Full text
PDF
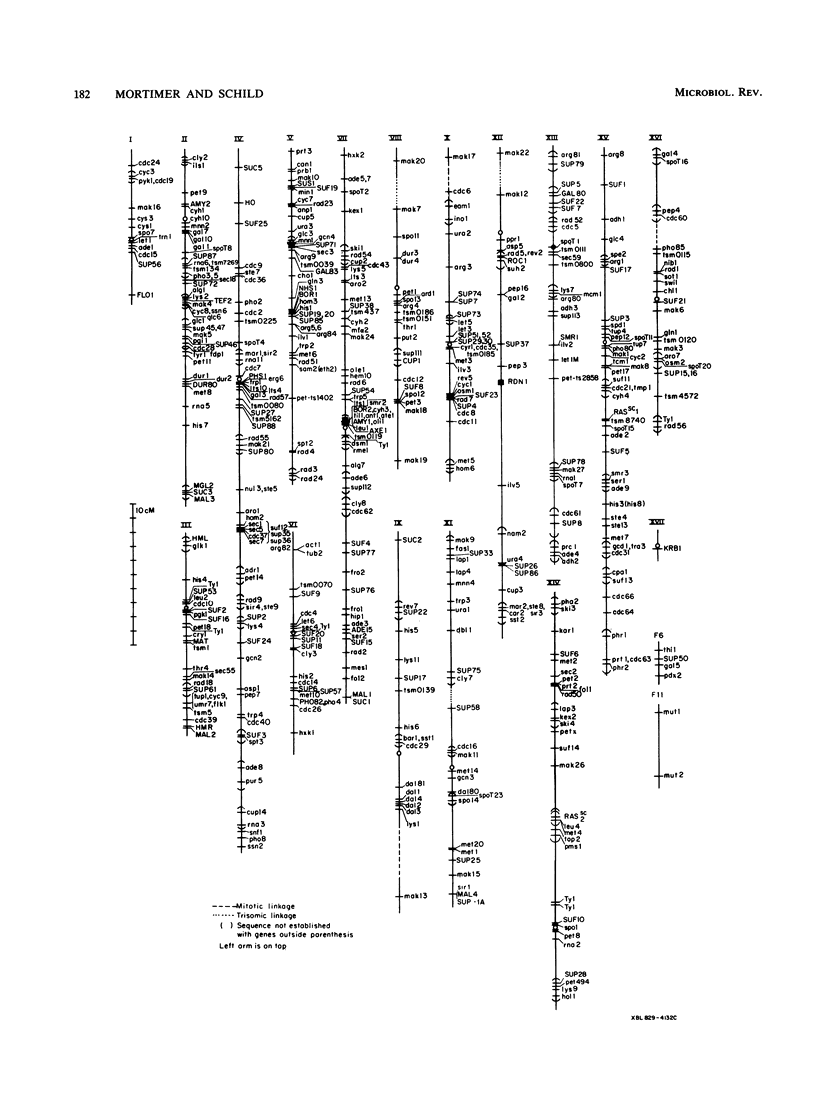
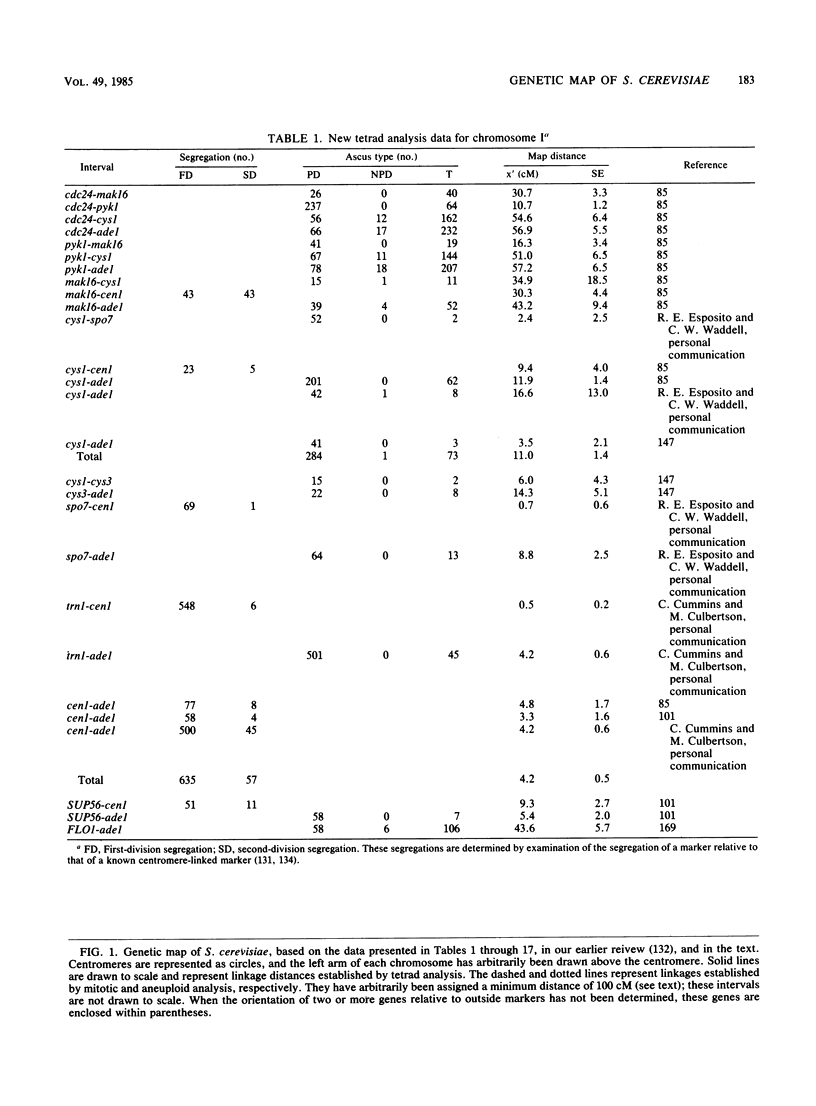
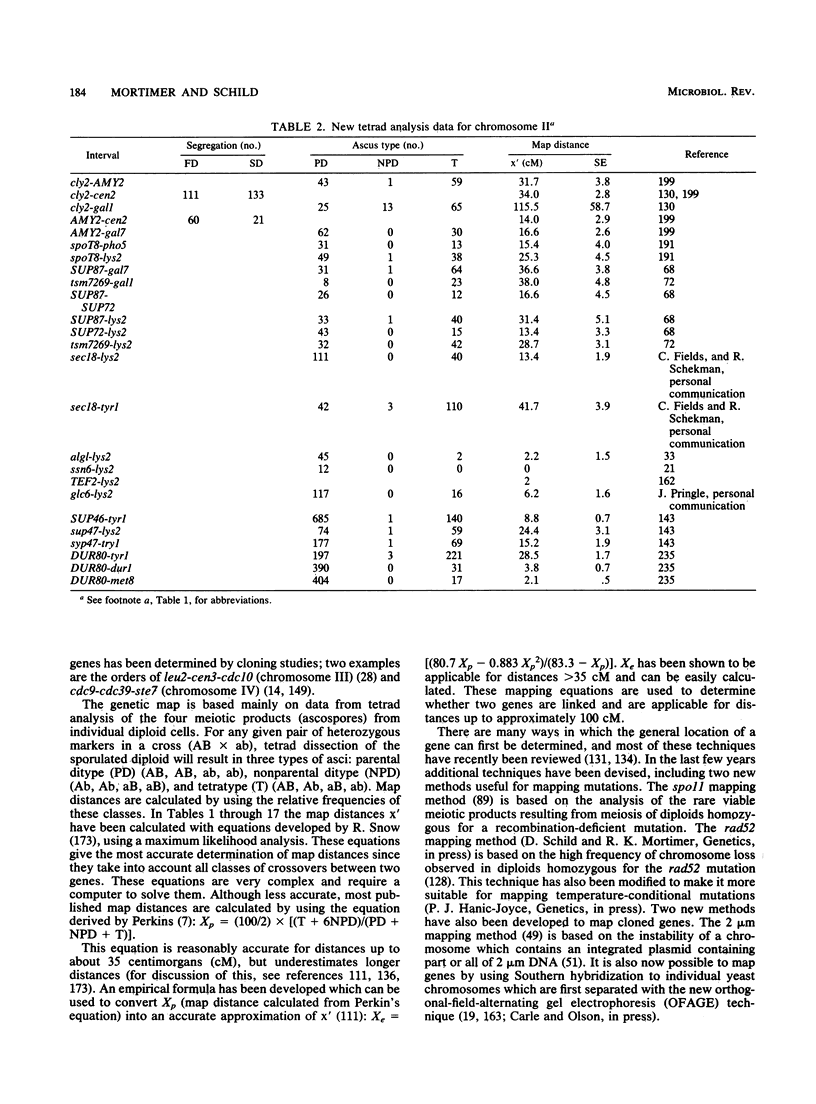
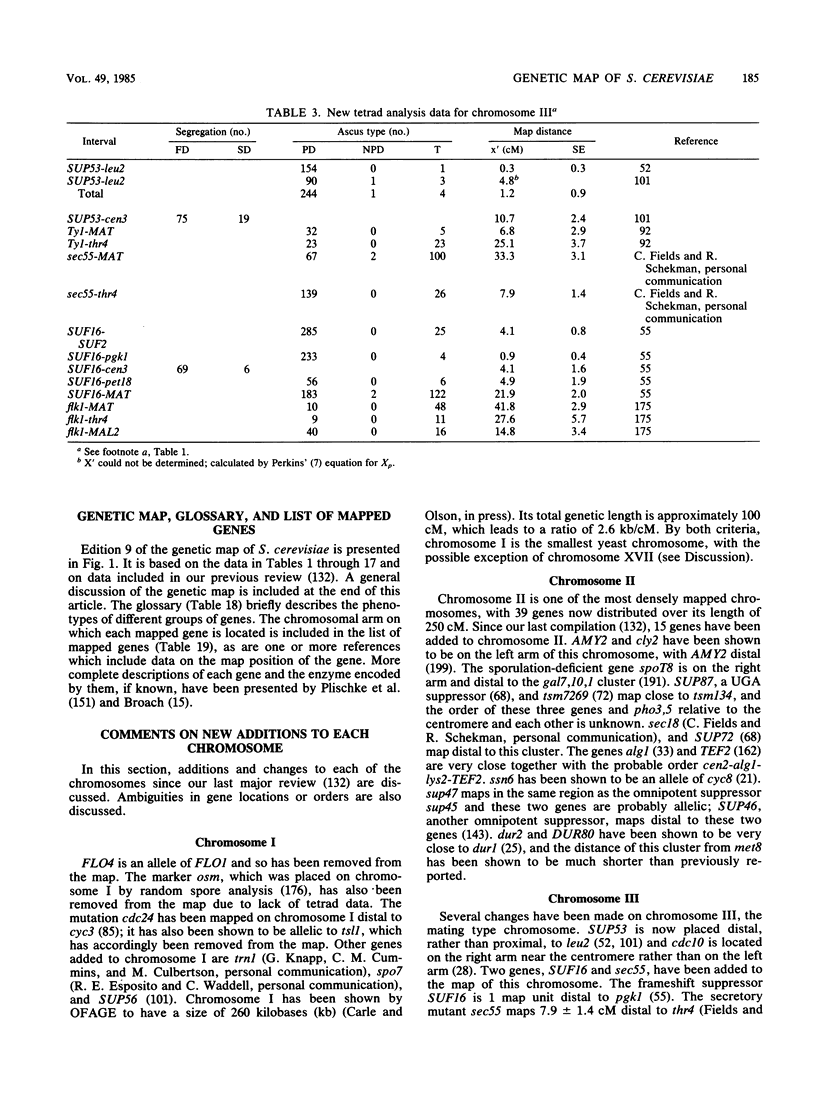









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antalis C., Fogel S., Ballou C. E. Genetic control of yeast mannan structure. Mapping the first gene concerned with mannan biosynthesis. J Biol Chem. 1973 Jul 10;248(13):4655–4659. [PubMed] [Google Scholar]
- Armitt S., Woods R. A. Purine-excreting mutants of Saccharomyces cerevisiae. I. Isolation and genetic analysis. Genet Res. 1970 Feb;15(1):7–17. doi: 10.1017/s0016672300001324. [DOI] [PubMed] [Google Scholar]
- Atkinson K. D., Jensen B., Kolat A. I., Storm E. M., Henry S. A., Fogel S. Yeast mutants auxotrophic for choline or ethanolamine. J Bacteriol. 1980 Feb;141(2):558–564. doi: 10.1128/jb.141.2.558-564.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson K. D. SACCHAROMYCES CEREVISIAE Recessive Suppressor That Circumvents Phosphatidylserine Deficiency. Genetics. 1984 Nov;108(3):533–543. doi: 10.1093/genetics/108.3.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARRATT R. W., NEWMEYER D., PERKINS D. D., GARNJOBST L. Map construction in Neurospora crassa. Adv Genet. 1954;6:1–93. doi: 10.1016/s0065-2660(08)60127-3. [DOI] [PubMed] [Google Scholar]
- Ballou D. L. Genetic control of yeast mannan structure: mapping genes mnn2 and mnn4 in Saccharomyces cerevisiae. J Bacteriol. 1975 Aug;123(2):616–619. doi: 10.1128/jb.123.2.616-619.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes G., Hansen W. J., Holcomb C. L., Rine J. Asparagine-linked glycosylation in Saccharomyces cerevisiae: genetic analysis of an early step. Mol Cell Biol. 1984 Nov;4(11):2381–2388. doi: 10.1128/mcb.4.11.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassel J., Mortimer R. Genetic order of the galactose structural genes in Saccharomyces cerevisiae. J Bacteriol. 1971 Oct;108(1):179–183. doi: 10.1128/jb.108.1.179-183.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisson L. F., Thorner J. Mutations in the pho80 gene confer permeability to 5'-mononucleotides in Saccharomyces cerevisiae. Genetics. 1982 Nov;102(3):341–359. doi: 10.1093/genetics/102.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandriss M. C. Proline utilization in Saccharomyces cerevisiae: analysis of the cloned PUT2 gene. Mol Cell Biol. 1983 Oct;3(10):1846–1856. doi: 10.1128/mcb.3.10.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandriss M. C., Stewart J. W., Sherman F., Botstein D. Substitution of serine caused by a recessive lethal suppressor in yeast. J Mol Biol. 1976 Apr 15;102(3):467–476. doi: 10.1016/0022-2836(76)90328-4. [DOI] [PubMed] [Google Scholar]
- Brendel M., Fäth W. W. Isolation and characterization of mutants of Saccharomyces cerevisiae auxotrophic and conditionally auxotrophic for 5'-dTMP. Z Naturforsch C. 1974 Nov-Dec;29(11-12):733–738. doi: 10.1515/znc-1974-11-1214. [DOI] [PubMed] [Google Scholar]
- Breter H. J., Ferguson J., Peterson T. A., Reed S. I. Isolation and transcriptional characterization of three genes which function at start, the controlling event of the Saccharomyces cerevisiae cell division cycle: CDC36, CDC37, and CDC39. Mol Cell Biol. 1983 May;3(5):881–891. doi: 10.1128/mcb.3.5.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkl G., Castorph H., Schweizer E. Mapping of a complex gene locus coding for part of the Saccharomyces cerevisiae fatty acid synthetase multienzyme complex. Mol Gen Genet. 1972;119(4):315–322. doi: 10.1007/BF00272089. [DOI] [PubMed] [Google Scholar]
- Calderon I. L., Contopoulou C. R., Mortimer R. K. Isolation of a DNA fragment that is expressed as an amber suppressor when present in high copy number in yeast. Gene. 1984 Jul-Aug;29(1-2):69–76. doi: 10.1016/0378-1119(84)90167-7. [DOI] [PubMed] [Google Scholar]
- Cameron J. R., Loh E. Y., Davis R. W. Evidence for transposition of dispersed repetitive DNA families in yeast. Cell. 1979 Apr;16(4):739–751. doi: 10.1016/0092-8674(79)90090-4. [DOI] [PubMed] [Google Scholar]
- Carle G. F., Olson M. V. Separation of chromosomal DNA molecules from yeast by orthogonal-field-alternation gel electrophoresis. Nucleic Acids Res. 1984 Jul 25;12(14):5647–5664. doi: 10.1093/nar/12.14.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson M., Osmond B. C., Botstein D. Genetic evidence for a silent SUC gene in yeast. Genetics. 1981 May;98(1):41–54. doi: 10.1093/genetics/98.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson M., Osmond B. C., Neigeborn L., Botstein D. A suppressor of SNF1 mutations causes constitutive high-level invertase synthesis in yeast. Genetics. 1984 May;107(1):19–32. doi: 10.1093/genetics/107.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celenza J. L., Carlson M. Cloning and genetic mapping of SNF1, a gene required for expression of glucose-repressible genes in Saccharomyces cerevisiae. Mol Cell Biol. 1984 Jan;4(1):49–53. doi: 10.1128/mcb.4.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celenza J. L., Carlson M. Rearrangement of the genetic map of chromosome VII of Saccharomyces cerevisiae. Genetics. 1985 Apr;109(4):661–664. doi: 10.1093/genetics/109.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan R. K., Otte C. A. Isolation and genetic analysis of Saccharomyces cerevisiae mutants supersensitive to G1 arrest by a factor and alpha factor pheromones. Mol Cell Biol. 1982 Jan;2(1):11–20. doi: 10.1128/mcb.2.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm G., Cooper T. cis-Dominant mutations which dramatically enhance DUR1,2 gene expression without affecting its normal regulation. Mol Cell Biol. 1984 May;4(5):947–955. doi: 10.1128/mcb.4.5.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke L., Carbon J. Isolation of a yeast centromere and construction of functional small circular chromosomes. Nature. 1980 Oct 9;287(5782):504–509. doi: 10.1038/287504a0. [DOI] [PubMed] [Google Scholar]
- Cohen J. D., Eaton N. R. Genetic analysis of multiple drug cross resistance in Saccharomyces cerevisiae: a nuclear-mitochondrial gene interaction. Genetics. 1979 Jan;91(1):19–33. doi: 10.1093/genetics/91.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn M. S., Tabor C. W., Tabor H. Isolation and characterization of Saccharomyces cerevisiae mutants deficient in S-adenosylmethionine decarboxylase, spermidine, and spermine. J Bacteriol. 1978 Apr;134(1):208–213. doi: 10.1128/jb.134.1.208-213.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper T. G., Gorski M., Turoscy V. A cluster of three genes responsible for allantoin degradation in Saccharomyces cerevisiae. Genetics. 1979 Jun;92(2):383–396. doi: 10.1093/genetics/92.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper T. G., Lam C., Turoscy V. Structural analysis of the dur loci in S. cerevisiae: two domains of a single multifunctional gene. Genetics. 1980 Mar;94(3):555–580. doi: 10.1093/genetics/94.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couto J. R., Huffaker T. C., Robbins P. W. Cloning and expression in Escherichia coli of a yeast mannosyltransferase from the asparagine-linked glycosylation pathway. J Biol Chem. 1984 Jan 10;259(1):378–382. [PubMed] [Google Scholar]
- Culbertson M. R., Charnas L., Johnson M. T., Fink G. R. Frameshifts and frameshift suppressors in Saccharomyces cerevisiae. Genetics. 1977 Aug;86(4):745–764. doi: 10.1093/genetics/86.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbertson M. R., Gaber R. F., Cummins C. M. Frameshift suppression in Saccharomyces cerevisiae. V. Isolation and genetic properties of nongroup-specific suppressors. Genetics. 1982 Nov;102(3):361–378. doi: 10.1093/genetics/102.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbertson M. R., Henry S. A. Genetic analysis of hybrid strains trisomic for the chromosome containing a fatty acid synthetase gene complex (fas1) in yeast. Genetics. 1973 Nov;75(3):441–458. doi: 10.1093/genetics/75.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbertson M. R., Underbrink K. M., Fink G. R. Frameshift suppression Saccharomyces cerevisiae. II. Genetic properties of group II suppressors. Genetics. 1980 Aug;95(4):833–853. doi: 10.1093/genetics/95.4.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins C. M., Gaber R. F., Culbertson M. R., Mann R., Fink G. R. Frameshift suppression in Saccharomyces cerevisiae. III. Isolation and genetic properties of group III suppressors. Genetics. 1980 Aug;95(4):855–879. doi: 10.1093/genetics/95.4.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS H. C., HAWTHORNE D. C. ENZYMATIC EXPRESSION AND GENETIC LINKAGE OF GENES CONTROLLING GALACTOSE UTILIZATION IN SACCHAROMYCES. Genetics. 1964 May;49:837–844. doi: 10.1093/genetics/49.5.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis C. L. Identification of new genes involved in the regulation of yeast alcohol dehydrogenase II. Genetics. 1984 Dec;108(4):833–844. doi: 10.1093/genetics/108.4.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNardo S., Voelkel K., Sternglanz R. DNA topoisomerase II mutant of Saccharomyces cerevisiae: topoisomerase II is required for segregation of daughter molecules at the termination of DNA replication. Proc Natl Acad Sci U S A. 1984 May;81(9):2616–2620. doi: 10.1073/pnas.81.9.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicarprio L., Hastings P. J. Gene conversion and intragenic recombination at the SUP6 locus and the surrounding region in Saccharomyces cerevisiae. Genetics. 1976 Dec;84(4):697–721. doi: 10.1093/genetics/84.4.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue T. F., Henry S. A. Inositol Mutants of SACCHAROMYCES CEREVISIAE: Mapping the ino1 Locus and Characterizing Alleles of the ino1, ino2 and ino4 Loci. Genetics. 1981 Jul;98(3):491–503. doi: 10.1093/genetics/98.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas H. C., Hawthorne C. D. Uninducible mutants in the gal i locus of Saccharomyces cerevisiae. J Bacteriol. 1972 Mar;109(3):1139–1143. doi: 10.1128/jb.109.3.1139-1143.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas H. C., Hawthorne D. C. Regulation of genes controlling synthesis of the galactose pathway enzymes in yeast. Genetics. 1966 Sep;54(3):911–916. doi: 10.1093/genetics/54.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutcher S. K., Hartwell L. H. The role of S. cerevisiae cell division cycle genes in nuclear fusion. Genetics. 1982 Feb;100(2):175–184. doi: 10.1093/genetics/100.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eibel H., Gafner J., Stotz A., Philippsen P. Characterization of the yeast mobile element Ty1. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 2):609–617. doi: 10.1101/sqb.1981.045.01.079. [DOI] [PubMed] [Google Scholar]
- Falco S. C., Botstein D. A rapid chromosome-mapping method for cloned fragments of yeast DNA. Genetics. 1983 Dec;105(4):857–872. doi: 10.1093/genetics/105.4.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falco S. C., Dumas K. S. Genetic analysis of mutants of Saccharomyces cerevisiae resistant to the herbicide sulfometuron methyl. Genetics. 1985 Jan;109(1):21–35. doi: 10.1093/genetics/109.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falco S. C., Li Y., Broach J. R., Botstein D. Genetic properties of chromosomally integrated 2 mu plasmid DNA in yeast. Cell. 1982 Jun;29(2):573–584. doi: 10.1016/0092-8674(82)90173-8. [DOI] [PubMed] [Google Scholar]
- Fischhoff D. A., Waterston R. H., Olson M. V. The yeast cloning vector YEp13 contains a tRNALeu3 gene that can mutate to an amber suppressor. Gene. 1984 Mar;27(3):239–251. doi: 10.1016/0378-1119(84)90069-6. [DOI] [PubMed] [Google Scholar]
- Fogel S., Hurst D. D. Meiotic gene conversion in yeast tetrads and the theory of recombination. Genetics. 1967 Oct;57(2):455–481. doi: 10.1093/genetics/57.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friis J., Ottolenghi P. The genetically determined binding of alcian blue by a minor fraction of yeast cell walls. C R Trav Lab Carlsberg. 1970;37(15):327–341. [PubMed] [Google Scholar]
- Gaber R. F., Culbertson M. R. Frameshift suppression in Saccharomyces cerevisiae. IV. New suppressors among spontaneous co-revertants of the Group II his4-206 and leu 2-3 frameshift mutations. Genetics. 1982 Jul-Aug;101(3-4):345–367. doi: 10.1093/genetics/101.3-4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaber R. F., Mathison L., Edelman I., Culbertson M. R. Frameshift Suppression in SACCHAROMYCES CEREVISIAE VI. Complete Genetic Map of Twenty-Five Suppressor Genes. Genetics. 1983 Mar;103(3):389–407. doi: 10.1093/genetics/103.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallwitz D., Donath C., Sander C. A yeast gene encoding a protein homologous to the human c-has/bas proto-oncogene product. Nature. 1983 Dec 15;306(5944):704–707. doi: 10.1038/306704a0. [DOI] [PubMed] [Google Scholar]
- Game J. C., Mortimer R. K. A genetic study of x-ray sensitive mutants in yeast. Mutat Res. 1974 Sep;24(3):281–292. doi: 10.1016/0027-5107(74)90176-6. [DOI] [PubMed] [Google Scholar]
- Game J. C. Yeast cell-cycle mutant cdc21 is a temperature-sensitive thymidylate auxotroph. Mol Gen Genet. 1976 Aug 2;146(3):313–315. doi: 10.1007/BF00701257. [DOI] [PubMed] [Google Scholar]
- Gottlieb D. J., von Borstel R. C. Mutators in Saccharomyces cerevisiae: MUT1-1, MUT1-2 and MUT2-1. Genetics. 1976 Aug;83(4):655–666. doi: 10.1093/genetics/83.4.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant P. G., Schindler D., Davies J. E. Mapping of trichodermin resistance in Saccharomyces cerevisiae: a genetic locus for a component of the 60S ribsomal subunit. Genetics. 1976 Aug;83(4):667–673. doi: 10.1093/genetics/83.4.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant P., Sánchez L., Jiménez A. Cryptopleurine resistance: genetic locus for a 40S ribosomal component in Saccharomyces cerevisiae. J Bacteriol. 1974 Dec;120(3):1308–1314. doi: 10.1128/jb.120.3.1308-1314.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HURST D. D., FOGEL S. MITOTIC RECOMBINATION AND HETEROALLELIC REPAIR IN SACCHAROMYCES CEREVISIAE. Genetics. 1964 Sep;50:435–458. doi: 10.1093/genetics/50.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HWANG Y. L., LINDEGREN G., LINDEGREN C. C. MAPPING THE ELEVENTH CENTROMERE IN SACCHAROMYCES. Can J Genet Cytol. 1963 Sep;5:290–298. doi: 10.1139/g63-040. [DOI] [PubMed] [Google Scholar]
- HWANG Y. L., LINDEGREN G., LINDEGREN C. C. THE TWELFTH CHROMOSOME OF SACCHAROMYCES. Can J Genet Cytol. 1964 Sep;6:373–380. doi: 10.1139/g64-047. [DOI] [PubMed] [Google Scholar]
- Haber J. E., Garvik B. A new gene affecting the efficiency of mating-type interconversions in homothallic strains of Saccharomyces cerevisiae. Genetics. 1977 Sep;87(1):33–50. doi: 10.1093/genetics/87.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansche P. E., Beres V., Lange P. Gene duplication in Saccharomyces cerevisiae. Genetics. 1978 Apr;88(4 Pt 1):673–687. [PMC free article] [PubMed] [Google Scholar]
- Harashima S., Oshima Y. Mapping of the homothallic genes, HM alpha and HMa, in Saccharomyces yeasts. Genetics. 1976 Nov;84(3):437–451. doi: 10.1093/genetics/84.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H., Mortimer R. K., Culotti J., Culotti M. Genetic Control of the Cell Division Cycle in Yeast: V. Genetic Analysis of cdc Mutants. Genetics. 1973 Jun;74(2):267–286. doi: 10.1093/genetics/74.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawthorne D C, Mortimer R K. Chromosome Mapping in Saccharomyces: Centromere-Linked Genes. Genetics. 1960 Aug;45(8):1085–1110. doi: 10.1093/genetics/45.8.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawthorne D C. The Use of Linear Asci for Chromosome Mapping in Saccharomyces. Genetics. 1955 Jul;40(4):511–518. doi: 10.1093/genetics/40.4.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawthorne D. C., Mortimer R. K. Genetic mapping of nonsense suppressors in yeast. Genetics. 1968 Dec;60(4):735–742. doi: 10.1093/genetics/60.4.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilger F., Mortimer R. K. Genetic mapping of arg1 and arg8 in Saccharomyces cerevisiae by trisomic analysis combined with interallelic complementation. J Bacteriol. 1980 Jan;141(1):270–274. doi: 10.1128/jb.141.1.270-274.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnebusch A. G., Fink G. R. Positive regulation in the general amino acid control of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5374–5378. doi: 10.1073/pnas.80.17.5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm C. Sensitivity to the Yeast Plasmid 2mu DNA is conferred by the nuclear allele nibl. Mol Cell Biol. 1982 Aug;2(8):985–992. doi: 10.1128/mcb.2.8.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs P., Jauniaux J. C., Grenson M. A cis-dominant regulatory mutation linked to the argB-argC gene cluster in Saccharomyces cerevisiae. J Mol Biol. 1980 Jun 5;139(4):691–704. doi: 10.1016/0022-2836(80)90055-8. [DOI] [PubMed] [Google Scholar]
- James A P, Lee-Whiting B. Radiation-Induced Genetic Segregations in Vegetative Cells of Diploid Yeast. Genetics. 1955 Nov;40(6):826–831. doi: 10.1093/genetics/40.6.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones E. W., Lam K. B. Mutations affecting levels of tetrahydrofolate interconversion enzymes in Saccharomyces cerevisiae. II. Map positions on chromosome VII of ade3-41 and ADE15. Mol Gen Genet. 1973 Jul 2;123(3):209–218. doi: 10.1007/BF00271239. [DOI] [PubMed] [Google Scholar]
- Jones G. E., Mortimer R. K. L-asparaginase-deficient mutants of yeast. Science. 1970 Jan 9;167(3915):181–182. doi: 10.1126/science.167.3915.181. [DOI] [PubMed] [Google Scholar]
- Kaback D. B., Halvorson H. O. Ribosomal DNA magnification in Saccharomyces cerevisiae. J Bacteriol. 1978 Apr;134(1):237–245. doi: 10.1128/jb.134.1.237-245.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaback D. B., Oeller P. W., Yde Steensma H., Hirschman J., Ruezinsky D., Coleman K. G., Pringle J. R. Temperature-sensitive lethal mutations on yeast chromosome I appear to define only a small number of genes. Genetics. 1984 Sep;108(1):67–90. doi: 10.1093/genetics/108.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko Y., Toh-e A., Oshima Y. Identification of the genetic locus for the structural gene and a new regulatory gene for the synthesis of repressible alkaline phosphatase in Saccharomyces cerevisiae. Mol Cell Biol. 1982 Feb;2(2):127–137. doi: 10.1128/mcb.2.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassir Y., Hicks J. B., Herskowitz I. SAD mutation of Saccharomyces cerevisiae is an extra a cassette. Mol Cell Biol. 1983 May;3(5):871–880. doi: 10.1128/mcb.3.5.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka T., Powers S., McGill C., Fasano O., Strathern J., Broach J., Wigler M. Genetic analysis of yeast RAS1 and RAS2 genes. Cell. 1984 Jun;37(2):437–445. doi: 10.1016/0092-8674(84)90374-x. [DOI] [PubMed] [Google Scholar]
- Klapholz S., Esposito R. E. A new mapping method employing a meiotic rec-mutant of yeast. Genetics. 1982 Mar;100(3):387–412. doi: 10.1093/genetics/100.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klapholz S., Esposito R. E. Chromosomes XIV and XVII of Saccharomyces cerevisiae constitute a single linkage group. Mol Cell Biol. 1982 Nov;2(11):1399–1409. doi: 10.1128/mcb.2.11.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klar A. J., Fogel S., Macleod K. MAR1-a Regulator of the HMa and HMalpha Loci in SACCHAROMYCES CEREVISIAE. Genetics. 1979 Sep;93(1):37–50. doi: 10.1093/genetics/93.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein H. L., Petes T. D. Genetic mapping of Ty elements in Saccharomyces cerevisiae. Mol Cell Biol. 1984 Feb;4(2):329–339. doi: 10.1128/mcb.4.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupiec M., Simchen G. Cloning and mapping of the RAD50 gene of Saccharomyces cerevisiae. Mol Gen Genet. 1984;193(3):525–531. doi: 10.1007/BF00382094. [DOI] [PubMed] [Google Scholar]
- Kuroiwa T., Kojima H., Miyakawa I., Sando N. Meiotic karyotype of the yeast Saccharomyces cerevisiae. Exp Cell Res. 1984 Jul;153(1):259–265. doi: 10.1016/0014-4827(84)90469-5. [DOI] [PubMed] [Google Scholar]
- LINDEGREN C. C., LINDEGREN G., SHULT E. E., DESBOROUGH S. Chromosome maps of Saccharomyces. Nature. 1959 Mar 21;183(4664):800–802. doi: 10.1038/183800a0. [DOI] [PubMed] [Google Scholar]
- LINDEGREN C. C., LINDEGREN G., SHULT E., HWANG Y. L. Centromeres, sites of affinity and gene loci on the chromosomes of Saccharomyces. Nature. 1962 Apr 21;194:260–265. doi: 10.1038/194260a0. [DOI] [PubMed] [Google Scholar]
- Lam K. B., Marmur J. Isolation and characterization of Saccharomyces cerevisiae glycolytic pathway mutants. J Bacteriol. 1977 May;130(2):746–749. doi: 10.1128/jb.130.2.746-749.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer G. D., Roberts T. M., Klotz L. C. Determination of the nuclear DNA content of Saccharomyces cerevisiae and implications for the organization of DNA in yeast chromosomes. J Mol Biol. 1977 Aug 25;114(4):507–526. doi: 10.1016/0022-2836(77)90175-9. [DOI] [PubMed] [Google Scholar]
- Lawrence C. W., Sherman F., Jackson M., Gilmore R. A. Mapping and gene conversion studies with the structural gene for iso-1-cytochrome C in yeast. Genetics. 1975 Dec;81(4):615–629. doi: 10.1093/genetics/81.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawther R. P., Riemer E., Chojnacki B., Cooper T. G. Clustering of the genes for allantoin degradation in Saccharomyces cerevisiae. J Bacteriol. 1974 Aug;119(2):461–468. doi: 10.1128/jb.119.2.461-468.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemontt J. F., Fugit D. R., Mackay V. L. Pleiotropic Mutations at the TUP1 Locus That Affect the Expression of Mating-Type-Dependent Functions in SACCHAROMYCES CEREVISIAE. Genetics. 1980 Apr;94(4):899–920. doi: 10.1093/genetics/94.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemontt J. F. Mutants of yeast defective in mutation induced by ultraviolet light. Genetics. 1971 May;68(1):21–33. doi: 10.1093/genetics/68.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebman S. W., Srodulski Z., Reed C. R., Stewart J. W., Sherman F., Brennan G. Yeast amber suppressors corresponding to tRNA3Leu genes. J Mol Biol. 1984 Sep 15;178(2):209–226. doi: 10.1016/0022-2836(84)90140-2. [DOI] [PubMed] [Google Scholar]
- Liebman S. W., Stewart J. W., Parker J. H., Sherman F. Leucine insertion caused by a yeast amber suppressor. J Mol Biol. 1977 Jan 5;109(1):13–22. doi: 10.1016/s0022-2836(77)80043-0. [DOI] [PubMed] [Google Scholar]
- Liebman S. W., Stewart J. W., Sherman F. Serine substitutions caused by an ochre suppressor in yeast. J Mol Biol. 1975 Jun 5;94(4):595–610. doi: 10.1016/0022-2836(75)90324-1. [DOI] [PubMed] [Google Scholar]
- Liljelund P., Losson R., Kammerer B., Lacroute F. Yeast regulatory gene PPR1. II. Chromosomal localization, meiotic map, suppressibility, dominance/recessivity and dosage effect. J Mol Biol. 1984 Dec 5;180(2):251–265. doi: 10.1016/s0022-2836(84)80003-0. [DOI] [PubMed] [Google Scholar]
- Liras P., McCusker J., Mascioli S., Haber J. E. Characterization of a mutation in yeast causing nonrandom chromosome loss during mitosis. Genetics. 1978 Apr;88(4 Pt 1):651–671. [PMC free article] [PubMed] [Google Scholar]
- Lobo Z., Maitra P. K. Genetics of yeast hexokinase. Genetics. 1977 Aug;86(4):727–744. doi: 10.1093/genetics/86.4.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchini G., Carbone M. L., Cocucci M., Sensi M. L. Nuclear inheritance of resistance to antimycin A in Saccharomyces cerevisiae. Mol Gen Genet. 1979;177(1):139–143. doi: 10.1007/BF00267263. [DOI] [PubMed] [Google Scholar]
- Ma C., Mortimer R. K. Empirical equation that can be used to determine genetic map distances from tetrad data. Mol Cell Biol. 1983 Oct;3(10):1886–1887. doi: 10.1128/mcb.3.10.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQuillan A. M., Herman A., Coberly J. S., Green G. A second photoreactivation-deficient mutation in Saccharomyces cerevisiae. Photochem Photobiol. 1981 Dec;34(6):673–677. [PubMed] [Google Scholar]
- Maitra P. K., Lobo Z. Genetic studies with a phosphoglucose isomerase mutant of Saccharomyces cerevisiae. Mol Gen Genet. 1977 Nov 4;156(1):55–60. doi: 10.1007/BF00272252. [DOI] [PubMed] [Google Scholar]
- Maitra P. K., Lobo Z. Genetics of yeast glucokinase. Genetics. 1983 Nov;105(3):501–515. doi: 10.1093/genetics/105.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitra P. K., Lobo Z. Pyruvate kinase mutants of Saccharomyces cerevisiae: biochemical and genetic characterisation. Mol Gen Genet. 1977 Apr 29;152(3):193–200. doi: 10.1007/BF00268817. [DOI] [PubMed] [Google Scholar]
- Masselot M., De Robichon-Szulmajster H. Methionine biosynthesis in Saccharomyces cerevisiae. I. Genetical analysis of auxotrophic mutants. Mol Gen Genet. 1975 Aug 5;139(2):121–132. doi: 10.1007/BF00264692. [DOI] [PubMed] [Google Scholar]
- Masselot M., Surdin-Kerjan Y. Methionine biosynthesis in Saccharomyces cerevisiae. II. Gene-enzyme relationships in the sulfate assimilation pathway. Mol Gen Genet. 1977 Jul 7;154(1):23–30. doi: 10.1007/BF00265572. [DOI] [PubMed] [Google Scholar]
- Masselot M., de Robichon-Szulmajster H. Methionine biosynthesis in Saccharomyces cerevisiae: mutations at the regulatory locus ETH2. I. Genetic data. Mol Gen Genet. 1974 Apr 3;129(4):339–348. doi: 10.1007/BF00265697. [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Toh-e A., Oshima Y. Isolation and characterization of dominant mutations resistant to carbon catabolite repression of galactokinase synthesis in Saccharomyces cerevisiae. Mol Cell Biol. 1981 Feb;1(2):83–93. doi: 10.1128/mcb.1.2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K., Uno I., Oshima Y., Ishikawa T. Isolation and characterization of yeast mutants deficient in adenylate cyclase and cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2355–2359. doi: 10.1073/pnas.79.7.2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCammon M. T., Hartmann M. A., Bottema C. D., Parks L. W. Sterol methylation in Saccharomyces cerevisiae. J Bacteriol. 1984 Feb;157(2):475–483. doi: 10.1128/jb.157.2.475-483.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight G. L., Cardillo T. S., Sherman F. An extensive deletion causing overproduction of yeast iso-2-cytochrome c. Cell. 1981 Aug;25(2):409–419. doi: 10.1016/0092-8674(81)90059-3. [DOI] [PubMed] [Google Scholar]
- McLaughlin C. S., Hartwell L. H. A mutant of yeast with a defective methionyl-tRNA synthetase. Genetics. 1969 Mar;61(3):557–566. doi: 10.1093/genetics/61.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade J. H., Riley M. I., Manney T. R. Expression of cryptopleurine resistance in Saccharomyces cerevisiae. J Bacteriol. 1977 Mar;129(3):1428–1434. doi: 10.1128/jb.129.3.1428-1434.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikus M. D., Petes T. D. Recombination between genes located on nonhomologous chromosomes in Saccharomyces cerevisiae. Genetics. 1982 Jul-Aug;101(3-4):369–404. doi: 10.1093/genetics/101.3-4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell A. P., Magasanik B. Regulation of glutamine-repressible gene products by the GLN3 function in Saccharomyces cerevisiae. Mol Cell Biol. 1984 Dec;4(12):2758–2766. doi: 10.1128/mcb.4.12.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer R. K., Contopoulou R., Schild D. Mitotic chromosome loss in a radiation-sensitive strain of the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5778–5782. doi: 10.1073/pnas.78.9.5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer R. K., Hawthorne D. C. Genetic Mapping in Saccharomyces IV. Mapping of Temperature-Sensitive Genes and Use of Disomic Strains in Localizing Genes. Genetics. 1973 May;74(1):33–54. doi: 10.1093/genetics/74.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer R. K., Hawthorne D. C. Genetic mapping in Saccharomyces. Genetics. 1966 Jan;53(1):165–173. doi: 10.1093/genetics/53.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer R. K., Hawthorne D. C. Genetic mapping in yeast. Methods Cell Biol. 1975;11:221–233. doi: 10.1016/s0091-679x(08)60325-8. [DOI] [PubMed] [Google Scholar]
- Mortimer R. K., Schild D. Genetic map of Saccharomyces cerevisiae. Microbiol Rev. 1980 Dec;44(4):519–571. doi: 10.1128/mr.44.4.519-571.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munz P. Clone size distribution among haploid yeast segregants carrying a UV-induced recessive lethal mutation. Arch Genet (Zur) 1972;45(3):173–180. [PubMed] [Google Scholar]
- Müller P. P., Fox T. D. Molecular cloning and genetic mapping of the PET494 gene of Saccharomyces cerevisiae. Mol Gen Genet. 1984;195(1-2):275–280. doi: 10.1007/BF00332759. [DOI] [PubMed] [Google Scholar]
- Nakai S., Mortimer R. K. Studies on the genetic mechanism of radiation-induced mitotic segregation in yeast. Mol Gen Genet. 1969;103(4):329–338. doi: 10.1007/BF00383483. [DOI] [PubMed] [Google Scholar]
- Nass G., Poralla K. Genetics of borrelidin resistant mutants of Saccharomyces cerivisiae and properties of their threonyl-tRNA-synthetase. Mol Gen Genet. 1976 Aug 10;147(1):39–43. doi: 10.1007/BF00337933. [DOI] [PubMed] [Google Scholar]
- Olson M. V., Loughney K., Hall B. D. Identification of the yeast DNA sequences that correspond to specific tyrosine-inserting nonsense suppressor loci. J Mol Biol. 1979 Aug 15;132(3):387–410. doi: 10.1016/0022-2836(79)90267-5. [DOI] [PubMed] [Google Scholar]
- Ono B. I., Stewart J. W., Sherman F. Serine insertion caused by the ribosomal suppressor SUP46 in yeast. J Mol Biol. 1981 Apr 15;147(3):373–379. doi: 10.1016/0022-2836(81)90489-7. [DOI] [PubMed] [Google Scholar]
- Ono B. I., Stewart J. W., Sherman F. Yeast UAA suppressors effective in psi+ strains serine-inserting suppressors. J Mol Biol. 1979 Feb 15;128(1):81–100. doi: 10.1016/0022-2836(79)90309-7. [DOI] [PubMed] [Google Scholar]
- Ono B. I., Stewart J. W., Sherman F. Yeast UAA suppressors effective in psi+ strains: leucine-inserting suppressors. J Mol Biol. 1979 Aug 15;132(3):507–520. doi: 10.1016/0022-2836(79)90272-9. [DOI] [PubMed] [Google Scholar]
- Ono B., Moriga N., Ishihara K., Ishiguro J., Ishino Y., Shinoda S. Omnipotent Suppressors Effective in psi Strains of SACCHAROMYCES CEREVISIAE: Recessiveness and Dominance. Genetics. 1984 Jun;107(2):219–230. doi: 10.1093/genetics/107.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono B., Suruga T., Yamamoto M., Yamamoto S., Murata K., Kimura A., Shinoda S., Ohmori S. Cystathionine accumulation in Saccharomyces cerevisiae. J Bacteriol. 1984 Jun;158(3):860–865. doi: 10.1128/jb.158.3.860-865.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson T. A., Prakash L., Prakash S., Osley M. A., Reed S. I. Regulation of CDC9, the Saccharomyces cerevisiae gene that encodes DNA ligase. Mol Cell Biol. 1985 Jan;5(1):226–235. doi: 10.1128/mcb.5.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petes T. D. Meiotic mapping of yeast ribosomal deoxyribonucleic acid on chromosome XII. J Bacteriol. 1979 Apr;138(1):185–192. doi: 10.1128/jb.138.1.185-192.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remer S., Sherman A., Kraig E., Haber J. E. Suppressor of deoxythmidine monophosphate uptake in Saccharomyces cerevisiae. J Bacteriol. 1979 May;138(2):638–641. doi: 10.1128/jb.138.2.638-641.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick M. A. Genetic control of radiation sensitivity in Saccharomyces cerevisiae. Genetics. 1969 Jul;62(3):519–531. doi: 10.1093/genetics/62.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick M. A., Mortimer R. K. Unsaturated fatty acid mutants of Saccharomyces cerevisiae. J Bacteriol. 1966 Sep;92(3):597–600. doi: 10.1128/jb.92.3.597-600.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rine J., Sprague G. F., Jr, Herskowitz I. rme1 Mutation of Saccharomyces cerevisiae: map position and bypass of mating type locus control of sporulation. Mol Cell Biol. 1981 Oct;1(10):958–960. doi: 10.1128/mcb.1.10.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein R. J., Sherman F. Genes affecting the expression of cytochrome c in yeast: genetic mapping and genetic interactions. Genetics. 1980 Apr;94(4):871–889. doi: 10.1093/genetics/94.4.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schild D., Johnston J., Chang C., Mortimer R. K. Cloning and mapping of Saccharomyces cerevisiae photoreactivation gene PHR1. Mol Cell Biol. 1984 Sep;4(9):1864–1870. doi: 10.1128/mcb.4.9.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D. C., Cantor C. R. Separation of yeast chromosome-sized DNAs by pulsed field gradient gel electrophoresis. Cell. 1984 May;37(1):67–75. doi: 10.1016/0092-8674(84)90301-5. [DOI] [PubMed] [Google Scholar]
- Sherman F., Stewart J. W., Helms C., Downie J. A. Chromosome mapping of the CYC7 gene determining yeast iso-2-cytochrome c: structural and regulatory regions. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1437–1441. doi: 10.1073/pnas.75.3.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shult E. E., Lindegren G., Lindegren C. C. Hybrid specific linkage relations in Saccharomyces. Can J Genet Cytol. 1967 Dec;9(4):723–759. doi: 10.1139/g67-079. [DOI] [PubMed] [Google Scholar]
- Shuster J. R. Mating-defective ste mutations are suppressed by cell division cycle start mutations in Saccharomyces cerevisiae. Mol Cell Biol. 1982 Sep;2(9):1052–1063. doi: 10.1128/mcb.2.9.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A., Manney T. R. Genetic analysis of mutations affecting growth of Saccharomyces cerevisiae at low temperature. Genetics. 1974 Aug;77(4):651–659. doi: 10.1093/genetics/77.4.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A., Sherman F. Deletions of the iso-1-cytochrome c and adjacent genes of yeast: discovery of the OSM1 gene controlling osmotic sensitivity. Genetics. 1978 Aug;89(4):653–665. doi: 10.1093/genetics/89.4.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skogerson L., McLaughlin C., Wakatama E. Modification of ribosomes in cryptopleurine-resistant mutants of yeast. J Bacteriol. 1973 Nov;116(2):818–822. doi: 10.1128/jb.116.2.818-822.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow R. Maximum likelihood estimation of linkage and interference from tetrad data. Genetics. 1979 May;92(1):231–245. doi: 10.1093/genetics/92.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow R. Mutants of yeast sensitive to ultraviolet light. J Bacteriol. 1967 Sep;94(3):571–575. doi: 10.1128/jb.94.3.571-575.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague G. F., Jr Isolation and characterization of a Saccharomyces cerevisiae mutant deficient in pyruvate kinase activity. J Bacteriol. 1977 Apr;130(1):232–241. doi: 10.1128/jb.130.1.232-241.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark H. C., Fugit D., Mowshowitz D. B. Pleiotropic properties of a yeast mutant insensitive to catabolite repression. Genetics. 1980 Apr;94(4):921–928. doi: 10.1093/genetics/94.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stateva L., Venkov P. Meiotic mapping of the nuclear determinant of cell lysis of the osmotic dependent Saccharomyces cerevisiae mutant VY1160 . Mol Gen Genet. 1981;181(3):414–415. doi: 10.1007/BF00425624. [DOI] [PubMed] [Google Scholar]
- Stewart G. G., Russell I. The identification, characterization, and mapping of a gene for flocculation in Saccharomyces sp. Can J Microbiol. 1977 Apr;23(4):441–447. doi: 10.1139/m77-065. [DOI] [PubMed] [Google Scholar]
- Strathern J. N., Newlon C. S., Herskowitz I., Hicks J. B. Isolation of a circular derivative of yeast chromosome III: implications for the mechanism of mating type interconversion. Cell. 1979 Oct;18(2):309–319. doi: 10.1016/0092-8674(79)90050-3. [DOI] [PubMed] [Google Scholar]
- Takano I., Oshima Y. Mutational nature of an allele-specific conversion of the mating type by the homothallic gene HO alpha in Saccharomyces. Genetics. 1970 Jul;65(3):421–427. doi: 10.1093/genetics/65.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatchell K., Chaleff D. T., DeFeo-Jones D., Scolnick E. M. Requirement of either of a pair of ras-related genes of Saccharomyces cerevisiae for spore viability. Nature. 1984 Jun 7;309(5968):523–527. doi: 10.1038/309523a0. [DOI] [PubMed] [Google Scholar]
- Thrash C., Voelkel K., DiNardo S., Sternglanz R. Identification of Saccharomyces cerevisiae mutants deficient in DNA topoisomerase I activity. J Biol Chem. 1984 Feb 10;259(3):1375–1377. [PubMed] [Google Scholar]
- To-E A., Ueda Y., Kakimoto S. I., Oshima Y. Isolation and characterization of acid phosphatase mutants in Saccharomyces cerevisiae. J Bacteriol. 1973 Feb;113(2):727–738. doi: 10.1128/jb.113.2.727-738.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh-E A. Genetic Mapping of the pho2, PHO82-pho4 and pho85 Loci of Yeast. Genetics. 1980 Apr;94(4):929–932. doi: 10.1093/genetics/94.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh-E A., Guerry P., Wickner R. B. Chromosomal superkiller mutants of Saccharomyces cerevisiae. J Bacteriol. 1978 Dec;136(3):1002–1007. doi: 10.1128/jb.136.3.1002-1007.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumbly R. J., Bradley G. Isolation and characterization of aminopeptidase mutants of Saccharomyces cerevisiae. J Bacteriol. 1983 Oct;156(1):36–48. doi: 10.1128/jb.156.1.36-48.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi M. The isolation and genetic analysis of sporulation-deficient mutants in Saccharomyces cerevisiae. Mol Gen Genet. 1983;191(1):17–21. doi: 10.1007/BF00330883. [DOI] [PubMed] [Google Scholar]
- Turoscy V., Chisholm G., Cooper T. G. Location of the genes that control induction of the allantoin-degrading enzymes in Saccharomyces cerevisiae. Genetics. 1984 Dec;108(4):827–831. doi: 10.1093/genetics/108.4.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban-Grimal D., Labbe-Bois R. Genetic and biochemical characterization of mutants of Saccharomyces cerevisiae blocked in six different steps of heme biosynthesis. Mol Gen Genet. 1981;183(1):85–92. doi: 10.1007/BF00270144. [DOI] [PubMed] [Google Scholar]
- Van Loon A. P., Vijn R. J., De Groot R. J., Polman J. E., Grivell L. A. Nuclear genes coding for four subunits of the yeast ubiquinol-cytochrome c reductase complex are present in single copies in the haploid genome and at least two of these are located on different chromosomes. Mol Gen Genet. 1984;197(2):219–224. doi: 10.1007/BF00330966. [DOI] [PubMed] [Google Scholar]
- Wickner R. B., Boutelet F., Hilger F. Evidence for a new chromosome in Saccharomyces cerevisiae. Mol Cell Biol. 1983 Mar;3(3):415–420. doi: 10.1128/mcb.3.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B., Leibowitz M. J. Chromosomal genes essential for replication of a double-stranded RNA plasmid of Saccharomyces cerevisiae: the killer character of yeast. J Mol Biol. 1976 Aug 15;105(3):427–443. doi: 10.1016/0022-2836(76)90102-9. [DOI] [PubMed] [Google Scholar]
- Wickner R. B., Leibowitz M. J. Dominant chromosomal mutation bypassing chromosomal genes needed for killer RNA plasmid replication in yeast. Genetics. 1977 Nov;87(3):453–469. doi: 10.1093/genetics/87.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B., Leibowitz M. J. Mak mutants of yeast: mapping and characterization. J Bacteriol. 1979 Oct;140(1):154–160. doi: 10.1128/jb.140.1.154-160.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B., Leibowitz M. J. Two chromosomal genes required for killing expression in killer strains of Saccharomyces cerevisiae. Genetics. 1976 Mar 25;82(3):429–442. doi: 10.1093/genetics/82.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B. Mapping chromosomal genes of Saccharomyces cerevisiae using an improved genetic mapping method. Genetics. 1979 Jul;92(3):803–821. doi: 10.1093/genetics/92.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B. Mutants of Saccharomyces cerevisiae that incorporate deoxythymidine-5'-monophosphate into deoxyribonucleic acid in vivo. J Bacteriol. 1974 Jan;117(1):252–260. doi: 10.1128/jb.117.1.252-260.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B., Ridley S. P., Fried H. M., Ball S. G. Ribosomal protein L3 is involved in replication or maintenance of the killer double-stranded RNA genome of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4706–4708. doi: 10.1073/pnas.79.15.4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B. Twenty-six chromosomal genes needed to maintain the killer double-stranded RNA plasmid of Saccharomyces cerevisiae. Genetics. 1978 Mar;88(3):419–425. doi: 10.1093/genetics/88.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston F., Chaleff D. T., Valent B., Fink G. R. Mutations affecting Ty-mediated expression of the HIS4 gene of Saccharomyces cerevisiae. Genetics. 1984 Jun;107(2):179–197. doi: 10.1093/genetics/107.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamb T. J., Petes T. D. Analysis of the junction between ribosomal RNA genes and single-copy chromosomal sequences in the yeast Saccharomyces cerevisiae. Cell. 1982 Feb;28(2):355–364. doi: 10.1016/0092-8674(82)90353-1. [DOI] [PubMed] [Google Scholar]
- Zubenko G. S., Mitchell A. P., Jones E. W. Mapping of the proteinase b structural gene PRB1, in Saccharomyces cerevisiae and identification of nonsense alleles within the locus. Genetics. 1980 Sep;96(1):137–146. doi: 10.1093/genetics/96.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Poll K. W., Schamhart D. H. Characterization of a regulatory mutant of fructose 1,6-bisphosphatase in Saccharomyces carlsbergensis. Mol Gen Genet. 1977 Jul 7;154(1):61–66. doi: 10.1007/BF00265577. [DOI] [PubMed] [Google Scholar]


