blood pressure and cerebral blood flow velocity. Cerebrovasc Dis. 2003;16:402–9. doi: 10.1159/000072564. [DOI] [PubMed] [Google Scholar]
- 11.Reinhard M, Roth M, Guschlbauer B, Harloff A, Timmer J, Czosnyka M, et al. Dynamic cerebral autoregulation in acute ischemic stroke assessed from spontaneous blood pressure fluctuations. Stroke. 2005;36:1684–9. doi: 10.1161/01.STR.0000173183.36331.ee. [DOI] [PubMed] [Google Scholar]
- 12.Dawson SL, Panerai RB, Potter JF. Serial changes in static and dynamic cerebral autoregulation after acute ischaemic stroke. Cerebrovasc Dis. 2003;16:69–75. doi: 10.1159/000070118. [DOI] [PubMed] [Google Scholar]
- 13.Immink RV, van Montfrans GA, Stam J, Karemaker JM, Diamant M, van Lieshout JJ. Dynamic cerebral autoregulation in acute lacunar and middle cerebral artery territory ischemic stroke. Stroke. 2005;36:2595–600. doi: 10.1161/01.STR.0000189624.06836.03. [DOI] [PubMed] [Google Scholar]
- 14.Reinhard M, Hetzel A, Lauk M, Lucking CH. Dynamic cerebral autoregulation testing as a diagnostic tool in patients with carotid artery stenosis. Neurol Res. 2001;23:55–63. doi: 10.1179/016164101101198299. [DOI] [PubMed] [Google Scholar]
- 15.Aaslid R. Transcranial Doppler Sonography. Springer-Verlag; New York: 1986. [Google Scholar]
- 16.Payne SJ, Mohammad J, Tisdall MM, Tachtsidis I. Effects of arterial blood gas levels on cerebral blood flow and oxygen transport. Biomed Opt Express. 2011;2:966–79. doi: 10.1364/BOE.2.000979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tachtsidis I, Elwell CE, Leung TS, Lee CW, Smith M, Delpy DT. Investigation of cerebral haemodynamics by near-infrared spectroscopy in young healthy volunteers reveals posture-dependent spontaneous oscillations. Physiol Meas. 2004;25:437–45. doi: 10.1088/0967-3334/25/2/003. [DOI] [PubMed] [Google Scholar]
- 18.Reinhard M, Roth M, Muller T, Guschlbauer B, Timmer J, Czosnyka M, et al. Effect of carotid endarterectomy or stenting on impairment of dynamic cerebral autoregulation. Stroke. 2004;35:1381–7. doi: 10.1161/01.STR.0000127533.46914.31. [DOI] [PubMed] [Google Scholar]
- 19.Kwan J, Lunt M, Jenkinson D. Assessing dynamic cerebral autoregulation after stroke using a novel technique of combining transcranial Doppler ultrasonography and rhythmic handgrip. Blood Press Monit. 2004;9:3–8. doi: 10.1097/00126097-200402000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Thomsen LL, Iversen HK. Experimental and biological variation of three-dimensional transcranial Doppler measurements. J Appl Physiol. 1993;75:2805–10. doi: 10.1152/jappl.1993.75.6.2805. [DOI] [PubMed] [Google Scholar]
- 21.Payne SJ, Selb J, Boas DA. Effects of autoregulation and CO2 reactivity on cerebral oxygen transport. Ann Biomed Eng. 2009;37:2288–98. doi: 10.1007/s10439-009-9763-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peng T, Rowley AB, Ainslie PN, Poulin MJ, Payne SJ. Multivariate system identification for cerebral autoregulation. Ann Biomed Eng. 2008;36:308–20. doi: 10.1007/s10439-007-9412-9. [DOI] [PubMed] [Google Scholar]
- 23.Schroeter ML, Schmiedel O, von Cramon DY. Spontaneous low-frequency oscillations decline in the aging brain. J Cereb Blood Flow Metab. 2004;24:1183–91. doi: 10.1097/01.WCB.0000135231.90164.40. [DOI] [PubMed] [Google Scholar]
- 24.Peng T, Ainslie PN, Cotter JD, Murrell C, Thomas K, Williams MJ, et al. The effects of age on the spontaneous low-frequency oscillations in cerebral and systemic cardiovascular dynamics. Physiol Meas. 2008;29:1055–69. doi: 10.1088/0967-3334/29/9/005. [DOI] [PubMed] [Google Scholar]