Abstract
The transcription factor Pax8, a member of the Paired-box gene family, is a critical regulator required for proper development and differentiation of thyroid follicular cells. Despite being Pax8 well characterized with respect to its role in regulating genes responsible for thyroid differentiation, its involvement in cell survival and proliferation has been hypothesized but remains unclear. Here, we show that Pax8 overexpression significantly increases proliferation and colony-forming efficiency of Fischer rat thyroid line 5 epithelial cells, although it is not sufficient to overcome their hormone dependence. More interestingly, we show that Pax8-specific silencing induces apoptosis through a p53-dependent pathway that involves caspase-3 activation and cleavage of poly(ADP)ribose polymerase. Our data indicate that tumor protein 53 induced nuclear protein 1 (tp53inp1), a positive regulator of p53-dependent cell cycle arrest and apoptosis, is a transcriptional target of Pax8 and is upregulated by Pax8 knockdown. Remarkably, tp53inp1 silencing significantly abolishes Pax8-induced apoptosis thus suggesting that tp53inp1 may be the mediator of the observed effects. In conclusion, our data highlight that Pax8 is required for the survival of differentiated epithelial cells and its expression levels are able to modulate the proliferation rate of such cells.
Keywords: Pax8, cell survival, tp53inp1, thyroid epithelial cells
The Pax genes encode evolutionary conserved transcription factors that act high up in the regulatory hierarchy controlling the development of various organs.1 In mammals, there are nine Pax genes, grouped into four different classes based on identity within their DNA-binding domain (the paired box), gene structure and expression pattern.2 The Pax genes are tightly regulated in both temporal and spatial expression patterns; most adult cells switch them off during late phases of terminal differentiation and this pattern is maintained in the mature organism. Recently, a substantial body of evidence demonstrated that even if the constitutive expression of the Pax gene in adult tissues may not be itself oncogenic, it may contribute to the malignant phenotype by sustaining abnormal cell proliferation.3, 4
Pax8, one of the Pax family members, is expressed in developing kidney, neural tube, and developing and adult thyroid.5 Specifically, Pax8 was shown to be required for both morphogenesis of the thyroid gland6 and maintenance of the thyroid differentiated phenotype.7 Together with another thyroid-specific transcription factor named TTF-1, Pax8 is involved in the regulation of thyroid-specific genes such as those encoding thyroglobulin (Tg), thyroperoxidase and sodium/iodide symporter.8, 9 Even though during embryogenesis Pax8 is expressed in different districts such as thyroid, metanephros and Mullerian duct,5, 10 knockout mice show only a thyroid phenotype, whereas they have no obvious defects in the other tissues.6 In particular, mice lacking Pax8 have a barely visible thyroid gland deprived of the follicular cells, suggesting that this transcription factor may be required for the survival of thyroid cell precursors.6 As a consequence, the knockout mice suffer from hypothyroidism and die within 2–3 weeks after weaning. In humans, congenital hypothyroidism is most often caused by absent, hypoplastic or ectopic thyroid11 and some human patients suffering from congenital hypothyroidism have been shown to carry mutations in the PAX8 gene.12, 13, 14
In addition to hypothyroidism, PAX8 has a role in a subset of renal,15 bladder,16 ovarian,17 pancreatic endocrine and thyroid neoplasm.18, 19, 20 A specific translocation t(2;3)(q13;p25) resulting in a fusion protein between PAX8 and peroxisome proliferator-activated receptor γ (PPARγ) acts as an oncogene21 and it is able to modulate the activating function of PAX8 on transcription units with thyroid-specific activity.22 This fusion protein has been detected in both benign and malignant thyroid tumors, with a high percentage in follicular thyroid carcinomas.23
Recently, PAX8 was reported as one of the top 40 genes specifically upregulated in different types of ovarian carcinoma.24 It was also found to be overexpressed in Wilms tumor25 and in the majority of gliomas, acting as an important regulator of telomerase activity.26
In order to investigate the role of Pax8 in cell proliferation and survival, we examined the in vitro effects of Pax8 overexpression and silencing in epithelial-differentiated cells. Our results provide strong evidences that Pax8 has a crucial role in the regulation of both biological processes. Moreover, we suggest that the tp53inp1 protein (tumor protein 53-induced nuclear protein 1) may function as the mediator of Pax8 control of epithelial cell survival. It is well known that tp53inp1 is a stress-induced nuclear protein, direct target of p53, that has a role in cell cycle arrest and in p53-mediated apoptosis.27 Tp53inp1 also strongly alters p53 transcriptional activity on several p53-dependent promoters thus stimulating its capacity to induce apoptosis and regulate cell cyle.28 Altogether, the currently available observations indicate that tp53inp1 has an important role in cellular homeostasis through its antiproliferative and pro-apoptotic activities. Our findings here reported indicate that tp53inp1 is a direct target of Pax8 and we propose that Pax8 could regulate cell survival of differentiated epithelial cells via the transcriptional regulation of tp53inp1.
Results
Pax8 overexpression leads to increased proliferation rate of differentiated epithelial cells
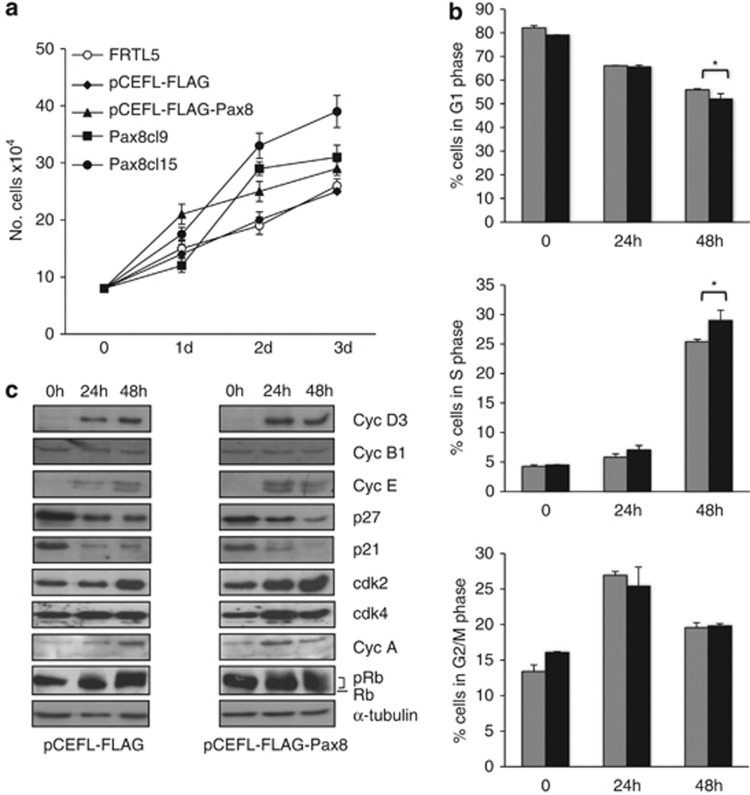
To get clues on the functional activity of Pax8 in differentiated epithelial cells, we used the Fischer rat thyroid line 5 (FRTL-5) cells as experimental model system. These cells have been extensively characterized:29 they are a differentiated thyroid cell line that expresses an endogenous Pax8 and depends on specific hormones for proliferation (see Materials and Methods). Individual FRTL-5 stable clones and a mass population stably overexpressing exogenous Pax8 were grown in normal medium conditions and counted each day for 4 days to establish cell proliferation curves. The analysis shows that the proliferation rate of Pax8 stable-transfected pool and individual clones is significantly higher than that of the parental or backbone vector-transfected FRTL-5 cells (Figure 1a). A similar experiment has been carried out with cells growing in medium deprived of hormones but neither the wild-type cells nor the Pax8 overexpressing ones were able to grow (Supplementary Figure 1a). Although the requirement of hormones for cell growth is well-known for wild-type FRTL-5 cells, the fact that the Pax8 overexpressing pool and clones are not able to grow without hormones indicates that these cells have a higher proliferation rate but do not lose their hormone dependance. In parallel, we have also characterized the experimental and control FRTL5-FLAG pools with respect to the differentiated gene expression profile of the FRTL-5 parental cell line and no significant changes were observed (Supplementary Figure 1b).
Figure 1.
Pax8 overexpression affects the cell growth and the cell cycle distribution of FRTL-5 cells. (a) Growth curves of wild-type FRTL-5 cells, individual Pax8 FRTL-5 stable clones and pools of clones expressing Pax8 or transfected with the empty vector. The results are the mean of three independent experiments. (b) Flow cytometry analysis of proliferating pCEFL-FLAG-Pax8 and pCEFL-FLAG cells starved for 3 days in 0.2% serum (t=0) and treated with complete medium at the indicated times. Gray and black bars correspond to pCEFL-FLAG and pCEFL-FLAG-Pax8 cells, respectively. Statistical analysis uses t-test (P=0.08). (c) Protein extracts of pCEFL-FLAG-Pax8 and pCEFL-FLAG cells starved for 3 days in 0.2% serum (t=0) and treated with complete medium at the indicated times were analyzed by western blot for the expression of cell cycle regulatory proteins. Filter was reblotted with α-tubulin to ensure protein loading and integrity
Next, we analyzed the kinetics of cell cycle entry and the expression of cell cycle regulators in the FRTL5-FLAG and FRTL5-FLAG-Pax8 pool populations. To this aim, both pools were starved for hormones (six hormones (6H)) and serum for 3 days, subsequently stimulated to re-enter into the cell cycle with serum and 6H, and analyzed by flow cytometry 24 and 48 h after treatment. The result of three independent experiments is reported in Figure 1b and shows that Pax8 overexpression slightly accelerates the cell exit from the G1 phase, as indicated by the reduced percentage of FRTL5-FLAG-Pax8 cells in G1 and the proportional increase in the S phase. This observation was further strengthen by comparing the expression level of cyclins, Cdks and Cdk inhibitors in the two pools by western blot. In the FRTL5-FLAG-Pax8 pool, upon stimulation with 6H and serum, we observed a small increase in the expression of cyclin D3, cyclin A and cyclin E and a similarly modest reduction in the expression of the cyclin-dependent kinase inhibitors p21 and p27 (Figure 1c).
As Rb phosphorylation controls S-phase entry, we also examined whether the observed alterations of the cell cycle correlated well with Rb phosphorylation levels. Upon starvation of the cells, FRTL5-FLAG and FRTL5-FLAG-Pax8 pools show Rb dephosphorylation; when re-stimulated with complete medium, Rb phosphorylation is accelerated in FRTL5-FLAG-Pax8 cells compared with the control pool (Figure 2c, see the 24 h time point) thus correlating with the earlier entry into the S phase of the cells overexpressing Pax8.
Figure 2.
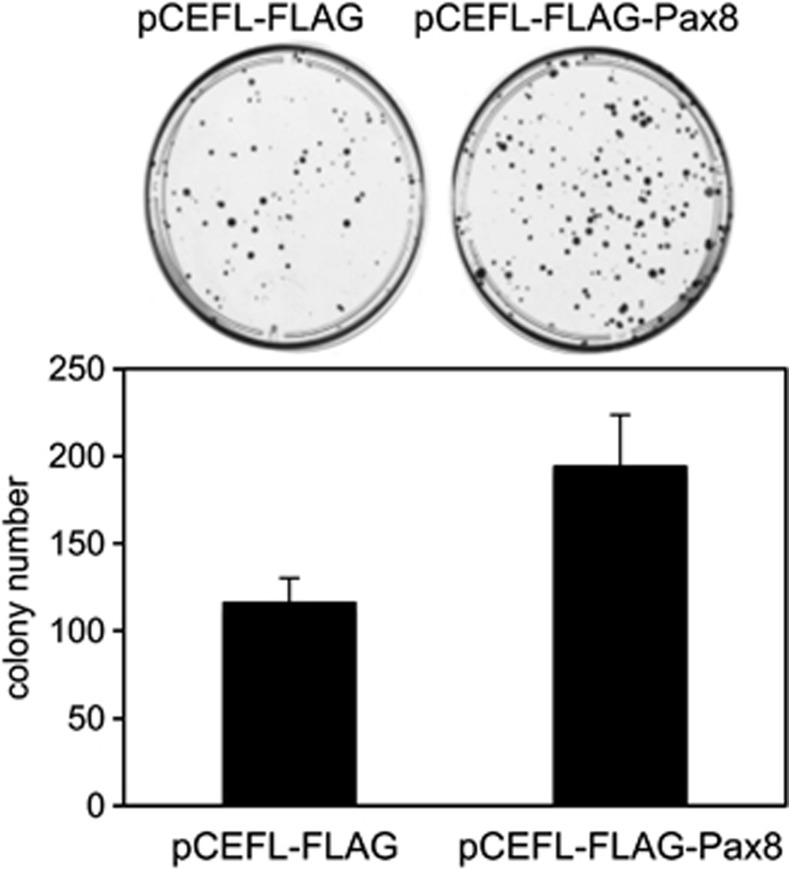
Colony-forming assays were performed with FRTL-5 cells transfected with a vector expressing Pax8 cDNA (pCEFL-FLAG-Pax8) or with the empty vector (pCEFL-FLAG). The results are the mean of two independent experiments. Cells transfected with Pax8 generated a higher number of colonies than that of cells transfected with the backbone vector
Subsequently, we carried out a colony-forming assay with the FRTL5-FLAG and FRTL5-FLAG-Pax8 pool populations. As shown in Figure 2, FRTL-5 cells transfected with Pax8 generated a higher number of colonies than cells transfected with the control vector, indicating that the overexpression of Pax8 increases the clonogenic ability of these cells.
Taken together, these data indicate that Pax8 overexpression is not sufficient per se to overcome hormone dependence but, in the presence of hormones, significantly increases both proliferation and colony formation capacity of differentiated epithelial cells.
Pax8 silencing results in apoptosis of FRTL-5 epithelial cells
The contribution of Pax8 to thyroid cell survival has been suggested by the analysis of the early steps of thyroid morphogenesis in Pax8 null embryos. In the mouse model, in the absence of this transcription factor, at E11.5 the thyroid primordium appears much smaller than the wild-type primordium and at E12.5 the follicular cells are essentially undetectable.6 Thus, Pax8 seems to be required for the survival of thyroid cell precursors but not for their specification.
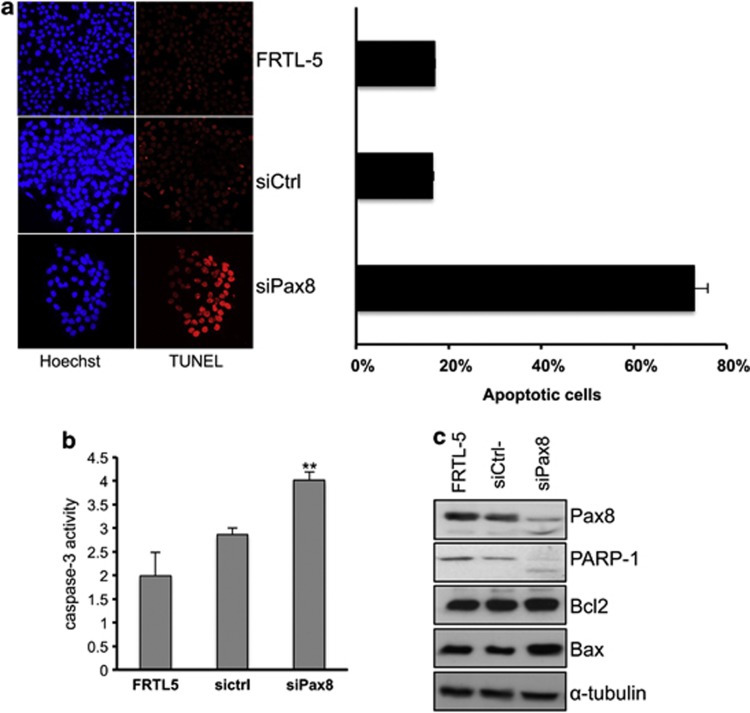
To investigate whether Pax8 has a pro-survival function also in a differentiated thyroid cell line, we silenced the expression of endogenous Pax8 in FRTL-5 cells by RNA interference. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assays were performed on FRTL-5 cells transiently transfected with Pax8 siRNA (experimental) or non-targeting siRNA (control). Forty-eight hours after transfection, Pax8 silencing strongly increased the number of TUNEL-positive cells compared with the control siRNA (Figure 3a, left panel), indicating that knockdown of Pax8 in differentiated epithelial cells leads to cell death. The average result of three independent experiments is reported in the right panel of Figure 3a.
Figure 3.
Pax8 knockdown promotes apoptosis of FRTL-5 cells. (a) Fluorescence microscopy representative images of TUNEL staining of FRTL-5 cells 48 h after Pax8 silencing. On the right side, the quantitative analysis of TUNEL-positive cells is shown. More than 500 cells were counted for each measurement, and experiments were repeated three times. Averages of TUNEL-positive cells (%) with standard error are shown in the graph. Statistical analysis uses t-test (P<0.01). (b) Pax8 silencing increases the caspase-3 activity. Cells were transfected with siRNA scramble (siCtrl-) and siPax8 and after 24 h the whole-cell lysates were subjected to caspase-3 activity assay. The results are the mean of three independent experiments (P<0.05). (c) Analysis by western blot of the expression levels of some genes involved in the control of apoptosis, upon Pax8 silencing. PARP1 was detected as a doublet indicative of its activation. The hybridization with α-tubulin is shown to ensure protein loading and integrity
Next, we evaluated the activity of caspase-3 by a colorimetric assay. The results shown in Figure 3b indicate that caspase-3 activity is significantly increased in siPax8-transfected FRTL-5 cells compared with the controls at 24 h after transfection. Moreover, Pax8 silencing induces the caspase-3 pathway and results in poly(ADP)ribose polymerase 1 (PARP-1) cleavage (Figure 3c). Western blot analyses also showed that Pax8 knockdown leads to a slightly increased expression of Bax but not of Bcl-2 (Figure 3c). Altogether, our data reveal that the loss of Pax8 in FRTL-5 epithelial cells strongly affects cell survival.
Molecular mechanisms underlying siPax8-induced apoptosis
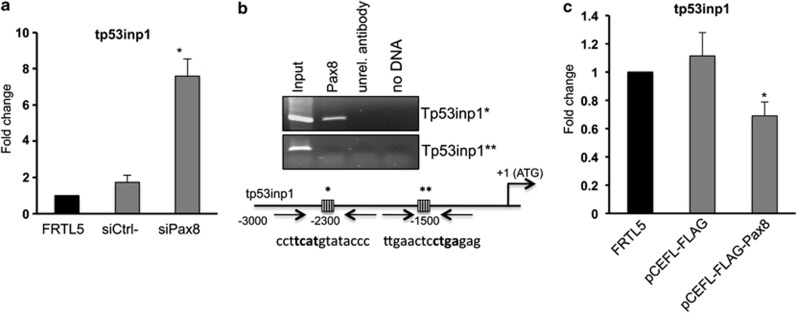
Recently, we reported a large-scale gene expression profile analysis following Pax8 silencing in FRTL-5 cells performed to identify new Pax8 target genes.30 The array data suggested that Pax8 regulates several pathways, mainly involved in the regulation of the cell cycle, cancer and apoptosis. Specifically, genes belonging to the cell cycle and apoptosis categories resulted most modified in our array. We focused our attention on tp53inp1, a positive regulator of p53-dependent cell cycle arrest and apoptosis28 that was significantly upregulated in our array. To validate the expression data obtained by the microarray analysis, we performed qRT-PCR on RNA samples prepared 72 h after Pax8 siRNA transfection and we confirmed tp53inp1 upregulation in the siPax8 RNA sample (Figure 4a). The results so far obtained prompted us to investigate more in details the 5′-flanking genomic region of tp53inp1 and specifically to localize possible Pax8-binding site(s) within this region. Therefore, we used the MatInspector analysis software (Genomatix) to search for transcription factor-binding sites and we found several Pax8 consensus sequences. To confirm the predictions of the MatInspector analysis, chromatin immunoprecipitation (ChIP) assays on FRTL-5 cells were carried out. The cross-linked chromatin was immunoprecipitated using a specific antibody recognizing Pax8 or an unrelated antibody as control. The location of selected PCR primers corresponding to Pax8 consensus sequences at higher score are shown in Figure 4b. The ChIP results indicate that in vivo Pax8 is able to bind one of the two high-score selected sites present on the rat tp53inp1 promoter (Figure 4b). In agreement with the data obtained interfering with Pax8 expression, we also show that FRTL-5 cells overexpressing Pax8 have a significant reduction of tp53inp1 mRNA (Figure 4c) thus suggesting that tp53inp1 is a transcriptional target of Pax8 and may be the mediator of Pax8 regulation of epithelial cell survival.
Figure 4.
Pax8 silencing directly modulates tp53inp1 gene expression. (a) Total RNA was isolated from FRTL-5 cells 72 h after transfection and subjected to qPCR analysis. The values are means±S.D. of three independent experiments in duplicate, normalized by the expression of β-actin and expressed as fold change with respect to FRTL-5 wild-type cells whose value was set at 1.0. Statistical analysis uses t-test (P=0.01). (b) Schematic representation of the upstream region of the rat tp53inp1 gene. Pax8-binding sites are represented as striped boxes and are marked by asterisks. Pax8 binding sequences are indicated below the boxes and the core sequences are in bold. ChIP analysis was performed using FRTL-5 cells. Crosslinked chromatin was immunoprecipitated with the antibody for Pax8 or with an unrelated antibody (α-tubulin). Immunoprecipitates from each sample were analyzed by PCR using primers specific for the tp53inp1 promoter (indicated by arrows). (c) RNA samples from wild-type FRTL-5 cells, pCEFL-FLAG and pCEFL-FLAG-Pax8 pools were used to perform qPCR to measure the expression of tp53inp1. The data shown are normalized mean values±S.D. of three independent experiments in duplicate. Statistical analyses were performed using t-test (P<0.05)
The absence of tp53inp1 impairs Pax8-induced apoptosis
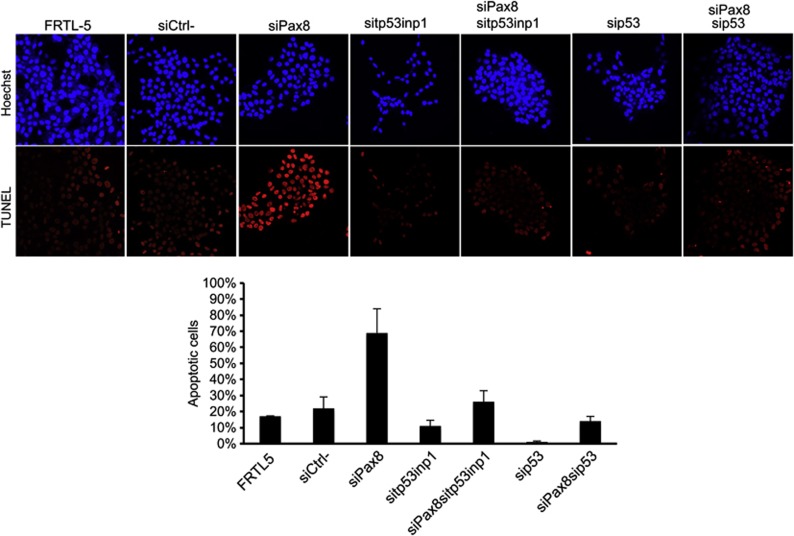
In the attempt to evaluate whether Pax8-induced apoptosis is mediated by tp53inp1, TUNEL assays were performed on FRTL-5 cells transiently transfected with Pax8 siRNA alone or in combination with siRNA specific for tp53inp1. The efficient and specific silencing of both genes was confirmed by qRT-PCR at 48 h after transfection (Supplementary Figure 2). Interestingly, Figure 5 shows that tp53inp1 knockdown significantly abolishes Pax8-induced apoptosis. At the same time, to evaluate whether p53 could influence such process, we silenced p53 in combination with Pax8. As before, the efficient and specific silencing of both genes was confirmed by qRT-PCR at 48 h after transfection (Supplementary Figure 2). TUNEL assays were performed 48 h after siRNAs transfection and showed that the number of apoptotic cells decreased significantly also in the absence of p53 (Figure 5), indicating that p53 contributes to Pax8-mediated apoptosis. At the same time, we performed the single silencing of tp53inp1 and p53 and we did not observe any effect.
Figure 5.
tp53inp1 depletion blocks Pax8-mediated apoptosis by a p53-dependent mechanism. TUNEL analysis of FRTL5 cells 48 h after silencing of Pax8 alone or in combination with tp53inp1 and p53. Averages of TUNEL-positive cells (%) with standard error are shown in the graph. Statistical analysis uses t-test (P<0.05)
Discussion
In addition to the essential role played by Pax8 in the morphogenesis of the thyroid gland and in the maintenance of the thyroid-differentiated phenotype, in this study, we describe a new role of Pax8 in the regulation of cell survival and proliferation. Such new role was somehow already suggested by studies on mouse models. One example is the Pax2 and Pax8 double mutant mouse that exhibits a complete lack of kidney formation, due to an increase in apoptosis during metanephros development.31, 32, 33
In addition, studies on Pax8 inactivation by antisense oligonucleotide strategy provided evidence that this transcription factor could have a role in thyroid cell growth in response to TSH and IGF-I stimuli.34
Here, we show that in differentiated epithelial cells Pax8 silencing strongly induces apoptosis, whereas its overexpression deregulates cell proliferation. Indeed, Pax8 null mice revealed that in the embryonic life the thyroid diverticulum is able to evaginate from the endoderm and migrate to its final position, but it requires Pax8 expression for further development. Infact, in the absence of this transcription factor the follicular cells are no longer detectable in the thyroid primordium at E12.5, suggesting that Pax8 is required for the survival of thyroid cell precursors.6 Accordingly, diffused presence of apoptotic cells has been shown at 1 day after birth in mice where Pax8 is conditionally ablated in the thyroid.35 Our data clearly demonstrate that Pax8 is critical also for the survival of FRTL-5 differentiated epithelial cells. We believe that at early stages of thyroid development Pax8 is needed for the survival of the cells during the migration process until the final localization is reached. Afterwards, when the thyroid bud is localized in the proper position, cells undergo differentiation and Pax8 is involved in the transcriptional regulation of the differentiation marker genes as already described.11 In addition, in line with studies on Pax8 inactivation suggesting that this transcription factor could have a role in thyroid cell growth,34 our study here reported indicate that Pax8 is also involved in the survival and proliferation of differentiated epithelial cells.
It is known that PAX gene expression confers a strong advantage to cancer cell growth and that Pax8 is overexpressed in a high proportion of tumors.4, 36, 37 Recently, a genome-wide approach to identify genetic alterations in cancer genomes has pinpointed Pax8 as essential for proliferation and survival of ovarian cancer cell lines.38 Moreover, our recent data relative to genome-wide expression profiling following Pax8 silencing in FRTL-5 differentiated epithelial cells indicated that many gene categories are affected by Pax8 knockdown, in particular, genes of the cell cycle category such as E2F1, E2F6, cyclin-A2 (CCNA2) and CDC6.30 In agreement with our data above mentioned, the capability of Pax8 to promote tumor cell proliferation through regulation of E2F1 and concomitant stabilization of the RB protein has been recently described.39 E2F1 is a member of the E2F gene family that includes eight members (E2F1–8), all of which has a critical role in the control of cell proliferation by transcriptionally regulating genes required for cell cycle progression and genes involved in apoptosis, DNA repair, differentiation and development.40
Our findings indicate that tp53inp1 is a direct target of Pax8 and is upregulated by Pax8 knockdown. Tp53inp1 is a p53-regulated gene27, 41 and has a pivotal role in cell cycle control and in p53-mediated apoptosis.27 Hence, we propose that Pax8 could regulate cell survival of differentiated epithelial cells via the transcriptional regulation of tp53inp1.
Pax8 overexpression has been implicated in several cancers; however, low level or loss of Pax8 has been reported specifically in thyroid tumors.42, 43 How can these two observations be compatible? A possible explanation for this apparent paradox might be suggested by recently published studies on the p53-HIPK2 (homeodomain-interacting protein kinase 2) pathway in thyroid tumors, which underlines a reduced HIPK2 expression or function in thyroid cancers.44 Moreover, when the loss of HIPK2 and/or its cytoplasmic relocalization occurs in thyroid tumors,45 p53-mediated apoptosis is inhibited and consequently this event is responsible for aberrant tumor growth. Therefore, we hypothesize that in pathological conditions, that is several tumor types, Pax8 is overexpressed and confers a proliferative advantage to the cells. At variance, in epithelial thyroid cells, Pax8 is downregulated in the malignant tissue and its reduced or lost expression does not trigger apoptosis in such condition because the p53-HIPK2 pathway is also altered in this tumor type. In conclusion, the role of Pax8 in cell cycle progression and cell survival in FRTL-5 differentiated epithelial cells that here we describe represents a new function of the Pax8 protein that is beginning to come out, but still much work remains to be performed. To improve our knowledge of the complex role of this transcription factor, of great interest will be the characterization of the entire molecular network that allows Pax8 to regulate such diverse cellular processes.
Materials and Methods
Materials
Coon's modified F-12 medium and calf serum (CS) were from Euroclone (Milano, Italy). Hormones (thyrotropic hormone, hydrocortisone, insulin, transferrin, somatostatin and glycyl-histidyl-lysine) were from Sigma-Aldrich Corp. (St. Louis, MO, USA). Geneticin were from Gibco (Grand Island, NY, USA). FuGENE HD transfection reagent was purchased from Promega Corp. (Madison, WI, USA). DharmaFECT 1 Transfection Reagent was obtained from Dharmacon Inc. (Chicago, IL, USA). Primary antibodies against p21waf1/cip1, cyclin D3, cyclin B1, cyclin E, cyclin A, cyclin B1, cdk2, cdk4 and α-tubulin were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Polyclonal antibody anti-Pax8, anti-Nkx2.1, anti-Tg and anti-Foxe1 were kindly provided by Professor R Di Lauro, the monoclonal antibody for p27kip1 was from BD Biosciences Transduction Laboratories (Milano, Italy). Primary antibodies for Bcl2, Bax and PARP1 were purchased from Cell Signaling Technology (Danvers, MA, USA). The primary antibody for Rb was purchased from BD Pharmigen (G3-245; San Jose, CA, USA). The secondary antibodies used were horseradish peroxidase-conjugated, anti-mouse and anti-rabbit antibodies (GE Healthcare, Piscataway, NJ, USA). Enhanced chemiluminescence was purchased from Pierce Chemical (Rockford, IL, USA). Culture dishes were purchased from Euroclone.
Cell culture and transfection
FRTL-5 cells were grown in Coon's modified F-12 medium supplemented with 5% CS and a 6H mixture as described by Ambesi-Impiombato and Coon (Ambesi-Impiombato, 1979). Transfections were carried out with the FuGENE reagent according to the manufacturer's instructions. The transfected cells were selected in a medium containing geneticin (G418). Several clones and the mass cell population were isolated and expanded for further analysis.
Plasmid constructs, colony-forming and cell proliferation assays
The mouse 3XFLAG-Pax8-coding region46 was subcloned in the BamHI-XbaI sites of the pCEFL vector. The expression of 3XFLAG-Pax8 was assessed by western blotting. FRTL-5 cells were transfected with 2 μg of FLAG-Pax8 or pCEFL backbone vector and selected with geneticin (G418). After 2 weeks of drug selection, the cells were fixed and stained with 1% crystal violet.
To measure cell growth parameters, FRTL5-FLAG and FRTL5-FLAG-Pax8 pools and FRTL5-FLAG-Pax8 individual clones were plated at 80 000 cells/60-mm plate. The cells were grown in F12 medium with 5% CS and the six-hormones mixture or in medium hormone-deprived and counted every 24 h.
Cell cycle analysis
Cells were starved in medium containing 0.2% CS for 72 h and then cultured in complete medium for 48 h. Cells were collected by trypsinization at different times and fixed in ice-cold 80% ethanol. After washing in PBS, cells were incubated with 25 μl/ml RNase and 50 μl/ml propidium iodide for 30 min. Cell-cycle distribution was analyzed with FACS-Calibur flow cytometer (Becton Dickinson, Milan, Italy), and the data were analyzed using a Mod-Fit cell cycle analysis program.
RNA interference
FRTL-5 cells were plated (8 × 104 well) in 24-well plates and transfected in triplicate with 100 nM Pax8 siGENOME siRNA, 100 nM tp53inp1 siGENOME SMART pool siRNA, 100 nM p53 siGENOME SMART pool siRNA or siGENOME Non-Targeting (Dharmacon, Inc.) using the DharmaFECT1 transfection reagent, as previously described.47 Cells were harvested 24, 48 and 72 h after transfection and the total RNA or total cell lysates were prepared.
RNA extraction and quantitative real-time RT-PCR
Total RNA was extracted using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and cDNAs were synthesized using the iScript cDNA Synthesis kit according to the manufacturer's instructions (Bio-Rad, Hercules, CA, USA). Real-time RT-PCR analysis was performed using IQ SYBR Green PCR Master Mix (Bio-Rad) in a iCycler IQ real-time detection system (Bio-Rad).
Caspase Activity Assay
Total cell lysates were prepared from FRTL-5 24 h after transfection and the activity of caspase-3 was assessed using the Caspase Fluorometric Assay kit (Alexis Biochemicals, San Diego, CA, USA) according to the manufacturer's instructions. Cells were harvested and lysed in 50 μl lysis buffer containing 2 mM DTT. After centrifugation, the supernatant containing 100 μg protein was incubated at 37 °C for 2 h with the caspase-3 substrate (Ac-DEVD-AFC) and the fluorescence from the cleaved substrate was measured. The activation of the caspase was expressed as fold change with respect to the absorbance of a blank control containing 50 μl 2 × reaction buffer and 50 μl lysis buffer.
TUNEL assay
In situ TUNEL was performed using in situ Cell Death Detection kit (Roche) as suggested by the manufacturer. All staining were counterstained with DRAQ5 (Vinci-Biochem Srl, Florence, Italy) before mounting. Microscopy and imaging were performed at a confocal laser scanner microscope (LSM 510; Zeiss, Gottingen, Germany). For quantification of apoptotic cells, a minimum of 100 cells, each from at least three microscope fields, was examined for TUNEL-positive nuclei among the DRAQ5-stained ones. The frequency of apoptosis was expressed as a percentage (means±S.E.) of apoptotic cells obtained from three independent experiments.
ChIP
ChIP was performed as previously described.30 Precleared chromatin from FRTL-5 cells was incubated with 1 μg of affinity-purified rabbit polyclonal anti-Pax8 antibody or monoclonal anti-tubulin antibody and rotated at 4 °C for 16 h. The immunoprecipitated DNA was analyzed by PCR using the following tp53inp1 primers: 5′-GTAGCTGTCTTCAGACACACC-3′ and 5′-TGACTGTGCAGTCTGCCTAAC-3′.
Protein extraction and western blotting
Protein extraction and western blotting procedures were carried out as reported elsewhere (Di Palma et al., 2008). Membranes were incubated with the specific antibodies as indicated in the figures; the secondary antibodies used were horseradish peroxidase-conjugated anti-mouse and anti-rabbit antibodies. The proteins were detected by enhanced chemiluminescence.
Acknowledgments
This work was supported by grants from the Italian Ministry of Education, University and Research (MIUR-PRIN 2009), from the Italian Ministry of Economy and Finance to the CNR for the Project FaReBio di Qualità and by the grant Medical Research in Italy (MERIT) RBNE08YFN3_001. We deeply thank M Crescenzi for helpful discussions.
Glossary
- FRTL-5
Fischer rat thyroid line 5
- PARP-1
poly(ADP)ribose polymerase 1
- tp53inp1
Tumor protein 53 induced nuclear protein 1
- Tg
thyroglobulin
- PPARγ
peroxisome proliferator-activated receptor gamma
- 6H
six hormones
- ChIP
chromatin immunoprecipitation
- TUNEL
Terminal deoxynucleotidyl transferase dUTP nick end labeling
- HIPK2
homeodomain-interacting protein kinase 2
- CS
calf serum
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Cell Death and Disease website (http://www.nature.com/cddis)
Edited by D Aberdam
Supplementary Material
References
- Dahl E, Koseki H, Balling R. Pax genes and organogenesis. Bioessays. 1997;19:755–765. doi: 10.1002/bies.950190905. [DOI] [PubMed] [Google Scholar]
- Walther C, Guenet JL, Simon D, Deutsch U, Jostes B, Goulding MD, et al. Pax: a murine multigene family of paired box-containing genes. Genomics. 1991;11:424–434. doi: 10.1016/0888-7543(91)90151-4. [DOI] [PubMed] [Google Scholar]
- Lang D, Powell SK, Plummer RS, Young KP, Ruggeri BA. PAX genes: roles in development, pathophysiology, and cancer. Biochem Pharmacol. 2007;73:1–14. doi: 10.1016/j.bcp.2006.06.024. [DOI] [PubMed] [Google Scholar]
- Muratovska A, Zhou C, He S, Goodyer P, Eccles MR. Paired-Box genes are frequently expressed in cancer and often required for cancer cell survival. Oncogene. 2003;22:7989–7997. doi: 10.1038/sj.onc.1206766. [DOI] [PubMed] [Google Scholar]
- Plachov D, Chowdhury K, Walther C, Simon D, Guenet JL, Gruss P. Pax8, a murine paired box gene expressed in the developing excretory system and thyroid gland. Development. 1990;110:643–651. doi: 10.1242/dev.110.2.643. [DOI] [PubMed] [Google Scholar]
- Mansouri A, Chowdhury K, Gruss P. Follicular cells of the thyroid gland require Pax8 gene function. Nat Genet. 1998;19:87–90. doi: 10.1038/ng0598-87. [DOI] [PubMed] [Google Scholar]
- Pasca di Magliano M, Di Lauro R, Zannini M. Pax8 has a key role in thyroid cell differentiation. Proc Natl Acad Sci USA. 2000;97:13144–13149. doi: 10.1073/pnas.240336397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno M, Zannini M, Levy O, Carrasco N, di Lauro R. The paired-domain transcription factor Pax8 binds to the upstream enhancer of the rat sodium/iodide symporter gene and participates in both thyroid-specific and cyclic-AMP-dependent transcription. Mol Cell Biol. 1999;19:2051–2060. doi: 10.1128/mcb.19.3.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zannini M, Francis-Lang H, Plachov D, Di Lauro R. Pax-8, a paired domain-containing protein, binds to a sequence overlapping the recognition site of a homeodomain and activates transcription from two thyroid-specific promoters. Mol Cell Biol. 1992;12:4230–4241. doi: 10.1128/mcb.12.9.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong GX, Memeo L, Colarossi C, Hamele-Bena D, Magi-Galluzzi C, Zhou M, et al. PAX8 and PAX2 immunostaining facilitates the diagnosis of primary epithelial neoplasms of the male genital tract. Am J Surg Pathol. 2011;35:1473–1483. doi: 10.1097/PAS.0b013e318227e2ee. [DOI] [PubMed] [Google Scholar]
- De Felice M, Di Lauro R. Thyroid development and its disorders: genetics and molecular mechanisms. Endocr Rev. 2004;25:722–746. doi: 10.1210/er.2003-0028. [DOI] [PubMed] [Google Scholar]
- Al Taji E, Biebermann H, Limanova Z, Hnikova O, Zikmund J, Dame C, et al. Screening for mutations in transcription factors in a Czech cohort of 170 patients with congenital and early-onset hypothyroidism: identification of a novel PAX8 mutation in dominantly inherited early-onset non-autoimmune hypothyroidism. Eur J Endocrinol. 2007;156:521–529. doi: 10.1530/EJE-06-0709. [DOI] [PubMed] [Google Scholar]
- Di Palma T, Zampella E, Filippone MG, Macchia PE, Ris-Stalpers C, de Vroede M, et al. Characterization of a novel loss-of-function mutation of PAX8 associated with congenital hypothyroidism. Clin Endocrinol (Oxf) 2010;73:808–814. doi: 10.1111/j.1365-2265.2010.03851.x. [DOI] [PubMed] [Google Scholar]
- Macchia PE, Lapi P, Krude H, Pirro MT, Missero C, Chiovato L, et al. PAX8 mutations associated with congenital hypothyroidism caused by thyroid dysgenesis. Nat Genet. 1998;19:83–86. doi: 10.1038/ng0598-83. [DOI] [PubMed] [Google Scholar]
- Tong GX, Yu WM, Beaubier NT, Weeden EM, Hamele-Bena D, Mansukhani MM, et al. Expression of PAX8 in normal and neoplastic renal tissues: an immunohistochemical study. Mod Pathol. 2009;22:1218–1227. doi: 10.1038/modpathol.2009.88. [DOI] [PubMed] [Google Scholar]
- Tong GX, Weeden EM, Hamele-Bena D, Huan Y, Unger P, Memeo L, et al. Expression of PAX8 in nephrogenic adenoma and clear cell adenocarcinoma of the lower urinary tract: evidence of related histogenesis. Am J Surg Pathol. 2008;32:1380–1387. doi: 10.1097/PAS.0b013e31816b1020. [DOI] [PubMed] [Google Scholar]
- Bowen NJ, Logani S, Dickerson EB, Kapa LB, Akhtar M, Benigno BB, et al. Emerging roles for PAX8 in ovarian cancer and endosalpingeal development. Gynecol Oncol. 2007;104:331–337. doi: 10.1016/j.ygyno.2006.08.052. [DOI] [PubMed] [Google Scholar]
- Nonaka D, Tang Y, Chiriboga L, Rivera M, Ghossein R. Diagnostic utility of thyroid transcription factors Pax8 and TTF-2 (FoxE1) in thyroid epithelial neoplasms. Mod Pathol. 2008;21:192–200. doi: 10.1038/modpathol.3801002. [DOI] [PubMed] [Google Scholar]
- Ros P, Rossi DL, Acebron A, Santisteban P. Thyroid-specific gene expression in the multi-step process of thyroid carcinogenesis. Biochimie. 1999;81:389–396. doi: 10.1016/s0300-9084(99)80086-8. [DOI] [PubMed] [Google Scholar]
- Sangoi AR, Ohgami RS, Pai RK, Beck AH, McKenney JK. PAX8 expression reliably distinguishes pancreatic well-differentiated neuroendocrine tumors from ileal and pulmonary well-differentiated neuroendocrine tumors and pancreatic acinar cell carcinoma. Mod Pathol. 2011;24:412–424. doi: 10.1038/modpathol.2010.176. [DOI] [PubMed] [Google Scholar]
- Eberhardt NL, Grebe SK, McIver B, Reddi HV. The role of the PAX8/PPARgamma fusion oncogene in the pathogenesis of follicular thyroid cancer. Mol Cell Endocrinol. 2010;321:50–56. doi: 10.1016/j.mce.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espadinha C, Cavaco BM, Leite V. PAX8PPARgamma stimulates cell viability and modulates expression of thyroid-specific genes in a human thyroid cell line. Thyroid. 2007;17:497–509. doi: 10.1089/thy.2006.0263. [DOI] [PubMed] [Google Scholar]
- Cheung L, Messina M, Gill A, Clarkson A, Learoyd D, Delbridge L, et al. Detection of the PAX8-PPAR gamma fusion oncogene in both follicular thyroid carcinomas and adenomas. J Clin Endocrinol Metab. 2003;88:354–357. doi: 10.1210/jc.2002-021020. [DOI] [PubMed] [Google Scholar]
- Schaner ME, Ross DT, Ciaravino G, Sorlie T, Troyanskaya O, Diehn M, et al. Gene expression patterns in ovarian carcinomas. Mol Biol Cell. 2003;14:4376–4386. doi: 10.1091/mbc.E03-05-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poleev A, Fickenscher H, Mundlos S, Winterpacht A, Zabel B, Fidler A, et al. PAX8, a human paired box gene: isolation and expression in developing thyroid, kidney and Wilms' tumors. Development. 1992;116:611–623. doi: 10.1242/dev.116.3.611. [DOI] [PubMed] [Google Scholar]
- Chen YJ, Campbell HG, Wiles AK, Eccles MR, Reddel RR, Braithwaite AW, et al. PAX8 regulates telomerase reverse transcriptase and telomerase RNA component in glioma. Cancer Res. 2008;68:5724–5732. doi: 10.1158/0008-5472.CAN-08-0058. [DOI] [PubMed] [Google Scholar]
- Okamura S, Arakawa H, Tanaka T, Nakanishi H, Ng CC, Taya Y, et al. p53DINP1, a p53-inducible gene, regulates p53-dependent apoptosis. Mol Cell. 2001;8:85–94. doi: 10.1016/s1097-2765(01)00284-2. [DOI] [PubMed] [Google Scholar]
- Tomasini R, Samir AA, Carrier A, Isnardon D, Cecchinelli B, Soddu S, et al. TP53INP1s and homeodomain-interacting protein kinase-2 (HIPK2) are partners in regulating p53 activity. J Biol Chem. 2003;278:37722–37729. doi: 10.1074/jbc.M301979200. [DOI] [PubMed] [Google Scholar]
- Fusco A, Berlingieri MT, Di Fiore PP, Portella G, Grieco M, Vecchio G. One- and two-step transformations of rat thyroid epithelial cells by retroviral oncogenes. Mol Cell Biol. 1987;7:3365–3370. doi: 10.1128/mcb.7.9.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Palma T, Conti A, de Cristofaro T, Scala S, Nitsch L, Zannini M. Identification of novel Pax8 targets in FRTL-5 thyroid cells by gene silencing and expression microarray analysis. PLoS One. 2011;6:e25162. doi: 10.1371/journal.pone.0025162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard M, Souabni A, Mandler M, Neubuser A, Busslinger M. Nephric lineage specification by Pax2 and Pax8. Genes Dev. 2002;16:2958–2970. doi: 10.1101/gad.240102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narlis M, Grote D, Gaitan Y, Boualia SK, Bouchard M. Pax2 and pax8 regulate branching morphogenesis and nephron differentiation in the developing kidney. J Am Soc Nephrol. 2007;18:1121–1129. doi: 10.1681/ASN.2006070739. [DOI] [PubMed] [Google Scholar]
- Torban E, Eccles MR, Favor J, Goodyer PR. PAX2 suppresses apoptosis in renal collecting duct cells. Am J Pathol. 2000;157:833–842. doi: 10.1016/S0002-9440(10)64597-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi DL, Acebron A, Santisteban P. Function of the homeo and paired domain proteins TTF-1 and Pax-8 in thyroid cell proliferation. J Biol Chem. 1995;270:23139–23142. doi: 10.1074/jbc.270.39.23139. [DOI] [PubMed] [Google Scholar]
- Fagman H, Amendola E, Parrillo L, Zoppoli P, Marotta P, Scarfo M, et al. Gene expression profiling at early organogenesis reveals both common and diverse mechanisms in foregut patterning. Dev Biol. 2011;359:163–175. doi: 10.1016/j.ydbio.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles MR, Yun K, Reeve AE, Fidler AE. Comparative in situ hybridization analysis of PAX2, PAX8, and WT1 gene transcription in human fetal kidney and Wilms' tumors. Am J Pathol. 1995;146:40–45. [PMC free article] [PubMed] [Google Scholar]
- Long KB, Srivastava A, Hirsch MS, Hornick JL. PAX8 Expression in well-differentiated pancreatic endocrine tumors: correlation with clinicopathologic features and comparison with gastrointestinal and pulmonary carcinoid tumors. Am J Surg Pathol. 2010;34:723–729. doi: 10.1097/PAS.0b013e3181da0a20. [DOI] [PubMed] [Google Scholar]
- Cheung HW, Cowley GS, Weir BA, Boehm JS, Rusin S, Scott JA, et al. Systematic investigation of genetic vulnerabilities across cancer cell lines reveals lineage-specific dependencies in ovarian cancer. Proc Natl Acad Sci USA. 2011;108:12372–12377. doi: 10.1073/pnas.1109363108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CG, Nyman JE, Braithwaite AW, Eccles MR. PAX8 promotes tumor cell growth by transcriptionally regulating E2F1 and stabilizing RB protein. Oncogene. 2011;30:4824–4834. doi: 10.1038/onc.2011.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGregori J, Johnson DG. Distinct and overlapping roles for E2F family members in transcription, proliferation and apoptosis. Curr Mol Med. 2006;6:739–748. doi: 10.2174/1566524010606070739. [DOI] [PubMed] [Google Scholar]
- Tomasini R, Samir AA, Pebusque MJ, Calvo EL, Totaro S, Dagorn JC, et al. P53-dependent expression of the stress-induced protein (SIP) Eur J Cell Biol. 2002;81:294–301. doi: 10.1078/0171-9335-00248. [DOI] [PubMed] [Google Scholar]
- Bishop JA, Sharma R, Westra WH. PAX8 immunostaining of anaplastic thyroid carcinoma: a reliable means of discerning thyroid origin for undifferentiated tumors of the head and neck. Hum Pathol. 2011;42:1873–1877. doi: 10.1016/j.humpath.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Zhang P, Zuo H, Nakamura Y, Nakamura M, Wakasa T, Kakudo K. Immunohistochemical analysis of thyroid-specific transcription factors in thyroid tumors. Pathol Int. 2006;56:240–245. doi: 10.1111/j.1440-1827.2006.01959.x. [DOI] [PubMed] [Google Scholar]
- Lavra L, Rinaldo C, Ulivieri A, Luciani E, Fidanza P, Giacomelli L, et al. The loss of the p53 activator HIPK2 is responsible for galectin-3 overexpression in well differentiated thyroid carcinomas. PLoS One. 2011;6:e20665. doi: 10.1371/journal.pone.0020665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierantoni GM, Rinaldo C, Mottolese M, Di Benedetto A, Esposito F, Soddu S, et al. High-mobility group A1 inhibits p53 by cytoplasmic relocalization of its proapoptotic activator HIPK2. J Clin Invest. 2007;117:693–702. doi: 10.1172/JCI29852. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Di Palma T, Nitsch R, Mascia A, Nitsch L, Di Lauro R, Zannini M. The paired domain-containing factor Pax8 and the homeodomain-containing factor TTF-1 directly interact and synergistically activate transcription. J Biol Chem. 2003;278:3395–3402. doi: 10.1074/jbc.M205977200. [DOI] [PubMed] [Google Scholar]
- de Cristofaro T, Di Palma T, Ferraro A, Corrado A, Lucci V, Franco R, et al. TAZ/WWTR1 is overexpressed in papillary thyroid carcinoma. Eur J Cancer. 2011;47:926–933. doi: 10.1016/j.ejca.2010.11.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.