Abstract
Background & Aims
Hepatorenal syndrome (HRS) type 1 is a progressive functional renal failure in subjects with advanced liver disease. The aim of this study was to evaluate the efficacy and safety of terlipressin, a systemic arterial vasoconstrictor, for cirrhosis type 1 HRS.
Methods
A prospective, randomized, double-blind, placebo-controlled clinical trial of terlipressin was performed. Subjects with type 1 HRS were randomized to terlipressin (1 mg intravenously every 6 hours) or placebo plus albumin in both groups. The dose was doubled on day 4 if the serum creatinine (SCr) level did not decrease by 30% of baseline. Treatment was continued to day 14 unless treatment success, death, dialysis, or transplantation occurred. Treatment success was defined by a decrease in SCr level to ≤1.5 mg/dL for at least 48 hours by day 14 without dialysis, death, or relapse of HRS type 1.
Results
Fifty-six subjects were randomized to each arm. Treatment success with terlipressin was double that with placebo (25% vs 12.5%, P = .093). SCr level improved from baseline to day 14 on terlipressin (−0.7 mg/dL) as compared with placebo (0 mg/dL), P < .009. Terlipressin was superior to placebo for HRS reversal (34% vs 13%, P= .008), defined by decrease in SCr level ≤1.5 mg/dL. Overall and transplantation-free survival was similar between study groups; HRS reversal significantly improved survival at day 180. One nonfatal myocardial infarction occurred with terlipressin, but the total adverse event rate was similar to placebo.
Conclusions
Terlipressin is an effective treatment to improve renal function in HRS type 1.
Hepatorenal syndrome (HRS) type 1 is a functional and potentially reversible form of progressive renal failure that occurs in advanced cirrhosis and is associated with a high mortality.1,2 The main pathophysiologic basis for the progression from cirrhosis with diuretic-sensitive ascites to diuretic-refractory ascites and eventually to HRS is progressive systemic arterial vasodilation.3 Arterial vasodilation leads to a decrease in effective arterial blood volume, which in turn activates renal sodium-retentive mechanisms and leads to renal vasoconstriction. In the face of effective hypovolemia, renal perfusion is maintained by autoregulatory mechanisms in the kidney.4,5 When these mechanisms are overwhelmed by the severity of effective hypovolemia, the glomerular filtration rate declines and renal failure ensues.6–8
Appreciation of the central role of arterial vasodilation in the pathogenesis of HRS has led to the use of arterial vasoconstrictors for the treatment of type 1 HRS. A variety of vasoconstrictors including terlipressin, ornipressin, midodrine plus octreotide, and norepinephrine have been used in uncontrolled trials9,10; of these, terlipressin, a 12-amino acid synthetic analogue of lysine-vasopressin,11 is the most studied.12–17 In 2 small, randomized, single-blind pilot studies with a brief period of follow-up, terlipressin plus albumin was shown to improve serum creatinine (SCr) more effectively as compared with placebo plus albumin.18,19 In a meta-analysis of the latter and other uncontrolled trials, terlipressin was reported to reverse HRS type 1 in up to 50% of subjects.20
We report the results of an international, randomized, double-blind, placebo-controlled, multicenter phase III trial of terlipressin for the treatment of HRS type 1. The specific aim was to define the efficacy and safety of terlipressin vs placebo in addition to supportive medical care including intravenous albumin administration in type 1 HRS.
Materials and Methods
Patients
Adult subjects (≥18 years of age) with acute or chronic liver disease and HRS type 1, as defined by the International Ascites Club criteria (rapidly progressive reduction in renal function, eg, a doubling of SCr to ≥2.5 mg/dL in less than 2 weeks and failure of renal function to improve following diuretic withdrawal and plasma volume expansion21), were included in this trial. Exclusion criteria included evidence of obstructive or parenchymal renal disease (eg, acute tubular necrosis, glomerular diseases, interstitial nephritis, and urinary obstruction), factors that would affect renal function independent of HRS (use of nephrotoxic drugs, shock, uncontrolled bacterial infection, uncorrected fluid losses, acute liver disease because of factors known to also be nephrotoxic), and the presence of severe cardiovascular disease as determined by the clinical judgment of individual investigators.
Study Design
The study was a prospective, randomized, double-blind, placebo-controlled, multicenter clinical trial conducted at 35 medical centers across the United States (n = 30), Germany (n = 2), and Russia (n = 3) from June 2004 through September 2006. The study was registered in the national clinical trials database (ClinicalTrials.gov identifier NCT00089570) and approved by the institutional review boards at each center. All patients or their legally authorized representatives gave written informed consent for participation. An independent data safety monitoring board (DSMB) was established to review safety data and make recommendations about continuing the trial. As defined in the DSMB charter, an interim semi-blinded safety analysis was performed by an independent statistician at approximately 50% enrollment. The DSMB reviewed these data and concluded that the study should proceed as planned. The DSMB also received blinded quarterly reports to monitor the safety of the trial. All data were recorded on standardized case report forms that were entered into a database at a data coordinating center. The study sites were regularly monitored to ensure that procedures were being followed and that data were accurate and consistent.
Subjects with acute or chronic liver disease and acute worsening of renal function were initially screened for the study. During this period, blood and urine cultures, urinalysis, renal imaging studies, and a fluid challenge (typically 1.5 L of normal saline and/or albumin) were performed to confirm the presence of HRS type 1 and rule out exclusionary factors. Once eligibility was confirmed, subjects were randomized through an interactive voice response system and computer-generated randomization scheme to receive either terlipressin or placebo in a 1:1 ratio, with stratification for the presence of alcoholic hepatitis.
Following randomization, patients received blinded study medication, either terlipressin at a dose of 1 mg administered by slow intravenous (IV) push every 6 hours or matching placebo. It was recommended that patients receive concomitant IV albumin (100 g on day 1 and 25 g daily until end of treatment) as per standard medical practice.14,15,22 If after 3 days of therapy, SCr level had not decreased by at least 30% from the baseline value, the dose of the study drug (terlipressin or placebo) was increased to 2 mg every 6 hours. Concomitant use of the following medications was prohibited during the period of study drug administration: vasoactive drugs (such as dopamine or noradrenaline), prostaglandin analogues and nonsteroidal anti-inflammatory drugs. Otherwise, patients were to receive treatment in accordance with standard medical practice at the institution. Serum electrolytes, blood urea nitrogen, and creatinine were evaluated daily during treatment.
Patients could receive study drug for a maximum of 14 days but were to be discontinued from the study earlier for treatment failure (see definitions below) or liver transplantation. Patients could also be withdrawn for an adverse event, withdrawal of consent, or physician decision/administrative reason. Patients who achieved treatment success could be discontinued or continue on therapy at the investigator’s discretion until the maximum of 14 days. If judged by the investigator to be potentially beneficial, patients who demonstrated at least a partial response during the initial 14-day treatment course and then developed recurrence of HRS type 1 during the follow-up period were eligible to be retreated with the initially assigned study drug for up to an additional 14 days.
Study End Points
The primary end point was incidence of treatment success at day 14 defined as the percentage of patients with resolution of HRS, as documented by SCr level ≤1.5 mg/dL on 2 occasions at least 48 hours apart, without dialysis, death, or recurrence of HRS type 1 on or prior to day 14. The treatment success end point was developed by the study investigators as a more stringent version of the traditional end point of “HRS reversal,” which is defined as a decrease in SCr level to ≤1.5 mg/dL during treatment without dialysis.14–17,23–25 An analysis of HRS reversal was also planned to provide a comparison between the current study and data published in the literature.
The secondary end points included change in SCr level from baseline to day 14, incidence of treatment failure at day 14 (defined as SCr level ≥baseline value after day 7, dialysis, or death), combined incidence of treatment success and partial response (ie, SCr level decreased by >50% from baseline but not ≤1.5 mg/dL, without dialysis or recurrence of HRS); transplant-free survival at day 60, and overall survival at day 60. Other end points included overall and transplant-free survival at day 180, Model for End-Stage Liver Disease (MELD) score, encephalopathy (West Haven Criteria), vital signs, laboratory results, and adverse events.
Statistical Analysis
All efficacy analyses, excluding overall and transplant-free survival, were based on both the modified intention-to-treat (MITT) population (defined as patients who did not receive a liver transplant on or prior to day 14) and the intention-to-treat (ITT) population (all randomized patients). The MITT population was utilized in particular for the analysis of treatment success at day 14 to avoid the bias introduced by liver transplantation, which could not be predicted at the time of randomization. Analyses of overall survival and transplant-free survival were based primarily on the ITT population and secondarily on the MITT population. Of the 112 randomized patients, 92 (82%) did not receive a liver transplant within the 14-day treatment period and comprised the MITT population. To report the most complete data, all results presented are for the ITT population; results were similar for the MITT population.
Treatment success at day 14 was analyzed using a Cochran-Mantel-Haenszel (CMH) χ2 test adjusted for baseline strata (alcoholic hepatitis present or not). Incidence of treatment failure and combined incidence of partial response and treatment success were analyzed similarly. Change from baseline in SCr level was analyzed using a repeated measures analysis based on the mixed-effect model using the Observed Case method and modeling the interaction between treatment and day.26 Transplant-free survival and overall survival were analyzed using a 2-sample log-rank test adjusted for baseline strata (alcoholic hepatitis present or not). A summary of product limit estimates of the survival distribution was also provided by treatment group, as estimated in Proc Lifetest of SAS (SAS Institute, Inc, Cary, NC). A univariate logistic regression analysis was conducted to determine baseline patient characteristics that were predictive of HRS reversal and overall survival up to day 60. The following factors were analyzed: age group (<65, ≥65 years), gender, race group (nonwhite, white), alcoholic hepatitis (present or not), baseline MELD score, baseline Child–Pugh score, and baseline SCr level. The frequency of adverse events and other safety data was summarized descriptively. SAS software version 8.2 was used to perform all statistical analyses and to prepare summary tables and data listings.
Sample Size Calculations
Sample size calculations were based on enrolling 90 patients in the MITT population (no liver transplantation within 14 days). This study was designed with a type I error of 0.05 and a power of 95% to detect a 30% difference in the primary efficacy end point (treatment success rate at day 14) between terlipressin and placebo. The estimated rate of treatment success at day 14 for the terlipressin group was based on largely uncontrolled studies published in the literature, which reported HRS reversal in approximately 50%– 60% of patients treated with terlipressin and albumin.14–17,23–25 Considering the more rigorous definition of treatment success used in this study (ie, SCr level ≤1.5 mg/dL on 2 occasions at least 48 hours apart, without dialysis, death, or recurrence of HRS within the 14 day treatment period), it was projected that 35% and 5% of patients in the terlipressin plus albumin and placebo plus albumin groups, respectively, would achieve the primary end point.
Role of Funding Source
The study was supported in part by a grant from the Food and Drug Administration (FDA) (Office of Orphan Products Grant 1R01FD003024-01) and in part by Orphan Therapeutics. Orphan Therapeutics worked with the OT-0401 Executive and Steering Committees (membership listed in the Appendix) in the design and conduct of the study and in the interpretation of the data and writing of the report. The FDA Office of Orphan Products reviewed the study protocol during the grant application process and monitored progress of the study; comments and guidance were provided to the OT-0401 Executive Committee.
Results
A total of 112 patients were enrolled at 35 sites (range, 1–9 patients per site); 56 patients were randomized to receive terlipressin and 56 to receive placebo. Four subjects (2 in each arm) had a SCr level <2.5 mg/dL prior to randomization; of these, both patients randomized to terlipressin, and 1 patient randomized to placebo had experienced doubling of their SCr level within the previous 2 weeks. As shown in Table 1, baseline data were similar between the 2 groups (all variables not significant). Approximately one third of the subjects (36%) in each arm had underlying alcoholic hepatitis. Baseline SCr level and MELD scores were similar between groups; however, there was an imbalance in the number of patients with a baseline SCr level >7.0 mg/dL randomized to the terlipressin group (6 in terlipressin vs none in placebo). Equal numbers of patients (88%) in both groups received intravenous albumin in addition to their randomized study drug. The mean daily albumin doses were similar between treatment groups (48.2 g/day in the terlipressin group vs 45.8 g/day in the placebo group).
Table 1.
Summary of Key Demographic and Baseline Characteristics
| Demographic measures | Terlipressin n = 56 | Placebo n = 56 |
|---|---|---|
| Age, mean, y (SD) | 50.6 (10.5) | 52.9 (11.4) |
| Sex, n (%) | 41 (73.2) Male | 39 (69.6) Male |
| Ethnic origin, n (%) | 51 (91.1) White | 49 (87.5) White |
| Child–Pugh score, mean (SD) | 11.7 (1.9) | 11.2 (1.8) |
| MELD score, mean (SD) | 33.4 (6.0) | 33.4 (6.3) |
| Serum creatinine, mean, mg/dL (SD) | 3.96 (2.19) | 3.85 (1.17) |
| Serum sodium, mean, mmol/L (SD) | 130.6 (6.9) | 132.4 (7.0) |
| Albumin, mean, g/dL (SD) | 2.6 (0.84) | 2.9 (0.79) |
| Bilirubin, mean, mg/dL (SD) | 15.0 (13.6) | 15.8 (15.1) |
| INR, mean (SD) | 2.3 (0.8) | 2.3 (1.1) |
| AST, mean, U/L (SD) | 104.8 (111.9) | 117.3 (143.2) |
| ALT, mean, U/L (SD) | 57.6 (76.4) | 63.1 (91.3) |
| Alcoholic hepatitis, n (%) | 20 (35.7) | 20 (35.7) |
| Factors associated with HRS development, n (%) | 30 (53.6) | 31 (55.4) |
| Infection | 14 (25.0) | 9 (16.1) |
| Gastrointestinal bleeding | 2 (3.6) | 3 (5.4) |
| Prior diuretic treatment | 12 (21.4) | 15 (26.8) |
| Large volume paracentesis | 5 (8.9) | 4 (7.1) |
| Cirrhosis, n (%) | 51 (91.1) | 51 (91.1) |
| Etiology of liver disease, n (%)a | ||
| Alcohol | 29 (51.8) | 29 (51.8) |
| Hepatitis C | 22 (39.3) | 19 (33.9) |
| Hepatitis B | 4 (7.1) | 1 (1.8) |
| Primary biliary cirrhosis | 2 (3.6) | 1 (1.8) |
| Hepatocellular carcinoma | 4 (7.1) | 6 (10.7) |
| Nonalcoholic steatohepatitis | 2 (3.6) | 5 (8.9) |
| History of esophageal variceal hemorrhage, n (%) | 27 (48.2) | 27 (48.2) |
| Ascites, n (%) | 54 (96.4) | 54 (96.4) |
| Diabetics, n (%) | 13 (23.2) | 13 (23.2) |
| Mean arterial pressure, mean, mm Hg (SD) | 75.5 (11.4) | 77.2 (13.6) |
| Prior octreotide, n (%) | 10 (17.9) | 11 (19.6) |
| Prior midodrine, n (%) | 11 (19.6) | 12 (21.4) |
INR, International normalized ratio; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Includes patients with more than 1 etiology of liver disease.
The mean duration of treatment was 6.3 days in the terlipressin group and 5.8 days in the placebo group. A significantly greater number of subjects receiving placebo required dose escalation because of failure to achieve a decrease in SCr level by at least 30% by day 4 (13 terlipressin vs 23 placebo patients, P = .037). Approximately one third of the subjects received less than 3 days of therapy because of lack of response/dialysis, choice of palliative care, death, transplantation, early termination because of adverse events, or other reasons, including withdrawal of consent and administrative reasons. Two patients (1 terlipressin-treated patient and 1 placebo-treated patient) experienced a relapse of HRS type 1 after an initial response and were retreated with the originally assigned blinded study drug as defined in the protocol.
Changes in Renal Function
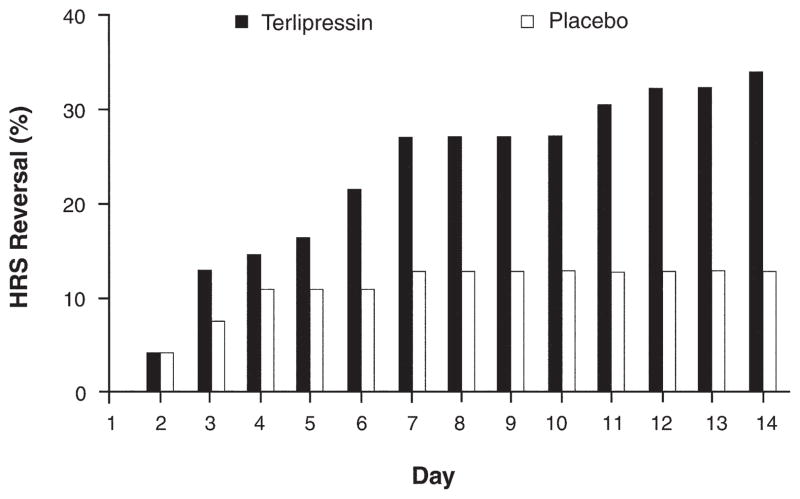
As shown in Table 2, the incidence of treatment success at day 14 in the terlipressin group was double that of placebo, but this difference only trended toward statistical significance (14/56, 25% vs 7/56, 12.5%, respectively, P = .093). The results were similar when including a surviving patient who was missing a day 14 SCr value (15/56, 26.8%, for terlipressin and 7/56, 12.5%, for placebo, P = .059). However, the results for HRS reversal, defined by a decrease in SCr level to ≤1.5 mg/dL, were significantly different between treatment groups (19/56, 33.9% for terlipressin and 7/56, 12.5% for placebo, P = .008) (Table 2). Figure 1 presents the cumulative incidence of HRS reversal. In the placebo/albumin arm, the benefit of therapy was seen only within the first 96 hours. In contrast, in the terlipressin group, the incidence of HRS reversal continued to increase throughout the 14 days of therapy.
Table 2.
Treatment Outcomes
| End point | Terlipressin n (%) | Placebo n (%) | P value |
|---|---|---|---|
| All patients | (n = 56) | (n = 56) | |
| Treatment success at day 14 | 14 (25.0) | 7 (12.5) | .093 |
| HRS reversal | 19 (33.9) | 7 (12.5) | .008 |
| Patients who received >3 days of treatment | (n = 36) | (n = 39) | |
| Treatment success at day 14 | 14 (38.9) | 7 (17.9) | .046 |
| HRS reversal | 19 (52.8) | 7 (17.9) | .002 |
Figure 1.
Cumulative incidence of HRS reversal by day. Treatment was begun on day 1.
Published literature indicates that at least 3 days of treatment with terlipressin is needed to improve renal function.14,16,27 Similarly in this trial, treatment success or HRS reversal was only observed in patients treated for more than 3 days, with significantly more terlipressin patients vs placebo patients achieving these end points (Table 2).
Two patients who responded to study treatment experienced a relapse of HRS type 1 (1/19, 5.3%, for terlipressin and 1/7, 14.3%, for placebo); both patients achieved a second treatment response when retreated with the originally assigned blinded study drug. The response in these 2 patients and in all others who achieved HRS reversal was durable, with no further patients relapsing during the follow-up period of either 30 (0/19) or 60 (0/12) days of observation.
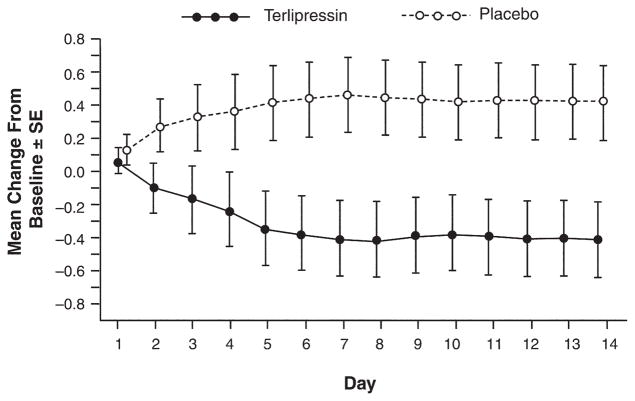
With regard to the secondary end point of change from baseline in SCr level to day 14, the terlipressin group experienced a highly significant decrease relative to placebo (−0.7 mg/dL with terlipressin vs 0 mg/dL with placebo, P = .009). Figure 2 depicts the change from baseline to the end of treatment using the last observation carried forward method to demonstrate the effect of treatment for the entire population. A greater number of terlipressin patients experienced combined partial response or treatment success as compared with placebo (16/56, 29% vs 10/56, 18%, respectively; P = .181). Conversely, a greater number of placebo patients experienced treatment failure as compared with terlipressin (37/56, 66% vs 31/56, 55%, respectively; P = .247). Consistent with an improvement in renal function in the terlipressin group, significant differences in MELD score (−2.5, P = .003) and mean arterial pressure (6.2 mm Hg, P = .017) from baseline were observed as compared with the placebo group. On average, a temporary increase in mean arterial pressure (mean, 3.4 mm Hg) occurred following each dose of terlipressin; this acute increase did not correlate with outcome. However, the overall increase in mean arterial pressure from baseline to end of treatment was correlated to response, ie, terlipressin responders experienced a greater increase in mean arterial pressure than nonresponders (7.3 vs −1.02 mm Hg, respectively, P = .025).
Figure 2.
Mean change from baseline in SCr (mg/dL) to end of treatment. Data include all patients in the ITT population at each time point. The mean change from baseline values represents the change from baseline to end of treatment using the last observation carried forward in the ITT population.
Survival
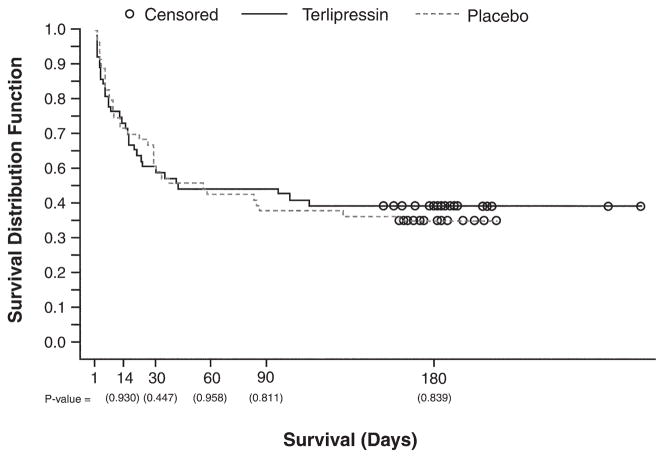
Overall survival at day 180, as shown in Figure 3, was 42.9% (n = 24/56) vs 37.5% (n = 21/56) for terlipressin and placebo, respectively (P = .839). Transplant-free survival up to day 180 also was similar in both groups. For patients who did not undergo liver transplantation, 7 patients (13%) in the terlipressin group and 5 patients (9%) in the placebo group survived to day 180. The causes of death for the 32 terlipressin patients and 35 placebo patients who died up to day 180 were hepatic failure/cirrhosis (15 terlipressin vs 15 placebo), HRS/renal failure (3 terlipressin vs 10 placebo), respiratory disorder (5 terlipressin vs 4 placebo), multiorgan failure (6 terlipressin vs 2 placebo), infections/systemic inflammatory response syndrome (SIRS) (7 terlipressin vs 2 placebo), gastrointestinal hemorrhage (0 terlipressin vs 3 placebo), cardiac event (0 terlipressin vs 2 placebo), and unspecified (1 in each group).
Figure 3.
Kaplan–Meier plot of overall survival up to day 180. Observations were censored at the last time a patient was known to be alive (represented by open circles).
Impact of Renal and Liver Function on Survival
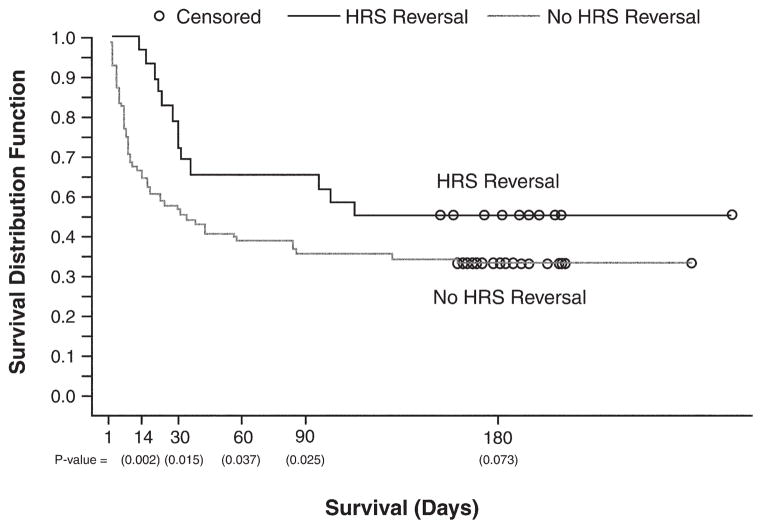
An analysis of the effect of overall survival as a function of HRS reversal was performed. Figure 4 presents a Kaplan–Meier plot of survival for HRS reversal responders vs nonresponders; there is a clear separation in the survival distribution, with patients achieving HRS reversal, irrespective of treatment, exhibiting longer survival through day 180.
Figure 4.
Overall survival for HRS reversal vs no HRS reversal. Observations were censored at the last time a patient was known to be alive (represented by open circles). All patients are included irrespective of treatment.
Baseline SCr concentration and baseline MELD score were found to be significant predictors of HRS reversal for the ITT population (P = .021 and P = .017, respectively) by univariate logistic regression analysis. Baseline characteristics predictive for overall survival up to day 60 were alcoholic hepatitis, MELD score, Child–Pugh score, and SCr level (P = .001, P = .015, and P < .001, respectively).
Adverse Events
Table 3 provides an overview of the safety profile for patients treated with terlipressin or placebo. Overall, the incidence of adverse events and serious adverse events were similar between treatment groups. Serious adverse events were considered to be related to study treatment in 5 (9%) terlipressin patients and 1 (2%) placebo patient. These related serious adverse events were (terlipressin patients unless otherwise noted) nonfatal myocardial infarction (n = 1), nonsustained supraventricular tachycardia (n = 1), respiratory distress (n = 2), respiratory acidosis (n = 1), arrhythmia (n = 1, placebo), atrial fibrillation (n = 1), and livedo reticularis (n = 1). Three terlipressin patients were withdrawn by the investigator because of related adverse events (nonfatal myocardial infarction, livedo reticularis, and cyanosis of the fingers), and 2 placebo patients were withdrawn because of unrelated adverse events (hypotension and respiratory failure). The mean encephalopathy score did not increase in either treatment group, and there were no significant differences between treatment groups during treatment or on day 14. There were no significant changes from baseline during treatment in international normalized ratio, bilirubin, alkaline phosphatase, aspartate amino-transferase, and alanine aminotransferase in either treatment group.
Table 3.
Overview of Safety Data
| Safety parameter (number of patients with AE) | Terlipressin (n = 56) n (%) | Placebo (n = 55) n (%) |
|---|---|---|
| AEs up to 7 days posttreatment | ||
| All | 52 (92.9) | 49 (89.1) |
| Related | 18 (32.1) | 12 (21.8) |
| Serious AEs up to 30 days posttreatment | ||
| All | 37 (66.1) | 36 (65.5) |
| Related | 5 (8.9) | 1 (1.8) |
| Deaths up to 30 days posttreatment | ||
| All | 27 (48.2) | 27 (49.1) |
| Related | 0 (0.0) | 0 (0.0) |
| Withdrawals because of AEs up to 7 days | ||
| All | 3 (5.4) | 2 (3.6) |
| Related | 3 (5.4) | 0 (0.0) |
AEs, adverse events.
Discussion
HRS type 1 has traditionally been considered to be a harbinger of death and liver transplantation the only viable hope for survival for afflicted individuals.28,29 However, not all subjects are candidates for liver transplantation, and liver transplantation is not universally available. In those who are transplant candidates, a suitable organ often cannot be found in time, and, even when an organ is available, the presence of uncorrected HRS type 1 worsens the outcomes of liver transplantation.24 These data underscore the need for effective medical therapy to reverse HRS type 1. Although many previous studies attempted unsuccessfully to reverse HRS type 1 with renal vasodilators, the focus of therapy has shifted in recent years to the use of systemic vasoconstrictors based on the realization that the principal driver of the pathogenesis of HRS type 1 is progressive systemic arterial vasodilation.30–32 The current phase III randomized clinical trial confirms the findings of previous uncontrolled and small controlled pilot studies that terlipressin, a systemic vasoconstrictor, improves renal function in HRS type 1.17–19
In this study, patients who received terlipressin had significant improvements in renal function relative to controls, although the difference between the treated (25%) and control groups (12.5%) did not reach statistical significance when the primary end point of treatment success at day 14 was used. The end point routinely applied in the literature, HRS reversal (SCr level ≤1.5 mg/dL),14–17,23–25 was, however, achieved in significantly more patients receiving terlipressin (33.9%) as compared with controls (12.5%). The response to treatment was also durable, with a HRS type 1 relapse rate of 5.3% among responders to terlipressin vs 14.3% for those who responded to placebo. Interestingly, in the 2 subjects who relapsed, retreatment with either terlipressin or placebo produced a durable improvement in renal function.
The lack of statistical significance for the newly designed treatment success end point appears to be due to a combination of (1) use of a stricter end point than HRS reversal, (2) a higher than expected placebo response, and (3) failure of all patients to receive the drug for an adequate length of time. Previous reports have suggested that improvements in renal function are only seen when terlipressin is administered for more than 3 days.14,16 In the current study, when patients who received treatment for more than 3 days were analyzed, the primary end point (terlipressin vs placebo: 38.9% vs 17.9%, respectively, P < .04) and HRS reversal (52.5% vs 17.9%, respectively, P < .002) were both achieved more frequently with terlipressin (Table 2). These results are similar to the HRS reversal data obtained in a recent meta-analysis.20
The results were also impacted by an imbalance in the number of patients with a baseline SCr level of >7.0 mg/dL (6 in the terlipressin arm vs zero in the control group). None of these patients responded to study treatment or survived (mean survival of 3 days), suggesting that after a certain point the renal failure is not reversible and that patients should be treated early in the course of HRS. Univariate logistic regression analysis clearly identified baseline SCr concentration as a significant predictor of HRS reversal for the ITT population.
Overall survival in both treatment groups was higher than in previously reported studies, primarily because of the fact that more than 30% of patients underwent liver transplantation. There were no significant differences in survival between those receiving terlipressin or placebo. As noted by previous investigators, terlipressin does not affect the underlying severe liver disease and therefore was not expected to have a major effect on survival.33 Consequently, the study was not designed to test for such effects. The chance allocation to the terlipressin group of all subjects with the most severe renal failure (SCr level >7 mg/dL), each of whom failed to respond and died, may have masked any potential benefits of terlipressin on survival. Analysis of those with a SCr level <7 mg/dL at baseline revealed a modest trend for improved survival for subjects on terlipressin (48% vs 37%, terlipressin vs placebo) by day 180. One must, however, be cautious about interpreting such post hoc analyses. At the same time, it is worth noting that it would take approximately 800 subjects to demonstrate such a difference with 80% power. Given the level of sickness of this population and the relative rarity of the condition, it is unlikely such a study will be undertaken.
Despite the failure to detect any differences in overall survival, a striking and significant improvement in both overall and transplant-free survival was noted in those who experienced HRS reversal regardless of treatment (Figure 4). These data confirm that improvement in renal function improves survival in subjects with HRS type 1.1,24 Improvement in renal function is also known to improve transplant outcomes in this population.24 Terlipressin was significantly superior to placebo in improving renal function and may thus be useful in the treatment of HRS type 1 because of its beneficial effects on renal function.
Patients in both arms of this trial also received albumin for plasma volume expansion, in accordance with current medical practice and to maximize the effect of terlipressin.14,15 When this study was designed, no controlled data on the efficacy of albumin alone or its optimal dose were available. The size of the albumin effect was estimated to be 5% based on an approximation from historic uncontrolled data. Subsequent to study initiation, 5 of 13 patients with HRS type 1 had an improvement in renal function with albumin and furosemide in a small uncontrolled trial.34 Intravenous albumin has been shown to be associated with increased vascular resistance in cirrhotic patients with ascites.35,36 These data, together with those from this trial, indicate that some patients may experience HRS reversal with albumin alone. When present, this effect is invariably obvious within 48 –72 hours. However, the probability of HRS reversal is significantly further enhanced by the concomitant use of terlipressin.
Overall, the incidence of adverse events and serious adverse events was similar between treatment groups. There was 1 case of nonfatal myocardial infarction in a patient with preexisting cardiovascular disease who received the higher terlipressin dose (2 mg every 6 hours). However, 3 other patients in the terlipressin group with known preexisting coronary artery disease did not experience any cardiac ischemia or other cardiac adverse event during treatment with low-dose terlipressin (1 mg every 6 hours). Terlipressin should be used with caution in patients with severe cardiovascular disease, and higher terlipressin doses are not recommended. The rate of adverse events leading to withdrawal in this study (5%) is consistent with findings in the literature and is reported to be lower with terlipressin as compared with vasopressin and other vasopressin analogues.37–39
In summary, terlipressin plus albumin is superior to placebo for reversal of type 1 HRS and produces a significant decrease in SCr compared with placebo and albumin. Administration of albumin by itself also had a modest salutary effect and reversed HRS in 13% of cases. Reversal of HRS using either terlipressin or placebo was associated with an improvement in both overall and transplant-free survival. Terlipressin is an effective treatment to improve renal function in HRS type 1.
Acknowledgments
Supported in part by a grant from the FDA (Office of Orphan Products Grant 1R01FD003024-01) and in part by Orphan Therapeutics.
The authors thank Vicente Arroyo, MD, for assistance with study design. The assistance of Prof. Dr. Klaus-D. Doehler with study design, trial conduct, and site monitoring in Germany is also gratefully acknowledged.
Abbreviations used in this paper
- HRS
hepatorenal syndrome
- MELD
Model for End-Stage Liver Disease
- MITT
modified intention-to-treat
- SCr
serum creatinine
Appendix
OT-0401 Executive Committee members: Arun Sanyal, MD, Thomas Boyer, MD, and Peter Teuber, PhD.
Steering Committee: Arun Sanyal, MD (principal investigator), Thomas Boyer, MD, Guadalupe Garcia-Tsao, MD, Frederic Regenstein, MD, Lorenzo Rossaro, MD, and Peter Teuber, PhD.
Data and Safety Monitoring Board: John Michael Henderson, MD, (chairman), Michael Lincoff, MD, Anthony Tavill, MD, Vincent Davis, MD, and Dan Anbar, PhD.
Twenty-four hour investigator hotline: Arun Sanyal, MD, and Velimir A Luketic, MD.
Drug Safety and Regulatory Affairs: Elizabeth Diaz, MD, Candice Teuber PharmD (Orphan Therapeutics LLC).
Statistics: Dror Rom, PhD (Prosoft Software).
Clinical Monitoring and Data Management: Peter Fratarcangelo, William Tobin, Susan Clement, Kevin Bickford, Marcie Shore (International Healthcare, LLC).
OT-0401 Investigators and Study Sites: Listed are site principal investigators.
United States (87 patients): St. Luke’s Texas Liver Institute, Houston, TX: V. Ankoma-Sey, S. Pappas; Albert Einstein Medical Center, Philadelphia, PA: V. Araya; Cleveland Clinic, Cleveland, OH: D. Barnes; Northwestern Memorial Hospital, Chicago, IL: A. Blei; University of Arizona, Tucson, AZ: T. Boyer; Washington University, St. Louis, MO: J. Crippin; University of Colorado Hospital, Denver, CO: G. Everson; California Pacific Medical Center, San Francisco, CA: T. Frederick; Yale University School of Medicine, New Haven, CT: G. Garcia-Tsao; Lahey Clinic Medical Center, Burlington, MA: F. Gordon; Mayo Clinic Transplant Center, Jacksonville, FL: A. Keaveny; University of Washington Medical Center, Seattle, WA: K, Kowdley; San Diego VA, San Diego, CA: D. Kravetz; UT Southwestern, Dallas, TX: M. Mayo; University of Nebraska, Omaha, NE: T. McCashland; University of Pennsylvania Hospital - Div. of GI, Philadelphia, PA: K. R. Reddy; Tulane University Health Sciences Center, New Orleans, LA: F. Regenstein; Medical University of South Carolina, Charleston, SC: A. Reuben; University of California Davis Medical Center, Sacramento, CA: L. Rossaro; UT Memphis, Memphis, TN: M. Sachdev; University Hospital-UMDNJ, Newark, NJ: A. Samanta; Medical College of Virginia, Richmond, VA: A. Sanyal, V. Luketic; Mount Sinai Medical Center, New York, NY: T. Schiano; Georgetown, Washington, DC: K. Shetty; Weill Cornell Medical Center & Columbia, New York, NY: S. Sigal; Johns Hopkins Hospital, Baltimore, MD: P. Thuluvath; Mayo Clinic, Scottsdale, Phoenix, AZ: H. Vargas; N. Carolina Memorial Hospital/UNC Hospital, Chapel Hill, NC: S. Zacks, R. Shrestha; Oregon Health and Science University, Portland, OR: A. Zaman.
Russia (13 patients): Russian People’s Friendship University, Moscow: V. Dvornikov; Centre of Applied Pharmacology, Moscow: V. Moiseev; Russian State Medical University, Moscow: G. Storozhakov.
Germany (12 patients): University Clinic Munich, Munich: V. Gulberg; University Clinic Bonn, Bonn: T. Sauer-bruch/B. Appenrodt.
Footnotes
This is original work and is not under consideration for publication elsewhere. This work has been presented in part at the annual meeting of the American Association for the Study of Liver Diseases (AASLD) in Boston in November 2006. The study was registered at www.clinicaltrials.gov (ClinicalTrials.gov identifier NCT00089570).
Conflicts of interest and financial disclosures: Arun J. Sanyal, MBBS, MD: Stipends paid by Orphan Therapeutics to maintain the 24-hour study Medical Hotline (June 2004, $12,000; May 2005, $12,000). Reimbursement for travel costs and data reviews for FDA meetings (2006, $501.90; April 2007, $251.67). Thomas Boyer, MD: Reimbursement for travel costs and data reviews for FDA meetings (February 2004, $1157.90; June 2006, $377.70; October 2006, $699.20; March 2007, $389.60). Honorarium for OT-0401 Steering Committee in 2004 ($1350.00). Guadalupe Garcia-Tsao, MD: Honorarium for OT-0401 Steering Committee in 2004 ($750.00). Frederick Regenstein, MD: Honorarium for OT-0401 Steering Committee in 2004 ($750.00). Lorenzo Rossaro, MD: Honorarium for OT-0401 Steering Committee in 2004 ($750.00). Andres Blei, MD: None. Veit Gülberg, MD: None. Beate Appenrodt, MD: None. Samuel Sigal, MD: None. Peter Teuber, PhD: President and Managing Partner of Orphan Therapeutics, LLC (OT-0401 study cosponsor).
References
- 1.Alessandria C, Ozdogan O, Guevara M, et al. MELD score and clinical type predict prognosis in hepatorenal syndrome: relevance to liver transplantation. Hepatology. 2005;41:1282–1289. doi: 10.1002/hep.20687. [DOI] [PubMed] [Google Scholar]
- 2.Gines A, Escorsell A, Gines P, et al. Incidence, predictive factors, and prognosis of the hepatorenal syndrome in cirrhosis with ascites. Gastroenterology. 1993;105:229–236. doi: 10.1016/0016-5085(93)90031-7. [DOI] [PubMed] [Google Scholar]
- 3.Arroyo V, Terra C, Gines P. Advances in the pathogenesis and treatment of type-1 and type-2 hepatorenal syndrome. J Hepatol. 2007;46:935–946. doi: 10.1016/j.jhep.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Rimola A, Gines P, Arroyo V, et al. Urinary excretion of 6-keto-prostaglandin F1 α, thromboxane B2 and prostaglandin E2 in cirrhosis with ascites. Relationship to functional renal failure (hepatorenal syndrome) J Hepatol. 1986;3:111–117. doi: 10.1016/s0168-8278(86)80154-4. [DOI] [PubMed] [Google Scholar]
- 5.Moller S, Abrahamsen J, Ring-Larsen H, et al. The hepatorenal syndrome. Ugeskrift for laeger. 1995;157:3185–3189. [PubMed] [Google Scholar]
- 6.Martin PY, Gines P, Schrier RW. Nitric oxide as a mediator of hemodynamic abnormalities and sodium and water retention in cirrhosis. N Engl J Med. 1998;339:533–541. doi: 10.1056/NEJM199808203390807. [DOI] [PubMed] [Google Scholar]
- 7.Dagher L, Moore K. The hepatorenal syndrome. Gut. 2001;49:729–737. doi: 10.1136/gut.49.5.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arroyo V, Guevara M, Gines P. Hepatorenal syndrome in cirrhosis: pathogenesis and treatment. Gastroenterology. 2002;122:1658–1676. doi: 10.1053/gast.2002.33575. [DOI] [PubMed] [Google Scholar]
- 9.Salerno F, Gerbes A, Gines P, et al. Diagnosis, prevention and treatment of the hepatorenal syndrome in cirrhosis. A consensus workshop of the international ascites club. Gut. 2007;56:1310–1318. doi: 10.1136/gut.2006.107789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arroyo V, Terra C, Gines P. New treatments of hepatorenal syndrome. Semin Liver Dis. 2006;26:254–264. doi: 10.1055/s-2006-947293. [DOI] [PubMed] [Google Scholar]
- 11.Cort JH, Albrecht I, Novakova J, et al. Rregional and systemic haemodynamic effect of some vasopressins: structural features of the hormone which prolong activity. Eur J Clin Invest. 1975;5:165–175. doi: 10.1111/j.1365-2362.1975.tb00443.x. [DOI] [PubMed] [Google Scholar]
- 12.Ganne-Carrie N, Hadengue A, Mathurin P, et al. Hepatorenal syndrome. Long-term treatment with terlipressin as a bridge to liver transplantation. Dig Dis Sci. 1996;41:1054–1056. doi: 10.1007/BF02088218. [DOI] [PubMed] [Google Scholar]
- 13.Cervoni JP, Lecomte T, Cellier C, et al. Terlipressin may influence the outcome of hepatorenal syndrome complicating alcoholic hepatitis. Am J Gastroenterol. 1997;92:2113–2114. [PubMed] [Google Scholar]
- 14.Uriz J, Gines P, Cardenas A, et al. Terlipressin plus albumin infusion: an effective and safe therapy of hepatorenal syndrome. J Hepatol. 2000;33:43–48. doi: 10.1016/s0168-8278(00)80158-0. [DOI] [PubMed] [Google Scholar]
- 15.Ortega R, Gines P, Uriz J, et al. Terlipressin therapy with and without albumin for patients with hepatorenal syndrome: results of a prospective, nonrandomized study. Hepatology. 2002;36:941–948. doi: 10.1053/jhep.2002.35819. [DOI] [PubMed] [Google Scholar]
- 16.Colle I, Durand F, Pessione F, et al. Clinical course, predictive factors and prognosis in patients with cirrhosis and type 1 hepatorenal syndrome treated with Terlipressin: a retrospective analysis. J Gastroenterol Hepatol. 2002;17:882–888. doi: 10.1046/j.1440-1746.2002.02816.x. [DOI] [PubMed] [Google Scholar]
- 17.Moreau R, Durand F, Poynard T, et al. Terlipressin in patients with cirrhosis and type 1 hepatorenal syndrome: a retrospective multicenter study. Gastroenterology. 2002;122:923–930. doi: 10.1053/gast.2002.32364. [DOI] [PubMed] [Google Scholar]
- 18.Solanki P, Chawla A, Garg R, et al. Beneficial effects of terlipressin in hepatorenal syndrome: a prospective, randomized placebo-controlled clinical trial. J Gastroenterol Hepatol. 2003;18:152–156. doi: 10.1046/j.1440-1746.2003.02934.x. [DOI] [PubMed] [Google Scholar]
- 19.Hadengue A, Gadano A, Moreau R, et al. Beneficial effects of the 2-day administration of terlipressin in patients with cirrhosis and hepatorenal syndrome. J Hepatol. 1998;29:565–570. doi: 10.1016/s0168-8278(98)80151-7. [DOI] [PubMed] [Google Scholar]
- 20.Fabrizi F, Dixit V, Martin P. Meta-analysis: terlipressin therapy for the hepatorenal syndrome. Aliment Pharmacol Ther. 2006;24:935–944. doi: 10.1111/j.1365-2036.2006.03086.x. [DOI] [PubMed] [Google Scholar]
- 21.Arroyo V, Gines P, Gerbes AL, et al. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. International Ascites Club Hepatology. 1996;23:164–176. doi: 10.1002/hep.510230122. [DOI] [PubMed] [Google Scholar]
- 22.Arroyo V, Terra C, Torre A. Circulatory support with albumin in liver cirrhosis. Gastroenterologia y hepatologia. 2005;28:74–79. doi: 10.1157/13070705. [DOI] [PubMed] [Google Scholar]
- 23.Duhamel C, Mauillon J, Berkelmans I, et al. Hepatorenal syndrome in cirrhotic patients: terlipressine is a safe and efficient treatment; propranolol and digitalic treatments: precipitating and preventing factors? Am J Gastroenterol. 2000;95:2984–2985. doi: 10.1111/j.1572-0241.2000.03214.x. [DOI] [PubMed] [Google Scholar]
- 24.Restuccia T, Ortega R, Guevara M, et al. Effects of treatment of hepatorenal syndrome before transplantation on posttransplantation outcome. A case-control study. J Hepatol. 2004;40:140–146. doi: 10.1016/j.jhep.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 25.Saner F, Kavuk I, Lang H, et al. Terlipressin and gelafundin: safe therapy of hepatorenal syndrome. Eur J Med Res. 2004;9:78–82. [PubMed] [Google Scholar]
- 26.Verbeke G, Molenberghs G. Verbeke 2000: Linear mixed models for longitudinal data. New York: Springer-Verlag; 2000. pp. 46–47.pp. 98–99.pp. 199–120. [Google Scholar]
- 27.Arroyo V. Review article: hepatorenal syndrome—how to assess response to treatment and nonpharmacological therapy. Aliment Pharmacol Ther. 2004;20(Suppl 3):49–56. doi: 10.1111/j.1365-2036.2004.02114.x. [DOI] [PubMed] [Google Scholar]
- 28.Moreau R. Hepatorenal syndrome in patients with cirrhosis. J Gastroenterol Hepatol. 2002;17:739–747. doi: 10.1046/j.1440-1746.2002.02778.x. [DOI] [PubMed] [Google Scholar]
- 29.Gines P. Diagnosis and treatment of hepatorenal syndrome. Baillieres Best Pract Res Clin Gastroenterol. 2000;14:945–957. doi: 10.1053/bega.2000.0140. [DOI] [PubMed] [Google Scholar]
- 30.Schrier RW, Arroyo V, Bernardi M, et al. Peripheral arterial vaso-dilation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology. 1988;8:1151–1157. doi: 10.1002/hep.1840080532. [DOI] [PubMed] [Google Scholar]
- 31.Gines P, Fernandez-Esparrach G, Arroyo V. Ascites and renal functional abnormalities in cirrhosis. Pathogenesis and treatment. Baillieres Clin Gastroenterol. 1997;11:365–385. doi: 10.1016/s0950-3528(97)90045-2. [DOI] [PubMed] [Google Scholar]
- 32.Knotek M, Rogachev B, Schrier RW. Update on peripheral arterial vasodilation, ascites and hepatorenal syndrome in cirrhosis. Can J Gastroenterol. 2000;14(Suppl D):D112–D121. doi: 10.1155/2000/340128. [DOI] [PubMed] [Google Scholar]
- 33.Mulkay JP, Louis H, Donckier V, et al. Long-term terlipressin administration improves renal function in cirrhotic patients with type 1 hepatorenal syndrome: a pilot study. Acta Gastroenterol Belg. 2001;64:15–19. [PubMed] [Google Scholar]
- 34.Peron JM, Bureau C, Gonzalez L, et al. Treatment of hepatorenal syndrome as defined by the International Ascites Club by albumin and furosemide infusion according to the central venous pressure: a prospective pilot study. Am J Gastroenterol. 2005;100:2702–2707. doi: 10.1111/j.1572-0241.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- 35.Fernandez J, Navasa M, Garcia-Pagan JC, et al. Effect of intravenous albumin on systemic and hepatic hemodynamics and vaso-active neurohormonal systems in patients with cirrhosis and spontaneous bacterial peritonitis. J Hepatol. 2004;41:384–390. doi: 10.1016/j.jhep.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 36.Fernandez J, Monteagudo J, Bargallo X, et al. A randomized unblinded pilot study comparing albumin versus hydroxyethyl starch in spontaneous bacterial peritonitis. Hepatology. 2005;42:627–634. doi: 10.1002/hep.20829. [DOI] [PubMed] [Google Scholar]
- 37.Guevara M, Gines P, Fernandez-Esparrach G, et al. Reversibility of hepatorenal syndrome by prolonged administration of ornipressin and plasma volume expansion. Hepatology. 1998;27:35–41. doi: 10.1002/hep.510270107. [DOI] [PubMed] [Google Scholar]
- 38.Gulberg V, Bilzer M, Gerbes AL. Long-term therapy and retreatment of hepatorenal syndrome type 1 with ornipressin and dopamine. Hepatology. 1999;30:870–875. doi: 10.1002/hep.510300430. [DOI] [PubMed] [Google Scholar]
- 39.Ioannou GN, Doust J, Rockey DC. Systematic review: terlipressin in acute oesophageal variceal hemorrhage. Aliment Pharmacol Ther. 2003;17:53–64. doi: 10.1046/j.1365-2036.2003.01356.x. [DOI] [PubMed] [Google Scholar]