Full text
PDF



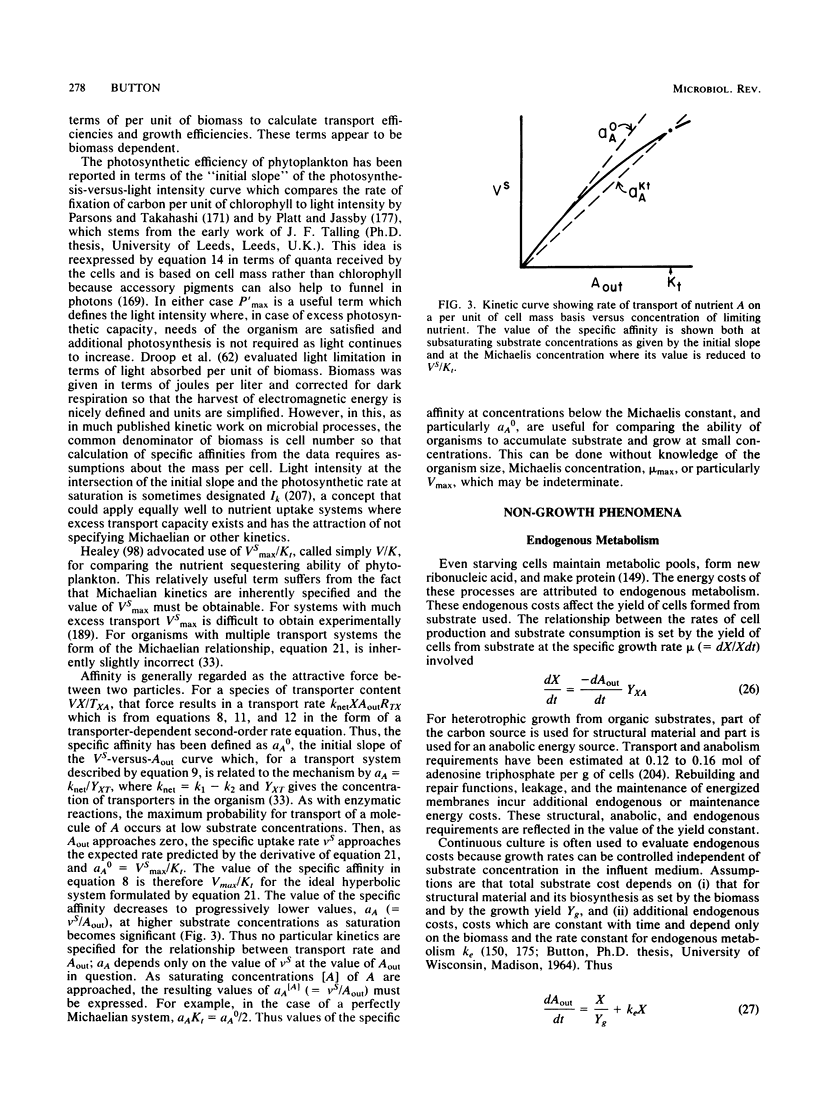
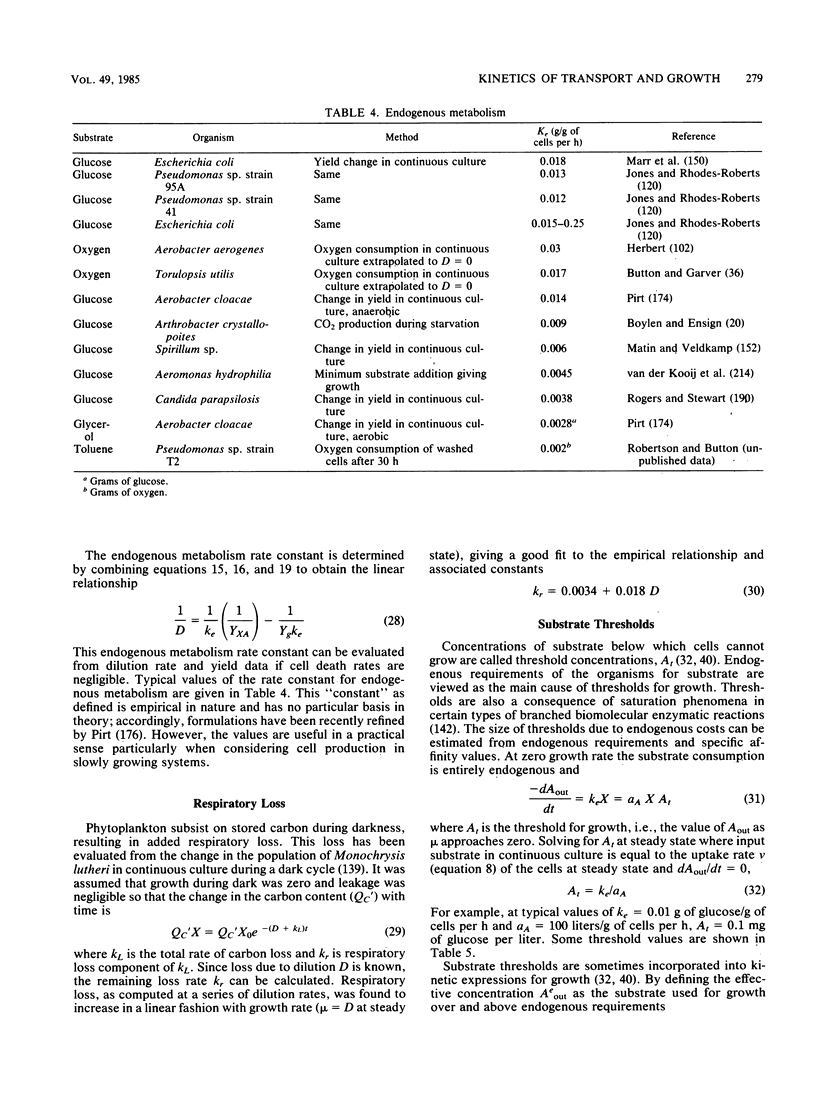

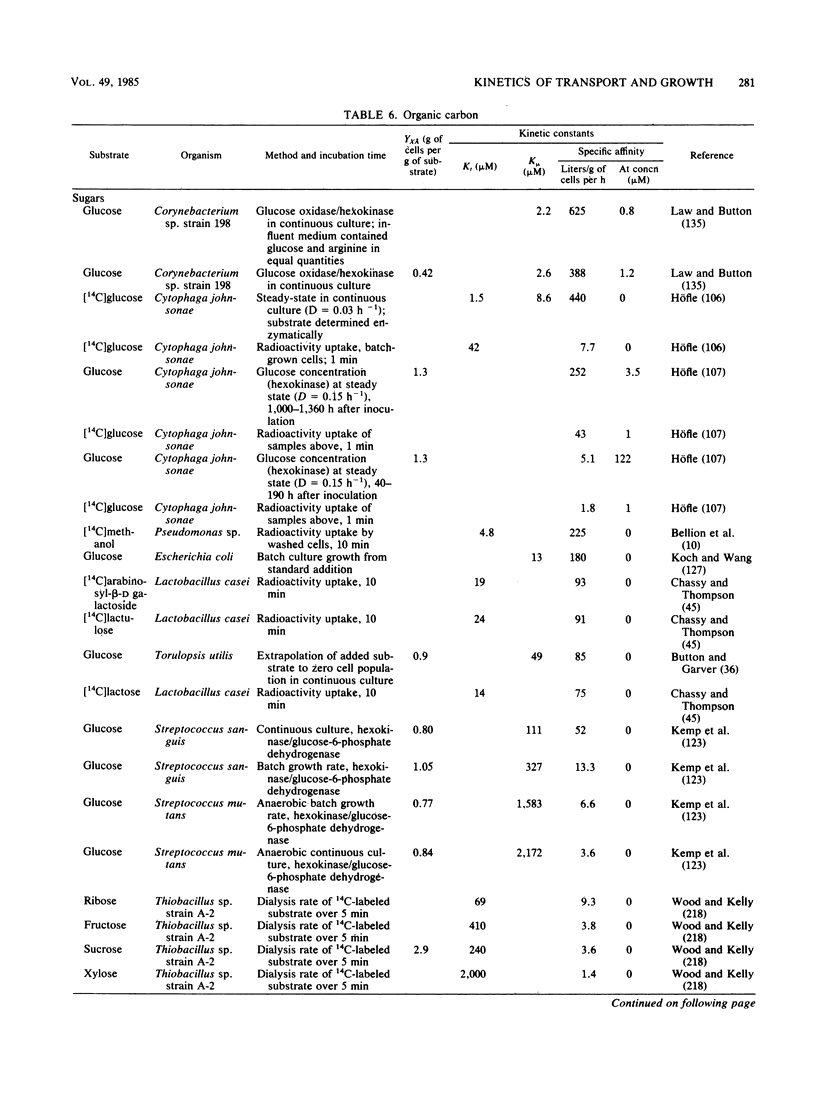
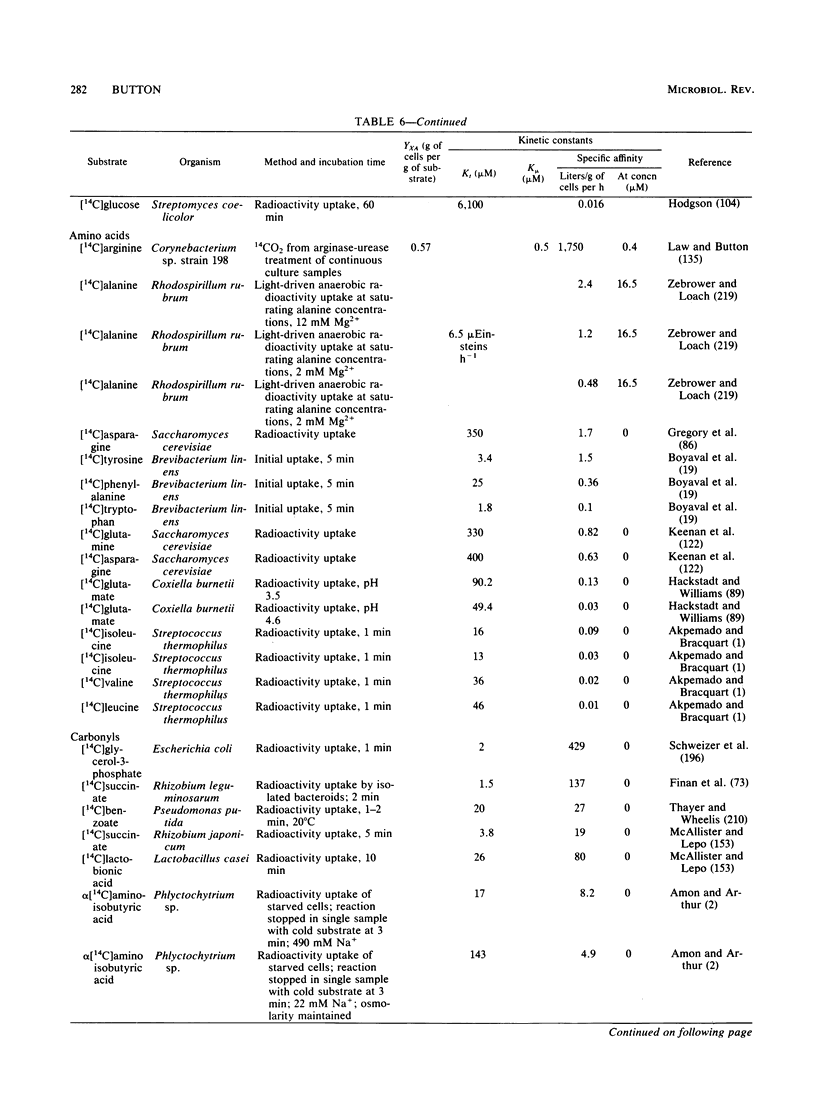







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akpemado K. M., Bracquart P. A. Uptake of Branched-Chain Amino Acids by Streptococcus thermophilus. Appl Environ Microbiol. 1983 Jan;45(1):136–140. doi: 10.1128/aem.45.1.136-140.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azov Y. Effect of pH on Inorganic Carbon Uptake in Algal Cultures. Appl Environ Microbiol. 1982 Jun;43(6):1300–1306. doi: 10.1128/aem.43.6.1300-1306.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRITTEN R. J., McCLURE F. T. The amino acid pool in Escherichia coli. Bacteriol Rev. 1962 Sep;26:292–335. doi: 10.1128/br.26.3.292-335.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUTTIN G., COHEN G. N., MONOD J., RICKENBERG H. V. La galactoside-perméase d'Escherichia coli. Ann Inst Pasteur (Paris) 1956 Dec;91(6):829–857. [PubMed] [Google Scholar]
- Bakken L. R., Olsen R. A. Buoyant densities and dry-matter contents of microorganisms: conversion of a measured biovolume into biomass. Appl Environ Microbiol. 1983 Apr;45(4):1188–1195. doi: 10.1128/aem.45.4.1188-1195.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellion E., Kent M. E., Aud J. C., Alikhan M. Y., Bolbot J. A. Uptake of methylamine and methanol by Pseudomonas sp. strain AM1. J Bacteriol. 1983 Jun;154(3):1168–1173. doi: 10.1128/jb.154.3.1168-1173.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson S., Fennewald M., Shapiro J., Huettner C. Fractionation of inducible alkane hydroxylase activity in Pseudomonas putida and characterization of hydroxylase-negative plasmid mutations. J Bacteriol. 1977 Nov;132(2):614–621. doi: 10.1128/jb.132.2.614-621.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasco F., Ducet G., Azoulay E. Mise en évidence de deux systèmes de transport du phosphate chez Candida tropicalis. Biochimie. 1976;58(3):351–357. doi: 10.1016/s0300-9084(76)80442-7. [DOI] [PubMed] [Google Scholar]
- Borkowski J. D., Johnson M. J. Experimental evaluation of liquid film resistance in oxygen transport to microbial cells. Appl Microbiol. 1967 Nov;15(6):1483–1488. doi: 10.1128/am.15.6.1483-1488.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst-Pauwels G. W. Ion transport in yeast. Biochim Biophys Acta. 1981 Dec;650(2-3):88–127. doi: 10.1016/0304-4157(81)90002-2. [DOI] [PubMed] [Google Scholar]
- Bowman B. J. Vanadate uptake in Neurospora crassa occurs via phosphate transport system II. J Bacteriol. 1983 Jan;153(1):286–291. doi: 10.1128/jb.153.1.286-291.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyaval P., Moreira E., Desmazeaud M. J. Transport of aromatic amino acids by Brevibacterium linens. J Bacteriol. 1983 Sep;155(3):1123–1129. doi: 10.1128/jb.155.3.1123-1129.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylen C. W., Ensign J. C. Intracellular substrates for endogenous metabolism during long-term starvation of rod and spherical cells of Arthrobacter crystallopoietes. J Bacteriol. 1970 Sep;103(3):578–587. doi: 10.1128/jb.103.3.578-587.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlee A. G., Arst H. N., Jr Nitrate uptake in Aspergillus nidulans and involvement of the third gene of the nitrate assimilation gene cluster. J Bacteriol. 1983 Sep;155(3):1138–1146. doi: 10.1128/jb.155.3.1138-1146.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucheder F., Broda E. Energy-dependent zinc transport by escherichia coli. Eur J Biochem. 1974 Jun 15;45(2):555–559. doi: 10.1111/j.1432-1033.1974.tb03581.x. [DOI] [PubMed] [Google Scholar]
- Burns D. J., Beever R. E. Kinetic characterization of the two phosphate uptake systems in the fungus Neurospora crassa. J Bacteriol. 1977 Nov;132(2):511–519. doi: 10.1128/jb.132.2.511-519.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns D. J., Beever R. E. Mechanisms controlling the two phosphate uptake systems in Neurospora crassa. J Bacteriol. 1979 Jul;139(1):195–204. doi: 10.1128/jb.139.1.195-204.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button D. K., Dunker S. S., Morse M. L. Continuous culture of Rhodotorula rubra: kinetics of phosphate-arsenate uptake, inhibition, and phosphate-limited growth. J Bacteriol. 1973 Feb;113(2):599–611. doi: 10.1128/jb.113.2.599-611.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button D. K., Egan J. B., Hengstenberg W., Morse M. L. Carbohydrate transport in Staphylococcus aureus. IV. Maltose accumulation and metabolism. Biochem Biophys Res Commun. 1973 Jun 8;52(3):850–855. doi: 10.1016/0006-291x(73)91015-2. [DOI] [PubMed] [Google Scholar]
- Button D. K., Garver J. C. Continuous culture of Torulopsis utilis: a kinetic study of oxygen limited growth. J Gen Microbiol. 1966 Nov;45(2):195–204. doi: 10.1099/00221287-45-2-195. [DOI] [PubMed] [Google Scholar]
- Button D. K., Robertson B. R., Craig K. S. Dissolved hydrocarbons and related microflora in a fjordal seaport: sources, sinks, concentrations, and kinetics. Appl Environ Microbiol. 1981 Oct;42(4):708–719. doi: 10.1128/aem.42.4.708-719.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button D. K. Thiamine limited steady state growth of the yeast Cryptococcus albidus. J Gen Microbiol. 1969 Sep;58(1):15–21. doi: 10.1099/00221287-58-1-15. [DOI] [PubMed] [Google Scholar]
- Cembella A. D., Antia N. J., Harrison P. J. The utilization of inorganic and organic phosphorous compounds as nutrients by eukaryotic microalgae: a multidisciplinary perspective: part 1. Crit Rev Microbiol. 1984;10(4):317–391. doi: 10.3109/10408418209113567. [DOI] [PubMed] [Google Scholar]
- Cembella A. D., Antia N. J., Harrison P. J. The utilization of inorganic and organic phosphorus compounds as nutrients by eukaryotic microalgae: a multidisciplinary perspective. Part 2. Crit Rev Microbiol. 1984;11(1):13–81. doi: 10.3109/10408418409105902. [DOI] [PubMed] [Google Scholar]
- Chan H. K., Campbell N. E. Phytoplankton uptake and excretion of assimilated nitrate in a small Canadian shield lake. Appl Environ Microbiol. 1978 Jun;35(6):1052–1060. doi: 10.1128/aem.35.6.1052-1060.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassy B. M., Thompson J. Regulation of lactose-phosphoenolpyruvate-dependent phosphotransferase system and beta-D-phosphogalactoside galactohydrolase activities in Lactobacillus casei. J Bacteriol. 1983 Jun;154(3):1195–1203. doi: 10.1128/jb.154.3.1195-1203.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke P. H. Adaptation. The Fifteenth Marjory Stephenson Memorial Lecture. J Gen Microbiol. 1981 Sep;126(1):5–20. doi: 10.1099/00221287-126-1-5. [DOI] [PubMed] [Google Scholar]
- Collins C. D., Boylen C. W. Ecological Consequences of Long-Term Exposure of Anabaena variabilis (Cyanophyceae) to Shifts in Environmental Factors. Appl Environ Microbiol. 1982 Jul;44(1):141–148. doi: 10.1128/aem.44.1.141-148.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne W. E., Magasanik B. Ammonia regulation of amino acid permeases in Saccharomyces cerevisiae. Mol Cell Biol. 1983 Apr;3(4):672–683. doi: 10.1128/mcb.3.4.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuhel R. L., Taylor C. D., Jannasch H. W. Assimilatory sulfur metabolism in marine microorganisms: characteristics and regulation of sulfate transport in Pseudomonas halodurans and Alteromonas luteo-violaceus. J Bacteriol. 1981 Aug;147(2):340–349. doi: 10.1128/jb.147.2.340-349.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuppoletti J., Segel I. H. Kinetic analysis of active membrane transport systems: equations for net velocity and isotope exchange. J Theor Biol. 1975 Sep;53(1):125–144. doi: 10.1016/0022-5193(75)90107-1. [DOI] [PubMed] [Google Scholar]
- Cuppoletti J., Segel I. H. Kinetics of sulfate transport by Penicillium notatum. Interactions of sulfate, protons, and calcium. Biochemistry. 1975 Oct 21;14(21):4712–4718. doi: 10.1021/bi00692a023. [DOI] [PubMed] [Google Scholar]
- Dean A. C., Rogers P. L. The cell size and macromolecular composition of Aerobacter aerogenes in various systems of continuous culture. Biochim Biophys Acta. 1967 Oct 9;148(1):267–279. doi: 10.1016/0304-4165(67)90302-9. [DOI] [PubMed] [Google Scholar]
- Dills S. S., Seno S. Regulation of hexitol catabolism in Streptococcus mutans. J Bacteriol. 1983 Feb;153(2):861–866. doi: 10.1128/jb.153.2.861-866.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccleston M., Kelly D. P. Oxidation kinetics and chemostat growth kinetics of Thiobacillus ferrooxidans on tetrathionate and thiosulfate. J Bacteriol. 1978 Jun;134(3):718–727. doi: 10.1128/jb.134.3.718-727.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker D. J., Emery T. Iron uptake from ferrichrome A and iron citrate in Ustilago sphaerogena. J Bacteriol. 1983 Aug;155(2):616–622. doi: 10.1128/jb.155.2.616-622.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Failla M. L., Benedict C. D., Weinberg E. D. Accumulation and storage of Zn2+ by Candida utilis. J Gen Microbiol. 1976 May;94(1):23–36. doi: 10.1099/00221287-94-1-23. [DOI] [PubMed] [Google Scholar]
- Finan T. M., Wood J. M., Jordan D. C. Symbiotic properties of C4-dicarboxylic acid transport mutants of Rhizobium leguminosarum. J Bacteriol. 1983 Jun;154(3):1403–1413. doi: 10.1128/jb.154.3.1403-1413.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg I. Phosphate transport in Micrococcus lysodeikticus. Biochim Biophys Acta. 1977 May 2;466(3):451–460. doi: 10.1016/0005-2736(77)90338-8. [DOI] [PubMed] [Google Scholar]
- Fuhrmann G. F., Rothstein A. The transport of Zn2+, Co2+ and Ni2+ into yeast cells. Biochim Biophys Acta. 1968 Nov 5;163(3):325–330. doi: 10.1016/0005-2736(68)90117-x. [DOI] [PubMed] [Google Scholar]
- Gains N. The determination of the kinetic parameters of a carrier mediated transport process in the presence of an unstirred water layer. J Theor Biol. 1980 Dec 7;87(3):559–568. doi: 10.1016/0022-5193(80)90235-0. [DOI] [PubMed] [Google Scholar]
- Gaudy A. F., Jr, Obayashi A., Gaudy E. T. Control of growth rate by initial substrate concentration at values below maximum rate. Appl Microbiol. 1971 Dec;22(6):1041–1047. doi: 10.1128/am.22.6.1041-1047.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gober J. W., Kashket E. R. Methylammonium uptake by Rhizobium sp. strain 32H1. J Bacteriol. 1983 Mar;153(3):1196–1201. doi: 10.1128/jb.153.3.1196-1201.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillo J. F., Gibson J. Regulation of phosphate accumulation in the unicellular cyanobacterium Synechococcus. J Bacteriol. 1979 Nov;140(2):508–517. doi: 10.1128/jb.140.2.508-517.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAROLD F. M., HAROLD R. L., ABRAMS A. A MUTANT OF STREPTOCOCCUS FAECALIS DEFECTIVE IN PHOSPHATE UPTAKE. J Biol Chem. 1965 Jul;240:3145–3153. [PubMed] [Google Scholar]
- HERBERT D., ELSWORTH R., TELLING R. C. The continuous culture of bacteria; a theoretical and experimental study. J Gen Microbiol. 1956 Jul;14(3):601–622. doi: 10.1099/00221287-14-3-601. [DOI] [PubMed] [Google Scholar]
- HOFSTEE B. H. J. On the evaluation of the constants Vm and KM in enzyme reactions. Science. 1952 Sep 26;116(3013):329–331. doi: 10.1126/science.116.3013.329. [DOI] [PubMed] [Google Scholar]
- Hackstadt T., Williams J. C. pH dependence of the Coxiella burnetii glutamate transport system. J Bacteriol. 1983 May;154(2):598–603. doi: 10.1128/jb.154.2.598-603.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M. Chemiosmotic interpretation of active transport in bacteria. Ann N Y Acad Sci. 1974 Feb 18;227:297–311. doi: 10.1111/j.1749-6632.1974.tb14395.x. [DOI] [PubMed] [Google Scholar]
- Harold F. M., Spitz E. Accumulation of arsenate, phosphate, and aspartate by Sreptococcus faecalis. J Bacteriol. 1975 Apr;122(1):266–277. doi: 10.1128/jb.122.1.266-277.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heefner D. L., Kobayashi H., Harold F. M. ATP-linked sodium transport in Streptococcus faecalis. II. Energy coupling in everted membrane vesicles. J Biol Chem. 1980 Dec 10;255(23):11403–11407. [PubMed] [Google Scholar]
- Hellingwerf K. J., Friedberg I., Lolkema J. S., Michels P. A., Konings W. N. Energy coupling of facilitated transport of inorganic ions in Rhodopseudomonas sphaeroides. J Bacteriol. 1982 Jun;150(3):1183–1191. doi: 10.1128/jb.150.3.1183-1191.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugouvieux-Cotte-Pattat N., Quesneau Y., Robert-Baudouy J. Aldohexuronate transport system in Erwinia carotovora. J Bacteriol. 1983 May;154(2):663–668. doi: 10.1128/jb.154.2.663-668.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman M. R., Wood P. M. Methane oxidation by Nitrosomonas europaea. Biochem J. 1983 Apr 15;212(1):31–37. doi: 10.1042/bj2120031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höfle M. G. Long-Term Changes in Chemostat Cultures of Cytophaga johnsonae. Appl Environ Microbiol. 1983 Nov;46(5):1045–1053. doi: 10.1128/aem.46.5.1045-1053.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INGRAHAM J. L. Growth of psychrophilic bacteria. J Bacteriol. 1958 Jul;76(1):75–80. doi: 10.1128/jb.76.1.75-80.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingvorsen K., Zehnder A. J., Jørgensen B. B. Kinetics of Sulfate and Acetate Uptake by Desulfobacter postgatei. Appl Environ Microbiol. 1984 Feb;47(2):403–408. doi: 10.1128/aem.47.2.403-408.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrell K. F., Sprott G. D. Nickel transport in Methanobacterium bryantii. J Bacteriol. 1982 Sep;151(3):1195–1203. doi: 10.1128/jb.151.3.1195-1203.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen B. B., Cox R. P. Direct measurements of steady-state kinetics of cyanobacterial n(2) uptake by membrane-leak mass spectrometry and comparisons between nitrogen fixation and acetylene reduction. Appl Environ Microbiol. 1983 Apr;45(4):1331–1337. doi: 10.1128/aem.45.4.1331-1337.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. J. Aerobic microbial growth at low oxygen concentrations. J Bacteriol. 1967 Jul;94(1):101–108. doi: 10.1128/jb.94.1.101-108.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp C. W., Robrish S. A., Curtis M. A., Sharer S. A., Bowen W. H. Application of a competition model to the growth of Streptococcus mutans and Streptococcus sanguis in binary continuous culture. Appl Environ Microbiol. 1983 Apr;45(4):1277–1282. doi: 10.1128/aem.45.4.1277-1282.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H. Second system for potassium transport in Streptococcus faecalis. J Bacteriol. 1982 May;150(2):506–511. doi: 10.1128/jb.150.2.506-511.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A. L. The adaptive responses of Escherichia coli to a feast and famine existence. Adv Microb Physiol. 1971;6:147–217. doi: 10.1016/s0065-2911(08)60069-7. [DOI] [PubMed] [Google Scholar]
- Koch A. L., Wang C. H. How close to the theoretical diffusion limit do bacterial uptake systems function? Arch Microbiol. 1982 Feb;131(1):36–42. doi: 10.1007/BF00451496. [DOI] [PubMed] [Google Scholar]
- Käppeli O., Finnerty W. R. Partition of alkane by an extracellular vesicle derived from hexadecane-grown Acinetobacter. J Bacteriol. 1979 Nov;140(2):707–712. doi: 10.1128/jb.140.2.707-712.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammers P. J., Sanders-Loehr J. Active transport of ferric schizokinen in Anabaena sp. J Bacteriol. 1982 Jul;151(1):288–294. doi: 10.1128/jb.151.1.288-294.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang D. S., Brown E. J. Phosphorus-limited growth of a green alga and a blue-green alga. Appl Environ Microbiol. 1981 Dec;42(6):1002–1009. doi: 10.1128/aem.42.6.1002-1009.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law A. T., Button D. K. Multiple-carbon-source-limited growth kinetics of a marine coryneform bacterium. J Bacteriol. 1977 Jan;129(1):115–123. doi: 10.1128/jb.129.1.115-123.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman J. T., Scavia D. Microscale nutrient patches produced by zooplankton. Proc Natl Acad Sci U S A. 1982 Aug;79(16):5001–5005. doi: 10.1073/pnas.79.16.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licko V. Some biochemical threshold mechanisms. Bull Math Biophys. 1972 Mar;34(1):103–112. doi: 10.1007/BF02477030. [DOI] [PubMed] [Google Scholar]
- Lusk J. E., Williams R. J., Kennedy E. P. Magnesium and the growth of Escherichia coli. J Biol Chem. 1968 May 25;243(10):2618–2624. [PubMed] [Google Scholar]
- Matin A., Veldkamp H. Physiological basis of the selective advantage of a Spirillum sp. in a carbon-limited environment. J Gen Microbiol. 1978 Apr;105(2):187–197. doi: 10.1099/00221287-105-2-187. [DOI] [PubMed] [Google Scholar]
- McAllister C. F., Lepo J. E. Succinate transport by free-living forms of Rhizobium japonicum. J Bacteriol. 1983 Mar;153(3):1155–1162. doi: 10.1128/jb.153.3.1155-1162.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers J., Graham J. R. The photosynthetic unit in chlorella measured by repetitive short flashes. Plant Physiol. 1971 Sep;48(3):282–286. doi: 10.1104/pp.48.3.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOVICK A. Growth of bacteria. Annu Rev Microbiol. 1955;9:97–110. doi: 10.1146/annurev.mi.09.100155.000525. [DOI] [PubMed] [Google Scholar]
- Neilands J. B. Microbial iron compounds. Annu Rev Biochem. 1981;50:715–731. doi: 10.1146/annurev.bi.50.070181.003435. [DOI] [PubMed] [Google Scholar]
- Paris D. F., Steen W. C., Baughman G. L., Barnett J. T. Second-order model to predict microbial degradation of organic compounds in natural waters. Appl Environ Microbiol. 1981 Mar;41(3):603–609. doi: 10.1128/aem.41.3.603-609.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirt S. J. Maintenance energy: a general model for energy-limited and energy-sufficient growth. Arch Microbiol. 1982 Dec 3;133(4):300–302. doi: 10.1007/BF00521294. [DOI] [PubMed] [Google Scholar]
- Pirt S. J. The maintenance energy of bacteria in growing cultures. Proc R Soc Lond B Biol Sci. 1965 Oct 12;163(991):224–231. doi: 10.1098/rspb.1965.0069. [DOI] [PubMed] [Google Scholar]
- Raven J. A. Nutrient transport in microalgae. Adv Microb Physiol. 1980;21:47–226. doi: 10.1016/s0065-2911(08)60356-2. [DOI] [PubMed] [Google Scholar]
- Ray W. J., Jr Rate-limiting step: a quantitative definition. Application to steady-state enzymic reactions. Biochemistry. 1983 Sep 27;22(20):4625–4637. doi: 10.1021/bi00289a003. [DOI] [PubMed] [Google Scholar]
- Rice C. W., Hempfling W. P. Oxygen-limited continuous culture and respiratory energy conservation in Escherichia coli. J Bacteriol. 1978 Apr;134(1):115–124. doi: 10.1128/jb.134.1.115-124.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivkin R. B., Voytek M. A., Seliger H. H. Phytoplankton division rates in light-limited environments: two adaptations. Science. 1982 Feb 26;215(4536):1123–1125. doi: 10.1126/science.215.4536.1123. [DOI] [PubMed] [Google Scholar]
- Robertson B. R., Button D. K. Phosphate-limited continuous culture of Rhodotorula rubra: kinetics of transport, leakage, and growth. J Bacteriol. 1979 Jun;138(3):884–895. doi: 10.1128/jb.138.3.884-895.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers P. J., Stewart P. R. Energetic efficiency and maintenance. Energy characteristics of Saccharomyces cerevisiae (wild type and petite) and Candida parapsilosis grown aerobically and micro-aerobically in continuous culture. Arch Microbiol. 1974;99(1):25–46. doi: 10.1007/BF00696220. [DOI] [PubMed] [Google Scholar]
- Roomans G. M., Kuypers G. A., Theuvenet A. P., Borst-Pauwels G. W. Kinetics of sulfate uptake by yeast. Biochim Biophys Acta. 1979 Feb 20;551(1):197–206. doi: 10.1016/0005-2736(79)90365-1. [DOI] [PubMed] [Google Scholar]
- Russell L. M., Rosenberg H. Linked transport of phosphate, potassium ions and protons in Escherichia coli. Biochem J. 1979 Oct 15;184(1):13–21. doi: 10.1042/bj1840013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer H., Argast M., Boos W. Characteristics of a binding protein-dependent transport system for sn-glycerol-3-phosphate in Escherichia coli that is part of the pho regulon. J Bacteriol. 1982 Jun;150(3):1154–1163. doi: 10.1128/jb.150.3.1154-1163.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehata T. E., Marr A. G. Effect of nutrient concentration on the growth of Escherichia coli. J Bacteriol. 1971 Jul;107(1):210–216. doi: 10.1128/jb.107.1.210-216.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver S., Johnseine P., King K. Manganese Active Transport in Escherichia coli. J Bacteriol. 1970 Dec;104(3):1299–1306. doi: 10.1128/jb.104.3.1299-1306.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simkins S., Alexander M. Models for mineralization kinetics with the variables of substrate concentration and population density. Appl Environ Microbiol. 1984 Jun;47(6):1299–1306. doi: 10.1128/aem.47.6.1299-1306.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonson C., Brener D., DeVoe I. W. Expression of a high-affinity mechanism for acquisition of transferrin iron by Neisseria meningitidis. Infect Immun. 1982 Apr;36(1):107–113. doi: 10.1128/iai.36.1.107-113.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slee A. M., Tanzer J. M. Sucrose transport by Streptococcus mutans. Evidence for multiple transport systems. Biochim Biophys Acta. 1982 Nov 22;692(3):415–424. doi: 10.1016/0005-2736(82)90392-3. [DOI] [PubMed] [Google Scholar]
- Thayer J. R., Wheelis M. L. Active transport of benzoate in Pseudomonas putida. J Gen Microbiol. 1982 Aug;128(8):1749–1753. doi: 10.1099/00221287-128-8-1749. [DOI] [PubMed] [Google Scholar]
- Winne D. Unstirred layer, source of biased Michaelis constant in membrane transport. Biochim Biophys Acta. 1973 Feb 27;298(1):27–31. doi: 10.1016/0005-2736(73)90005-9. [DOI] [PubMed] [Google Scholar]
- Zebrower M., Loach P. A. Efficiency of light-driven metabolite transport in the photosynthetic bacterium Rhodospirillum rubrum. J Bacteriol. 1982 Jun;150(3):1322–1328. doi: 10.1128/jb.150.3.1322-1328.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries G. E., van Brussel A. A., Quispel A. Mechanism of regulation of glucose transport in Rhizobium leguminosarum. J Bacteriol. 1982 Mar;149(3):872–879. doi: 10.1128/jb.149.3.872-879.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kooij D., Visser A., Hijnen W. A. Growth of Aeromonas hydrophila at Low Concentrations of Substrates Added to Tap Water. Appl Environ Microbiol. 1980 Jun;39(6):1198–1204. doi: 10.1128/aem.39.6.1198-1204.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]


