Abstract
Multiple factors affect the structural development of the skeleton; in particular, estrogen levels during growth are an important factor in the pathogenesis of bone fragility. The delay of menarche and infrequent menstrual cycles decrease estrogen levels during adolescence and decrease peak bone mass. The aim of this study was to determine if delayed puberty through administration of a GnRH antagonist initiated prior to the onset of the first estrus cycle would delay the increase in estrogen levels and impede bone strength development in female rats. Twenty-three-day-old female Sprague–Dawley rats were randomly assigned to one of four groups; 1) short-term control group (C-ST) (n = 12), 2) long-term control (C-LT) (n = 12), 3) short-term GnRH antagonist group (G-ST) (n = 12) and 4) long-term GnRH antagonist group (G-LT) (n = 12). Injections (0.2 ml) of either saline or GnRH antagonist (100 μg/day) (Cetrotide™, Serono, Inc) were given intraperitoneally for a duration of 18 days. Pubertal and gonadal development was retarded as indicated by a delay in vaginal opening (an indicator of pubertal onset), lower ovarian and uterine weights and lower estradiol levels in the short-term experimental animals (G-ST). However, at maturity (G-LT), there were no significant differences found in these measures. A delay in the timing of puberty significantly attenuated the development of femoral bone strength at 6 weeks of age. Peak moment, yield moment and stiffness in the G-ST group were all significantly less than the C-ST group. Cortical width was significantly attenuated due to the increased percentage of marrow area per total bone area in the G-ST group. However, femoral bone strength was recovered at maturity (G-LT). In summary, a transient delay in pubertal timing has short-term effects on bone strength development. In the current animal model of delaying puberty through GnRH antagonist injections, there appears to be no long-term effects on bone strength.
Keywords: Delayed puberty, Estrogen, Bone adaptation, Bone strength, Rat model
Introduction
Osteoporosis, once thought to be a natural part of aging among women, is no longer considered age- or gender-dependent. Low bone mass in young women has been associated with an increased incidence of stress fractures that may also increase their risk of osteoporotic fractures later in life. Therefore, factors during the developmental years may have a crucial effect on the accrual of bone mass and the risk of fracture. Optimizing peak bone mass during late adolescence may be effective in reducing the effects of osteoporosis.
Multiple factors affect the structural development of the skeleton; in particular, estrogen levels during growth are an important factor in the pathogenesis of bone fragility [1]. The delay of menarche and infrequent menstrual cycles decrease estrogen levels during adolescence and decrease peak bone mass [2–4]. Investigators have identified bone densities in young (17–35 years) athletic women with decreased estrogen levels similar to the bone densities of 51-year-old women [2,5]. On the structural level, cortical width is established during puberty in females by endosteal apposition. Low estrogen levels during growth may result in a thinner cortex if the increased endosteal resorption [6] is not offset by an increased periosteal apposition [7]. The relative cellular activity on these bone surfaces affects bone size, a critical element of bone strength.
The timing of puberty has emerged as a crucial factor in bone strength development. Peak bone mineral accrual rate occurs at puberty [8], with an accrual of 26% of adult total bone mineral within 2 years [9]. However, a delay in the timing of puberty is one factor among many that correlate with low bone mass in young women [10–12]. Warren et al. (2002) [13] found the age of menarche to be more correlated to stress fracture occurrence than bone mineral density (BMD). Yet, the effect of a delayed puberty on the development of peak bone strength remains unclear.
The purpose of this study was to investigate the effect of a delay in puberty on bone strength immediately post-puberty and at maturity in female rats. This model offers an opportunity to reproduce an environment of delayed puberty and to investigate the effect on bone strength at a critical time point in bone development. The investigative hypothesis predicts that administration of a GnRH antagonist prior to the onset of the first estrus cycle would suppress the increase in estrogen levels associated with the onset of puberty and impede the development of cortical bone strength in female rat femurs (6 weeks) and the peak bone strength at maturity (6 months).
Materials and methods
Forty-eight female Sprague–Dawley rats (23 days-of-age) (Charles Rivers Laboratories, Wilmington, MA, USA) were housed 3 per cage and provided standard rat chow and water ad libitum. The animals were housed in a 12 h light–dark cycle. All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at Brooklyn College (City University of New York).
The animals were randomly assigned to one of four groups; 1) short-term control group (C-ST) (n = 12), 2) long-term control (C-LT) (n = 12), 3) short-term GnRH antagonist group (G-ST) (n = 12) and 4) long-term GnRH antagonist group (G-LT) (n = 12). At 25 days-of-age, daily injections of a gonadotropin-releasing hormone antagonist (GnRH-a) (Cetrotide™, Serono, Inc.) were used to delay the onset of puberty. Gonadotropin-releasing hormone antagonists (GnRH-a) have successfully delayed the onset of puberty in female rats and have the advantage that normal hypothalamic–pituitary function is restored after cessation of injections [14]. Injections (0.2 ml) of either saline or the GnRH-a (100 μg/day) (Cetrotide™, Serono, Inc.) were given intraperitoneally. Both short-term and long-term groups received the GnRH-a for a duration of 18 days (day 25–42). However, the short-term groups were sacrificed after the last injection (day 43) and the long-term groups at 6 months of age. All animals were sacrificed during the proestrus phase of their cycle as determined by cytology of vaginal smears. The proestrus phase is predominated by cells with a very high nuclear to cytoplasm ratio. The 5 short-term animals that did not reach puberty as determined by vaginal opening during the injection period were sacrificed on day 43.
All animals were monitored daily for vaginal opening, an indicator of pubertal onset, and vaginal swabs were taken to confirm the day of the first estrous cycle. Body weights were measured every 5 days during the 18-day injection period and weekly thereafter. On the day of sacrifice, animals were anesthetized by intraperitoneal injection of ketamine (80 mg/kg) and xylazine (16 mg/kg)). Blood was taken through cardiac puncture, after which the animals were killed by overdose of pentobarbital. Serum estradiol was measured using a radioimmunoassay (3rd Generation Estradiol RIA, DSL-39100, Diagnostic Systems Laboratories, Inc. Webster, TX, USA). Inter-assay coefficient of variation was less than 6%, and sensitivity was 0.6 pg/ml. After sacrifice, uterine and ovarian tissues were harvested and weighed. The right and left femurs were removed and cleaned of soft tissue. Right femurs were tested for mechanical strength, and left femurs were processed for histomorphometric analysis.
Histomorphometry and bone geometry
Left femurs were fixed in 10% buffered formalin and embedded in methyl methacrylate with 15% dibutyl phthalate. Undecalcified cross-sections (200 μm thickness) were cut at the mid-diaphysis using an Isomet 1000 precision saw with a diamond wafering blade (Buehler, Lake Bluff, IL. USA). The slices were mounted on white acrylic slides and hand-polished to a final thickness of 100 μm (Personal Communication: Damien Laudier/Mount Sinai School of Medicine). The slides were then stained with von Kossa method [15] and cover-slipped for histomorphometric analysis.
Cortical bone changes were assessed using bright field microscopy (magnification 10×). Histomorphometry was performed using the OsteoMeasure system (Osteometrics, Atlanta, GA, USA) following standard measures described by Parfitt et al. (1987) [16]. The structural (static) properties measured included total subperiosteal area (T.Ar; mm2), marrow area (Ma.Ar; mm2), cortical area (Ct.Ar = [T.Ar−Ma.Ar]; mm2), periosteal perimeter (Ps.Pm; mm) and endosteal perimeter (Ec.Pm; mm). All measurements were made by a single observer who was blinded to the specimen identity. The mean polar moment of inertia (Jo; mm4), medial–lateral moment of inertia (Iml; mm4) and cortical width (Ct.Wi; μm) were measured from mid-diaphysis femoral cross-sections using image processing software (SCION Image, Scion Corp., Frederick, MD, USA). Magnified sections were imaged directly with a camera mounted on a microscope (Nikon Eclipse E400, Morrell, Melville, NY, USA) and connected to a computer (Dell, TX, USA).
Mechanical testing
Breaking strength of each femur was measured under 3-point bending using a materials testing machine (Instron, Canton, MA, USA) fitted with a 1000 N load cell. Femurs were placed on the loading fixture anterior side down and loaded in the anterior–posterior plane. Due to the difference in femoral length between the short-term and long-term animals and in order to minimize the effect of shear loading, the distance between the lower support points was maximized for each group: 12 mm for the short-term animals and 16 mm for the long-term animals. Prior to testing, the right femurs were thawed in saline at room temperature to ensure hydration. The femurs were loaded to failure at a rate of 0.05 mm/s, during which displacement and force were collected (100 Hz) and normalized for comparison between short-term and long-term groups. The force and displacement values were normalized using terms derived from engineering analysis of three-point bending [17]. Bending moments were calculated from the force data (M = FL/4) (N mm). Displacement data were divided by (L2/12) (mm/mm2), where L is the distance between the lower supports (short-term groups: 12 mm, long-term groups: 16 mm). Structural properties were then determined from the moment versus normalized displacement curves: peak moment (N mm), yield moment (N mm), stiffness (N mm2), post-yield displacement (mm/mm2), and energy to failure (N mm mm/mm2). The yield moment was calculated as the point where a 10% change in slope of the moment versus normalized displacement curve occurred [18]. Young’s Modulus (E) was calculated from structural and cross-sectional properties using the equation [19]: E = Stiffness/Iml.
Data analysis
A Student’s t test assessed differences between the control and experimental groups at a significance level of P <0.05 (Sigma Stat 3.0, SPSS Chicago, IL. USA) in both the short-term and long-term age groups. If the normality or equal variance assumptions were violated, Mann–Whitney Rank Sum Test assessed group differences (vaginal opening, estradiol, post-yield deformation (ST) and cortical width (LT)). Prior to statistical evaluations, the majority of outcome measures were found to scale with body weight and were normalized with a linear regression-based correction [20]. All variables with an R2 level greater than 0 were normalized to avoid choosing an arbitrary R2 value as a cut-off for normalization. The correction decreased the variability in the data. Results are presented as mean (SD) values.
Results
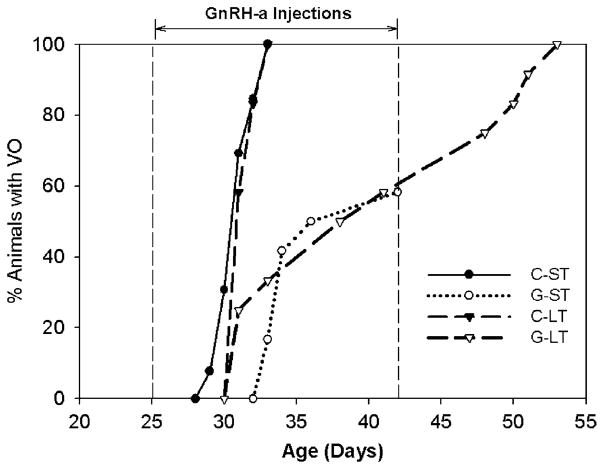
There were no differences in body weights between the groups at sacrifice in both the short-term and at maturity (Table 1). There was no effect of the GnRH-a injections on femoral length, however, the femurs were 25% longer at maturity compared to day 42 (Table 1). Eighteen days of GnRH-a injections resulted in a significant delay in the timing of vaginal opening. Pubertal onset was delayed an average of 8 days (2 estrus cycles) in the short-term GnRH-a (G-ST) group and 10 days (2.5 estrus cycles) in the long-term (G-LT) group (Fig. 1, Table 1). (Five animals in the G-ST group never had a vaginal opening, therefore 42 days was the date of vaginal opening for statistical analysis.) The delay in pubertal development was also confirmed by decreased uterine weights (74%) and ovarian weights (77%) in the G-ST group. The uterine and ovarian weights were normal in the G-LT group (Table 1).
Table 1.
Summary of group differences in uterine and ovarian weights, day of vaginal opening (VO), femur length and body weights at sacrifice in both short-term and long-term groups
| Parameters | Groups
|
|||
|---|---|---|---|---|
| C-ST | G-ST | C-LT | G-LT | |
| Uterine weight (g) | 0.608 ± 0.105 | 0.156 ± 0.059 a | 0.676 ± 0.090 | 0.654 ± 0.082 |
| Ovary weight (g) | 0.235 ± 0.227 | 0.053 ± 0.030 a | 0.165 ± 0.027 | 0.167 ± 0.053 |
| VO (day) | 31.1 ± 1.2 | 38 ± 4.2 b | 31.6 ± 0.8 | 41.1 ± 8.6 c |
| Femur length (mm) | 27.2 ± 0.4 | 27.0 ± 0.7 | 33.8 ± 1.2 | 34.4 ± 1.1 |
| Body weight at sacrifice (g) | 183.8 ± 16.3 | 195.1 ± 17.0 | 337.6 ± 27.9 | 366.2 ± 57.7 |
Mean (SD).
P < 0.001 versus C-ST.
P < 0.05 versus C-ST.
P < 0.05 versus C-LT.
Fig. 1.
Comparison of the timing of vaginal opening (VO) of the short-term and long-term groups. The cumulative percent of animals with VO is displayed per age (days). The G-ST and G-LT have a lower percentage of animals with VO at later ages. The C-ST and C-LT groups reached 100% VO by day 33. Five animals in the G-ST group did not have a vaginal opening by sacrifice (day 42).
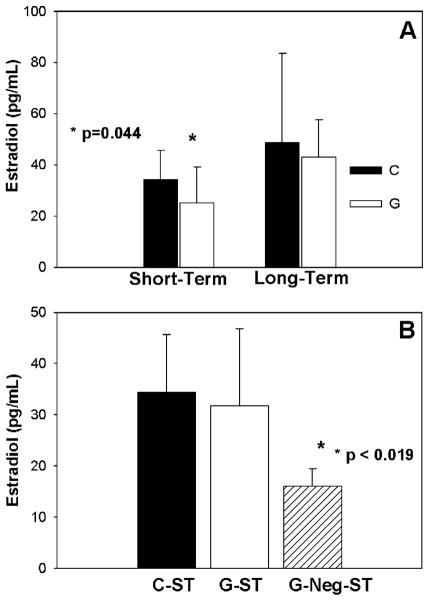
Estrogen levels were 27% lower after the GnRH-a injections in the G-ST group (P = 0.044) (Fig. 2A). Specifically, in those 5 animals that did not reach puberty at sacrifice (42 days of age) (G-Neg-ST), estradiol levels were 53% lower compared to C-ST (Fig. 2B). Estradiol levels recovered at 6 months of age in the long-term groups after the cessation of the injections at 42 days of age (Fig. 2A).
Fig. 2.
(A) Estradiol levels (pg/ml) were lower in the short-term GnRH antagonist (G-ST) group (white bar) at 6 weeks of age. Estradiol levels were not significantly different at maturity (long term). (B) Estradiol levels were predominately lower in the G-Neg-ST group (lined bar). G-Neg-ST represents the 5 animals in the G-ST group that did not reach puberty by the end of the 42-day experimental period.
After the 18 days of GnRH-a injections and the lower estrogen levels, there was a deficit in the mechanical strength of the cortical diaphysis in the G-ST group (Table 2). Specifically, the peak moment (N mm) of the G-ST group was 15% lower, and the stiffness (N mm2) was 22% lower than the C-ST group. The yield moment (N mm) was 21% lower than the C-ST group, but there was a trend (P = 0.109) towards an increase in the post-yield displacement in the G-ST group (Table 2). This trend remained at maturity, the post-yield displacement of the G-LT group was 69% greater than the C-LT group (P = 0.057). The energy at failure was also greater in the G-LT group compared to the C-LT group (36%, P = 0.074). However, the peak moment, yield moment and stiffness values recovered in the G-LT group at maturity (6 months of age) (Table 2).
Table 2.
Whole bone mechanical parameters measured from three-point bending of the femoral diaphysis for the short-term and long-term groups
| Parameters | Groups
|
|||
|---|---|---|---|---|
| C-ST | G-ST | C-LT | G-LT | |
| Peak moment (N mm) | 247.2 ± 20.3 | 210.8 ± 32.1 a | 746.3 ± 66.0 | 768.2 ± 73.5 |
| Yield moment (N mm) | 210.7 ± 28.7 | 166.1 ± 39.7 a | 673.7 ± 47.3 | 687.2 ± 89.8 |
| Stiffness (N mm 2) | 8473.7 ± 1300.9 | 6569.6 ± 1046.9 a | 50,226.0 ± 6259.6 | 51,346.8 ± 9295.1 |
| Post-yield displacement (mm/mm2) | 0.0198 ± 0.0143 | 0.0322 ± 0.0213 b | 0.0084 ± 0.0071 | 0.0142 ± 0.0069 c |
| Energy (N mm mm/mm2) | 7.99 ± 2.61 | 8.57 ± 2.04 | 12.29 ± 5.09 | 16.66 ± 6.06 d |
| Young’s Modulus (GPa) | 2.96 ± 0.53 | 2.34 ± 0.66 a | 10.19 ± 2.91 | 9.70 ± 2.79 |
Mean (SD).
P < 0.05 versus C-ST.
P = 0.109 versus C-ST.
P = 0.057 versus C-LT.
P = 0.074 versus C-LT.
The cortical width (Ct.Wi, mm) was thinner in the G-ST group with a trend towards an increase in marrow area (Ma. Ar, mm2) (P = 0.085) compared to C-ST group (Table 3). The ratio of Ma.Ar/T.Ar (%) was significantly larger in the G-ST group, however, this increase in marrow area in relation to the total bone area was not maintained at maturity (6 months of age). There were no significant differences in the polar moment of inertia (J) or the medial–lateral moment of inertia (Iml) between the groups. Young’s modulus (E) was significantly lower (21%) in the G-ST group compared to C-ST, however, there was no difference at maturity.
Table 3.
Static histomorphometry measures at the femoral diaphysis for the short-term and long-term groups
| Parameters | Groups
|
|||
|---|---|---|---|---|
| C-ST | G-ST | C-LT | G-LT | |
| Ct.Wi (mm) | 0.5846 ± 0.0538 | 0.5358 ± 0.0633 a | 0.7398 ± 0.0724 | 0.7523 ± 0.0451 |
| Ma.Ar (mm2) | 3.0315 ± 0.4359 | 3.3867 ± 0.5483 b | 3.3374 ± 0.5139 | 3.4316 ± 0.3689 |
| T.Ar (mm2) | 7.7063 ± 0.6693 | 7.7645 ± 0.6156 | 9.8533 ± 1.0730 | 10.1551 ±0.9188 |
| Ma.Ar/T.Ar (%) | 39.3 ± 4.2 | 43.5 ± 5.7 a | 33.9 ± 3.9 | 33.8 ± 2.0 |
| Ct.Ar (mm2) | 4.6748 ± 0.4785 | 4.3781 ± 0.5084 | 6.5096 ± 0.8137 | 6.7235 ± 0.6475 |
| Ec.Pm (mm) | 6.4973 ± 0.5708 | 6.8865 ± 0.6589 | 6.7666 ± 0.9990 | 6.9656 ± 0.3768 |
| Ps.Pm (mm) | 10.457 ± 0.7879 | 10.378 ± 0.4769 | 11.735 ± 0.7887 | 11.808 ± 0.7408 |
| J (mm4) | 8.402 ± 1.390 | 8.090 ± 1.088 | 15.725 ± 3.873 | 15.569 ± 3.516 |
| Iml (mm4) | 2.920 ± 0.526 | 2.889 ± 0.416 | 5.193 ± 1.157 | 5.242 ± 1.061 |
Mean (SD).
P < 0.05 versus C-ST.
P = 0.085 versus C-ST.
Discussion
A delay in the timing of puberty through GnRH antagonist injections administered to animals prior to the first estrus cycle significantly attenuated the development of femoral bone strength at 6 weeks of age. The short-term deficit in peak moment and stiffness may have resulted from a thinner cortex due to a larger marrow area per total area ratio in the G-ST group. The lower yield moment and the trend towards a greater post-yield displacement may be due to the material properties of the bone. Post-yield displacement has been inversely related to bone quality [18]. The calculated Young’s modulus (E) was significantly lower in the G-ST group, however, no differences existed at maturity. The increase in post-yield displacement was sustained into maturity, and the energy to failure was greater in the G-LT groups, however, these results are trends and warrant more investigation. Femoral bone strength including peak moment, yield moment and stiffness values recovered at maturity (6 months of age) in the current study. These data suggest that a suboptimal bone strength post-puberty does not affect peak bone strength at maturity. A study of prepubertal glucocorticoid induced low bone mass in rabbits reported a complete recovery in bone density and bone strength at maturity due to “catch-up growth” [21]. Even bone of lesser quality may not affect bone strength at maturity since the growing skeleton relies on modeling for bone formation not just remodeling [21]. The results of the current study reflect results reported in human studies. Delayed menarche has been correlated with low bone mass (BMD) [10–12] and an increased incidence of stress fractures [13]. Delayed pubertal onset in humans has been found to delay skeletal maturation, however, this was compensated by a late acceleration of linear growth [22]. Low estrogen levels are a hypothesized mechanism for altered bone strength. Estradiol levels were lower in the G-ST following the 18-day GnRH-a injection period, however, other mechanisms may be responsible for the deficit in bone strength. The GnRH-a injection model may be an effective model to investigate alternate mechanisms.
GnRH antagonist injections at a dosage of 100 μg/day retard pubertal and gonadal development as indicated by the delay in vaginal opening, lower ovarian and uterine weights and lower estradiol levels in the experimental animals in the current and in previous studies [14]. However, the GnRH antagonist injections did not completely suppress the onset of puberty in all animals. Fifty-eight percent of the GnRH-a animals had vaginal openings by day 42 while receiving a dosage of 100 μg/day of the GnRH antagonist (Cetrotide™), similar to the findings of Roth et al. (2000) [14] that report that 41% of the GnRH-a animals reached puberty by 37 days of age. The animals that did not reach puberty by day 42 had significantly lower estradiol levels compared to the other experimental animals (Fig. 2A). However, a comparison of both mechanical and cross-sectional variables did not reveal any differences in bone phenotype between the animals not reaching puberty (G-Neg-ST) compared to those who reached puberty prior to day 42 but still received GnRH-a injections (G-ST). The mechanism of the GnRH antagonist is likely to be a direct inhibition of pituitary gonadotropin secretion [14]. Therefore, a mechanism other than low estrogen levels may have an effect on bone strength development.
To our knowledge, only one other study investigated the effect of delayed puberty by GnRH antagonist injections on bone properties. Rakover et al. [23] reported a suppression of estradiol after 4 weeks of GnRH antagonist injections at a dosage of 125 μg/day. Femoral BMD, bone mineral content (BMC), trabecular density and cortical width were all lower after the 4-week injection period in the experimental group compared to the control group. However, Rakover et al. [23] did not measure bone strength and began the GnRH antagonist injections at 42 days of age which may have been after the onset of puberty. In our laboratory, the average day of vaginal opening is 31 days of age, suggesting that a delay in the onset of puberty was not achieved by Rakover et al. [23] but a suppression of estrogen levels post-puberty (secondary amenorrhea) was achieved.
During bone growth, the endocortical perimeter increases during prepuberty, and, at the onset of puberty, endocortical apposition occurs to thicken the cortex [1], therefore a delayed puberty may extend the resorptive prepubertal phase on the endocortical surface. The decreased cortical width due to the increased percentage of marrow area per total bone area in the current study suggests an increase or a lengthening of the duration of resorption activity at the endosteal surface. A study comparing the age at menarche and cortical bone geometry in humans also reported a significant increase in endosteal cortical perimeter [24]. Ovariectomy studies in young animals have reported similar increases in medullary area and increased endocortical bone resorption [6]. However, a significant increase in total area or periosteal perimeter in the G-ST group did not result from decreased estradiol levels as has been reported in previous studies [7]. The age at which estradiol levels are suppressed may have an effect on the resultant periosteal expansion. Further studies looking at the formation and resorption rates at the periosteal and endosteal surfaces during delayed puberty are needed. Estrogen levels also account for the cessation of longitudinal growth [1,25]. In the current study, no differences were found in femoral length as a result of GnRH-a injections. One study reported differences in femoral length 4 months post-ovariectomy [25], yet, at 3 weeks post-surgery, Baldock et al. [26] reported no differences in femur length. The dosage of GnRH-a and the duration of the injection period in the current study may have contributed to the lack of periosteal expansion and longitudinal growth.
Recent studies involving inbred mice strains have also linked the timing of puberty with the mechanical properties and morphology of bone [18,27]. Krewson et al. [27] reported that A/J mice had a significantly earlier age of vaginal opening compared to B6 (C57BL/6J) mice. The A/J strain had stiffer femurs, increased cortical thickness, smaller polar moment of inertia and less post-yield deformation compared to the B6 strain. These results are similar to the results of the C-ST group compared to the G-ST group in the current study; greater strength in control animals compared to delayed puberty animals. An interesting result of the current study was a complete recovery of the bone strength parameters after the injection protocol was ended, suggesting that modifications to bone structure initiated during puberty can be overcome by maturity. Compensation for a delay in pubertal growth by a late acceleration of linear growth has been found in elite athletes [22], however, other studies involving athletes suggest that bone mass cannot be regained after delayed puberty and amenorrhea during young adulthood [13,28]. Specifically, amenorrheic dancers receiving hormone replacement for 2 years had no increase in BMD compared to the placebo or control groups. Although a genetic component exists in the determination of the age of pubertal onset and the optimization of peak bone strength, what remains unclear is the interaction of lifestyle factors including exercise, nutrition and delayed puberty on these different phenotypes.
The effect of loading on bone development may also be affected by a delay in pubertal timing and lower estradiol levels. Decreased estrogen levels have been hypothesized to increase the minimum effective strain threshold increasing the strain level necessary to depress bone remodeling [29]. Local loading on bone affects local bone architecture, yet systemic influences may modify mechanically adaptive processes [30]. The increase in sensitivity of the skeleton to mechanical loading during early puberty has been attributed to the simultaneous elevation of estradiol [31]. Evidence suggests that estrogen receptors (ER) are involved with the effectiveness of the adaptation of bone to mechanical loading [32]. Mechanical strain had a similar effect on cellular response as increased exposure to estrogen [33]. A suppression of estradiol during a delay in puberty may yield bone less sensitive to loading, and thus the bone may be in a perceived disuse state. In the current study, there were no differences in the body weight between the experimental and control groups, suggesting that they experienced similar loading conditions. Therefore, another mechanism of suboptimal bone strength during delayed puberty may be a decreased sensitivity to external loading on the bone. However, further studies of loading protocols during the delayed puberty period are necessary.
In summary, the timing of puberty has short-term effects on bone strength development. In the current animal model of delaying puberty through GnRH antagonist injections, there appears to be no long-term effects on bone strength. This GnRH-a model of delayed puberty is not confounded by low body weight, nutritional factors or energy deficiency. The decreased estradiol levels were due to altered hypothalamic functioning that was not completely suppressed as would occur following ovariectomy surgery. GnRH antagonists (Cetrotide™) have successfully delayed the onset of puberty in female rats [14], and the withdrawal of GnRH antagonists restores normal hypothalamic–pituitary function, allowing control over estrogen levels over a finite period of time. Further investigation is necessary to better understand the multiple factors that contribute to the long-term suppression of bone strength that may lead to increased fracture risk in humans.
Acknowledgments
This study was funded by the National Institutes of Health (R15 AG19654-01A1) and The City University of New York PSC-CUNY Research Award Program (64293-00 33). The authors would like to thank Dr. Karl J. Jepsen, Dr. Mitchell B. Schaffler and Philip Nasser, M.S.M.E., M.S.E.E (The Leni and Peter W. May Department of Orthopaedics, Mount Sinai School of Medicine) for the use of the materials testing machine. Damien Laudier for his histological expertise. Dr. Matthew J. Silva and Michael D. Brodt, MS (Department of Orthopaedic Surgery, Washington University in St. Louis, School of Medicine) for their continued support.
References
- 1.Seeman E. Pathogenesis of bone fragility in women and men. Lancet. 2002;359:1841–50. doi: 10.1016/S0140-6736(02)08706-8. [DOI] [PubMed] [Google Scholar]
- 2.Drinkwater BL, Nilson K, Chesnut CH, III, Bremner WJ, Shainholtz S, Southworth MB. Bone mineral content of amenorrheic and eumenorrheic athletes. N Engl J Med. 1984;311:277–81. doi: 10.1056/NEJM198408023110501. [DOI] [PubMed] [Google Scholar]
- 3.Pettersson U, Stalnacke B, Ahlenius G, Henriksson-Larsen K, Lorentzon R. Low bone mass density at multiple skeletal sites, including the appendicular skeleton in amenorrheic runners. Calcif Tissue Int. 1999;64:117–25. doi: 10.1007/s002239900589. [DOI] [PubMed] [Google Scholar]
- 4.Warren MP, Brooks-Gunn J, Fox RP, Lancelot C, Newman D, Hamilton WG. Lack of bone accretion and amenorrhea: evidence for a relative osteopenia in weight-bearing bones. J Clin Endocrinol Metab. 1991;72:847–53. doi: 10.1210/jcem-72-4-847. [DOI] [PubMed] [Google Scholar]
- 5.Myerson M, Gutin B, Warren MP, Wang J, Lichtman S, Pierson RN., Jr Total body bone density in amenorrheic runners. Obstet Gynecol. 1992;79:973–8. [PubMed] [Google Scholar]
- 6.Turner RT, Vandersteenhoven JJ, Bell NH. The effects of ovariectomy and 17 beta-estradiol on cortical bone histomorphometry in growing rats. J Bone Miner Res. 1987;2:115–22. doi: 10.1002/jbmr.5650020206. [DOI] [PubMed] [Google Scholar]
- 7.Turner RT, Wakley GK, Hannon KS. Differential effects of androgens on cortical bone histomorphometry in gonadectomized male and female rats. J Orthop Res. 1990;8:612–7. doi: 10.1002/jor.1100080418. [DOI] [PubMed] [Google Scholar]
- 8.Bailey DA. The Saskatchewan Pediatric Bone Mineral Accrual Study: bone mineral acquisition during the growing years. Int J Sports Med. 1997;18(Suppl 3):S191–4. doi: 10.1055/s-2007-972713. [DOI] [PubMed] [Google Scholar]
- 9.Bailey DA, Martin AD, McKay HA, Whiting S, Mirwald R. Calcium accretion in girls and boys during puberty: a longitudinal analysis. J Bone Miner Res. 2000;15:2245–50. doi: 10.1359/jbmr.2000.15.11.2245. [DOI] [PubMed] [Google Scholar]
- 10.Anai T, Miyazaki F, Tomiyasu T, Matsuo T. Risk of irregular menstrual cycles and low peak bone mass during early adulthood associated with age at menarche. Pediatr Int. 2001;43:483–8. doi: 10.1046/j.1442-200x.2001.01442.x. [DOI] [PubMed] [Google Scholar]
- 11.Dhuper S, Warren MP, Brooks-Gunn J, Fox R. Effects of hormonal status on bone density in adolescent girls. J Clin Endocrinol Metab. 1990;71:1083–8. doi: 10.1210/jcem-71-5-1083. [DOI] [PubMed] [Google Scholar]
- 12.Valentino R, Savastano S, Tommaselli AP, D’Amore G, Dorato M, Lombardi G. The influence of intense ballet training on trabecular bone mass, hormone status, and gonadotropin structure in young women. J Clin Endocrinol Metab. 2001;86:4674–8. doi: 10.1210/jcem.86.10.7908. [DOI] [PubMed] [Google Scholar]
- 13.Warren MP, Brooks-Gunn J, Fox RP, Holderness CC, Hyle EP, Hamilton WG. Osteopenia in exercise-associated amenorrhea using ballet dancers as a model: a longitudinal study. J Clin Endocrinol Metab. 2002;87:3162–8. doi: 10.1210/jcem.87.7.8637. [DOI] [PubMed] [Google Scholar]
- 14.Roth C, Leonhardt S, Seidel C, Luft H, Wuttke W, Jarry H. Comparative analysis of different puberty inhibiting mechanisms of two GnRH agonists and the GnRH antagonist cetrorelix using a female rat model. Pediatr Res. 2000;48:468–74. doi: 10.1203/00006450-200010000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Carter DH, Barnes JM, Aaron JE. Histomorphometry of fresh frozen iliac crest bone biopsies. Calcif Tissue Int. 1989;44:387–92. doi: 10.1007/BF02555966. [DOI] [PubMed] [Google Scholar]
- 16.Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, et al. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 17.Turner CH, Burr DB. Basic biomechanical measurements of bone: a tutorial. Bone. 1993;14:595–608. doi: 10.1016/8756-3282(93)90081-k. [DOI] [PubMed] [Google Scholar]
- 18.Jepsen KJ, Akkus OJ, Majeska RJ, Nadeau JH. Hierarchical relationship between bone traits and mechanical properties in inbred mice. Mamm Genome. 2003;14:97–104. doi: 10.1007/s00335-002-3045-y. [DOI] [PubMed] [Google Scholar]
- 19.Brodt MD, Ellis CB, Silva MJ. Growing C57Bl/6 mice increase whole bone mechanical properties by increasing geometric and material properties. J Bone Miner Res. 1999;14:2159–66. doi: 10.1359/jbmr.1999.14.12.2159. [DOI] [PubMed] [Google Scholar]
- 20.Di Masso RJ, Font MT, Capozza RF, Detarsio G, Sosa F, Ferretti JL. Long-bone biomechanics in mice selected for body conformation. Bone. 1997;20:539–45. doi: 10.1016/s8756-3282(97)00055-0. [DOI] [PubMed] [Google Scholar]
- 21.Gafni RI, McCarthy EF, Hatcher T, Meyers JL, Inoue N, Reddy C, et al. Recovery from osteoporosis through skeletal growth: early bone mass acquisition has little effect on adult bone density. FASEB J. 2002;16:736–8. doi: 10.1096/fj.01-0640fje. [DOI] [PubMed] [Google Scholar]
- 22.Georgopoulos NA, Markou KB, Theodoropoulou A, Vagenakis GA, Benardot D, Leglise M, et al. Height velocity and skeletal maturation in elite female rhythmic gymnasts. J Clin Endocrinol Metab. 2001;86:5159–64. doi: 10.1210/jcem.86.11.8041. [DOI] [PubMed] [Google Scholar]
- 23.Rakover Y, Lu P, Briody JN, Tao C, Weiner E, Ederveen AG, et al. Effects of delaying puberty on bone mineralization in female rats. Hum Reprod. 2000;15:1457–61. doi: 10.1093/humrep/15.7.1457. [DOI] [PubMed] [Google Scholar]
- 24.Rauch F, Klein K, Allolio B, Schonau E. Age at menarche and cortical bone geometry in premenopausal women. Bone. 1999;25:69–73. doi: 10.1016/s8756-3282(99)00104-0. [DOI] [PubMed] [Google Scholar]
- 25.Zhang XZ, Kalu DN, Erbas B, Hopper JL, Seeman E. The effects of gonadectomy on bone size, mass, and volumetric density in growing rats are gender-, site-, and growth hormone-specific. J Bone Miner Res. 1999;14:802–9. doi: 10.1359/jbmr.1999.14.5.802. [DOI] [PubMed] [Google Scholar]
- 26.Baldock PA, Morris HA, Moore RJ, Need AG, Durbridge TC. Prepubertal oophorectomy limits the accumulation of cancellous bone in the femur of growing rats with long-term effects on metaphyseal bone architecture. Calcif Tissue Int. 1998;62:244–9. doi: 10.1007/s002239900424. [DOI] [PubMed] [Google Scholar]
- 27.Krewson TD, Supelak PJ, Hill AE, Singer JB, Lander ES, Nadeau JH, et al. Chromosomes 6 and 13 harbor genes that regulate pubertal timing in mouse chromosome substitution strains. Endocrinology. 2004;145:4447–51. doi: 10.1210/en.2004-0543. [DOI] [PubMed] [Google Scholar]
- 28.Warren MP, Brooks-Gunn J, Fox RP, Holderness CC, Hyle EP, Hamilton WG, et al. Persistent osteopenia in ballet dancers with amenorrhea and delayed menarche despite hormone therapy: a longitudinal study. Fertil Steril. 2003;80:398–404. doi: 10.1016/s0015-0282(03)00660-5. [DOI] [PubMed] [Google Scholar]
- 29.Frost HM. On the estrogen–bone relationship and postmenopausal bone loss: a new model. J Bone Miner Res. 1999;14:1473–7. doi: 10.1359/jbmr.1999.14.9.1473. [DOI] [PubMed] [Google Scholar]
- 30.Lanyon L, Skerry T. Postmenopausal osteoporosis as a failure of bone’s adaptation to functional loading: a hypothesis. J Bone Miner Res. 2001;16:1937–47. doi: 10.1359/jbmr.2001.16.11.1937. [DOI] [PubMed] [Google Scholar]
- 31.Khan K, McKay HA, Haapasalo H, Bennell KL, Forwood MR, Kannus P, et al. Does childhood and adolescence provide a unique opportunity for exercise to strengthen the skeleton? J Sci Med Sport. 2000;3:150–64. doi: 10.1016/s1440-2440(00)80077-8. [DOI] [PubMed] [Google Scholar]
- 32.Damien E, Price JS, Lanyon LE. The estrogen receptor’s involvement in osteoblasts’ adaptive response to mechanical strain. J Bone Miner Res. 1998;13:1275–82. doi: 10.1359/jbmr.1998.13.8.1275. [DOI] [PubMed] [Google Scholar]
- 33.Zaman G, Cheng MZ, Jessop HL, White R, Lanyon LE. Mechanical strain activates estrogen response elements in bone cells. Bone. 2000;27:233–9. doi: 10.1016/s8756-3282(00)00324-0. [DOI] [PubMed] [Google Scholar]