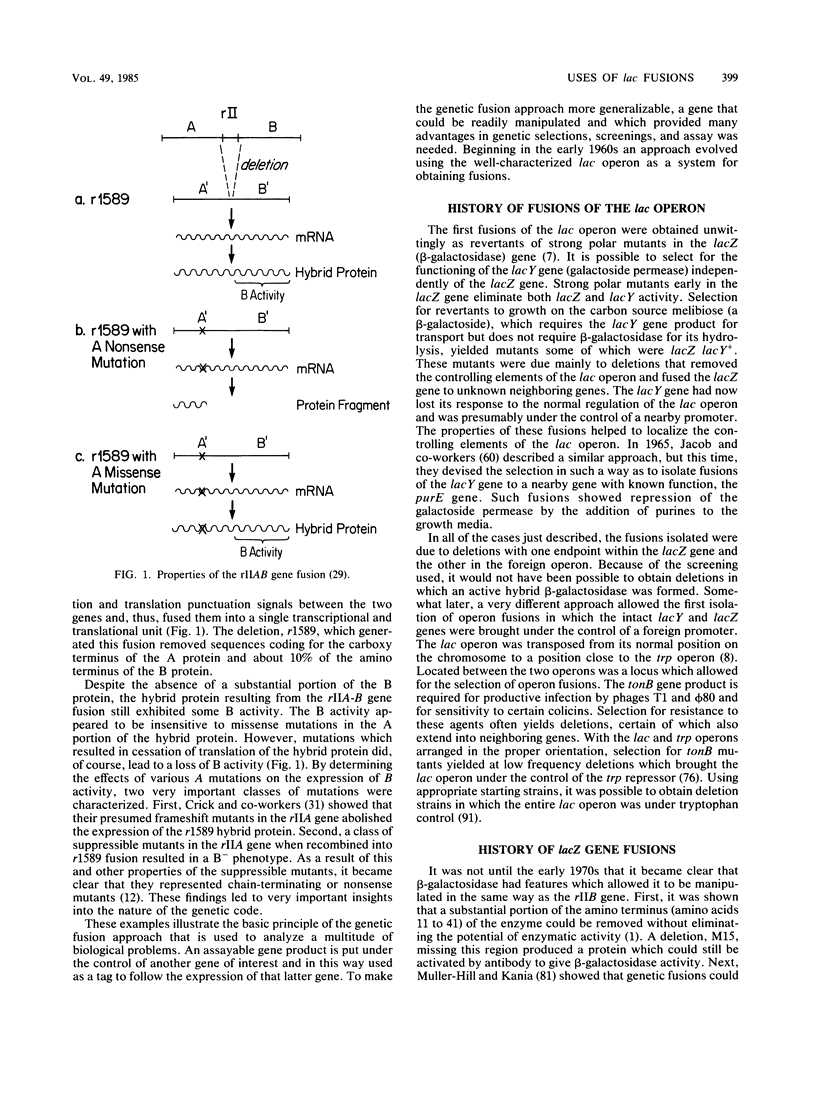
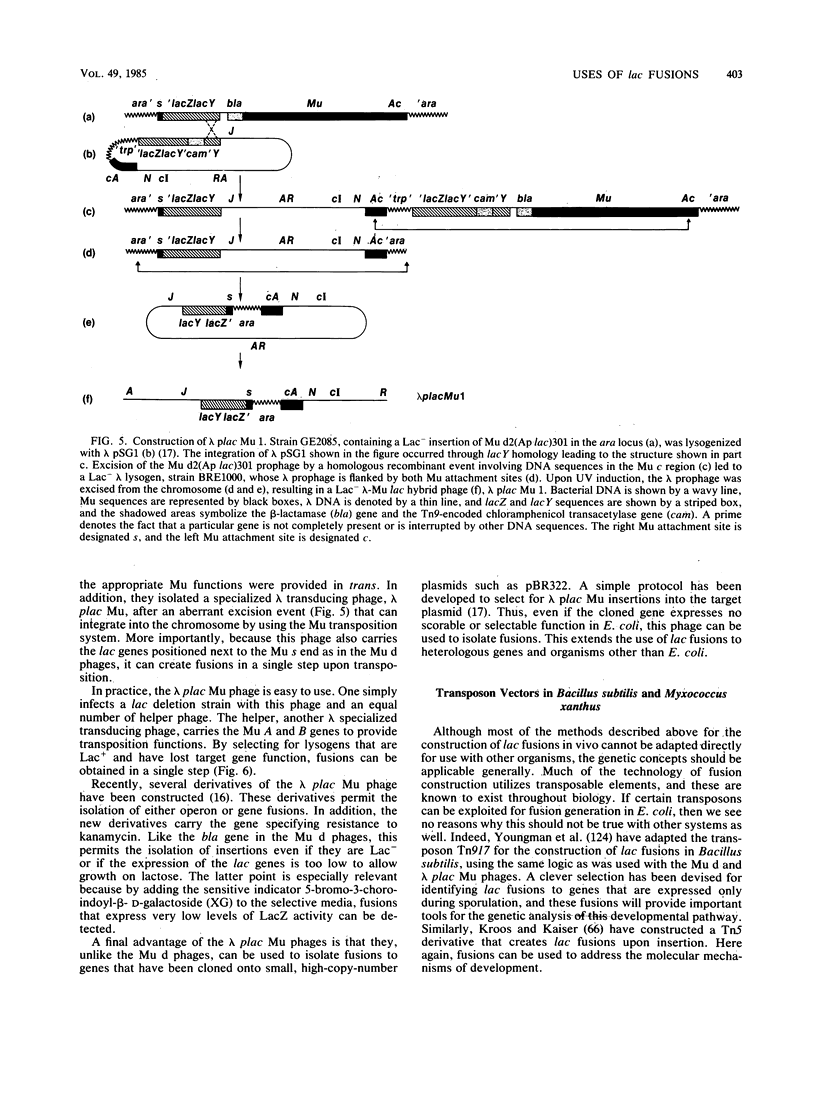
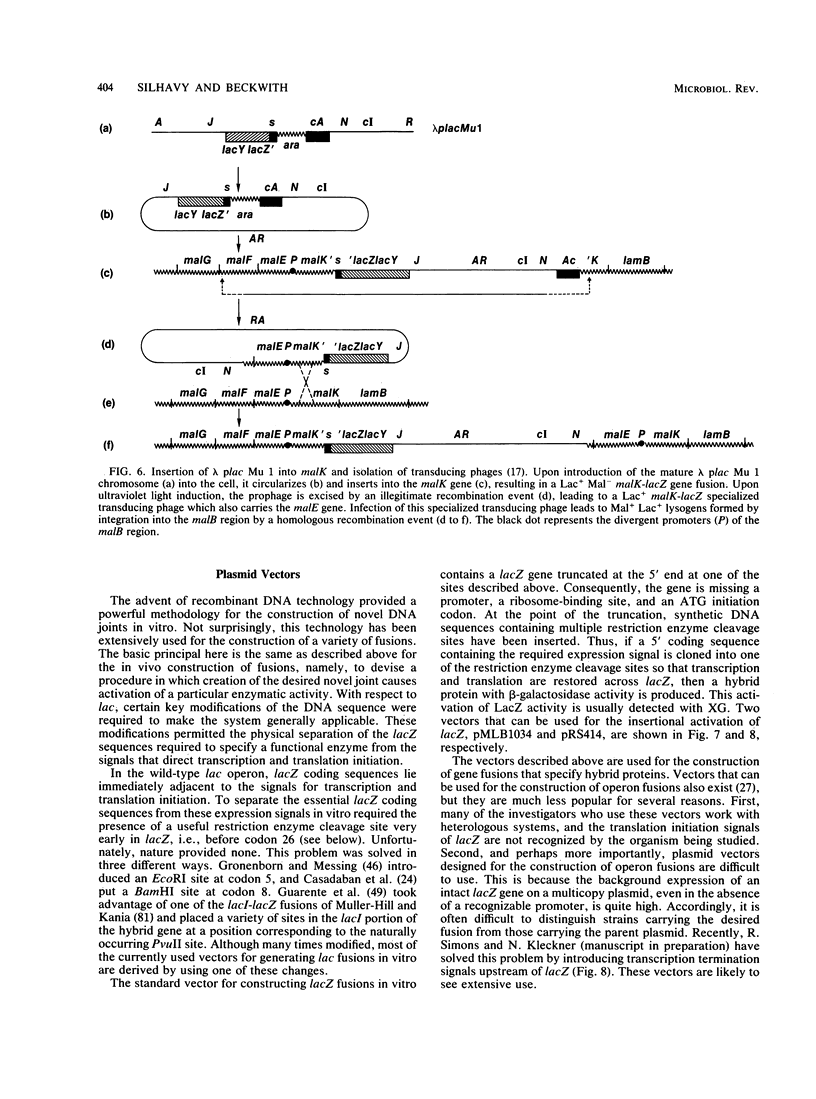
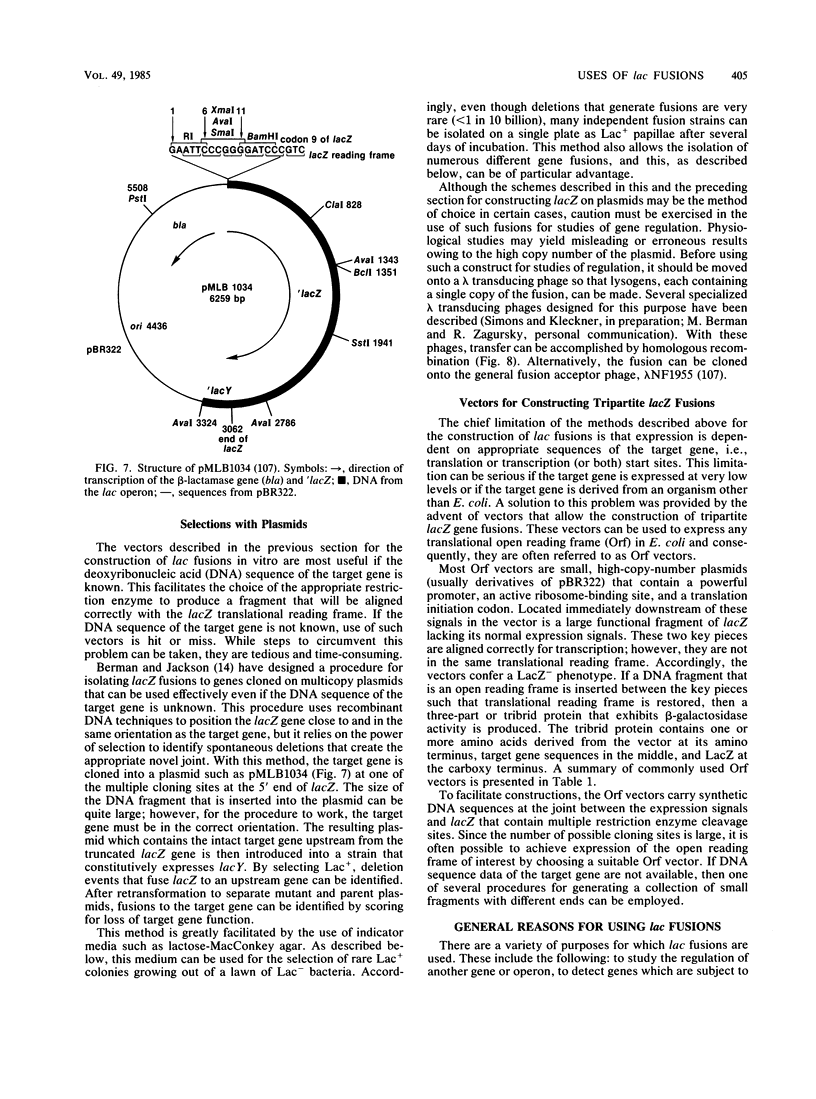
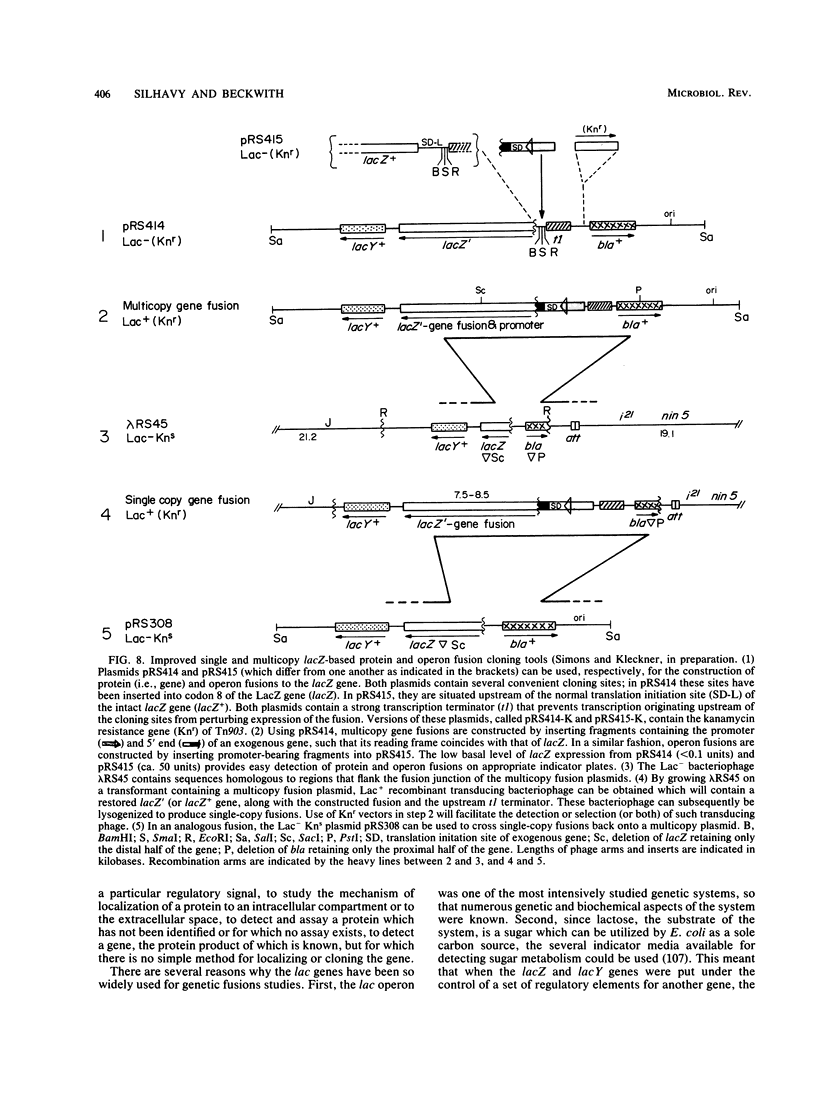
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Accolla R. S., Celada F. Antibody-mediated activation of a deletion-mutant beta-galactosidase defective in the alpha region. FEBS Lett. 1976 Sep 1;67(3):299–302. doi: 10.1016/0014-5793(76)80551-0. [DOI] [PubMed] [Google Scholar]
- Aksoy S., Squires C. L., Squires C. Translational coupling of the trpB and trpA genes in the Escherichia coli tryptophan operon. J Bacteriol. 1984 Feb;157(2):363–367. doi: 10.1128/jb.157.2.363-367.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BECKWITH J. R. A DELETION ANALYSIS OF THE LAC OPERATOR REGION IN ESCHERICHIA COLI. J Mol Biol. 1964 Mar;8:427–430. doi: 10.1016/s0022-2836(64)80206-0. [DOI] [PubMed] [Google Scholar]
- BENZER S., CHAMPE S. P. A change from nonsense to sense in the genetic code. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1114–1121. doi: 10.1073/pnas.48.7.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker H. V., 2nd, Wolf R. E., Jr Essential site for growth rate-dependent regulation within the Escherichia coli gnd structural gene. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7669–7673. doi: 10.1073/pnas.81.24.7669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker H. V., 2nd, Wolf R. E., Jr Growth rate-dependent regulation of 6-phosphogluconate dehydrogenase level in Escherichia coli K-12: beta-galactosidase expression in gnd-lac operon fusion strains. J Bacteriol. 1983 Feb;153(2):771–781. doi: 10.1128/jb.153.2.771-781.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankaitis V. A., Bassford P. J., Jr The synthesis of export-defective proteins can interfere with normal protein export in Escherichia coli. J Biol Chem. 1984 Oct 10;259(19):12193–12200. [PubMed] [Google Scholar]
- Beckwith J. R., Signer E. R., Epstein W. Transposition of the Lac region of E. coli. Cold Spring Harb Symp Quant Biol. 1966;31:393–401. doi: 10.1101/sqb.1966.031.01.051. [DOI] [PubMed] [Google Scholar]
- Beckwith J., Silhavy T. J. Genetic analysis of protein export in Escherichia coli. Methods Enzymol. 1983;97:3–11. doi: 10.1016/0076-6879(83)97114-8. [DOI] [PubMed] [Google Scholar]
- Bedouelle H. Mutations in the promoter regions of the malEFG and malK-lamB operons of Escherichia coli K12. J Mol Biol. 1983 Nov 15;170(4):861–882. doi: 10.1016/s0022-2836(83)80192-2. [DOI] [PubMed] [Google Scholar]
- Benson S. A., Bremer E., Silhavy T. J. Intragenic regions required for LamB export. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3830–3834. doi: 10.1073/pnas.81.12.3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson S. A., Hall M. N., Silhavy T. J. Genetic analysis of protein export in Escherichia coli K12. Annu Rev Biochem. 1985;54:101–134. doi: 10.1146/annurev.bi.54.070185.000533. [DOI] [PubMed] [Google Scholar]
- Berman M. L., Beckwith J. Use of gene fusions to isolate promoter mutants in the transfer RNA gene tyrT of Escherichia coli. J Mol Biol. 1979 May 25;130(3):303–315. doi: 10.1016/0022-2836(79)90543-6. [DOI] [PubMed] [Google Scholar]
- Berman M. L., Jackson D. E. Selection of lac gene fusions in vivo: ompR-lacZ fusions that define a functional domain of the ompR gene product. J Bacteriol. 1984 Aug;159(2):750–756. doi: 10.1128/jb.159.2.750-756.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer E., Beck E., Hindennach I., Sonntag I., Henning U. Cloned structural gene (ompA) for an integral outer membrane protein of Escherichia coli K-12: localization on hybrid plasmid pTU100 and expression of a fragment of the gene. Mol Gen Genet. 1980;179(1):13–20. doi: 10.1007/BF00268440. [DOI] [PubMed] [Google Scholar]
- Bremer E., Silhavy T. J., Weinstock G. M. Transposable lambda placMu bacteriophages for creating lacZ operon fusions and kanamycin resistance insertions in Escherichia coli. J Bacteriol. 1985 Jun;162(3):1092–1099. doi: 10.1128/jb.162.3.1092-1099.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer E., Silhavy T. J., Weisemann J. M., Weinstock G. M. Lambda placMu: a transposable derivative of bacteriophage lambda for creating lacZ protein fusions in a single step. J Bacteriol. 1984 Jun;158(3):1084–1093. doi: 10.1128/jb.158.3.1084-1093.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman E., Silhavy T. J., Bassford P. J., Jr, Shuman H. A., Beckwith J. R. Sites within gene lacZ of Escherichia coli for formation of active hybrid beta-galactosidase molecules. J Bacteriol. 1979 Jul;139(1):13–18. doi: 10.1128/jb.139.1.13-18.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAMPE S. P., BENZER S. An active cistron fragment. J Mol Biol. 1962 Apr;4:288–292. doi: 10.1016/s0022-2836(62)80006-0. [DOI] [PubMed] [Google Scholar]
- CRICK F. H., BARNETT L., BRENNER S., WATTS-TOBIN R. J. General nature of the genetic code for proteins. Nature. 1961 Dec 30;192:1227–1232. doi: 10.1038/1921227a0. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Chou J., Cohen S. N. Overproduction of the Tn3 transposition protein and its role in DNA transposition. Cell. 1982 Feb;28(2):345–354. doi: 10.1016/0092-8674(82)90352-x. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Chou J. In vivo formation of gene fusions encoding hybrid beta-galactosidase proteins in one step with a transposable Mu-lac transducing phage. Proc Natl Acad Sci U S A. 1984 Jan;81(2):535–539. doi: 10.1073/pnas.81.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980 Apr;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Lactose genes fused to exogenous promoters in one step using a Mu-lac bacteriophage: in vivo probe for transcriptional control sequences. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4530–4533. doi: 10.1073/pnas.76.9.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Martinez-Arias A., Shapira S. K., Chou J. Beta-galactosidase gene fusions for analyzing gene expression in escherichia coli and yeast. Methods Enzymol. 1983;100:293–308. doi: 10.1016/0076-6879(83)00063-4. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J. Regulation of the regulatory gene for the arabinose pathway, araC. J Mol Biol. 1976 Jul 5;104(3):557–566. doi: 10.1016/0022-2836(76)90120-0. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976 Jul 5;104(3):541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- Cerretti D. P., Dean D., Davis G. R., Bedwell D. M., Nomura M. The spc ribosomal protein operon of Escherichia coli: sequence and cotranscription of the ribosomal protein genes and a protein export gene. Nucleic Acids Res. 1983 May 11;11(9):2599–2616. doi: 10.1093/nar/11.9.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J., Casadaban M. J., Lemaux P. G., Cohen S. N. Identification and characterization of a self-regulated repressor of translocation of the Tn3 element. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4020–4024. doi: 10.1073/pnas.76.8.4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas M. G., Geller B. L., Emr S. D. Intracellular targeting and import of an F1-ATPase beta-subunit-beta-galactosidase hybrid protein into yeast mitochondria. Proc Natl Acad Sci U S A. 1984 Jul;81(13):3983–3987. doi: 10.1073/pnas.81.13.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Débarbouillé M., Shuman H. A., Silhavy T. J., Schwartz M. Dominant constitutive mutations in malT, the positive regulator gene of the maltose regulon in Escherichia coli. J Mol Biol. 1978 Sep 15;124(2):359–371. doi: 10.1016/0022-2836(78)90304-2. [DOI] [PubMed] [Google Scholar]
- Ebright R. H., Cossart P., Gicquel-Sanzey B., Beckwith J. Mutations that alter the DNA sequence specificity of the catabolite gene activator protein of E. coli. Nature. 1984 Sep 20;311(5983):232–235. doi: 10.1038/311232a0. [DOI] [PubMed] [Google Scholar]
- Emr S. D., Schauer I., Hansen W., Esmon P., Schekman R. Invertase beta-galactosidase hybrid proteins fail to be transported from the endoplasmic reticulum in Saccharomyces cerevisiae. Mol Cell Biol. 1984 Nov;4(11):2347–2355. doi: 10.1128/mcb.4.11.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emr S. D., Silhavy T. J. Mutations affecting localization of an Escherichia coli outer membrane protein, the bacteriophage lambda receptor. J Mol Biol. 1980 Jul 25;141(1):63–90. doi: 10.1016/s0022-2836(80)80029-5. [DOI] [PubMed] [Google Scholar]
- Fowler A. V., Zabin I. Purification, structure, and properties of hybrid beta-galactosidase proteins. J Biol Chem. 1983 Dec 10;258(23):14354–14358. [PubMed] [Google Scholar]
- Freitag C. S., Eisenstein B. I. Genetic mapping and transcriptional orientation of the fimD gene. J Bacteriol. 1983 Dec;156(3):1052–1058. doi: 10.1128/jb.156.3.1052-1058.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froshauer S., Beckwith J. The nucleotide sequence of the gene for malF protein, an inner membrane component of the maltose transport system of Escherichia coli. Repeated DNA sequences are found in the malE-malF intercistronic region. J Biol Chem. 1984 Sep 10;259(17):10896–10903. [PubMed] [Google Scholar]
- Garrett S., Taylor R. K., Silhavy T. J., Berman M. L. Isolation and characterization of delta ompB strains of Escherichia coli by a general method based on gene fusions. J Bacteriol. 1985 May;162(2):840–844. doi: 10.1128/jb.162.2.840-844.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germino J., Bastia D. Interaction of the plasmid R6K-encoded replication initiator protein with its binding sites on DNA. Cell. 1983 Aug;34(1):125–134. doi: 10.1016/0092-8674(83)90142-3. [DOI] [PubMed] [Google Scholar]
- Germino J., Bastia D. Rapid purification of a cloned gene product by genetic fusion and site-specific proteolysis. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4692–4696. doi: 10.1073/pnas.81.15.4692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germino J., Bastia D. The replication initiator protein of plasmid R6K tagged with beta-galactosidase shows sequence-specific DNA-binding. Cell. 1983 Jan;32(1):131–140. doi: 10.1016/0092-8674(83)90503-2. [DOI] [PubMed] [Google Scholar]
- Germino J., Gray J. G., Charbonneau H., Vanaman T., Bastia D. Use of gene fusions and protein-protein interaction in the isolation of a biologically active regulatory protein: the replication initiator protein of plasmid R6K. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6848–6852. doi: 10.1073/pnas.80.22.6848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray M. R., Colot H. V., Guarente L., Rosbash M. Open reading frame cloning: identification, cloning, and expression of open reading frame DNA. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6598–6602. doi: 10.1073/pnas.79.21.6598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronenborn B., Messing J. Methylation of single-stranded DNA in vitro introduces new restriction endonuclease cleavage sites. Nature. 1978 Mar 23;272(5651):375–377. doi: 10.1038/272375a0. [DOI] [PubMed] [Google Scholar]
- Guan C. D., Wanner B., Inouye H. Analysis of regulation of phoB expression using a phoB-cat fusion. J Bacteriol. 1983 Nov;156(2):710–717. doi: 10.1128/jb.156.2.710-717.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarente L. P., Beckwth J., Wu A. M., Platt T. A mutation distal to the messenger RNA endpoint reduces transcription termination in the tryptophan operon in Escherichia coli. J Mol Biol. 1979 Sep 5;133(1):189–197. doi: 10.1016/0022-2836(79)90258-4. [DOI] [PubMed] [Google Scholar]
- Guarente L. P., Mitchell D. H., Beckwith J. Transcription termination at the end of the tryptophan operon of Escherichia coli. J Mol Biol. 1977 May 25;112(3):423–436. doi: 10.1016/s0022-2836(77)80190-3. [DOI] [PubMed] [Google Scholar]
- Guarente L., Lauer G., Roberts T. M., Ptashne M. Improved methods for maximizing expression of a cloned gene: a bacterium that synthesizes rabbit beta-globin. Cell. 1980 Jun;20(2):543–553. doi: 10.1016/0092-8674(80)90640-6. [DOI] [PubMed] [Google Scholar]
- Guarente L., Mason T. Heme regulates transcription of the CYC1 gene of S. cerevisiae via an upstream activation site. Cell. 1983 Apr;32(4):1279–1286. doi: 10.1016/0092-8674(83)90309-4. [DOI] [PubMed] [Google Scholar]
- Guarente L., Ptashne M. Fusion of Escherichia coli lacZ to the cytochrome c gene of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2199–2203. doi: 10.1073/pnas.78.4.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez C., Raibaud O. Point mutations that reduce the expression of malPQ, a positively controlled operon of Escherichia coli. J Mol Biol. 1984 Jul 25;177(1):69–86. doi: 10.1016/0022-2836(84)90058-5. [DOI] [PubMed] [Google Scholar]
- Hall M. N., Gabay J., Débarbouillé M., Schwartz M. A role for mRNA secondary structure in the control of translation initiation. Nature. 1982 Feb 18;295(5850):616–618. doi: 10.1038/295616a0. [DOI] [PubMed] [Google Scholar]
- Hall M. N., Hereford L., Herskowitz I. Targeting of E. coli beta-galactosidase to the nucleus in yeast. Cell. 1984 Apr;36(4):1057–1065. doi: 10.1016/0092-8674(84)90055-2. [DOI] [PubMed] [Google Scholar]
- Hall M. N., Schwartz M., Silhavy T. J. Sequence information within the lamB genes in required for proper routing of the bacteriophage lambda receptor protein to the outer membrane of Escherichia coli K-12. J Mol Biol. 1982 Mar 25;156(1):93–112. doi: 10.1016/0022-2836(82)90461-2. [DOI] [PubMed] [Google Scholar]
- Heidecker G., Müller-Hill B. Synthetic multifunctional proteins: isolation of covalently linked tryptophan synthetase alpha-subunit-lac-repressor-beta-galactosidase chimeras. Mol Gen Genet. 1977 Oct 24;155(3):301–307. doi: 10.1007/BF00272809. [DOI] [PubMed] [Google Scholar]
- Hoffman C. S., Wright A. Fusions of secreted proteins to alkaline phosphatase: an approach for studying protein secretion. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5107–5111. doi: 10.1073/pnas.82.15.5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins J. D. A new class of promoter mutations in the lactose operon of Escherichia coli. J Mol Biol. 1974 Aug 25;87(4):715–724. doi: 10.1016/0022-2836(74)90080-1. [DOI] [PubMed] [Google Scholar]
- Huisman O., D'Ari R. Effect of suppressors of SOS-mediated filamentation on sfiA operon expression in Escherichia coli. J Bacteriol. 1983 Jan;153(1):169–175. doi: 10.1128/jb.153.1.169-175.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kania J., Müller-Hill B. Construction, isolation and implications of repressor-galactosidase - beta-galactosidase hybrid molecules. Eur J Biochem. 1977 Oct 3;79(2):381–386. doi: 10.1111/j.1432-1033.1977.tb11819.x. [DOI] [PubMed] [Google Scholar]
- Kenyon C. J., Walker G. C. DNA-damaging agents stimulate gene expression at specific loci in Escherichia coli. Proc Natl Acad Sci U S A. 1980 May;77(5):2819–2823. doi: 10.1073/pnas.77.5.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N., Roth J., Botstein D. Genetic engineering in vivo using translocatable drug-resistance elements. New methods in bacterial genetics. J Mol Biol. 1977 Oct 15;116(1):125–159. doi: 10.1016/0022-2836(77)90123-1. [DOI] [PubMed] [Google Scholar]
- Koenen M., Rüther U., Müller-Hill B. Immunoenzymatic detection of expressed gene fragments cloned in the lac Z gene of E. coli. EMBO J. 1982;1(4):509–512. doi: 10.1002/j.1460-2075.1982.tb01199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komeda Y., Iino T. Regulation of expression of the flagellin gene (hag) in Escherichia coli K-12: analysis of hag-lac gene fusions. J Bacteriol. 1979 Sep;139(3):721–729. doi: 10.1128/jb.139.3.721-729.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroos L., Kaiser D. Construction of Tn5 lac, a transposon that fuses lacZ expression to exogenous promoters, and its introduction into Myxococcus xanthus. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5816–5820. doi: 10.1073/pnas.81.18.5816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuritzkes D. R., Zhang X. Y., Lin E. C. Use of phi(glp-lac) in studies of respiratory regulation of the Escherichia coli anaerobic sn-glycerol-3-phosphate dehydrogenase genes (glpAB). J Bacteriol. 1984 Feb;157(2):591–598. doi: 10.1128/jb.157.2.591-598.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laimins L. A., Rhoads D. B., Epstein W. Osmotic control of kdp operon expression in Escherichia coli. Proc Natl Acad Sci U S A. 1981 Jan;78(1):464–468. doi: 10.1073/pnas.78.1.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leathers T. D., Noti J., Umbarger H. E. Physical characterization of ilv-lac fusions. J Bacteriol. 1979 Oct;140(1):251–260. doi: 10.1128/jb.140.1.251-260.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lis J. T., Simon J. A., Sutton C. A. New heat shock puffs and beta-galactosidase activity resulting from transformation of Drosophila with an hsp70-lacZ hybrid gene. Cell. 1983 Dec;35(2 Pt 1):403–410. doi: 10.1016/0092-8674(83)90173-3. [DOI] [PubMed] [Google Scholar]
- MacNeil D. General method, using Mu-Mud1 dilysogens, to determine the direction of transcription of and generate deletions in the glnA region of Escherichia coli. J Bacteriol. 1981 Apr;146(1):260–268. doi: 10.1128/jb.146.1.260-268.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloy S. R., Roth J. R. Regulation of proline utilization in Salmonella typhimurium: characterization of put::Mu d(Ap, lac) operon fusions. J Bacteriol. 1983 May;154(2):561–568. doi: 10.1128/jb.154.2.561-568.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenney K., Shimatake H., Court D., Schmeissner U., Brady C., Rosenberg M. A system to study promoter and terminator signals recognized by Escherichia coli RNA polymerase. Gene Amplif Anal. 1981;2:383–415. [PubMed] [Google Scholar]
- Michaelis S., Beckwith J. Mechanism of incorporation of cell envelope proteins in Escherichia coli. Annu Rev Microbiol. 1982;36:435–465. doi: 10.1146/annurev.mi.36.100182.002251. [DOI] [PubMed] [Google Scholar]
- Michaelis S., Guarente L., Beckwith J. In vitro construction and characterization of phoA-lacZ gene fusions in Escherichia coli. J Bacteriol. 1983 Apr;154(1):356–365. doi: 10.1128/jb.154.1.356-365.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. H., Reznikoff W. S., Silverstone A. E., Ippen K., Signer E. R., Beckwith J. R. Fusions of the lac and trp Regions of the Escherichia coli Chromosome. J Bacteriol. 1970 Dec;104(3):1273–1279. doi: 10.1128/jb.104.3.1273-1279.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. L., Mekalanos J. J. Synthesis of cholera toxin is positively regulated at the transcriptional level by toxR. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3471–3475. doi: 10.1073/pnas.81.11.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell D. H., Reznikoff W. S., Beckwith J. R. Genetic fusions defining trp and lac operon regulatory elements. J Mol Biol. 1975 Apr 15;93(3):331–350. doi: 10.1016/0022-2836(75)90281-8. [DOI] [PubMed] [Google Scholar]
- Miura A., Krueger J. H., Itoh S., de Boer H. A., Nomura M. Growth-rate-dependent regulation of ribosome synthesis in E. coli: expression of the lacZ and galK genes fused to ribosomal promoters. Cell. 1981 Sep;25(3):773–782. doi: 10.1016/0092-8674(81)90185-9. [DOI] [PubMed] [Google Scholar]
- Mizuno T., Chou M. Y., Inouye M. A unique mechanism regulating gene expression: translational inhibition by a complementary RNA transcript (micRNA). Proc Natl Acad Sci U S A. 1984 Apr;81(7):1966–1970. doi: 10.1073/pnas.81.7.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Hill B., Kania J. Lac repressor can be fused to beta-galactosidase. Nature. 1974 Jun 7;249(457):561–563. doi: 10.1038/249561a0. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Wu H. C. Amino acid sequence homology among the major outer membrane proteins of Escherichia coli. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1048–1052. doi: 10.1073/pnas.81.4.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor M. B., Malamy M. H. A new insertion sequence, IS121, is found on the Mu dI1 (Ap lac) bacteriophage and the Escherichia coli K-12 chromosome. J Bacteriol. 1983 Nov;156(2):669–679. doi: 10.1128/jb.156.2.669-679.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver D. B., Beckwith J. E. coli mutant pleiotropically defective in the export of secreted proteins. Cell. 1981 Sep;25(3):765–772. doi: 10.1016/0092-8674(81)90184-7. [DOI] [PubMed] [Google Scholar]
- Oliver D. Protein secretion in Escherichia coli. Annu Rev Microbiol. 1985;39:615–648. doi: 10.1146/annurev.mi.39.100185.003151. [DOI] [PubMed] [Google Scholar]
- Oppenheim D. S., Yanofsky C. Translational coupling during expression of the tryptophan operon of Escherichia coli. Genetics. 1980 Aug;95(4):785–795. doi: 10.1093/genetics/95.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall L. L., Hardy S. J. Export of protein in bacteria. Microbiol Rev. 1984 Dec;48(4):290–298. doi: 10.1128/mr.48.4.290-298.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznikoff W. S., Miller J. H., Scaife J. G., Beckwith J. R. A mechanism for repressor action. J Mol Biol. 1969 Jul 14;43(1):201–213. doi: 10.1016/0022-2836(69)90089-8. [DOI] [PubMed] [Google Scholar]
- Rose M., Casadaban M. J., Botstein D. Yeast genes fused to beta-galactosidase in Escherichia coli can be expressed normally in yeast. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2460–2464. doi: 10.1073/pnas.78.4.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rymond B. C., Zitomer R. S., Schümperli D., Rosenberg M. The expression in yeast of the Escherichia coli galK gene on CYC1::galK fusion plasmids. Gene. 1983 Nov;25(2-3):249–262. doi: 10.1016/0378-1119(83)90229-9. [DOI] [PubMed] [Google Scholar]
- Saint-Girons I. New regulatory mutations affecting the expression of the threonine operon in Escherichia coli K-12. Mol Gen Genet. 1978 Jun 1;162(1):95–100. doi: 10.1007/BF00333855. [DOI] [PubMed] [Google Scholar]
- Sallus L., Haselbeck R. J., Nunn W. D. Regulation of fatty acid transport in Escherichia coli: analysis by operon fusion. J Bacteriol. 1983 Sep;155(3):1450–1454. doi: 10.1128/jb.155.3.1450-1454.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarthy A., Fowler A., Zabin I., Beckwith J. Use of gene fusions to determine a partial signal sequence of alkaline phosphatase. J Bacteriol. 1979 Sep;139(3):932–939. doi: 10.1128/jb.139.3.932-939.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarthy A., Michaelis S., Beckwith J. Deletion map of the Escherichia coli structural gene for alkaline phosphatase, phoA. J Bacteriol. 1981 Jan;145(1):288–292. doi: 10.1128/jb.145.1.288-292.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer I., Emr S., Gross C., Schekman R. Invertase signal and mature sequence substitutions that delay intercompartmental transport of active enzyme. J Cell Biol. 1985 May;100(5):1664–1675. doi: 10.1083/jcb.100.5.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A., McDonald G. A. Regulation of outer membrane protein synthesis in Escherichia coli K-12: deletion of ompC affects expression of the OmpF protein. J Bacteriol. 1984 Aug;159(2):555–563. doi: 10.1128/jb.159.2.555-563.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro J. A., Adhya S. L. The galactose operon of E. coli K-12. II. A deletion analysis of operon structure and polarity. Genetics. 1969 Jun;62(2):249–264. doi: 10.1093/genetics/62.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattuck-Eidens D. M., Kadner R. J. Exogenous induction of the Escherichia coli hexose phosphate transport system defined by uhp-lac operon fusions. J Bacteriol. 1981 Oct;148(1):203–209. doi: 10.1128/jb.148.1.203-209.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada K., Weisberg R. A., Gottesman M. E. Prophage lambda at unusual chromosomal locations. II. Mutations induced by bacteriophage lambda in Escherichia coli K12. J Mol Biol. 1973 Oct 25;80(2):297–314. doi: 10.1016/0022-2836(73)90174-5. [DOI] [PubMed] [Google Scholar]
- Shultz J., Silhavy T. J., Berman M. L., Fiil N., Emr S. D. A previously unidentified gene in the spc operon of Escherichia coli K12 specifies a component of the protein export machinery. Cell. 1982 Nov;31(1):227–235. doi: 10.1016/0092-8674(82)90422-6. [DOI] [PubMed] [Google Scholar]
- Shuman H. A., Silhavy T. J., Beckwith J. R. Labeling of proteins with beta-galactosidase by gene fusion. Identification of a cytoplasmic membrane component of the Escherichia coli maltose transport system. J Biol Chem. 1980 Jan 10;255(1):168–174. [PubMed] [Google Scholar]
- Shuman H. A., Silhavy T. J. Identification of the malK gene product. A peripheral membrane component of the Escherichia coli maltose transport system. J Biol Chem. 1981 Jan 25;256(2):560–562. [PubMed] [Google Scholar]
- Silhavy T. J., Beckwith J. Isolation and characterization of mutants of Escherichia coli K12 affected in protein localization. Methods Enzymol. 1983;97:11–40. doi: 10.1016/0076-6879(83)97115-x. [DOI] [PubMed] [Google Scholar]
- Silhavy T. J., Benson S. A., Emr S. D. Mechanisms of protein localization. Microbiol Rev. 1983 Sep;47(3):313–344. doi: 10.1128/mr.47.3.313-344.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silhavy T. J., Brickman E., Bassford P. J., Jr, Casadaban M. J., Shuman H. A., Schwartz V., Guarente L., Schwartz M., Beckwith J. R. Structure of the malB region in Escherichia coli K12. II. Genetic map of the malE,F,G operon. Mol Gen Genet. 1979 Jul 24;174(3):249–259. doi: 10.1007/BF00267797. [DOI] [PubMed] [Google Scholar]
- Silver P. A., Keegan L. P., Ptashne M. Amino terminus of the yeast GAL4 gene product is sufficient for nuclear localization. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5951–5955. doi: 10.1073/pnas.81.19.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons R. W., Kleckner N. Translational control of IS10 transposition. Cell. 1983 Sep;34(2):683–691. doi: 10.1016/0092-8674(83)90401-4. [DOI] [PubMed] [Google Scholar]
- Smith T. F., Sadler J. R. The nature of lactose operator constitive mutations. J Mol Biol. 1971 Jul 28;59(2):273–305. doi: 10.1016/0022-2836(71)90051-9. [DOI] [PubMed] [Google Scholar]
- Sodergren E. J., Davidson J., Taylor R. K., Silhavy T. J. Selection for mutants altered in the expression or export of outer membrane porin OmpF. J Bacteriol. 1985 Jun;162(3):1047–1053. doi: 10.1128/jb.162.3.1047-1053.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. K., Garrett S., Sodergren E., Silhavy T. J. Mutations that define the promoter of ompF, a gene specifying a major outer membrane porin protein. J Bacteriol. 1985 Jun;162(3):1054–1060. doi: 10.1128/jb.162.3.1054-1060.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. K., Hall M. N., Enquist L., Silhavy T. J. Identification of OmpR: a positive regulatory protein controlling expression of the major outer membrane matrix porin proteins of Escherichia coli K-12. J Bacteriol. 1981 Jul;147(1):255–258. doi: 10.1128/jb.147.1.255-258.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiedeman A. A., Smith J. M. Isolation and characterization of regulatory mutations affecting the expression of the guaBA operon of Escherichia coli K-12. Mol Gen Genet. 1984;195(1-2):77–82. doi: 10.1007/BF00332727. [DOI] [PubMed] [Google Scholar]
- Van den Broeck G., Timko M. P., Kausch A. P., Cashmore A. R., Van Montagu M., Herrera-Estrella L. Targeting of a foreign protein to chloroplasts by fusion to the transit peptide from the small subunit of ribulose 1,5-bisphosphate carboxylase. 1985 Jan 31-Feb 6Nature. 313(6001):358–363. doi: 10.1038/313358a0. [DOI] [PubMed] [Google Scholar]
- Wanner B. L., McSharry R. Phosphate-controlled gene expression in Escherichia coli K12 using Mudl-directed lacZ fusions. J Mol Biol. 1982 Jul 5;158(3):347–363. doi: 10.1016/0022-2836(82)90202-9. [DOI] [PubMed] [Google Scholar]
- Wanner B. L., Wieder S., McSharry R. Use of bacteriophage transposon Mu d1 to determine the orientation for three proC-linked phosphate-starvation-inducible (psi) genes in Escherichia coli K-12. J Bacteriol. 1981 Apr;146(1):93–101. doi: 10.1128/jb.146.1.93-101.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock G. M., ap Rhys C., Berman M. L., Hampar B., Jackson D., Silhavy T. J., Weisemann J., Zweig M. Open reading frame expression vectors: a general method for antigen production in Escherichia coli using protein fusions to beta-galactosidase. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4432–4436. doi: 10.1073/pnas.80.14.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis J. H., Enquist L. W., Salstrom J. S., Watson R. J. An immunologically active chimaeric protein containing herpes simplex virus type 1 glycoprotein D. Nature. 1983 Mar 3;302(5903):72–74. doi: 10.1038/302072a0. [DOI] [PubMed] [Google Scholar]
- Weisemann J. M., Funk C., Weinstock G. M. Measurement of in vivo expression of the recA gene of Escherichia coli by using lacZ gene fusions. J Bacteriol. 1984 Oct;160(1):112–121. doi: 10.1128/jb.160.1.112-121.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisemann J. M., Weinstock G. M. Direct selection of mutations reducing transcription or translation of the recA gene of Escherichia coli with a recA-lacZ protein fusion. J Bacteriol. 1985 Aug;163(2):748–755. doi: 10.1128/jb.163.2.748-755.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngman P., Zuber P., Perkins J. B., Sandman K., Igo M., Losick R. New ways to study developmental genes in spore-forming bacteria. Science. 1985 Apr 19;228(4697):285–291. doi: 10.1126/science.228.4697.285. [DOI] [PubMed] [Google Scholar]
- Zabeau M., Stanley K. K. Enhanced expression of cro-beta-galactosidase fusion proteins under the control of the PR promoter of bacteriophage lambda. EMBO J. 1982;1(10):1217–1224. doi: 10.1002/j.1460-2075.1982.tb00016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabin I., Fowler A. V., Beckwith J. R. Position of the mutation in beta-galactosidase ochre mutant U118. J Bacteriol. 1978 Jan;133(1):437–438. doi: 10.1128/jb.133.1.437-438.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabin I. beta-Galactosidase alpha-complementation. A model of protein-protein interaction. Mol Cell Biochem. 1982 Nov 26;49(2):87–96. doi: 10.1007/BF00242487. [DOI] [PubMed] [Google Scholar]


