Abstract
Parkia speciosa Hassk., or stink bean, is a plant indigenous to Southeast Asia. It is consumed either raw or cooked. It has been used in folk medicine to treat diabetes, hypertension, and kidney problems. It contains minerals and vitamins. It displays many beneficial properties. Its extracts from the empty pods and seeds have a high content of total polyphenol, phytosterol, and flavonoids. It demonstrates a good antioxidant activity. Its hypoglycemic effect is reported to be attributable to the presence of β-sitosterol, stigmasterol, and stigmast-4-en-3-one. The cyclic polysulfide compounds exhibit antibacterial activity, while thiazolidine-4-carboxylic acid possesses anticancer property. The pharmacological properties of the plant extract are described in this review. With ongoing research conducted on the plant extracts, Parkia speciosa has a potential to be developed as a phytomedicine.
1. Introduction
Parkia speciosa Hassk., or stink bean, is a plant that is abundantly found in the tropical regions like Malaysia, Indonesia, Thailand, and Philippines [1, 2]. It is a plant that belongs to the genus Parkia and species speciosa in the family Fabaceae (also placed in Leguminosae and Mimosaceae). It is known as petai in Malaysia, Singapore, and Indonesia [1, 3], sator or sataw in Thailand [4, 5], u'pang in Philippines [3], and yongchak in India [6]. It grows up to 40 meter high [6]. It bears green long and flat beans which are called pods in stalks. The stalks are 2 to 6 cm wide and 30 to 45 cm long. The light green stink bean seeds with seed coats are encapsulated in these pods (Figure 1). The seeds have a peculiar smell and can be eaten raw as “ulam” (a Malay word for uncooked) or cooked. The seeds are the most consumed “ulam” in Malaysia [7]. Half-riped seeds are also usually pickled in brine. The plant seeds have been used by the locals to treat various diseases and symptoms like diabetes, kidney disorder, and headache [1, 8, 9].
Figure 1.

The pods and seeds with (grey, A) or without (green, B) seed coats of Parkia speciosa. The plant materials were collected from a plantation at Batang Kali, Selangor, Malaysia, in January 2013.
2. Nutritional Values
P. speciosa seeds contain many nutritional values such as protein, fat, and carbohydrate. They are also a good source for minerals (Table 1). The seeds have a considerable amount of vitamin C [10] and α-tocopherol (vitamin E) [11]. Among fourteen types of vegetable which were commonly consumed by the southern Thais, P. speciosa seeds had relatively the highest content of thiamin (vitamin B1, 2.8 μg/g) but insignificant antithiamine factor [12]. High concentration of tannin was detected in its seed coats and pods compared to other fruit vegetables [5]. Tannin has been reported to decrease protein and amino acid digestibility [13]. Therefore, it is not advisable for children to consume the seeds in high amounts as good protein absorption is necessary for good body development.
Table 1.
Nutritional value of Parkia speciosa Hassk. seeds.
| Component | Composition (per 100 g edible portion) |
|---|---|
| Ash (g) | 1.2–4.6 |
| Protein (g) | 6.0–27.5 |
| Fat (g) | 1.6–13.3 |
| Carbohydrate (g) | 13.2–52.9 |
| Crude fiber (g) | 1.7–2.0 |
| Energy (kcal) | 91.0–441.5 |
| Calcium (mg) | 108.0–265.1 |
| Iron (mg) | 2.2–2.7 |
| Phosphorus (mg) | 115.0 |
| Potassium (mg) | 341.0 |
| Magnesium (mg) | 29.0 |
| Manganese (ppm) | 42.0 |
| Copper (ppm) | 36.7 |
| Zinc (ppm) | 8.2 |
| Vitamin C (mg) | 19.3 |
| α-Tocopherol (mg) | 4.15 |
| Thiamin (mg) | 0.28 |
3. Chemical Compounds
Table 2 tabulates the phytochemicals screening in various parts of P. speciosa. Almost all major chemical compounds are present in the seeds. Phenolic compounds are also present in almost all parts of the plant. To date, not many studies have been done to elucidate the chemical properties in the pods. In the seeds, the terpenoids detected using gas chromatography were β-sitosterol, stigmasterol, lupeol, campesterol, and squalene [14, 15]. Interestingly, lupeol was found to possess anticarcinogenic [16], antinociceptive, and anti-inflammatory properties [17]. No flavonoid like quercetin, myricetin, luteolin, kaempferol, or apigenin was detected in the methanolic extract of P. speciosa seeds using a reversed-phase high performance liquid chromatography [18], but it was noted to be present in the ethanolic extract when screened using a colorimetric assay [10]. Besides, alkaloids and saponins were also found in the plant. The seeds also contain cyclic polysulfides, namely, hexathionine, tetrathiane, trithiolane, pentathiopane, pentathiocane, and tetrathiepane [19] which are responsible for its strong pungent smell and taste, while the presence of djenkolic acid in the seeds is thought to cause blockage of the ureter [20].
Table 2.
Phytochemical substances in P. speciosa.
| Parts | Alkaloid | Saponin | Terpenoids | Phenolic | Flavonoid | Tannin |
|---|---|---|---|---|---|---|
| Seeds | + | − | + | + | + | − |
| Barks | + | − | − | + | − | ND |
| Leaves | − | − | + | + | + | ND |
| Seed coats | + | + | − | − | + | + |
| Pods | ND | ND | ND | + | ND | + |
Chromatography analysis of the stink bean seeds had identified presence of fatty acids which were undecanoic, myristic, palmitic, oleic, linoleic, elaidic, stearic, stearoic, lauric, arachidonic, and linoleic acids [14]. In the seeds, formation of thiazolidine-4-carboxylic acid, a thioproline, was remarkably increased after boiling when detected using gas chromatography-thermal energy analyzer [21]. This compound was reported to have an anticarcinogenic property [22]. The chemical structures of lupeol, thiazolidine-4-carboxylic acid, and some cyclic polysulfides (tetrathiane, tetrathiepane, trithiolane, and pentathiocane) are shown in Figure 2.
Figure 2.
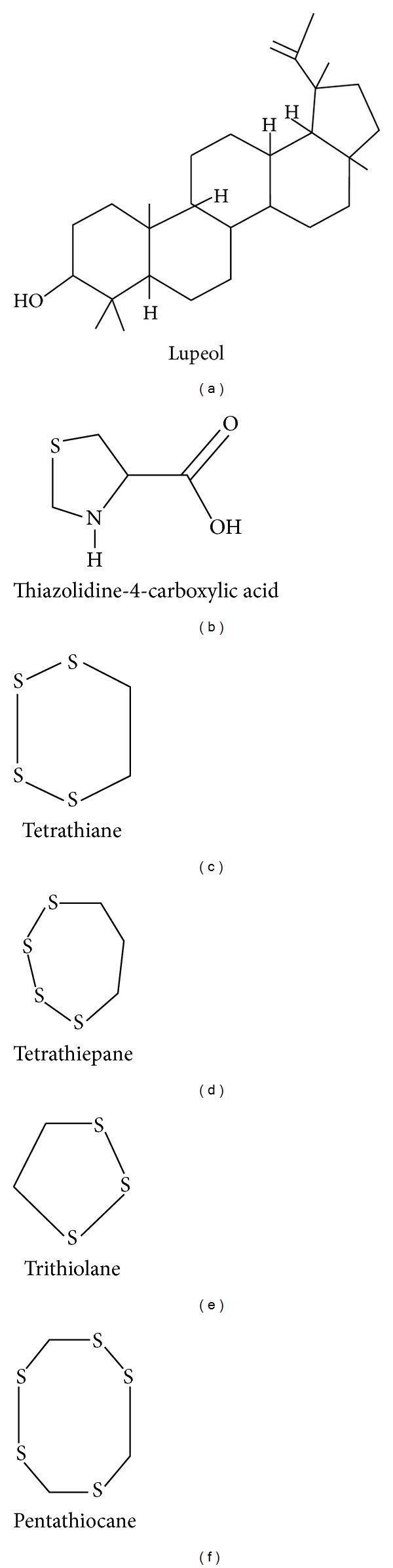
Chemical structures of lupeol (a), thiazolidine-4-carboxylic acid (b), and some cyclic polysulfides (c-f) found in Parkia speciosa.
4. Properties of P. speciosa
Studies conducted on P. speciosa have revealed many potential properties of the plant. However, it needs to be ventured further to understand and to identify its mechanism of actions for future use in humans. Each property of the plants is described and discussed.
4.1. Antioxidant Activity
Oxidative stress has been implicated to play an important role in many pathological conditions such as hypertension [23], hyperbilirubinemia [24], stress-induced gastric lesion [25], hyperhomocysteinemia [26], cancer [27], atherosclerosis [28], and diabetes [29]. Therefore, there is a growing interest to study plants with potential antioxidant property to treat various diseases. P. speciosa is not an exception.
Commonly, the simple ways to measure natural antioxidant in plant extracts are the total phenolic content, reducing ferric ion antioxidant potential (FRAP) and 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical-scavenging assays. Plants are a major source of phenolic compounds such as cinnamic, p-coumaric, caffeic, ferulic, chlorogenic, protocatechuic, and gallic acids [10]. The total phenolic content assay commonly uses the gallic acid as a standard. The FRAP assay is done to determine the antioxidant activity of a compound by measuring its redox property to reduce the ferric ion by single electron donation, while the DPPH radical-scavenging assay measures the ability of the compound to donate hydrogen to DPPH radical. There is a strong positive correlation between the FRAP and DPPH assays [30, 31] and between both assays and the total phenolic content [31].
Table 3 summarizes screening studies of the antioxidant properties of P. speciosa. The antioxidant capacity was relatively very high in the pods and seeds mixture where the methanolic extract had larger capacities than the aqueous extract for all the three assays [32]. Methanolic extract contains hydrophilic and intermediate hydrophilic compounds, whereas the aqueous extract contains hydrophilic constituents only. This difference explains the greater capacity of the methanolic extract. Antioxidant activity was also present in the seeds and leaves of the P. speciosa but with lower activities when compared to the activity in the pod and seed mixtures. This suggests that the pods retain greater antioxidant content than the other parts of the plant. Flavonoids which possess antioxidant property were detected in the ethanolic extract of the seeds [10], but none was found in its methanolic extract [18]. The antioxidant content and activity of P. speciosa is considered high amongst edible plants [10, 32, 33] especially its total phenolic contents. There was a difference in the total phenolic and flavonoid contents from the seeds extracted in ethanol and methanol. Both compounds were found to be higher in the ethanolic extract than the methanolic extract [10, 18], which could be due to more hydrophobic compounds being retained in the ethanol compared to the methanol extract. This is further shown in two different studies [18, 34] where the total phenolic content was totally absent in the former study [18] but detected at a larger content in the latter study [34]. There are many factors that may affect the chemical content or composition in plants such as species, method of extraction, storage condition, and season and age of the plant parts at the time of harvest as well as geographical factors.
Table 3.
Antioxidant activity in various P. speciosa extracts.
| Plant part | Extract | Total phenolic content (mg GAE/g)a | DPPH assay (μmol Trolox/g)a | FRAP assay (μmol Trolox/g)a | Total flavonoids (mg RE/g)a | Tannin (mg/g)a | Reference |
|---|---|---|---|---|---|---|---|
| Pod and seed | Aqueous | 1557.6b,c | 7418.3b,d | 1617.3b,d | — | — | Ayub Ali et al. [32] |
| Pod and seed | Methanol | 2464.3b,c | 5936.9b,d | 1898.0b,d | — | — | Ayub Ali et al. [32] |
| Pod | Ethanol | — | — | — | — | 250 | Tunsaringkarn et al. [5] |
| Seed | Ethanol | 51.9a | — | — | 20.3a | — | Maisuthisakul et al. [10] |
| Seed | Methanol | — | — | — | 0 | — | Miean and Mohamed [18] |
| Seed | Methanol | 120b,c | 40b,e | — | — | — | Tangkanakul et al. [34] |
| Seed | Aqueous | 6.5 | 67.62 | 44.67 | — | — | Reihani and Azhar [30] |
| Seed coat | Ethanol | — | — | — | — | 350 | Tunsaringkarn et al. [5] |
| Leaf | Ethanol | 44.7 | 89.26f | — | — | — | Tangkanakul et al. [4] |
| Leaf | Aqueous | 22.7 | 57.4f | — | — | — | Tangkanakul et al. [4] |
| Leaf | Aqueous | 32.73 | 22.7 | 49.9 | — | — | Wong et al. [33] |
aDry weight basis, bfresh weight basis, c(mg GAE/100 g), d(mg Trolox/100 g), e(mg vitamin C equivalent/g), and f(mg BHA equivalent/g).
The DPPH radical-scavenging activity of the plant was also determined based on median inhibition concentration (IC50). The pod ethanolic extract of the plant exhibited mean IC50 of 10.03 μg/mL compared to those of standard antioxidants used, quercetin (0.45 μg/mL), and butylated hydroxytoluene (BHT, 3.47 μg/mL). The finding indicated that the extract possessed a relatively high antioxidant property when compared to other plant extracts which gave IC50 in the range of 0.06 to 2016.64 μg/mL [35]. Another similar study showed a lower DPPH radical-scavenging mean IC50 of which was 0.667 μg/mL in the ethanolic extract of the seeds [10], suggesting that this extract also has higher antioxidant activity in the seeds.
The antioxidant activity of the plant was also assessed using a Heinz body induction using an in vitro model [5]. In the study, packed red cells that were mixed with acetylphenylhydrazine (a hemolytic agent) and incubated with the extracts from P. speciosa seed coat and pericarp (pods) showed lower Heinz body formation than other plant extracts. This indicated that the extracts were able to inhibit oxidative destruction to the erythrocytes. The seed coat extract exhibited the highest inhibitory activity, while the pericarp extract was the third highest amongst the twenty-one plants tested. The IC50 for the former was 3.90 mg/mL and the latter was 46.29 mg/mL. The inhibitory activity was found to be positively correlated to the tannin concentration in the plants (r = 0.658, P < 0.01). This proved that tannin which was found in the plant extracts had a strong Heinz body inhibitory ability and antioxidant ability [5]. The findings of the study showed that P. speciosa has a potential to be used as an agent to reduce hemolytic jaundice.
4.2. Hypoglycemic Activity
Diabetes is a general term that describes a disease with an elevation of blood glucose more than normal or known as hyperglycemia, when the body is unable to metabolize glucose properly. There are two types of diabetes mellitus; insulin-dependent (IDDM, type 1) and noninsulin-dependent (NIDDM, type 2). The former type is manifested by a low insulin release due to destruction of the pancreatic cells and it can be controlled by regular administrations of insulin [36]. In type 2 NIDDM, the body fails to use insulin properly or becomes less responsive to insulin [37].
Many plants have been screened for its hypoglycemic property. Studies regarding the hypoglycemic property of P. speciosa had started in early 1990s [38, 39]. The plant showed good hypoglycemic activity in in vivo and in vitro experiments. Many crude P. speciosa extracts from the empty pods (pericarp) and seeds were tested for the activity. Research works done by Jamaluddin and Mohamed [38] and Jamaluddin et al. [40] showed that the antidiabetic activity was only observed in the chloroform extract, either in seeds or empty pods, and none in other extracts such as petroleum ether, dichloromethane, ethyl acetate, ammoniacal chloroform, and methanol. Oral administration of the chloroform extract of the empty pods and seeds significantly decreased glucose level 2 hours after ingestion and the effect lasted for at least 24 hours in alloxan-induced diabetic rats. The activity was higher in the seeds than in the empty pods [38] with a minimum effective dose of 25 mg/kg and 50 mg/kg, respectively [39, 40]. β-Sitosterol and stigmasterol, the two major phytosterols present in the seeds of P. speciosa were responsible for the hypoglycemic activity. They acted synergistically but no hypoglycemic effect was observed when they were tested individually [39], while in the empty pods, stigmast-4-en-3-one was identified and elucidated to be the active compound that produced the effect. It reduced blood glucose level by 84% at 100 mg/kg body weight compared to 111% reduction by glibenclamide (5 mg/kg body weight) [40]. However, the possible hypoglycemic mechanism of the pure compounds whether they had any effect on insulin release or glucose absorption from the gut was not elucidated in these studies.
Other than alloxan-induced diabetic rat model, the hypoglycemic property of the plant was also tested in in vitro experiments by measuring the activities of α-amylase and α-glucosidase. α-Amylase is an enzyme that is involved in carbohydrates breakdown to produce simpler saccharides, whereas α-glucosidase is the enzyme involved in the carbohydrates intestinal absorption [41]. Therefore, inhibition of both enzymes would be beneficial in NIDDM treatment due to the delayed digestion and uptake of glucose from the intestinal tract. Decreased postprandial hyperglycemia is one of therapeutic approaches in the management of diabetes. Strong inhibitor of both enzymes like Acarbose [42] produces common side effects such as abdominal distention and flatulence, due to its strong inhibition on pancreatic α-amylase [43], thus results in colonic bacterial fermentation of undigested carbohydrates [44]. Therefore, a drug with a weak α-amylase inhibition but a good inhibitory property against α-glucosidase would be a good alternative.
The hexane and dichloromethane extracts of P. speciosa seeds showed no inhibitory effect on α-amylase activity [45]. However, the aqueous extract of the plant demonstrated a good α-glucosidase inhibitory activity with the empty pods having almost 15 times higher activity than the seeds [46, 47]. The α-glucosidase inhibitory activity was also observed in the petroleum ether, dichloromethane, and ethanolic extracts. Similar to the aqueous extract, the empty pods of these extracts possessed better activity than the seeds about twofold [48]. However, the aqueous extract of the seeds was able to increase insulin release [49].
P. speciosa has the potential to be developed as an oral hypoglycemic agent. Nevertheless, it was not shown to have any effect on blood glucose level in normal healthy experimental animals [38, 40]. Several possible mechanistic approaches need to be carried out before its possible use in humans.
4.3. Antitumor and Antimutagenicity
Cancer is one of the leading causes of death worldwide. Much attention has been paid to explore any potential antitumor agents in edible plants, for future use in humans. Amongst many medicinal plants screened, the methanolic extract of P. speciosa seeds demonstrated a moderate antimutagenic activity in the Ames test [34], while in inhibition assay of Epstein-Barr virus (EBV), the antitumor promoting activity of the seeds was considered as weakly active [50]. Nevertheless, it had been reported that consumption of the raw seeds reduced the incidence of esophageal cancer in Southern Thailand [51].
The methanolic extract of the seed coats exhibited selective cytotoxicity on breast cancer (MCG-7 and T47D), colon cancer (HCT-116), and hepatocarcinoma (HepG2) cells [52]. The methanolic extract of the seeds on the other hand did not show significant cytotoxic effect on any cancer cell lines. However, the ethyl acetate subextract of the methanolic extract showed selective cytotoxicity on hormone sensitive breast cancer cells, MCF-7 [53]. These findings are not conclusive yet due to the nature of the studies that used crude extract rather than pure compounds.
Development of a tumor is always associated with host immune response. Increased immune system enhances the ability of the host to resist tumor development as well as infectious diseases. Compounds with the ability to increase mitogenesis of lymphocytes may have a potential to be used as antitumor agents [54]. Lectin isolated from the P. speciosa seeds had shown mitogenic effect on human lymphocytes and rat thymocytes. The lectin stimulated incorporation of [3H]-thymidine into cell DNA [55, 56]. Its activity increased with the increasing dose, before declining to an optimum point. The effect was comparable to other known T-cell mitogens such as concanavalin A, pokeweed mitogen, and phytohemagglutinin [55]. Lectins with mitogenic activity usually exert antiproliferative, immunomodulatory, and antitumor properties [54]. Similar to lectins from other sources, the P. speciosa lectin also had a strong hemagglutinating activity for rabbit, goat, rat, and human erythrocytes [47, 56, 57]. These findings indicate that lectin from the seeds may increase DNA synthesis and, therefore, may enhance immune response against infections and tumors. The effect of natural products on tumor cell viability was found to be negatively associated with their mitogenic activity [58].
Angiogenesis is a critical process which is involved in many physiological and pathological conditions such as metastasis of solid tumors. The methanolic extract of the fresh pods of the P. speciosa demonstrated antiangiogenic activity. In vitro, the extract inhibited microvessel outgrowth in rat aortae more than 50%, which was not observed in the water and hexane extracts. It also inhibited the ability of human umbilical vein endothelial cells (HUVEC) to form capillary-like structures in matrigel matrix [53]. Both extracts of hexane and methanol of the seed coats showed antiangiogenic activity in rat aortic rings with vessels outgrowth inhibition of 74% and 82%, respectively [52]. The effect might be due to the formation of many vacuoles in the endothelial cells observed in light microscopy [53]. The presence of the vacuoles indicates cellular starvation due to nutritional deprivation which is an essential characteristic to maintain the viability of the cells [59]. This property is beneficial in the treatment of cancer due to its ability to prevent neovascularization of the tumors.
Thiazolidine-4-carboxylic acid, a thioproline, might be responsible for the antitumor effect of the seeds. It was shown to possess antiproliferative effects against cancer cells [22]. It was found in cooked seeds of P. speciosa but was undetectable in uncooked seeds [21]. The compound is an effective nitrite-trapping agent which can inhibit the endogenous formation of carcinogenic N-nitroso compounds [60]. Its derivatives were previously designed and synthesized as novel influenza neuraminidase inhibitors [61].
4.4. Antimicrobial Activity
The seeds of P. speciosa have been used by the Orang Asli in West Malaysia to treat kidney disorder which is believed to be urinary tract infection [1]. Studies regarding the antimicrobial property of P. speciosa are still lacking where, so far, only the seeds of the plant have been screened for its antimicrobial activity. The extracts of the seeds in petroleum ether, chloroform, and methanol demonstrated antibacterial activity against Helicobacter pylori but none was found in the water extract. The activity was the highest in the chloroform extract followed by methanol and petroleum ether. Comparatively, the chloroform extract showed a moderate inhibition zone diameter to mg extract ratio (25.0), while the ratio of other plant extracts was in the range of 1.5 to 117.5 [62]. A previous study also showed the ability of the seed extract in methanol to inhibit H. pylori growth, while the ethyl acetate extract was effective against Escherichia coli. These extracts, however, had no inhibitory effect on Salmonella typhimurium, Salmonella typhi, and Shigella sonnei growth [63]. An aqueous suspension of the seeds displayed an ability to inhibit the growth of Aeromonas hydrophila, Staphylococcus aureus, Streptococcus agalactiae, Streptococcus anginosus, and Vibrio parahaemolyticus. However, the suspension was ineffective against Citrobacter freundii, Edwardsiella tarda, Escherichia coli, Vibrio alginolyticus, and Vibrio vulnificus. These bacteria were isolated from moribund fishes and shrimps [64].
Collectively, it may be assumed that the seeds of P. speciosa are more effective against Gram-negative bacteria. However, the spectrum of the activity depends on the type of the extract. The antibacterial property is due to the seed content of hexathionine and trithiolane, two cyclic polysulfide compounds [19]. It was also screened for antiplasmodial activity against Plasmodium falciparum, but no activity was found [65].
4.5. Effects on Cardiovascular System
Decoction of the roots of P. speciosa has been used in folk medicine in Malaysia to treat hypertension [9, 66]. To date, however, no scientific data regarding the plant effects on hypertension is available. Hypertension increases the risk of atherosclerosis, an artery clogging process which leads to heart attacks and strokes. Angiogenesis plays an important role in atherosclerosis. As mentioned earlier, the methanolic extract of the empty pods also possessed antiangiogenic property. It is suggestive that the plant extract may inhibit or reduce the development of atherosclerosis, thus needs to be explored further. Vascular endothelial growth factor (VEGF) is a factor that is involved in pathological angiogenesis or hypervascularization [67] which also plays a crucial role in atherosclerotic lesions [68]. The methanolic extract was shown to inhibit the expression of VEGF and neovascularization in rat aortic rings [53].
Its possible fibrinolytic activity was screened among other Thai indigenous plants using an in vitro experiment by measuring the clear zone area of fibrinogen and thrombin mixture. Relatively, P. speciosa had the lowest fibrinolytic activity which was only 1.5 mm2 (the plant with the highest activity was 50.2 mm2) [69]. Thus, it can be considered that the plant has no significant fibrinolytic activity.
5. Pharmacokinetics and Toxicity
To date there is no single study conducted on the pharmacokinetics of P. speciosa. This could be due to research being carried out on this plant so far only used its crude extracts rather than the pure compounds. A few studies conducted had detected the active compounds responsible for its hypoglycemic effects (β-sitosterol, stigmasterol, and stigmast-4-en-3-one), antibacterial activity (hexathionine and trithiolane), and antitumor (lectin and thiazolidine-4-carboxylic acid) [19, 39, 40, 56], but to date no study had used the pure compounds for the respective effects. Other studies performed were still at the stage of activity screening. Pharmacokinetic data of P. speciosa is important and vital in order to better understand its pharmacodynamic effects. The extent of its absorption would affect the amount of dosage needs to be administered. Its metabolism pathway should also be studied to determine whether the produced metabolites are toxic or otherwise. Its excretion is believed to be through the kidneys due to the odorized urine after its consumption. Other possible routes are not known.
Its toxicity study is also lacking. No in vivo toxicity study has been carried out. Only Aisha et al. [53] had performed a cytotoxicity study of the plant using HUVEC. In their study, the methanolic extract of the fresh pods (100 μg/mL) did not show any significant cytotoxic effect on the cell lines. Information gathered from the locals, consumption of the seeds up to 30 seeds (two long pods) in a serving almost everyday does not cause any adverse effect.
6. Conclusion
Parkia speciosa Hassk. which is rich in antioxidant content especially total phenolic has many potentials to be developed as a phytomedicine. The properties are attributable to the presence of β-sitosterol, stigmasterol, stigmastenone, thiazolidine-4-carboxylic acid, hexathionine, and trithiolane in the plant. Traditionally, it is used to treat hypertension, diabetes, and headache, but with no scientific evidence so far. Many scientific studies have been performed on its hypoglycemic, antitumor, antimicrobial, and antiangiogenic properties. This still warrants further studies to explore the potential properties of the plant including its antihypertensive, analgesic, or anti-inflammatory (due to its lupeol and flavonoid contents) properties. Further toxicity studies and research in humans should also be conducted.
Acknowledgment
The financial fund was provided by the Universiti Kebangsaan Malaysia (UKM-GUP-2011-296).
References
- 1.Samuel AJSJ, Kalusalingam A, Chellappan DK, et al. Ethnomedical survey of plants used by the Orang Asli in Kampung Bawong, Perak, West Malaysia. Journal of Ethnobiology and Ethnomedicine. 2010;6, article 5 doi: 10.1186/1746-4269-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rozaq P, Sofriani N. Organic pesticide from urine and spices modification. Asian Journal of Food and Agro-Industry. 2009:S105–S111. [Google Scholar]
- 3.Orwa C, Mutua A, Kindt R, Jamnadass R, Simons A. Agroforestree Database: a tree reference and selection guide. Version 4.0, 2009, http://www.worldagroforestry.org/af/treedb/
- 4.Tangkanakul P, Trakoontivakorn G, Jariyavattanavijit C. Extracts of Thai indigenous vegetables as rancid inhibitor in a model system. Kasetsart Journal. 2005;39(2):274–283. [Google Scholar]
- 5.Tunsaringkarn T, Soogarun S, Rungsiyothin A, Palasuwan A. Inhibitory activity of Heinz body induction in vitro antioxidant model and tannin concentration of Thai mimosaceous plant extracts. Journal of Medicinal Plants Research. 2012;6(24):4096–4101. [Google Scholar]
- 6.Lim TK. Edible Medicinal and Non-Medicinal Plants: Fruits. Vol. 2. Springer Science Business Media; 2012. Parkia speciosa; pp. 798–803. [Google Scholar]
- 7.Nurul Izzah A, Aminah A, Md Pauzi A, Lee YH, Wan Rozita WM, Siti Fatimah D. Patterns of fruits and vegetable consumption among adults of different ethnics in Selangor, Malaysia. International Food Research Journal. 2012;19(3):1095–1107. [Google Scholar]
- 8.Milow P, Ghazali NH, Mohammad NS, Ong HC. Characterization of plant resource at Kampung Parit Tok Ngah, Perak, Malaysia. Scientific Research and Essays. 2011;6(13):2606–2618. [Google Scholar]
- 9.Azliza MA, Ong HC, Vikineswary S, Noorlidah A, Haron NW. Ethno-medicinal resources used by the Temuan in Ulu Kuang Village. Ethno Medicine. 2012;6(1):17–22. [Google Scholar]
- 10.Maisuthisakul P, Pasuk S, Ritthiruangdej P. Relationship between antioxidant properties and chemical composition of some Thai plants. Journal of Food Composition and Analysis. 2008;21(3):229–240. [Google Scholar]
- 11.Ching LS, Mohamed S. Alpha-tocopherol content in 62 edible tropical plants. Journal of Agricultural and Food Chemistry. 2001;49(6):3101–3105. doi: 10.1021/jf000891u. [DOI] [PubMed] [Google Scholar]
- 12.Taungbodhitham AK. Thiamin content and activity of antithiamin factor in vegetables of southern Thailand. Food Chemistry. 1995;52(3):285–288. [Google Scholar]
- 13.Sarwar Gilani G, Wu Xiao C, Cockell KA. Impact of antinutritional factors in food proteins on the digestibility of protein and the bioavailability of amino acids and on protein quality. British Journal of Nutrition. 2012;108(2, supplement):S315–S332. doi: 10.1017/S0007114512002371. [DOI] [PubMed] [Google Scholar]
- 14.Mohd Azizi CY, Salman Z, Nik Norulain NA, Mohd Omar AK. Extraction and identification of compounds from Parkia speciosa seeds by supercritical carbon dioxide. Journal of Chemical and Natural Resources Engineering. 2008;2:153–163. [Google Scholar]
- 15.Nik Norulaini NAR, Zhari S, Sarker MZI, Ferdosh S, Che Yunus MA, Abd Kadir MO. Profile of Parkia speciosa Hassk metabolites extracted with SFE using FTIR- PCA Method. Journal of Chinese Chemical Society. 2011;58(6):1–9. [Google Scholar]
- 16.Manoharan S, Palanimuthu D, Baskaran N, Silvan S. Modulating effect of lupeol on the expression pattern of apoptotic markers in 7, 12-dimethylbenz(a)anthracene induced oral carcinogenesis. Asian Pacific Journal of Cancer Prevention. 2012;13(11):5753–5757. doi: 10.7314/apjcp.2012.13.11.5753. [DOI] [PubMed] [Google Scholar]
- 17.Chen YF, Ching C, Wu TS, Wu CR, Hsieh WT, Tsai HY. Balanophora spicata and lupeol acetate possess antinociceptive and anti-inflammatory activities in vivo and in vitro . Evidence-Based Complementary and Alternative Medicine. 2012;2012:20 pages. doi: 10.1155/2012/371273.371273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miean KH, Mohamed S. Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. Journal of Agricultural and Food Chemistry. 2001;49(6):3106–3112. doi: 10.1021/jf000892m. [DOI] [PubMed] [Google Scholar]
- 19.Gmelin R, Susilo R, Fenwick GR. Cyclic polysulphides from Parkia speciosa . Phytochemistry. 1981;20(11):2521–2523. [Google Scholar]
- 20.Susilo R, Gmelin R. Precursor of cyclic polysulphides in seeds of Parkia speciosa . Zeitschrift für Naturforschung. 1982;37:584–586. [Google Scholar]
- 21.Suvachittanont W, Kurashima Y, Esumi H, Tsuda M. Formation of thiazolidine-4-carboxylic acid (thioproline), an effective nitrite-trapping agent in human body, in Parkia speciosa seeds and other edible leguminous seeds in Thailand. Food Chemistry. 1996;55(4):359–363. [Google Scholar]
- 22.Chen J, Wang Z, Lu Y, Dalton JT, Miller DD, Li W. Synthesis and antiproliferative activity of imidazole and imidazoline analogs for melanoma. Bioorganic and Medicinal Chemistry Letters. 2008;18(11):3183–3187. doi: 10.1016/j.bmcl.2008.04.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leong X-F, Salimon J, Mustafa MR, Jaarin K. Effect of repeatedly heated palm olein on blood pressure-regulating enzymes activity and lipid peroxidation in rats. Malaysian Journal of Medical Sciences. 2012;19(1):20–29. [PMC free article] [PubMed] [Google Scholar]
- 24.Kamisah Y, Norhayati MY, Zakri B, Asmadi AY. The effects of palmvitee on δ-aminolevulinic acid-induced hyperbilirubinaemia in suckling rats. Archives of Medical Science. 2009;5(3):329–334. [Google Scholar]
- 25.Mohd Fahami NA, Ibrahim IA, Kamisah Y, Mohd Ismail N. Palm vitamin E reduces catecholamines, xanthine oxidase activity and gastric lesions in rats exposed to water-immersion restraint stress. BMC Gastroenterology. 2012;12, article 54 doi: 10.1186/1471-230X-12-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Norsidah KZ, Asmadi AY, Azizi A, Faizah O, Kamisah Y. Palm tocotrienol-rich fraction reduced plasma homocysteine and heart oxidative stress in rats fed with a high-methionine diet. Journal of Physiology and Biochemistry. 2012 doi: 10.1007/s13105-012-0226-3. [DOI] [PubMed] [Google Scholar]
- 27.Zitka O, Skalickova S, Gumulec J, et al. Redox status expressed as GSH : GSSG ratio as a marker for oxidative stress in paediatric tumour patients. Oncology Letters. 2012;4(6):1247–1253. doi: 10.3892/ol.2012.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lacroix S, Rosiers CD, Tardif JC, Nigam A. The role of oxidative stress in postprandial endothelial dysfunction. Nutrition Research Reviews. 2012;25(2):288–301. doi: 10.1017/S0954422412000182. [DOI] [PubMed] [Google Scholar]
- 29.Kaneto H, Matsuoka TA. Involvement of oxidative stress in suppression of insulin biosynthesis under diabetic conditions. International Journal of Molecular Sciences. 2012;13(10):13680–13690. doi: 10.3390/ijms131013680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reihani SFS, Azhar ME. Antioxidant activity and total phenolic content in aqueous extracts of selected traditional Malay salads (Ulam) International Food Research Journal. 2012;19(4):1439–1444. [Google Scholar]
- 31.Razab R, Aziz AA. Antioxidants from tropical herbs. Natural Product Communications. 2010;5(3):441–445. [PubMed] [Google Scholar]
- 32.Ayub Ali M, Victoria Chanu K, Inaotombi Devi L. Antioxidant capacities of vegetables consumed in north east india assessed by three different in vitro assays. International Journal of Research in Pharmaceutical Sciences. 2011;2(2):118–123. [Google Scholar]
- 33.Wong SP, Leong LP, William Koh JH. Antioxidant activities of aqueous extracts of selected plants. Food Chemistry. 2006;99(4):775–783. [Google Scholar]
- 34.Tangkanakul P, Trakoontivakorn G, Saengprakai J, et al. Antioxidant capacity and antimutagenicity of thermal processed Thai foods. Japan Agricultural Research Quarterly. 2011;45(2):211–218. [Google Scholar]
- 35.Ramli S, Bunrathep S, Tansaringkarn T, Ruangrungsi N. Screening for free radical scavenging activity from ethanolic extract of mimosaceous plants endemic to Thailand. Journal of Health Research. 2008;22(2):55–59. [Google Scholar]
- 36.Pickup JC. Insulin-pump therapy for type 1 diabetes mellitus. New England Journal of Medicine. 2012;366(17):1616–1624. doi: 10.1056/NEJMct1113948. [DOI] [PubMed] [Google Scholar]
- 37.Little A, Feldman E, Hughes RAC. Enhanced glycemic control for preventing and treating diabetic neuropathy. Cochrane Database of Systematic Reviews. 2012;6 doi: 10.1002/14651858.CD007543.pub2.CD007543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jamaluddin F, Mohamed S. Hypoglycemic effect of extracts of petai papan (Parkia speciosa, Hassk) Pertanika Journal of Tropical Agricultural Science. 1993;16(3):161–165. [Google Scholar]
- 39.Jamaluddin F, Mohamed S, Lajis MN. Hypoglycaemic effect of Parkia speciosa seeds due to the synergistic action of β-sitosterol and stigmasterol. Food Chemistry. 1994;49(4):339–345. [Google Scholar]
- 40.Jamaluddin F, Mohamed S, Lajis Md. N. Hypoglycaemic effect of stigmast-4-en-3 one, from Parkia speciosa empty pods. Food Chemistry. 1995;54(1):9–13. [Google Scholar]
- 41.Al-Zuhair S, Dowaidar A, Kamal H. Inhibitory effects of dates-extract on α-amylase and α-glucosidase enzymes relevant to non-insulin dependent diabetes mellitus. Journal of Biochemical Technology. 2010;2(2):158–160. [Google Scholar]
- 42.Williamson G. Possible effects of dietary polyphenols on sugar absorption and digestion. Molecular Nutrition and Food Research. 2013;57(1):48–57. doi: 10.1002/mnfr.201200511. [DOI] [PubMed] [Google Scholar]
- 43.Chiasson J-L, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet. 2002;359(9323):2072–2077. doi: 10.1016/S0140-6736(02)08905-5. [DOI] [PubMed] [Google Scholar]
- 44.Bischoff H. Pharmacology of α-glucosidase inhibition. European Journal of Clinical Investigation. 1994;24(3, supplement):3–10. [PubMed] [Google Scholar]
- 45.Ali H, Houghton PJ, Soumyanath A. α-Amylase inhibitory activity of some Malaysian plants used to treat diabetes; with particular reference to Phyllanthus amarus . Journal of Ethnopharmacology. 2006;107(3):449–455. doi: 10.1016/j.jep.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 46.Tunsaringkarn T, Rungsiyothin A, Ruangrungsi N. α-Glucosidase inhibitory activity of water soluble extract from Thai mimosaceous plants. The Public Health Journal of Burapha University. 2009;4(2):54–63. [Google Scholar]
- 47.Chankhamjon K, Petsom A, Sawasdipuksa N, Sangvanich P. Hemagglutinating activity of proteins from Parkia speciosa seeds. Pharmaceutical Biology. 2010;48(1):81–88. doi: 10.3109/13880200903046195. [DOI] [PubMed] [Google Scholar]
- 48.Tunsaringkarn T, Rungsiyothin A, Ruangrungsi N. α-Glucosidase inhibitory activity of Thai mimosaceous plant extracts. Journal of Health Research. 2008;22(1):29–33. [Google Scholar]
- 49.Houghton PJ, Howes M-J, Lee CC, Steventon G. Uses and abuses of in vitro tests in ethnopharmacology: visualizing an elephant. Journal of Ethnopharmacology. 2007;110(3):391–400. doi: 10.1016/j.jep.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 50.Murakami A, Ali AM, Mat-Salleh K, Koshimizu K, Ohigashi H. Screening for the in vitro anti-tumor-promoting activities of edible plants from Malaysia. Bioscience, Biotechnology and Biochemistry. 2000;64(1):9–16. doi: 10.1271/bbb.64.9. [DOI] [PubMed] [Google Scholar]
- 51.Chanvitan A, Unolcholket S, Chongsuvivatwong V, Geater A. Risk factors for squamous cell carcinoma in southern Thailand. In: Chanvitan A, editor. Esophageal Canver Studies in Southern Thailand. Bangkok, Thailand: Medical Media; 1990. pp. 81–100. [Google Scholar]
- 52.Aisha AFA, Abu-Salah KM, Darwis Y, Abdul Majid AMS. Screening of antiangiogenie activity of some tropical plants by rat aorta ring assay. International Journal of Pharmacology. 2009;5(6):370–376. [Google Scholar]
- 53.Aisha AFA, Abu-Salah KM, Alrokayan SA, Ismail Z, Abdul Majid AMS. Evaluation of antiangiogenic and antoxidant properties of Parkia speciosa Hassk extracts. Pakistan Journal of Pharmaceutical Sciences. 2012;25(1):7–14. [PubMed] [Google Scholar]
- 54.Singh RS, Bhari R, Kaur HP. Mushroom lectins: current status and future perspectives. Critical Reviews in Biotechnology. 2010;30(2):99–126. doi: 10.3109/07388550903365048. [DOI] [PubMed] [Google Scholar]
- 55.Suvachittanont W, Jaranchavanapet P. Mitogenic effect of Parkia speciosa seed lectin on human lymphocytes. Planta Medica. 2000;66(8):699–704. doi: 10.1055/s-2000-9565. [DOI] [PubMed] [Google Scholar]
- 56.Suvachittanont W, Peutpaiboon A. Lectin from Parkia speciosa seeds. Phytochemistry. 1992;31(12):4065–4070. [Google Scholar]
- 57.Schertz KF, Boyd WC, Jurgelsky W, Cabanillas E. Seed extracts with agglutinating activity for human blood. Economic Botany. 1958;14(3):232–240. [Google Scholar]
- 58.Lee S-H, Lillehoj HS, Heckert RA, et al. Immune enhancing properties of safflower leaf (Carthamus tinctorius) on chicken lymphocytes and macrophages. Journal of Poultry Science. 2008;45(2):147–151. [Google Scholar]
- 59.Nakatogawa H, Ohsumi Y. Starved cells eat ribosomes. Nature Cell Biology. 2008;10(5):505–507. doi: 10.1038/ncb0508-505. [DOI] [PubMed] [Google Scholar]
- 60.Suo M, Mukaisho K-I, Shimomura A, Sugihara H, Hattori T. Thioproline prevents carcinogenesis in the remnant stomach induced by duodenal reflux. Cancer Letters. 2006;237(2):256–262. doi: 10.1016/j.canlet.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 61.Liu Y, Jing F, Xu Y, et al. Design, synthesis and biological activity of thiazolidine-4-carboxylic acid derivatives as novel influenza neuraminidase inhibitors. Bioorganic and Medicinal Chemistry. 2011;19(7):2342–2348. doi: 10.1016/j.bmc.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 62.Uyub AM, Nwachukwu IN, Azlan AA, Fariza SS. In-vitro antibacterial activity and cytotoxicity of selected medicinal plant extracts from penang island Malaysia on metronidazole-resistant Helicobacter pylori and some pathogenic bacteria. Ethnobotany Research and Applications. 2010;8:95–106. [Google Scholar]
- 63.Sakunpak A, Panichayupakaranant P. Antibacterial activity of Thai edible plants against gastrointestinal pathogenic bacteria and isolation of a new broad spectrum antibacterial polyisoprenylated benzophenone, chamuangone. Food Chemistry. 2012;130(4):826–831. [Google Scholar]
- 64.Musa N, Wei LS, Seng CT, Wee W, Leong LK. Potential of edible plants as remedies of systemic bacterial disease infection in cultured fish. Global Journal of Pharmacology. 2008;2(2):31–36. [Google Scholar]
- 65.Leaman DJ, Arnason JT, Yusuf R, et al. Malaria remedies of the Kenyah of the Apo Kayan, East Kalimantan, Indonesian Borneo: a quantitative assessment of local consensus as an indicator of biological efficacy. Journal of Ethnopharmacology. 1995;49(1):1–16. doi: 10.1016/0378-8741(95)01289-3. [DOI] [PubMed] [Google Scholar]
- 66.Ong HC, Ahmad N, Milow P. Traditional medicinal plants used by the Temuan villagers in Kampung Tering, Negeri Sembilan, Malaysia. Studies on Ethno-Medicine. 2011;5(3):169–173. [Google Scholar]
- 67.Gabhann FM, Qutub AA, Annex BH, Popel AS. Systems biology of pro-angiogenic therapies targeting the VEGF system. Wiley Interdisciplinary Reviews. 2010;2(6):694–707. doi: 10.1002/wsbm.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sang H, Yuan N, Yao S, et al. Inhibitory effect of the combination therapy of simvastatin and pinocembrin on atherosclerosis in apoE-deficient mice. Lipids in Health and Disease. 2012;11(1):p. 166. doi: 10.1186/1476-511X-11-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hong JH, Manochai B, Trakoontivakorn G, Thalang VN. Fibrinolytic activity of Thai indigenous vegetables. Kasetsart Journal. 2004;38:241–246. [Google Scholar]
- 70.Hoe VB, Siong KH. The nutritional value of indigenous fruits and vegetables in Sarawak. Asia Pacific Journal of Clinical Nutrition. 1999;8(1):24–31. doi: 10.1046/j.1440-6047.1999.00046.x. [DOI] [PubMed] [Google Scholar]
- 71.Liliwirianis N, Musa NLW, Zain WZWM, Kassim J, Karim SA. Premilinary studies on phytochemical screening of ulam and fruit from malaysia. E-Journal of Chemistry. 2011;8(1):S285–S288. [Google Scholar]


